Abstract
Background
Peritoneal metastases from gynecologic and gastrointestinal cancer is of increasing interest to surgical and medical oncologists because of newly recognized benefits of treatment. In contrast to prior outcomes, prolonged disease-free survival and cure have been reported.
Methods
To date, the benefits are to use complete surgical removal of the peritoneal metastases combined with hyperthermic intraperitoneal chemotherapy (HIPEC) delivered in the operating room. To supplement the local-regional control, normothermic intraperitoneal chemotherapy used long term (NIPEC-LT) and delivered by an intraperitoneal port has been explored.
Results
In three high grade malignancies with the preponderance of cytoreductive surgery (CRS) and HIPEC treatment failures within the peritoneal space, NIPEC-LT has been favorably reported in the oncology literature. In ovarian cancer and malignant peritoneal mesothelioma the NIPEC-LT is used an adjuvant treatment in an attempt to preserve a surgical complete response of CRS. In gastric cancer, NIPEC-LT is given as a neoadjuvant treatment with responders going on to radical surgical resection. Responses are monitored by laparoscopy.
Conclusions
This overview highlights benefits of NIPEC-LT in three diseases where benefits from CRS and HIPEC have been recognized but that local-regional failures persist. Improved results with NIPEC-LT have been reviewed for ovarian cancer, gastric cancer, and peritoneal mesothelioma.
Keywords: appendix cancer, gastric cancer, hyperthermic intraperitoneal chemotherapy (HIPEC), normothermic intraperitoneal chemotherapy long-term (NIPEC-LT), ovarian cancer, peritoneal mesothelioma, peritoneal metastases
Introduction
Twenty years ago, identification of peritoneal metastases indicated a terminal outcome in a great majority of abdominal and pelvic malignancies. A single exception was ovarian cancer peritoneal metastases; in this disease the systemic chemotherapy is, in a proportion of patients, so effective that some patients with peritoneal disease experience long term survival [1]. The combined treatment that has resulted in long term survival in patients who in the past lacked curative treatment options is cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) [2].
Although these new treatments have resulted in remarkable increased benefits to large numbers of patients, there remain many failures. Most commonly the disease progression is within the peritoneal space. The HIPEC treatment is unable to sustain the surgical complete response.
Perhaps it is not surprising that HIPEC fails to maintain cancer control within the abdomen and pelvis even though the cytoreductive surgery is able to visibly remove all evidence of disease. The HIPEC is only used once at the completion of the CRS. A single application of a chemotherapy agent is unlikely to permanently eradicate the disease. This treatment failure occurs even though there is a small volume of residual cancer and even though the cytotoxicity is augmented by heat [3].
A second shortcoming of HIPEC is the lack of a consistent cytotoxic effect on cancer cells. None of the cancer chemotherapy agents are universally effective; to the contrary, most are consistently unable to eradicate a population of cancer cells. Response rates with gastrointestinal cancer rarely exceed 30 %.
CRS and HIPEC have shown themselves to be a large advance in the management of peritoneal metastases. Yet this strategy results in treatment failures in a large proportion of patients. The return of peritoneal surface malignancy after CRS and HIPEC occurs more frequently as the peritoneal cancer index (PCI) is elevated. Knowing that success with CRS and HIPEC is most likely with a small extent of peritoneal metastases, it follows that the most reliable use of HIPEC may be prevention of peritoneal metastases in a primary malignancy. The cell kill necessary to show benefit is minimal. And if the HIPEC is delivered with the primary cancer resection, non-uniform distribution of the heat and chemotherapy is less likely to occur [4, 5].
In this article the authors accept HIPEC alone as the adjuvant treatment to be explored for prevention of recurrence of peritoneal metastases after CRS. Also, HIPEC is indicated to prevent the reseeding of peritonectomized surfaces in patients with established disease who have a complete CRS. This article explores the use of NIPEC-LT not as a substitute for CRS and HIPEC. Rather it is a supplement to improve the end result in peritoneal metastases patients after CRS and HIPEC have been maximally utilized. The results to date of NIPEC-LT with ovarian cancer, gastric cancer, and malignant peritoneal mesothelioma will be explored.
