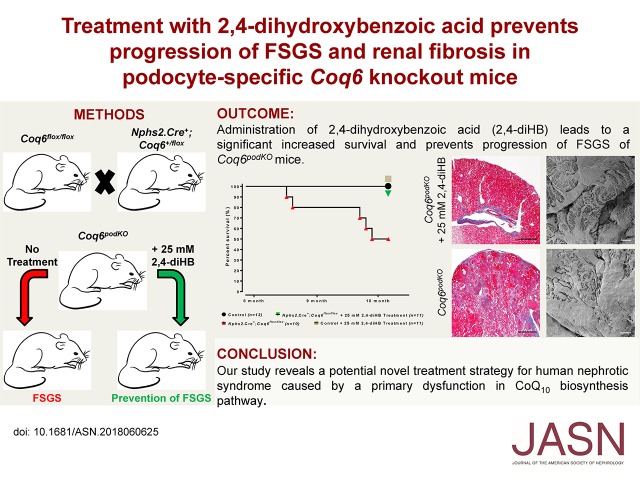
Significance Statement
Studies have identified mutations in >55 genes that cause steroid-resistant nephrotic syndrome (SRNS) and localized SRNS pathogenesis to podocytes. The authors previously reported that individuals with mutations in COQ6, a coenzyme Q (CoQ10, CoQ, or ubiquinone) biosynthesis pathway enzyme, develop SRNS, and demonstrated that CoQ can reduce kidney dysfunction. In this study, they generated a podocyte-specific Coq6 knockout mouse and showed that abrogating Coq6 in mouse podocytes caused FSGS and proteinuria. In vitro studies revealed an impaired podocyte migration rate in COQ6 knockdown human podocytes. Treating affected mice or cells with 2,4-dihydroxybenzoic acid, an analog of a CoQ precursor molecule, prevented renal dysfunction and reversed migration rate impairment. This suggests a potential therapeutic approach for those cases of human nephrotic syndrome that result from a primary dysfunction in the CoQ10 biosynthesis pathway.
Keywords: nephrotic syndrome, podocyte, focal segmental glomerulosclerosis
Visual Abstract
Abstract
Background
Although studies have identified >55 genes as causing steroid-resistant nephrotic syndrome (SRNS) and localized its pathogenesis to glomerular podocytes, the disease mechanisms of SRNS remain largely enigmatic. We recently reported that individuals with mutations in COQ6, a coenzyme Q (also called CoQ10, CoQ, or ubiquinone) biosynthesis pathway enzyme, develop SRNS with sensorineural deafness, and demonstrated the beneficial effect of CoQ for maintenace of kidney function.
Methods
To study COQ6 function in podocytes, we generated a podocyte-specific Coq6 knockout mouse (Coq6podKO) model and a transient siRNA-based COQ6 knockdown in a human podocyte cell line. Mice were monitored for development of proteinuria and assessed for development of glomerular sclerosis. Using a podocyte migration assay, we compared motility in COQ6 knockdown podocytes and control podocytes. We also randomly assigned 5-month-old Coq6podKO mice and controls to receive no treatment or 2,4-dihydroxybenzoic acid (2,4-diHB), an analog of a CoQ precursor molecule that is classified as a food additive by health authorities in Europe and the United States.
Results
Abrogation of Coq6 in mouse podocytes caused FSGS and proteinuria (>46-fold increases in albuminuria). In vitro studies revealed an impaired podocyte migration rate in COQ6 knockdown human podocytes. Treating Coq6podKO mice or cells with 2,4-diHB prevented renal dysfunction and reversed podocyte migration rate impairment. Survival of Coq6podKO mice given 2,4diHB was comparable to that of control mice and significantly higher than that of untreated Coq6podKO mice, half of which died by 10 months of age.
Conclusions
These findings reveal a potential novel treatment strategy for those cases of human nephrotic syndrome that are caused by a primary dysfunction in the CoQ10 biosynthesis pathway.
