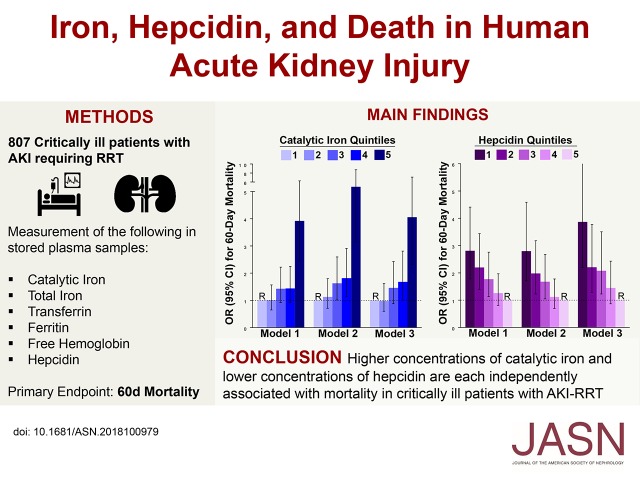
Significance Statement
Dysregulated iron metabolism plays an important pathogenic role in AKI in animal models, but limited data are available on circulating iron parameters in human AKI. To assess the association of plasma catalytic iron, total iron, transferrin, TSAT, ferritin, free hemoglobin, and hepcidin with 60-day mortality, the authors examined observations in a cohort study of 807 critically ill patients with AKI (all requiring RRT) who were enrolled the ARF Trial Network study. They found that higher concentrations of catalytic iron and lower concentrations of hepcidin are each monotonically and independently associated with increased mortality. These findings identify plasma catalytic iron and hepcidin as potentially useful prognostic markers or therapeutic targets in patients with AKI requiring RRT.
Keywords: acute renal failure, mortality risk, nephrotoxicity, outcomes
Visual Abstract
Abstract
Background
Iron is a key mediator of AKI in animal models, but data on circulating iron parameters in human AKI are limited.
Methods
We examined results from the ARF Trial Network study to assess the association of plasma catalytic iron, total iron, transferrin, ferritin, free hemoglobin, and hepcidin with 60-day mortality. Participants included critically ill patients with AKI requiring RRT who were enrolled in the study.
Results
Of the 807 study participants, 409 (51%) died by day 60. In both unadjusted and multivariable adjusted models, higher plasma concentrations of catalytic iron were associated with a significantly greater risk of death, as were lower concentrations of hepcidin. After adjusting for other factors, patients with catalytic iron levels in the highest quintile versus the lowest quintile had a 4.06-fold increased risk of death, and patients with hepcidin levels in the lowest quintile versus the highest quintile of hepcidin had a 3.87-fold increased risk of death. These findings were consistent across multiple subgroups. Other iron markers were also associated with death, but the magnitude of the association was greatest for catalytic iron and hepcidin. Higher plasma concentrations of catalytic iron and lower concentrations of hepcidin are each independently associated with mortality in critically ill patients with AKI requiring RRT.
Conclusions
These findings suggest that plasma concentrations of catalytic iron and hepcidin may be useful prognostic markers in patients with AKI. Studies are needed to determine whether strategies to reduce catalytic iron or increase hepcidin might be beneficial in this patient population.
Iron is essential for life, because it plays a key role in oxygen transport, DNA repair, cell proliferation, and many other critical biologic processes. However, when iron is present in excess, it is toxic to the kidneys,1–5 heart,6,7 endothelium,8–10 and other tissues due to its ability to cause oxidative damage to cellular membranes and proteins.8,11 Dysregulated iron metabolism plays a particularly important pathogenic role in AKI in experimental animal models,1–5 but limited data are available on iron in the context of human AKI.
Iron status can be assessed through a variety of parameters. Serum iron is the total amount of iron in the circulation. Transferrin is the main protein that binds iron in the blood and transports it to sites on the basis of physiologic demands. Transferrin saturation (TSAT) is calculated by dividing serum iron by transferrin, and it indicates the percentage of iron binding sites of transferrin occupied by iron. Ferritin is the primary intracellular iron storage protein, but small amounts are also secreted into the circulation, and they are often used as an indirect marker of total body iron stores.
Beyond the above parameters, which are commonly measured in clinical practice, other markers provide more specificity regarding iron that may directly cause tissue injury. Plasma free hemoglobin, the amount of hemoglobin present in the circulation outside of red blood cells, reflects the severity of intravascular hemolysis. Free hemoglobin can cause renal and extrarenal injury through a variety of mechanisms,12 including sequestration of nitric oxide, which results in arteriolar vasoconstriction and impaired tissue perfusion. Catalytic iron, also known as labile iron, is a transitional pool of extracellular and intracellular iron that is not transferrin bound, but rather loosely bound to albumin or low mol wt complexes.13 Catalytic iron can cause oxidative injury by facilitating the conversion of hydrogen peroxide to free radical ions—a process known as the Fenton reaction—and is a key mediator of AKI in animal models.1–5 Finally, the liver-derived hormone, hepcidin, is the main regulator of systemic iron homeostasis, and it may also have renoprotective functions in experimental AKI through its effects on cellular iron distribution.14,15 Specifically, hepcidin binds to the iron exporter ferroportin, which is present on the basolateral aspect of duodenal enterocytes, on the cell surface of macrophages, and elsewhere, and it induces its internalization and degradation.16 Hepcidin thereby limits iron absorption from the gut and iron export from storage sites, whereas suppression of hepcidin upregulates ferroportin and facilitates iron export into the circulation.
In humans, small studies have identified abnormalities in circulating iron parameters as risk factors and potential contributors to AKI.17–20 However, no study has examined a comprehensive panel of circulating iron parameters in patients with established AKI. We measured plasma concentrations of total iron, transferrin, ferritin, free hemoglobin, catalytic iron, and hepcidin in a large cohort of critically ill patients with severe AKI requiring RRT to assess their associations with death and renal recovery.
Methods
Study Design
We performed a cohort study to assess the associations between plasma iron marker concentrations, hepcidin, and death among patients enrolled in the ARF Trial Network (ATN) study. The ATN study enrolled 1124 critically ill patients with AKI requiring RRT from 27 major United States medical centers into a randomized trial of intensive versus less intensive RRT. Patients were eligible if they had AKI requiring RRT and failure of at least one nonrenal organ (defined as a nonrenal Sequential Organ Failure Assessment21 score of two or more) or sepsis. Additional inclusion and exclusion criteria are described elsewhere in detail.22
Subject enrollment began in November 2003, and it was completed July 2007. At the time of randomization (day 1), blood samples (n=817) were obtained in EDTA-containing vacutainers, immediately placed on ice, and centrifuged at 4°C, and the plasma was aliquoted and stored in a biorepository at −80°C. Additional samples (n=556) were collected and stored 1 week later (day 8) for patients who were still alive. Patients who provided samples for the biorepository (n=817) had similar baseline characteristics and outcomes compared with the overall cohort (n=1124) (Supplemental Table 1). We measured iron parameters using available samples from the biorepository (n=807 from day 1 and n=556 from day 8).
