Abstract
Purpose
Several researchers have suggested that the rs243865 (16q13-q21) polymorphism in the promoter region of the metalloproteinase-2 (MMP-2) gene could be associated with an increased risk of developing age-related macular degeneration (AMD). However, previous results remain inconclusive. To clarify this controversy, we conducted a meta-analysis of the relationship between rs243865 of MMP-2 and AMD.
Methods
We included 6 independent case-control studies involving 1,682 AMD patients and 2,295 healthy subjects. The association between the rs243865 polymorphism and AMD was examined by the overall odds ratio (OR) with a 95% confidence interval (CI). We used a recessive genetic model analysis, sensitivity analysis, and assessment of bias in our meta-analysis.
Results
Our results showed that there was no significant association between the variant T allele (p-value = 0.10, OR [95%CI] = 0.95 [0.82–1.10]) or the CT+TT genotype (p-value = 0.16, OR [95%CI] = 0.92 [0.76–1.12]) of rs243865 MMP-2 polymorphism and the presence of AMD.
Conclusions
The rs243865 MMP-2 polymorphism was not associated with an increased risk of developing AMD. The MMP-2 (-1306 C>T) promoter variant is unlikely to have a major role in AMD risk susceptibility.
Introduction
Age-related macular degeneration (AMD) is the leading cause of severe vision loss and blindness worldwide, and the incidence in developed countries ranges from 9% to 25% at ages 65 to 75 years [1,2]. The risk of developing advanced AMD lesions increases with age and is ten-fold greater in people over 85 years compared those 65 to 75 years old [3]. According to the severity of the disease, AMD is classified as early, intermediate, and advanced. The advanced form includes atrophic and/or neovascular conditions [4].
The development of AMD is influenced by lifestyle (smoking), dietary factors, drusen accumulation, and parainflammation. In addition, the incidence of patients with AMD is also affected by genetic components [5,6]. Current data suggests that the rs243865 matrix metalloproteinase-2 (MMP-2) polymorphism within the 16q13-q21 chromosomal region is associated with an increased risk of developing AMD [7–12]. The rs243865 polymorphism is located in the promoter transcription region of the MMP-2 gene, and it consists of a C>T change (-1306C>T). This modifies the promoter activity of the MMP-2 gene, altering the Sp1-type promoter site [13]. The MMP-2 gene is crucial for the regulation of extracellular matrix components [14,15], and extracellular matrix dysregulation could be associated with the development of AMD [5]. In the studies of allelic association with AMD, the rs243865 MMP-2 polymorphism has been especially analyzed because it modifies MMP-2 activity. Several groups have reported the association between the rs243865 MMP-2 polymorphism and the risk of AMD [7–12]. However, the results from previous studies have been inconclusive, partly because each study was underpowered due to inadequate sample sizes. We have chosen to analyze the rs243865 polymorphism for two main reasons: (1) it modifies the MMP-2 gene promoter activity and, thus, the extracellular matrix regulation, which may be linked to the development of AMD [5]; and (2) because to the best of our knowledge, the rs243865 MMP-2 polymorphism has not been included in any genome-wide association (GWAS) or meta-analysis studies for AMD. Thus, we conducted this meta-analysis based on the published literature to characterize more precisely the potential association between the rs243865 polymorphism and AMD.
Materials and methods
The current study conformed to the checklist for meta-analysis of genetic association studies specified for PLOS One approach (S1 Table) [16].
Search strategy and selection criteria
To identify eligible studies, we searched in the PubMed, Web of Science, Scopus, and Embase electronic databases. Potentially relevant articles were sought by using the search terms in combination as Medical Subject Headings (MeSH) terms and text words: “matrix metalloproteinase-2”, “polymorphism”, “rs243865”, “-1306C>T”, “age-related macular degeneration”, “AMD”, “macular degeneration”, and “maculopathy”. We also scanned the reference lists of the retrieved publications to identify additional relevant articles (S2 Table).
The following inclusion criteria were used to identify published articles for our meta-analysis: (1) associations between the rs243865 MMP-2 polymorphism and AMD published in the last 18 years; (2) study populations included only individuals not related to one another; (3) sufficient genotype data were presented to allow calculation of the odds ratios (ORs); (4) each study clearly described the diagnosis of AMD, which was limited to the National Institute for Health and Care Excellence (NICE) Age-Related Maculopathy grading system or the International Classification and Grading system [17,18]; (5) the sources of the cases and controls were identified; and (6) genotype distributions complied with the Hardy–Weinberg equilibrium (HWE). In addition, we excluded reviews and redundant studies. Only English-language articles were analysed.
