Abstract
Background
Recurrent, platin-resistant ovarian cancer (rPROC) has a poor survival. Even with the AURELIA trial, which is the best available treatment today, progression-free survival (PFS) is still only 6.7 months from the start of the second-line chemotherapy. Innovative, effective therapies are urgently needed. Pressurized Intra-Peritoneal Aerosol Chemotherapy (PIPAC) is a novel drug delivery system for administering drugs into the abdomen. PIPAC with cisplatin and doxorubicin (PIPAC C/D) may be safely used at an intraperitoneal dose of 10.5 mg/m2 and 2.1 mg/m2, respectively. Systemic toxicity of this therapy is low. In a phase II trial with 53 women, 62 % patients had an objective tumor response. Tumor regression on histology was observed in 76 % patients who underwent all three PIPACs. Randomized phase III studies are now required to evaluate the effect of PIPAC C/D compared to other standard treatments (sequential or simultaneous applications with systemic chemotherapy).
Methods
The present phase III study is a prospective, open, randomized, multicentric pivotal trial. A total of 244 patients will be randomly assigned (1:1) to the control (A) or to the experimental (B) group. Group A: Systemic palliative chemotherapy, physician’s best choice (monotherapy consisting of pegylated liposomal doxorubicin or topotecan or gemcitabine or paclitaxel weekly. Bevacizumab can be used in combination with paclitaxel, topotecan, or pegylated liposomal doxorubicin). Group B: Intraperitoneal chemotherapy, 3×PIPAC C/D, performed every 6 weeks. Combination with systemic therapy is not allowed. Treatment is continued until disease progression, death, or patient refusal. In case of progression, no recommendation for further therapy is given by protocol. Patients are allowed to receive PIPAC C/D or systemic chemotherapy after study termination. The primary endpoint is PFS (according to RECIST v1.1) or death from any cause. The co-primary endpoint is the health-related quality of life (HRQoL) measured as the global health status (GHS, QLQ-30 of EORTC). Secondary outcomes comprise overall survival, safety (CTCAE 5.0), and tumor response according to peritoneal regression grading score (PRGS).
Discussion
We expect PIPAC C/D to control peritoneal disease and preserve the QoL on this subset of patients.
Trial registration
The EudraCT number 2018-003664-31
Keywords: chemotherapy, platin-resistant ovarian cancer (rPROC), Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC), randomized phase III trial
Introduction
Rationale: context and hypothesis
With 295 000 new cases and 184 000 deaths worldwide in 2018 [1], ovarian cancer is a rare but lethal disease. Despite a high response rate to initial treatment [2], most patients will present with disease recurrence within 2 years. Those patients are currently treated with new combinations of systemic chemotherapy. Iterative laparotomy with complete cytoreductive surgery (CRS) is also an option with a median overall survival (OS) of over 30 months [3, 4, 5]. Extent of peritoneal metastases, completeness of CRS, and platinum resistance are major prognostic factors [6, 7]. Platinum-resistant recurrence represents 25 % of all recurrent cases and has a very poor prognosis with a progression-free survival (PFS) of 3.4 months, which may be extended to 6.7 months with the addition of bevacizumab as shown in the AURELIA trial [8].
Thus, there is an urgent need for innovative, better therapies for platinum-resistant, recurrent ovarian cancer. Pressurized Intraperitoneal Chemotherapy (PIPAC) is a new way of administration of intraperitoneal chemotherapy. This innovative approach to treat peritoneal metastases has been assessed in women with gynecologic malignancies [9]. Tempfer et al. showed that PIPAC is feasible and has a safe local and systemic safety profile in women with peritoneal metastases from recurrent ovarian cancer. We assumed that intraabdominal application of pressurized chemotherapy can be as effective as palliative chemotherapy with less side effects [10].
Originality and innovative aspects
Patients with a platinum-resistant recurrence of ovarian cancer have a poor prognosis. The benefit of systemic chemotherapy and bevacizumab remains modest. PARP inhibitors benefit patients with platinum-sensitive recurrent ovarian cancer whatever the BRCA status is but not patients with platin-resistant ovarian cancer [11, 12]. The phase II PIPAC-OV3 trial will compare best physician’s choice palliative chemotherapy (CT) to three cycles of PIPAC with cisplatin and doxorubicin (PIPAC C/D) at a dose of doxorubicin 2.1 mg/m2 and cisplatin 10.5 mg/m2. The dose-escalation study of Tempfer et al. (Gynecol Oncol 2018) has delivered an evidence-based dose which is used in this study protocol [13].
