Abstract
Background
Pseudomyxoma peritonei (PMP) is difficult to treat. Intraperitoneal delivery of mucolytic solutions might potentially improve therapy, in addition to surgical cytoreduction and hyperthermic intraperitoneal chemotherapy.
Methods
Comparison of mucolytic effect of two formulations (control: bromelain 300 µg/mL+N-Acetylcystein 250 mM; test: bromelain 200 µg/mL+200 mM cysteamine) in vitro on a mucin producing cell lines (HT29) and ex vivo on mucus obtained from 18 PMP patients. Mucin plugs were classified according to their density into three categories: hard, semi hard and soft. Simulation of peritoneal washing ex vivo using a closed heated circulating pump.
Results
Solubilisation was faster with the test vs. the control formulation (90 vs. 180 min) for dissolving the soft mucin plugs (p < 0.05). The test solution was also more effective in dissolving the hard mucus plugs compared to control (82.5±2.74 % vs. 36.33±3.27 %). All mucin types disintegrated in simulated peritoneal washing. Cytotoxicity of the test solution on HT29 cell line was time-dependent.
Conclusions
The test formulation is more effective and faster than the control formulation in dissolving mucus plugs of various densities. Mucus plugs were all solubilised after 40 min in simulated peritoneal washing. This novel mucolytic formulation might pave the way for an effective and less invasive therapy of PMP in the future.
Keywords: bromelain, cysteamine, disintegration, hardness index, mucin, mucolytic agents and intraperitoneal chemotherapy, pseudomyxoma peritonei
Introduction
Pseudomyxoma peritonei (PMP) is a rare disease with secretion of mucinous ascities in the peritoneal cavity. Excess peritoneal mucin accumulation and intestinal blockage often lead to nutritional compromise with subsequent patient demise [1, 2]. PMP mucin secreting tumour cells originate commonly from ruptured appendix tumours although they may also arise from colorectal, ovarian, pancreas, lungs and other sources [3, 4]. Current treatment methods involving laparotomy, cytoreduction and hyperthermic intraperitoneal chemotherapy, as first instituted by Sugarbaker [5], has improved patient 5 year survival to > 80 % [6]. However, owing to the invasive nature of the treatment, patients requiring subsequent treatment often suffer from various compromises [7, 8]. If the mucin can be solubilised, in situ, treatment may only involve an abdominal puncture with mucin removal through a catheter. This is a much less invasive process that can be repeated.
Our previous mucolytic (bromelain+N-acetyl cysteine) was efficient in both in vitro and in vivo model [9, 10]. Mucin is composed of numerous units of glycoproteins which polymerises using both glycosidic and disulfide linkages [11]. Bromelain hydrolyses glycosidic linkages [12], whilst NAC reduces disulphide bonds [13]. NAC and cysteamine are antioxidant thiols, the former being used for treating acetaminophen overdose [14]. Cysteamine prevents selenite induced cataract in rats [15], reduce invasion and metastasis in mouse model of pancreatic cancer [16]. Cysteamine is a reducing aminothiol [17], used for treating cystinosis [18, 19]. We hypothesised that since cysteamine (mol. wt.=77) is a much smaller molecule compared to NAC (mol. wt.=163), it may have a better penetration through the mucinous mass. Therefore, we investigated the mucolytic efficiacy of cysteamine in combination with bromelain that contains a number proteolytic enzymes [20, 21]. Since there is an inter-patient variability in hardness of PMP mucin, we have classified patient mucin samples into three categories (soft, semi hard and hard)[10]. Using these three categories of mucin, we compared the in vitro mucolytic efficacy of cysteamine+bromelain (test formulation) to that of NAC+bromelain (control formulation). Finally, we also used a formula with minimal concentration of cysteamine and bromelain for treating the three grades of mucin in a simulated peritoneal wash since a large volume of solution will be used in the latter process.
Materials and methods
Materials
Pharmaceutical grade bromelain, cysteamine and other chemicals of analytical grade were purchased from Sigma Aldrich, Sydney, Australia. With approval from the ethics committee of St. George Hospital, pseudomyxoma peritonei mucin samples were collected from patients undergoing treatment and frozen under sterile condition at – 20 °C. They mucin samples were thawed at room temperature before use.
