Abstract
Background
Repeated intraperitoneal (IP) administration of paclitaxel (PTX) with concurrent systemic chemotherapy is clinically effective for the treatment of peritoneal metastases (PM) from gastric cancer. However, it is unclear how biochemical modifications may affect the pharmacokinetics and bioavailability of IP administered PTX.
Methods
In a xenograft PM model using human gastric cancer cells, MKN45, fluorescein-conjugated PTX (OG-PTX) was given IP and the intra-tumor distribution of PTX examined with fluorescein microscopy.
Results
After IP injection, PTX was seen to directly infiltrate up to several hundred micrometers from the surface of the PM. Co-injection with 5 % non-animal stabilized hyaluronic acid increased PTX infiltration and suppressed the development of PM more efficiently than PTX alone. PTX solubilized with amphiphilic polymer composed of 2-methacryloyloxyethyl phosphorylcholine (MPC) and n-butyl methacrylate (BMA) efficiently formed a micellar formation 50–100 nm in diameter. IP injection of the nanomicellar PTX (PTX-30W) also showed significantly enhanced tumor infiltration and further inhibition of the growth of PM compared with PTX solubilized with Cremophor–ethanol (PTX-Cre). Finally, IP administration of NK105, another nanomicellar PTX, inhibited the growth of subcutaneous tumors as well as PM, compared with conventional PTX-Cre in the same murine model.
Conclusions
PTX administered IP directly infiltrates PM and are thus a useful strategy for the treatment of PM. Drug modification with nanotechnology may further enhance penetration of PM resulting in improved clinical efficacy.
Keywords: hyaluronic acid, intraperitoneal chemotherapy, MKN45, nanodrug, paclitaxel, peritoneal metastasis
Introduction
Peritoneal metastases (PM) frequently occur in patients with recurrent abdominal malignancies, such as gastrointestinal and ovarian cancer. Despite recent advances in chemotherapeutic drugs, systemic chemotherapy alone has shown limited efficacy for the treatment of PM, possibly because of the peritoneum-plasma barrier which prevents effective drug delivery from the systemic circulation into the peritoneal cavity [1].
Intraperitoneal (IP) chemotherapy appears to be an easy way to deliver drugs to peritoneal lesions. The theoretical rationale of IP chemotherapy was first described in 1978 by Dedrick et al. who showed that IP administration results in a higher drug concentration and longer half-life in the peritoneal cavity, compared with systemic administration [2]. Since then, many studies of the pharmacokinetic and antitumor effects of IP chemotherapy have been conducted, which have shown variable results. Usually, IP drugs are given under hyperthermic conditions (heated intraperitoneal chemotherapy; HIPEC) which may increase cytotoxicity in the peritoneal cavity, without an increase in systemic toxicity [3]. In fact, HIPEC combined with cytoreductive surgery (CRS) has been shown to improve the outcome of patients with PM from some malignancies [4, 5, 6]. However, these demanding procedures are often associated with high mortality and morbidity [7, 8]. Therefore, it is now considered that CRS+HIPEC should be administered to selected patients in specialized facilities, which prohibits the widespread use of this strategy.
Theoretically, an anti-cancer agent used for IP chemotherapy would slowly exit the peritoneal cavity, which would allow optimal penetration of the tumor surface [9]. Thus, taxanes, such as paclitaxel (PTX) or docetaxel (DOC), are supposed to be ideal drugs for IP chemotherapy because of high local concentrations over a long period of time due to their hydrophobic properties [10]. In fact, we and others have recently shown that IP administration of PTX or DOC can exhibit remarkable effects against PM from gastric cancer (GC) [11, 13, 14]. However, the underlying mechanism to explain the marked shrinkage of PM is still unknown.
The route of peritoneal absorption and pharmacokinetics following IP administration varies a great deal based on the biophysical characteristics of drugs such as their formulation, solvent, and molecular structure. Therefore, it is suggested that biochemical modification may favorably alter the pharmacokinetics and bioavailability of IP-administered taxanes for the treatment of PM.
In fact, to improve the therapeutic efficacy, new techniques to prolong the retention of drugs in the peritoneal cavity have been examined. Many in vivo studies have shown that the addition of high molecular weight agents such as 4 % icodextrin [15], 6 % hydroxyethyl starch (hetastarch) [16], or various hydrogels [17, 18, 19, 20, 21] can prolong the retention time in the abdominal cavity.
