Abstract
Background
Pressurised intraperitoneal aerosol chemotherapy (PIPAC) is a novel technique of intraperitoneal chemotherapy devoted to unresectable peritoneal metastasis (PM). The first results obtained with PIPAC in preclinical models of colon cancer are presented here.
Methods
In vitro, PIPAC (normotherm oxaliplatin at 0.028 mg/mL for 10 min at 1.6 bars) and HIPEC (hyperthermic oxaliplatin at 0.14 mg/mL for 30 min) were compared using the apoptosis and proliferation assay on two colon cancer cell lines (LS 174 and CT 26); ex vivo tumours from an orthotopic mouse model of PM and non-tumour peritoneum from a patient treated according to the two modalities were assessed, investigating the percentage of penetration of oxaliplatin in the tumour and oxaliplatin concentration below the peritoneum. In vivo, a mouse model of colon (CT 26) PM was used to create a PIPAC model (same modalities) for the comparison of IV oxaliplatin (at 5 mg/mL).
Results
In vitro, the rate of apoptotic and proliferative cells as well as the level of oxaliplatin penetration in tumour nodes was higher in PIPAC groups with less systemic passage through the peritoneum. In vivo, in the colon PM mouse model, the peritoneal cancer index (PCI) was decreased to the same level using PIPAC or IV oxaliplatin. Systemic passage was lower in the PIPAC group.
Conclusions
PIPAC with low-dose oxaliplatin is efficient in both in vitro and in vivo models of colon PM. Lower concentrations of chemotherapy are needed in PIPAC to achieve the same effect as IV chemotherapy on PCI. With a very low systemic oxaliplatin passage, this technique of drug delivery seems to be as effective as IV delivery for PM control.
Keywords: chemotherapy, colon cancer, hyperthermic intraperitoneal chemotherapy (HIPEC), oxaliplatin, peritoneal metastasis, pressurised intraperitoneal aerosol chemotherapy (PIPAC)
Introduction
Pressurised intraperitoneal aerosol chemotherapy (PIPAC) has been recently proposed as a new technique for delivering intraperitoneal chemotherapy. PIPAC is reported as a new palliative treatment for different kinds of peritoneal metastasis, arising from colon, ovarian or gastric cancer [1]. In the initial description, PIPAC is used in an isolated form, without being combined with intravenous chemotherapy.
This new concept is based on association of hyper-pressure and aerosol formation to increase drug delivery using an aerosol as a carrier system and pressure as the tissue penetration increases. The concept of increasing drug penetration under pressure had been previously demonstrated in other animal models [2]. Moreover, it is postulated that hyper-pressure obtained by gas administration during laparoscopy decreases the venous blood flow in abdominal organs, resulting in an increased time of drug tissue contact [3].
The concept proposed by the first surgeons to develop PIPAC is maintaining local peritoneal control of the disease with the administration of limited drug concentrations, equivalent to the intravenous treatment associated with a decrease of the secondary effect because of the administration of a low dose of locoregional treatment [1]. Another secondary aim is obtaining a major tumour response offering a solution for cytoreductive secondary surgery associated with a hyperthermic intraperitoneal chemotherapy (HIPEC) procedure. That situation, where PIPAC is used as neoadjuvant chemotherapy, has only been reported very recently [4]. Exploring the second concept will require clinical trials. PIPAC is just starting to be controlled and different technical aspects, such as single port access, have recently been proposed for controlling safety and efficacy [5]. Further scientific information is required to help with technical development and offer evidence for efficacy.
Only one team have reported limited experimental data regarding pharmacokinetic drug delivery using PIPAC, or tumour control analysis [6]. However, the authors analysed the PIPAC device rather than the technical concept [7]. Second, they performed the majority of their study on ex vivo peritoneum animal explants, resulting in a limited impact of their results.
Therefore, we decided to evaluate some major specific points of the PIPAC process using an experimental pharmacokinetic animal model: (i) Is systemic drug diffusion limited and (ii) Is the anti-tumour effect similar to that achieved with intravenous treatment but with a lower drug concentration?