Normothermic intraperitoneal chemotherapy long term for ovarian cancer
Tewari and colleagues summarized the long term survival advantage associated with the normothermic intraperitoneal chemotherapy treatment used long term in patients with advanced ovarian cancer [6]. They pooled the data from all patients enrolled onto Gynecologic Oncology Group (GOG) protocols 114 and 172 [7, 8]. There were 876 patients included in the two protocols. Both protocols compared treatment of stage III epithelial ovarian cancer patients with exclusively intravenous (IV) chemotherapy or treatment that utilized both IV and intraperitoneal (IP) chemotherapy. Median follow-up was 10.7 years. In the combined data the progression-free survival (PFS) was 20 and 25 months for the IV vs. IV plus IP patients (p=0.019). The corresponding overall survival (OS) was 51.4 vs. 61.8 months respectively (p=0.042). The authors conclude that IV plus IP chemotherapy used long term was associated with a 21 % decreased risk of progression and a 23 % decreased risk of death from epithelial ovarian cancer. The survival long term of IV vs. IV plus IP chemotherapy is shown in Figure 1.
Figure 1:
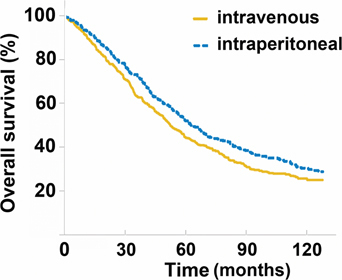
Long-term survival of ovarian cancer patients treated with intravenous (IV) vs. intraperitoneal (IP) chemotherapy (p=0.04).
From reference [6] with permission.
As might be expected, optimal utilization of NIPEC-LT improved the benefits of IV plus IP treatments. For example, patients with IV plus IP treatment did not complete all of the cycles of chemotherapy to be given IP. However, the more IP drugs that were delivered (between 1 and 6 cycles) the more favorable the patient’s outcome. Also, younger patients survived better probably because young age was associated with a higher likelihood of completing IP therapy.
In conclusion, Tewari et al. provides the first data with survival benefit extending beyond 10 years. What they have not investigated is the possible benefits of NIPEC-LT if the cytoreduction of ovarian cancer is augmented with HIPEC. Already, HIPEC has been shown to be of benefit in patients with recurrent ovarian cancer by Spiliotis et al. [9]. HIPEC should minimize reseeding but also extensive washing of the peritoneal space with a dilute chemotherapy solution may facilitate distribution of the IP component of IV plus IP chemotherapy regimens.
Normothermic intraperitoneal chemotherapy long term for gastric cancer
Although ovarian cancer has direct access to the peritoneal space from its earliest beginnings, gastric cancer has the epithelial lining of the stomach as the primary site. In order to show peritoneal metastases, this cancer inside a thick-walled tubular structure must cause cancer cells to access the peritoneal space. This migration occurs frequently because local recurrence and peritoneal metastases are the most common sites of first recurrence in gastric cancer after curative resection [10, 11, 12]. This high incidence of local and regional disease recurrence is seen whether patients received neoadjuvant chemotherapy or postoperative adjuvant treatment as compared to surgical resection alone [13]. Less localized recurrence is observed when extended lymphadenectomy is utilized as compared to limited surgery [14, 15, 16].
Causes of peritoneal metastases after curative resection are [1] spontaneous dissemination from the primary tumor and [2] traumatic dissemination of cancer cells during the surgical procedure. If cancer has penetrated the wall of the stomach resulting in serosal invasion, spontaneous dissemination is common prior to or at the time of gastrectomy and patients are frequently found to have viable intraperitoneal cancer cells (positive cytology) [17]. Tumor cells can also find access to the peritoneal space during surgery according to the tumor cell entrapment hypothesis [18]. During surgery there is disruption of lymphatics, trauma to narrow margins of resection, and tumor-contaminated blood spillage. Iatrogenically disseminated tumor cells adhere spontaneously within minutes and vascularization is facilitated by fibrin entrapment and the wound healing process. Cytokines, such as growth factors important for wound healing, may also promote tumor progression.
Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for primary gastric cancer with peritoneal metastases
There is a small but real potential for long-term survival for patients with gastric cancer and peritoneal metastases if complete resection of the primary disease including wide resection of regional lymph nodes and the peritoneal metastases is combined with HIPEC. There are several single institution data and phase II studies that support use of this strategy but the patients must be highly selected. Glehen et al. studied 159 patients with a median follow-up of 20.4 months. There was a median overall survival of 9.2 months but the 5-year survival rate was 13 % [19]. Although the proportion of patients was limited, gastric cancer patients with peritoneal metastases treated with CRS and HIPEC were the only patients that experienced any 5-year survivals [20].
