Abstract
The effects on Giardia duodenalis of Slab51 probiotic supernatants were evaluated in vitro and ex vivo. In vitro, Slab51 (101 UFC) was cultured and the obtained supernatant was filtered, adjusted at pH 7, and added (100μl/ml) as such (Slab51 FS) or after heat-treatment, to G. duodenalis cultures to evaluate its effects on G. duodenalis trophozoites growth and adherence. For comparison, negative and metronidazole (20μg/ml) treated controls were used. The morphological and ultrastructural alterations of G. duodenals trophozoites following treatment with Slab51 FS supernatant were investigated by transmission electron microscopy. Ex vivo, mice duodenal portions were cultivated in standard conditions with 5x105 G. duodenalis trophozoites/ml, while to further five duodenal portions similarly cultured and infected, Slab51 FS 200μl was added. After 12 and 18h, samples were fixed in 10% buffered formalin and histologically processed to score Giardia infection and cell damage. Cell proliferation/apoptosis was scored by Ki67, TUNEL and Caspase–3 tests. All experiments were conducted in triplicate throughout the study. All data were statistically evaluated (P< 0.05). Results showed that Slab51 FS significantly reduced Giardia growth and adherence respect to negative controls, but its efficacy was overall lower than that of metronidazole. Moreover, the effects of Slab51 FS were significantly lowered by heat-treatment and this reduction was statistically higher at 90°C than at 56°C, indicating a heat-sensitive nature of active Slab51 FS compounds. At the ultrastructural level, Slab51 FS treated Giardia trophozoites were swelling, increased in size and showed alterations of their cellular membrane and vacuole patterns, loss of the nuclear envelope and nuclear architecture. In ex vivo trials, viable G. duodenalis trophozoites and enterocyte TUNEL+ and Caspase-3 expression were significantly reduced in intestinal sections added with Slab51 FS, while enterocyte Ki67 expression was significantly increased, confirming the anti-G. duodenalis activity of Slab51 FS observed in vitro. In conclusion, results from this study showed that the fresh culture supernatant of the commercial probiotic Slab51 has anti-G. duodenalis properties both in vitro and ex vivo in a mouse model.
Introduction
Flagellated protozoans of the genus Giardia are found in the digestive tract of vertebrate hosts worldwide in which they are the cause of giardiasis [1]. Giardia duodenalis (syn. Giardia intestinalis, Giardia lamblia) is the only species found in humans and in many other wild and domestic mammals worldwide [1, 2]. Based on genetic analysis, G. duodenalis is considered a species complex, which includes at least eight distinct genetic groups or assemblages, from A to H [1]. Assemblages A and B are usually isolated from humans but can also infect other animals, being considered zoonotic [1, 3].
The localization site of G. duodenalis is the small intestine, mainly duodenum and jejunum, and it may be responsible for asymptomatic, acute and chronic clinical forms [4, 5]. Diarrhea, malabsorption and weight loss, as well as numerous post-infectious pathologies and extra-intestinal complications are the main clinical signs of symptomatic infections [4–6]. The life cycle of G. duodenalis is direct and involves two stages, the trophozoite and the cyst. Mammal hosts may acquire G. duodenalis infections via ingestion of infectious cysts in contaminated food or water sources, or directly via the fecal-oral route [1, 2].
Giardiasis is one of the most common intestinal protozoal infections reported in humans, pet and farm animals [7, 8]. Moreover, human giardiasis has been included in the World Health Organization's (WHO) Neglected Diseases Initiative since 2006, estimating that 280 million people are infected each year [9, 10]. The control of giardiasis is dependent on chemotherapy, and treatments are based mainly on the use of nitroimidazoles, such as metronidazole and tinidazole, and benzimidazoles, mainly fenbendazole and albendazole; furazolidone, acridine, quinacrine, nitazoxanide and paromomycin are also used in some situations [5; 11–15]. However, most of the therapeutically used anti-Giardia drugs, including metronidazole, may cause severe side effects and are not well tolerated by many human and animal patients or cannot be used in farm animals [11, 16]. Moreover, the use of these drugs is often associated with clinical failure and drug resistance [16–18]. Hence, identifying new anti-Giardia agents is an important consideration for the control of giardiasis in human and veterinary medicine [16, 19].
Some data from recent in vitro and in vivo studies, largely from mice and humans, have shown that probiotic treatment may possibly ameliorate G. duodenalis symptoms or reduce infection with G. duodenalis [6, 20]. These compounds have attracted the attention as potential substitutes for, or as combined therapy to currently used anti-Giardia drugs due to their powerful activity, stability and low toxicity to humans and other mammal hosts [11, 21].
In the present study, potential negative effects of the supernatant of a commercial probiotic on G. duodenalis were evaluated in vitro and ex vivo.
Materials and methods
Slab51 (SivoMixx)
Slab51 (sold in Europe today under the trademark SivoMixx, Ormendes SA, Jouxtens-Mézery, CH) is a commercial multi-strain probiotic containing 200 billion lactic acid bacteria per 1.5 grams of product, comprised of the following strains: Streptococcus thermophilus DSM 32245, Bifidobacterium lactis DSM 32246, Bifidobacterium lactis DSM 32247, Lactobacillus acidophilus DSM 32241, Lactobacillus helveticus DSM 32242, Lactobacillus paracasei DSM 32243, Lactobacillus plantarum DSM 32244, Lactobacillus brevis DSM 27961.
Parasite and cultures
Trophozoites (5x104) of G. duodenalis WB strain (genotype A1) were maintained in axenic culture at 37°C in 8 ml of TYI-S-33 medium in screw-cap cell culture vials. Penicillin G (250μg/ml), streptomycin sulfate (250μg/ml), gentamicin sulfate (50μg/ml) and amphotericin B (0.25μg/ml) were added during routine culture [22, 23]. After two days, log-phase cultures were harvested after cooling the culture vials at 4°C for 15 min and centrifugation at 700 × g for 10 min. Trophozoites were washed three times, counted by using a Neubauer cell-counter chamber under light microscope (Nikon Eclipse 80i) and used as inoculum to study the in vitro effects of fresh and heat-treated Slab51 supernatants on growth and adherence of G. duodenalis trophozoites, to evaluate the morphological and ultrastructural alterations of G. duodenalis trophozoites following treatment with fresh Slab51 supernatant and to evaluate possible ex vivo differences between mice intestinal portions cultured with G. duodenalis trophozoites and with G. duodenalis trophozoites plus 200μl of fresh Slab51 supernatant.
Effects of Slab51 supernatant in vitro
The effects of Slab51 supernatant on growth and adherence of G. duodenalis trophozoites were evaluated in vitro by using previously reported methods [23–25].