Materials and methods
Cell culture
LS174 (human colon cancer cells) and CT26 (murine colon cancer cells) were obtained from the American Type Culture Collection. The CT26 cell line is a murine colon adenocarcinoma cell line derived from BALB/c mice treated with N-nitroso-N-methylurethane. The two cell lines were maintained in Dulbecco’s modified Eagle’s medium, supplemented with 10 % heat-inactivated foetal bovine serum/penicillin (50 U/mL)/streptomycin (50 g/mL) (Gibco BRL Life Technologies Inc., Grand Island, NY) in a humidified atmosphere of 95 % air and 5 % CO2 at 37 °C.
Cell apoptosis assays
To evaluate in vitro tumour cell proliferation and apoptosis, LS174 and CT26 cells were maintained under normal growth conditions, described previously herein. Fifteen 104 cells were seeded in triplicate into each well of a four-well flat-bottom plate. The cells were allowed to attach and grow overnight.
After removing media, cells were treated with oxaliplatin using the HIPEC (0.14 mg/mL for 30 min at 43 °C) or PIPAC (0.028 mg/mL for 30 min at 37 °C) modalities.
Using a terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling (TUNEL) kit (Roche Diagnostics), the apoptosis of cultured LS174 and CT26 cells was determined just after treatment. The ratio of the surface occupied by TUNEL staining to total surface examined for the cross-sections and the percentage of TUNEL-stained cells to DAPI-stained cells for the cultured LS174 and CT26 cells in vitro were determined using a customised application (Histolab).
Oxaliplatin tumour concentration by Inductively Coupled Plasma mass spectrometry (ICP-MS)
Tumour nodes obtain 15 days after the intraperitoneal injection of CT26 were explanted and treated with oxaliplatin with the HIPEC (0.14 mg/mL for 30 min at 43 °C) or PIPAC (0.028 mg/mL for 30 min at 37 °C) modalities.
After treatment, tumour nodes were cut into three parts: two external parts and one internal part.
The oxaliplatin concentration of the three tumour nodes parts was assessed using Inductively Coupled Plasma mass spectrometry (ICP-MS).
To reflect the oxaliplatin depth of penetration, a ratio of oxaliplatin was obtained by comparing the internal and external parts of the PM tumour nodes.
Systemic passage of oxaliplatin
Human non-tumour peritoneum was obtained from hernia repair patients and treated with oxaliplatin according to the two modalities, HIPEC (0.14 mg/mL for 30 min at 43 °C) or PIPAC (0.028 mg/mL for 30 min at 37 °C).
Liquid that went through the peritoneum was obtained and the concentration of oxaliplatin was analysed.
Animal model of orthotopic peritoneal carcinomatosis
All of the experimental protocols met the standards required by the European Community guidelines for the care and use of laboratory animals. The laboratory number for the national accreditation for animal experimentation is C75-10-03 (November 27, 2012). The registration number of the Ethics Committee for the carcinomatosis model experiments is APAFIS-3944-2015122813591563v3. This study has been lead in accordance with the ARRIVE guidelines checklist [8].
Five-week-old female BALB/C mice (Charles River, Arbresle, France) were acclimatised for 1–2 weeks before tumour transplantation (8–10 animals per experimental group).
For the peritoneal carcinomatosis orthotopic model, the animals were anaesthetised. Under sterile conditions, CT26 (3 x 104) cells were injected into the peritoneal cavity.
At day 7 after tumour transplantation for the CT26 colon cancer model, the mice received oxaliplatin intravenously (5 mg/mL, Ox IV group) or with PIPAC (0.028 mg/mL, Ox PIPAC group). Two separate control (CT) groups were created: CT IV and CT PIPAC groups, applying saline solution intravenously or using PIPAC, respectively. The animals were monitored daily and were sacrificed at day 14. Blood was obtained and the level of oxaliplatin was analysed. The incision was then extended to allow photography of the peritoneal cavity and determination of the extent of peritoneal carcinomatosis using a modified Peritoneal Cancer Index (PCI). The PCI (range, 1–39) allows assessment of the distribution of cancer in the abdomen and pelvis and is calculated by totalling the lesion size scores (0 to 3) in the abdominopelvic regions (0 to 13). The PCI was adapted to tumour sizes in mice with the following lesion size scores: tumour smaller than 2.0 mm (lesion size 1), 2.1 to 4.0 mm (lesion size 2), and greater than 4.0 mm or confluence (lesion size 3), as previously described [9].