Informed Consent
All patients or their surrogates provided written informed consent, and the study was approved by the Human Rights Committee at the West Haven Veterans Affairs Cooperative Studies Program Coordinating Center and the institutional review boards at each of the participating sites.
Exposures
The primary exposures were plasma concentrations of catalytic iron, total iron, transferrin, TSAT, ferritin, free hemoglobin, and hepcidin, each measured on day 1. In secondary analyses, we also assessed the change in iron parameters from day 1 to day 8 both overall and according to RRT treatment assignment. Unless otherwise specified, data presented refer to iron parameters measured on day 1.
Outcomes
The primary end point was 60-day all-cause mortality. Secondary end points were durations of RRT and mechanical ventilation. To avoid the confounding effect of mortality, we calculated the number of RRT- and ventilator-free days. Data on RRT and mechanical ventilation were available through day 28 and day 14, respectively. Accordingly, the number of RRT-free days was calculated as 28 minus the number of RRT-dependent days, and the number of ventilator-free days was calculated as 14 minus the number of ventilator-dependent days. For both outcomes, patients who died before 28 or 14 days, respectively, were assigned a score of zero.23
Laboratory Analyses
Catalytic iron, total iron, transferrin, ferritin, and free hemoglobin were measured in plasma samples obtained on days 1 and 8, whereas hepcidin was measured on day 1 only. Catalytic iron concentrations were measured using the modified bleomycin assay.24 In brief, the antitumor agent, bleomycin, reacts with and degrades DNA in the presence of catalytic iron and suitable reducing agent. The DNA degradation products react with thiobarbituric acid to form a chromogen, the intensity of which is measured at 532 nm using a Beckman (DU800) spectrophotometer. The assay measures only free iron content in the sample that is capable of taking part in the generation of reactive oxygen species. The assay is not affected by the presence of free hemoglobin, transferrin, catalase, or lactoferrin in the sample.
We also measured plasma levels of total iron, transferrin, ferritin, free hemoglobin, and hepcidin. Total iron binding capacity was estimated from the transferrin concentration. TSAT (percentage) was calculated as the ratio of total iron (micrograms per deciliter) to total iron binding capacity (micrograms per deciliter) multiplied by 100. Hepcidin was measured using a monoclonal ELISA (Intrinsic LifeSciences, La Jolla, CA). Additional data on measurements and assay characteristics, including interassay coefficients of variation, are provided in Supplemental Material and Supplemental Table 2.
Serum levels of creatinine and albumin were measured for clinical purposes by the hospital laboratory. Details regarding measurement of plasma IL-6 levels are reported elsewhere.25
Statistical Analyses
We performed the statistical analyses with SAS version 9.4 (Cary, NC) and R version 3.2.3 (Vienna, Austria). We compared baseline characteristics and iron parameters between patients alive versus dead at day 60 using the Wilcoxon rank sum and chi-squared tests for continuous and categoric variables, respectively.
We used univariate (model 1) and multivariable (models 2 and 3) logistic regression to test the associations between iron parameters and 60-day mortality. In these models, iron parameters were natural log transformed and normalized to 1 SD to allow for comparison across markers. To account for multiple iron parameters being tested, we only considered P values <0.01 to be significant for these analyses. For all other analyses, we considered P values <0.05 to be significant. We adjusted for covariates according to biologic relevance and univariate associations with 60-day mortality. Accordingly, model 2 was adjusted for demographics and comorbidities (age, sex, race, baseline eGFR, diabetes mellitus, congestive heart failure [CHF], chronic liver disease, and chronic lung disease). Model 3 was further adjusted for severity of illness (intensive care unit type; mechanical ventilation; Acute Physiology and Chronic Health Evaluation II [APACHE II] score26; RRT before randomization; treatment randomization group; oliguria; sepsis; shock; and serum/plasma levels of albumin, creatinine, and IL-6).
In addition to the three models described above, we also tested the associations between catalytic iron and hepcidin with death across quartiles of APACHE II scores and plasma IL-6, TSAT, and ferritin levels. Finally, we assessed for effect modification according to baseline characteristics by testing the significance of interaction terms (subgroup × iron parameter) introduced into the model.
We also assessed catalytic iron and hepcidin in quintiles to test for nonlinear associations with mortality. We used logistic regression and adjusted for the same covariates as above. We also depicted the associations between quintiles of catalytic iron and hepcidin with death using Kaplan–Meier curves, and we used the log rank test to compare rates of death across quintiles.
For secondary end points, we assessed the associations between catalytic iron and hepcidin with ventilator-free and RRT-free days using Spearman correlation coefficients. Similarly, we used Spearman correlation coefficients to assess the associations between catalytic iron, other iron parameters, and hepcidin, and we depicted these relationships using restricted cubic splines. Finally, in exploratory analyses, we assessed longitudinal changes in iron parameters (from day 1 to 8) using the Wilcoxon signed rank test, and we tested for effect modification according to intensive versus less intensive RRT treatment assignment.
Overall, 1.7% of demographic and severity of illness data were missing, and they were imputed using regression switching with predictive mean matching.27 Five datasets were multiply imputed, and results were pooled using Rubin rules.28
Results
Baseline Characteristics
Among the 807 participants, 409 (51%) died by day 60. On average, patients who died were older, had higher APACHE II scores, and were more likely to be oliguric and have shock. Additional demographic, clinical, and laboratory parameters are shown in Table 1.
Table 1.