Data extraction
To improve the reliability of our results, two investigators (ALP, MML) used a standardised form to independently extract data. Any divergences of opinion were resolved by consensus with the senior authors (RGS, JCP). Second and third literature searches were conducted but did not contribute additional studies for the analyses. Citations were first scanned at the title/abstract level. Shortlisted studies were then retrieved in full text.
From each study, the following information was extracted: the author´s name, year of publication, location, and ethnicity and demographic information (age and sex) of the study population. Allele and genotype frequencies were extracted or calculated. HWE was verified for each eligible study. The corresponding authors of the original studies were contacted if further data were needed.
Quality assessment
Study quality was ranked as high, moderate, or low (score categories 7–9, 4–6, 0–3, respectively) by the Newcastle-Ottawa Quality Assessment Scale (NOS) [19]. Two independent reviewers conducted the data extraction and quality assessment, and disagreements were resolved by discussion (S3 Table).
Statistical analyses
The main meta-analysis compared the presence of the variant T allele of rs243865 MMP-2 polymorphism among AMD patients as cases versus healthy subjects as controls. If three or more studies were available, further meta-analyses were performed comparing: (1) the presence of CT+TT genotype of rs243865 MMP-2 polymorphism among AMD patients and healthy subjects; (2) the presence of CT+TT genotype of rs243865 MMP-2 polymorphism among AMD patients and healthy subjects by age; and (3) the presence of CT+TT genotype of rs243865 MMP-2 polymorphism among AMD patients > 65 years old and AMD patients < 65 years old.
For each study, we used a random-effects model [20] to calculate the OR and the 95% confidence interval (CI) along with the respective p-values. P-values < 0.05 were considered statistically significant. To evaluate heterogeneity, Cochran´s Q-statistic was used. For estimation of inconsistency in meta-analysis, the I2 statistic was determined, representing the percentage of the observed study variability attributed to heterogeneity rather than to chance [21]. Begger´s funnel plots were drawn to assess publication bias. Sensitivity analysis was performed by excluding individual studies. The meta-analysis was performed using RevMan 5.0 software [22]. Deviation of genotype frequencies from the HWE in healthy subjects was assessed by X2 test.
Results
Characteristics of the included studies
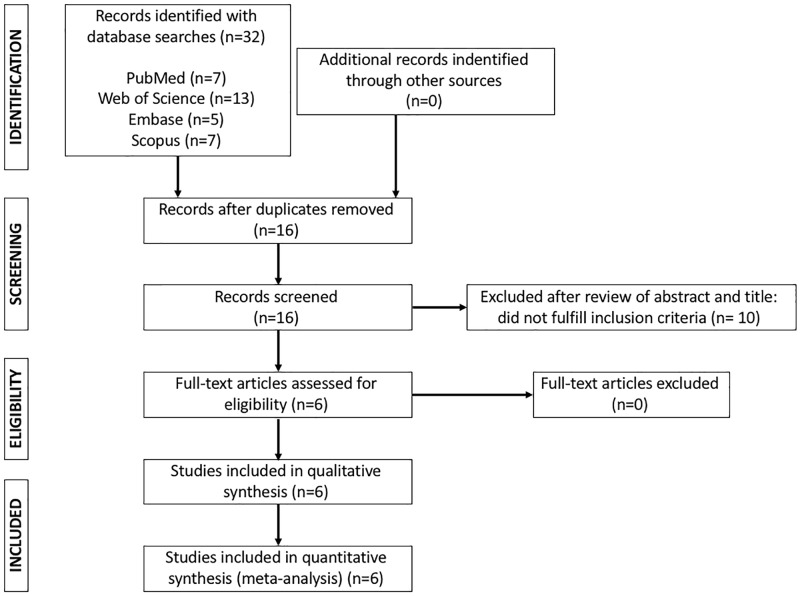
A flow diagram of the study selection process is shown in Fig 1. The initial search identified 32 references for possible inclusion in our meta-analysis. After removing duplicates, 16 studies remained, and 10 of these were excluded after abstract examination revealed that they failed to meet the inclusion criteria (S2 Table). This step left 6 full articles that were assessed for eligibility [7–12], and none of them had to be removed. The corresponding author of three articles [9,11,12] was contacted due to doubts about a possible overlap of the patients and controls included in the articles. The corresponding author in each case confirmed to us that the patients and controls included in each article were different, so that the three articles were included in the meta-analysis. Therefore, a total of 6 studies were ultimately included (Fig 1). These studies included 1,682 AMD patients and 2,295 healthy subjects, and the population demographics are shown Table 1.