The supernatant was obtained by culturing Slab51 (at 101 UFC) in TYI-S-33 medium without antibiotics at 37°C for 24h and the supernatant (Slab51S) obtained from these cultures was collected, centrifuged at 4,000g x 10min [24], filtered by using filters with pore size of 0.22μm Pes and adjusted at pH 7 by using 5N NaOH. The supernatant was used as fresh (Slab51 FS) and after heat-treatment at 56°C (Slab51S 56°C) and at 90°C (Slab51S 90°C) for 30 minutes.
In all assays, 100μl of Slab51 supernatants were added to 900μl of fresh TYI-S-33 medium in 1.5 ml eppendorf vials with 5x104 log-phase trophozoites (FS-treated groups).
Negative controls (NC) were performed in similar experimental conditions without any supernatants, while positive controls (PC) were performed in similar conditions but adding metronidazole at 20μg/ml to G. duodenalis culture medium.
To verify the growth of Slab51 lactobacilli in TYI-S-33 medium, Slab51 (101 UFC) was cultured in this medium without antibiotics at 37°C. After 24 h, 100 μl of bacterial colonies grown onto TYI-S-33 media were cultured on MRS agar plates at 37°C. After 24 h, 5 colonies for each plate were identified with API 50CHL (Biomerieux, France).
Growth inhibition assay
The growth of G. duodenalis trophozoites was evaluated at 24 and 48h in cultures treated with fresh (Slab51 FS) and heat-treated (Slab51S 56°C and Slab51S 96°C) Slab51 supernatants, and in negative and positive controls. After each different incubation periods, the culture vials were placed at 4°C for 15min, the trophozoites were resuspended and the total number of cells was counted using a Neubauer cell-counter chamber under light microscope in triplicates (Nikon Eclipse 80i).
Adhesion inhibition assay
The effects of Slab51 supernatants (Slab51 FS, Slab51S 56°C and Slab51S 96°C) on the adhesion ability of G. duodenalis trophozoites were evaluated after 24 and 48h and compared to that observed in negative and positive controls. After inverting to mix, from each culture 10 μl of the medium were removed and the number of unattached cells was counted using a Neubauer cell-counter chamber under light microscope in triplicates (Nikon Eclipse 80i). After exposure to 4°C for 15min, the total cell number was calculated as described in the growth assay. Results were expressed as the percentage of attached trophozoites in relation to the total number of G. duodenalis trophozoites counted in each culture. More specifically, these percentages were obtained by dividing the difference between the number of trophozoites counted in the medium after exposure to 4°C for 15min (total cells) and the number of trophozoites counted in the medium after mixing at 37°C (non-adhering cells) on the total cells [23].
Transmission electron microscopy
After the treatment with the Slab51 supernatant for 24h, the morphological and ultrastructural alterations of G. duodenalis trophozoites were investigated by transmission electronic microscopy (TEM). To this aim, trophozoite samples were fixed in phosphate-buffered 0.1M of 2% glutaraldehyde (pH 7.4), post-fixed in phosphate-buffered 1% OsO4 and, after dehydration, embedded in Epon/Araldite (Polyscience Inc., Warrington, PA, USA). Ultrathin sections (70 nm) were placed on 200-mesh nickel grids supplied with formvar-carbon film (Agar Scientific Ltd, Stansted, UK). Grids were then stained with lead citrate and uranyl acetate and examined with a JEOL 1200-EX transmission electron microscope (JEOL, Peabody, MA, USA).
Effects of Slab51 ex vivo
With the aim to evaluate the anti-Giardia activity of Slab51 FS supernatant in controlled conditions but with the minimum alteration of natural conditions, some ex vivo trials were conducted on mice gut. Intestinal tracts were taken from healthy mice used as negative control in a study approved by the institutional research ethics board of the Italian Ministry of Health (authorization n°244/2017-PR). More specifically, thirty– 1 cm long duodenal portions were taken from CD-1(ICR)BR mice obtained from Charles River GmbH, Sulzfeld, Germany. Animals were kept according to the Italian regulations of animal experiments with free access to germfree food and sterile water. All mice were considered negative for Giardia spp. infection based on the absence of Giardia trophozoites, cysts and fecal antigens in three fecal samples collected from each mouse in three non-consecutive days and examined by fresh and Lugol stained fecal smears, flotation test and a commercial rapid immune-chromatographic assay (RIDA QUICK Cryptosporidium/Giardia Combi, R-Biopharm, Darmstadt, Germany) [26]. Mice duodenal portions were cultivated in vitro for 12 to 18h in RPMI 1640 medium containing 10% v/v heat-inactivated fetal bovine serum added with 100 units/ml of an antibiotic-antimycotic solution (Antibiotic Antimycotic Solution, Sigma-Aldrich, Saint Louis, MO, USA). Specimens were then placed in 25ml Falcon’s tubes and incubated at 37°C with 5% CO2 until examination. Five intestinal fragments were cultured with 1.8ml of medium containing 5x105 G. duodenalis WB strain trophozoites/ml plus 200μl of ultrafiltered Slab51 FS, while further five intestinal fragments were cultured with 1.8ml of the same medium containing 5x105 trophozoites/ml plus 200μl of sterile saline solution (negative controls, NC). Samples were stopped at different times (12 and 18h, respectively) and the tissues fixed in 10% buffered formalin for a period of 8h, then washed in sterile saline solution, dehydrated and paraffin embedded.
Histological examination
Two-μm paraffin sections were placed on Superfrost Plus slides (Histoline, Milan, Italy). The slides were then dewaxed and stained with hematoxylin & eosin stain (H&E) for microscopic examination, primarily to score the intensity of infection and the morphology of the intestinal mucosa at different time periods both in Slab51 FS-treated and in untreated samples, as reported afterwards. Histological examination included assessment of inflammation by scoring the number of inflammatory cells (mononuclear cells, such as macrophages, lymphocytes, and plasma cells, and neutrophils) at a magnification of ×400. The number of inflammatory cells was evaluated by using a visual analogue scale modified for murine gastrointestinal specimens, and results were reported as the mean for the entire specimen. When considerable variation of intensity of infiltration was evident in the same specimen, the mean for several areas was determined and the specimen was scored accordingly. Neutrophils and mononuclear cells were classified as absent (score of 0) when at a magnification of ×400, there were no or fewer than 5 cells per high-power field (HPF), mild (score of 1) for 5 to 19 cells per HPF, moderate (score of 2) for 20 to 49 cells per HPF, marked (score of 3) for 50 to 99 cells per HPF, and severe (score of 4) for 100 to 200 cells or more per HPF.