PIPAC device for small animal model
We decided to use a specific material to obtain aerosol. The Capnomed system, used in human PIPAC, is not accurate in our animal laboratory. We require an airflow control and a pump for hyper-pressure. For that, we decided to use a nebuliser from the DIHEN process (Direct Injection High Efficiency – MEINHARD Colorado USA). The DIHEN is a pneumatic micronebuliser designed to introduce micro-volumes of sample solutions directly into any container without a spray chamber. DIHEN can introduce microvolumes into plasma at a rate of 1–100 μL/min, with a relatively small aerosol droplet size, and with nearly 100 % use of the sample. The droplet formation is optimal at around five bars.
A commercial, hermetically sealable plastic box with a total volume of 2 l, mimicking the abdominal cavity, was used. In the centre of the top cover of the plastic box, 5-mm holes were made to place the nebuliser. Mice were place in the box, anaesthetised with isoflurane (0.5 %) and monitored to prevent any cardiorespiratory depression. The animals were placed in the decubitus position on a heating-blanket (38 °C); the superior part of the mice, including the thorax and head, was protected from nebulisation by sterile gauze.
The plastic box was then tightly sealed, and a constant O2 instillation of 12 mmHg was applied throughout the entire PIPAC procedure and monitored with a manometer. Oxaliplatin (0.028 mg/mL) in 2 mL of 0.9 % NaCl at room temperature (23 °C) was aerosolised with a flow rate of 0.4 mL/min for 5 min. After the aerosol phase, the tissue specimens were exposed to another 10 min of aerosolised oxaliplatin (exposure phase).
Immunohistological analysis
For the evaluation of CD31 and Ki-67 staining, each frozen tumour sample was sectioned at a thickness of 5-µm using a cryostat (Leica Microsystems GmbH, Wetzlar, Germany). The sections were incubated with anti-CD31 antibody and anti–Ki-67 antibody (BD Biosciences) for 1 hour at room temperature and then with the second antibody. Images were acquired using a fluorescence microscope equipped with appropriate filters (Observer.Z1; Carl Zeiss Inc.). Histo-Lab software (version 7.0; Microvision Instruments, Evry, France) was used to quantify the results. Tumour tissue was then harvested and frozen for subsequent immunohistochemical analysis.
Statistical analysis
The results are expressed as mean ± SEM. The unpaired t-test was used to compare two different experimental groups. The comparisons between more than two different experimental groups were performed using one-way analysis of variance followed by a Bonferroni post-test. A p-value <0.05 was considered significant.
Results
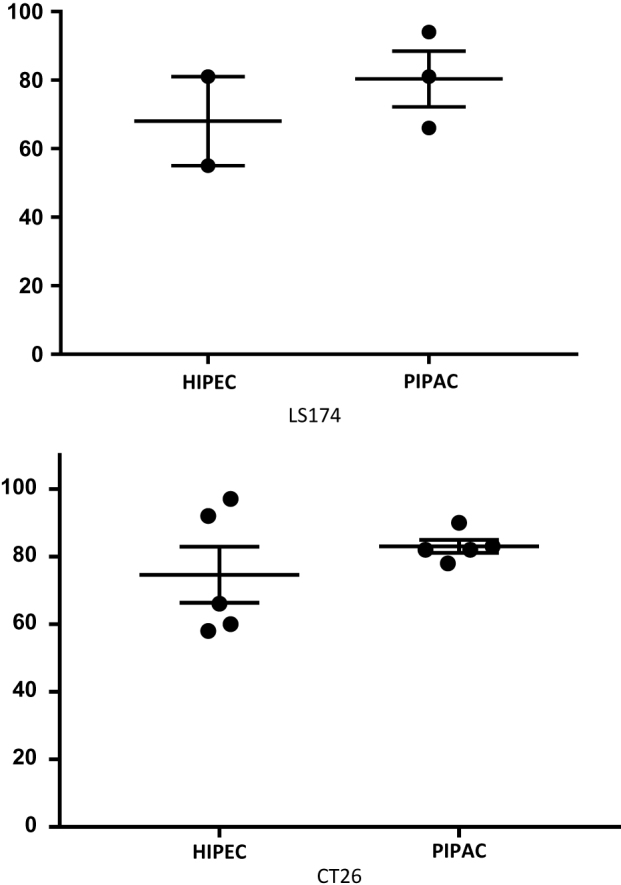
PIPAC affects tumour cell apoptosis in a drug exposure three-dimensional xenograft model with colorectal cancer cell lines
To ascertain whether oxaliplatin affects the apoptosis of colon cancer cells, we investigated the effect of HIPEC and PIPAC on apoptosis in LS174 and CT26 cells in vitro. There was a trend in PIPAC increasing apoptosis in CT26 (mean±SEM: 83 1.9 and 75±8.3 for the PIPAC and HIPEC groups, respectively; p=0.65) and LS174 cell lines (mean±SEM: 80 8.1 and 68±13 for the PIPAC and HIPEC groups, respectively, p=0.50; Figure 1).