Enrollment characteristics
| Characteristic | All, n=807 | Survivors, n=398 | Nonsurvivors, n=409 | P Value |
|---|---|---|---|---|
| Demographics | ||||
| Age, yr, median (IQR) | 62 (51–72) | 59 (48–68) | 64 (54–75) | <0.001 |
| Men, no. (%) | 561 (70) | 272 (68) | 289 (71) | 0.47 |
| White race, no. (%) | 621 (77) | 297 (75) | 324 (79) | 0.12 |
| Comorbidities, no. (%) | ||||
| Diabetes mellitus | 198 (25) | 108 (28) | 90 (23) | 0.10 |
| Congestive heart failure | 197 (25) | 93 (24) | 104 (27) | 0.49 |
| Chronic liver disease | 94 (12) | 37 (10) | 57 (14) | 0.04 |
| Chronic lung diseasea | 102 (13) | 43 (11) | 59 (15) | 0.12 |
| CKDb | 293 (39) | 137 (38) | 156 (40) | 0.56 |
| Malignancy | 156 (19) | 68 (17) | 88 (22) | 0.11 |
| Baseline renal function | ||||
| Creatinine, mg/dl, median (IQR)c | 1.1 (0.9–1.4) | 1.1 (0.8–1.4) | 1.1 (0.9–1.4) | 0.80 |
| eGFR, median (IQR)d | 70 (50–93) | 72 (52–96) | 69 (49–89) | 0.07 |
| Medical ICU, no. (%) | 356 (44) | 174 (44) | 182 (45) | 0.82 |
| Severity of illness | ||||
| Sepsis, no. (%) | 518 (64) | 248 (62) | 270 (66) | 0.27 |
| APACHE II score, median (IQR)e | 26 (21–31) | 23 (19–29) | 28 (24–33) | <0.001 |
| Mechanical ventilation, no. (%) | 652 (81) | 290 (73) | 362 (89) | <0.001 |
| Shock, no. (%)f | 508 (63) | 215 (54) | 293 (72) | <0.001 |
| Oliguria, no. (%)g | 645 (80) | 296 (74) | 349 (85) | <0.001 |
| RRT before randomization, no. (%) | 558 (69) | 274 (69) | 284 (69) | 0.86 |
| Days in ICU, median (IQR)h | 4 (3–8) | 4 (3–7) | 4 (3–9) | 0.002 |
| Enrollment laboratory values, median (IQR) | ||||
| White cell count, per mm3 | 13 (9–19) | 13 (9–18) | 14 (9–20) | 0.31 |
| Hemoglobin, g/dl | 9.8 (8.9–10.8) | 9.7 (8.8–10.6) | 9.9 (9.1–10.9) | 0.03 |
| Creatinine, mg/dl | 3.9 (2.9–5.1) | 4.3 (3.2–5.6) | 3.6 (2.8–4.6) | <0.001 |
| Albumin, g/dl | 2.3 (1.9–2.8) | 2.3 (1.9–3.0) | 2.3 (1.8–2.8) | 0.03 |
| IL-6, pg/ml | 166 (74–540) | 126 (59–281) | 231 (103–911) | <0.001 |
Percentages are on the basis of the number of patients without missing data. IQR, interquartile range; ICU, intensive care unit; APACHE II, Acute Physiology and Chronic Health Evaluation II.
Defined as chronic hypoxemia, hypercapnea, pulmonary hypertension, or ventilator dependence.
Defined as baseline eGFR <60 ml/min per 1.73 m2.
Baseline serum creatinine was defined as the premorbid serum creatinine at the time of screening or if unavailable, the lowest serum creatinine within 4 days before screening.
eGFR is reported in milliliters per minute per 1.73 m2 and was determined using the Chronic Kidney Disease Epidemiology Collaboration equation.40
APACHE II score is an ICU severity of illness scoring system ranging from zero to 71, with higher scores indicating more severe disease.
Shock was defined as requirement for vasopressor support for >1 hour.
Oliguria was defined as an average urine output <20 ml/h for >24 hours.
Before randomization.
Univariate Analyses of Iron Parameters (Assessed Continuously) and Mortality
Iron parameters on days 1 and 8 according to 60-day survival status are shown in Table 2 and Supplemental Table 3. Higher concentrations of catalytic iron, TSAT, ferritin, and free hemoglobin and lower concentrations of transferrin and hepcidin on day 1 were each associated with higher 60-day mortality.
Table 2.
Iron marker levels on day 1 according to 60-day survival status
| Iron Parameter | All, n=807 | Survivors, n=398 | Nonsurvivors, n=409 | P Valuea |
|---|---|---|---|---|
| Catalytic iron, μmol/L | 0.62 (0.36–1.47) | 0.49 (0.34–1.07) | 0.76 (0.41–2.62) | <0.001 |
| Total iron, μg/dl | 65 (54–82) | 63 (53–79) | 66 (55–83) | 0.05 |
| Transferrin, mg/dl | 133 (108–163) | 138 (112–167) | 127 (106–156) | 0.003 |
| TSAT, % | 26 (22–32) | 25 (21–31) | 27 (23–33) | <0.001 |
| Ferritin, μg/L | 843 (385–2361) | 689 (338–1659) | 1078 (436–3308) | <0.001 |
| Free hemoglobin, mg/dl | 12 (7–22) | 11 (6–20) | 13 (7–23) | 0.001 |
| Hepcidin, ng/ml | 124 (46–230) | 158 (65–268) | 97 (33–202) | <0.001 |
Data are shown as median and interquartile range (25th to 75th percentiles). TSAT, transferrin saturation.
P values refer to the comparison between iron parameters in patients alive versus dead at 60 days. For these analyses, only P values <0.01 are considered significant to account for multiple comparisons.
Multivariable Analyses of Iron Parameters and Mortality
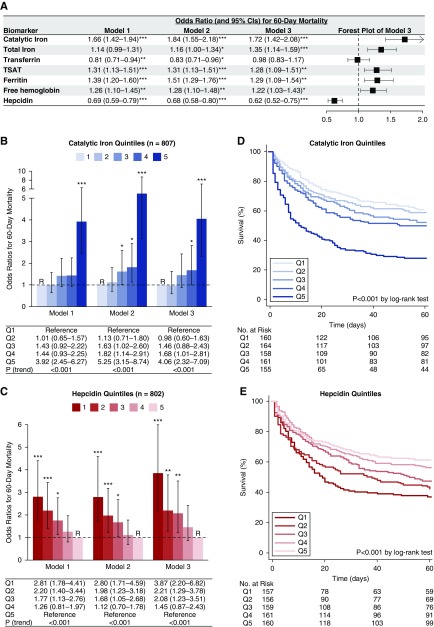
We assessed each iron parameter on day 1 using three models: model 1 was unadjusted, model 2 was adjusted for demographics and comorbidities, and model 3 was further adjusted for acute severity of illness. In fully adjusted analyses (model 3), catalytic iron, total iron, TSAT, ferritin, and hepcidin were each associated with death (Figure 1A). The magnitude of association was greatest for catalytic iron and hepcidin. Specifically, higher concentrations of catalytic iron and lower concentrations of hepcidin were each associated with higher 60-day mortality (adjusted odds ratio per 1-SD higher ln[catalytic iron], 1.72; 95% confidence interval [95% CI], 1.42 to 2.08; adjusted odds ratio per 1-SD higher ln[hepcidin], 0.62; 95% CI, 0.52 to 0.75). In exploratory analyses, we assessed the effect of including all iron parameters in our models in addition to the model 2 and 3 covariates. In these models, catalytic iron and hepcidin, but not other iron parameters, remained significantly associated with death, although the strength of these associations was slightly attenuated (Supplemental Table 4).