Histological criteria for normal intestinal characteristics included detection of no or only a few mononuclear cells per HPF and no or only a few scattered neutrophils in sub-epithelial areas and/or in peri-glandular area of duodenal mucosa, without tissue changes, i.e. no interstitial thickening, Peyer’s patches/gut associated lymphoid tissue [GALT] enlargement or epithelial-associated lymphocytes increase).
Immuno-histochemical tests
Paraffin sections were used for immuno-histochemical tests. Rehydrated sections were treated for endogenous peroxidases neutralization with 3% hydrogen peroxide for 1h followed by rinsing for 5min in deionized water. Antigen retrieval was achieved by incubating slides in antigen retrieval solution in a steamer (Black & Decker, Towson, MD, USA) for 20min. Nonspecific immunoglobulin binding was blocked by incubation of slides for 10min with a protein-blocking agent (Dako, Carpinteria, CA, USA) before application of the primary antibody. Slides were incubated overnight in a moist-chamber with polyclonal rabbit anti-human Ki67 antibody (Santa Cruz Biotechnology, Inc., Dallas, TX, USA), used as primary antibody at dilutions of 1:50. A goat anti-rabbit byotinilated antibody (Dako), was used as secondary antibody at standard dilution of 1:250 in buffer. A streptavidin–immunoperoxidase staining procedure (Dako, Carpinteria, CA, USA) was used for immunolabeling. The immunoreaction was observed with 3,3'-diaminobenzidine (DAB) or VIP substrate (Vector Laboratories, Inc., Burlingame, CA, USA). Sections were counterstained with Mayer's hematoxylin. Positive immunohistochemical controls included mouse mammary carcinoma sections. Negative immunohistochemical controls were known mouse mammary carcinoma or intestinal sections, treated identically as routine sections with 20min antigen retrieval and 10min protein blocking, except that the overnight incubation with primary antibodies was replaced by an overnight incubation with buffer. Expression of cleaved Caspase-3 in paraffin-embedded tissue sections was investigated using the Anti-active Caspase-3 antibody (Promega Corporation, Madison, WI, USA) directed against a peptide from the p17 fragment of the active (cleaved) human Caspase-3, and after an O/N incubation with this antibody, sections were treated routinely as described above. Sections from mouse mesenteric lymph nodes were subsequently selected as the positive control for further tests. The primary antibody was replaced by phosphate buffered saline solution (PBS) as a negative control. In small intestinal sections, pro-apoptotic effect induced by Giardia in crypts, and mucosal lining epithelial cells were highlighted through a TUNEL colorimetric staining (DeadEnd, Promega Corporation, Madison, WI, USA) according to the manufacturer’s instructions. To score the intensity of G. duodenalis trophozoites, and Ki67 positive cells at different times in treated and untreated samples, ten random fields of the sample were examined under a dry-X40 objective. The total number of protozoans and Ki67 stained epithelial cells was recorded. The mean value obtained per histological section per time was considered. To enumerate the TUNEL positive nuclei and cleaved Caspase-3 expression, tissues were graded in five categories by two independent blinded observers according to the number of detected apoptotic cells as follows: 0: without any apoptotic signal; 1: low level of apoptotic signal (<5%); 2: moderate level of apoptotic signal (5–10%); 3: high level of apoptotic signal (10–20%); 4: very high level of apoptotic signal (>20%). For the evaluation of these parameters indicating the apoptotic rate, 10 random fields of the sample were examined under a dry-x40 objective. The number of positive enterocytes was normalized to the number of enterocytes per field and expressed as a percentage of these values. Similarly, Ki-67 slides were visually scanned and scored: 0, negative (<5% positive cells); 1, sporadic (5%-20% positive cells); 2, moderate (20–50%); 3, diffuse (50–75%), and 4, strongly diffuse (>75% cells). For all parameters, cells on the margins of the tissue sections were not considered for evaluation to avoid possible artifactual staining.
Statistical analysis
All in vitro experiments were repeated in triplicate in two independent assays. Values were expressed as mean ± sd and compared by repeated measures analysis of variance, followed by Bonferroni’s multiple comparison [23].
All ex vivo experiments were repeated in triplicate. Descriptive and comparative statistical analyses of ex vivo tests were performed and results (mitotic and apoptotic cell numbers and number of G. duodenalis trophozoites in fresh supernatant-treated or untreated tri-dimensional culture cells) were described and tested for normal distribution with the Kolmogorov–Smirnov test and normal probability plots. As they were not normally distributed, the non-parametric Wilcoxon Signed-Rank test was used to compare median values for these variables between the treated and untreated control biopsies. Correlations between degrees of expressions of these different variants in the two groups of samples were analysed with Spearman rank tests [27].
The level of statistical significance was set at P <0.05 throughout the study.
Results
Effects of Slab51 in vitro
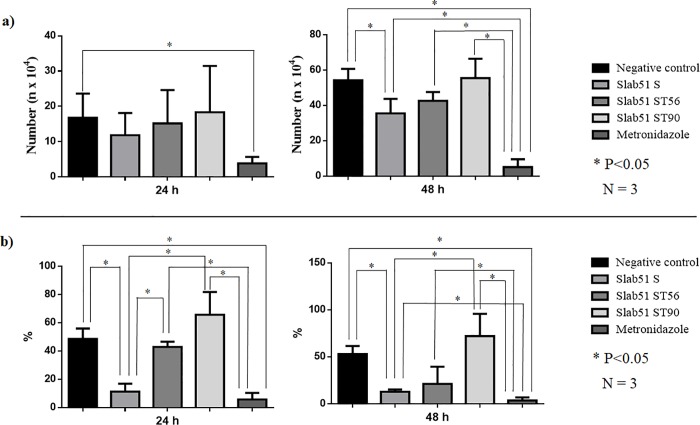
Slab51FS was able to inhibit the growth and the adhesion ability of G. duodenalis trophozoites respect to untreated controls, but its effects were generally lower than that of metronidazole, the reference drug. In fact, in the growth inhibition assay the number of trophozoites (35.48±8.16x104) found after 48h in cultures treated with fresh Slab51 supernatant was significantly (P <0.05) reduced respect to that counted in untreated cultures (54.15±6.58x104). However, this reduction was significantly lower (P <0.05) respect to that observed in cultures treated with metronidazole where the number of trophozoites was extremely low (5.05±4.47x104) (Table 1; Fig 1). In the adhesion assays, after 24h the number of adherent G. duodenalis trophozoites (9.51± 7.08%) observed in Slab51 FS-treated cultures was significantly reduced (P <0.05) respect to that observed in untreated cultures (48.58± 7.31%) but similar to that counted in culture treated with metronidazole (5.64±4.75). However, after 48h the number of adherent G. duodenalis trophozoites (12.85±2.26%) counted in Slab51 FS-treated cultures was still significantly lower (P<0.05) respect to that observed in untreated cultures (52.91±8.64%), but significantly higher (P<0.05) than in culture treated with metronidazole (3.43±3.44%) (Table 2; Fig 1). On the contrary, the cultures treated with metronidazole showed a significant reduction of the growth and adhesion of G. duodenalis trophozoites in comparison with untreated cultures, both at 24h and at 48h (Tables 1 and 2; Fig 1).