Fig 1. Flow chart of the selection of studies for inclusion in the meta-analysis.
Table 1. Characteristics of the studies included in the meta-analysis.
| Authors, year | Country | Ethnicity | Female/male ratio | Age, y [mean ± SD or median (min—max)] | HWE p-value | ||
|---|---|---|---|---|---|---|---|
| AMD patients | Healthy subjects | AMD patients | Healthy subjects | ||||
| Cheng et al., 2017 | China | Asian | 42/84 | 50/91 | 62.4±9.6 | 63.8±10.2 | 0.117 |
| Liutkeviciene et al., 2016 | Lithuania | Caucasian | 186/201 | 330/352 | 65 (57–93) | 66 (55–97) | 0.664 |
| Ortak et al., 2013 | Turkey | N/A | 79/65 | 84/88 | 67.1 ± 6.9 | 66.1 ± 6.5 | >0.05 |
| Seitzman et al., 2008 | USA | Caucasian | 434/0 | 456/0 | > 60 | >60 | >0.05 |
| Liutkeviciene et al., 2017 | Lithuania | Caucasian | 164/126 | 316/210 | 67 (59.9–67.8) | 68 (60.8–68.3) | 0.528 |
| Liutkeviciene et al., 2018 | Lithuania | Caucasian | 168/99 | 205/113 | 75.4±7.6 | 74.7±8.4 | 0.382 |
AMD: Age-related macular degeneration; N/A: not available; y: years; SD: standard deviation; HEW: Hardy-Weinberg equilibrium.
Meta-analysis databases: Association between rs243865 MMP-2 polymorphism and AMD
Each of the articles analysed in our meta-analysis included the genotypic and allelic frequencies of rs243865 MMP-2 polymorphism in AMD patients and healthy controls (Table 2). All of the studies used genomic DNA extracted from nucleated peripheral blood cells, and genotyping was performed with polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP). There were no statistically significant deviations from the HWE in the control groups of any of the studies.
Table 2. Genotype and allelic distribution of rs243865 MMP-2 polymorphism in age-related macular degeneration patients and healthy subjects.
| Authors, year | Subject groups | N | Genotype distribution | Allele distribution | ||||
|---|---|---|---|---|---|---|---|---|
| CC | CT | TT | CT+TT | C | T | |||
| Cheng et al., 2017 | AMD patients | 126 | 94 | 27 | 5 | 32 | 215 | 37 |
| Healthy subjects | 141 | 89 | 42 | 10 | 52 | 220 | 62 | |
| AMD patients < 65 y | 53 | 40 | N/A | N/A | 13 | N/A | N/A | |
| Healthy controls < 65 y | 76 | 49 | N/A | N/A | 27 | N/A | N/A | |
| AMD patients > 65 y | 73 | 54 | N/A | N/A | 19 | N/A | N/A | |
| Healthy controls > 65 y | 65 | 40 | N/A | N/A | 25 | N/A | N/A | |
| Liutkeviciene et al., 2016 | AMD patients | 387 | 229 | 132 | 26 | 158 | 590 | 184 |
| Healthy controls | 682 | 385 | 252 | 45 | 297 | 1022 | 342 | |
| AMD patients < 65 y | 181 | 118 | 48 | 15 | 63 | 284 | 78 | |
| Healthy controls < 65 y | 306 | 186 | 103 | 17 | 120 | 475 | 137 | |
| AMD patients > 65 y | 206 | 111 | 84 | 11 | 95 | 306 | 106 | |
| Healthy controls > 65 y | 376 | 199 | 149 | 28 | 177 | 547 | 205 | |
| Ortak et al., 2013 | AMD patients | 144 | 80 | 60 | 4 | 64 | 220 | 68 |
| Healthy controls | 172 | 108 | 64 | 0 | 64 | 280 | 64 | |