Figure 1:
Effects of HIPEC and PIPAC on colorectal tumour cell apoptosis.
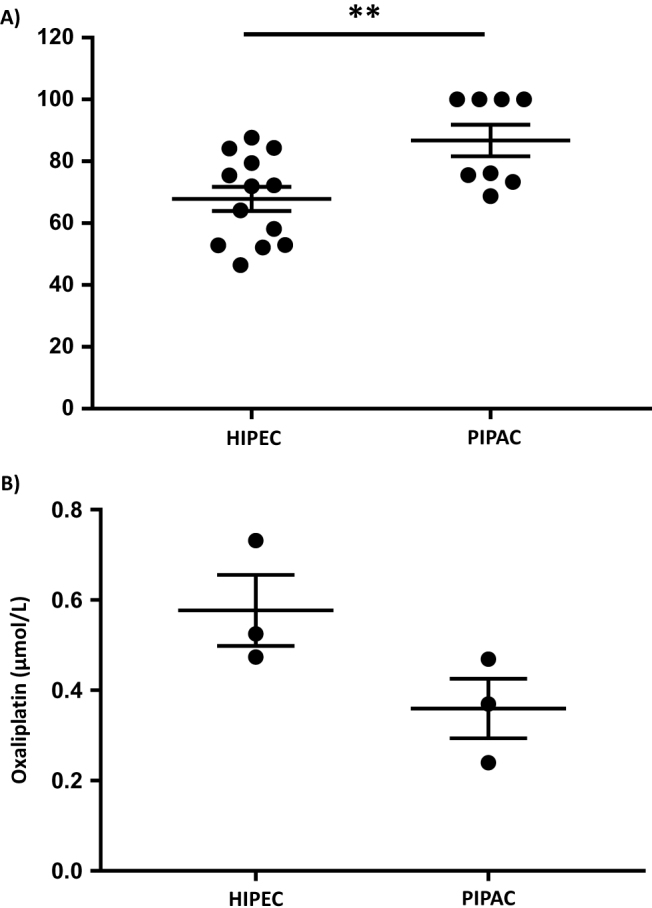
PIPAC increase oxaliplatin tissue uptake with less systemic passage in ex vivo models of peritoneal carcinomatosis
The depth ratio of oxaliplatin was higher in the PIPAC group compared with HIPEC treatment (mean±SEM: 86.7 5.1 (n=8) vs. 67.8±3.9 (n=13), p=0.0082; Figure 2A), attesting to a better drug penetration in tumour nodes.
Figure 2:
Effect of HIPEC and PIPAC in (A) oxaliplatin tissue uptake (to reflect the oxaliplatin depth of penetration, a ratio of oxaliplatin was obtained comparing internal and external nodule parts) and (B) systemic passage in ex vivo models of peritoneal carcinomatosis.
The concentration of oxaliplatin through the non-tumour peritoneum has shown a trend towards being lower in the PIPAC group compared with the HIPEC group (mean±SEM: 0.36 0.11 vs. 0.53±1.13, p=0.1; Figure 2B).