Table 1. Growth inhibition of Giardia duodenalis trophozoites by fresh (Slab51 FS) and 56°C (Slab51S 56°C) and 90°C (Slab51S 96°C) heat-treated Slab51 supernatants.
The number (n x104) of Giardia duodenalis trophozoites are expressed as average and standard deviation of trophozoites counted in three replicates after 24 and 48 h observation periods.
| Growth assay | ||||
|---|---|---|---|---|
| 24 h | 48h | |||
| Mean | SD | Mean | SD | |
| Negative control | 16.74b | 6.88 | 54.15c | 6.58 |
| Slab51 FS | 11.82ab | 6.26 | 35.48b | 8.16 |
| Slab51 S 56°C | 15.14ab | 9.46 | 42.54bc | 5.02 |
| Slab51 S 90°C | 18.32ab | 13.16 | 55.42bc | 10.96 |
| Metronidazole | 3.82a | 1.82 | 5.05a | 4.47 |
a,b,c: P < 0.05
Fig 1.
(a) Growth inhibition of Giardia duodenalis trophozoites by fresh (Slab51 FS) and 56°C (Slab51S 56°C) and 90°C (Slab51S 96°C) heat-treated Slab51 supernatants. The number (n x104) of G. duodenalis trophozoites are expressed as average and standard deviation of trophozoites counted in three replicates after 24 and 48 h observation periods.; (b) Adhesion inhibition of G. duodenalis trophozoites by fresh (Slab51 FS) and 56°C (Slab51S 56°C) and 90°C (Slab51S 96°C) heat-treated Slab51 supernatants. Attached trophozoites have been expressed as the mean percentage of attached G. duodenalis trophozoites in relation to the total number of G. duodenalis trophozoites counted after 24 and 48 hours in each culture and in three replicates.
Table 2. Adhesion inhibition of Giardia duodenalis trophozoites by fresh (Slab51 FS) and 56°C (Slab51S 56°C) and 90°C (Slab51S 96°C) heat-treated Slab51 supernatants.
Attached trophozoites have been expressed as the mean percentage of attached G. duodenalis trophozoites in relation to the total number of G. duodenalis trophozoites counted after 24 and 48 hours in each culture and in three replicates.
| Adhesion assay | ||||
|---|---|---|---|---|
| 24 h | 48h | |||
| Mean | SD | Mean | SD | |
| Negative control | 48.58b | 7.31 | 52.91c | 8.64 |
| Slab51S | 9.51a | 7.08 | 12.85b | 2.26 |
| Slab51S 56°C | 42.84b | 3.70 | 21.15bc | 18.33 |
| Slab51S 90°C | 65.65b | 16.10 | 72.06c | 23.79 |
| Metronidazole | 5.64a | 4.75 | 3.43a | 3.44 |
a,b,c: P < 0.05
The heat-treatment reduced the negative effects on G. duodenalis growth and adhesion ability of fresh Slab51 supernatant. In fact, while after 48 h the inhibiting activity of 56°C heat-treated Slab51 supernatant (42.54± 5.02%) was significantly different (P<0.05) both from treated (5.05±4.47x104) and untreated controls (54.15±6.58 x104), as well as from Slab51 FS-treated cultures (35.48±8.16x104). After the same time-period, results observed for 90°C heat-treated Slab51 supernatant cultures (55.42±10.96 x104) were comparable to that of untreated controls (Table 1, Fig 1). Moreover, in the adhesion assay no statistical difference with untreated cultures (52.91±8.64%) was observed both for 56°C (21.15±18.33%) and 90°C heat-treated Slab51 supernatant cultures (72.06±10.96%) (Table 2).
All the colonies from Slab51 cultures in TYI-S-33 medium with and without antibiotics and cultured in MRS agar plates were identified as L. plantarum.
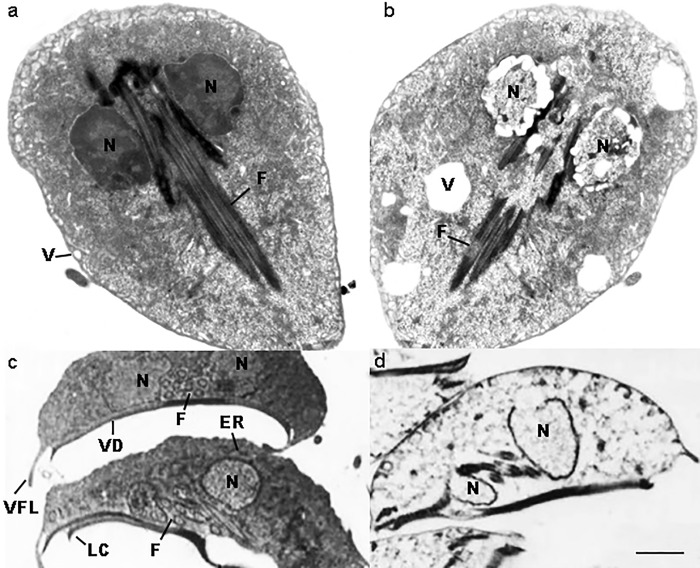
At the ultrastructural level, untreated trophozoites showed normal structure and morphology (Fig 1A and 1C), while treated parasites were swelling and increased in size (Fig 2B and 2D). Moreover, trophozoites showed alterations of their cellular membrane and vacuole patterns. Inside the cells, an ostensibly low electron density and granules grouped in clusters were evidenced. In the nucleus, the loss of the nuclear envelope and nuclear architecture and the presence of structures resembling holes or lacunas were clearly visible (Fig 2B and 2D).
Fig 2. Ultrastructure of Giardia duodenalis by TEM.
Untreated G. duodenalis trophozoites showing normal structure and morphology (a). Trophozoite coronal section (c). A coronal view of a trophozoite demonstrates the nuclei (N), endoplasmic reticulum (ER), flagella (F), vacuoles (V), ventral disk (VD), lateral crest (LC) and ventrolateral flange (VLF). The same sections (b, d) of treated parasites show swelling trophozoites, with an increased size, and evident alterations of their cellular membrane and with a vacuolar degenerative pattern (x6,700). Note in the coronal section the severely damaged nuclei, nuclear membrane rupture, loss of the chromatin, flanges and ventral disk rupture (x6,700).
