Abstract
Background
Peritoneal metastasis is a common and dismal evolution of several gastrointestinal (GI) tumors, including gastric, colorectal, hepatobiliary, pancreatic, and other cancers. The therapy of peritoneal metastasis is largely palliative; with the aim of prolonging life and preserving its quality. In the meantime, a significant pharmacological advantage of intraperitoneal chemotherapy was documented in the preclinical model, and numerous clinical studies have delivered promising clinical results.
Methods
This is a prospective, open, randomized multicenter phase III clinical study with two arms that aims to evaluate the effects of pressurized intraperitoneal aerosol chemotherapy (PIPAC) combined with systemic chemotherapy vs. intravenous systemic chemotherapy alone on patients with metastatic upper GI tumors with a peritoneal seeding. Upper GI-adenocarcinomas originated from biliary tract, pancreas and stomach, or esophago- gastric junction are eligible. Patients in the study are treated with standard of care systemic palliative chemotherapy (mFOLFOX6) vs. PIPAC with intravenous (i.v.) chemotherapy (mFOLFOX6). Patients in first line with first diagnosed peritoneal seeding are eligible. Primary outcome is progression free survival (PFS).
Conclusions
PIPAC-procedure is explicit a palliative method but it delivers cytotoxic therapy like in hyperthermic intraperitoneal chemotherapy (HIPEC)-procedure directly to the tumor in a minimally invasive technique, without the need for consideration of the peritoneal-plasma barrier. The technique of PIPAC is minimally invasive and very gentle and the complete procedure takes only round about 45 min and, therefore, optimal in a clearly palliative situation where cure is not the goal. It is also ideal for using this approach in a first line situation, where deepest response should be achieved. The symbiosis of systemic therapy and potentially effective surgery has to be well-planned without deterioration of the patient due to aggressive way of surgery like in cytoreductive surgery (CRS)+HIPEC.
Trial registration
EudraCT: 2018-001035-40.
Keywords: intraperitoneal therapy, peritoneal carcinomatosis, pressurized intraperitoneal aerosol chemotherapy (PIPAC), upper gastrointestinal cancer.
Introduction
Peritoneal metastasis is a common and dismal evolution of several GI tumors, including gastric, colorectal, hepatobiliary, pancreatic, and other cancers [1]. The therapy of peritoneal metastasis is largely palliative; with the aim of prolonging life and preserving its quality. Most patients receive Platin-based, combination systemic chemotherapy. Despite this guideline-recommended therapy, they die within months after diagnosis of peritoneal dissemination [2]. Almost 70 years ago, intraperitoneal chemotherapy has been discovered as an alternative therapeutic option in peritoneal metastasis [3]. In the meantime, a significant pharmacological advantage of intraperitoneal chemotherapy was documented in the preclinical model, and numerous clinical studies have delivered promising clinical results [4]. In the last 30 years, cytoreductive surgery (CRS) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) has been increasingly used. On the basis of long-term survivors, some authors see a curative role for this combined therapy [5]. However, the level of evidence of CRS and HIPEC is still relatively low, and the complication rate remains significant so that this therapy is not accepted by all oncologists [6].
In spite of the above controversies, there is a broad agreement that CRS and HIPEC should only be offered to highly selected patients, taking into consideration the tumor type, the extent of disease, and the general condition of the patient [7]. In particular, diffuse invasion of the small bowel represent a contraindication for CRS and HIPEC because of the dilemma between complete cytoreduction and extensive resection of the small bowel—which is not compatible with life [8]. Thus, there is an urgent need for novel therapies for the majority of peritoneal metastasis patients especially for those not eligible for CRS and HIPEC.
PIPAC is an innovative approach delivering chemotherapy into the peritoneal cavity without crop damage. It is easy to handle and several applications via laparoscopy (minor surgery) are possible without the need for major surgical manipulation [9, 10, 11, 12, 13, 14, 15, 16].
