Abstract
Background
The aim of this systematic review was to investigate the accuracy of additional staging laparoscopy (SL) in advanced epithelial ovarian cancer (AEOC) to predict futile laparotomy (FL).
Methods
Systematic review according to preferred reporting items for systematic reviews and meta-analyses statement (PRISMA) criteria. Clinical studies investigating the role of SL in selecting women with AEOC for primary debulking surgery (PDS) were included. Index test: SL. Reference test: laparotomy. Target condition: incomplete cytoreduction (CR) with remaining disease<1 cm.
Results
Nine prospective and retrospective studies reporting on eight cohorts totalizing 778 LS were included. Reference test was completed in 76 % cases. PPV for FL was between 0.69 and 1.0. In three studies examining the value of a predictive index value (PIV) for predicting FL, sensitivity of the index test (LS with PIV ≥8) was between 46% and 70 %, and specificity between 89 % and 100 %. The proportion of patients that received CR during PDS differed widely between studies (from 50 to 91). Using a PIV did not increase the sensitivity and might result in more patients receiving FL. In the only randomized trial, FL occurred in 10 (10 %) of 102 patients in the LS group versus 39 (39 %) of 99 patients in the primary PDS group (relative risk, 0.25; 95 % CI, 0.13–0.47; p<0.001). Port-site recurrences occurred in 2%–6 % patients. Overall costs of with or without SL were comparable.
Conclusions
The evidence available from this systematic review supports the inclusion of an additional LS to the conventional initial diagnostic workup in women with AEOC.
Keywords: advanced epithelial ovarian cancer, diagnostic test, laparoscopy, staging
Introduction
Epithelial ovarian cancer (EOC) is the leading cause of gynaecologic cancer death in Western countries [1]. A majority of women with EOC are diagnosed at advanced stage of disease. Conventional preoperative staging in EOC consists of medical history, complete physical examination, assessment of CA125 and CEA serum levels, chest X-ray and contrast-enhanced abdominopelvic CT.
The guideline-recommended treatment for these patients is primary debulking surgery (PDS) followed by combination platinum-based chemotherapy [2, 3]. In a Cochrane review, there were no RCTs identified that were designed to evaluate the effectiveness of surgery when performed as a primary procedure in advanced stage ovarian cancer. There was a high risk of bias due to the retrospective nature of these studies where, despite statistical adjustment for important prognostic factors, selection bias was still likely to be of particular concern [4].
owever, there is large agreement that completeness of cytoreduction (CR) during PDS is the most important prognostic factor in EOC [5] and that complete resection of all macroscopic disease is the objective whenever PDS is performed [6]. However, CR during PDS may require extensive surgery with a subsequent higher risk of mortality and morbidity [7]. Against this background, it has to be noted that, in the Cochrane review [8], adverse events, quality of life and cost-effectiveness were not reported by treatment arm or to a satisfactory level in any of the studies [4]. Moreover, despite maximal surgical effort, complete PDS is not always possible and 30%–70 % of patients are left with gross residual disease after PDS, dep both on extent of disease and quality of CR [9]. In those cases, neoadjuvant chemotherapy followed by interval debulking surgery is currently recommended by US SGO and ASCO guidelines [10].
Futile (“open and close”) laparotomy (FL) should be prevented in women with EOC: not only such exploration has no prognostic benefit for the patient, but it might be harmful since it is associated with pain, complications, intraoperative blood loss, hospital stay, interruption of diet intake, delays the start of neoadjuvant/palliative chemotherapy and significant costs [11].
Unfortunately, in spite of recent technological progress, preoperative contrast-enhanced CT of the abdomen and pelvis is still not able to demonstrate small volume (<5 mm) extra-ovarian deposits on bowel serosa, mesentery and peritoneum especially in the absence of ascites [12]. Predictive value of CT for CR has been shown to be limited in EOC, because it underestimates extent of peritoneal disease, in particular in those women with expected early-stage EOC. Overall area under the receiver-operating characteristic (ROC) curve for score predicting suboptimal CR was 0.761 with sensitivity, specificity, and positive and negative predictive values of 70.6 %, 73.2 %, 68.7 %, and 91.9 %, respectively [13].
Since CT-scan alone is not able to prevent effectively futile laparotomies, laparoscopic staging (LS) is increasingly incorporated into the early management of EOC (Figure 1) in order to better predict feasibility of complete PDS. The hope is that LS might better predict the feasibility of CR in EOC than CT-scan alone, and thus prevent futile explorative laparotomies [14]. The rationale for this approach is that LS is minimally invasive, has a low complication rate, is not related to significant pain and does not cause significant blood loss. Moreover, LS is a short-stay procedure [15]. Finally, biopsies taken during LS allow confirmation of diagnosis and molecular analysis [16]. Thus, LS appears to many gynaecologists to be a reasonable additional option in the initial staging of women with AEOC. On the other hand, LS is an additional surgical intervention requiring general anaesthesia. The overall risk of complications of LS is reported between 1 % and 5 % [17], port-site recurrences have been reported in up to 31 % cases [18] and LS causes additional costs for the health system.