Figure 1.
Higher catalytic iron and lower hepcidin concentrations associate with death. Odds ratios (ORs; and 95% confidence intervals [95% CIs]) for death according to iron marker concentrations. A shows ORs for 60-day mortality according to natural log-transformed iron parameters standardized to 1 SD. Model 1 is unadjusted, model 2 is adjusted for demographics and comorbidities (age, sex, race, baseline eGFR, diabetes mellitus, congestive heart failure, chronic liver disease, and chronic lung disease), and model 3 is further adjusted for severity of illness (intensive care unit type; mechanical ventilation; Acute Physiology and Chronic Health Evaluation II score; RRT before randomization; treatment randomization group; oliguria; sepsis; shock; and serum/plasma levels of albumin, creatinine, and IL-6). For the analyses shown in A, only P values <0.01 are considered significant to account for multiple comparisons. TSAT, transferrin saturation. B shows ORs for 60-day mortality according to quintiles of catalytic iron. Quintile 1 was the reference (R) group in all models. Catalytic iron levels by quintile: quintile 1, 0.18–0.33 μmol/L; quintile 2, 0.34–0.46 μmol/L; quintile 3, 0.47–0.80 μmol/L; quintile 4, 0.81–2.12 μmol/L; and quintile 5, 2.16–47.01 μmol/L. C shows ORs for 60-day mortality according to quintiles of hepcidin (the y axis is cut at six). Quintile 5 was the reference (R) group in all models. Hepcidin levels by quintile: quintile 1, 4–33 ng/ml; quintile 2, 33–86 ng/ml; quintile 3, 86–163 ng/ml; quintile 4, 163–266 ng/ml; and quintile 5, 266–2415 ng/ml. *P<0.05; **P<0.01; ***P<0.001. D and E show Kaplan–Meier survival curves according to quintiles of catalytic iron and hepcidin, respectively.
Next, we assessed catalytic iron and hepcidin by quintiles. We found that higher quintiles of catalytic iron were associated with a monotonic increase in the risk of death in both unadjusted and adjusted analyses (Figure 1B). In fully adjusted analyses (model 3), patients with catalytic iron concentrations in the highest quintile compared with the lowest quintile had a 4.06 greater odds of death (95% CI, 2.32 to 7.09). Similarly, we found that lower quintiles of hepcidin were associated with a monotonic increase in the risk of death in both unadjusted and adjusted analyses (Figure 1C). In fully adjusted analyses (model 3), patients with hepcidin concentrations in the lowest quintile compared with the highest quintile had a 3.87 greater odds of death (95% CI, 2.20 to 6.82). We found similar results for quintiles of catalytic iron and hepcidin using time-to-event analyses, with the majority of deaths occurring in the first 30 days (Figure 1, D and E, enlarged Kaplan–Meier survival curves are shown in Supplemental Figure 1).
Catalytic Iron and Hepcidin—Stratified and Subgroup Analyses
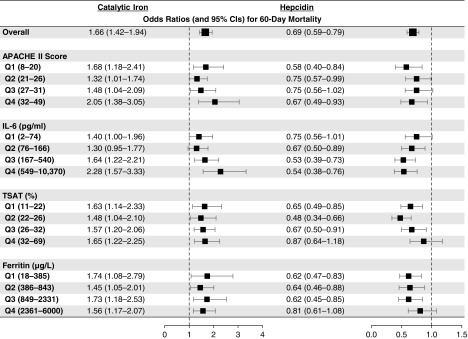
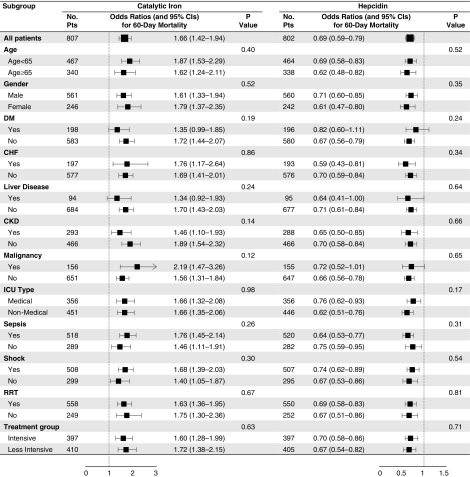
Higher concentrations of catalytic iron and lower concentrations of hepcidin were each associated with increased mortality across nearly all quartiles of APACHE II scores and plasma IL-6, TSAT, and ferritin levels (Figure 2). Additionally, we found no differences in the magnitude of associations between catalytic iron and hepcidin with death across a variety of subgroups. Specifically, we found no differences on the basis of age, sex, diabetes mellitus, CHF, chronic liver disease, CKD, malignancy, intensive care unit type, sepsis, shock, RRT use before randomization, or treatment group (Figure 3).
Figure 2.
Catalytic iron and hepcidin concentrations associate with death across stratified analyses. Odds ratios for 60-day mortality according to catalytic iron and hepcidin levels across quartiles of Acute Physiology and Chronic Health Evaluation II (APACHE II) scores and plasma IL-6, transferrin saturation (TSAT), and ferritin levels. Catalytic iron and hepcidin concentrations were natural log transformed and standardized to 1 SD.
Figure 3.
Catalytic iron and hepcidin concentrations associate with death across subgroups. Odds ratios for 60-day mortality according to catalytic iron and hepcidin concentrations across subgroups. Catalytic iron and hepcidin concentrations were natural log transformed and standardized to 1 SD. P values refer to the significance of interaction terms testing for effect modification by subgroup. CHF, congestive heart failure; DM, diabetes mellitus; ICU, intensive care unit.
Secondary End Points
We assessed the associations between catalytic iron and hepcidin and the duration of RRT and mechanical ventilation. We found that higher concentrations of catalytic iron correlated with fewer RRT-free days (rs=−0.18; P<0.001) and fewer ventilator-free days (rs=−0.18; P<0.001), implying longer duration of RRT and mechanical ventilation. Similarly, we found that lower concentrations of hepcidin correlated with fewer RRT-free days (rs=0.16; P<0.001) and fewer ventilator-free days (rs=0.14; P<0.001).
