Abstract
Background
Peritoneal metastasis (PM) is the second most common site of recurrence in colon cancer (CC) patients and accounts for approximately one-third of all recurrences. Patients with T4 or intraperitoneal perforated colon cancers have an increased risk of developing PM, and since manifest PM is difficult to treat, high-risk patients should be offered prophylactic treatment. Here, we propose a study of adjuvant oxaliplatin administered as pressurized intraperitoneal aerosol chemotherapy (PIPAC OX) in patients with high-risk colon cancer (T4, perforated tumors, ovarian metastasis).
Methods
PIPAC-OPC3 CC is a non-randomized, non-blinded phase 2 cohort study designed to treat high-risk colon cancer patients with adjuvant PIPAC-directed therapy. Based on an expected 90 % peritoneal recurrence-free survival with adjuvant PIPAC against the estimated 75 % without, 60 patients are needed (α: 0.05, power: 0.8). Eligible patients will receive two PIPAC treatments with oxaliplatin (92 mg/m2) at 4–6 week intervals. During laparoscopy, the peritoneum is biopsied at two locations, and peritoneal lavage with 500 mL of saline and laparoscopic ultrasound is performed. The patients are screened for adverse medical events and surgery-related complications after each PIPAC procedure. After the second PIPAC procedure, the patients will be examined in the outpatient clinic and followed with CT scans 12, 24 and 36 months after resection. The primary outcome of the PIPAC-OPC3 CC trial is to evaluate if PIPAC-directed adjuvant therapy can reduce the risk of PM. Secondary outcomes include the number of conversions from positive to negative peritoneal lavage cytology after one PIPAC procedure, completion rate of two adjuvant PIPAC treatments, toxicity and complication rate and recurrence-free and overall survival rates after 1, 3 and 5 years.
Results
It is expected that PIPAC-directed adjuvant therapy can provide an absolute risk reduction of 15 % regarding the development of PM in high-risk colon cancer patients, and that this may result in increased survival rates. We expect that free intraperitoneal tumor cells (FITC) may be detected by peritoneal lavage performed just prior to the administration of PIPAC-directed therapy, and that this treatment may convert FITC-positive patients to a FITC-negative status.
Conclusions
This study may provide important knowledge to be used in designing additional studies on PIPAC in the adjuvant setting of other primary cancers.
Trial registration
ClinicalTrials.gov Identifier NCT03280511 (2017-09-12). European Clinical Trials Database (EudraCT) 2017-002637-37.
Keywords: adjuvant PIPAC, high-risk, colon cancer, resection, peritoneal metastasis.
Introduction
Despite curative intended surgery and perioperative chemotherapy in patients with colon cancer, relapse is often encountered, and across tumor stages recurrence is found in 18–26 % of the resected patients [1, 2]. Eighty-six percent of the recurrences are diagnosed within 3 years after resection and 36 % within the 1 year regardless of tumor stage [1]. The route of dissemination varies between different cancer types, but a common denominator is the risk of peritoneal metastasis (PM). PM is the second most common site of recurrence in colon cancer patients and accounts for 25–35 % of all recurrences [3, 4]. Patients with PM – both in the synchronous and in the metachronous setting – will often have a very short life expectancy [5] and traditionally, treatments have been nihilistic due to poor performance status and response rates, and most patients with peritoneal recurrence will only be treated with best supportive care [4]. During the last two decades, more aggressive treatment strategies have been implemented, including extensive surgery (cytoreductive surgery, CRS) followed by hyperthermic intraperitoneal chemotherapy (HIPEC) [6, 7]. However, CRS and HIPEC are only used in selected patients with limited PM.
High-risk factors for relapse with PM have been identified, and patients with T4 colon cancers have a risk of relapse with metachronous PM between 12 and 50 % [8, 9, 10, 11], while patients with intraperitoneally perforated colorectal cancers relapse with PM in 14–58 % of the cases [12, 13, 14]. The risk of metachronous PM in perforated or T4 colorectal cancers has been validated [15] and widely recognized in the ongoing European trials, where adjuvant HIPEC is being investigated to reduce the risk of metachronous PM [16] (www.clinicaltrials.gov: NCT02231086, NCT02965248, NCT02614534, NCT02974556, NCT01226394).