Even if completely cytoreduced, HIPEC is less effective for patients with a large extent of peritoneal metastases. Glehen et al. showed that one of the strongest prognostic factors was extent of carcinomatosis [19]. When the peritoneal cancer index (PCI) was greater than 12, despite a complete cytoreduction, there were no survivors greater than 3 years [21]. Fujimoto, et al reported 40 to 50 % 5-year survival for limited peritoneal metastases but only an 18 % 1-year survival for patients with extensive peritoneal metastases [22]. Cytoreduction with HIPEC in gastric cancer patients with a PCI score greater than 12 may be contraindicated.
Yang et al. reported the only phase III study regarding CRS and HIPEC in gastric cancer presenting with peritoneal metastases [23]. They used cisplatin (120 mg) and mitomycin C (30 mg) in 6000 mL of normal saline at 43 °C for 60–90 min. Median follow-up was 32 months; 97.1 % (33 of 34) of patients after CRS died as compared to 85.3 % (29 of 34) of CRS and HIPEC patients died. Median survival was 6.5 months (95 % CI 4.8 to 8.2 months) after CRS and 11 months (95 % CI; 10.0 to 11.9 months) in CRS and HIPEC group (p=0.046) [23]. There was similar morbidity between the groups. The independent predictors in a multivariate analysis for improved survival were synchronous peritoneal metastases, CC 0 – 1 cytoreduction, more than 6 cycles of systemic chemotherapy, and no adverse events. This randomized controlled study with the other single and multi-institutional reports confirms that CRS and HIPEC for patients with peritoneal metastases can result in a survival benefit but should be restricted to a limited patient population.
NIPEC-LT prior to surgery to select patients for resection of gastric cancer with peritoneal metastases
In a phase II study, Yonemura and coworkers treated patients with biopsy-proven peritoneal metastases identified by laparoscopy, laparotomy or cytology from ascites. To be treated with NIPEC-LT, patients must have [1] proven peritoneal seeding by histology or cytology [2], no hematogenous or remote lymph node metastases [3], be less than or equal to 65 years [4], an Eastern Clinical Oncology Group score of 2 or less [5], adequate bone marrow, liver, cardiac, and renal function, and [6] no other severe medical comorbidities or synchronous malignancies. Yonemura and coworkers referred to this strategy as neoadjuvant intraperitoneal and systemic chemotherapy (NIPS). In this manuscript this plan of management is referred to as NIPEC-LT.
At the time of laparoscopy, qualifying patients had a peritoneal port system (Bard Port, C.R. Bard Inc., USA) inserted into the abdominal cavity under local anesthesia with the catheter tip placed within the cul-de-sac of Douglas.
Neoadjuvant intraperitoneal and systemic chemotherapy regimen
Prior to administration of chemotherapy, 500 mL of saline was instilled into the peritoneal cavity and fluid was removed for cytology. Docetaxel 40 mg and carboplatin 150 mg were used for intraperitoneal chemotherapy in addition to 1000 mL of saline over 30 min. Methotrexate 100 mg/m2 and 5-fluorouracil 600 mg/m2 in 100 mL of saline over 15 min were administered intravenously the same day. This regimen was administered weekly for two cycles. After the second cycle, peritoneal wash cytology was again performed. If cytology was positive, neoadjuvant chemotherapy was continued for 2 more cycles. Peritoneal cytology testing was repeated after the fourth cycle and the process was continued as long as cytology was positive [24].
If cytology became negative, upper endoscopy, repeat laparoscopy and CT scan were performed. If the primary gastric cancer showed no response, then 2 more cycles of NIPEC-LT were administered. The number of chemotherapy cycles was controlled by the effect on the primary cancer and peritoneal cytology. Complete cytoreduction was required for prolonged survival in prior studies of peritoneal metastases. Therefore, the goal of the NIPEC-LT regimen was complete or near complete response of metastases on small bowel surfaces so that gastrectomy plus cytoreduction resulted in complete visible clearing of the abdomen and pelvis.
Surgery for gastric cancer with peritoneal metastases after successful neoadjuvant NIPEC-LT
Gastrectomy and peritonectomy were performed if peritoneal wash cytology became negative or there was a complete or partial response to NIPEC-LT. Patients with progressive disease or who continue to have positive cytology despite 6 cycles of NIPEC-LT were not candidates for surgery.
If peritoneal metastases on small bowel surfaces were eliminated by NIPEC-LT, there was a possibility that gastrectomy and parietal peritonectomy could achieve a complete cytoreduction. Sugarbaker and Yonemura reported the use of peritonectomy for peritoneal metastases to cytoreduce the peritoneal surface and facilitate total resection of the primary gastric cancer [25].