Associations between Catalytic Iron and Hepcidin with Baseline Characteristics
Next, we sought to determine the baseline/clinical characteristics that were associated with catalytic iron and hepcidin. We observed higher concentrations of catalytic iron in younger patients, patients with fewer comorbidities (except for liver disease), and patients with greater acute severity of illness. Additional associations with catalytic iron are shown in Supplemental Tables 5 and 6. We observed lower concentrations of hepcidin in patients with chronic liver disease, CHF, shock, and oliguria. Additional associations with hepcidin are shown in Supplemental Tables 7 and 8.
Associations between Catalytic Iron and Hepcidin with Other Iron Parameters
The associations between catalytic iron and hepcidin with other iron parameters are shown in Supplemental Figure 2. Higher concentrations of catalytic iron were associated with higher concentrations of total iron (rs=0.11; P=0.003), TSAT (rs=0.23; P<0.001), free hemoglobin (rs=0.23; P<0.001), and ferritin (rs=0.46; P<0.001), and they were associated with lower concentrations of transferrin (rs=−0.19; P<0.001). Higher concentrations of hepcidin were associated with lower transferrin and higher TSAT levels, and they showed a “J”-shaped association with free hemoglobin. Finally, lower concentrations of hepcidin were associated with higher concentrations of catalytic iron (rs=−0.20; P<0.001). Additional correlations between iron parameters are shown in Supplemental Table 9.
Changes in Iron Parameters over Time
Finally, in exploratory analyses, we assessed whether the change in iron marker concentrations over time was affected by treatment assignment to intensive versus less intensive RRT. These analyses were limited to the 556 participants with samples available on days 1 and 8. Overall, concentrations of catalytic iron, total iron, TSAT, and ferritin decreased, whereas concentrations of transferrin increased from day 1 to 8 (Supplemental Table 10). The decline in catalytic iron was greater among participants assigned to intensive versus less intensive RRT (P=0.03 for interaction). Additionally, we observed a downward trend in concentrations of free hemoglobin over time in the intensive RRT group but an upward trend in the less intensive RRT group (P=0.01 for interaction). Other iron parameters showed similar trends over time between groups.
Discussion
In this cohort study, we assessed a comprehensive panel of circulating iron parameters in critically ill patients with AKI requiring RRT, and we found significant associations between several iron parameters and death. Notably, we report that higher plasma concentrations of catalytic iron and lower concentrations of hepcidin are each associated with increased mortality. These relationships were monotonic and independent of other known risk factors, including other iron parameters. Furthermore, although other iron parameters, such as TSAT and ferritin, were also associated with death, the magnitude of association was strongest for catalytic iron and hepcidin.
Our findings are consistent with and expand on prior studies conducted in animals, in which catalytic iron was shown to have an important pathologic role across a wide spectrum of AKI models, including AKI caused by ischemia-reperfusion, aminoglycosides, cisplatin, rhabdomyolysis, hemoglobinuria, and iodinated radiocontrast.1–5 We previously extended these findings to humans by reporting an association between higher concentrations of plasma catalytic iron and a greater risk of incident AKI.17,18 However, these pilot studies were limited by small samples sizes and a low event rate for hospital mortality, which precluded detailed multivariable analyses; varying degrees of AKI severity on enrollment, which could have confounded the results; and lack of data on hepcidin. Here, we present the largest study to date assessing dysregulated iron homeostasis in the context of human AKI.
Although this study was not designed to establish the sources of catalytic iron in this population, potential mechanisms by which critically ill patients may develop elevated concentrations of catalytic iron include both ex vivo hemolysis (e.g., from transfusion of packed red blood cells) and in vivo hemolysis (e.g., from sepsis). Supporting the notion of hemolysis as a major source of catalytic iron in critical illness, we found a significant positive correlation between concentrations of catalytic and free hemoglobin. Because all patients in this study had severe AKI requiring RRT, an alternative possibility is that decreased filtration, rather than increased production, could have resulted in increased catalytic iron. However, iron is excreted primarily through sloughed mucosal cells in the gastrointestinal tract rather than in the urine, and urinary catalytic iron levels are increased, not decreased, in patients with nonoliguric AKI.29 Nonetheless, we cannot entirely exclude the possibility that decreased urinary excretion of catalytic iron could have contributed to our findings, particularly given the high prevalence of oliguria in this cohort. Notably, we found that the association between catalytic iron and death was independent of oliguria.
Additional potential sources of catalytic iron include elevated concentrations of ferritin, the main iron storage protein, and decreased concentrations of transferrin, the main iron transport protein. Ferritin may release iron in the presence of superoxide, which is produced under proinflammatory conditions.30 However, circulating ferritin is relatively iron poor.31 Thus, a more likely possibility is that ferritin was elevated nonspecifically, reflecting its role as an acute-phase reactant. As with catalytic iron, we found that plasma ferritin levels were elevated and associated with increased mortality in our cohort. However, the magnitude of association with mortality was strongest for catalytic iron. Furthermore, the association between catalytic iron and death persisted despite adjustment for ferritin and other iron parameters, whereas the same was not true for ferritin. Additionally, the association between catalytic iron and death was significant across all four quartiles of ferritin. Thus, measurement of catalytic iron seems to provide additional prognostic data beyond ferritin. Moreover, catalytic iron, not ferritin, is the biologically active form of iron that is capable of inducing tissue injury. Alternatively, low circulating concentrations of transferrin could represent an important source of catalytic iron. Transferrin is a negative acute-phase reactant that declines rapidly in critical illness,32 and we observed transferrin concentrations on day 1 that were well below reference ranges in healthy adults. Lower transferrin concentrations in critically ill patients with AKI could increase the amount of nontransferrin-bound iron and, thus, the amount of catalytic iron in the circulation as a consequence of reduced iron-carrying capacity. Supporting this notion, we found that lower transferrin associated with higher catalytic iron concentrations (Supplemental Figure 2B).