Free intraperitoneal tumor cells (FITCs) are perceived as a precursor of PM, and it is expected that patients with perforated/T4 colon cancer will have FITC prior to visible recurrence with PM. However, despite the reported impact on the risk of recurrence and poor survival data [17], the presence of FITC is not routinely investigated, and this may be due to the lack of treatment options regarding the eradication of free tumor cells. In a systematic review of resected patients with stage I-IV colorectal cancer, 13.7 % had FITC detected at perioperative peritoneal lavage. The rate of FITC increased with increasing T-stage [18] and FITC held a significantly increased risk of both local and overall recurrence, plus a significantly increased mortality [19].
Due to the above-mentioned rates of recurrences after resection of high-risk colon cancer and the limited treatment options, new treatment strategies are needed.
Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC)
PIPAC is a new drug delivery system that may be used in patients with manifest PM [20]. PIPAC is a safe procedure with a very low adverse event profile [21, 22, 23, 24, 25] and without any risk to healthcare personnel as long as certain safety precautions are used [25]. Preliminary clinical experience shows promising results in PIPAC-directed treatment of patients with PM from several different types of primary cancer including colorectal [20]. However, the potential use of PIPAC-directed therapy in the adjuvant setting (i. e. following R0 resection) has not been evaluated.
Detection of FITC
FITC is usually detected by analyzing smears of sediment from the peritoneal lavage fluid collected during a staging laparoscopy or during the course of surgery. As FITC is routinely investigated in especially Asian gastric cancer patients, the current experience on the detection of FITC arises mainly from studies in gastric cancer patients. A recent meta-analysis described the risk of peritoneal recurrence of gastric cancer, based on analysis of peritoneal lavage fluid [26]. In this study, peritoneal lavage cytology predicted peritoneal recurrence with a sensitivity of only 0.45, while the specificity was 0.92–0.98. By the detection of carcinoembryonic antigen (CEA) in the peritoneal lavage fluid, the sensitivity was 0.77 and the specificity 0.89, whereas the analysis of CEA mRNA raised the sensitivity to 0.87, with a specificity of 0.80 [26]. In a systematic review, peritoneal lavage cytology predicted the peritoneal recurrence of gastric cancer patients with a sensitivity of 0.11–0.80 and a specificity of 0.86–1.00 [27]. By analyzing the lavage fluid with immunoassays, immunohistochemistry or reverse transcriptase polymerase chain reaction (RT-PCR) mainly towards CEA, the sensitivity and specificity still varied significantly (sensitivity 0.23–1.00, specificity 0.81–0.98). Despite the variable results, the included studies confirmed a significantly reduced median overall survival in patients with FITC. However, there is no standard definition of the techniques or cut-off points used to detect FITC – neither in gastric/gastroesophageal junction (GEJ) cancer patients nor in colon cancer patients.
Aim
Primary and secondary outcomes
The primary outcome of this trial is the proportion of patients with peritoneal recurrence diagnosed with contrast enhanced CT of the thorax and abdomen 36 months after resection of their high-risk colon cancer. Secondary outcomes include (1) the number of conversions from positive to negative peritoneal lavage cytology after one PIPAC procedure, (2) completion rate of two adjuvant PIPAC treatments, (3) treatment-related toxicity and complication rate, (4) 1- and 2-year peritoneal recurrence-free survival, based on CT of the thorax and abdomen, (5) 1-, 2- and 3-year recurrence-free survival based on CT of the thorax and abdomen and (6) 1-, 3- and 5-year overall survival rate.