Despite initial chemosensitivity with response rates of around 70 % to first-line platinum-based chemotherapy, ovarian cancer (OC) almost invariably recurs. Yet, little is known about the mechanisms of primary or acquired resistance. In addition, most genomic studies to date have focused on profiling the primary tumor at diagnosis. We would therefore propose to conduct the detailed genomic characterizations of the recurred tumor to describe candidate mechanisms of resistance and uncover novel therapeutic targets.
Expected patient or public health benefits
Patients with a platinum-resistant recurrence have a poor prognosis. Even with the AURELIA trial, which is the best available treatment today, PFS is still only 6.7 months from the start of the second-line chemotherapy. We expect PIPAC to control peritoneal disease and preserve the quality of life (QoL) on this subset of patients [8].
Objectives of the study
Principal objectives
The main objective of this study is to compare the PFS in patients treated with PIPAC vs. palliative systemic chemotherapy according to the best physician’s choice and to compare the QoL assessed by the Functional Assessment of Cancer Therapy-Ovarian.
Secondary objectives
Efficacy:
-
–
To compare the median OS duration in both arms
-
–
To assess the tumor response
-
–
To assess the time to deterioration of ascites
Safety:
-
–
To report potential treatment-related mortality during the first 30 days
-
–
To determine the nature, frequency, and maximum degree of toxicity as assessed by common terminology criteria for adverse events (CTCAE 5.0)
-
–
To assess the time of discontinuation of the protocol treatment
Endpoints
Primary outcome measure
PFS is defined as the interval between the date of randomization and the first radiologically documented disease progression or death, whichever occurs first. Disease progression will be based on RECIST V1.1 criteria performed on thoracoabdominopelvic computed tomography scans [10]. Patients who have not progressed or died at the time of analysis will be censored at the time of the latest date of assessment from their last evaluable RECIST assessment. However, if the patient progresses or dies after two or more missed visits, the patient will be censored at the time of the latest evaluable RECIST assessment. If the patient has no evaluable visits or does not have a baseline assessment she will be censored at day 1 unless the patient dies within two visits of baseline. The PFS time will always be derived from imagery assessment dates, not from visit dates. The primary analysis will be based on investigator-recorded assessments. If a new anticancer treatment or surgery is used before the data cutoff and if there is no documentation of progressive disease before the start of the new anticancer treatment, then the PFS is censored at the last adequate tumor assessment before the new anticancer treatment started, regardless of whether there was a progressive disease or death after the start of the new anticancer treatment.
The co-primary endpoint is quality of life
QoL and symptom control are assessed by the European Organization for Research and Treatment of Cancer (EORTC) QLQ-C30 every 6 weeks until deterioration. The time to deterioration was defined as the time from randomization to a score increased (i. e. worsened) by at least 10 points from baseline (0–100 point scale). If the respective score is missing, and the patient died within 30 days after scheduled time for completion, the patient will be considered to have deteriorated with respect to this score. In this case, time to deterioration is time to death (time to deterioration in fatigue, time to deterioration in pain, time to deterioration in nausea/vomiting, time to deterioration in appetite loss).
Secondary outcome measures
OS will be measured from the date of randomization to the date of death or to the end of follow-up.
Adverse events (AEs) will be assessed according to CTCAE 5.0 weekly during the protocol treatment, then every 6 weeks. The time of discontinuation is defined as the time from randomization to therapy change or dose reduction because of progression of disease or intolerance or adverse effects or patient refusal or death.
Tumor response will be assessed by histology using the peritoneal grading regression score (PRGS), in patients with evaluable disease, at every laparoscopy.
The PRGS is a 4-tied histological regression score with a maximum of 4 (no tumor response) and a minimum of 1 (complete tumor response, no tumor cells remaining). A mean score is calculated from four peritoneal biopsies. A tumor response is defined as a decrease of 0.5 in the mean PRGS [10].
Characteristics of the study
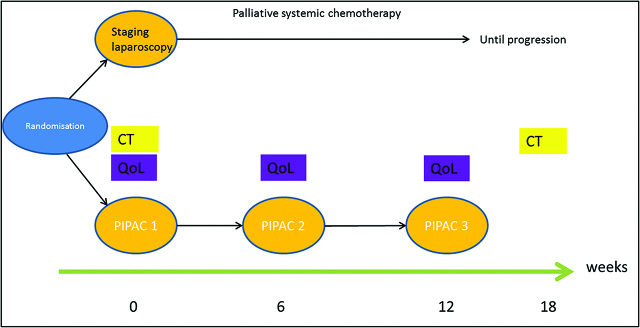
Trial design (see Figure 1)
Figure 1:
PIPAC-OV3 study flowchart.
The present phase III study is a prospective, open, randomized, and multicentric trial. Patients will be randomly assigned (1:1) to the experimental group (Group B) or to the control group (Group A).