Finally, we measured plasma concentrations of hepcidin, the master regulator of systemic iron homeostasis. We found that lower concentrations of hepcidin are strongly associated with increased mortality. Because hepcidin is upregulated, not downregulated, by inflammation, it is less likely that the association between hepcidin and death is simply a reflection of acute severity of illness. Rather, our findings are more suggestive of a direct effect of hepcidin on iron homeostasis. Consistent with this notion, we found that lower concentrations of hepcidin correlated with higher concentrations of catalytic iron. Because the major action of hepcidin is to downregulate the expression of the iron exporter ferroportin, which is present on the cell surface of macrophages, the basolateral aspect of duodenal enterocytes, and elsewhere, these findings raise the possibility that decreased hepcidin levels in AKI could contribute to elevated circulating catalytic iron via increased export of iron from intracellular stores (Figure 4). Supporting this notion, the association between lower concentrations of hepcidin and higher mortality was attenuated by adjustment for catalytic iron and other iron parameters (Supplemental Table 4), suggesting that catalytic iron could at least partially mediate this association. Lower concentrations of hepcidin could also contribute to increased mortality in critically ill patients via reduced pathogen clearance leading to increased sepsis (Figure 4), because hepcidin also functions as an antimicrobial peptide with broad antibacterial and antifungal activity.33 These antimicrobial activities, in turn, could be either dependent or independent of hepcidin’s effects on reducing circulating concentrations of iron, although most evidence suggests that these effects are iron dependent.34,35 Supporting the biologic plausibility of lower concentrations of hepcidin contributing to adverse outcomes in AKI, administration of exogenous hepcidin attenuates the severity of both ischemia-reperfusion– and hemoglobin-mediated kidney injury in mice.14,15
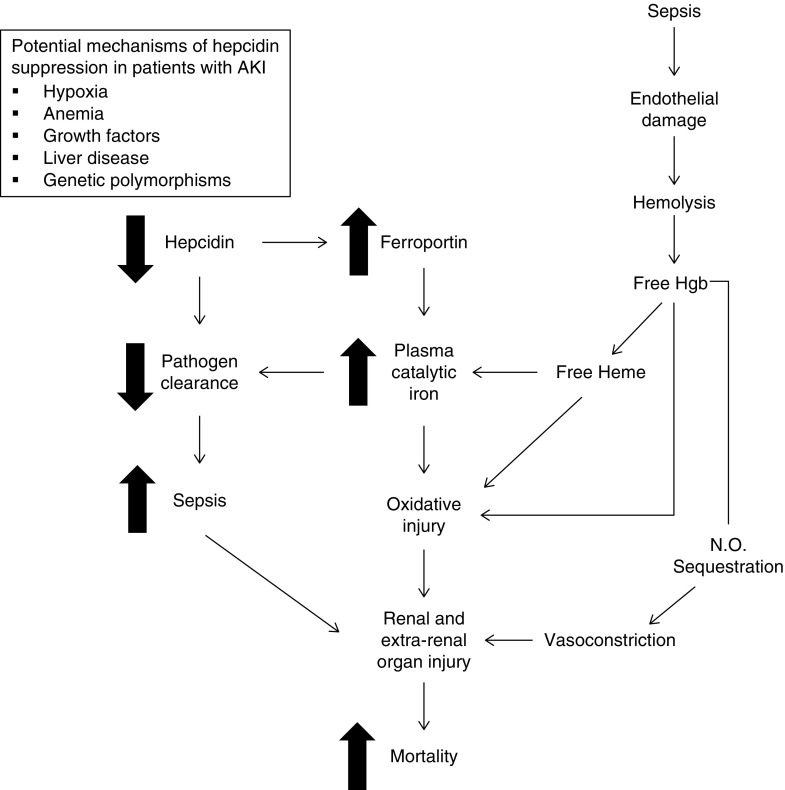
Figure 4.
Potential pathways linking hepcidin and catalytic iron with death in AKI. Critically ill patients with AKI may have decreased circulating concentrations of hepcidin due to a variety of physiologic stimuli (e.g., hypoxia or anemia), comorbidities (e.g., liver disease), or genetic polymorphisms. Decreased hepcidin upregulates expression of the iron exporter, ferroportin, on the cell surface of monocytes/macrophages (and elsewhere), resulting in increased iron export into the blood, which may result in increased plasma catalytic iron. The latter results in oxidative injury and promotes renal and extrarenal organ injury. Hepcidin also functions as an antimicrobial peptide; thus, decreased circulating hepcidin may also result in impaired pathogen clearance. The latter could promote sepsis and lead to additional renal and extrarenal organ injury. Finally, hemolysis represents an additional potential source of catalytic iron that may be independent of hepcidin. Free hemoglobin (Hgb) released into the circulation may also directly cause renal and extrarenal organ injury through sequestration of nitric oxide (N.O.), which causes arteriolar vasoconstriction and impaired tissue perfusion.
Potential mechanisms contributing to decreased circulating levels of hepcidin in critically ill patients with AKI include hypoxia/shock, anemia, and liver disease, each of which was associated with lower hepcidin levels. The presence of liver disease in particular showed a strong association with both lower hepcidin and higher catalytic iron concentrations. In addition to producing hepcidin, the liver is also the main source of transferrin, and thus, liver dysfunction could contribute to higher circulating concentrations of catalytic iron due to less transferrin availability. The liver is also the main storage site for iron and a scavenger for catalytic iron, and therefore, impaired uptake of catalytic iron in the context of liver dysfunction could also contribute to higher circulating levels. Notably, the associations between both catalytic iron and hepcidin with mortality persisted despite adjustment for liver disease. Furthermore, the number of patients with liver disease was relatively low, and catalytic iron and hepcidin were associated with death even in patients without liver disease (Figure 3). Thus, liver disease alone is unlikely to account for our findings.
Our study has several strengths. We assessed multiple iron parameters in a large number of patients who enrolled in the ATN study, a population with high event rates for hard clinical outcomes, including death. Thus, we had ample statistical power to test our hypotheses both overall and in subgroups of patients. Additionally, all patients enrolled in the ATN study had AKI requiring RRT on enrollment, thereby minimizing confounding from varying degrees of AKI severity. Finally, the ATN study collected detailed data on demographics, comorbid conditions, and laboratory data, which allowed us to adjust our analyses to account for a large number of covariates.
We also acknowledge several limitations, including observational design and lack of data on cause of death. Although we performed measurements on multiple iron parameters, the contributions of changes in unmeasured variables, including markers of hemolysis (such as haptoglobin, hemopexin, lactate dehydrogenase, and reticulocyte count), are unknown and require additional study. Data on intravenous iron administration and transfusion of packed red blood cells were not collected in the ATN study, and thus, they could not be investigated as a potential source of catalytic iron. Similarly, only limited data were collected on the use of erythropoiesis-stimulating agents, and thus, exogenous erythropoietin could not be investigated as a potential mechanism of hepcidin suppression. Serum creatinine phosphokinase levels were not recorded, and thus, muscle injury could not be investigated as a potential source of catalytic iron. Although we adjusted for multiple covariates, we cannot exclude potential residual confounding by variables that may not have been ascertained. On the basis of the observational nature of the study, we cannot determine whether elevated catalytic iron or decreased hepcidin is the more important variable potentially responsible for excess mortality. Finally, we presented data on the associations between iron parameters and death using odds ratios, which overestimate the relative risk when the event of interest is common.36 Accordingly, the odds ratios should be interpreted cautiously.