Subjects and methods
Protocol overview
Study design
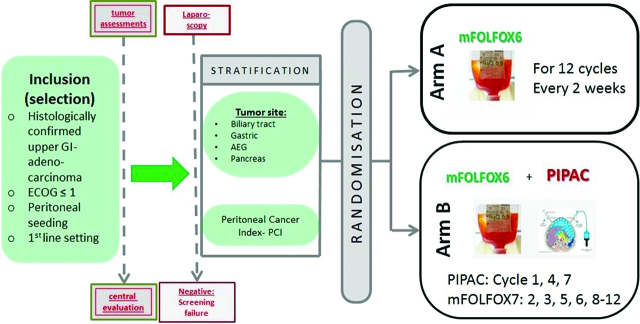
This is a prospective, open, randomized multicenter phase III clinical study with two arms that aims to evaluate the effects of PIPAC combined with systemic chemotherapy vs. intravenous systemic chemotherapy alone on patients with metastatic upper GI tumors with a peritoneal seeding. Upper GI-adenocarcinomas originated from biliary tract, pancreas and stomach, or esophago- gastric junction are eligible. Patients in the study are treated with standard of care systemic palliative chemotherapy (mFOLFOX6) vs. PIPAC with intravenous (i.v.) chemotherapy (mFOLFOX6). Patients in first line with first diagnosed peritoneal seeding are eligible.
Intraoperatively, at the time diagnostic laparoscopy to confirm peritoneal seeding, patients will be randomized after preoperatively written consent has been given for participation.
The scope of the trial is to evaluate the efficacy as well the safety and tolerability of the combination of PIPAC combined with systemic therapy vs. the same i.v. chemotherapy alone. Primary endpoint will be progression free survival (PFS) from randomization (the first PIPAC application, diagnostic laparoscopy, resp.) until disease progression or death of any cause. Secondary endpoint will be overall survival (OS), site of recurrence, morbidity, and quality of life (QoL).
Patients with peritoneal seeding of adenocarcinoma of upper GI (definition see upon) could be included into the trial if they fulfill the inclusion parameters after a central review.
All enrolled patients will receive a standard of care chemotherapy (mFOLFOX6)±PIPAC.
Randomization
At the time of diagnostic laparoscopy to verify clinically or radiologically suspect peritoneal seeding, if patient has given written informed consent and meets inclusion criteria, patient will be randomized, using an interactive Web response system. Randomization will be balanced and stratified according to stratification criteria defined in the protocol.
Pre-therapeutic work-up
Patients eligible for the study (clinical and radiological evidence of peritoneal seeding) will be seen in clinics to check the inclusion and exclusion criteria. The patient will be required to give written informed consent to participate to this clinical study before any nonroutine screening tests or evaluations are conducted and before the explorative laparoscopy. The following assessments should be performed: Performance Status, Thoraco-Abdomino-Pelvic CT scan, PET Scan (optional), laboratory exams: serum CEA, CA19.9, and CA72.4 (optional marker according to tumor origin); hematology and serum chemistry; quality of life assessment (EORTC QLQ-C30). Staging video-laparoscopy of the abdominal cavity will be performed after written informed consent
Patients with no macroscopic peritoneal carcinomatosis, not visible during the laparoscopic examination or patient where a laparoscopic access failed during surgery will be excluded from the study and treated as screening failure.
Patient fulfilling the inclusion criteria, with written informed consent and visible proven peritoneal seeding according to the laparoscopy will be treated according to randomization result as Arm A or Arm B.
Arm A (mFOLFOX6)
Patients with clinically and radiologically signs of peritoneal seeding get a laparoscopic examination, if the peritoneal seeding is confirmed patients will be randomized. After randomization to Arm A laparoscopy will be finished after completion of 12 mFOLFOX6 doses without PIPAC, because patients in Arm A only receive intravenous therapy. Intravenous mFOLFOX6 standard systemic chemotherapy for the upper GI-cancers (SOC) will be administered. Patients will receive i.v. therapy only.