Methods
Ex vivo
Time profile dissolution study on soft mucin comparing 200 µg/mL bromelain+200 mM cysteamine (test formulation) to 300 µg/mL bromelain+250 mM N-acetyl cysteine (control formulation)
To a 50ml centrifuge tube containing 1 g of mucin was added 10 mL of Tris buffer pH.7.0 containing 300 µg/mL bromelain+250 mM NAC. Similarly, to 1 g of mucin was added 10 mL of TRIS buffer containing 200 µg/mL bromelain+200 mM cysteamine. For each time interval (½, 1.0, 1 ½, 2.0, 2 ½ and 3.0 hours), a total of 6 tubes (for each time interval) in triplicates were prepared. The controls contained, TRIS, Bromelain (300 µg/mL and 200 µg/mL), NAC (250 mM) and cysteamine (200 mM) prepared in TRIS at pH.7.0 with mucin (Table 1).The tubes were incubated at 37 °C, in a shaker water bath and from sample tubes, at time interval of ½ hour, remanent mucin was carefully retrieved and weighed. Percentage weight lost was calculated as follows:
Table 1:
Chemical composition of the control and test formulation.
| Composition (chemical) | Control formulation | Test formulation |
|---|---|---|
| Bromelain | 300 µg/mL | 200 µg/mL |
| N-Acetylcystein | 250 mM | – |
| Cysteamine | – | 200 µg/mL |
Percentage weight lost (disintegration)=[mucin weight (before) – mucin weight (after)]/mucin weight (before).
Comparison of disintegration of different grades of PMP mucin (soft, semi hard and hard) treated to 200 µg/mL bromelain+200 mM cysteamine (test formulation) or 300 µg/mL bromelain+250 mM N-acetyl cysteine (control formulation) for 3 hours
The experimental set up was similar to that as described above. Experiments were performed using the 3 grades of mucin samples, soft, semi hard and hard as shown in (Figure 1A). Six samples of mucin were chosen from each mucin category (grades) and subjected to mucolytics over 3 hours. At the end of 1 1/2 hours and 3 hours hours, samples were retrieved and weighed.
Figure 1:
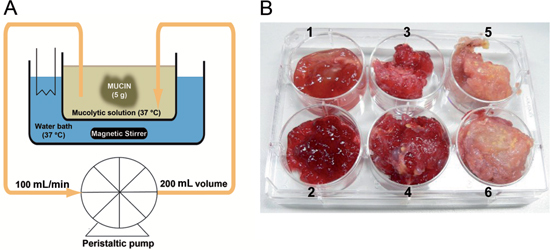
(A) Diagrammatic version of the peritoneal wash with representative equipments used. (B) Appearance of the three grades of mucin collected from PMP patients.
1-2 represents soft mucin; 3-4 represents semi hard mucin and 5-6 represents hard mucin.
Calculations:
Weight lost/min (disintegration rate)=Initial weight (g) – Final weight (g) /Time taken (min)
Simultation study
Peritoneal wash simulation to disintegrate PMP mucin with 50 µg/mL bromelain+50 mM cysteamine
A mass of 5 g of mucin from each mucin grade was deposited into a 400 mL beaker that has an inflowing and out flowing polypropylene tube and containing either 200 mL of Tris buffer (pH.7.0), 200 mL of Tris with 50 µg/mL bromelain, 200 mL of Tris with 50 µg/mL cysteamine or 200 mL Tris containing 50 µg/mL bromelain+50 mM cysteamine. Using a peristaltic pump, the solutions were circulated (100 mL/min), with the mucin completely soaked in the solution at 37 °C. (Figure 1B). At an interval of 20 and 40 min, the weight of the remaining mucin sample from the different grades of mucin was recorded.
Calculations:
Disintegration rate: Initial weight (g) – Final weight (g) /time (min)
In vitro
In vitro cytotoxicity effect of bromelain+cysteamine on mucin producing colorectal adenocarcinoma cell line (HT29)
5000 cells/well were cultured in a 96 well plate containing 200 µL of culture media (RPMI) at 37 °C for three days until 50 % confluence with evidence of mucin production. The cells were treated with RPMI (control), RPMI containing 50 µg/mL bromelain+50 mM cysteamine or 200 µg/mL bromelain, +200 mM cysteamine and incubated for 1, 2 and 3 hours at 37 °C, after which the media was removed and washed twice with RPMI media. The wells were then filled with 200 µL. RPMI culture media and followed at 37 °C over a period of 1–2 days, at each day interval; the cells were fixed in 10 % TCA (trichloroacetic acid) in ice and then subjected to standard sulfhordamine assay [22] to access cell viability.