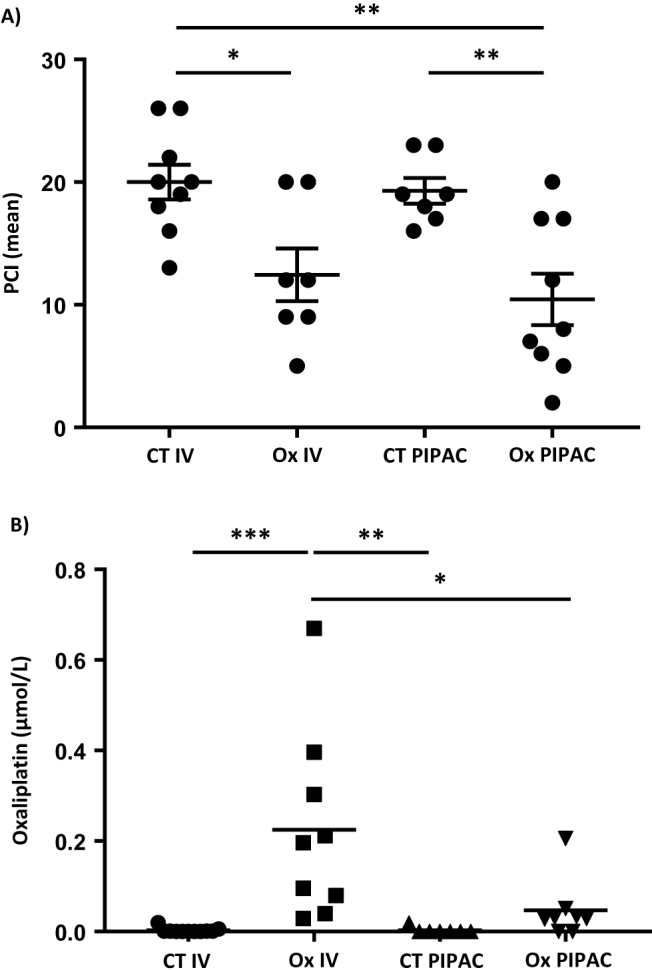
PIPAC decreases CRC carcinomatosis and its related oxaliplatin systemic passage
We next examined the effects of oxaliplatin with PIPAC compared with IV oxaliplatin, on CRC carcinomatosis using a modified PCI assessment. Day 14 after cancer cell transplantation, the PCI was significantly decreased in animals receiving oxaliplatin either with IV or the PIPAC delivery mode (mean±SEM: 12.43 2.1 (n=7) vs. 10.44±2.1 (n=9)) compared to saline fluid with IV or PIPAC (mean±SEM: 20±1.4 (n=9) vs. 19.29±1.04 (n=7), p=0.02 and 0.008, respectively; Figure 3A).
Figure 3:
Effect of IV and PIPAC oxaliplatin on (A) CRC PC burden and (B) systemic passage.
We then analysed the systemic passage of oxaliplatin by blood concentration. We confirmed that in the control groups, application by IV or using the PIPAC procedure generated no systemic passage of oxaliplatin (mean±SEM: 0.0027±0.0018 vs. 0.0027±0.0027, respectively). In the Ox IV group, the blood concentration of oxaliplatin was higher than in the Ox PIPAC group (mean±SEM: 0.225±0.069 vs. 0.047±0.024, respectively, p=0.008; Figure 3A), reflecting the lower systemic passage of oxaliplatin with the PIPAC procedure.