Arm B (mFOLFOX6 ± PIPAC)
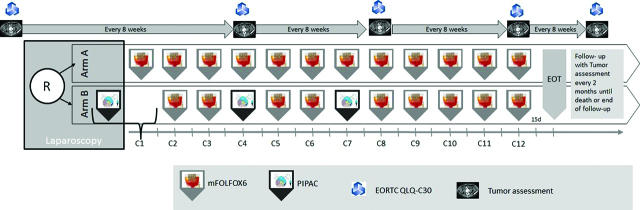
Patients with clinically and radiologically signs of peritoneal seeding get a laparoscopic examination, if the peritoneal seeding is confirmed patients will be randomized. After randomization to arm B patient will get a combination of i.v. chemotherapy with mFOLFOX6+PIPAC. Systemic i.v. chemotherapy (mFOLFOX6) will be administered at the ward, independent of the PIPAC. 3 days after PIPAC patients are able to leave hospital if there are no signs of medical or surgical complications. Patients will be evaluated with clinical examination daily. Laboratory exams will be performed in order to assess hematological, renal, and hepatic function. Locoregional toxicity and systemic toxicity will be evaluated according to the Common Terminology Criteria for Adverse Events (CTC-AE v4.0) from the National Cancer Institute. PIPAC procedure is repeated every 6 weeks for three times and nine mFOLFOX6 doses.
In both of the arms, tumor assessments (CT or MRI) are performed prior (max. 28 days) to randomization and then every 8 weeks thereafter until progression/relapse, death or end of follow-up. During treatment, clinical visits (blood cell counts, detection of toxicity) occur prior to every treatment dose. Safety will be monitored continuously by careful monitoring of all adverse events (AEs) and serious adverse events (SAEs) reported.
QoL will be measured via EORTC QLQ-C30 v3.0 questionnaire in both arms, after written informed consent, before randomization and then every 8 weeks at the time of radiologically tumor assessments.
Measures of outcomes and assessments
Objectives
-
–
To compare OS, PFS, Disease Control Rate (DCR), QoL in the two trial arms
-
–
To determine the safety of PIPAC combined with standard systemic chemotherapy
Safety objectives
-
–
To evaluate the efficacy, safety, and tolerability of PIPAC combined with intravenous mFOLFOX6 compared with i.v. mFOLFOX6 alone in patients with primarily untreated chemo naïve upper- GI gastrointestinal adenocarcinomas originating from pancreatiobiliary tract or esophagogastric origin with laparoscopically proven peritoneal carcinomatosis and indication to receive first line standard chemotherapy. Main efficacy objective is OS and safety objectives focus on surgical SAEs, National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE v4.03) Grade≥3 adverse events, and Grade≥3 laboratory toxicities
-
–
To evaluate the perioperative morbidity and mortality of PIPAC combined with systemic chemotherapy vs. i.v. chemotherapy alone
Endpoints
Primary outcome
PFS will be measured from randomization (the first PIPAC application, diagnostic laparoscopy, resp.) until disease progression or death of any cause.
Secondary outcomes
Efficacy (1 year PFS, 1 year and 2 years OS), pathological response rates and localization of recurrence, morbidity, and QoL. OS and PFS according to different subgroup, such as tumor entity (will be defined in the study analysis plan). DCR defined as the percentage of patients who have achieved complete response, partial response, and stable disease to a therapeutic intervention.
Main inclusion criteria
Subjects with histologically confirmed unresectable locally advanced or metastatic upper GI- adenocarcinoma (originating from biliary tract, pancreas, stomach, or esophago- gastric junction) with peritoneal seeding. No prior chemotherapy in palliative indication. Proven peritoneal carcinomatosis by CT/MRI and laparoscopy. Medically operable – fit for laparoscopy, ECOG≤1.
Main exclusion criteria
Concurrent anticancer treatment (for example, cytoreductive therapy, radiotherapy [with the exception of palliative bone-directed radiotherapy], immune therapy, or cytokine therapy, except for erythropoietin) including irradiation. Prior chemotherapy for unresectable locally advanced or metastatic adenocarcinoma of the stomach or gastroesophageal (GEJ), biliary tract or pancreas.
Treatments
PIPAC-procedure
Shortly, after insufflation of a 12 mmHg CO2 pneumoperitoneum with open access or with Veres needle, two balloon safety trocars (5 and 12 mm, Applied Medical, Düsseldorf, Germany) are inserted into the abdominal wall. The extent of peritoneal carcinomatosis (PCI score) is determined based on lesion size and distribution [17]. Peritoneal biopsies are taken in all four quadrants for histological examination, and a local partial peritonectomy of several square centimeters was performed routinely to improve accuracy of anatomopathology.