Statistics
Comparison of mean values between two groups were carried out using non-parametric test with 95 % confidence limit denoted as p < 0.05. Graph pad prism software 5.0 version was used for drawing of graphs and statistical analysis. (Version–5.0, Graph pad software, La Jolla, CA, USA).
Results
Time profile dissolution study on soft mucin, comparing 300 µg/mL bromelain + 250 mM N-acetyl cysteine (control formulation) to 200 µg/mL bromelain + 200 mM cysteamine (test formulation)
Only 200 µg/mL bromelain+200 mM cysteamine showed a 100 %±0.00 disintegration at 1 ½±0.00 hours. In contrast, a combination of 300 µg/mL bromelain+250 mM N-acetyl cysteine took 3.0±0.00 hours (twice as long) (Figure 2)
Figure 2:
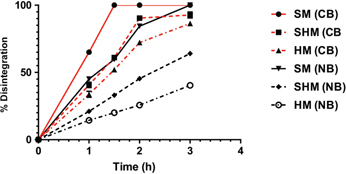
Time (hours) taken for the three grades of mucin to have a 50% disintegration and greater when treated with 200 µg/mL bromelain +200 mM cysteamine or 300 µg/mL bromelain+250 mM N-acetyl cysteine (NAC).
Only soft mucin has 100 % disintegration when treated with both the formulation. The semi hard and hard mucin also have a substantial (>86 %) disintegration when treated with bromelain+cysteamine. SM=soft mucin; SHM=semi hard mucin; HM=hard mucin.
Individual agents only showed hydration initially, with subsequent slight disintegration and hydration. At 3 hours, all the individual agents, except TRIS buffer showed no gain in weight. Further, the time to 50 % disintegration for cysteamine+bromelain was 25±1.2 min whilst for NAC+bromelain; it was 60±3.3 min (twice as long).
Comparative disintegration of different grades of PMP mucin (soft, semi hard and hard) treated to 200 µg/mL bromelain + 200 mM cysteamine (test formulation) over 3 hours
Soft mucin treated with 200 µg/mL bromelain+200 mM cysteamine produced 100 %±0.00 dissolution within 1 1/2 hours, however only 85.33±3.50 % semi soft and 72.18±1.95 % hard mucin disintegrated during this time (Table 2, Figure 3A). At 3 hours the dissolution was 90.3±2.73 % for semi hard and 82.5±2.74 % for hard mucin. Hence, at 3 hours almost all semi hard mucin disintegrated whilst only a small percentage of the hard mucin remained. On the other hand 300 µg/mL bromelain+250 mM NAC produced 79±2.86 % dissolution of soft mucin within 1 1/2 hours and considerably less for semi hard (41.5±3.08 %) and hard mucin (24±2.28 %) (Table 2, Figure 3B). However, at 3 hours soft mucin disintegrated completely, whilst 60.8 %±4.02 % of semi hard and 36.3±3.27 % of hard mucin disintegrated. Hence, the novel formula outperforms the previous formulation.
Table 2:
Percentage disintegration in the three grades of PMP mucin at 1 ½ and 3 hours when subjected to either 200 µg/mL bromelain+200 mM cysteamine or 300 µg/mL bromelain+250 mM NAC at 37 °C.
| Test formula | Control formula | |||
|---|---|---|---|---|
| Cysteamine+bromelain | NAC+bromelain | |||
| Mucin type | 1 1/2 hours | 3 hours | 1 1/2 hours | 3 hours |
| Soft mucin | 100±0.00 | 100±0.00 | 79.0±2.86 | 100±0.00 |
| Semi hard mucin | 85.3±3.50 | 90.3±2.173 | 41.5±3.08 | 60.8±4.02 |
| Hard mucin | 72.18±1.95 | 82.5±1.95 | 24.0±2.18 | 36.3±3.27 |
Figure 3:
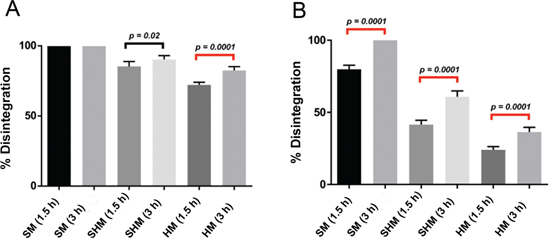
(A) Comparative percentage disintegration of different grades of mucin when treated with test formulation (200 µg/mL bromelain+200 mM cysteamine) with statistical significance.Noticeably at 1 ½ hours, all soft mucin disintegrated. A similar comparison is made for the treatment with 300 µg/mL bromelain+250 mM N-acetyl cysteine (B).