Another approach is to use the nanoparticles as vesicles for the delivery of anti-cancer drugs which are known as nanodrugs with a molecular diameter of 10–100 nm. The nanodrugs are considered to be effectively accumulated in tumor tissue due to enhanced permeability and retention (EPR) and elicit enhanced antitumor effects with less toxicity in normal tissues [22]. The EPR effect is based on the particular characteristics of solid tumors, such as incomplete vascular architecture, hyperpermeability of tumor vessel walls, and immature lymphatic drainage [23]. Based on this concept, various kinds of nanodrugs have been developed for the treatment of cancer [24]. However, while the versatility and clinical effects of nanodrugs have been evaluated after systemic administration, few studies have focused on the clinical efficacy of nanodrugs following IP administration.
In previous studies, we established a murine xenograft model to visualize the distribution of fluorescein-conjugated PTX in peritoneal tumors and examined the anti-tumor effects in vivo [25, 26]. Here, in this review, we summarize our previous results obtained with this murine model and discuss the relationship between the modification of various forms of PTX and the clinical efficacy for the treatment of PM.
Materials and methods
Cells and reagents
MKN45 which was originally established from poorly differentiated gastric carcinoma tissue was obtained from RIKEN CELL BANK (Tsukuba, JAPAN), and a highly metastatic subclone, MKN45P, was generated in our laboratory [27]. Oregon-green 488-conjugated PTX (OG-PTX) was purchased from Molecular Probe (Portland, OR, USA). Rat monoclonal antibody to mouse platelet/endothelial cell adhesion molecule 1 (PECAM-1) and DAPI were purchased from BD PharMingen (San Diego, CA, USA) and Wako Pure Chemical Industries Ltd. (Osaka Japan), respectively. Non-animal stabilized hyaluronic acid (NASHA) was a generous gift from Q-Med (Uppsala, Sweden). NASHA with median molecular weight (MW>106), Restylane® was used for experiments. PMB polymer which is a water-soluble, amphiphilic polymer composed of 2-methacryloyloxyethyl phosphorylcholine (MPC) and n-butyl methacrylate (BMA) was produced in our collaborating laboratory, Department of Materials Engineering and Department of Bioengineering, School of Engineering, University of Tokyo. Using dynamic light scattering method, it is confirmed that the mixture of PTX with this polymer constructs PTX-containing nanoparticles with mean diameter of 50 nm, which was named PTX-30W [28]. Finally, NK105 was a gift from Nippon Kayaku (Tokyo, Japan).
In vivo experiment
Four-week-old specific-pathogen-free conditioned female BALB/c nude mice were fed in a temperature-controlled, light cycled room. Five weeks after birth, the mice were inoculated with 1–3×106 MKN45-P suspended in 1 mL of PBS. On days 7, 14, and 21 after inoculation of the MKN45-P cells, the mice were given the respective drugs IP, in a total volume of 1 mL to allow optimal distribution in the peritoneal cavity. The dose of PTX was fixed at 20 mg/kg. On day 28, all mice were sacrificed, laparotomy performed, and the number of peritoneal nodules counted.
Evaluation of PTX infiltration in peritoneal tumor under the fluorescein microscopy
To investigate the distribution of PTX, OG-PTX, dissolved in various media was given on day 21 after tumor inoculation. As a control, OG-PTX dissolved in Cremophor:ethanol (1:1, v/v) was given. After the assigned interval, peritoneal nodules were excised, fixed for 1 h in 10 % neutral buffered formalin at room temperature, washed overnight in PBS containing 10 % sucrose at 4℃, embedded in optimal cutting temperature compound and 10 µm cryostat sections of post-fixed frozen samples were examined for green fluorescein of OR-PTX using a fluorescence stereomicroscope (BZ8000, Keyence, Osaka, Japan). All experiments related to animal comply with World Medical Association Declaration of Helsinki and institutional policies for the care and use of animals and approved by the Institutional Review Board of the University of Tokyo.
Results
IP-administrated PTX infiltrates into the periphery of peritoneal tumor [25, 26]
Spatial distribution of IP administrated PTX was clearly visualized by this method. As shown in Figure 1A, IP-PTX accumulated in the peripheral area of peritoneal tumors, suggesting that PTX infiltrated from both sides of the tumor surface. The fluorescein intensities of every 10 μm on a certain line in the tumor were measured, indicating that IP PTX infiltrates to 670 and 520 μm depth from tumor surface after IP administration. In contrast, after IV administration, fluorescein intensities were much lower without a specific accumulation pattern.