Figure 1:
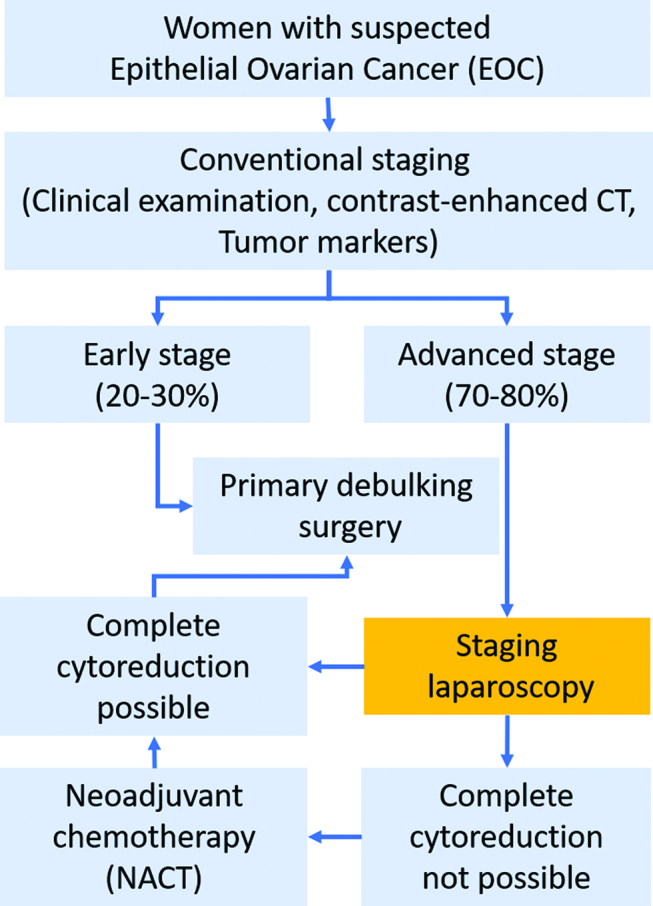
Clinical pathway and diagnostic test (staging laparoscopy).
A Cochrane review published in 2014 (last edited in June 2016) investigated if performing a LS after the conventional diagnostic workup of patients suspected of advanced EOC (AEOC) is accurate in predicting the resectability of disease [19]. This review did not allow to draw firm conclusions: first, the number of studies (seven) was fairly limited. Second, due to the difference in prevalence in CR between studies, there was a wide range in negative predictive values between studies. Third, five studies did not perform the reference standard (explorative laparotomy) in all patients.
Thus, the jury is still out and, at the present point of time, discrepancy exists between guidelines: Whereas the US NCCN current guidelines recommends LS as part of the diagnostic workup in AEOC (Evidence level 2B) [20], the current German S3-guideline explicitly discourages the use of LS in EOC [3]. Since 2014, publication of a randomized controlled trial [14] and of a larger cohort study [21] contributed additional information on this question. This systematic review will review systematically data available, and suggest that LS should now be recommended as part of the initial diagnostic workup in women with advanced EOC (AEOC).
Materials and methods
This systematic review agrees to the guidelines outlined in the preferred reporting items for systematic reviews and meta-analyses statement (PRISMA), which is designed to improve manuscript quality [22]. The PRISMA statement was used in conjunction with the PRISMA explanation and elaboration document [23] and PRISMA abstracts guidelines [24]. A PRISMA flow chart is included, depicting the flow of information through the different phases of this systematic review.
Search strategy and screening
For identifying potentially relevant literature, we searched the MEDLINE database from 1996 to 27 February 2018. Following search terms were used: “ovarian cancer” AND “laparoscopy” AND “staging”. An initial screening of the title of every record found was performed and nonrelevant studies excluded. Then, abstracts were reviewed for meeting inclusion and exclusion criteria, and potential relevant trials selected. Finally, full texts of these trials were downloaded and information extracted.
Eligibility and inclusion
The inclusion criteria for studies to be included in this systematic review were as following: (a) study design: randomized controlled trials OR matched-pair analysis OR well-designed cohort studies; (b) patients: women with advanced EOC; (c) intervention: LS (experimental group) vs. primary laparotomy (control); (d) primary outcome: number of futile laparotomies (FL); (e) secondary outcomes (exploratory): intraoperative and postoperative complication rate, survival, costs.
Following studies were excluded: (a) studies in early epithelial ovarian cancer; (b) studies in languages other than English; non-human studies; (c) case-control studies; case reports or series with less than 10 patients; (d) editorials, (e) previous reviews and meta-analysis; (f) no full-text available.
Data extraction
For each study, information extracted included the following: (1) Study design; (2) General study information (author and year of publication, number of patients, criteria used for defining CR, scoring system and cut-off value) (3) Main results (number of reference tests (PDS); number of complete CR; number of futile laparotomies, complications of LS).