Statistical significance (p < 0.05) existed when the difference in percentage disintegration for semi hard and hard mucin was examined for bromelain+cysteamine at 1 1/2 and 3 hours. A similar comparison for bromelain+NAC also showed statistical significance for all mucin types. Hence, time is a factor that affects disintegration.
Peritoneal wash simulation with 50 µg/mL bromelain + 50 mM cysteamine
The weight of residual mucin in each class of mucin (Table 3, Figure 4A) indicates that at the end of 20 min, all soft mucin was completely disintegrated whilst 51±3.16 % of semi hard and 53.25±3.77 % hard mucin disintegrated. However, after a further 20 min wash, 86±1.29 % of semi hard and 86±3.20 % hard mucin disintegrated. This clearly indicates that all the three classes of mucin can be effectively disintegrated in situ using a peritoneal wash.
Table 3:
Mean weight (g) of mucin at the end of 20 and 40 min in a simulated peritoneal wash. Initial weight of mucin was 5.0 g.
| Twenty minutes wash | Forty minutes wash | |||||
|---|---|---|---|---|---|---|
| Reagents | Soft mucin, g | Semi hard mucin, g | Hard mucin, g | Soft mucin, g | Semi-hard mucin, g | Hard mucin, g |
| Tris | 14.2±1.90 | 9.2±0.9 | 8.0 | 17.2±1.8 | 11.6±1.4 | 10.6±1.9 |
| CYS | 5.0±0.70 | 5.6±0.5 | 7.1 | 5.2±0.6 | 5.9±0.6 | 8.0±0.9 |
| BR | 7.0±0.60 | 6.2±0.4 | 15.6 | 7.6±0.8 | 6.8±0.8 | 16.0±2.1 |
| CYS+BR | 0 | 2.5±0.3 | 2.5 | 0 | 0.7±0.1 | 0.8±1.0 |
Tris, Tris-hydrochloric buffer (pH.7.0); CYS, cysteamine; BR, bromelain.
Figure 4:
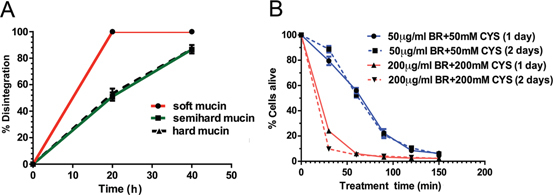
(A) Percentage disintegration of the tree grades of mucin over a time period of 40 minutes when treated with 50 µg/mL bromelain+50 mM cysteamine in a simulated peritoneal wash at 37 °C. (B) Cytotoxic effect on colorectal cell line (HT29) with treatment of either 50 µg/mL bromelain+50 mM cysteamine or 200 µg/mL bromelain+200 mM cysteamine at 37 °C, over 2 ½ hours (150 min).
Comparison of disintegration efficiency of non-perfusion model to perfusion model
Based on 50 % disintegration of mucin, mucolysis proceeded at a much higher rate in the perfusion model (peritoneal wash). Comparing the 50 % disintegration (D50 %) of perfusion model to non-perfusion model, the disintegration rate is approximately about 20 times faster (Table 4, Figure 4A).
Table 4:
Comparison of disintegration efficiency of non perfusion model to perfusion model. Mean disintegration rate (mg/min) for 50 % disintegration.
| Mucin Type | PM | NPM | Ratio: PM/NPM |
|---|---|---|---|
| Soft | 250 | 11.11 | 22.7 |
| Semi hard | 125 | 7.14 | 17.5 |
| Hard | 125 | 6.02 | 20.8 |
PM, perfusion model; NPM, non-perfusion model; disintegration rate, initial weight (mg) – final weight (mg) for 50 % disintegration/time (minutes).