Group A: Systemic palliative chemotherapy, physician’s best choice (monotherapy consisting of but not exclusively: pegylated liposomal doxorubicin or topotecan or gemcitabine or paclitaxel weekly). Bevacizumab can be used in combination with paclitaxel, topotecan, or pegylated liposomal doxorubicin until disease progression (RECIST 1.1 performed on a thoracoabdominopelvic computed tomography scan).
Group B: Intraperitoneal chemotherapy, 3×PIPAC, performed every 6 weeks and then until disease progression (RECIST 1.1).
Cisplatin 10.5 mg/m2 body surface and doxorubicin 2.1 mg/m2 body surface administered as PIPAC for 30 min at a pressure of 12–15 mmHg and a temperature of 37 °C (normothermia). Combination with systemic therapy is not allowed. The investigational drug is provided in solution as a fixed-drug combination. The 6 week period (42 days) is 1 cycle. Protocol treatment basically comprises at least three cycles. Treatment is continued until disease progression, death, or patient refusal.
In case of progression, no recommendation for further therapy is given by protocol. Patients are allowed to receive PIPAC therapy or systemic chemotherapy after study termination (outside the study protocol). The analysis of efficacy will be performed on all randomized subjects in accordance with the intention-to-treat (ITT) principle. In order to assess the robustness of the results, the same analyses will be done using all randomized subjects who satisfy the eligibility criteria. The analysis of safety will be performed on all subjects who have received at least one dose of study treatment.
Number of patients
A total of n=244 patients will be randomized into this trial.
Selection of the study population
Inclusion criteria
The inclusion criteria are the following:
-
–
Histologically confirmed epithelial ovarian, tubal, or primary peritoneal platinum-resistant (clinical recurrence or persistent within 6 months of last treatment) carcinoma International Federation of Gynecology and Obstetrics classification (FIGO) stages I to IVa
-
–
Ages eligible for study: 18 Years and older (Adult, Senior)
-
–
Sexes eligible for study: Female
-
–
Accepts healthy volunteers: No
-
–
Visible intraabdominal lesions in CT-scanner or PET-scanner
-
–
ECOG performance status 0–2.
-
–
Adequate bone marrow, liver and kidney function, and coagulation parameters (within 7 days of start of treatment).
-
–
a. ANC≥1.5×109/L
-
–
b. Thrombocytes≥100×109/L
-
–
c. Hemoglobin (Hb)≥6 mmol/l
-
–
d. Serum bilirubin (BR)≤1.5×ULN (upper limit of normal)
-
–
e. Serum transaminase≤2.5×ULN
-
–
f. Serum creatinin≤1.5×ULN
-
–
g. Urin stix for protein <2+
-
–
h. INR≤1.5
-
–
i. APTT≤1.5×ULN
-
–
Covered by a Health System where applicable, and/or in compliance with the recommendations of the national laws in force relating to biomedical research;
-
–
Signed written informed consent obtained prior to any study-specific screening procedures.
Exclusion criteria
Patients are not eligible for this study if any of the following exclusion criteria apply:
-
–
Extraperitoneal metastasis (with the exception of retroperitoneal lymph nodes and pleural effusion);
-
–
Any prior malignancy not considered in complete remission for at least 2 years;
-
–
Pregnancy or breastfeeding;
-
–
Untreated central nervous system disease or symptomatic central nervous system metastasis, history or evidence of thrombotic or hemorrhagic disorders not considered currently in complete remission;
-
–
Contraindication to any drug contained in the chemotherapy regimen;
-
–
Medical, geographical, sociological, psychological, or legal conditions that would prevent the patient from completing the study or signing the informed consent;
-
–
Any significant disease which, in the investigator’s opinion, excludes the patient from the study;
-
–
Patients who have received other types of experimental treatment or participated in a clinical study less than 28 days prior to this study;
-
–
Bowel obstruction or parenteral nutrition or gastric tube;
-
–
Underlying medical disease not adequately treated (diabetes, cardiovascular disease);
-
–
Surgery apart from staging laparoscopy less than 4 weeks before inclusion;
-
–
Hypersensitivity to the active substance or one or more of the other substances contained in the protocol drugs (cisplatin, doxorubicin);
-
–
Maximal previous dose of doxorubicin or other anthracyclines;
-
–
Active infection requiring antibiotics;
-
–
Patients for whom completion of this study and/or follow-up is deemed inappropriate for any reason;
-
–
Not under any administrative or legal supervision.