| Seitzman et al., 2008 | ADM patients | 434 | N/A | N/A | N/A | N/A | 660 | 208 |
| Healthy controls | 456 | N/A | N/A | N/A | N/A | 678 | 234 | |
| Liutkeviciene et al., 2017 | ADM patients | 324 | 191 | 112 | 21 | 133 | 494 | 154 |
| Healthy controls | 526 | 289 | 198 | 39 | 237 | 776 | 276 | |
| Liutkeviciene et al., 2018 | AMD patients | 267 | 157 | 84 | 26 | 110 | 398 | 136 |
| Healthy controls | 318 | 190 | 108 | 20 | 128 | 488 | 148 | |
| AMD patients < 65 y | 99 | 56 | 35 | 8 | 43 | 147 | 51 | |
| Healthy controls < 65 y | 113 | 69 | 39 | 5 | 44 | 177 | 49 | |
| AMD patients > 65 y | 168 | 101 | 49 | 18 | 67 | 251 | 85 | |
| Healthy controls > 65 y | 205 | 121 | 69 | 15 | 84 | 311 | 99 | |
MMP-2, matrix metalloproteinase-2 gene; AMD, age-related macular degeneration; y, years of age; N/A, not available
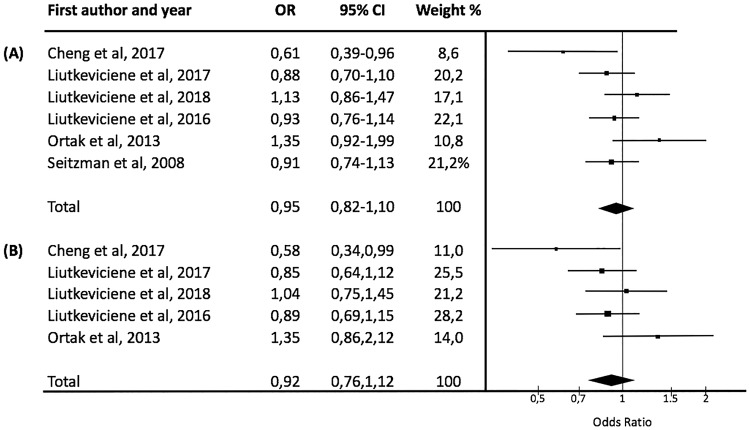
After analysis of the pooled selected studies, there were no significant associations between the variant T allele (Fig 2A, p-value = 0.10, OR [95%CI] = 0.95 [0.82–1.10]) or the CT+TT genotype (Fig 2B, p-value = 0.16, OR [95%CI] = 0.92 [0.76–1.12]) of the rs243865 MMP-2 polymorphism and the presence of AMD (Fig 2). There were also no significant differences based on study population ethnicity. The sensitivity analysis, which excluded individual studies, did not modify these results. Further, there were no significant differences in the genotypic distribution of rs243865 MMP-2 polymorphism based on age between AMD patients and healthy subjects (Table 3). Nor was there a difference in the genotypic distribution between AMD patients > 65 years old and those < 65 years old (Table 3).
Fig 2. Meta-analysis of the association between rs243865 MMP-2 polymorphism and age-related macular degeneration.
(A) C vs. T allele. Test for overall effect: Z = 0.67 (P = 0.50). Test for heterogeneity: v2 = 9.17 (P = 0.10). Test for inconsistency: I2 = 46%. (B) CC vs. CT+TT genotype. Test for overall effect: Z = 0.82 (P = 0.41). Test for heterogeneity: v2 = 6.55 (P = 0.16), Test for inconsistency: I2 = 39%. Each study is shown by an OR estimate with the corresponding 95% CI.
Table 3. Summary of meta-analysis results for the association of rs243865 MMP-2 polymorphism with age-related macular degeneration by age.
| OR | 95% CI | P (random-effects model) | I2 | P (heterogeneity) | |
|---|---|---|---|---|---|
| AMD patients < 65 y vs healthy subjects < 65 y (N = 3) | |||||
| CC vs CT+TT | 0.88 | 0.66–1.17 | 0.44 | 15% | 0.31 |
| AMD patients > 65 y vs healthy subjects > 65 y (N = 3) | |||||
| CC vs CT+TT | 0.90 | 0.70–1.15 | 0.41 | 0% | 0.40 |
| AMD patients > 65 y vs AMD patients < 65 y (N = 3) | |||||
| CC vs CT+TT | 1.19 | 0.78–1.81 | 0.43 | 44% | 0.43 |
OR, odds ratio; CI, confidence interval; AMD, age-related macular degeneration; y, age in years; N, number of studies included in each meta-analysis.