In vitro cytotoxicity effect of a mixture of bromelain and cysteamine on mucin-producing colorectal cancer cell line (HT29)
Treatment of HT29 cells (mucin producing colorectal tumour cells) with 50 µg/mL bromelain+50 mM cysteamine over 30–150 min and re-culturing in normal culture media (RPMI) over 1–2 days shows a similar pattern of cell death. Like wise at higher concentration of both the agents also showed a similar pattern although with a steeper curve of cell death indicating an accelerated effect (Figure 4B). The time to achieve IC50 value with exposure to 200 µg/mL bomelain+200 mM cysteamine was about 22 min, whilst exposure to lower concentrations of the agents was about 65 min (about 3 times as long).
Discussion
Although initial studies indicated that a combination of 50 – 200 mM cysteamine to 100–300 µg/mL bromelain disintegrated soft mucin completely, a combination of 200 mM cysteamine+200 µg/mL bromelain was chosen for further studies on the different grades of mucin. This combination disintegrated soft mucin within 1 ½ hours. Subsequent comparison of time to complete disintegration for soft mucin with 300 µg/mL bromelain +250 mM NAC (control formulation) to 200 µg/mL bromelain+200 mM cysteamine (test formulation) showed 3±0.00 hours and 1 ½±0.00 hours, respectively. Hence, the test formulation was twice as efficient.
The test formulation also disintegrated the semi hard mucin almost completely (90.33±2.733 %) and 82.5± 2.74 % of hard mucin within 3 hours. A similar comparison for control formulation only showed 79.83±2.858 % for soft mucin, and much less for semi hard mucin (41.5±3.08 %) and hard mucin (24±2.28 %). However at 3 hours, the control formula disintegrated the soft mucin completely whilst the effect on semi hard mucin was 60.83±4.02 % and 36.33±3.27 % on hard mucin. Examining the effect of mucin variability (hardness) on percentage disintegration, the test formulation gave a value of 3.36, indicating that the extension of another 1 ½ hours produced substantial disintegration on semi hard and hard mucin. Previous formula showed no such effect.
Both 50 % disintegration and subsequent 50 % mucin disintegration were similar (11.11 mg/min) for the soft mucin with the test formulation, however with the control formulation, initial 50 % disintegration proceeded at 7.14 mg/min with subsequent disintegration proceeded at 4.54 mg/min. Using the test formulation, the 50 % disintegration of semi hard was 7.14 mg/mL (similar to control formulation for soft mucin) and 6.02 mg/mL for hard mucin. However, similar comparison for control formulation was 3.3 mg/mL and 2.77 mg/mL. Hence, the disintegration rate is far superior with the test formulation. Further comparison of 50 % disintegration for the two formula (comparison of the slopes of the graphs) indicated that the new formula was about 4.5 times faster (125/17.5), indicative of its superior efficacy.
For use in a peritoneal wash a milder formulation comprising of 50 µg/mL bromelain+50 mM cysteamine only disintegrated the soft mucin within 3 hours. Subsequent investigation using a mixture of bromelain (50 µg/mL)+cysteamine (50 mM) in a simulated peritoneal wash involving mucolytic volume: mucin weight of 40: 1, has shown that all soft mucin can be disintegrated in 20 min whilst the semi hard and hard mucin takes a little longer time (40 min) with very small quantities of residues that appeared to be of cellular in nature. Hence, this study indicated that using a milder formulation, mucolysis can be achieved quite completely for all mucin types. This enhanced mucolysis can be accounted firstly in terms of cysteamine and bromelain used. Original dissolution work used a ratio of 1 g of mucin: 10 mL of mucolytic; to account for the low concentration of the reagents, the total mucolytic volume was increased to 4 fold, hence using 40ml: 1 g of mucin. Secondly, the perfusion that delivered 100ml/min produced a shear force in the solution that affected the dissolution of mucin. HIPEC has been reported to be performed with a flow speed of 1 L/min [23], and at this speed, tremendous turbulence and shear force will be generated that may further affect mucin dissolution. The comparison of 50 % disintegration rate between the non-perfusion and perfusion model (peritoneal wash) indicated that the latter model was about 20 times more efficient.
The treatment of HT29, a common mucin producing tumour for 30–150 min (1/2–2 ½ hours) to a mixture containing either 50 µg/mL bromelain+50 mM cysteamine or 200 µg/mL bromelain+200 mM cysteamine showed a dramatic reduction of viable cells. Subsequent exposure of cells to normal media (RPMI) did not rehabilitate these cells at either 1 or 2 days, indicating that damage to cells was permanent during this short exposure to the mucolytic. It also indicates that exposure to the lower concentrations may require longer exposure time i. e. 150 min, whilst with the stronger formula (200 µg/mL bromelain+200 mM cysteamine, about 90 min exposure was sufficient to kill all the cells. The time to 50 % inhibition of cell proliferation indicates that the cell viability as assessed after 24 hours was sufficient. Further, relating the cell viability studies to the peritoneal wash, the higher concentration of bromelain and cysteamine (200 µg/mL+200 mM) used in the cell viability study correlates with the peritoneal wash which used 4 times the volume at a lower concentration of the agents.