Figure 1:
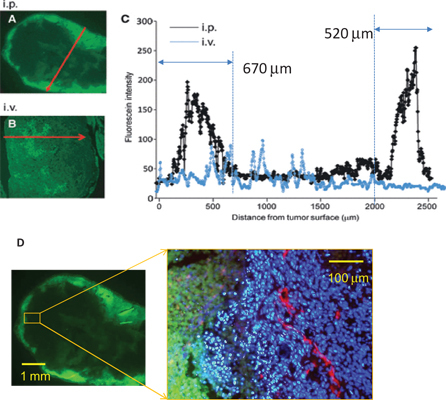
Objective measurement of PTX infiltration in peritoneal nodules.(A–C) Fluorescein intensity (FI) in peritoneal tumors at 24 h after intraperitoneal (IP) and intravenous (iv) administration of Oregon-green conjugated PTX. A representative line which appears to reflect the deepest infiltration was set as red arrows in sections of peritoneal tumors, and FI on the lines were automatically measured and plotted. (D) The same section after IP administration of Oregon-green conjugated PTX was co-stained with PE conjugated anti-CD31 mAb (red) and DAPI (blue). Tumor cells in peripheral area showed massive apoptosis.
The same sections were then immunostained with a rat monoclonal antibody to mouse PECAM-1 to detect blood vessels. Subsequently, specimens were incubated with corresponding secondary antibodies labeled with Alexa Fluor 594. Cell nuclei were also counterstained with DAPI. OG-PTX, PECAM-1, and DAPI were imaged using green, red, and blue filters, respectively, and then, all images were superimposed. Figure 1D shows a typical image, clearly indicating that peripheral tumor cells close to the PTX infiltration have highly condensed nuclei, suggesting massive apoptosis.
Drug modification of PTX critically affects the intratumorous distribution and anti-tumor effect
Mixture with hyaluronic acid [29]
Hyaluronic acid is a simple disaccharide polymer with an extremely high molecular weight and a physiological function as an efficient space filler which maintains hydration, and thus works as a shock absorber in joints or a protective barrier around mesothelial cells which makes the peritoneal membrane a non-adhesive surface and prevents abrasion.
We examined the effect of the mixture of HA on the antitumor effects of PTX on peritoneal tumor. We used NASHA, a preferred type of HA manufactured by bacterial fermentation from specific strains of streptococci, since it is less allergenic and widely used in plastic surgery [30, 31].
As shown in Figure 2A, IP-PTX significantly inhibits the development of PM in nude mice. However, IP injection of the same amount of PTX together with 5 % NASHA further reduced the number of PM. Observation by fluorescein microscopy showed that a much larger amount of PTX infiltrated into peritoneal nodules at 3–24 h after IP injection if NASHA was mixed with PTX (Figure 2B). HA is known to modify the pharmacokinetics of drugs possibly due to the high viscoelasticity, suggesting that HA can prolong the retention of PTX in the peritoneal cavity, which may enhance the direct infiltration of PTX and antitumor effects for PM.
Figure 2:
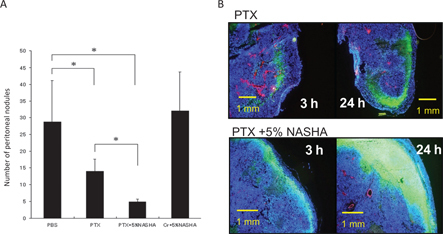
Addition of HA enhanced anti-tumor effects of IP injected PTX.(A) MKN45-P (3×106) were injected IP in nude mice and PTX given at 7, 14, and 21 days after inoculation. The numbers of disseminated peritoneal nodules on day 28 are shown. Although PTX alone can suppress the development of peritoneal metastases, the addition of 5 % NASHA to PTX further reduced the number of metastatic nodules. Data are shown as the mean±SD in five mice in a representative experiment. (B) Representative figures of peritoneal tumors stained with the same method as in Figure 1. Addition of NASHA resulted in much higher accumulation of PTX in peritoneal nodules.