Bias diagnostics
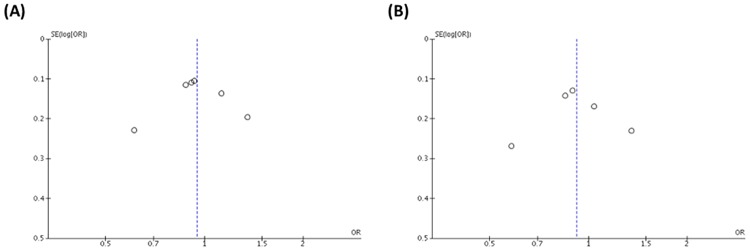
When there are fewer than 10 studies in a meta-analysis, the power of tests for funnel plot asymmetry is too low to distinguish chance from real asymmetry [23]. Even so, we examined publication bias by visual inspection using a Begger’s funnel plot with the effect size, i.e., the OR, on the x-axis and the inverse of variance of the effect on the y-axis (Fig 3). The shape of the funnel plot for publication bias appeared to be symmetrical (Fig 3A and 3B), although there was some uncertainty regarding the degree of symmetry. Thus, the estimate of the effect was considered to be biased. Nevertheless, the results based on the Begger’s test of the allele (C vs. T, Fig 3A) and the recessive (CC vs. CT/TT, Fig 3B) models showed no evidence of publication bias.
Fig 3. Funnel plot of studies included in the meta-analysis assessing the association of the rs243865 MMP-2 polymorphism with age-related macular degeneration.
The effect size (OR) was plotted on the x-axis, and the inverse of variance of the effect was plotted on the y-axis. (A) C vs. T allele. (B) CC vs. CT+TT genotype.
Sensitivity analyses
Sensitivity analyses were performed after sequentially removing each eligible study. This approach is regarded as an indispensable step for analysing multiple criteria. The significance of the pooled ORs was not influenced by any single study in the recessive genetic model, indicating that our results were statistically robust.
Discussion
The aetiology of AMD is unknown; nevertheless, several observations suggest that genetic alterations may be crucial in the development of the disease [5,6]. To date, through GWAS, more than 50 independently associated common genetic variants distributed across more than 30 loci have been associated with a significantly increased susceptibility to developing AMD [24–26]. To our knowledge, none of them included an analysis of the rs243865 MMP-2 polymorphism. In particular, variations in the complement factor H gene and the ARMS2/HTRA1 genes have been considered to be the most consistent genetic risk factors for AMD development [27,28]. The precise genetic factors, as well as their interactions, remain unclear in AMD. Therefore, in an attempt to prevent AMD, the identification of other potential genetic factors is crucial due to the lack of effective treatments at present.
AMD is characterised by drusen formation, which are accumulations of abnormal extracellular matrix deposits that cause visual function impairment [29,30]. A growing number of studies suggest that the rs243865 MMP-2 polymorphism could be associated with increased susceptibility to AMD development [7–12]. However, the results from past studies remain inconclusive, with some studies supporting a significant association, while others claim the opposite. Also, caution should be undertaken when interpreting the results from these single studies as they are often underpowered due to inadequate sample sizes. The present meta-analysis, which incorporated 6 independent case-control studies, was designed to evaluate whether or not rs243865 MMP-2 polymorphism modifies the risk of developing AMD. In comparison to past single studies, our analysis included a large sample size, 1,682 AMD patients and 2,295 healthy subjects, with attendant increased statistical power. Also, a quality evaluation system was included to minimise the potential for bias. Based on this approach, our most significant finding was that the 16q13-q21 rs243865 MMP-2 polymorphism is not a risk factor for developing AMD.