Steroid-resistant nephrotic syndrome (SRNS) is a genetically heterogeneous, incurable renal disease that is the second most frequent cause of ESRD in the first two decades of life.1 Mutations in >55 genes have been identified as causing SRNS,2 which is clinically defined as nephrotic syndrome that is resistant to standard steroid treatment.3,4 FSGS appears to be most frequent histological feature of SRNS.5 Although the blood-filtrating component of the glomerulus consists of three cell types (endothelium, mesangium, and podocytes), identification of >55 monogenic causes of SRNS6 has revealed that podocytes are the primary cell type affected in SRNS.7,8 Podocytes are specialized epithelial cells with a complex structure that have elaborate interdigitating foot processes, which form the slit diaphragm, a filtration barrier that consists of various cytoskeletal proteins. Maintenance of podocyte structure and function requires a highly regulated amount of energy, suggesting a high sensitivity to alteration of oxidative metabolism.9,10 Gene identification has revealed that mutations in genes encoding cytoskeletal components11–17 and components of the mitochondrial enzymes18–24 cause SRNS. Coenzyme Q (CoQ), or ubiquinone, is a redox-active lipophilic molecule and a critical component of the mitochondrial inner membrane, where it functions in the electron transport chain by transferring electrons from complexes I and II to complex III.25,26 CoQ acts also in nucleotide biosynthesis.25 It displays a potent antioxidant activity in its reduced form, thus protecting cellular membranes from lipid peroxidation.27,28 De novo CoQ production involves a complex but poorly understood biochemical pathway, depending on the activity of at least ten different enzymes. Recently, mutations in genes encoding CoQ biosynthesis pathway enzymes PDSS2, COQ2, COQ6, and ADCK4 have been reported to cause SRNS.21–23,29 Discovery of monogenic forms of SRNS that represent primary mitochondrial diseases due to deficiency in CoQ10 levels has identified a subpopulation of patients with SRNS, who may benefit from treatment with dietary CoQ10 or its precursor analogs. In order to test this hypothesis, we generated a podocyte-specific knockout Coq6 mouse line and a transient human podocyte knockdown cell line.
Methods
Mouse Breeding and Maintenance
The Nphs2.Cre+;Coq6loxP/loxP mouse model on C57BL/6 genetic background used in this study was generated from targeted Coq6 ES cells obtained from the Knockout Mouse Project (EUCOMM) Repository. Coq6 ES cells were injected into blastocysts to generate Coq6-transgenic mice. Coq6+/loxP mice were crossed with Nphs2.Cre+ mice30 and double heterozygous mice were backcrossed to generate podocyte-specific Nphs2.Cre+;Coq6loxP/loxP knockout mice and littermate controls (Supplemental Figure 1A). Genotypes of animals were assessed by PCR (Supplemental Figure 1, B and C). Genotyping primer sequences are available upon request. Nphs2.Cre+ mice were kindly provided by Dr. Holzman (University of Pennsylvania). The experimental protocol was reviewed and approved by the Animal Care Committee of the Boston Children’s Hospital. All animals were housed in pathogen-free conditions with lights on at 7:00 am and off at 7:00 pm. Mice had unlimited access to water and rodent chow. Animals were randomly assigned to experimental groups.
Urine Analysis
Urine was collected by housing the mice overnight (12 hours) in metabolic cages. All samples were frozen straight away and stored at −80°C. The samples were thawed on ice before performing the urine albumin and creatinine measurements. Urinary albumin and urinary creatinine were measured using a fluorometric albumin kit (Progen) and a colorimetric creatinine kit (R&D Systems) per manufacturer instructions. Proteinuria was expressed as milligrams of albumin per milligram of creatinine.
Histologic and Ultrastructural Analyses
Kidneys were harvested immediately after euthanasia and submerged in fixative—4% paraformaldehyde for histology, or 2.5% glutaraldehyde, 1.25% paraformaldehyde, and 0.03% picric acid in 0.1 M sodium cacodylate buffer (pH 7.4) for transmission electron microscopy (TEM) and scanning electron microscopy (SEM). Kidney serial sections were subjected to staining with hematoxylin and eosin, periodic acid–Schiff, Masson’s trichrome, and Jones' methenamine silver following standard protocols, as well as histologic analyses. TEM and SEM were carried out using standard techniques.