In conclusion, we found that dysregulated iron homeostasis is a prognostically important factor in critically ill patients with AKI requiring RRT. The strongest parameters appear to be higher concentrations of catalytic iron and lower concentrations of hepcidin, and the latter could be at least partially responsible for the former as a result of increased iron export into the circulation. The associations between catalytic iron and hepcidin with death could be mediated through toxic effects of iron on the kidneys,1–3 heart,6,7 endothelium,8–10 brain,37 or other tissues. Interventional studies are needed to determine whether strategies aimed at reducing plasma concentrations of catalytic iron, such as administration of iron chelators38 or hepcidin agonists,39 could provide therapeutic benefit in this patient population.
Disclosures
M.R. and S.S.L. report holding a United States patent for the methods and kit for the measurement of serum catalytic iron for early detection of acute coronary syndrome and prediction of adverse cardiac events. J.L.B. has ownership interest in Ferrumax Pharmaceuticals and has received consulting fees from Keryx Biopharmaceuticals and Disc Medicine.
Supplementary Material
Acknowledgments
D.E.L. designed the study, performed the statistical analyses, interpreted the data, and wrote the manuscript. M.R., S.S.L., and B.M. performed the iron measurements. E.A.S.B. assisted with sample processing and data interpretation. M.F.E. and K.S. assisted with data interpretation and statistical analysis. J.A.K. designed and performed analyses of plasma IL-6, and assisted with data interpretation. M.C. performed the hepcidin measurements. P.M.P. was responsible for overseeing patient enrollment and sample collection. S.S.W. participated in study design, statistical analysis, and data interpretation. All authors provided assistance in critically revising the manuscript, approve the final version to be published, and agree to be accountable for all aspects of the work.
This work was supported by National Institute of Diabetes and Digestive Kidney Diseases (NIDDK) Grants K23DK106448 (to D.E.L.); P30-DK079337 (pilot and feasibility award to D.E.L.) from the University of Alabama at Birmingham–University of California, San Diego O’Brien Center for AKI Research; R01DK070910 (to J.A.K.); K08DK093608 (to M.C.); U01DK085660 (to S.S.W.); and R01DK093574 (to S.S.W.). This work was also supported by an American Society of Nephrology Foundation for Kidney Research Carl W. Gottschalk Research Scholar Grant (to D.E.L.) and the Westchester Community Foundation Renal Clinical Fund (M.C.). The Veterans Affairs/National Institutes of Health ARF Trial Network study was supported by the Cooperative Studies Program of the Department of Veterans Affairs Office of Research and Development (CSP 530) and the NIDDK through interagency agreement Y1-DK-3508. Funding for measurement of iron markers and free hemoglobin was provided by the Muljibhai Patel Society for Research in Nephrology (Gujarat, India).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018100979/-/DCSupplemental.
Supplemental Figure 1. Kaplan–Meier survival curves according to quintiles of catalytic iron and hepcidin.
Supplemental Figure 2. Associations between catalytic iron and hepcidin with other iron parameters.
Supplemental Material. Supplemental methods.
Supplemental Table 1. Enrollment characteristics in the ATN study: all patients versus the biorepository subcohort.
Supplemental Table 2. Assay characteristics.
Supplemental Table 3. Iron marker levels on day 8 according to 60-day survival status.
Supplemental Table 4. Odds ratios (and 95% CIs) for death according to iron marker levels—exploratory analysis adjusted for all model 2 and model 3 covariates and all iron parameters.
Supplemental Table 5. Enrollment characteristics according to quintiles of catalytic iron.
Supplemental Table 6. Univariate and multivariable associations between enrollment characteristics and catalytic iron concentrations.
Supplemental Table 7. Enrollment characteristics according to quintiles of hepcidin.
Supplemental Table 8. Univariate and multivariable associations between enrollment characteristics and hepcidin concentrations.
Supplemental Table 9. Associations between iron and inflammatory parameters.
Supplemental Table 10. Changes in iron markers from day 1 to 8 overall and according to treatment assignment.
References
- 1.Baliga R, Ueda N, Shah SV: Increase in bleomycin-detectable iron in ischaemia/reperfusion injury to rat kidneys. Biochem J 291: 901–905, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baliga R, Zhang Z, Baliga M, Ueda N, Shah SV: In vitro and in vivo evidence suggesting a role for iron in cisplatin-induced nephrotoxicity. Kidney Int 53: 394–401, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Walker PD, Shah SV: Evidence suggesting a role for hydroxyl radical in gentamicin-induced acute renal failure in rats. J Clin Invest 81: 334–341, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baliga R, Zhang Z, Baliga M, Shah SV: Evidence for cytochrome P-450 as a source of catalytic iron in myoglobinuric acute renal failure. Kidney Int 49: 362–369, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Paller MS: Hemoglobin- and myoglobin-induced acute renal failure in rats: Role of iron in nephrotoxicity. Am J Physiol 255: F539–F544, 1988 [DOI] [PubMed] [Google Scholar]
- 6.Ambrosio G, Zweier JL, Jacobus WE, Weisfeldt ML, Flaherty JT: Improvement of postischemic myocardial function and metabolism induced by administration of deferoxamine at the time of reflow: The role of iron in the pathogenesis of reperfusion injury. Circulation 76: 906–915, 1987 [DOI] [PubMed] [Google Scholar]
- 7.van der Kraaij AM, Mostert LJ, van Eijk HG, Koster JF: Iron-load increases the susceptibility of rat hearts to oxygen reperfusion damage. Protection by the antioxidant (+)-cyanidanol-3 and deferoxamine. Circulation 78: 442–449, 1988 [DOI] [PubMed] [Google Scholar]
- 8.Jacob AK, Hotchkiss RS, DeMeester SL, Hiramatsu M, Karl IE, Swanson PE, et al.: Endothelial cell apoptosis is accelerated by inorganic iron and heat via an oxygen radical dependent mechanism. Surgery 122: 243–253, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Won SM, Lee JH, Park UJ, Gwag J, Gwag BJ, Lee YB: Iron mediates endothelial cell damage and blood-brain barrier opening in the hippocampus after transient forebrain ischemia in rats. Exp Mol Med 43: 121–128, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Eijk LT, Heemskerk S, van der Pluijm RW, van Wijk SM, Peters WH, van der Hoeven JG, et al.: The effect of iron loading and iron chelation on the innate immune response and subclinical organ injury during human endotoxemia: A randomized trial. Haematologica 99: 579–587, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halliwell B, Gutteridge JM: Role of free radicals and catalytic metal ions in human disease: An overview. Methods Enzymol 186: 1–85, 1990 [DOI] [PubMed] [Google Scholar]