Methods
Recruitment process
Radically resected patients with colonic adenocarcinoma or signet ring cell carcinoma and high-risk tumors (perforated/pT4NanyM0 (UICC 8th edition)/pTanyNanyM1 with radically resected PM including ovarian metastases) are eligible for inclusion (Table 1). Prior to inclusion, the patients will be discussed at a dedicated PIPAC multi-disciplinary tumor conference (MDT). The patients will be treated according to international guidelines with 3 or 6 months adjuvant systemic chemotherapy, and then scheduled for adjuvant PIPAC OX 2–4 weeks after completion of the systemic chemotherapy. In selected cases, systemic chemotherapy is not an option (patient refusal, allergies etc.), and then the patients will be scheduled for PIPAC 2 months after resection.
Table 1:
Inclusion and exclusion criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
| – Radically resected patients with colonic adenocarcinoma or signet ring cell carcinoma with high-risk tumors defined as: perforated/pT4NanyM0 (UICC 8th edition)/pTanyNanyM1 with radically resected PM including ovarian metastases – Performance status 0–1 – Age>18 years – Fertile women must use contraceptives – Written informed consent |
– Radiologically or clinically proven relapse – Previous CRS with HIPEC – Other malignant diagnoses within the last 2 years – Contraindications to laparoscopy (e. g. severe adhesions, peritonitis) – A history of allergic reaction to oxaliplatin- or other platinum-containing compounds – Renal impairment, defined as GFR<50 mL/min, (Cockcroft-Gault Equation) |
| – Myocardial insufficiency, defined as NYHA class>2 | |
| – Impaired liver function defined as bilirubin ≥ 1.5×UNL (upper normal limit) | |
| – Inadequate hematological function defined as ANC ≤ 1.5 x 109/L and platelets ≤ 100×109/L | |
| – Any other condition or therapy that in the investigator’s opinion may pose a risk to the patient or interfere with the study objectives |
Study intervention and procedures
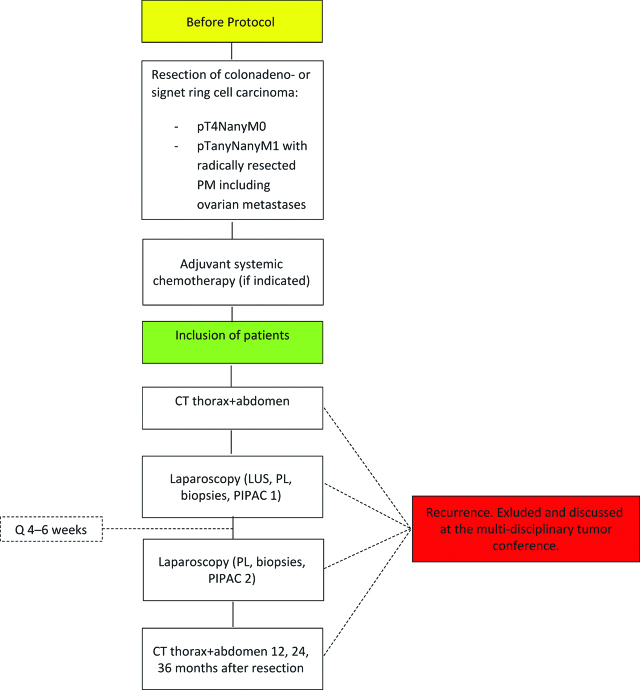
Included patients will be scheduled for two adjuvant PIPAC procedures with intervals of 4–6 weeks (Figure 1). When the patient is enrolled, a baseline CT of the thorax and abdomen is performed to rule out recurrence before intervention. A laparoscopic ultrasound (LUS) is also performed during the first PIPAC procedure to rule out any (abdominal) recurrence that may have been missed during baseline CT [28].
Figure 1:
Patient flow chart. LUS: laparoscopic ultrasound, PL: peritoneal lavage, PM: peritoneal metastasis.
Diagnostic laparoscopy
In general anesthesia and following prophylactic antibiotics, a diagnostic laparoscopy is performed through two standard trocars, where insufflation of normothermic CO2 maintains an intraabdominal pressure of 12 mmHg. Unexpected PM or suspicious lesions will be biopsied and sent for frozen section evaluation.