Results after NIPEC-LT plus gastrectomy with cytoreduction
Several single institution reports have focused on NIPEC-LT with cytologic and laparoscopic monitoring for selection of patients for gastrectomy with cytoreduction. A recent report by Canbay et al. is a phase II study of NIPEC-LT including 194 patients. Average age was 51.5 years. One-hundred-four patients had primary gastric cancer and 90 patients had recurrent peritoneal metastases [26]. Prior to NIPEC-LT peritoneal fluid cytology was positive in 137 patients and negative in 57 patients. There was complete resolution of peritoneal metastases after NIPEC-LT chemotherapy in 24.3 % of patients. After induction treatment, 152 patients were judged to respond sufficiently to undergo surgery.
Operative interventions were total gastrectomy (n=94, 62 %), subtotal gastrectomy (n=17, 12 %), small bowel resection (n=44, 29 %). Left and right sub diaphragmatic peritonectomy and pelvic peritonectomy was completed in 44 (17 %), 31 (20 %), and 61 (40 %) patients, respectively. Complete cytoreduction was achieved in 103 (67.7 %) of patients.
Figure 2 demonstrates overall survival of the 194 patients. Median survival was 15.8 months for the 152 patients who had received surgical intervention vs. 7.5 months for patients who did not have an operation. Median survival of the 194 patients was 14.4 months. One-year survival was 54 % for all patients. There was a significant survival difference (p=0.03) between patients who underwent operative intervention vs. those who did not. There was a higher median survival of 18 months for patients who received a complete cytoreduction. There was no difference between primary and recurrent disease after cytoreduction with a median survival of 17.6 months vs. 14.1 months, respectively (p=0.39) (see discussion below).
Figure 2:
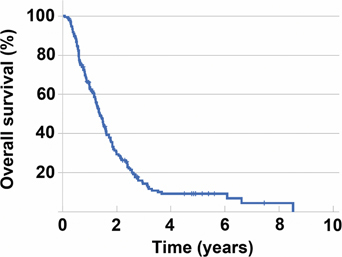
Overall survival in 194 gastric cancer patients with peritoneal carcinomatosis.
From reference [26] with permission.
Adverse events from neoadjuvant intraperitoneal and systemic chemotherapy and gastrectomy plus cytoreductive surgery
The most common chemotherapy-related grade 3 or 4 adverse events were bone marrow suppression, diarrhea and renal failure. Less common adverse events were port site infection (n=2) and renal failure (n=1). After cytoreduction with peritonectomy, in 152 patients, 36 (23.6 %) developed complications. The overall operative mortality rate was 3.9 % (6 of 152 patients). Thirteen patients developed sepsis associated with anastomotic leakage [26].
Other strategies for normothermic intraperitoneal chemotherapy long term for gastric cancer with peritoneal metastases
Another approach to the concept of NIPEC-LT has been reported by Kitayama and colleagues [27]. They used oral S1 combined with intraperitoneal paclitaxel (20 mg/m2) and intravenous paclitaxel (50 mg/m2) on days 1 and 8. S1 was administered at 80 mg/m2 per day for 14 consecutive days. Gastrectomy but not HIPEC was considered in fit patients if 1) no distant metastases occurred except in the peritoneal cavity, 2) peritoneal cytology became negative, 3) by laparoscopy peritoneal nodules were reduced or under control. Of 60 patients treated approximately half [28] were able to have gastrectomy. Median overall survival for 60 patients was 16.6 months. For the 34 having gastrectomy it was 26.4 months and for the 30 who did not have gastrectomy, it was only 12.1 months.
Yet another approach to NIPEC-LT was reported by Fujiwara and coworkers [29]. They used intraperitoneal Docetaxel (40–60 mg/m2) combined with oral S1 at 40 mg/m2 twice daily. After 2 cycles repeat laparoscopy was performed. At post-NIPEC-LT evaluation, 14 patients had negative peritoneal cytology and no macroscopic peritoneal metastases. Sixteen patients had gastrectomy. Median overall survival for all patients was 24.6 months. These data strongly suggest a benefit from neoadjuvant NIPEC-LT combined with gastrectomy plus cytoreduction.