- 12.Rother RP, Bell L, Hillmen P, Gladwin MT: The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: A novel mechanism of human disease. JAMA 293: 1653–1662, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Hider RC, Silva AM, Podinovskaia M, Ma Y: Monitoring the efficiency of iron chelation therapy: The potential of nontransferrin-bound iron. Ann N Y Acad Sci 1202: 94–99, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Scindia Y, Dey P, Thirunagari A, Liping H, Rosin DL, Floris M, et al.: Hepcidin mitigates renal ischemia-reperfusion injury by modulating systemic iron homeostasis. J Am Soc Nephrol 26: 2800–2814, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Swelm RP, Wetzels JF, Verweij VG, Laarakkers CM, Pertijs JC, van der Wijst J, et al.: Renal handling of circulating and renal-synthesized hepcidin and its protective effects against hemoglobin-mediated kidney injury. J Am Soc Nephrol 27: 2720–2732, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al.: Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306: 2090–2093, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Leaf DE, Rajapurkar M, Lele SS, Mukhopadhyay B, Waikar SS: Plasma catalytic iron, AKI, and death among critically ill patients. Clin J Am Soc Nephrol 9: 1849–1856, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leaf DE, Rajapurkar M, Lele SS, Mukhopadhyay B, Rawn JD, Frendl G, et al.: Increased plasma catalytic iron in patients may mediate acute kidney injury and death following cardiac surgery. Kidney Int 87: 1046–1054, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vermeulen Windsant IC, Snoeijs MG, Hanssen SJ, Altintas S, Heijmans JH, Koeppel TA, et al.: Hemolysis is associated with acute kidney injury during major aortic surgery. Kidney Int 77: 913–920, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Choi N, Whitlock R, Klassen J, et al.: Early intraoperative iron-binding proteins are associated with acute kidney injury after cardiac surgery. J Thorac Cardiovasc Surg 157: 287–297.e2, 2019 [DOI] [PubMed] [Google Scholar]
- 21.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al.: The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22: 707–710, 1996 [DOI] [PubMed] [Google Scholar]
- 22.Palevsky PM, Zhang JH, O’Connor TZ, Chertow GM, Crowley ST, Choudhury D, et al.: VA/NIH Acute Renal Failure Trial Network : Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med 359: 7–20, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubenfeld GD, Angus DC, Pinsky MR, Curtis JR, Connors AF Jr., Bernard GR: Outcomes research in critical care: Results of the American Thoracic Society critical care assembly workshop on outcomes research. The members of the outcomes research workshop. Am J Respir Crit Care Med 160: 358–367, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Lele S, Shah S, McCullough PA, Rajapurkar M: Serum catalytic iron as a novel biomarker of vascular injury in acute coronary syndromes. EuroIntervention 5: 336–342, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Murugan R, Wen X, Shah N, Lee M, Kong L, Pike F, et al.: Biological Markers for Recovery of Kidney (BioMaRK) Study Investigators : Plasma inflammatory and apoptosis markers are associated with dialysis dependence and death among critically ill patients receiving renal replacement therapy. Nephrol Dial Transplant 29: 1854–1864, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knaus WA, Draper EA, Wagner DP, Zimmerman JE: APACHE II: A severity of disease classification system. Crit Care Med 13: 818–829, 1985 [PubMed] [Google Scholar]
- 27.White IR, Royston P, Wood AM: Multiple imputation using chained equations: Issues and guidance for practice. Stat Med 30: 377–399, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Rubin DB: Multiple Imputation for Nonresponse in Surveys, New York, Wiley, 1987 [Google Scholar]
- 29.Akrawinthawong K, Shaw MK, Kachner J, Apostolov EO, Basnakian AG, Shah S, et al.: Urine catalytic iron and neutrophil gelatinase-associated lipocalin as companion early markers of acute kidney injury after cardiac surgery: A prospective pilot study. Cardiorenal Med 3: 7–16, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biemond P, van Eijk HG, Swaak AJ, Koster JF: Iron mobilization from ferritin by superoxide derived from stimulated polymorphonuclear leukocytes. Possible mechanism in inflammation diseases. J Clin Invest 73: 1576–1579, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arosio P, Yokota M, Drysdale JW: Characterization of serum ferritin in iron overload: Possible identity to natural apoferritin. Br J Haematol 36: 199–207, 1977 [DOI] [PubMed] [Google Scholar]
- 32.Piagnerelli M, Cotton F, Herpain A, Rapotec A, Chatti R, Gulbis B, et al.: Time course of iron metabolism in critically ill patients. Acta Clin Belg 68: 22–27, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Park CH, Valore EV, Waring AJ, Ganz T: Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem 276: 7806–7810, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Stefanova D, Raychev A, Deville J, Humphries R, Campeau S, Ruchala P, et al.: Hepcidin protects against lethal Escherichia coli sepsis in mice inoculated with isolates from septic patients. Infect Immun 86: 86, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stefanova D, Raychev A, Arezes J, Ruchala P, Gabayan V, Skurnik M, et al.: Endogenous hepcidin and its agonist mediate resistance to selected infections by clearing non-transferrin-bound iron. Blood 130: 245–257, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knol MJ, Le Cessie S, Algra A, Vandenbroucke JP, Groenwold RH: Overestimation of risk ratios by odds ratios in trials and cohort studies: Alternatives to logistic regression. CMAJ 184: 895–899, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakamura T, Xi G, Park JW, Hua Y, Hoff JT, Keep RF: Holo-transferrin and thrombin can interact to cause brain damage. Stroke 36: 348–352, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Fraga CM, Tomasi CD, Damasio DC, Vuolo F, Ritter C, Dal-Pizzol F: N-acetylcysteine plus deferoxamine for patients with prolonged hypotension does not decrease acute kidney injury incidence: A double blind, randomized, placebo-controlled trial. Crit Care 20: 331, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Casu C, Nemeth E, Rivella S: Hepcidin agonists as therapeutic tools. Blood 131: 1790–1794, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al.: CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.