Peritoneal lavage (PL)
Five-hundred milliliters of saline will be administered into the peritoneal cavity through a standard irrigation and suction device with 100 mL delivered in the right subphrenic area, 100 mL in the right paracolic gutter, 100 mL in the left paracolic gutter, 100 mL in the epigastrium and 100 mL in the pelvis. The patient is then rotated to the anti-Trendelenburg position and 200 mL fluid is collected, and referred for further examination, while the remaining fluid will be collected and disposed. The fluid will be centrifuged, and smears of the sediment will be analyzed by conventional cytology (Papanicolaou and May–Giemsa Grünwald staining). Leftovers of the sediment are embedded in paraffin wax. If appropriate, as judged by the pathologist and based on the findings at conventional cytology, sections from the paraffin-embedded material will be used for immunocytochemical analyses for tumor markers, such as CEA, EpCAM, CDX2 and/or CK20 as well as markers for mesothelial cells, such as calretinin and vimentin, on 4 μm thick sections from the paraffin block. Moreover, mRNA from the sediment will be analyzed using RT-PCR for mRNA of markers such as CEA, CK20 and Ep-CAM. To minimize the risk of false-positive detection of FITC, positive peritoneal lavage cytology is crucial in the diagnosis of FITC. Still, if cytology with immunocytochemical analyses demonstrates atypical cells and mRNA CEA levels are increased, the patient is categorized as having FITC, since increased mRNA CEA levels alone lead to a significantly increased risk of developing PM [26].
Histology
Two peritoneal biopsies (<5 g) will be analyzed by a dedicated gastrointestinal pathologist, to rule out visually undetected malignancy. No protocol-specific analyses will be made, and if malignancy is detected, the tissue will be evaluated according to the department’s PIPAC guidelines, which are based on evaluation of the Peritoneal Regression Grading Score (PRGS), using H&E-stained slides and immunostaining of EpCAM [29]. If necessary, immunostaining for other markers will be performed, based on the judgement of the pathologist, which also is in accord with the department’s standard PIPAC guidelines.
PIPAC
PIPAC is performed through a CE-certified nebulizer (CapnoPen, Gothia Medical, Billdal, Sweden), using oxaliplatin at a dose of 92 mg/m2. The chemotherapy is installed at a rate of 30 mL/min with a maximum pressure of 200 pound-force per square inch and after 5 min, the chemotherapy has been delivered, and the injector is turned off. After an additional 25 min of simple diffusion, intraabdominal CO2 is evacuated in a closed system, and the patient is closed according to the department’s PIPAC guidelines. Dose and administration is based on available studies where PIPAC with oxaliplatin have been used for treating PM of colorectal origin. Dose modification can be made in case of adverse reactions (Table 2).
Table 2:
Dose modification during PIPAC therapy.
| Adverse reaction | Action |
|---|---|
| Grade 1 | No modification |
| Grade 2 | If the toxicity is tolerable for the patient, the planned treatment will continue. If the toxicity is poorly tolerated, the dose of oxaliplatin will be reduced to 75 %. |
| Grade 3 | The treatment will be postponed until the toxicity is below grade 2. After this, oxaliplatin can be given in a dose of 75 %. |
| Grade 4 | Stop treatment. Treatment can be reinstituted at the discretion of the responsible physician, if the adverse reaction falls below grade 2 |
Post-procedure monitoring
Post-operative monitoring and treatment of nausea, vomiting and pain will be according to departmental guidelines. The patient will be discharged the same day or the first day after the PIPAC procedure. After the first PIPAC procedure, the patient will be contacted by telephone after approximately 2 weeks, to screen for adverse reactions and give information on the cytology and histology and to plan the second PIPAC procedure. Adverse medical events will be graded according to the Common Terminology Criteria for Adverse Events (CTCAE, version 4.0) and surgery-related complications according to the Dindo-Clavien classification. The second PIPAC treatment will be given after 4–6 weeks. During this laparoscopy, biopsies and PL will be repeated. After two PIPAC procedures, the patients will be examined in the outpatient clinic, to screen for adverse reactions and give information on the cytology and histology. After this visit, the patients will be scheduled for follow-up with CT of the thorax and abdomen 12, 24 and 36 months after resection.