Statistical methods and sample size determination
Sample size calculation
A total of n=244 patients will be randomized into this trial over a 12 month period. Patients will be stratified at randomization into three groups according to the time point of the recurrence (first, second, and third and beyond). Assuming a median PFS time of 4 months on PC and 6 months on PIPAC, following these patients for a minimum of 6 months after the last patient is entered will generate approximately 191 PFS events. At the end of the follow-up period, both noninferiority (NI) and superiority of PIPAC to PC will be assessed in a closed test procedure as follows: NI will be assessed first by comparison of the upper two-sided 95 % CL of the hazard ratio for PIPAC : PC to 1.2; if the CL is<1.2 then NI will be declared (note to give an upper CL<1.2 with 191 events would require the observation of a small advantage for PIPAC vs PC of 10 %). If NI is declared, superiority will be assessed by comparison of the upper two-sided 95 % CL to unity; if the CL is<1, then superiority will have been demonstrated. With the number of patients stated and PFS events expected, the trial has 80 % power at the two-sided 0.05 alpha level.
Definition of the study population
Intention-to-treat (ITT) population
The ITT population is defined as all patients randomized in the trial, regardless of whether they actually received treatment. The treatment groups will be analyzed as randomized.
Per protocol population
The PP population is a subgroup of the ITT population containing all patients who do not have any major protocol violation. Major protocol violations will be defined in the statistical analysis plan.
Safety analysis population
All patients randomized who are known to have received at least one dose of study treatment and one safety follow-up, whether withdrawn prematurely or not.
Analysis populations
All efficacy analyses will be performed on the ITT population including all randomized patients analyzed according to the randomization scheme. Additional sensitivity analyses will be performed on PP population. The safety data will be analyzed on the safety analysis set.
Statistical analysis
Analysis of the primary outcomes
The primary endpoint is PFS defined as the time from the date of randomization to the first documented disease progression (according to RECIST v1.1 [10]) or death from any cause, whichever occurs first. PFS will be estimated using the Kaplan–Meier method and will be described in terms of median PFS per arm. PFS distributions will be compared between the two study arms using a two-sided log-rank test (significance level of 5 %). Hazard ratio for progression between the two arms was estimated by Cox proportional hazard model as for the log-rank test. Associated two-sided 95 % confidence intervals for the estimates will be provided (NI design).
The co-primary endpoint is the health-related quality of life (HRQoL) measured as the global health status (GHS). GHS is based on EORTC QLQ-C30 questionnaires filled in by the patient on the day before therapy. The time frame is every 6 weeks until disease progression or death, coinciding with the clinical care schedule dictated by the trial regimen (superiority design).
Analysis of secondary outcomes
Overall survival analysis
OS will be analyzed similarly to the PFS (Kaplan–Meier method, log-rank test between the two arms supported by a stratified Cox regression). An interim analysis of OS will be performed at the time of the final PFS analysis. A final update of OS results will be performed when the OS data are approximately 80 % mature.
Safety data analysis
The assessment of safety will be based mainly on the frequency of AEs. Descriptive statistics will be provided for characterizing and assessing patient tolerance to treatment. A focus will be done on postoperative morbidity. The period of safety analysis will be defined from the first dose of any study treatment to 30 days after end of the last study treatment.
Feasibility of the present study
The feasibility of the present study is based on the following arguments:
Referral networks and investigator/site experience
Patients will be recruited by expert centers in the care management of EOC and in the PIPAC techniques (French Oncologic and Gynecologic HIPEC – FROGHI Group). All centers approached to participate in this study have already been collaborating actively for many years to implement national and international research protocols.
Safety
Definitions
Adverse events
An AE is any untoward medical occurrence in a patient or clinical investigation patient which does not necessarily have to have a causal relationship with the medicinal product. An AE can therefore be any unfavorable and unintended sign (including an abnormal laboratory finding, for example), symptom, or disease temporally associated with the research, whether or not considered related to the medicinal product.
Serious adverse events
An event will be considered as serious adverse event (SAE) if it presents as any untoward medical occurrence that at any dose:
-
–
Results in death
-
–
Is life-threatening
-
–
Requires in-patient hospitalization or prolongation of existing hospitalization
-
–
Results in persistent or significant disability/incapacity
-
–
Is a congenital anomaly/birth defect
-
–
Is a medically significant event.
Medical and scientific judgment should be exercised in deciding whether expedited reporting is appropriated in situations, such as important medical events that may not be immediately life-threatening or result in death or hospitalization but may jeopardize the patient or may require intervention to prevent one of the outcomes listed in the definition above.
The term “severe” is a measure of intensity; thus a severe adverse event is not necessarily serious. For example, “nausea of several hours” duration may be severe but may not be clinically serious.