MMPs and tissue regulators have a central role in the deposition of extracellular matrix accumulations in several diseases [31–34]. It has been reported that MMP-2, MMP-3, and MMP-9 could be related to the pathogenesis of AMD. MMP-2, also known as gelatinase A, participates in the degradation of extracellular matrix components like collagen and laminin [34–36]. The rs243865 MMP-2 variant is located in the promoter region of the gene, where it causes a modification in MMP-2 transcription due to the variant T allele alteration in the Sp1-type promoter site [13]. The C to T allelic variation disrupts the Sp1 binding site in the promoter region and leads to significantly lower transcriptional activity. Therefore, the T allele has a markedly lower activity (50%) than the C allele. Several authors have reported that the dysregulation of MMP-2 synthesis leads to the formation of deposits in the basement membrane of the retinal pigment epithelium that, in turn, could contribute to drusen formation, and therefore to AMD development [34–36]. Moreover, an increased amount of inactive MMP-2 has been found in the subretinal space of AMD patients compared to that in normal human donor eyes [37]. This diminished level of active MMP-2 could be related to a reduction in the remodelling and accumulation of basal laminar deposits in AMD subjects [38].
Although the MMP pathway could play an important role in AMD development, the results of our study suggest that the rs243865 MMP-2 polymorphism was not associated with an increased risk of developing AMD. In addition, no statistically significant differences were attributable to either ethnicity or age. Therefore, other genetic and/or environmental factors might be more crucial than the rs243865 MMP-2 polymorphism in the susceptibility of AMD development. In this study, we analysed only one selected polymorphism within the MMP-2 gene; therefore, our results do not exclude the involvement of other MMP-2 gene variations in AMD pathogenesis.
It is necessary to emphasize the limitations in our meta-analysis. First, only English-language articles were analysed, which may have limited the number of studies included in our meta-analysis. Second, not all the studies reported adjusted ORs, and adjustments for the level of confounders such as geographic distribution, type of AMD, ethnicity, age, and gender varied among them. Third, we did not specify our results according to AMD type due to the small sample size that could have reduced the statistical power. Finally, our meta-analysis was focused mainly on the Caucasian population as the Asian population provided only a relatively small sample size. Nevertheless, we have been able to overcome the statistical limitations of small sample size and homogeneous sample composition that is commonly encountered in allelic genetic association studies. Even with these limitations, this meta-analysis contributes significantly to our understanding of AMD because it is the first to clarify the risk of development associated with the rs243865 MMP-2 polymorphism. Multiple GWAS and meta-analysis studies for AMD have been published, but to our knowledge, none of them included in an analysis of the rs243865 MMP-2 polymorphism.
Conclusions
In conclusion, our meta-analysis provides reliable evidence that rs243865 MMP-2 polymorphism is unlikely to be a risk factor for AMD development. Further investigations analysing the combined effect of genes and the environment may improve our current understanding of the association between the rs243865 or other MMP-2 polymorphisms and the risk of developing AMD, as well as the clinical and biological implications of other risk factors.
Supporting information
(DOC)
(DOCX)
(DOCX)
Acknowledgments
The authors thank the participants and researchers of the primary studies identified for this meta-analysis. We are grateful to Dr. Rasa Liutkeviciene for providing extra data regarding their studies. We thank Karla Loss, Keiko Ueda, and Gerhard da Paz, PPCR teaching assistant coordinators from the Harvard T. H. Chan School of Public Health, for their critical comments and helpful suggestions.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported in part by a grant from the Consejería de Educación, Junta de Castilla y León, Fondos FEDER (VA077P17) (JCP) and from Gerencia Regional de Salud de Castilla y León (INT/M/06/18) (AJC).