A patented nebulizer device (Capnopen®) is then inserted via a 12 mm trocar into the abdominal cavity. The nebulizer unit is then connected with a high pressure line to a high-pressure injector. The liquid chemotherapeutic drugs (Cisplatin 7.5 mg/m2 body surface in a total of 150 mL NaCl 0.9 %; Doxorubicin 1.5 mg/m2 body surface in a total of 50 mL NaCl 0.9 %) are then injected with a flow rate of 30 mL/min into the constant capnoperitoneum of 12 mm Hg. After an aerosol exposure phase of 30 min, the aerosol is evacuated via a closed aerosol waste system. Prior to the application of chemotherapy peritoneal biopsies are routinely taken from all four abdominal quadrants (if possible) taken both for conventional histological analysis and for gene expression testing. The laboratory team will be blinded to the clinical outcome. If present, ascites will be removed at the same time and the volume documented. PIPAC and PC sampling will be repeated every 6 weeks for three times or stopped earlier in cases of progression, death, or unacceptable toxicity [10, 11, 12, 13, 14] (Figure 1).
Figure 1:
Trial flow chart.
Treatment schedule
Arm A (mFOLFOX6 only)–Control(s)/comparator(s)
mFOLFOX6 till PD or inaccepatble toxicity, start of next cycle on day 15 (d15):
-
–
Oxaliplatin 85 mg/m2, d1, i.v. over 2 h PIAC
-
–
Leucovorin* 400 mg/m2, d1 i.v. over 2 h
-
–
5-FU 400 mg/m2, d1, Bolus
-
–
5-FU 2.400 mg/m2, d1, i.v. over 46 h
Arm B (mFOLFOX6/PIPAC) – Intervention
mFOLFOX6/PIPAC till PD or inacceptable toxicity, start of next cycle on day 15 (d15):
PIPAC (Cycles 1, 4 and 7):
-
–
Cisplatin 7.5 mg/m2
-
–
Doxorubicin 1.5 mg/m2
mFOLFOX6 (Cycles 2, 3, 5, 6, 8–12):
-
–
Oxaliplatin 85 mg/m2, d1, i.v. over 2 h
-
–
Leucovorin* 400 mg/m2, d1, i.v. over 2 h
-
–
5-FU 400 mg/m2, d1, Bolus
-
–
5-FU 2.400 mg/m2, d1, i.v. over 46 h
See scheme therapy-timepoints (Figure 2).
Figure 2:
Scheme therapy – timepoints.
Sample size calculation
The present trial is designed as a randomized phase III study which aims at estimating the therapeutic efficacy in terms of OS and PFS of the PIPAC- therapy including systemic therapy in relation to the standard systemic therapy. The assumptions derived from the historical data on patients in the described entity with a pronounced peritoneal seeding are verified by a randomized reference group without PIPAC.
The phase II part is exploratory. The primary endpoint of the phase II part of the trial is PFS as calculated by the hazard ratio for survival.
The assumptions are as follows
Median PFS with mFOLFOX6 is 4 months [18, 19, 20] in the population. The expected median PFS for the PIPAC arm is 5.5 months. The recruitment duration is 2 years and the total duration of the phase II part of the study is 30 months (that means the 24 months enrolment time plus 6 months follow-up after last patient in). Based on these assumptions a total patient number (phase II) of n=206 was determined to observe, which are required to detect the improvement in PFS mentioned above with a power of 80 % and a significance level of 0.2 (two-sided) using a log-rank test. A 5 % drop out rate is included in the sample size. The software used for sample size calculation is SAS v9.3. Other secondary endpoints such as 5-year PFS and 5-year OS rates will be evaluated based on time to event outcome using Kaplan-Meier (KM) rates at 5 years over all patients for analyses. A sample size calculation of the phase III part will be performed based on the most current data available [21, 22, 23, 24, 25, 26, 27, 28].
Study duration
Recruitment period will last 2 years (approximately 100 patients per year). Total study duration is 2.5–3 years (2 years recruitment plus 6 months follow-up after last patient in). The study can be analyzed earlier or later depending on the number of events observed.