PTX-30W [25, 26]
First, we used a newly produced material, a PMB polymer which is a water-soluble, amphiphilic polymer composed of 2-MPC and n-BMA as a formula to generate nanomicellar PTX (Figure 3A). The in vitro cytotoxicity of PTX-30W was almost same as PTX-Cre, which is PTX dissolved in a conventional formula (Figure 3B). However, if administered IP in nude mice, PTX-30W shows markedly deeper infiltration into tumors compared with PTX-Cre at all time points up to 24 h after IP injection (Figure 4). If the PTX concentration in the nodules is measured using high-performance liquid chromatography (HPLC), the concentration of PTX in peritoneal tumors is significantly higher using PTX-30W compared with PTX-Cre (Figure 3C). Finally, PTX-30W showed greater anti-tumor effects than PTX-Cre in vivo as evaluated by total weight of PM (Figure 3D). These facts indicate that PTX-30W has greater potential to directly infiltrate into peritoneal tumors, which may further enhance the anti-tumor effects of PTX in vivo.
Figure 3:
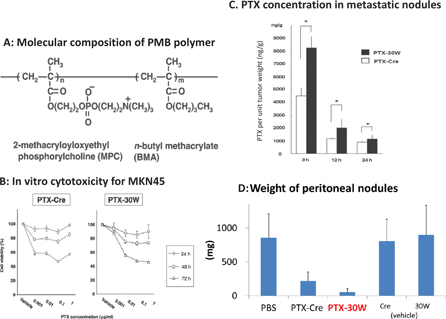
Solubilazation in PMB polymer enhanced anti-tumor effects of IP injected PTX.(A) Chemical structure of PMB polymer. (B) In vitro cytotoxicity of PTX formulated with PMB polymer (PTX-30W) or conventional Cremophor+ethanol (PTX-Cre) against MKN45-P. (C) The intratumoral concentrations of PTX calculated were significantly higher in mice receiving PTX-30W compared with PTX-Cre. Each PTX was given IP to nude mice with PM, and the peritoneal nodules excised from peritoneum of each mouse were homogenized and PTX concentration of the supernatant after the centrifugation of the homogenate solution was measured with HPLC. Data show the mean±SD. *: p<0.05 (D) MKN45-P were IP injected in nude mice and PTX were given as in Figure 2. The total weight of peritoneal tumors on day 28 in each group was shown. PTX-Cre significantly suppressed the development of peritoneal metastases. However, PTX-30W further reduced the tumor volume of PM compared to PTX-Cre (*p<0.05). Data shown are mean±SD in 8 mice.
Figure 4:
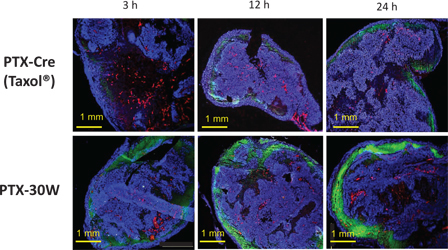
Spatial distribution of PTX-Cre or PTX-30W in PM. Peritoneal nodules around 3~5 mm in diameter were excised at 3, 12, and 24 h after IP injection of OG-PTX diluted in (PTX-Cre) or PMB30W (PTX-30W).
The intratumor distribution pattern of PTX in PM is shown in three representative color composite images as in Figure 1.
NK105 [32]
NK105 is another PTX-incorporating “core-shell-type” polymeric micellar nanoparticle formulation [33]. Previous studies have shown that NK105 is clinically useful against several malignancies including GC [34, 35]. Then, we examined the effect of the IP administration of NK105 in the same xenograft model. As shown in Figure 5, IP administration of NK105 also shows enhance antitumor effects against PM compared with PTX-Cre (Figure 5A). In this series of experiments, MKN45 was injected in the subcutaneous (SC) lesions of nude mice and the response of the SC tumor evaluated. Interestingly, IP-NK105 is also more effective to suppress the growth of SC tumors than PTX-Cre (Figure 5B). This would appear to be due to a higher serum concentration of PTX in NK105 compared with PTX-Cre, possibly due to EPR effects. These data suggest that IP administration of NK105 is useful not only for the treatment of PM but also for the control of extraperitoneal lesions.
Figure 5:
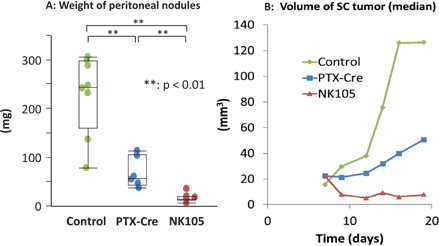
In vivo antitumor effects of paclitaxel solubilized in Cremophor–ethanol (PTX-Cre) and the PTX-incorporating micellar nanoparticle (NK105).