The patient file and radiology reports will be evaluated after 1, 3 and 5 years according to primary and secondary outcomes. The active treatment period is 2 months and then the patient will be followed for a total of 3 years, meaning that the last contact will be after the 36-month post-operative CT scan.
Study design
This is a non-randomized, non-blinded phase 2 cohort study designed to treat high-risk colon cancer patients with adjuvant PIPAC.
Ethical declarations
This study is GCP monitored and has been approved by the Regional Scientific Ethical Committees for Southern Denmark (IRB S-20170106) and the Danish Medicines Agency (case no 2017063403, EudraCT 2017-002637-37). Oral and written consent from participants are mandatory.
Statistics
Values are given as means or medians where appropriate. Categorical data will be specified with 95 % confidence intervals, comparisons will be performed using non-parametric tests, all p values are two-tailed and a p value of 0.05 will be considered statistically significant. Survival will be modelled in Kaplan–Meier analyses.
Results and discussion
Hypothesis and power calculation
As PIPAC seems to have an effect on visible PM in colon cancer patients, we hypothesize that PIPAC can minimize the risk of PM recurrence in resected high-risk colon cancer patients. Furthermore, we expect that PIPAC can eradicate FITC in these patients. An estimated 25 % of patients with a pT4 or perforated primary colon tumor are expected to develop PM. Based on the preliminary results of PIPAC, we expect an absolute risk reduction of 15 % in the adjuvant setting. To evaluate this difference (90 % peritoneal recurrence-free survival with adjuvant PIPAC against the estimated 75 % without) a total of 54 patients are needed (two-sided, α: 0.05, power: 0.8). With an expected dropout rate of 10 %, a total of 60 patients will be included.
Interim analysis
An interim analysis will be performed when 20 patients have completed both PIPAC treatments to test if PIPAC has been able to eradicate FITC detected by peritoneal lavage cytology and/or RT-PCR. If FITC is eradicated in at least one patient, it will be interpreted as a proof of efficacy and the study will continue. If there are no patients with FITC after the treatment of 20 patients, it will be evaluated whether the timing of intervention after resection or the methods of peritoneal lavage fluid analysis have to be changed. If FITC is detected, but eradication does not occur in at least one patient after treatment of 20 patients, the type or dosage of intraperitoneal chemotherapy will be changed or the study will be terminated.
Footnotes
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Study schedule: The trial started inclusion of patients in December 2017. Inclusion and treatment of the last patient is expected by March 2020. All data generated or analyzed during this study will be included in the published article(s).
Trial organization: The trial is organized by Odense PIPAC Center (OPC) which is a multi-disciplinary cancer group based at Odense University Hospital.
Research funding: This study was funded by Odense PIPAC Center (OPC) and Odense Pancreas Center (OPAC), Region of Southern Denmark, Odense University Hospital, 5000 Odense, Denmark.
Employment or leadership: None declared.
Honorarium: None declared.
Competing interests: The funding organization(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
Contributor Information
Martin Graversen, Email: martin.graversen@rsyd.dk.
Sönke Detlefsen, Email: sonke.detlefsen@rsyd.dk.
Claus Fristrup, Email: claus.wilki.fristrup@rsyd.dk.
Per Pfeiffer, Email: per.pfeiffer@rsyd.dk.
Michael Bau Mortensen, Email: michael.bau.mortensen@rsyd.dk.