Immunofluorescence Analyses
Mice kidneys were frozen in optimal cutting temperature compound and sectioned at 5 μm. These sections were fixed with PBS containing 4% paraformaldehyde, blocked with PBS containing 10% donkey serum and 1% BSA for 90 minutes at room temperature, then incubated with primary antibody at 4°C overnight. Sections were washed several times with PBS and incubated with fluorophore-conjugated secondary antibodies (Invitrogen) for 45 minutes at room temperature. A Leica SP5X confocal microscope was used for imaging. Image processing was done using Leica AF, ImageJ, and Adobe Photoshop CS6 software.
Isolation and Characterization of Mouse Glomeruli
Mouse glomeruli were isolated as described previously in a modified fashion due to perfusion.31 Briefly, kidneys were harvested immediatly after euthanasia and perfused via renal artery with HBSS containing Dynabeads M-450, digested in Collagenase 1A and DNAse I solution, glomeruli were separated on DynaMag-2 magnet and glass-glass homogenized in Pierce RIPA lysis buffer containing Halt Protease Inhibitor and Halt Phosphatase Inhibitor cocktails. Protein concentration was determined by DC Protein assay (Bio-Rad). An equal amount of protein was subjected to SDS–PAGE followed by western blotting. Band intensity was assessed with Image Lab software.
Mouse Drinking Water Supplementation with 2,4-Dihydroxybenzoic Acid
2,4-Dihydroxybenzoic acid (2,4-diHB) was administered to the mice in the drinking water at 25-mM concentration and changed twice a week. The treatment was started at 5 months of age and continued up to 10.5 months of age.
Cell Lines and Cell Culture
Experiments shown in this publication were performed in HEK293T cells and immortalized human podocytes. HEK293T cells were purchased from the ATCC biologic resource center. Human immortalized podocytes were a kind gift from Moin Saleem, University of Bristol, Bristol, UK, and were cultured as previously described.32 HEK293T cells were maintained in DMEM supplemented with 10% FBS, 50 IU/ml penicillin, and 50 μg/ml streptomycin. Podocytes were maintained in RPMI plus GlutaMAX-I (Gibco) supplemented with 10% FBS, 50 IU/ml penicillin/50 μg/ml streptomycin, and 1% insulin-transferrin-selenium-X. Cell lines were tested for mycoplasma contamination on a biweekly basis.
RNAi Knockdown in Human Podocyte Cell Line
To achieve a transient knockdown human, podocytes were transfected with siRNA using RNAiMAX per manufacturer instructions.
| Gene | siRNA targets | siRNA target sequence |
|---|---|---|
| COQ6 (NM_182476.2) | siRNA# 9 | GGACUUAGGUUCCGUGAGC |
| siRNA# 10 | GAGCAAAGCCAUUCGCUAU | |
| siRNA# 11 | CCACAGAGUCCAUCCGCUU | |
| siRNA# 12 | CCUAAAGAAAGAUUACGUU |
Isolation of Mitochondria
Mitochondria from HEK293T and human podocyte cell lines were isolated using a mitochondria isolation kit(ThermoFisher) per manufacturer instructions. Isolated mitochondria were lysed in Pierce RIPA lysis buffer containing Halt Protease Inhibitor and Halt Phosphatase Inhibitor cocktails. Equal amounts of isolated mitochondria were subjected to SDS–PAGE and western blotting. Band intensity was assessed with Image Lab software.
Podocyte Migration Assays
The podocyte migration assay using xCELLigence system was performed as previously described.33 Briefly, real-time migration assays were performed using the xCELLigence system (Roche Applied Science) with CIM-plate 16 per manufacturer instructions. Immortalized human podocytes were transfected either with scrambled siRNA or with siRNAs targeting human COQ6. Thirty-six hours after transfection, 4×104 cells were seeded in serum-free medium in the upper chambers of the migration plate. The lower chambers were filled with medium containing 10% FBS as chemo attractant, or with serum-free medium as control. Indicated supplements were added to the media in the lower and upper chambers before the assay was started. Changes in impedance were analyzed using the RTCA software. Results were plotted as cell index (relative podocyte migration) versus time.