(A) MKN45-P cells were inoculated simultaneously both subcutaneously and intraperitoneally, and PTX-Cre or NK105 was administered IP on days 7 and 14 and the weights of the peritoneal nodules were measured at day 21. (B) In the same experiments, the volume of the subcutaneous tumor was calculated as an ellipsoid sphere and the median volume is shown for each group. NK105 inhibited the tumor growth more effectively than PTX-Cre for both peritoneal and subcutaneous tumors. *p<0.05, **p<0.01.
Discussion
IP chemotherapy is an attractive strategy for the treatment of PM, since it enables to subject high concentration of anticancer drugs directly to each metastatic lesion. However, it has not been a standard treatment for PM, presumably because of the relatively week scientific evidence. The effectiveness of IP chemotherapy critically depends on pharmacokinetics. For effective IP chemotherapy, two major biological characteristics are supposed to be required for IP drugs, i.e., long retention in abdominal cavity and highly infiltrating activity into disseminated tumor tissue.
Thus far, PTX is considered to be the most suitable drug for IP chemotherapy, due to its prolonged retention time. In fact, recent clinical studies have suggested the usefulness of IP-PTX for peritoneal lesions in ovarian [36] or gastric [11, 37] cancers. However, it remains unclear how deep the IP-PTX actually infiltrates into a tumor nodule on the peritoneal surface in these patients. Previous studies have suggested that IP administrated drugs can directly penetrate into rat peritoneal metastasis using radiolabeled drugs [38, 39] or theoretical calculation method [40]. Our results using the fluorescein-labeled PTX are mostly consistent with theirs and provide more convincing evidence that IP-PTX can infiltrate into disseminated tumor, which induce massive apoptosis of malignant cells in tumor periphery. This accumulation pattern is a marked contrast with that of systemic administrated PTX and thus suggested the clinical usefulness of the simultaneous administration of PTX via IV and IP routes (bidirectional chemotherapy).
Then, we have evaluated the drug distribution as well as anti-tumor effects of various types of PTX. First, the addition of high molecular weight HA can elongate the resident time of PTX in abdominal cavity, resulting in the enhanced anti-tumor effects. Similar results have been already reported on polysorbate-80 [41], Pluronic F127 [42], chitosan [43], or injectable hydrogels [20] for PTX. Those facts strongly suggest the addition of biomaterials to PTX formulation is a promising strategy to enhance the effects of IP chemotherapy. Optimal and practical method should be selected for future clinical trials.
Next, we found that two forms of nanomicellar PTX, PTX-30W and NK105 can induce more prominent antitumor effects as well as higher intratumor accumulation in PM as compared with conventional PTX-Cre. In general, nanoscale drugs are supposed to be highly penetrative in tumor tissue. On the other hand, nanoparticles are generally considered to be absorbed rapidly from abdominal cavity as compared with microspheres with larger size, which might be a disadvantage for IP chemotherapy. However, recent studies have shown that some nanodrugs can interact various protein components in biofluids and form larger aggregates, which can critically affect the pharmacokinetics [44, 45]. It is possible that those nanodrugs in ascitic fluids have a longer retention time in abdomen which enables the efficient penetration in peritoneal nodules. Similar result is shown in PTX-polymersome recently [46].
Conclusions
Mixture with HA or nanomicellar PTX greatly increase retention time and enhance the depth of drug penetration in PM, which may lead to improved clinical results. Many biophysiological factors are related to drug permeability in solid tumors, and the mechanisms are still largely unknown [47]. Modification of nanomicellar structures, such as molecular size, surface electronic charge, or chemical characteristics of solvent polymers may be critically related to the clinical efficacy of IP-PTX [48, 49]. Meanwhile, we have no chemotherapeutic reagents specifically approved for IP chemotherapy and clinical studies have been performed by off-label use of drugs for IV applications. However, development of ideal nanodrugs for IP administration may be a major breakthrough in the treatment of PM.
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: None declared.
Employment or leadership: None declared.
Honorarium: None declared.