Treatment of metachronous peritoneal metastases
Peritoneal metastases diagnosed in follow-up after definitive treatment of the primary gastric cancer have always been regarded as a terminal condition. Palliative systemic chemotherapy is usually recommended but is only of small short-term benefit. Yang and coworkers in the phase III study of HIPEC in gastric cancer with peritoneal metastases treated 10 patients with metachronous disease [23]. The median overall survival of these patients was 5.5 months. For 24 patients with synchronous peritoneal metastases treated with CRS and HIPEC the median survival was 12.0 months. These two groups were statistically significantly different, with a p-value of 0.02. In the trial of Yang and coworkers, metachronous peritoneal metastases were not successfully treated by CRS and HIPEC.
In sharp contrast the study using NIPEC-LT reported by Canbay et al., 104 patients had synchronous peritoneal metastases and 90 had metachronous peritoneal metastases [26]. Median overall survival of the two groups was 17.6 vs. 14.1 months; the difference was not significant with a p-value of 0.39. This report would indicate that NIPEC-LT followed by cytoreductive surgery plus gastrectomy is a treatment option for peritoneal metastases diagnosed in follow-up. Cytoreductive surgery plus HIPEC in the absence of NIPEC-LT was not shown to be effective.
All clinical data supports complete cytoreduction as the goal in management of gastric cancer patients with peritoneal seeding
Complete cytoreduction is crucial in the surgical treatment for peritoneal metastases from gastric cancer. If there is P2 or P3 dissemination, complete cytoreduction should not be attempted unless NIPEC-LT results in markedly diminished disease on intestinal surface and thereby facilitate complete cytoreduction.
Normothermic intraperitoneal chemotherapy long term for malignant peritoneal mesothelioma
Malignant peritoneal mesothelioma (MPM) is a disease that progresses within the peritoneal space throughout its natural history. On occasion, direct extension of the disease into the right hemidiaphragm and right pleural space is observed. As this characteristic pattern of local-regional dissemination of MPM was accepted, peritoneal surface oncology centers around the world recognized the possible benefits of combined cytoreductive surgery and HIPEC. Cytoreductive surgery was employed in an attempt to remove all visible evidence of disease from the abdomen and pelvis. Surgery was combined with HIPEC which was to maintain the surgical complete or near complete response. The early efforts of this multinational coalition to treat peritoneal mesothelioma should be recognized as the initial success of a global attack on this rare malignancy. This literature includes a systematic review and a meta-analysis [30, 31]. A multidisciplinary conference in 2006 at the National Institutes of Health sponsored by the National Organization for Rare Diseases concluded that CRS plus perioperative chemotherapy may be considered by the multidisciplinary team as an initial treatment plan for patients with MPM [32]. The Peritoneal Surface Oncology Group International (PSOGI) consensus conference in 2008 declared CRS and perioperative chemotherapy as the standard of care for this disease realizing that knowledgeable selection of patients for such an aggressive treatment plan is necessary [33]. Currently, an Peritoneal Surface Oncology Group International (PSOGI) registry exists to track the management of this disease around the world [34].
Strategies for surgical treatment of malignant peritoneal mesothelioma patients
All patients for the 20 years of this study underwent a cytoreductive surgical procedure, the goal of which was to remove all or nearly all visible disease. The overall strategy was to achieve a complete response through the use of surgery and then maintain that response through the use of regional chemotherapy. The surgery required a series of five parietal peritonectomy procedures used plus visceral resections as required to remove visible evidence of disease [28, 35]. MPM layered out on the visceral peritoneal surface of small bowel, colon, or rectum usually required visceral resections. A single surgeon (PHS) performed all of the cytoreductions throughout the 20 years of this clinical effort. All peritonectomy procedures and major visceral resections were prospectively recorded over the 20 years of this effort. Greater omentectomy, cholecystectomy and appendectomy were performed in all patients and were excluded from data analysis.
HIPEC and NIPEC-LT treatments for 3 groups of patients
In this report there were 3 groups of patients. All patients had maximal cytoreductive surgery. Group 1 had adjuvant HIPEC. Group 2 had HIPEC plus early postoperative intraperitoneal chemotherapy (EPIC) with paclitaxel. In the third group of 29 patients, the perioperative chemotherapy was the same as in group 2. However, prior to closure of the abdomen, an intraperitoneal port (Smiths Medical ASD Inc., St. Paul, MN) was implanted. At 4–6 weeks postoperatively, the intraperitoneal port was accessed. The first 8 patients received long-term six months of IP paclitaxel given as NIPEC. Paclitaxel dose was 20 mg/m2 five days in a row, one week of every month. The subsequent 21 patients were treated with IP pemetrexed at 1000 mg/m2 in 1 liter of 1.5 % dextrose peritoneal dialysis solution infused at 1000 ml/min over 1 hour. Following IP pemetrexed, cisplatin at 75 mg/m2 was infused IV in 250 ml of normal saline over 120 min. These treatments were repeated for a total of 6 cycles with 3 weeks between each treatment [36].