Effects of Slab51 ex vivo
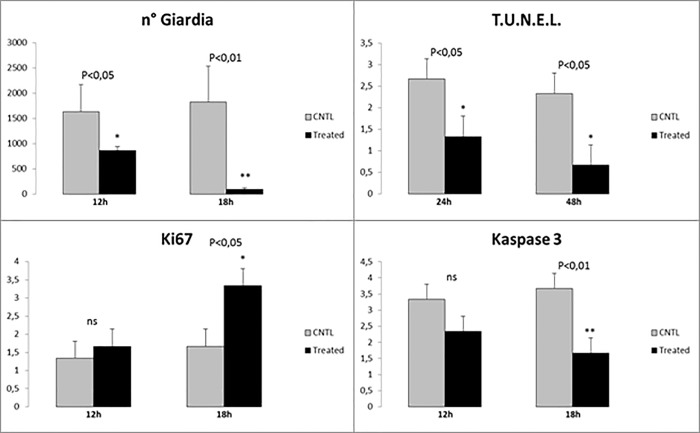
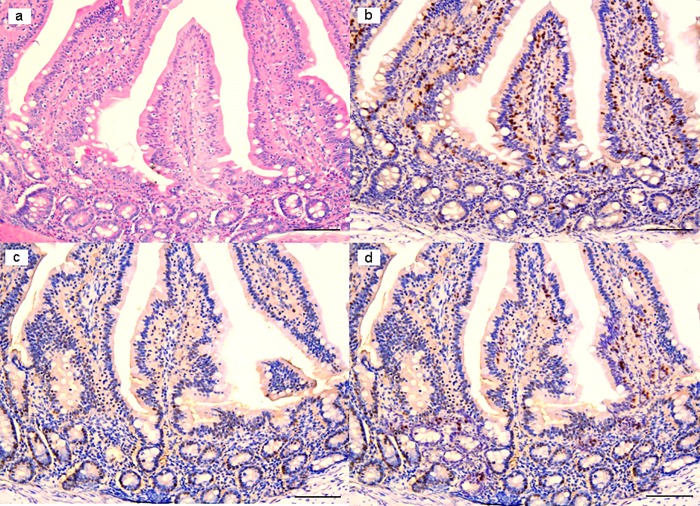
In ex vivo trials, significant results were observed in treated samples at 18h post-infection (PI). Indeed, at this time treated with Slab51 ultrafilterd fresh supernatant showed a significant reduction of viable G. duodenalis trophozoites at the end of the observation period, as evidenced in Fig 3. In these same samples, a progressive and significant decrease in TUNEL+ enterocytes was observed (Fig 4C), while at the same time apoptotic activity peaked in untreated samples (Fig 5C) when compared to Slab51 FS-treated samples. Similar results were obtained in sections stained for Caspase-3 (Figs 4D and 5D). In fact, as shown in Fig 4D, also for Caspase-3 the peak in decrease of expression was observed in ex vivo intestinal tissue cultures after 18h of incubation with Slab51 ultrafilterd fresh supernatant. In untreated controls, G. duodenalis trophozoites showed an intact morphology also after 18h (Fig 6A) while the apoptosis rate of enterocytes in untreated samples increased progressively in these groups throughout the experiment (Fig 6B). As shown in Figs 3B and 4B, Ki67 enterocyte nuclear staining was observed in mice intestinal mucosa still after 18h in both Slab51 FS-treated groups and untreated control groups, suggesting that even after this period of cultivation, the epithelium covering the intestinal mucosa is still able to live and proliferate ex vivo. These observations were also supported by the general morphology of bioptic samples. In fact, even if well preserved, untreated samples showed a clear loss of epithelial cells with a different pattern of inflammatory cell distribution throughout the mucosal corion, as evidenced in haematoxylin-eosin stained tissues (Figs 4A and 5A).
Fig 3. Statistical evaluation of different parameters in ex-vivo duodenal tissue cultures, before and after treatment.
(a) Significant reduction in the number of viable Giardia cells counted in biopsies treated with Slab51 ultrafiltered supernatant at 12h and 18h. (b) Statistical comparison of TUNEL positive nuclei before and after the same treatment at 24h and 48h. (c) Statistical comparison of level of cellular viability and replication by Ki67 nuclear assessment at 12h and 18h. (d) Statistical confrontation of Caspase3 positive cells before and after the same treatment at 12h and 18h. Caspase3 expression in association with the level of the TUNEL expression, as showed in Fig 2B, indicate the entire fraction of apoptotic cell because these two apoptotic markers are expressed in subsequent time.
Fig 4. Ex-vivo intestinal tissue, from mice treated with Slab51 ultrafiltered fresh supernatant.
At 18h post-infection with Giardia duodenalis, biopsies showed a preserved morphology and viability as demonstrated by H&E stain (a) and Ki67 enterocytes expression (b). In these samples, a low number of TUNEL+ enterocytes is observed (c). A similar pattern of expression of Caspase-3 indicates a low apoptotic rate in these samples (d). Presence of inflammatory cells with a diffuse and non-polarized pattern of infiltration is also observed in these biopsies (H&E, and IHC with Mayer Haematoxilin nuclear counterstain, scale bar 400 μm).
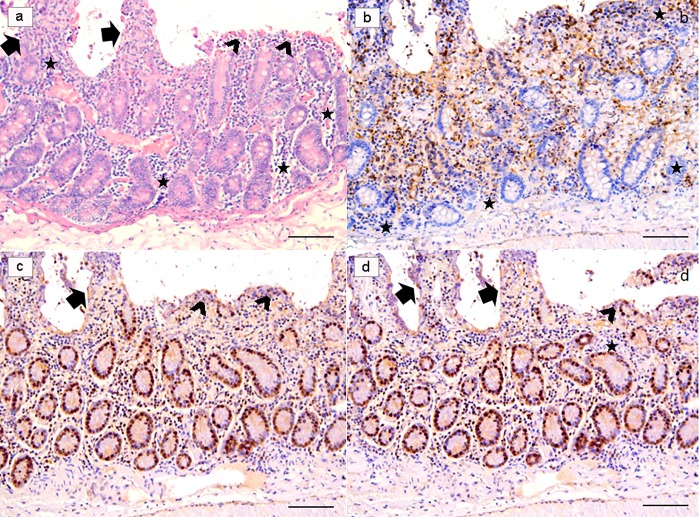
Fig 5. Ex-vivo intestinal tissue, from negative control (NC) group.