Competing interests: The funding organization(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
References
- 1.Jacquet P, Sugarbaker PH, Peritoneal-plasma barrier. Cancer Treat Res 1996;82:53–63. [DOI] [PubMed]
- 2.Dedrick RL, Myers CE, Bungay PM, DeVita VT, Jr., Pharmacokinetic rationale for peritoneal drug administration in the treatment of ovarian cancer. Cancer Treat Rep 1978;62:1–11. [PubMed]
- 3.Sugarbaker PH, Mora JT, Carmignani P, Stuart OA, Yoo D, Update on chemotherapeutic agents utilized for perioperative intraperitoneal chemotherapy. Oncologist 2005;10:112–22. [DOI] [PubMed]
- 4.Glehen O, Kwiatkowski F, Sugarbaker PH, Elias D, Levine EA, De Simone M, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: A multi-institutional study. J Clin Oncol 2004;22:3284–92. [DOI] [PubMed]
- 5.Yan TD, Black D, Savady R, Sugarbaker PH, Systematic review on the efficacy of cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal carcinoma. J Clin Oncol 2006;24:4011–9. [DOI] [PubMed]
- 6.Kitayama J. Intraperitoneal chemotherapy against peritoneal carcinomatosis: Current status and future perspective. Surg Oncol 2014;23:99–106. [DOI] [PubMed]
- 7.Yonemura Y, Canbay E, Li Y, Coccolini F, Glehen O, Sugarbaker PH, et al. A comprehensive treatment for peritoneal metastases from gastric cancer with curative intent. Eur J Surg Oncol 2016;42:1123–31. [DOI] [PubMed]
- 8.Munoz-Casares FC, Medina-Fernandez FJ, Arjona-Sanchez A, Casado-Adam A, Sanchez-Hidalgo JM, Rubio MJ, et al. Peritonectomy procedures and HIPEC in the treatment of peritoneal carcinomatosis from ovarian cancer: Long-term outcomes and perspectives from a high-volume center. European J Surg Oncol 2016;42:224–33. [DOI] [PubMed]
- 9.Markman M, Intraperitoneal antineoplastic drug delivery: Rationale and results. Lancet Oncol 2003;4:277–83. [DOI] [PubMed]
- 10.Eiseman JL, Eddington ND, Leslie J, MacAuley C, Sentz DL, Zuhowski M, et al. Plasma pharmacokinetics and tissue distribution of paclitaxel in CD2F1 mice. Cancer Chemother Pharmacol 1994;34:465–71. [DOI] [PubMed]
- 11.Ishigami H, Kitayama J, Kaisaki S, Hidemura A, Kato M, Otani K, et al. Phase II study of weekly intravenous and intraperitoneal paclitaxel combined with S-1 for advanced gastric cancer with peritoneal metastasis. Ann Oncol 2010;21:67–70. [DOI] [PubMed]
- 12.Fushida S, Kinoshita J, Kaji M, Hirono Y, Goda F, Yagi Y, et al. Phase I/II study of intraperitoneal docetaxel plus S-1 for the gastric cancer patients with peritoneal carcinomatosis. Cancer Chemother Pharmacol 2013;71:1265–72. [DOI] [PMC free article] [PubMed]
- 13.Fujiwara Y, Takiguchi S, Nakajima K, Miyata H, Yamasaki M, Kurokawa Y, et al. Intraperitoneal docetaxel combined with S-1 for advanced gastric cancer with peritoneal dissemination. J Surg Oncol 2012;105:38–42. [DOI] [PubMed]
- 14.Yamaguchi H, Kitayama J, Ishigami H, Emoto S, Yamashita H, Watanabe T, A phase 2 trial of intravenous and intraperitoneal paclitaxel combined with S-1 for treatment of gastric cancer with macroscopic peritoneal metastasis. Cancer 2013;119:3354–8. [DOI] [PubMed]
- 15.Hosie K, Gilbert JA, Kerr D, Brown CB, Peers EM, Fluid dynamics in man of an intraperitoneal drug delivery solution: 4 % icodextrin. Drug Deliv 2001;8:9–12. [DOI] [PubMed]
- 16.Mohamed F, Marchettini P, Stuart OA, Sugarbaker PH, Pharmacokinetics and tissue distribution of intraperitoneal paclitaxel with different carrier solutions. Cancer Chemother Pharmacol 2003;52:405–10. [DOI] [PubMed]
- 17.Luo Y, Kirker KR, Prestwich GD, Cross-linked hyaluronic acid hydrogel films: New biomaterials for drug delivery. J Control Release 2000;69:169–84. [DOI] [PubMed]