Statistical comparisons of the treatments administered in group 1 (CRS+HIPEC) vs. group 2 (CRS+HIPEC+EPIC) vs. group 3 (CRS+HIPEC+EPIC+NIPEC) showed a significant impact on survival with a p-value of 0.0374. A comparison of patients without NIPEC (groups 1 and 2) and with NIPEC (group 3) shows a p-value of 0.0108 (Figure 3).
Figure 3:
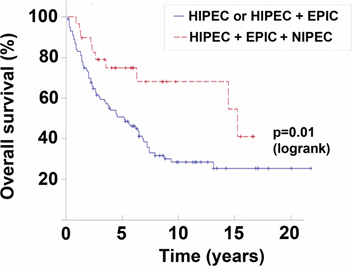
Survival of patients with malignant peritoneal mesothelioma.
Solid line is HIPEC or HIPEC+EPIC. Dashed line is HIPEC+EPIC+NIPEC-LT. From reference [36] with permission.
A multivariate analysis was performed and showed treatment group and completeness of cytoreduction were significantly associated to survival. For comparison of treatment group, HIPEC+EPIC+NIPEC was used as the reference group and there is evidence of increasing trend over time in the risk ratio (3.47 fold, p=0.0039) for patients treated with HIPEC only, while moderately increased risk ratio (2.50, p=0.0206) for patients treated with HIPEC+EPIC. For completeness of cytoreduction comparison, patents in groups 0 and 1 were used as the reference group; patients with CC=2 have 3.12 fold (p=0.0035) risk ratio while patients of CC=3 have 3.98 fold (p<0.0038) risk ratio.
With the insertion of the intraperitoneal port at the time of cytoreductive surgery one might expect from prior literature a considerable proportion of problems with long-term intraperitoneal chemotherapy administration. Extensive irrigation of the peritoneal space with a chemotherapy solution of hetastarch for the first five postoperative days occurred. This irrigation would minimize adhesions and then maximize the distribution of drug at later time through the intraperitoneal port. Although the paclitaxel is given in hetastarch in order to improve its retention of this drug within the peritoneal space, it is possible that this hetastarch irrigation postoperatively is of help in long-term adhesion prevention.
These sequential but increasingly prolonged cancer chemotherapy treatments seem to be well tolerated by the peritoneal space. All surviving patients are currently nutritionally sound, not requiring enteral or parenteral nutrition, and not being treated for bowel obstruction. None of these patients required a reoperative procedure for bowel obstruction (data not shown). The adverse events associated with the treatment of MPM have been previously published [37].
In summary, over a 20 year time span these 3 groups of patients adding EPIC to HIPEC showed no significant improvement; however, a statistically significant increase in survival resulted when multiple cycles of NIPEC-LT were initiated. These data may be interpreted to show that addition of NIPEC-LT regional chemotherapy resulted in an improved maintenance of the surgical complete or near-complete response.
There are obvious limitations to the interpretation of these data that a long-term regional chemotherapy treatment is solely responsible for the significant improvement in survival. The numbers of patients treated with NIPEC are limited and the regional chemotherapy regimens used are somewhat inconsistent. Changes in the disease and an alteration in referral patterns are possible. These benefits have only been demonstrated at a single institution. Because this is a rare disease and no established referral centers exist, it took 20 years to establish the possible significance of these chemotherapy treatments. An evolution in the surgical approach to this disease may have unknowingly occurred.
Footnotes
Author contributions: The author has accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: None declared.
Employment or leadership: None declared.
Honorarium: None declared.
Competing interests: The funding organization(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
References
- 1.Halkia E, Sugarbaker PH. Neoadjuvant, adjuvant and long-term IP/IV chemotherapy, HIPEC, EPIC for ovarian cancer. J Peritoneum in press.