Gene Expression Analyses
Total cellular RNA from immortalized human podocytes was extracted using the RNeasy Plus kit following manufacturer instructions. Total RNA was quantified and an equal amount of 1 µg was subjected to RT-PCR using the iScript cDNA synthesis kit. Gene expression was assessed using TaqMan probes and normalized to expression of GAPDH.
Primary Antibodies
| Target protein | Company | Catalogue # | Species |
|---|---|---|---|
| COQ6 | Santa Cruz Biotechnology | sc-393932 | Mouse |
| αTubulin | Abcam | ab15246 | Rabbit |
| CoxIV | Cell Signaling | 4850 | Rabbit |
| βActin | Abcam | ab20272 | Mouse |
| Podocin | Sigma Aldrich | P0372 | Rabbit |
| Nephrin | Progen | GP-N2 | Guinea pig |
| WT1 | Cell Signaling | 83535 | Rabbit |
| Synaptopodin | ThermoFisher | PA5–56997 | Rabbit |
| Nidogen | Novus Biologicals | NBP1–97701 | Rat |
| Desmin | Cell Signaling | 5332 | Rabbit |
| αSMA | Cell Signaling | 19245 | Rabbit |
| ColIV | Abcam | ab6586 | Rabbit |
| 58K | Abcam | ab27043 | Mouse |
Statistical Analyses
Statistical analyses were performed using Graph Pad Prism 7 software. Significance was determined at P<0.05. Particular tests performed in the experiments are indicated in the figure legends.
Results
Podocyte-Specific Knockout of Coq6 Leads to Increased Mortality in Adult Mice
To study the role of Coq6 in normal kidney function, we generated a transgenic Coq6 (Coq6tm1a) mouse line using embryonic stem cells from EUCOMM (Supplemental Figure 1A). Proper targeting of the Coq6 gene was confirmed by gene-specific genotyping (Supplemental Figure 1B). Whole body loss of Coq6 in Coq6tm1a mice was found by us to be not compatible with life. To circumvent Coq6tm1a embryonic lethality, we generated podocyte-specific Coq6 knockout mice Nphs2.Cre+;Coq6flox/flox (hereafter referred to as Coq6podKO), by crossing Podocin-Cre mice with Coq6floxlflox mice in which two loxP sites surround exon 6 in the Coq6 gene. We confirmed by PCR that Podocin-Cre–dependent Coq6 inactivation occurs only in the kidneys, and not in other organs (Supplemental Figure 1C). Successful deletion of Coq6 expression in podocytes was confirmed by western blotting of glomerular lysates from Coq6podKO kidneys, where Coq6 protein levels were reduced compared with Podocin-Cre− controls (Supplemental Figure 2A). Although Coq6podKO mice appeared grossly normal, we noticed increased morbidity (hunched posture, scruffy fur) (Supplemental Figure 3A) and increased mortality (Figure 1A) in older (>10-month-old) Coq6podKO mice, features that were not observed in littermate controls. Necropsy of 10-month-old Coq6podKO mice revealed that the mutant kidneys were pale and smaller than normal (Supplemental Figure 3B), indicating that podocyte-specific deletion of Coq6 leads to structural and functional kidney defects in Coq6podKO mice.
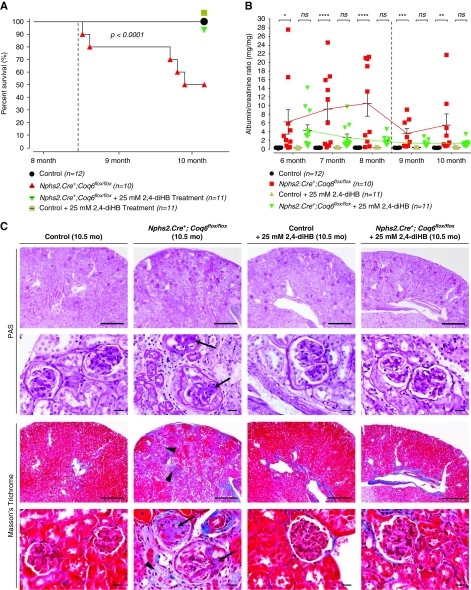
Figure 1.