Target conditions
The target condition was the number of futile laparotomies, defined as ovarian cancer residual disease with a diameter larger than 1 cm in diameter.
Test index
Beforehand, physical and ultrasound examination, serum CA 125 measurement and CT or MRI scan were performed. The test index under evaluation was an additional SL before PDS.
Reference standards
A positive index test (LS) result was the prediction that the tumour could not be completely resected; a negative index test (LS) result was the prediction that complete CR would be possible. Laparotomy was the reference standard to determine presence of absence of macroscopic tumour deposits after PDS.
Definitions
Sensitivity was defined as the number of patients with incomplete CR after PDS who were correctly identified (true positives) divided by the total number of patients with CR (true positives, TP+false negatives, FN). Specificity was defined as the number of patients with incomplete CR after PDS who were correctly identified (true negatives, TN) divided by the total number of women with CR after PDS (TN+false positives, FP). PPV was calculated as the number of TP divided by the total number of positive results (TP+FP), and negative predicting values (NPV) was defined as the number of TN divided by the total number of negative results (TN+TN). The percentage of women unnecessarily explored was 1-NPV, and the percentage of women inappropriately unexplored was 1-PPV.
Methodological quality assessment
QUADAS-2 (quality assessment of diagnostic accuracy studies) guidelines were applied for assessing the methodological quality of the studies included: (a) patient selection, (b) index test, (c) reference standard, and (d) flow and timing. For seven out of nine included studies analysis was based on and in accordance with the quality assessment previously performed by Rutten et al. [14].
Data analysis
The analysis was performed using RevMan 5.3 [25]. TP, FP, TN, FN for each study were summarised in tables and used to calculate sensitivity (and specificity when possible). Studies providing insufficient data to be shown on tables were used to present on NPV. Studies are presented graphically by plotting the estimates of sensitivity and – when possible – specificity. Negative predictive values, and when possible, positive predictive values are presented with their 95 % confidence intervals. True positive rate (TPR) and false positive rate (FPR) were used to draw a ROC graph depicting relative trade-offs between true positive (benefits) and false positive (costs). TPR is equivalent to sensitivity and FPR is equal to 1 – specificity. Points above the diagonal dividing the ROC space represent better than random classification results.
Results
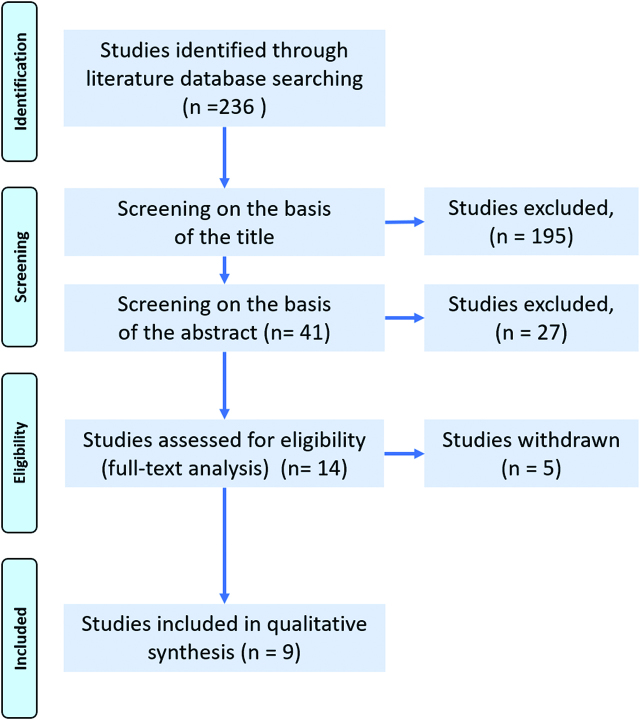
The literature search using the search strategy described above identified 236 studies. The search process is summarized in Figure 2. Ultimately, nine articles were included in these review [14, 21, 22, 23, 26, 27, 28, 29, 30] and summarized in Table 1. Of the articles included, a single one was a randomized controlled trial and two were prospective studies. The remaining trials were retrospective studies or their design was not fully reported. Two studies reported about the same patient cohort, the first one for analysing accuracy of LS, the second one for validating a scoring system [28, 29].
Figure 2:
Flow diagram according to PRISMA guidelines: results of the search for clinical studies evaluating the diagnostic accuracy of Staging Laparoscopy (LS) to predict complete cytoreduction (CR) during Primary Debulking Surgery (PDS) in women with Advanced Epithelial Ovarian Cancer (AEOC).