Section stained with H&E revealed a diffuse epithelial loss with inflammatory cells infiltration polarized under the destroyed intestinal epithelium. Note the reinforcement of inflammatory cells around intestinal glands (a). Villi are partially damaged (arrows), while in part they are totally flat or in any case strongly tuned (arrowheads) due to the effect of the strong colonization-adhesion of Giardia duodenalis trophozoites on the surface of the epithelium, which has become detached in many areas of the mucosa. Note the reinforcement of inflammatory cells around intestinal glands (stars). Ki67 nuclear staining evidenced an apparently higher number of positive cells because many inflammatory cells showed a strongly nuclear positivity (b). Note that TUNEL (c) and Caspase-3 (d) are over-expressed in these explanted intestinal samples. (H&E, and IHC with Mayer Haematoxilin nuclear counterstain, scale bar 400 μm).
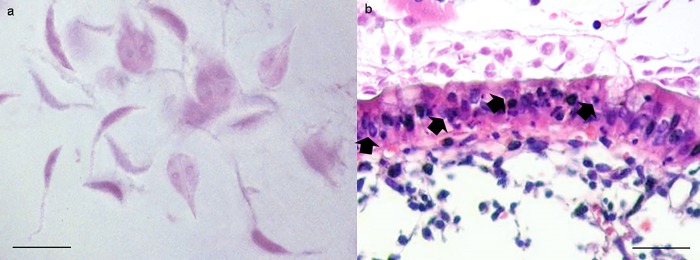
Fig 6. Giardia duodenalis in negative control (NC) group.
G. duodenalis trophozoites showed an intact morphology also after 18h (a), and the apoptosis rate of enterocytes (arrows), as demonstrated in b, increased progressively during the experiment, by the combined effect of the infection and the ex-vivo condition. (H&E, scale bar: a, 10 μm–b, 50 μm).
Discussion
G. duodenalis is a common fecal-oral parasite of the small intestine and one of the most important causes of human and animal diarrheal disease worldwide. Indeed, G. duodenalis infection can be asymptomatic, or cause acute or chronic diarrhea, dehydration, intestinal malabsorption, malnutrition and steatorrhea [2, 4–7]. Chronic fatigue, post-infectious irritable bowel syndrome and intestinal dysbiosis have also been documented in humans as possible consequences of G. duodenalis infections, [28, 29], while growth retardation and cognitive malfunction have been reported in children from endemic areas [2, 30]. Probiotics may interfere with G. duodenalis infection through different mechanisms, including competition for limited adhesion sites, competition for nutrients that would otherwise be utilized by G. duodenalis, stimulation of the host immune response and by producing substances that may inhibit G. duodenalis [6, 7, 11]. Probiotic bacteria can produce compounds, which have inhibitory effects directed against pathogens, as viruses, bacteria, fungi, parasites, as well as against cancer cells [11]. Among them, the anti-G. duodenalis activity of probiotic compounds, mainly derived from Lactobacilli, has been demonstrated [11, 20, 24]. In fact, bacteriocins derived from Lactobacillus acidophilus were found able to inhibit in vitro the adhesion and the growth of G. duodenalis trophozoites [11]. Moreover, these negative effects were found associated with severe morphological changes of G. duodenalis trophozoites, a decline of the intestinal parasite density and amelioration of intestinal pathology in infected mice treated with L. acidophilus bacteriocins [11]. More recently, results from some studies suggested that the ability to deconjugate bile salts showed by some lactobacilli, as L. johnsonii strain LA1 and Lactobacillus gasseri CNCM I-4884, may represent a further mechanism contributing to the inhibition of Giardia trophozoite growth in vitro [31, 32].
Negative effects on G. duodenalis showed by the fresh supernatant of the commercial probiotic evaluated in vitro and ex vivo in the present study, are similar or higher to those reported in most of these previous studies. In fact, the fresh Slab51 supernatant was able to inhibit in vitro the adhesion and the growth of G. duodenalis trophozoites, although this inhibition was significantly lower than that of metronidazole. In the study of Perez et al. [24], the culture supernatant of the probiotic strain LA1 of Lactobacillus johnsonii was able to control G. duodenalis growth in vitro but it was unable to inhibit the adhesion of the parasite, while six Lactobacillus acidophilus strains tested did not show any noticeable effects. These data could be indicative that Slab51 constituent probiotic strains, mainly L. plantarum DSM 32244, possibly produce more effective active anti-G. duodenalis compounds with respect to those produced by L. johnsonii LA1. Negative effects on G. duodenalis adherence here observed are consistent with the ability reported for some lactobacilli to modulate G. duodenalis infection in vivo by minimizing or preventing the adherence of trophozoites to the intestinal mucosal surface [33, 34].
In agreement with previous studies in which some probiotic compounds were found able to induce morphological changes of G. duodenalis trophozoites [11, 24], important morphological alterations of this protozoan parasite were herein observed in G. duodenalis trophozoites from in vitro cultures treated with Slab51 fresh supernatant, including profound alterations of cellular and nuclear membranes, nuclear disorganization and formation of intra-cytoplasmic cavities. These in vitro cytopathic effects are very similar to those caused by L. acidophilus bacteriocins in vivo, possibly indicating a similar mode of action [11] and may be one of the main factors responsible for the inhibition of the trophozoite proliferation and adhesion here observed in vitro.
Trials performed in this study showed that the in vitro inhibiting effects on G. duodenalis showed by Slab51 fresh supernatant were greatly reduced by heat treatment at 56°C and completely annulled at 90°C, indicating that active metabolites contained in Slab51 supernatant are likely term labile compounds. These results agree with those of a previous report [24] and encourage further studies aimed to identify the extracellular factors responsible for the anti-Giardia effects of fresh Slab51 supernatant observed in the present study.