Intensity
The intensity of the event will be graded according to the four-point system below:
-
–
Mild (Grade 1): Discomfort noticed but no disruption of normal daily activity
-
–
Moderate (Grade 2): Discomfort sufficient to reduce or affect normal daily activity
-
–
Severe (Grade 3): Incapacitating with inability to work or perform normal daily activity
-
–
Life-threatening (Grade 4): Substantial risk of dying at time of event
-
–
Death (Grade 5)
Obligations of the investigator
Adverse events reporting
All AEs regardless of seriousness or relationship to the investigational product/study protocol that occurred after the informed consent up to the end of the research are to be recorded in the AE pages of the electronic Case Report Form (eCRF – Clinsight© Web application). All events that meet one or more criteria of seriousness will be reported as SAE.
General AE/SAE reporting rules
-
–
Any episode of any grade of toxicities, related to a SAE, must be reported as “adverse event” in the appropriate eCRF pages;
-
–
Planned hospital admissions or surgical procedures for an illness or disease which existed before the patient was enrolled in the study or before study drug was given are not to be considered SAEs unless the condition deteriorated in an unexpected manner during the study (e. g. surgery was performed earlier than planned);
-
–
Whenever possible, symptoms should be grouped as a single syndrome or diagnosis. The investigator should specify the date of onset, intensity, action taken regarding trial medication, outcome of all AEs, and his opinion as to whether the AE can be related to the study drug and/or to concomitant drugs.
Serious adverse events reporting
All SAEs must also be reported on the AE page of the eCRF. An SAE that occurs after the end of the study, if considered related to the investigational product/study protocol, will be reported. All SAE forms must be dated and signed by the responsible investigator or one of his/her authorized staff members.
-
–
Group, whenever possible, symptoms as a single syndrome or diagnosis.
-
–
Attach the photocopy of all examinations carried out and the dates on which these examinations were performed. Care should be taken to ensure that the patient’s identity is protected and the patient’s identifiers in the clinical study are properly mentioned on any copy of source document. For laboratory results, include the laboratory normal ranges.
-
–
Assess the relationship to investigational product/study protocol, action taken, and outcome to date. The relatedness with concomitant treatment must also be evaluated. The causality can be one of two possibilities: unrelated or related.
-
–
Follow-up of any SAE that is fatal or life-threatening should be provided within one calendar week.
Follow-up of AE and SAE
Any SAE should be monitored until they are resolved or are clearly determined to be due to a patient’s stable or chronic condition or underlying condition. All AEs must be documented and the outcome must be followed up until the return to normal or consolidation of the patient’s condition.
Obligations of the sponsor
During the course of the study, the sponsor will report in an expedited manner all SAEs that are both unexpected and at least reasonably related to the research, to Eudravigilance, Health Authorities, Ethic Committee in accordance with European Directive 2001/20/EC and local regulations, and to the investigators.
The expectedness of an adverse reaction will be determined by the sponsor according to the Investigator’s Brochure. The sponsor will report all safety information from the trial in the Annual Safety Reports and notify it to the Health Authorities and Ethics Committees in accordance with local regulations.
Responsibilities of the investigator(s)
Patient informed consent
A patient must provide written consent before undergoing any protocol-required assessments. Written informed consent in compliance with local regulatory authority will be obtained from each patient prior to entering the trial. It is the responsibility of the investigator to obtain such consent. Sample informed consent form (ICF) documents will be provided to each center. The patient and the investigator will date and sign the ICF. The investigator shall provide a copy of the signed consent to the study patient; a copy shall be maintained in the investigator’s study file.
Protocol adherence
Each investigator must adhere to the protocol as detailed in this document. Each investigator will be responsible for allowing only those who have met protocol eligibility criteria to be randomized. Modifications to the protocol should not be made without agreement of the investigators and sponsor. Changes to the protocol will require written Ethics Committee, Regulatory Authority approval/favorable opinion prior to implementation. The sponsor will submit all protocol modifications to the appropriate regulatory authorities in accordance with the governing regulations.
Monitoring/audit
A representative of the sponsor or designee will visit the investigator periodically for the purpose of monitoring the progress of this study in accordance with good clinical practice (GCP) regulations. It is the responsibility of the investigator to be present or available for consultation during such scheduled monitoring visits. During these routine visits, all data pertaining to a patient’s participation in this clinical investigation must be made available to the monitor. On-site review of the eCRF for completeness and clarity, cross-checking with sources documents, and reconciliation and clarification of administrative matters will be performed.
An audit may be performed at any time during after completion of the clinical study by the sponsor or designee. All study-related documentation must be made available to the designated auditor(s).