- 18.Wang Y, Gong C, Yang L, Wu Q, Shi S, Shi H, et al. 5-FU-hydrogel inhibits colorectal peritoneal carcinomatosis and tumor growth in mice. BMC Cancer 2010;10:402. [DOI] [PMC free article] [PubMed]
- 19.Yu J, Lee HJ, Hur K, Kwak MK, Han TS, Kim WH, et al. The antitumor effect of a thermosensitive polymeric hydrogel containing paclitaxel in a peritoneal carcinomatosis model. Invest New Drugs 2012;30:1–7. [DOI] [PubMed]
- 20.Bajaj G, Kim MR, Mohammed SI, Yeo Y, Hyaluronic acid-based hydrogel for regional delivery of paclitaxel to intraperitoneal tumors. J Control Release 2012;158:386–92. [DOI] [PMC free article] [PubMed]
- 21.Emoto S, Yamaguchi H, Kamei T, Ishigami H, Suhara T, Suzuki Y, et al. Intraperitoneal administration of cisplatin via an in situ cross-linkable hyaluronic acid-based hydrogel for peritoneal dissemination of gastric cancer. Surg Today 2014;44(5):919–26. [DOI] [PubMed]
- 22.Matsumura Y, Maeda H, A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res 1986;46:6387–92. [PubMed]
- 23.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K, Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J Control Release 2000;65:271–84. [DOI] [PubMed]
- 24.Bregoli L, Movia D, Gavigan-Imedio JD, Lysaght J, Reynolds J, Prina-Mello A, Nanomedicine applied to translational oncology: A future perspective on cancer treatment. Nanomedicine 2016;12:81–103. [DOI] [PubMed]
- 25.Kamei T, Kitayama J, Yamaguchi H, Soma D, Emoto S, Konno T, et al. Spatial distribution of intraperitoneally administrated paclitaxel nanoparticles solubilized with poly (2-methacryloyloxyethyl phosphorylcholine-co n-butyl methacrylate) in peritoneal metastatic nodules. Cancer Sci 2011;102:200–05. [DOI] [PMC free article] [PubMed]
- 26.Soma D, Kitayama J, Konno T, Ishihara K, Yamada J, Kamei T, et al. Intraperitoneal administration of paclitaxel solubilized with poly(2-methacryloyloxyethyl phosphorylcholine-co n-butyl methacrylate) for peritoneal dissemination of gastric cancer. Cancer Sci 2009;100:1979–85. [DOI] [PMC free article] [PubMed]
- 27.Sako A, Kitayama J, Koyama H, Ueno H, Uchida H, Hamada H, et al. Transduction of soluble Flt-1 gene to peritoneal mesothelial cells can effectively suppress peritoneal metastasis of gastric cancer. Cancer Res 2004;64:3624–8. [DOI] [PubMed]
- 28.Konno T, Watanabe J, Ishihara K, Enhanced solubility of paclitaxel using water-soluble and biocompatible 2-methacryloyloxyethyl phosphorylcholine polymers. J Biomed Mater Res A 2003;65:209–14. [DOI] [PubMed]
- 29.Yamada J, Kitayama J, Tsuno NH, Yamashita H, Miyato H, Soma D, et al. Intra-peritoneal administration of paclitaxel with non-animal stabilized hyaluronic acid as a vehicle–a new strategy against peritoneal dissemination of gastric cancer. Cancer Lett 2008;272:307–15. [DOI] [PubMed]
- 30.Andre P, Evaluation of the safety of a non-animal stabilized hyaluronic acid (NASHA – Q-Medical, Sweden) in European countries: A retrospective study from 1997 to 2001. J Eur Acad Dermatol Venereol 2004;18:422–5. [DOI] [PubMed]
- 31.Friedman PM, Mafong EA, Kauvar AN, Geronemus RG, Safety data of injectable nonanimal stabilized hyaluronic acid gel for soft tissue augmentation. Dermatol Surg 2002;28:491–4. [DOI] [PubMed]
- 32.Emoto S, Yamaguchi H, Kishikawa J, Yamashita H, Ishigami H, Kitayama J, Antitumor effect and pharmacokinetics of intraperitoneal NK105, a nanomicellar paclitaxel formulation for peritoneal dissemination. Cancer Sci 2012;103:1304–10. [DOI] [PMC free article] [PubMed]
- 33.Hamaguchi T, Matsumura Y, Suzuki M, Shimizu K, Goda R, Nakamura I, et al. NK105, a paclitaxel-incorporating micellar nanoparticle formulation, can extend in vivo antitumour activity and reduce the neurotoxicity of paclitaxel. Br J Cancer 2005;92:1240–6. [DOI] [PMC free article] [PubMed]