Table 1:
Summary of studies included.
| Author | Year | Type | FIGO stage |
N pat | N PDS | N CR | CR/ PDS (%) |
N futile lap | Futile lap/patients | Cut-off for futile laparotomy | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Rutten | 2017 | Randomized controlled trial | IIb and higher | *102 | 63 | 53 | 84% | 10 | 10 % | Tumour > 1 cm |
| 2 | Petrillo | 2015 | Retrospective | AOC | 234 | 234 | 189 | 81 % | 45 | 19 % | Tumour > 1 cm |
| 3 | Fagotti | 2005 | Prospective | AOC (18 % I-II) | 95 | 64 | 38 | 59 % | 26† | 27 % | Tumour > 1 cm |
| 4 | Fagotti | 2008 | Prospective | AOC | 113 | 113 | 56 | 50 % | 57 | 50 % | Tumour > 1 cm |
| 5 | Brun | 2008 | Retrospective | AOC | 55 | 26 | 18 | 69 % | 8 | 15 % | Tumour > 1 cm |
| 6 | Brun | 2009 | Same population as Brun 2008 | ||||||||
| 7 | Vergote | 1998 | Retrospective | AOC | 77 | 28 | 21 | 75 % | 7 | 9 % | Tumour > 0.5 cm |
| 8 | Angioli | 2005 | Not reported | IIIc/IV | 87 | 53 | 51 | 96 % | 2 | 2 % | No tumour visible |
| 9 | Deffieux | 2006 | Not reported | IIIc/IV | 15 | 11 | 10 | 91 % | 1 | 7 % | Tumour > 1 cm |
| 778 | 592 (76 % patients) | 436 (56 % PDS) | 50 to 91 % | 156 (20 % patients) | 2 to 50 % | ||||||
Legend: AOC: Advanced ovarian cancer; LS: laparoscopic staging; PDS: Primary debulking surgery; CR: complete cytoreduction; *: LS group only; 39 % futile laparotomy after conventional staging, 10 % after LS, RR 0.25 (95 % CI: 0.13–0.47, p<0.001); in Fagotti 2004 CR status remained undefined in 13 patients; †: N/A: not available; TP: true positive; FP: false positive; FN: false negative; TN: true negative.
Table 2 shows the methodological quality and applicability of the selected studies. Two studies had a high level of quality with low risk of bias and no significant concerns regarding applicability of results [14, 21]. An applicability concern in the study of Rutten could be ruled out by selecting for analysis only the women with FIGO stage IIIc/IV EOC [14]. Two additional studies had a low risk of bias but some applicability concerns, because early-stage EOC patients were included [27, 30]. The other studies had a significant risk of bias due to patient selection, or to applicability concerns (e. g. in the study of Vergote et al. [26] a cut-off for CR of 0.5 cm, whereas all other studies had a cut-off of 1 cm).
Table 2:
Methodological quality and applicability of the selected studies.
| Risk of bias | Applicability concerns | ||||||
|---|---|---|---|---|---|---|---|
| Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference standard | |
| Angioli 2005 | + | ? | + | – | + | + | + |
| Brun 2008 | ? | + | + | – | + | + | + |
| Brun 2009 | ? | ? | + | – | + | + | + |
| Deffieux 2006 | ? | + | + | – | + | + | + |
| Fagotti 2004 | + | + | + | + | –* | + | + |
| Fagotti 2008 | + | + | + | + | –* | + | + |
| Petrillo 2015 | + | + | + | + | + | + | + |
| Rutten 2017 | + | + | + | + | + | + | + |
| Vergote 1998 | + | – | + | – | + | – | + |
Altogether, these nine studies included 778 patients who received LS (index test). Out of these patients, explorative laparotomy was completed in 592 (76 % patients), so that the reference test (laparotomy) was not performed in 21 % patients, or the reference test was performed in only three out of nine studies. Therefore, in six studies, it was not possible to determine sensitivity, specificity or positive predictive value of LS.
In six studies focussing on the value of LS for predicting incomplete CR, PPV was between 0.69 and 1.0 (Table 3). However, it has to be noted that, in the study with the best result [27], PPV was overestimated since 13 undetermined cases were excluded from analysis.
Table 3:
Diagnostic accuracy of LS to predict unresectable disease in the studies included.
| Author | Year | Study type | FIGO stage | N pat |
Reference standard performed | Index test # | Specificity | Sensitivity | NPV | PPV | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Rutten | 2017 | Randomized controlled trial | IIIc/IV | *71 | 68 | TP N/A | FP N/A | N/A | N/A | N/A | 0.91 |
| FN 6 | TN 62 | |||||||||||
| 2 | Fagotti | 2005 | Prospective | AEOC | 64 | 64 | TP 12 | FP 0 | 1.0‡ (95 % CI: 0.90–1.0) | 0.71‡ (95 % CI: 0.44–0.90) | 0.87‡ | 1.0‡ |
| FN 5‡ | TN 34‡ | |||||||||||
| 3 | Vergote | 1998 | Retrospective | AEOC | 285 | 28 | TP N/A | FP N/A | N/A | N/A | N/A | 0.75 |
| FN 7 | FP 21 | |||||||||||
| 4 | Angioli | 2005 | Not reported | IIIc/IV | 87 | 53 | TP N/A | FP N/A | N/A | N/A | N/A | 0.96 |
| FN 2 | TN 51 | |||||||||||
| 5 | Brun | 2009 | Retrospective | AEOC | 55 | 26 | TP N/A | FP N/A | N/A | N/A | N/A | 0.69 |
| FN 8 | TN 18 | |||||||||||
| 6 | Deffieux | 2006 | Not reported | IIIc/IV | 15 | 11 | TP N/A | FP N/A | N/A | N/A | N/A | 0.91 |
| FN 1 | TN 10 | |||||||||||
| Total | 522 | 224 | 0.75 to 1.0 | |||||||||
There is a high risk of bias since 3 out of 5 studies (Vergote, Angioli, and Ruffieux) did not perform the reference standard in all patients; in Rutten, reference standard was performed primarily or after NACT.