Ethical considerations, information giving, and written informed consent
The authors state that they have obtained appropriate Institutional Review Board approval and have followed the principles outlined in the Declaration of Helsinki for all human experimental investigations. In addition, informed consent has been obtained from the participants involved.
Discussion
There is an urgent need for novel therapies for most peritoneal metastasis patients not eligible for CRS and HIPEC therapy. CRS and HIPEC is a possible option for some colorectal cancers, even though the level of evidence even in CRC is low but CRS combined with HIPEC is not established in upper GI cancer types, because of the more aggressive nature of disease in most kinds of upper- GI- cancers. An already existing peritoneal seeding in upper GI- cancers it is a clearly palliative situation with OS less than 1 year in most cases [22]. There is an urgent need for improvement of PFS, OS in this kind of patients without compromising the QoL.
PIPAC-procedure is explicit a palliative method but it delivers cytotoxic therapy like in HIPEC- procedure directly to the tumor in a minimally invasive technique, without the need for consideration of the peritoneal-plasma barrier.
The peritoneal-plasma barrier is a pharmacologic entity of importance for treatment planning in patients with malignant tumors confined to the abdominal cavity. This physiologic barrier limits the resorption of drugs from the peritoneal cavity into the blood. The sequestration of chemotherapeutic agents improves their locoregional cytotoxicity and reduces their systemic toxicity.
The technique of PIPAC is minimally invasive and very gentle and the complete procedure takes only round about 45 min and, therefore, optimal in a clearly palliative situation where cure is not the goal. It is also ideal for using this approach in a first line situation, where deepest response should be achieved. The symbiosis of systemic therapy and potentially effective surgery must be well-planned without deterioration of the patient due to aggressive way of surgery like in CRS+HIPEC.
Participating centers of the current trial are pioneering the potential fields of the application of PIPAC, including defining indications and contraindications, chances and risks, as well as success and failures of this therapy.
They have observed repeatedly that some patients who were primarily not eligible for CRS and HIPEC, most often because of small bowel involvement, could be treated after repeated PIPAC application with CRS and HIPEC.
Struller et al. showed that PIPAC with cisplatin and doxorubicin in patients with gastric cancer is well tolerated and active and concluded that randomized controlled trials should now be designed [29].) According to Khomyakov V. et al. a combination of systemic chemotherapy with XELOX and PIPAC with cisplatin and doxorubicin can induce objective tumor regression and is associated with a promising survival [30].
There is an unmet need for upper GI cancer patients with a leading peritoneal carcinomatosis for an improvement of therapy due to using the most direct way of application. According to the literature there are only publications of individual cases and small cohorts of patients describing a benefit for the patients with PIPAC, but to the knowledge of the authors there are no randomized phase III data comparing PIPAC combined with systemic therapy versus the SOC of systemic therapy alone in this kind of cancer population.
The general aim of this trial is to improve progression free- as well OS of these patients receiving this sequential therapy, in association with systemic standard of care palliative chemotherapy.
Acknowledgments
The authors thank the AIO: Arbeitsgemeinschaft Internistische Onkologie (Working group of Medical Oncologists), CAOGI: Chirurgischen Arbeitsgemeinschaft für den Oberen Gastrointestinaltrakt (Surgical Working group of upper gastrointestinal tract), ACO: Assoziation chirurgische Onkologie (Working group of Surgical Oncologists) for study support.
Footnotes
Author contributions: TG is the study coordinator, and is responsible for the present paper. TG have been involved in drafting the manuscript; TG, AB, and PP have been involved in the study conception and design, assisted in writing the manuscript and have given final approval of the version to be published. All authors read and approved the final manuscript. All authors of the manuscript made substantial contributions in acquisition of data and have been involved in revising the manuscript critically for important intellectual content. Each of the authors have given final approval to the version to be published and have participated sufficiently in the work to take public responsibility for appropriate portions of the content and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: The current study is currently under evaluation for funding by the DKH – Deutsche Krebshilfe (German Cancer Aid).
Employment or leadership: None declared.
Honorarium: None declared.