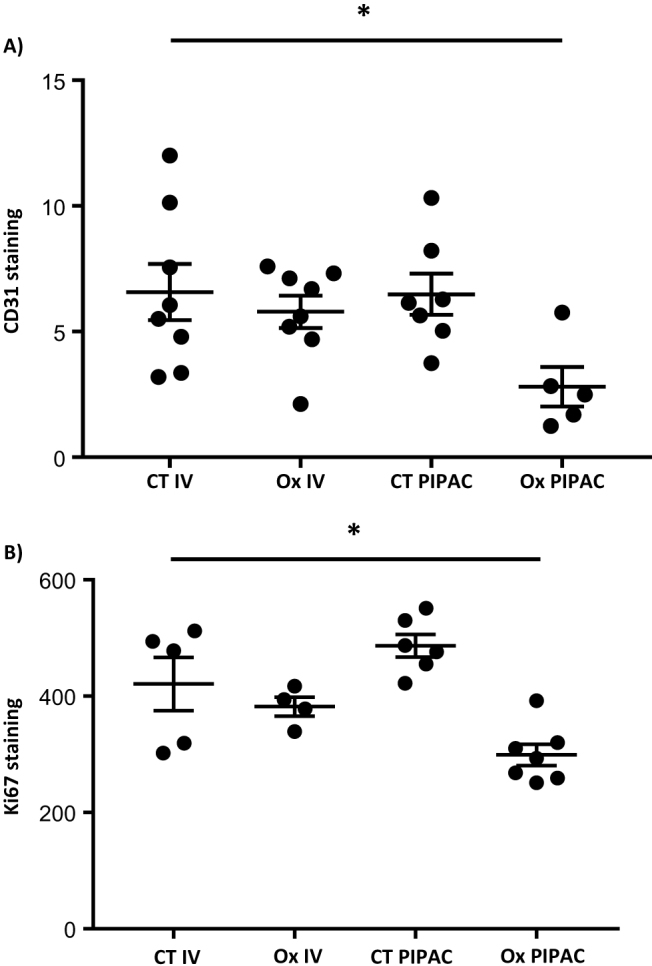
Role of PIPAC on tumour angiogenesis and tumour cell proliferation in CRC carcinomatosis xenograft model
Decreased tumour progression was related to a trend to a reduction in the staining surface of CD31 in animals in the Ox PIPAC group, suggesting that PIPAC exerts an inhibitory effect on tumour angiogenesis (mean±SEM: 5.79±0.05 and 0.47±0.06 for the Ox IV and Ox PIPAC groups, respectively, p=0.15; Figure 4A).
Figure 4:
Effect of IV and PIPAC oxaliplatin on (A) CD31 staining and (B) Ki67 staining.
Moreover, PIPAC has a trend to decrease the staining of Ki-67 in the tumour (mean±SEM: 382±16.42 and 299±18.33 for the Ox IV and Ox PIPAC groups, respectively, p=0.18; Figure 4B).
Discussion
Our experimental study was designed to examine possible advantages of PIPAC in colorectal PM compared to intravenous or HIPEC treatment. We showed an increased penetration in human tumour nodule of PM when ex vivo treated with PIPAC, which support the additional effect of increased intra-abdominal pressure of 12 mmHg. Jacquet et al. have already reported this finding in rat models with a maximal tissue concentration after an exposure time of 10 min [10].
This exposure time is the same as that used in our PIPAC model. We postulated that the PIPAC mortality will be too elevated if we performed a 30 min treatment for the mice. The duration of the open abdomen procedure had to be limited for small animals. This is the first limitation of our study. However, we postulated that, as for rats, the maximal tumour nodule concentration would be obtained at 10 min.
For the first time, we created a reproducible murine model of peritoneal metastasis treated with PIPAC application. Our model allowed us to compare the two modalities (intravenous and PIPAC chemotherapy) that could be proposed in palliative settings for peritoneal metastasis. We observed a comparable effect on tumour burden, analysed by PCI, of the two kinds of chemotherapy delivery. We deliberately chose a dose of chemotherapy adapted from human clinical practice with a very low dose in PIPAC instillation. The dose of oxaliplatin used in the intravenous treatment was previously utilised in other experiments in the lab unit [11]. The dose for PIPAC was decided using a five-times dose decrease, as reported for humans.
Interestingly, as the effect on peritoneal tumour was comparable, the systemic passage of oxaliplatin in blood was lower in the PIPAC group; attesting to the lower dose needed in PIPAC and the promising effect on toxicities.