In addition, a representative of the regulatory agency may choose to inspect a study center at any time prior to, during, or after completion of the clinical study. A sponsor representative or designee will be available to assist in the preparation for such an inspection. All pertinent study data should be made available as requested to the regulatory authority for verification, audit, or inspection purposes.
Criteria for premature withdrawal patient’s participation in the study
Circumstances that lead to premature withdrawal of a patient from the trial must be clearly reported by the investigator on the appropriate eCRF page.
Patients can be withdrawn from the study under the following circumstances:
-
–
Death;
-
–
Toxicity;
-
–
Intercurrent illness;
-
–
Noncompliance (including loss of patient to follow-up);
-
–
Voluntary withdrawal;
-
–
Failure to meet the eligibility criteria.
Patients are free to withdraw from the study at any time. When a patient decides to withdraw from the study, he should always be contacted in order to obtain information about the reason for withdrawal and to record any AEs.
Every effort will be made to contact patients who fail to return for scheduled visits. A patient is considered lost to follow-up if no information has been obtained when the last patient has completed the clinical phase of the study. During this time, there must be documented attempts to contact the patient either by phone or letter.
Data collection and follow-up for withdrawn subjects
Subjects may withdraw from the study at any time at their own request, or they may be withdrawn at any time at the discretion of the investigator or sponsor for safety, behavioral, or administrative reasons. If a subject does not return for a scheduled visit, every effort should be made to contact the subject. In any circumstance, every effort should be made to document subject outcome, if possible.
The investigator should inquire about the reason for withdrawal, request the subject to return for a follow-up visit, if applicable, and follow-up with the subject regarding any unresolved AEs. If the subject withdraws from the study, and also withdraws consent for disclosure of future information, no further evaluations should be performed, and no additional data should be collected. The sponsor may retain and continue to use any data collected before such withdrawal of consent.
Premature closure of the study
Study participation by individual sites or the entire study may be prematurely terminated, if in the opinion of the sponsor, there is sufficient reasonable cause. Any investigator who wants to discontinue his/her participation to the study must immediately inform the sponsor in writing of this decision.
Written notification documenting the reason for study termination will be provided to the investigator by the terminating party.
Examples of circumstances that may warrant termination include:
-
–
Failure to enter patients at an acceptable rate;
-
–
Insufficient adherence to protocol requirements;
-
–
Insufficient complete and/or evaluable data;
-
–
Frequency and/or unexpected severity of the toxicity;
-
–
Unacceptable toxicity.
Quality control
Responsibilities of the investigators
The investigator(s) undertake(s) to perform the study in accordance with GCP and specifically either GCP for trials on medicinal products in the European Community. The investigators are required to ensure compliance with respect to the investigational drug schedule, visit schedule required by the protocol, and all study procedures provided by the sponsor. The investigators agree to provide reliable data and all information requested in the eCRF in an accurate and legible manner according to the instructions provided and to ensure direct access to source documents to sponsor representatives. The investigator may appoint such other individual as he/she may deem appropriate as subinvestigators to assist in the conduct of the study in accordance with the protocol. All subinvestigators shall be appointed and listed in a timely manner. The subinvestigator will be supervised by and work under the responsibility of the investigator.
Patient compliance to the study treatment is the investigator’s responsibility and will be checked during site monitoring visits by a representative of the sponsor.
Responsibilities of the sponsor
The sponsor of this study has responsibilities to Health Authorities to take all reasonable steps to ensure the proper conduct of the study as regards ethics, protocol adherence, integrity, and validity of the data recorded on the eCRF. During monitoring visits, the following points will be scrutinized with the investigator: patient informed consent, patient recruitment and follow-up, patient compliance to the study treatment, study treatment accountability, concomitant therapy use, AE documentation and reporting, and quality of data.
Data quality assurance
Source document requirements
According to the guidelines for GCP, the study monitor has to check the CRF entries against the source documents. The ICF will include a statement by which the patients allow the sponsor’s duly authorized personnel (trial monitoring team) to have direct access to original medical records which support data on the eCRF (e. g. patient’s medical file, appointment books, original laboratory records, etc.). These personnel, bound by professional secrecy, will not disclose any personal identity or personal medical information (according to confidentiality rules).
Electronic case report form
Study data for each randomized subject will be entered into an eCRF (Clinsight© Web application) by site personnel using a secure, validated web-based electronic data capture (EDC) application. Coordination center will have view-only access to all data upon entry in the EDC application. Selected data for subjects who are not randomized will be collected in the EDC.
Instances of missing, discrepant, or uninterpretable data will be queried with the investigator for resolution. Any changes to study data will be made to the eCRF and documented in an audit trail, which will be maintained within the clinical database.