Competing interests: The funding organization(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
References
- 1.Lambert LA. Looking up: recent advances in understanding and treating peritoneal carcinomatosis. CA Cancer J Clin 2015;65:284–98. [DOI] [PubMed]
- 2.Sadeghi B, Arvieux C, Glehen O, Beaujard AC, Rivoire M, Baulieux J, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer 2000;88:358–63. [DOI] [PubMed]
- 3.Economou SG, Mrazek R, Mc DG, Slaughter D, Cole WH. The intraperitoneal use of nitrogen mustard at the time of operation for cancer Ann N Y Acad Sci 1958;68:1097–102. [DOI] [PubMed]
- 4.Ceelen WP, Levine E. Intraperitoneal cancer therapy: principles and practice. CRC Press, Boca Raton, Fl: 2015 December 9.
- 5.Chia CS, You B, Decullier E, Vaudoyer D, Lorimier G, Abboud K, et al. Patients with peritoneal carcinomatosis from gastric cancer treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: is cure a possibility? Ann Surg Oncol 2016;23:1971–9. [DOI] [PubMed]
- 6.Sugarbaker PH, Ryan DP. Cytoreductive surgery plus hyperthermic perioperative chemotherapy to treat peritoneal metastases from colorectal cancer: standard of care or an experimental approach? Lancet Oncol 2012;13:e362–9. [DOI] [PubMed]
- 7.Beckert S, Struller F, Grischke EM, Glatzle J, Zieker D, Königsrainer A, et al. surgical management of peritoneal surface malignancy with respect to tumour type, tumour stage and individual tumour biology. Zentralbl Chir 2016;141:415–20. [DOI] [PubMed]
- 8.Marmor RA, Kelly KJ, Lowy AM, Baumgartner JM. Laparoscopy is safe and accurate to evaluate peritoneal surface metastasis prior to cytoreductive surgery. Ann Surg Oncol 2016;23:1461–7. [DOI] [PubMed]
- 9.Demtroder C, Solass W, Zieren J, Strumberg D, Giger-Pabst U, Reymond MA. Pressurized intraperitoneal aerosol chemotherapy with oxaliplatin in colorectal peritoneal metastasis. Colorectal Dis 2016;18:364–71. [DOI] [PubMed]
- 10.Solass W, Giger-Pabst U, Zieren J, Reymond MA. Pressurized intraperitoneal aerosol chemotherapy (PIPAC): occupational health and safety aspects. Ann Surg Oncol 2013;20:3504–11. [DOI] [PMC free article] [PubMed]
- 11.Solass W, Herbette A, Schwarz SJS, Dutreix M, Ma R. Therapeutic approach of human peritoneal carcinomatosis with dbait in combination with capnoperitoneum: proof of concept. Surg Endosc 2012;26:847–52. [DOI] [PMC free article] [PubMed]
- 12.Solass W, Hetzel A, Nadiradze G, Sagynaliev E, Reymond MA. Description of a novel approach for intraperitoneal drug delivery and the related device. Surg Endosc 2012;26:1849–55. [DOI] [PubMed]
- 13.Solass W, Kerb R, Murdter T, Giger-Pabst U, Strumberg D, Tempfer C, et al. Intraperitoneal chemotherapy of peritoneal carcinomatosis using pressurized aerosol as an alternative to liquid solution: first evidence for efficacy. Ann Surg Oncol 2014;21:553–9. [DOI] [PMC free article] [PubMed]
- 14.Tempfer CB, Winnekendonk G, Solass W, Horvat R, Giger-Pabst U, Zieren J, et al. Pressurized intraperitoneal aerosol chemotherapy in women with recurrent ovarian cancer: A phase 2 study. Gynecol Oncol 2015;137:223–8. [DOI] [PubMed]
- 15.Giger-Pabst U, Demtröder C, Falkenstein TA, Ouaissi M, Götze TO, Rezniczek GA, et al. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) for the treatment of malignant mesothelioma. BMC Cancer 2018;18:442. [DOI] [PMC free article] [PubMed]
- 16.Falkenstein TA, Götze TO, Ouaissi M, Tempfer CB, Giger-Pabst U, Demtröder C. First clinical data of pressurized intraperitoneal aerosol chemotherapy (PIPAC) as salvage therapy for peritoneal metastatic biliary tract cancer. Anticancer Res 2018;38:373–8. [DOI] [PubMed]