Legend: AEOC: Advanced epithelial ovarian cancer; FIGO: Fédération International de Gynécologie Oncologique; NACT: neoadjuvant chemotherapy; N pat: number of patients; PDS: primary debulking surgery; Reference standard=PDS; *: laparoscopy group only, FIGO stage IIIC/IV only; ‡: 13 undetermined cases were excluded, therefore sensitivity, specificity, NPV and PPV overestimated; N/A: not available; # Index test positive=unresectable disease at PDS (futile laparotomy); Index test negative=resectable disease at PDS. TP: true positive; FP: false positive; FN: false negative; TN: true negative.
In the three studies examining the value of a PIV for predicting incomplete CR (FL), sensitivity of the index test (LS with PIV ≥8) was between 46% and 70 %, and specificity between 89 % and 100 % (Figure 3). To allow this analysis, another cut-off value (PIV ≥8) than the original value (PIV ≥10) was selected in the study of Petrillo et al. [21].
Figure 3:
Accuracy of Predictive Index Value (PIV) with a cut-off ≥ 8 to predict unresectable disease in 402 women with Advanced Epithelial Ovarian Cancer (AEOC).
Legend: TP: true positive; FP: faLSe positive; FN: faLSe negative; TN: true negative.
Importantly, the proportion of patients that received CR during PDS differed widely between studies (from 50% to 91 %, Table 1), which might explain in part the large differences observed in sensitivity and specificity between studies (Figure 4). Further factors might be bias in patient selection (depending on the quality of the radiological work-up), the nonavailability of the reference test in about one-fourth patients and differences in the definition of the target condition (various definitions of residual disease after PDS).
Figure 4:
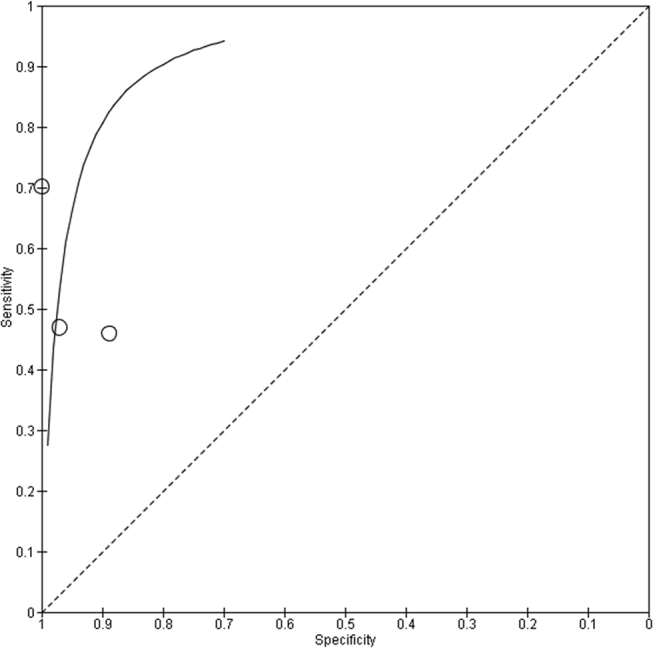
ROC curve of the accuracy (sensitivity vs. specificity) of Predictive Index Value (PIV) with a cut-off value of ≥ 8 in Advanced Epithelial Ovarian Cancer (AEOC) for predicting non-resectability (futile laparotomies) during Primary Debulking Surgery (PDS). Calculation based on 3 studies (Brun 2009, Fagotti 2008, Petrillo 2015).
Only the patients having received LS in the prospective randomized trial of Rutten were included in this review. Against this framework, it is important to note that, in this study, FL occurred in 10 (10 %) of 102 patients in the laparoscopy group versus 39 (39 %) of 99 patients in the primary surgery group (relative risk, 0.25; 95 % CI, 0.13–0.47; p<0.001).
Discussion
Determining the best therapeutic strategy is essential in women with EOC: One the one hand, complete CRS should be achieved but, on the other hand, the number of futile laparotomies should be minimal. Thus, the key is to select patients in whom complete CRS can be achieved, first in primary and if not possible, in the interval setting [31, 32, 33, 34].