Hence, this study indicates a potential for the test formulation to be used in the treatment of PMP. Although cysteamine which reduces the intra-lysosomal cysteine concentration was first introduced for treatment of cystinosis in 1976, it was approved by the US Food and Drug Administration in 1994 and has been used safely in the treatment of cystonosis by oral route [18, 24]. Bromelain on the other hand has recently been approved for wound debridement by European Medicines Agency that is marketed as gel or powder. However, the therapeutic potential of bromelain has not been fully exploited since it has a number of biochemical properties in treatment of cancer [25]. It has been mainly used orally and administration of 12 g/day has been shown to have no major side effects [26]. Indeed the combination of bromelain and cysteamine has never been used in therapies and this needs further investigation for both efficacy and safety with in vivo models. In reality, the peritoneal wash may be conveniently instituted through a catheter, prior to chemotherapy, in order to remove mucinous ascities from the peritoneal cavity with sensitization of tumour cells to subsequent chemotherapeutic drugs thereby improving therapeutic efficacy of the treatment [27, 28, 29] This is foreseeable, since the mucolytic, in the first instance has the capacity to remove mucinous barrier to chemotherapeutic agent and secondly may reduce the number of viable tumour cells for action by the chemotherapeutic agents.
Footnotes
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: None declared.
Employment or leadership: None declared.
Honorarium: None declared.
Competing interests: The funding organization(s) played no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
References
- 1.Moran BJ, Cecil TD. The etiology, clinical presentation, and management of pseudomyxoma peritonei. Surg Oncol Clin N Am 2003;12:585–603. [DOI] [PubMed]
- 2.Gough DB, Donohue JH, Schutt AJ, Gonchoroff N, Goellner JR, Wilson TO, et al. Pseudomyxoma peritonei. Long-term patient survival with an aggressive regional approach. Ann Surg 1994;219:112–9. [DOI] [PMC free article] [PubMed]
- 3.Smeenk RM, van Velthuysen ML, Verwaal VJ, Zoetmulder FA. Appendiceal neoplasms and pseudomyxoma peritonei: a population based study. Eur J Surgical Oncol 2008;34:196–201. [DOI] [PubMed]
- 4.Bevan KE, Mohamed F, Moran BJ. Pseudomyxoma peritonei. World J Gastrointest Oncol 2010;2:44–50. [DOI] [PMC free article] [PubMed]
- 5.Sugarbaker PH. Cytoreductive surgery and peri-operative intraperitoneal chemotherapy as a curative approach to pseudomyxoma peritonei syndrome. Eur J Surgical Oncol 2001;27:239–43. [DOI] [PubMed]
- 6.Chua TC, Moran BJ, Sugarbaker PH, Levine EA, Glehen O, Gilly FN, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clinical Oncol 2012;30:2449–56. [DOI] [PubMed]
- 7.Murphy EM, Sexton R, Moran BJ. Early results of surgery in 123 patients with pseudomyxoma peritonei from a perforated appendiceal neoplasm. Dis Colon Rectum 2007;50:37–42. [DOI] [PubMed]
- 8.Miner TJ, Shia J, Jaques DP, Klimstra DS, Brennan MF, Coit DG. Long-term survival following treatment of pseudomyxoma peritonei: an analysis of surgical therapy. Ann Surg 2005;241:300–8. [DOI] [PMC free article] [PubMed]
- 9.Pillai K, Akhter J, Chua TC, Morris DL. A formulation for in situ lysis of mucin secreted in pseudomyxoma peritonei. International journal of cancer 2014;134:478–86. [DOI] [PubMed]
- 10.Akhter J, Pillai K, Chua TC, Alzarin N, Morris DL. Efficacy of a novel mucolytic agent on pseudomyxoma peritonei mucin, with potential for treatment through peritoneal catheters. Am J Cancer Res 2014;4:495–507. [PMC free article] [PubMed]