- 17.Harmon RL, Sugarbaker PH. Prognostic indicators in peritoneal carcinomatosis from gastrointestinal cancer. Int Semin Surg Oncol 2005;2:3. [DOI] [PMC free article] [PubMed]
- 18.Seshadri RA, Glehen O. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in gastric cancer. World J Gastroenterol 2016;22:1114–30. [DOI] [PMC free article] [PubMed]
- 19.Yang XJ, Huang CQ, Suo T, Mei LJ, Yang GL, Cheng FL, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol 2011;18:1575–81. [DOI] [PMC free article] [PubMed]
- 20.Yonemura Y, Kawamura T, Bandou E, Takahashi S, Sawa T, Matsuki N. Treatment of peritoneal dissemination from gastric cancer by peritonectomy and chemohyperthermic peritoneal perfusion. Br J Surg 2005;92:370–5. [DOI] [PubMed]
- 21.Nadiradze G, Giger-Pabst U, Zieren J, Strumberg D, Solass W, Reymond MA. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) with low-dose cisplatin and doxorubicin in gastric peritoneal metastasis. J Gastrointest Surg 2016;20:367–73. [DOI] [PMC free article] [PubMed]
- 22.Khomyakov V, Ryabov A, Ivanov A, Utkina A, Cheremisov V, Kolobaev I, et al. Bidirectional chemotherapy in gastric cancer with peritoneal metastasis combining intravenous XELOX with intraperitoneal chemotherapy with low-dose cisplatin and doxorubicin administered as a pressurized aerosol: an open-label. Phase-2 Study (PIPAC-GA2) Pleura and Peritoneum 2016;1:159–66. [DOI] [PMC free article] [PubMed]
- 23.Hong SH, Shin YR, Roh SY, Jeon EK, Song KY, Park CH, et al. Treatment outcomes of systemic chemotherapy for peritoneal carcinomatosis arising from gastric cancer with no measurable disease: retrospective analysis from a single center. Gastric Cancer 2013;16:290–300. [DOI] [PubMed]
- 24.Thomassen I, Van Gestel YR, Van Ramshorst B, Luyer MD, Bosscha K, Nienhuijs SW, et al. Peritoneal carcinomatosis of gastric origin: A population-based study on incidence, survival and risk factors. Int J Cancer 2014;134:622–8. [DOI] [PubMed]
- 25.Seshadri RA, Glehen O. The role of hyperthermic intraperitoneal chemotherapy in gastric cancer. Indian J Surg Oncol 2016;7:198–207. [DOI] [PMC free article] [PubMed]
- 26.Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol 2006;24:2903–9. [DOI] [PubMed]
- 27.Lemmens VE, Klaver YL, Verwaal VJ, Rutten HJ, Coebergh JW, de Hingh IH. Predictors and survival of synchronous peritoneal carcinomatosis of colorectal origin: a population-based study. Int J Cancer 2011;128:2717–25. [DOI] [PubMed]
- 28.Kuijpers AM, Mehta AM, Boot H, Van Leerdam ME, Hauptmann M, Aalbers AG, et al. Perioperative systemic chemotherapy in peritoneal carcinomatosis of lymph node positive colorectal cancer treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Oncol 2014;25:864–9. [DOI] [PubMed]
- 29.Struller F, Horvath P, Solass W, Weinreich FJ, Konigsrainer A, Reymond MA. Pressurized intraperitoneal aerosol chemotherapy with low-dose cisplatin and doxorubicin (PIPAC C/D) in patients with gastric cancer and peritoneal metastasis (PIPAC-GA1). J Clin Oncol 2017;35:99–99:JCO.2017.35.4_suppl.99. suppl. [DOI] [PMC free article] [PubMed]
- 30.Khomiakov V, Ryabov A, Bolotina LV, Utkina A, Cheremisov V, Kolobaev I, et al. Bidirectional chemotherapy in gastric cancer (GC) with peritoneal carcinomatosis (PC) combining intravenous chemotherapy with intraperitoneal chemotherapy with low-dose cisplatin and doxorubicin administered as a pressurized aerosol: an open-label, phase II study. J Clin Oncol 35:JCO.2017.35.15_suppl.e15532. [DOI] [PMC free article] [PubMed]