References
- 1.Holmes AC, Riis AH, Erichsen R, Fedirko V, Ostenfeld EB, Vyberg M, et al. Descriptive characteristics of colon and rectal cancer recurrence in a Danish population-based study. Acta Oncol 2017;56:1111–9. [DOI] [PMC free article] [PubMed]
- 2.Van Gestel YR, De Hingh IH, Van Herk-Sukel MP, Van Erning FN, Beerepoot LV, Wijsman JH, et al. Patterns of metachronous metastases after curative treatment of colorectal cancer. Cancer Epidemiol 2014;38:448–54. [DOI] [PubMed]
- 3.Brodsky JT, Cohen AM. Peritoneal seeding following potentially curative resection of colonic carcinoma: implications for adjuvant therapy. Dis Colon Rectum 1991;34:723–7. [DOI] [PubMed]
- 4.Elferink MA, De Jong KP, Klaase JM, Siemerink EJ, De Wilt JH. Metachronous metastases from colorectal cancer: a population-based study in North-East Netherlands. Int J Colorectal Dis 2015;30:205–12. [DOI] [PubMed]
- 5.Sadeghi B, Arvieux C, Glehen O, Beaujard AC, Rivoire M, Baulieux J, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer 2000;88:358–63. [DOI] [PubMed]
- 6.Losa F, Barrios P, Salazar R, Torres-Melero J, Benavides M, Massuti T, et al. Cytoreductive surgery and intraperitoneal chemotherapy for treatment of peritoneal carcinomatosis from colorectal origin. Clin Transl Oncol 2014;16:128–40. [DOI] [PubMed]
- 7.Sugarbaker PH. Intraperitoneal chemotherapy and cytoreductive surgery for the prevention and treatment of peritoneal carcinomatosis and sarcomatosis. Semin Surg Oncol 1998;14:254–61. [DOI] [PubMed]
- 8.Segelman J, Granath F, Holm T, Machado M, Mahteme H, Martling A. Incidence, prevalence and risk factors for peritoneal carcinomatosis from colorectal cancer. Br J Surg 2012;99:699–705. [DOI] [PubMed]
- 9.Hompes D, Tiek J, Wolthuis A, Fieuws S, Penninckx F, Van Cutsem E, et al. HIPEC in T4a colon cancer: a defendable treatment to improve oncologic outcome? Ann Oncol 2012;23:3123–9. [DOI] [PubMed]
- 10.Sammartino P, Sibio S, Biacchi D, Cardi M, Accarpio F, Mingazzini P, et al. Prevention of peritoneal metastases from colon cancer in high-risk patients: preliminary results of surgery plus prophylactic HIPEC. Gastroenterol Res Pract 2012;2012:141585. [DOI] [PMC free article] [PubMed]
- 11.Kerscher AG, Chua TC, Gasser M, Maeder U, Kunzmann V, Isbert C, et al. Impact of peritoneal carcinomatosis in the disease history of colorectal cancer management: a longitudinal experience of 2406 patients over two decades. Br J Cancer 2013;108:1432–9. [DOI] [PMC free article] [PubMed]
- 12.Cheynel N, Cortet M, Lepage C, Ortega-Debalon P, Faivre J, Bouvier AM. Incidence, patterns of failure, and prognosis of perforated colorectal cancers in a well-defined population. Dis Colon Rectum 2009;52:406–11. [DOI] [PubMed]
- 13.Elias D, Honore C, Dumont F, Ducreux M, Boige V, Malka D, et al. Results of systematic second-look surgery plus HIPEC in asymptomatic patients presenting a high risk of developing colorectal peritoneal carcinomatosis. Annals of Surgery 2011;254:289–93. [DOI] [PubMed]
- 14.Van Santvoort HC, Braam HJ, Spekreijse KR, Koning NR, De Bruin PC, De Vries Reilingh TS, et al. Peritoneal carcinomatosis in t4 colorectal cancer: occurrence and risk factors. Ann Surg Oncol 2014;21:1686–91. [DOI] [PubMed]
- 15.Segelman J, Akre O, Gustafsson UO, Bottai M, Martling A. External validation of models predicting the individual risk of metachronous peritoneal carcinomatosis from colon and rectal cancer. Colorectal Dis 2016;18:378–85. [DOI] [PubMed]