Whereas the evidence for inclusion of LS in the initial staging of AEOC is now given, interpretation of this evidence should remain careful.
Progress in CT imaging technology allows better identification and characterization of metastatic lesions in clinical practice. For predicting incomplete CR (FL in AEOC, contrast-enhanced CT has recently been reported to have a sensitivity, specificity, PPV and NPV of 70.6 %, 73.2 %, 68.7 %, and 91.9 %, respectively) [13]. Specific radiologic criteria predicting incomplete CR were: lesions in the splenic hilum/ligaments (OR 1.36), retroperitoneal lymph nodes above the renal hilum (including supradiaphragmatic), (OR 1.31), gastrohepatic ligament/porta hepatis lesions (OR 1.44), diffuse small bowel adhesions/thickening (OR 1.12), lesions in the gallbladder, fossa/liver intersegmental fissure (OR 2), moderate-severe abdominal ascites (OR 2.21), lesser sac lesions>1 cm (OR 2.24) and lesions in the root of the SMA (OR 4.06). All ORs were significant with p<0.01. ROC curveswere generated to compare differentmodels’ accuracy in predicting gross RD. The eight CT criteria combined showed an AUC of 0.694. These figures do not to differ considerably from the figures obtained with LS (Table 4). It has to be noted, however, that, predictive value of CT for CR appears highly variable, depending on the stage of disease (NPV between 45 % and 96 %) [35] and probably on the technology available and on the expertise available.
Table 4:
Comparing Sensitivity, Specificity, PPV and NPV between CT and LS.
| Sensitivity | Specificity | PPV type | NPV | |
|---|---|---|---|---|
| CT | 71% | 73% | 69% | 92% |
| LS | 46–70% | 89–100% | 69–100% | 87% |
Although evidence available suggests that SL is largely equivalent to explorative laparotomy for staging the extent of intraperitoneal spread in EOC, a significant number of women cannot be evaluated by laparoscopy. For example, the presence of adhesions can prevent the access to the abdomen, or impair complete exploration of the peritoneal cavity. For example, in the study of Fagotti et al. [27], definitive conclusions on the possibility of optimal CR could not be drawn in 13/64 patients. Moreover, PIV score is focused on the assessment of intraperitoneal diffusion of the disease, not being able to properly evaluate the retroperitoneal space, which prevents complete CR in many cases.
The rate of complications after LS is low, but some of these complications can be severe [36]. The critical phase of LS is the abdominal access, and vessel and bowel injuries have been reported. The rate of abdominal access injuries varies from 5 to 30 per 10,000 procedures: 4.4 per 10,000 for bowel injuries, 3.1 per 10,000 for vascular injuries, and 3 per 100 for injuries related to umbilical trocar insertion [37].
A specific adverse event after LS are port site tumour recurrences, with a reported clinical incidence of 2 % [29], 3 % [26], 3 % [14] and 6 % [23] in this systematic review. Subclinical, histological port site recurrences might be more frequent [18]. Port-site recurrences after LS in AEOC probably have no impact on survival [38]. Preventive measures such as closure of the fascia might reduce their incidence [39]. Finally, placement of the abdominal access ports on the midline could help to control this potential complication of LS.
At least seven systems with different cut-offs (PIV) have been proposed for scoring the extent of intraperitoneal disease (reviewed in 41). However, the only randomized study showed superiority of LS over conventional staging alone in predicting incomplete CR without any PIV [14]. In fact, using a prediction model does not increase the sensitivity and might result in more patients unsuccessful debulking surgery [19]. Finally, completeness of might be is more related to the surgical effort than to the extent of the disease [40].
A recent randomized study evaluated the cost-effectiveness of SL prior to primary cytoreductive surgery to prevent futile primary cytoreductive surgery in 201 patients suspected of advanced stage ovarian cancer. SL generated costs of € 1400 per intervention but reduced the proportion of futile laparotomies from 39 % to 10 %. Overall costs of both strategies were found to be comparable. No significant difference in quality adjusted life years(QALYs) (utility=0.01; 95 % CI 0.006–0.02) was observed [15].
Taken together, the evidence available from this systematic review supports the inclusion of additional LS to the conventional initial diagnostic workup in women with AEOC. The conclusion on this systematic review is in accordance with the conclusion of the only RCT available in the field [14], and with the recommendation of the current US NCCN guideline [20].
Footnotes
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: None declared.
Employment or leadership: None declared.
Honorarium: None declared.