Taken together, our results confirm that the concept supposed to offer a pharmacological advantage of the PIPAC process could be identified in an animal PM model for colon cancer cells. Our results are very preliminary and many biases are present. The best model did not exist for PM. Large animals may be able to offer some information regarding gas diffusion and tissue concentration. However, no large animals, such as pig, have presented as an animal model for PM [12]. Regarding these major limitations, the human translation of our experiment is limited to major concepts. Our animal model could not to be considered to offer a replacement solution for human clinical trials.
Two different conclusions could be drawn from these findings for human clinical practice. The first refers to the idea that a dose escalation is needed to improve PIPAC results in terms of tissue depth penetration and tissue drug concentrations. This idea has been tested by increasing the drug concentration and pressure, in Petri dishes with a positive effect on cellular proliferation rate [13]. However, that ex vivo model is too far from human reality to impact human practice.
However, our in vivo model is also too far from the human physical situation regarding tissue pressure, drug exposure and volume distribution. Our PIPAC model is constructed in a plastic box, using animals weighing 25 g. For that reason, it could be postulated that our model is not adapted to find the exact dosage for pressure or oxaliplatin concentration required. For that reason, human clinical trials are mandatory. The idea will be tested in France, in the “PIPOX” trial, a phase I-II dose escalation protocol for peritoneal metastasis arising from all origins (ovarian, colon or gastric cancer). This trial will analyse the pharmacokinetic and toxicity aspect of dose escalation and has been financed by the French National Cancer Institute (INCa). In contrast to the concept of dose escalation, 92 mg/m2 body surface oxaliplatin in a 150 mL dextrose solution has been proposed for patients with colorectal cancer for PIPAC procedure by many surgical teams, as in the Italian PIPAC phase 2 trials for PM colon cancer.
The second hypothesis that we tested in our model was the use of PIPAC as an equivalent to standard carcinomatosis treatment. In that concept, PIPAC had to offer high tumour control without affecting the quality of life because of a limited secondary effect. We postulated that our animal results offer the first evidence for the efficacy for oxaliplatin.
To demonstrate those results, clinical trials are mandatory. A first report described isolated PIPAC or PIPAC associated with systemic chemotherapy to control PM [14]. Different studies are under discussion in Europe and for different PM, such as ovarian, gastric or colon cancer. We have constructed a phase II randomised study “PIPAC EstoK 01” on non-optimally resectable gastric peritoneal metastasis comparing IV chemotherapy alone or in combination with PIPAC. Neurotoxicity and quality of life will be carefully analysed. We hope that the reduced dose of oxaliplatin in the PIPAC arm will confer positive oncological results, as described by Demtröder et al. in colon cancer, without creating damageable systemic passage, as shown in our preclinical results, resulting in minor neurotoxicity [15]. Our study has also been financed by the French National Cancer Institute (INCa) and will start shortly.
Taking together our results, this study offers the first evidence of PIPAC efficacy in colon cancer PM using oxaliplatin. Animal models offer an opportunity to test different drugs for PIPAC delivery regarding different PM for different primary cancers. However, rodent animal models can only propose a limited level of efficiency regarding a very important model limitation because of the small volume which could affect PIPAC results.
Acknowledgments
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: This work was supported in part by a grand (RMA-2015-032) of the charity foundation “Fondation de l’Avenir”.
Employment or leadership: None declared.
Honorarium: None declared.
Competing interests: The funding organization(s) played no role in thestudy design; in thecollection, analysis, and interpretationof data; in the writing of the report; or in the decision tosubmit the report for publication.