Results from ex vivo trials confirmed the inhibiting effects of the fresh supernatant of Slab51 on G. duodenalis trophozoites observed in vitro. The best observations were obtained after an 18h incubation period. In fact, after this period a significant reduction of G. duodenalis trophozoites and a significantly greater vitality of intestinal epithelial cells was evidenced in treated intestinal cultures respect to the untreated controls. Moreover, G. duodenalis was found capable of slowing or damaging the intestinal epithelial cell turnover in untreated ex vivo cultures, since in these cultures the apoptotic rate was increased. On the other side, obtained results showed that the number of G. duodenalis trophozoites was significantly lowered by the fresh Slab51 supernatant. Moreover, in ex vivo cultures the apoptosis and the death of intestinal epithelial cells was higher in G. duodenalis-inoculated cultures respect to those inoculated with G. duodenalis and the fresh supernatant of Slab51, indicating that the damage to the epithelial cells induced by G. duodenalis was reduced by the fresh supernatant of this commercial probiotic. Considering that the increase in the rate of enterocyte apoptosis and enterocyte damage are included among the main pathogenic mechanisms of G. duodenalis [34], results obtained in ex vivo trials are promising about possible in vivo protective effects of the fresh culture supernatant of Slab51 against G. duodenalis. This is the first study in which negative effects on G. duodenalis by metabolites from lactobacilli were demonstrated both in vitro and by using a murine ex vivo model. In previous studies, a variety of different systems have been used to evaluate the adherence and growth of G. duodenalis, including synthetic surfaces, human cells and non-human cells, as isolated rat enterocytes and rat enterocyte cell lines [35–37]. Among them, the human colonic adenocarcinoma derived epithelial cell line Caco-2, functionally and structurally may resemble small bowel enterocytes [38]. Therefore, this cell line model is considered useful and appropriate for studies of host intestine-pathogen interactions, and it is frequently used as a model to study the attachment and other effects of G. duodenalis trophozoites under different conditions [35]. However, this model does not allow a proper evaluation of the damages caused by G. duodenalis trophozoites to the intestinal mucosa and of associated inflammatory cells, while the murine ex vivo model performed in this study allowed these evaluations, by preserving the tissue architecture and the cellular complexity over several days. Indeed, alterations, damage and inflammation herein observed in ex vivo negative controls, i.e. intestinal tracts inoculated with G. duodenalis trophozoites only, were not so different from that observed in in vivo rodent models and at histopathological examination of intestinal biopsies taken from symptomatic human patients [30, 35]. Moreover, the ex vivo mouse model used in this study allowed to assess the anti-Giardia activity of the fresh supernatant of Slab51 by the evaluation of several positive effects on intestines inoculated with G. duodenalis trophozoites, although this model cannot be able to mimic the complexity of whole living organisms.
In conclusion, results from this study showed that the fresh culture supernatant of the commercial probiotic Slab51 has negative effects on G. duodenalis both in vitro and ex vivo in a mouse model. These antagonistic effects may suggest that this probiotic may likely represent a further and interesting approach for the prevention of giardiasis and/or the reduction of the pathogenic effects and proliferation of this protozoan parasite in infected hosts. However, further studies aimed to evaluate its efficacy in vivo on experimentally and/or naturally infected animals, are needed.
Data Availability
All relevant data are within the paper.
Funding Statement
The authors declare that this study was partly supported by Actial Farmaceutica Lda, Praça Severino Ferraz, 258 082 Funchal, Madeira, Portugal. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript and no authors received a salary from this funder.
References
- 1.Ryan U, Cacciò SM. Zoonotic potential of Giardia. Int J Parasitol. 2013;43: 943–956. 10.1016/j.ijpara.2013.06.001 [DOI] [PubMed] [Google Scholar]
- 2.Manko A, Motta JP, Cotton JA, Feener T, Oyeyemi A, Vallance BA, et al. Giardia co-infection promotes the secretion of antimicrobial peptides beta-defensin 2 and trefoil factor 3 and attenuates attaching and effacing bacteria-induced intestinal disease. PloS ONE, 2017;12: e0178647 10.1371/journal.pone.0178647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marangi M, Berrilli F, Otranto D, Giangaspero A. Genotyping of Giardia duodenalis among children and dogs in a closed socially deprived community from Italy. Zoonoses Public Health 2010;57: 54–58. [DOI] [PubMed] [Google Scholar]
- 4.Allain T, Amat CB, Motta JP, Manko A, Buret AG. Interactions of Giardia sp. with the intestinal barrier: Epithelium, mucus, and microbiota, Tissue Barriers 2017;5:1, e1274354. 10.1080/21688370.2016.1274354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minetti C, Chalmers RM, Beeching NJ, Probert C, Lamden K. Giardiasis. BMJ 2016;27:355:i5369 10.1136/bmj.i5369 [DOI] [PubMed] [Google Scholar]
- 6.Hawrelak J. 2003. Giardiasis: Pathophysiology and Management. Altern Med Rev. 2003;8: 129–142. [PubMed] [Google Scholar]
- 7.Tangtrongsup S, Scorza V. Update on the diagnosis and management of Giardia spp. infections in dogs and cats. Top Companion Anim Med. 2010;25: 155–162. 10.1053/j.tcam.2010.07.003 [DOI] [PubMed] [Google Scholar]
- 8.Geurden T, Vanderstichel R, Pohle H, Ehsan A, von Samson-Himmelstjerna G, Morgan ER, et al. 2012. A multicentre prevalence study in Europe on Giardia duodenalis in calves, with molecular identification and risk factor analysis. Vet Parasitol. 2012;190: 383–90. 10.1016/j.vetpar.2012.06.039 [DOI] [PubMed] [Google Scholar]
- 9.Lalle M. Giardiasis in the Post Genomic Era: Treatment, Drug Resistance and Novel Therapeutic Perspectives. Infect Disord Drug Targets. 2010;10: 283–294. [DOI] [PubMed] [Google Scholar]
- 10.Savioli L, Smith H, Thompson A. Giardia and Cryptosporidium join the 'Neglected Diseases Initiative'. Trends Parasitol. 2006;22: 203–208. 10.1016/j.pt.2006.02.015 [DOI] [PubMed] [Google Scholar]
- 11.Amer EI, Mossallam SF, Mahrous H. Therapeutic enhancement of newly derived bacteriocins against Giardia lamblia. Exp Parasitol. 2014;146: 52–63. 10.1016/j.exppara.2014.09.005 [DOI] [PubMed] [Google Scholar]