Nphs2.Cre+;Coq6flox/flox mice develop severe progressive proteinuria and consecutive death in adulthood. Treatment with 2,4-diHB prevents disease progression, resulting in normal survival range. (A) Nphs2.Cre+;Coq6flox/flox mutant mice exhibit reduced life span with a median survival of 306 days, whereas Nphs2.Cre+;Coq6flox/flox mutant mice under treatment with 2,4-diHB have similar survival to healthy and healthy treated littermate controls. Dotted line displays onset of renal failure. n=10–12 animals in each group. Log-rank (Mantel–Cox) test, P<0.001. (B) Urinary albumin-to-creatinine ratio serial analysis at indicated ages and genotypes (n=10–12 animals in each group) reveals progressive proteinuria in Nphs2.Cre+;Coq6flox/flox mutant mice (red square) but not in littermate controls (black circle). Nphs2.Cre+;Coq6flox/flox mutant mice under treatment with 2,4-diHB (green triangles) are protected from developing proteinuria. Dotted line displays onset of renal failure. Note that once chronic renal failure ensues, urinary albumin excretion is reduced as is known to occur in SRNS. One-way ANOVA P values, calculated using Tukey’s multiple comparisons test, are shown in the figure; *P<0.05, **P<0.01, ***P<0.001, ****P<0.001; each data point represents mean value of technical duplicates, error bars represent SEM. (C) Kidney serial sections and representative images of 10.5-month-old mice with indicated genotypes were stained according to indicated conditions. The Nphs2.Cre+;Coq6flox/flox mutant mice exhibit FSGS (arrows) with focal interstitial fibrosis, tubular atrophy (arrow heads), and proteinaceous casts in dilated tubules (asterisks). In contrast, wild-type littermate control mice and Nphs2.Cre+;Coq6flox/flox mutant mice under treatment with 2,4-diHB display normal histologic kidney morphology. Scale bars, upper rows 500 μm and lower rows 20 μm. PAS, periodic acid–Schiff.
Coq6podKO Mice Develop Progressive Glomerular Sclerosis and Proteinuria
To characterize the progression of kidney functional decline we followed Coq6podKO mice by monthly urinalysis for 10 months (Figure 1B). The first significant increase in the albumin-to-creatinine ratio (7.4-fold, P<0.04) in Coq6podKO mice was observed at 5 months of age (Supplemental Figure 2B). The onset of kidney functional decline in Coq6podKO mice was associated with mild focal glomerular sclerosis and occasional protein casts in the proximal tubule (Supplemental Figure 2C). Staining with Jones' methenamine silver stain did not reveal drastic alterations in the glomerular basement membrane at this stage (Supplemental Figure 2C). However, kidney histologic analysis in 10-month-old Coq6podKO mice using periodic acid–Schiff and Masson’s trichrome staining revealed progressive and more pronounced focal glomerular sclerosis, obliteration of capillary lumens, and thickening of the basement membrane (Figure 1C). In addition, Masson’s trichrome staining revealed extensive tubulointerstitial fibrosis in the mutant kidneys (Figure 1C). Interestingly, male Coq6podKO mice were more susceptible to proteinuria compared with female mice. The increase over time in albuminuria was up to 46.9-fold in Coq6podKO mice compared with littermate controls (Figure 1B).