References
- 1.Solass W, Kerb R, Mürdter T, Giger-Pabst U, Strumberg D, Tempfer C, et al. Intraperitoneal chemotherapy of peritoneal carcinomatosis using pressurized aerosol as an alternative to liquid solution: first evidence for efficacy. Ann Surg Oncol 2014 Feb;21:553–9. [DOI] [PMC free article] [PubMed]
- 2.Esquis P, Consolo D, Magnin G, Pointaire P, Moretto P, Ynsa MD, et al. High intra-abdominal pressure enhances the penetration and anti-tumour effect of intraperitoneal cisplatin on experimental peritoneal carcinomatosis. Ann Surg 2006 Jul;244:106–12. [DOI] [PMC free article] [PubMed]
- 3.Tempfer CB. Pressurized intraperitoneal aerosol chemotherapy as an innovative approach to treat peritoneal carcinomatosis. Med Hypotheses 2015 Oct;85:480–4. [DOI] [PubMed]
- 4.Girshally R, Demtröder C, Albayrak N, Zieren J, Tempfer C, Reymond MA. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) as a neoadjuvant therapy before cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. World J Surg Oncol 2016 Sep 27;14:253. [DOI] [PMC free article] [PubMed]
- 5.Vaira M, Robella M, Borsano A, De Simone M. Single – Port Access for Pressurised IntraPeritoneal Aerosol Chemotherapy (PIPAC): technique, feasibility and safety. Pleura Peritoneum 2016;1:217–22. [DOI] [PMC free article] [PubMed]
- 6.Khosrawipour V, Khosrawipour T, Kern AJ, Osma A, Kabakci B, Diaz-Carballo D, et al. Distribution pattern and penetration depth of doxorubicin after pressurized intraperitoneal aerosol chemotherapy (PIPAC) in a post-mortem swine model. J Cancer Res Clin Oncol 2016 Nov;142:2275–80. [DOI] [PMC free article] [PubMed]
- 7.Göhler D, Khosrawipour V, Khosrawipour T, Diaz-Carballo D, Falkenstein TA, Zieren J, et al. Technical description of the microinjection pump (MIP®) and granulometric characterisation of the aerosol applied for pressurized intraperitoneal aerosol chemotherapy (PIPAC). Surg Endosc 2016 Sep 8. Epub ahead of print. [DOI] [PubMed]
- 8.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. Plos Biol 2010 Jun 29;8:e1000412. [DOI] [PMC free article] [PubMed]
- 9.Eveno C, Broqueres-You D, Feron JG, Rampanou A, Tijeras-Raballand A, Ropert S, et al. Netrin-4 delays colorectal cancer carcinomatosis by inhibiting tumour angiogenesis. Am J Pathol 2011 Apr;178:1861–9. [DOI] [PMC free article] [PubMed]
- 10.Jacquet P, Stuart OA, Chang D, Sugarbaker PH. Effects of intra-abdominal pressure on pharmacokinetics and tissue distribution of doxorubicin after intraperitoneal administration. Anticancer Drugs 1996 Jul;7:596–603. [DOI] [PubMed]
- 11.Jouvin I, Najah H, Pimpie C, Canet Jourdan C, Kaci R, Mirshahi M, et al. Reduction of carcinomatosis risk using icodextrin as a carrier solution of intraperitoneal oxaliplatin chemotherapy. Eur J Surg Oncol 2017 Jan 9. pii: S0748-7983(17)30036-7. [DOI] [PubMed]
- 12.Gremonprez F, Willaert W, Ceelen W. Intraperitoneal chemotherapy (IPC) for peritoneal carcinomatosis: review of animal models. J Surg Oncol 2014 Feb;109:110–6. [DOI] [PubMed]
- 13.Khosrawipour V, Diaz-Carballo D, Ali-Haydar A, Khosrawipour T, Falkenstein TA, Wu D, et al. Giger-Pabst U1,2. Cytotoxic effect of different treatment parameters in pressurised intraperitoneal aerosol chemotherapy (PIPAC) on the in vitro proliferation of human colonic cancer cells. World J Surg Oncol 2017 Feb 10;15:43. [DOI] [PMC free article] [PubMed]
- 14.Robella M, Vaira M, De Simone M. Safety and feasibility of pressurised intraperitoneal aerosol chemotherapy (PIPAC) associated with systemic chemotherapy: an innovative approach to treat peritoneal carcinomatosis. World J Surg Oncol 2016 Apr 29;14:128. [DOI] [PMC free article] [PubMed]
- 15.Demtröder C, Solass W, Zieren J, Strumberg D, Giger-Pabst U, Reymond MA. Pressurised intraperitoneal aerosol chemotherapy with oxaliplatin in colorectal peritoneal metastasis. Colorectal Dis 2016 Apr;18:364–71. [DOI] [PubMed]