References
- 1.Wang JJ, Foran S, Mitchell P. Age-specific prevalence and causes of bilateral and unilateral visual impairment in older Australians: the Blue Mountains Eye Study. Clin Experiment Ophthalmol. 2000. August;28(4):268–73. [DOI] [PubMed] [Google Scholar]
- 2.Wong WL, Su X, Li X, Cheung CMG, Klein R, Cheng C-Y, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014. February;2(2):e106–116. 10.1016/S2214-109X(13)70145-1 [DOI] [PubMed] [Google Scholar]
- 3.Jonasson F, Arnarsson A, Eiríksdottir G, Harris TB, Launer LJ, Meuer SM, et al. Prevalence of age-related macular degeneration in old persons: Age, Gene/environment Susceptibility Reykjavik Study. Ophthalmology. 2011. May;118(5):825–30. 10.1016/j.ophtha.2010.08.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferris FL, Wilkinson CP, Bird A, Chakravarthy U, Chew E, Csaky K, et al. Clinical classification of age-related macular degeneration. Ophthalmology. 2013. April;120(4):844–51. 10.1016/j.ophtha.2012.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zarbin MA. Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol. 2004. April;122(4):598–614. 10.1001/archopht.122.4.598 [DOI] [PubMed] [Google Scholar]
- 6.Ratnapriya R, Chew EY. Age-related macular degeneration-clinical review and genetics update. Clin Genet. 2013. August;84(2):160–6. 10.1111/cge.12206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seitzman RL, Mahajan VB, Mangione C, Cauley JA, Ensrud K, Stone KL, et al. Estrogen receptor alpha and matrix metalloproteinase 2 polymorphisms and age-related maculopathy in older women. American Journal of Epidemiology. 2008. May 15;167(10):1217–25. 10.1093/aje/kwn024 [DOI] [PubMed] [Google Scholar]
- 8.Ortak H, Demir S, Ates O, Benli I, Sogut E, Sahin M. The Role of MMP2 (-1306C > T) and TIMP2 (-418 G > C) Promoter Variants in Age-related Macular Degeneration. Ophthalmic Genetics. 2013. December;34(4):217–22. 10.3109/13816810.2013.781192 [DOI] [PubMed] [Google Scholar]
- 9.Liutkeviciene R, Lesauskaite V, Zaliaduonyte-Peksiene D, Sinkunaite-Marsalkiene G, Zaliuniene D, Mizariene V, et al. Role of MMP-2 (-1306 C/T) Polymorphism in Age-Related Macular Degeneration. Ophthalmic Genet. 2016;37(2):170–6. 10.3109/13816810.2015.1020556 [DOI] [PubMed] [Google Scholar]
- 10.Cheng J, Hao X, Zhang Z. Risk of macular degeneration affected by polymorphisms in Matrix metalloproteinase-2: A case-control study in Chinese Han population. Medicine (Baltimore). 2017. November;96(47):e8190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liutkeviciene R, Lesauskaite V, Sinkunaite-Marsalkiene G, Simonyte S, Zemaitiene R, Kriauciuniene L, et al. MMP-2 Rs24386 (C—>T) gene polymorphism and the phenotype of age-related macular degeneration. Int J Ophthalmol. 2017;10(9):1349–53. 10.18240/ijo.2017.09.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liutkeviciene R, Vilkeviciute A, Borisovaite D, Miniauskiene G. Association of exudative age-related macular degeneration with matrix metalloproteinases-2 (-1306 C/T) rs243865 gene polymorphism. Indian Journal of Ophthalmology. 2018. April;66(4):551–7. 10.4103/ijo.IJO_1050_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Price SJ, Greaves DR, Watkins H. Identification of novel, functional genetic variants in the human matrix metalloproteinase-2 gene: role of Sp1 in allele-specific transcriptional regulation. J Biol Chem. 2001. March 9;276(10):7549–58. 10.1074/jbc.M010242200 [DOI] [PubMed] [Google Scholar]
- 14.Vu TH, Werb Z. Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev. 2000. September 1;14(17):2123–33. [DOI] [PubMed] [Google Scholar]
- 15.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007. March;8(3):221–33. 10.1038/nrm2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Little J, Higgins JPT, Ioannidis JPA, Moher D, Gagnon F, von Elm E, et al. STrengthening the REporting of Genetic Association Studies (STREGA): an extension of the STROBE statement. PLoS Med. 2009. February 3;6(2):e22 10.1371/journal.pmed.1000022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Age-related macular degeneration | Guidance and guidelines | NICE [Internet]. [cited 2018 Nov 2]. https://www.nice.org.uk/guidance/ng82/chapter/recommendations
- 18.Bird AC, Bressler NM, Bressler SB, Chisholm IH, Coscas G, Davis MD, et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study Group. Surv Ophthalmol. 1995. April;39(5):367–74. [DOI] [PubMed] [Google Scholar]