- 12.Morrone F, Carneiro J, Reis C, Cardozo C, Ubal C, De Carli G. Study of enteroparasites infection frequency and chemotherapeutic agents used in pediatric patients in a community living in Porto Alegre, RS, Brazil. Rev Inst Med Trop Sao Paulo. 2004;46: 77–80. [DOI] [PubMed] [Google Scholar]
- 13.Sullayman I, Nolder D, Warchurst D, Rossignol J. In vitro activity of nitazxanide and related compounds against isolates of Giardia intestinalis, Entamoeba histolytica and Trichomonas vaginalis. J Antimicrob Chemother. 2002;49: 103–111. [DOI] [PubMed] [Google Scholar]
- 14.Escobedo AA, Cimerman S. Giardiasis: a pharmacotherapy review. Expert Opin Pharmcother. 2007;8: 1885–902. [DOI] [PubMed] [Google Scholar]
- 15.Fiechter R, Deplazes P, Schnyder M. Control of Giardia infections with ronidazole and intensive hygiene management in a dog kennel. Vet Parasitol. 2012;187: 93–98. 10.1016/j.vetpar.2011.12.023 [DOI] [PubMed] [Google Scholar]
- 16.Hart CJS, Munro T, Andrews KT, Ryan JH, Riches AG, Skinner-Adams TS. A novel in vitro image-based assay identifies new drug leads for giardiasis. Int J Parasitol Drugs Drug Resist. 2017;7: 83–89. 10.1016/j.ijpddr.2017.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian HF, Chen B, Wen JF. Giardiasis, drug resistance, and new target discovery. Infect Disord Drug Targets. 2010;10: 295–302. [DOI] [PubMed] [Google Scholar]
- 18.Ansell BR, McConville MJ, Ma'ayeh SY, Dagley MJ, Gasser RB, Svard SG, Jex AR. Drug resistance in Giardia duodenalis. Biotechnol. Adv. 2015;33: 888–901. 10.1016/j.biotechadv.2015.04.009 [DOI] [PubMed] [Google Scholar]
- 19.Tejman-Yarden N, Eckmann L. New approaches to the treatment of giardiasis. Curr Opin Infect Dis. 2011;24: 451–456. 10.1097/QCO.0b013e32834ad401 [DOI] [PubMed] [Google Scholar]
- 20.Travers MA, Florent I, Kohl L, Grellier P. Probiotics for the control of parasites: an overview. J Parasitol Res. 2011: 610769 10.1155/2011/610769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hassan M, Kjos M, Nes IF, Diep DB, Lotfipour F. Natural antimicrobial peptides from bacteria: characteristics and potential applications to fight against antibiotic resistance. J. Appl. Microbiol. 2012;113: 723–736. 10.1111/j.1365-2672.2012.05338.x [DOI] [PubMed] [Google Scholar]
- 22.Keister DB. Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans. R. Soc. Trop. Med. Hyg. 1983;77: 487–488. [DOI] [PubMed] [Google Scholar]
- 23.Machado M, Dinis AM, Salgueiro L, Cavaleiro C, Custódio JBA, Sousa CM. Anti-Giardia activity of phenolic-rich essential oils: effects of Thymbra capitata, Origanum virens, Thymus zygis subsp. sylvestris, and Lippia graveolens on trophozoites growth, viability, adherence, and ultrastructure. Parasitol. Res. 2010;106: 1205–1215. 10.1007/s00436-010-1800-7 [DOI] [PubMed] [Google Scholar]
- 24.Perez PF, Minnard J, Rouvet M, Knabenhans C, Brassart D, De Antoni GL, et al. Inhibition of Giardia intestinalis by extracellular factors from lactobacilli: an in vitro study. Appl. Environ. Microbiol. 2001;67: 5037–5042. 10.1128/AEM.67.11.5037-5042.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bénéré E, da Luz RA, Vermeersch M, Cos P, Maes L. A new quantitative in vitro microculture method for Giardia duodenalis trophozoites. J Microbiol Methods. 2007;71: 101–106. 10.1016/j.mimet.2007.07.014 [DOI] [PubMed] [Google Scholar]
- 26.Sauda F, Malandrucco L, Macrì G, Scarpulla M, De Liberato C, Terracciano G, et al. Leishmania infantum, Dirofilaria spp. and other endoparasite infections in kennel dogs in central Italy. Parasite. 2018;25: 2 10.1051/parasite/2018001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rossi G, Pengo G, Caldin M, Palumbo Piccionello A, Steiner JM, Cohen ND, et al. Comparison of Microbiological, Histological and Immunomodulatory Parameters in Response to Treatment with Either Combination Therapy with Prednisone and Metronidazole or Probiotic VSL#3 Strains in Dogs with Idiopathic Inflammatory Bowel Disease. PLOS ONE 2014;9: e94699 10.1371/journal.pone.0094699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Persson R, Wensaas KA, Hanevik K, Eide GE, Langeland N, Rortveit G. The relationship between irritable bowel syndrome, functional dyspepsia, chronic fatigue and overactive bladder syndrome: a controlled study 6 years after acute gastrointestinal infection. BMC Gastroenterol. 2015;15: 66 10.1186/s12876-015-0296-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beatty JK, Akierman SV, Motta JP, Muise S, Workentine ML, Harrison JJ et al. Giardia duodenalis induces pathogenic dysbiosis of human intestinal microbiota biofilms. Int J Parasitol. 2017;47: 311–326. 10.1016/j.ijpara.2016.11.010 [DOI] [PubMed] [Google Scholar]
- 30.Halliez MC, Buret AG. Extra-intestinal and longterm consequences of Giardia duodenalis infections. World J Gastroenterol. 2013;19: 8974–8985. 10.3748/wjg.v19.i47.8974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Travers MA, Sow C, Zirah S, Deregnaucourt C, Chaouch S, Queiroz RM. et al. Deconjugated bile salts produced by extracellular bile-salt hydrolase-like activities from the probiotic Lactobacillus johnsonii La1 Inhibit Giardia duodenalis In vitro Growth. Front. Microbiol. 2016; 7:1453 10.3389/fmicb.2016.01453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allain T, Chaouch S, Thomas M, Travers MA, Valle I, Langella P, Grellier P, Polack B, Florent I, Bermúdez-Humarán LG. Bile salt hydrolase activities: a novel target to screen anti-Giardia Lactobacilli? Front. Microbiol. 2018; 9:89 10.3389/fmicb.2018.00089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shukla G, Devi P, Sehgal R. Effect of Lactobacillus casei as a probiotic on modulation of giardiasis. Dig Dis Sci. 2008;53: 2671–2679. 10.1007/s10620-007-0197-3 [DOI] [PubMed] [Google Scholar]
- 34.Certad G, Viscogliosi E, Chabé M, Cacciò SM. Pathogenic Mechanisms of Cryptosporidium and Giardia. Trends Parasitol. 2017;33: 561–576. 10.1016/j.pt.2017.02.006 [DOI] [PubMed] [Google Scholar]
- 35.Kraft MR, Klotz C, Bücker R, Schulzke JD, Aebischer T. Giardia’s Epithelial Cell Interaction In Vitro: Mimicking Asymptomatic Infection? Front. Cell. Infect. Microbiol. 2017;7: 421 10.3389/fcimb.2017.00421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inge PMG, Edson CM, Farthing MJG. Attachment of Giardia lamblia to rat intestinal epithelial cells. Gut 1988; 29: 795–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Favennec L, Chochillon C, Meillet D, Magne D, Savel J, Raichvarg D, et al. Adherence and multiplication of Giardia intestinalis on human enterocyte-like differentiated cells in vitro. Parasitol Res 1990; 76: 581–4. [DOI] [PubMed] [Google Scholar]
- 38.Pinto M, Robine-Leon S, Appay MD, Kerdinger M, Triadou N, Dussaulx E, et al. Enterocyte-like differentiation and polarisation of the human colon carcinoma cell line Caco-2 culture. Biol Cell 1983; 47: 323–30. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.