- 2.Sugarbaker PH. Comprehensive management of peritoneal surface malignancy using cytoreductive surgery and perioperative intraperitoneal chemotherapy. Curr Res in Cancer 2009;3:179–203. [DOI] [PubMed]
- 3.Sugarbaker PH, van der Speeten K. Surgical technology and pharmacology of hyperthermic perioperative chemotherapy. J Gastrointest Oncol 2016;7:29–44. [DOI] [PMC free article] [PubMed]
- 4.Sugarbaker PH. Improving oncologic outcomes for colorectal cancer at high risk for local-regional recurrence with novel surgical techniques. Expert Rev Gastroenterol Hepatol 2016;10:205–13. [DOI] [PubMed]
- 5.Sugarbaker PH. Optimization of patient selection for surgical approach to peritoneal metastases from gastrointestinal cancer using cytoreductive surgery and perioperative cancer. Curr Colorectal Cancer Rep 2014;10:272–8.
- 6.Tewari D, Java JJ, Salani R, Armstrong DK, Markman M, Herzog T, et al. Long-term survival advantage and prognostic factors associated with intraperitoneal chemotherapy treatment in advanced ovarian cancer: a gynecologic oncology group study. J Clin Oncol 2015;33:1460–6. [DOI] [PMC free article] [PubMed]
- 7.Alberts DS, Liu PY, Hannigan EV, O’Toole R, Williams SD, Young JA, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med 1996;335:1950–5. [DOI] [PubMed]
- 8.Markman M, Bundy BN, Alberts DS, Fowler JM, Clark-Pearson DL, Carson LF, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the gynecologic oncology group, southwestern oncology group, and eastern cooperative oncology group. J Clin Oncol 2001;19:1001–7. [DOI] [PubMed]
- 9.Spiliotis J, Halkia E, Lianos E, Kalantzi N, Grivas A, Efstathiou E, et al. Cytoreductive surgery and HIPEC in recurrent epithelial ovarian cancer: a prospective randomized phase III study. Ann Surg Oncol 2015 May;22:1570–5. [DOI] [PubMed]
- 10.Gunderson LL, Sosin H. Adenocarcinoma of the stomach: areas of failure in a re-operation series clinicopathologic correlation and implications for adjuvant therapy. Int J Radiat Oncol Biol Phys 1982;8:1–11. [DOI] [PubMed]
- 11.Wisbeck WM, Becher EM, Russell AH. Adenocarcinoma of the stomach: autopsy observations with therapeutic implications for the radiation oncologist. Radiother Oncol J Eur Soc Ther Radiol Oncol 1986;7:13–8. [DOI] [PubMed]
- 12.Landry J, Tepper JE, Wood WC, Moulton EO, Koerner F, Sullinger J. Patterns of failure following curative resection of gastric carcinoma. Int J Radiat Oncol Biol Phys 1990;19:1357–62. [DOI] [PubMed]
- 13.Wils J, Meyer HJ, Wilke H. Current status and future directions in the treatment of localized gastric cancer. Ann Oncol 1994;5:69–72. [DOI] [PubMed]
- 14.Maruyama K, Okabayashi K, Kinoshita T. Progress in gastric cancer surgery in Japan and its limits of radicality. World J Surg 1987;11:418–25. [DOI] [PubMed]
- 15.Kaibara N, Sumi K, Yonekawa M, Ohta M, Makino M, Kimura O, et al. Does extensive dissection of lymph nodes improve the results of surgical treatment of gastric cancer? Am J Surg 1990;159:218–21. [DOI] [PubMed]
- 16.Korenaga D, Moriguchi S, Orita H, Kakeji Y, Haraguchi M, Maehara Y, et al. Trends in survival rates in Japanese patients with advanced carcinoma of the stomach. Surg Gynecol Obstet 1992;174:387–93. [PubMed]
- 17.Boku T, Nakane Y, Minoura T, Takada H, Yamamura M, Hioki K, et al. Prognostic significance of serosal invasion and free intraperitoneal cancer cells in gastric cancer. Br J Surg 1990;77:436–9. [DOI] [PubMed]
- 18.Sethna KS, Sugarbaker PH. New prospects for the control of peritoneal surface dissemination of gastric cancer using perioperative intraperitoneal chemotherapy. Cancer Ther 2004;2:79–84.