Archiving clinical trial files
The investigator shall maintain the essential clinical study documents (including source documents, clinical drug-disposition records, signed subject ICF, AE reports, and other regulatory documents) as required by the applicable regulatory requirements. The investigator should take adequate measures to prevent accidental or premature destruction of these documents. In the event of accidental destruction, the investigator must notify the sponsor immediately. The following essential clinical study documents must be maintained:
-
–
Signed informed consent documents for all subjects;
-
–
Subject identification code list, screening log (if applicable), and enrollment log;
-
–
Record of all communication between the investigator and Ethical Committee;
-
–
Composition of the Ethical Committee or other applicable statement;
-
–
Record of all communications between the investigator and the sponsor;
-
–
List of subinvestigators and other appropriately qualified persons to whom the principal investigator has delegated significant trial-related duties, together with their roles in the study and their signatures;
-
–
Copies of documentation of corrections for all subjects;
-
–
Drug-accountability records;
-
–
All other source documents (i. e. subject records, hospital records, laboratory records, etc.);
-
–
All other documents as listed in Section 8 of the consolidated guidelines on GCP (Essential Documents for the Conduct of a Clinical Trial). Essential clinical study documents shall be retained for at least 15 years following the date of the end of the study.
These documents shall be retained for a longer period, however, if required by additional applicable regulatory requirements or by an agreement with the sponsor. The investigator must, therefore, obtain approval in writing from the sponsor prior to destruction of any records.
The investigator shall notify the sponsor to any change in the location or status of any essential, clinical study documents. The sponsor shall be responsible for informing the investigator when these documents no longer need to be retained.
Ethics, regulatory, and legal considerations
Ethical conduct of the study
This study will be conducted in accordance with:
-
–
The Declaration of Helsinki on Ethical Principles for Medical Research Involving Human Subjects, adopted by the General Assembly of the World Medical Association (Fortaleza 2013, revised);
-
–
The International Conference on Harmonisation Guidance on GCP (Topic E6) (CPMP/ICH/135/95);
-
–
The European Clinical Trials Directive 2001/20/EC that provide greater protection to subjects participating in clinical trials, ensure quality of conduct, and harmonize regulation and conduct of clinical trials throughout Europe;
-
–
The Law N°78–17 of 06/01/1978, modified in relation to computer science, to databases, and to data collection;
-
–
The Law 2004–806 of August 9, 2004, defines the scope of the law as all biomedical research involving human beings, with the aim of increasing biological or medical knowledge;
-
–
The Bioethics Law N°2004–800 of August 6, 2004.
Regulatory authority approvals/authorizations
Regulatory authority approvals/authorizations/notifications, where required, must be in place and fully documented prior to study start In France, the sponsor will submit the study protocol for authorization to the competent authority “Agence Française de Sécurité du Médicament et des produits de santé ANSM”.
Subject information and consent
It is the responsibility of the investigator to obtain informed consent in compliance with national requirements from each patient prior to entering the trial or, where relevant, prior to evaluating the patient’s suitability for the study.
It must be made completely and unambiguously clear to each patient that they are free to refuse to participate in the study, or that they can withdraw their consent at any time and for any reason, without incurring any penalty or withholding of treatment on the part of the investigator.
The informed consent document used by the investigator for obtaining patient’s informed consent must be reviewed and approved by the sponsor prior to Ethical Committee submission.
Administrative procedures
Insurance
The sponsor certifies having taken out a liability insurance policy which covers the investigator and his co-workers and which is in accordance with the local laws and requirements. Specific statements will be contained in appendix where is needed. A certificate of insurance will be provided to the investigator.
Inspections by regulatory authorities
For the purpose of ensuring compliance with GCP and regulatory agency guidelines, it may be necessary to conduct a site audit or an inspection.
By signing this protocol, the investigator agrees to allow the sponsor and its representative and drug regulatory agencies to have direct access to his study records for review. These personnel, bound by professional secrecy, will not disclose any personal identity or personal medical information.
These audits involve review of source documents supporting the adequacy and accuracy of data gathered in CRF, review of documentation required to be maintained, and checks on drug accountability.
The sponsor will in all cases help the investigator prepare for an inspection by any regulatory authority.
Protocol amendments
No changes or amendments to this protocol may be made by the investigator or by the sponsor after the protocol has been agreed to and signed by both parties unless such change(s) or amendment(s) have been fully discussed and agreed upon by the investigator and the sponsor.
Any change agreed upon will be recorded in writing, the written amendment will be signed by the investigator and by the sponsor, and the signed amendment will be appended to this protocol.
Approval/authorization of amendments by Health Authorities (ANSM/Ethical Committee) is required prior to their implementation, unless there are overriding safety reasons.