Competing interests: The funding organization(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29. [DOI] [PubMed]
- 2.NCCN Clinical Practice Guidelines in Oncology. Ovarian cancer including fallopian tube cancer and primary peritoneal cancer Version I. 2015. www.nccn.org
- 3.https://www.ago-online.de/fileadmin/downloads/leitlinien/ovar/2016/032-035-OLl_Ovarialkarzinom_2016-10.pdf#page=1&zoom=auto,-158,843, consulted on Feb 25, 2018
- 4.Al Rawahi T, Lopes AD, Bristow RE, Bryant A, Elattar A, Chattopadhyay S, et al. Surgical cytoreduction for recurrent epithelial ovarian cancer. Cochrane Database Syst Rev 2013;(2):CD008765. [DOI] [PMC free article] [PubMed]
- 5.Ataseven B, Grimm C, Harter P, Heitz F, Traut A, Prader S, et al. Prognostic impact of debulking surgery and residual tumor in patients with epithelial ovarian cancer FIGO stage IV. Gynecol Oncol 2016;140:215–20. [DOI] [PubMed]
- 6.Chang SJ, Bristow RE, Chi DS, Cliby WA Role of aggressive surgical cytoreduction in advanced ovarian cancer. J Gynecol Oncol 2015;26:336–42. [DOI] [PMC free article] [PubMed]
- 7.Kehoe S, Hook J, Nankivell M, Jayson GC, Kitchener H, Swart AM, et al.: Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet 386:249–57, 2015 [DOI] [PubMed]
- 8.Elattar A, Bryant A, Winter-Roach BA, Hatem M, Naik R Optimal primary surgical treatment for advanced epithelial ovarian cancer. Cochrane Database Syst Rev 2011;(8):CD007565. [DOI] [PMC free article] [PubMed]
- 9.Mahner S, Heitz F, Burges A, Reuss A, Kraemer B, Schmalfeldt B TRUST: trial of radical upfront surgical therapy in advanced ovarian cancer (ENGOT ov33/AGO‐OVAR OP7). J Clin Oncol 2017;35:15_suppl, TPS5602-TPS5602.
- 10.Wright AA, Bohlke K, Armstrong DK, Bookman MA, Cliby WA, Coleman RL et al. Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: society of gynecologic oncology and American society of clinical oncology clinical practice guideline. J Clin Oncol 2016;34:3460–73. [DOI] [PMC free article] [PubMed]
- 11.Lee M, Kim SW, Paek J, Lee SH, Yim GW, Kim JH, et al. Comparisons of surgical outcomes, complications, and costs between laparotomy and laparoscopy in early-stage ovarian cancer. Int J Gynecol Cancer 2011;2:251–6. [DOI] [PubMed]
- 12.Sahdev A CT in ovarian cancer staging: how to review and report with emphasis on abdominal and pelvic disease for surgical planning. Cancer Imaging 2016;16:19. [DOI] [PMC free article] [PubMed]
- 13.Son HM, Kim SH, Kwon BR, Kim MJ, Kim CS, Cho SH Preoperative prediction of suboptimal resection in advanced ovarian cancer based on clinical and CT parameters. Acta Radiol 2017;58:498–504. [DOI] [PubMed]
- 14.Rutten MJ, Van Meurs HS, Van De Vrie R, Gaarenstroom KN, Naaktgeboren CA, Van Gorp T et al. Laparoscopy to predict the result of primary cytoreductive surgery in patients with advanced ovarian cancer: a randomized controlled trial. J Clin Oncol 2017;35:613–21. [DOI] [PubMed]
- 15.Van De Vrie R, Van Meurs HS, Rutten MJ, Naaktgeboren CA, Opmeer BC, Gaarenstroom KN et al. Cost-effectiveness of laparoscopy as diagnostic tool before primary cytoreductive surgery in ovarian cancer. Gynecol Oncol 2017;146:449–56. [DOI] [PubMed]
- 16.Gómez-Hidalgo NR, Martinez-Cannon BA, Nick AM, Lu KH, Sood AK, Coleman RL, et al. Predictors of optimal cytoreduction in patients with newly diagnosed advanced-stage epithelial ovarian cancer: time to incorporate laparoscopic assessment into the standard of care. Gynecol Oncol 2015;137:553–58. [DOI] [PMC free article] [PubMed]
- 17.Chi DS, Abu-Rustum NR, Sonoda Y, Awtrey C, Hummer A, Venkatraman ES, et al. Ten-year experience with laparoscopy on a gynecologic oncology service: analysis of risk factors for complications and conversion to laparotomy. Am J Obstet Gynec 2004;191:1138–45. [DOI] [PubMed]
- 18.Heitz F, Ognjenovic D, Harter P, Kommoss S, Ewald-Riegler N, Haberstroh M, et al. Abdominal wall metastases in patients with ovarian cancer after laparoscopic surgery: incidence, risk factors, and complications. Int J Gynecol Cancer 2010;20:41–46. [DOI] [PubMed]
- 19.Rutten MJ, Leeflang MM, Kenter GG, Mol BW, Buist M Laparoscopy for diagnosing resectability of disease in patients with advanced ovarian cancer. Cochrane Database Syst Rev 2014;(2):CD009786. [DOI] [PMC free article] [PubMed]
- 20.Guidelines in Oncology for Ovarian Cancer. https://www.tri-kobe.org/nccn/guideline/gynecological/english/ovarian.pdf, consulted on Feb 25, 2018.