- 19.Glehen O, Gilly FN, Arvieux C. Peritoneal carcinomatosis from gastric cancer: a multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann Surg Oncol 2010;17:2370–7. [DOI] [PubMed]
- 20.Yonemura Y, Canbay E, Li Y, Coccolini F, Glehen O, Sugarbaker PH, et al. A comprehensive treatment for peritoneal metastases from gastric cancer with curative intent. Eur J Surg Oncol 2016;42:1123–31. [DOI] [PubMed]
- 21.Magge D, Zenati M, Mavanur A, Winer J, Ramalingam L, Jones H, et al. Aggressive locoregional surgical therapy for gastric peritoneal carcinomatosis. Ann Surg Oncol 2014;21:1448–55. [DOI] [PMC free article] [PubMed]
- 22.Fujimoto S, Takahashi M, Mutou T, Kobayashi K, Toyosawa T, Isawa E, et al. Improved mortality rate of gastric carcinoma patients with peritoneal carcinomatosis treated with intraperitoneal hyperthermic chemoperfusion combined with surgery. Cancer 1997;79:884–91. [DOI] [PubMed]
- 23.Yang X-J, Huang C-Q, Suo T. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol 2011;18:1575–81. [DOI] [PMC free article] [PubMed]
- 24.Yonemura Y, Elnemr A, Endou Y, Hirano M, Mizumoto A, Takao N, et al. Multidisciplinary therapy for treatment of patients with peritoneal carcinomatosis from gastric cancer. World J Gastrointest Oncol 2010;2:85–97. [DOI] [PMC free article] [PubMed]
- 25.Sugarbaker PH, Yu W, Yonemura Y. Gastrectomy, peritonectomy and perioperative intraperitoneal chemotherapy: the evolution of treatment strategies for advanced gastric cancer. Semin Surg Oncol 2003;21:233–48. [DOI] [PubMed]
- 26.Canbay E, Mizumoto A, Ichinose M. Outcome data of patients with peritoneal carcinomatosis from gastric origin treated by a strategy of bidirectional chemotherapy prior to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in a single specialized center in Japan. Ann Surg Oncol 2014;21:1147–52. [DOI] [PubMed]
- 27.Kitayama J, Ishigami H, Yamaguchi H. Salvage gastrectomy after intravenous and intraperitoneal paclitaxel (PTX) administration with oral S-1 for peritoneal dissemination of advanced gastric cancer with malignant ascites. Ann Surg Oncol 2014;21:539–46. [DOI] [PubMed]
- 28.Sugarbaker PH. Peritonectomy procedures. Ann Surg 1995;221:29–42. [DOI] [PMC free article] [PubMed]
- 29.Fujiwara Y, Takiguchi S, Nakajima K, Miyata H, Yamasaki M, Kurokawa Y, et al. Intraperitoneal docetaxel combined with S-1 for advanced gastric cancer with peritoneal dissemination. J Surg Oncol 2012;105:38–42. [DOI] [PubMed]
- 30.Yan TD, Welch L, Black D, Sugarbaker PH. A systematic review on the efficacy of cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for diffuse malignant peritoneal mesothelioma. Ann Oncol 2007;18:827–34. [DOI] [PubMed]
- 31.Helm JH, Miura JT, Glenn JA, Marcus RK, Larrieux G, Jayakrishnan TT, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: a systematic review and meta-analysis. Ann Surg Oncol 2015;22:1686–93. [DOI] [PubMed]
- 32.Hassan R, Alexander R, Antman K, Boffetta P, Churg A, Coit D, et al. Current treatment options and biology of peritoneal mesothelioma: meeting summary of the first NIH peritoneal mesothelioma conference. Ann Oncol 2006;17:1615–19. [DOI] [PubMed]
- 33.Deraco M, Bartlett D, Kusamura S, Baratti D. Consensus statement on peritoneal mesothelioma. J Surg Oncol 2008;98:268–72. [DOI] [PubMed]
- 34.International PSOGI Registry on Peritoneal Mesothelioma. Available at: http://test.dinamoweb.net/p_mesotelioma/
- 35.Deraco M, Baratti D, Kusamura S, Laterza B, Balestra MR. Surgical technique of parietal and visceral peritonectomy for peritoneal surface malignancies. J Surg Oncol 2009;100:321–8. [DOI] [PubMed]
- 36.Sugarbaker PH, Chang D. Long-term intraperitoneal chemotherapy for patients with malignant peritoneal mesothelioma results in improved survival. Eur J Surg Oncol 2017 Jan 29. pii: S0748-7983(17)30059-8. doi: 10.1016/j.ejso.2017.01.009. [Epub ahead of print] [DOI] [PubMed]
- 37.Yan TD, Edwards G, Alderman R, Marquardt CE, Sugarbaker PH. Morbidity and mortality assessment of cytoreductive surgery and perioperative intraperitoneal chemotherapy for diffuse malignant peritoneal mesothelioma – a prospective study of 70 consecutive cases. Ann Surg Oncol 2007;14:515–25. [DOI] [PubMed]