- 11.Mantle M, Stewart G, Zayas G, King M. The disulphide-bond content and rheological properties of intestinal mucins from normal subjects and patients with cystic fibrosis. Biochem J 1990;266:597–604. [PMC free article] [PubMed]
- 12.Inagami T, Murachi T. Kinetic studies of bromelain catalysis. Biochemistry 1963;2:1439–44. [DOI] [PubMed]
- 13.Rubin BK. Mucolytics, expectorants, and mucokinetic medications. Respir Care 2007;52:859–65. [PubMed]
- 14.Heard KJ. Acetylcysteine for acetaminophen poisoning. N Engl J Med 2008;359:285–92. [DOI] [PMC free article] [PubMed]
- 15.Lee SM, Jeong EM, Jeong J, Shin DM, Lee HJ, Kim HJ, et al. Cysteamine prevents the development of lens opacity in a rat model of selenite-induced cataract. Invest Ophthalmol Vis Sci 2012;53:1452–9. [DOI] [PubMed]
- 16.Fujisawa T, Rubin B, Suzuki A, Patel PS, Gahl WA, Joshi BH, et al. Cysteamine suppresses invasion, metastasis and prolongs survival by inhibiting matrix metalloproteinases in a mouse model of human pancreatic cancer. Plos One 2012;7:e34437. [DOI] [PMC free article] [PubMed]
- 17.Dickinson DA, Forman HJ. Glutathione in defense and signaling: lessons from a small thiol. Ann N Y Acad Sci 2002;973:488–504. [DOI] [PubMed]
- 18.Besouw M, van den Heuvel L, van Eijsden R, Bongaers I, Kluijtmans L, Dewerchin M, et al. Increased human dermal microvascular endothelial cell survival induced by cysteamine. J Inherit Metab Dis 2013;36:1073–7. [DOI] [PubMed]
- 19.Hayes JD, McLellan LI. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radic Res 1999;31:273–300. [DOI] [PubMed]
- 20.Rowan AD, Buttle DJ, Barrett AJ. The cysteine proteinases of the pineapple plant. Biochem J 1990;266:869–75. [PMC free article] [PubMed]
- 21.Hatano K, Sawano Y, Miyakawa T, Tanokura M. Characterization of the acidic and basic limbs of a bell-shaped pH profile in the inhibitory activity of bromelain inhibitor VI. Biopolymers 2006;81:309–19. [DOI] [PubMed]
- 22.Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc 2006;1:1112–6. [DOI] [PubMed]
- 23.Macri A. Hyperthermic intraperitoneal chemotherapy: rationale and technique. World J Gastrointest Oncol 2010;2:68–75. [DOI] [PMC free article] [PubMed]
- 24.Charrier C, Rodger C, Robertson J, Kowalczuk A, Shand N, Fraser-Pitt D, et al. Cysteamine (Lynovex(R)), a novel mucoactive antimicrobial & antibiofilm agent for the treatment of cystic fibrosis. Orphanet J Rare Dis 2014;9:189. [DOI] [PMC free article] [PubMed]
- 25.Chobotova K, Vernallis AB, Majid FA. Bromelain activity and potential as anti-cancer agent: current evidence and perspectives. Cancer Lett 2010;290:148–56. [DOI] [PubMed]
- 26.Castell JV, Fredrick G, Kuhn CS, Poppe GE. Intestinal absorption of undegraded proteins in men: presence of bromelain in plasma after oral intake. Am J Physiol 1997;273:G139–46. [DOI] [PubMed]
- 27.Amini A, Ehteda A, Masoumi Moghaddam S, Akhter J, Pillai K, Morris DL. Cytotoxic effects of bromelain in human gastrointestinal carcinoma cell lines (MKN45, KATO-III, HT29-5F12, and HT29-5M21). Onco Targets Ther 2013;6:403–9. [DOI] [PMC free article] [PubMed]
- 28.Pillai KJA, Etheda A, Badar S, Chua TC, David DL. Anti-tumor and chemosensitising effect of a combination of bromelain+N-acetyl cysteine with cisplatin or 5-FU on malignant peritoneal mesothelioma cells. J Glycobiology 2013:1–10.
- 29.Pillai K, Ehteda A, Akhter J, Chua TC, Morris DL. Anticancer effect of bromelain with and without cisplatin or 5-FU on malignant peritoneal mesothelioma cells. Anti-Cancer Drugs 2014;25:150–60. [DOI] [PubMed]