Data and safety monitoring board (DSMB)
An independent Data and Safety Monitoring Board (DSMB) external to the trial investigators will be established specifically to monitor data throughout the life of the study to determine if it is appropriate, from both the scientific and ethical standpoint, to continue the PIPAC-OV3 as planned. The DSMB will meet to review SAEs and propose to continue or stop the study. The DSMB will be made up of experts in medical oncology, surgery and gynecology, and methodology in clinical research.
Their role will be to protect the interests of eligible patients for PIPAC-OV3 study and of those still to be entered by review of accumulating safety and tolerability data generated in the study. The sponsor will provide the DSMB with data by treatment arm to include demographic data, number of patients in study, duration of study drug exposure, AEs, SAEs, laboratory data, and response/relapse. Based upon their review, the DSMB will advise the Sponsor and the Steering Committee of any actions that should be taken in the study.
Study Steering Committee
A Steering Committee will be established to provide scientific guidance for this clinical study. This committee is comprised of experts who will assist the sponsor in revolving issues and/or questions encountered during the conduct of the study, and will consult with the sponsor regarding changes to the protocol as necessary.
Footnotes
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: None declared.
Employment or leadership: None declared.
Honorarium: None declared.
Competing interests: The funding organization(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
References
- 1.International Agency for Research on Cancer. GLOBOCAN 2018: estimated cancer incidence, mortality and prevalence worldwide in 2012. Available at: http://gco.iarc.fr/today/data/factsheets/populations/900-world-fact-sheets.pdf.
- 2.du Bois A, Quinn M, Thigpen T, Vermorken J, Avall-Lundqvist E, Bookman M, et al. 2004 Consensus statements on the management of ovarian cancer: final document of the 3rd International Gynecologic Cancer Intergroup Ovarian Cancer Consensus Conference (GCIG OCCC 2004). Ann Oncol. 2005;16:viii7–12. [DOI] [PubMed]
- 3.Harter P, du Bois A, Hahmann M, Hasenburg A, Burges A, Loibl S, et al. Surgery in recurrent ovarian cancer: the Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) DESKTOP OVAR trial. Ann Surg Oncol. 2006;13:1702–10. [DOI] [PubMed]
- 4.Bristow RE, Puri I, Chi DS. Cytoreductive surgery for recurrent ovarian cancer: a meta-analysis. Gynecol Oncol. 2009;112:265–74. [DOI] [PubMed]
- 5.Benedetti Panici P, De Vivo A, Bellati F, Manci N, Perniola G, Basile S, et al. Secondary cytoreductive surgery in patients with platinum-sensitive recurrent ovarian cancer. Ann Surg Oncol. 2007;14:1136–42. [DOI] [PubMed]
- 6.Helm CW, Randall-Whitis L, Martin RS, 3rd, Metzinger DS, Gordinier ME, Parker LP, et al. Hyperthermic intraperitoneal chemotherapy in conjunction with surgery for the treatment of recurrent ovarian carcinoma. Gynecol Oncol. 2007;105:90–6. [DOI] [PubMed]
- 7.Bakrin N, Bereder JM, Decullier E, Classe JM, Msika S, Lorimier G, et al. Peritoneal carcinomatosis treated with cytoreductive surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for advanced ovarian carcinoma: a French multicentre retrospective cohort study of 566 patients. Eur J Surg Oncol. 2013;39:1435–43. [DOI] [PubMed]
- 8.Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32:1302–8. [DOI] [PubMed]
- 9.Tempfer CB, Solass W, Reymond MA. Pressurized Intraperitoneal Chemotherapy (PIPAC) in women with gynecologic malignancies: a review. Wien Med Wochenschr. 2014;164:519–28. [DOI] [PubMed]
- 10.Tempfer CB, Winnekendonk G, Solass W, Horvat R, Giger-Pabst U, Zieren J, et al. Pressurized Intraperitoneal Aerosol Chemotherapy in women with recurrent ovarian cancer: a phase 2 study. Gynecol Oncol. 2015;137:223–8. [DOI] [PubMed]
- 11.Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375:2154–64. [DOI] [PubMed]
- 12.Pujade-Lauraine E, Ledermann JA, Selle F, Gebski V, Penson RT, Oza AM, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1274–84. [DOI] [PubMed]
- 13.Tempfer CB, Giger-Pabst U, Seebacher V, Petersen M, Dogan A, Rezniczek GA. A phase I, single-arm, open-label, dose escalation study of intraperitoneal cisplatin and doxorubicin in patients with recurrent ovarian cancer and peritoneal carcinomatosis. Gynecol Oncol. 2018;150:23–30. [DOI] [PubMed]