- 16.Klaver CE, Musters GD, Bemelman WA, Punt CJ, Verwaal VJ, Dijkgraaf MG, et al. Adjuvant hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with colon cancer at high risk of peritoneal carcinomatosis; the COLOPEC randomized multicentre trial. BMC Cancer 2015;15:428. [DOI] [PMC free article] [PubMed]
- 17.Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016;27:1386–422. [DOI] [PubMed]
- 18.Kanellos I, Zacharakis E, Kanellos D, Pramateftakis MG, Betsis D. Prognostic significance of CEA levels and positive cytology in peritoneal washings in patients with colorectal cancer. Colorectal Dis 2006;8:436–40. [DOI] [PubMed]
- 19.Mohan HM, O’Connor DB, O’Riordan JM, Winter DC. Prognostic significance of detection of microscopic peritoneal disease in colorectal cancer: a systematic review. Surgical Oncology 2013;22:e1–6. [DOI] [PubMed]
- 20.Grass F, Vuagniaux A, Teixeira-Farinha H, Lehmann K, Demartines N, Hubner M. Systematic review of pressurized intraperitoneal aerosol chemotherapy for the treatment of advanced peritoneal carcinomatosis. Br J Surg 2017;104:669–78. [DOI] [PubMed]
- 21.Hubner M, Teixeira Farinha H, Grass F, Wolfer A, Mathevet P, Hahnloser D, et al. Feasibility and safety of pressurized intraperitoneal aerosol chemotherapy for peritoneal carcinomatosis: a retrospective cohort study. Gastroenterol Res Pract 2017;2017:6852749. [DOI] [PMC free article] [PubMed]
- 22.Robella M, Vaira M, De Simone M. Safety and feasibility of pressurized intraperitoneal aerosol chemotherapy (PIPAC) associated with systemic chemotherapy: an innovative approach to treat peritoneal carcinomatosis. World J Surg Oncol 2016;14:128. [DOI] [PMC free article] [PubMed]
- 23.Solass W, Giger-Pabst U, Zieren J, Reymond MA. Pressurized intraperitoneal aerosol chemotherapy (PIPAC): occupational health and safety aspects. Ann Surg Oncol 2013;20:3504–11. [DOI] [PMC free article] [PubMed]
- 24.Teixeira Farinha H, Grass F, Labgaa I, Pache B, Demartines N, Inflammatory Response HM. Toxicity after pressurized intraperitoneal aerosol chemotherapy. J Cancer 2018;9:13–20. [DOI] [PMC free article] [PubMed]
- 25.Graversen M, Pedersen PB, Mortensen MB. Environmental safety during the administration of pressurized intraperitoneal aerosol chemotherapy (PIPAC). Pleura and Peritoneum 2016;1:203–8. [DOI] [PMC free article] [PubMed]
- 26.Xiao Y, Zhang J, He X, Ji J, Wang G. Diagnostic values of carcinoembryonic antigen in predicting peritoneal recurrence after curative resection of gastric cancer: a meta-analysis. Ir J Med Sci 2014;183:557–64. [DOI] [PubMed]
- 27.Leake PA, Cardoso R, Seevaratnam R, Lourenco L, Helyer L, Mahar A, et al. A systematic review of the accuracy and utility of peritoneal cytology in patients with gastric cancer. Gast Cancer 2012;15:S27–37. [DOI] [PubMed]
- 28.Ellebaek SB, Fristrup CW, Mortensen MB. Intraoperative ultrasound as a screening modality for the detection of liver metastases during resection of primary colorectal cancer – a systematic review. Ultrasound Int Open 2017;3:E60–E8. [DOI] [PMC free article] [PubMed]
- 29.Solass W, Sempoux C, Detlefsen S, Carr NJ, Bibeau F. Peritoneal sampling procedures and histological assessment of therapeutic response in peritoneal metastasis: proposal of the peritoneal regression grading score (PRGS). Pleura and Peritoneum 2016;1:99–107. [DOI] [PMC free article] [PubMed]