- 19.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010. September;25(9):603–5. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 20.Cochran WG. The Combination of Estimates from Different Experiments. Biometrics. 1954;10(1):101–29. [Google Scholar]
- 21.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003. September 6;327(7414):557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- 23.Cochrane Handbook for Systematic Reviews of Interventions [Internet]. [cited 2018 Nov 2]. http://handbook-5-1.cochrane.org
- 24.Fritsche LG, Chen W, Schu M, Yaspan BL, Yu Y, Thorleifsson G, et al. Seven new loci associated with age-related macular degeneration. Nat Genet. 2013. April;45(4):433–9, 439–2. 10.1038/ng.2578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fritsche LG, Igl W, Bailey JNC, Grassmann F, Sengupta S, Bragg-Gresham JL, et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat Genet. 2016. February;48(2):134–43. 10.1038/ng.3448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Black JRM, Clark SJ. Age-related macular degeneration: genome-wide association studies to translation. Genet Med. 2016. April;18(4):283–9. 10.1038/gim.2015.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Francis PJ, Schultz DW, Hamon S, Ott J, Weleber RG, Klein ML. Haplotypes in the Complement Factor H (CFH) Gene: Associations with Drusen and Advanced Age-Related Macular Degeneration. PLOS ONE. 2007. November 28;2(11):e1197 10.1371/journal.pone.0001197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grassmann F, Heid IM, Weber BHF, International AMD Genomics Consortium (IAMDGC). Recombinant Haplotypes Narrow the ARMS2/HTRA1 Association Signal for Age-Related Macular Degeneration. Genetics. 2017;205(2):919–24. 10.1534/genetics.116.195966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson LV, Ozaki S, Staples MK, Erickson PA, Anderson DH. A potential role for immune complex pathogenesis in drusen formation. Exp Eye Res. 2000. April;70(4):441–9. 10.1006/exer.1999.0798 [DOI] [PubMed] [Google Scholar]
- 30.Hageman GS, Mullins RF. Molecular composition of drusen as related to substructural phenotype. Mol Vis. 1999. November 3;5:28 [PubMed] [Google Scholar]
- 31.Belkhiri A, Richards C, Whaley M, McQueen SA, Orr FW. Increased expression of activated matrix metalloproteinase-2 by human endothelial cells after sublethal H2O2 exposure. Lab Invest. 1997. November;77(5):533–9. [PubMed] [Google Scholar]
- 32.Dollery CM, Libby P. Atherosclerosis and proteinase activation. Cardiovasc Res. 2006. February 15;69(3):625–35. 10.1016/j.cardiores.2005.11.003 [DOI] [PubMed] [Google Scholar]
- 33.Peten EP, Garcia-Perez A, Terada Y, Woodrow D, Martin BM, Striker GE, et al. Age-related changes in alpha 1- and alpha 2-chain type IV collagen mRNAs in adult mouse glomeruli: competitive PCR. Am J Physiol. 1992. November;263(5 Pt 2):F951–957. 10.1152/ajprenal.1992.263.5.F951 [DOI] [PubMed] [Google Scholar]
- 34.Atkinson SJ, Patterson ML, Butler MJ, Murphy G. Membrane type 1 matrix metalloproteinase and gelatinase A synergistically degrade type 1 collagen in a cell model. FEBS Lett. 2001. March 2;491(3):222–6. [DOI] [PubMed] [Google Scholar]
- 35.Elliot SJ, Catanuto P, Espinosa-Heidmann DG, Fernandez P, Hernandez E, Saloupis P, et al. Estrogen receptor beta protects against in vivo injury in RPE cells. Exp Eye Res. 2010. January;90(1):10–6. 10.1016/j.exer.2009.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liutkeviciene R, Lesauskaite V, Sinkunaite-Marsalkiene G, Zaliuniene D, Zaliaduonyte-Peksiene D, Mizariene V, et al. The Role of Matrix Metalloproteinases Polymorphisms in Age-Related Macular Degeneration. Ophthalmic Genet. 2015. June;36(2):149–55. 10.3109/13816810.2013.838274 [DOI] [PubMed] [Google Scholar]
- 37.Plantner JJ, Jiang C, Smine A. Increase in interphotoreceptor matrix gelatinase A (MMP-2) associated with age-related macular degeneration. Exp Eye Res. 1998. December;67(6):637–45. 10.1006/exer.1998.0552 [DOI] [PubMed] [Google Scholar]
- 38.Guo L, Hussain AA, Limb GA, Marshall J. Age-dependent variation in metalloproteinase activity of isolated human Bruch’s membrane and choroid. Invest Ophthalmol Vis Sci. 1999. October;40(11):2676–82. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.