- 34.Mukai H, Kato K, Esaki T, Ohsumi S, Hozomi Y, Matsubara N, et al. Phase I study of NK105, a nanomicellar paclitaxel formulation, administered on a weekly schedule in patients with solid tumors. Invest New Drugs 2016;34:750–9. [DOI] [PMC free article] [PubMed]
- 35.Kato K, Tahara M, Hironaka S, Muro K, Takiuchi H, Hamamoto Y, et al. A phase II study of paclitaxel by weekly 1-h infusion for advanced or recurrent esophageal cancer in patients who had previously received platinum-based chemotherapy. Cancer Chemother Pharmacol 2011;67:1265–72. [DOI] [PubMed]
- 36.Armstrong DK, Fleming GF, Markman M, Bailey HH, A phase I trial of intraperitoneal sustained-release paclitaxel microspheres (Paclimer) in recurrent ovarian cancer: A Gynecologic Oncology Group study. Gynecol Oncol 2006;103:391–6. [DOI] [PubMed]
- 37.Kitayama J, Ishigami H, Yamaguchi H, Yamashita H, Emoto S, Kaisaki S, et al. Salvage gastrectomy after intravenous and intraperitoneal paclitaxel (PTX) administration with oral S-1 for peritoneal dissemination of advanced gastric cancer with malignant ascites. Ann Surg Oncol 2014;21:539–546. [DOI] [PubMed]
- 38.Los G, Mutsaers PH, Van Der Vijgh WJ, Baldew GS, De Graaf PW, McVie JG, Direct diffusion of cis-diamminedichloroplatinum(II) in intraperitoneal rat tumors after intraperitoneal chemotherapy: A comparison with systemic chemotherapy. Cancer Res 1989;49:3380–4. [PubMed]
- 39.Flessner MF, Fenstermacher JD, Blasberg RG, Dedrick RL, Peritoneal absorption of macromolecules studied by quantitative autoradiography. Am J Physiol 1985;248:H26–32. [DOI] [PubMed]
- 40.El-Kareh AW, Secomb TW, A theoretical model for intraperitoneal delivery of cisplatin and the effect of hyperthermia on drug penetration distance. Neoplasia 2004;6:117–27. [DOI] [PMC free article] [PubMed]
- 41.Yokogawa K, Jin M, Furui N, Yamazaki M, Yoshihara H, Nomura M, et al. Disposition kinetics of taxanes after intraperitoneal administration in rats and influence of surfactant vehicles. J Pharm Pharmacol 2004;56:629–34. [DOI] [PubMed]
- 42.De Smet L, Colin P, Ceelen W, Bracke M, Van Bocxlaer J, Remon JP, et al. Development of a nanocrystalline Paclitaxel formulation for HIPEC treatment. Pharm Res 2012;29:2398–406. [DOI] [PubMed]
- 43.Lim Soo P, Cho J, Grant J, Ho E, Piquette-Miller M, Allen C, Drug release mechanism of paclitaxel from a chitosan-lipid implant system: Effect of swelling, degradation and morphology. Eur J Pharm Biopharm 2008;69:149–57. [DOI] [PubMed]
- 44.Walkey CD, Chan WC, Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chem Soc Rev 2012;41:2780–99. [DOI] [PubMed]
- 45.Dakwar GR, Braeckmans K, Demeester J, Ceelen W, De Smedt SC, Remaut K, Disregarded effect of biological fluids in sirna delivery: human ascites fluid severely restricts cellular uptake of nanoparticles. ACS Appl Mater Interfaces 2015;7:24322–9. [DOI] [PubMed]
- 46.Simon-Gracia L, Hunt H, Scodeller PD, Gaitzsch J, Braun GB, Willmore AM, et al. Paclitaxel-loaded polymersomes for enhanced intraperitoneal chemotherapy. Mol Cancer Ther 2016;15:670–9. [DOI] [PMC free article] [PubMed]
- 47.Minchinton AI, Tannock IF, Drug penetration in solid tumours. Nat Rev Cancer 2006;6:583–92. [DOI] [PubMed]
- 48.Ma P, Mumper RJ, Paclitaxel nano-delivery systems: A comprehensive review. J Nanomed Nanotechnol 2013;4:1000164. [DOI] [PMC free article] [PubMed]
- 49.Dakwar GR, Shariati M, Willaert W, Ceelen W, De Smedt SC, Remaut K, Nanomedicine-based intraperitoneal therapy for the treatment of peritoneal carcinomatosis - mission possible?. Adv Drug Deliv Rev 2017;108:13–24. [DOI] [PubMed]