- 21.Petrillo M, Vizzielli G, Fanfani F, Gallotta V, Cosentino F, Chiantera V, et al. Definition of a dynamic laparoscopic model for the prediction of incomplete cytoreduction in advanced epithelial ovarian cancer: proof of a concept. Gynecol Oncol 2015;139:5–9. [DOI] [PubMed]
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Open Med 2009;3:123–30 [PMC free article] [PubMed]
- 23.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Gøtzsche PC, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009;6:e1000100 [DOI] [PMC free article] [PubMed]
- 24.Beller EM, Glasziou PP, Altman DG, Hopewell S, Bastian H, Tovey D, et al. PRISMA for abstracts: reporting systematic reviews in journal and conference abstracts. PLoS Med 2013;10:e1001419. [DOI] [PMC free article] [PubMed]
- 25.Review manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. 2014.
- 26.Vergote I, De Wever I, Tjalma W, Van Gramberen M, Decloedt J, van Dam P Neoadjuvant chemotherapy or primary debulking surgery in advanced ovarian carcinoma: a retrospective analysis of 285 patients. Gynecol Oncol 1998;71:431–36. [DOI] [PubMed]
- 27.Fagotti A, Fanfani F, Ludovisi M, Lo Voi R, Bifulco G, Testa AC, et al. Role of laparoscopy to assess the chance of optimal cytoreductive surgery in advanced ovarian cancer: a pilot study. Gynecol Oncol 2005;96:729–35. [DOI] [PubMed]
- 28.Brun JL, Rouzier R, Uzan S, Daraï E External validation of a laparoscopic-based score to evaluate resectability of advanced ovarian cancers: clues for a simplified score. Gynecol Oncol 2008;110:354–59. [DOI] [PubMed]
- 29.Brun JL, Rouzier R, Selle F, Houry S, Uzan S, Daraï E Neoadjuvant chemotherapy or primary surgery for stage III/IV ovarian cancer: contribution of diagnostic laparoscopy. BMC Cancer 2009;9:171. [DOI] [PMC free article] [PubMed]
- 30.Fagotti A, Ferrandina G, Fanfani F, Garganese G, Vizzielli G, Carone V, et al. Prospective validation of a laparoscopic predictive model for optimal cytoreduction in advanced ovarian carcinoma. Am J Obstet Gynecol 2008;199:642.e1–6. [DOI] [PubMed]
- 31.Hoskings WJ, McGuire WP, Brady MF, Homesley HD, Creasman WT, Berman M The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial ovarian canrcinoma. Am J Obstet Gynecol 1994;170:974–79. [DOI] [PubMed]
- 32.Chi DS, Eisenhauer EL, Lang J, Huh J, Haddad L, Abu-Rustum NR, et al. What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)? Gynecol Oncol 2006;103:559–64. [DOI] [PubMed]
- 33.Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol 2002;20:1248–59. [DOI] [PubMed]
- 34.Kang S, Kim TJ, Nam BH, Seos SS, Kim BG, Bae DS, et al. Preoperative serum CA-125 levels and risk of suboptimal cytoreduction in ovarian cancer: a meta-analysis. J Surg Oncol 2010;101:13–17. [DOI] [PubMed]
- 35.Suidan RS, Ramirez PT, Sarasohn DM, Teitcher JB, Iyer RB, Zhou Q et al. multicenter assessment of the ability of preoperative computed tomography scan and CA-125 to predict gross residual disease at primary debulking for advanced epithelial ovarian cancer. Gynecol Oncol 2017;145:27–31. [DOI] [PMC free article] [PubMed]
- 36.Ahmad G, Gent D, Henderson D, O’Flynn H, Phillips K, Watson A Laparoscopic entry techniques. Cochrane Database Syst Rev 2015;8:CD006583. [DOI] [PubMed]
- 37.Pryor A, Mann WJ, Gracia G, Marks J, Falcone T, Chen W Complications of laparoscopic surgery. UpToDate 2013. www.uptodate.com/contents/complications-of-laparoscopic-surgery#
- 38.Ataseven B, Du Bois A, Harter P, Prader S, Grimm C, Kurzeder C, et al. Impact of abdominal wall metastases on prognosis in epithelial ovarian cancer. Int J Gynecol Cancer 2016;26:1594–600. [DOI] [PubMed]
- 39.Agostini A, Mattei S, Ronda I, Banet J, Lécuru F, Blanc B Prevention of port-site metastasis after laparoscopy. Gynecol Obstet Fertil 2002;30:878–81. Review. [DOI] [PubMed]
- 40.Chéreau E, Ballester M, Selle F, Cortez A, Daraï E, Rouzier R Comparison of peritoneal carcinomatosis scoring methods in predicting resectability and prognosis in advanced ovarian cancer. Am J Obstet Gynecol 2010;202:178.e1–10. [DOI] [PubMed]