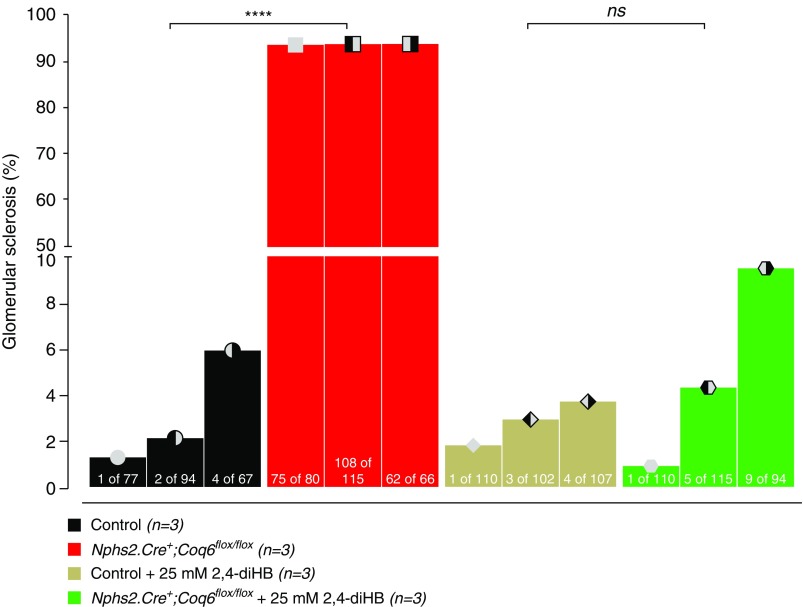
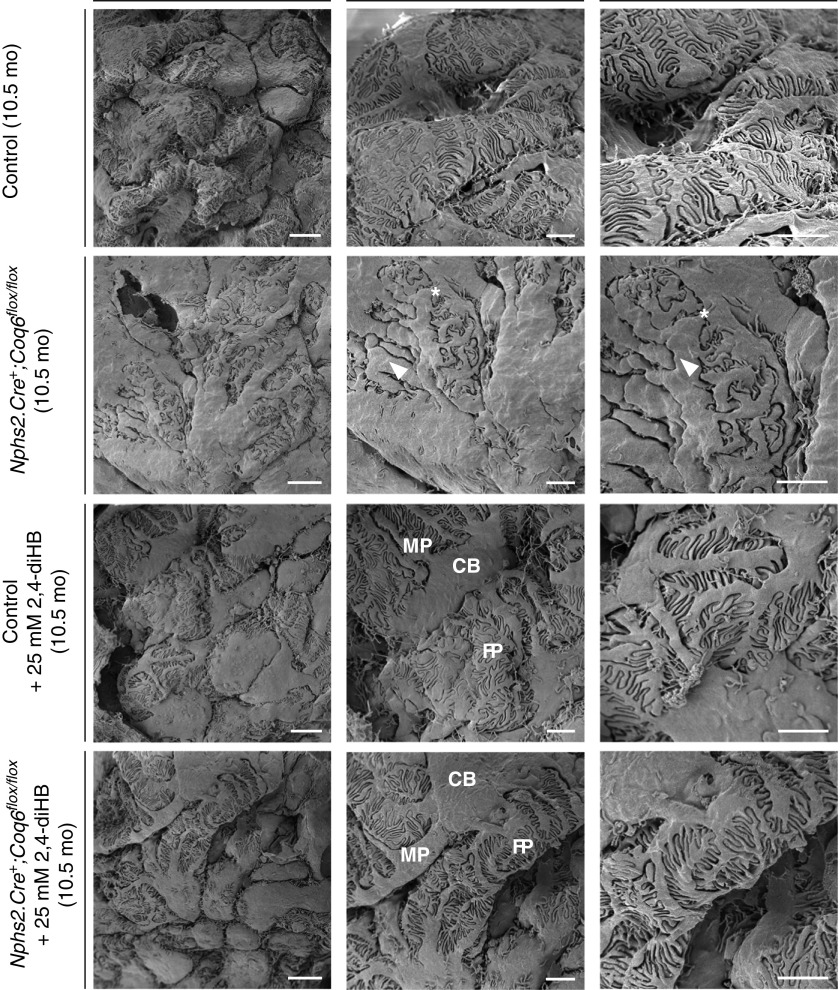
Recently, 2,4-diHB, a metabolic intermediate of CoQ10, has been successfully applied to ameliorate disparate phenotypes in mouse models caused by heterogeneous enzymatic defects in the CoQ10 biosynthesis pathway.34,35 Given that renal structural abnormalities and functional decline manifest only relatively late in Coq6podKO mice, we decided to initiate the treatment in 5-month-old mice. Mice were randomly assigned to study groups. Administration of 2,4-diHB led to significantly increased survival of Coq6podKO mice compared with untreated Coq6podKO mice (P<0.001), who presented with 50% mortality by 10 months of age (Figure 1A). Decline in mortality was associated with significantly improved kidney function and normal glomerular histology in 10-month-old treated Coq6podKO mice (Figure 1, B and C, Supplemental Figure 4). Supplementing 2,4-diHB did not have any effect on control mouse kidney function or histology (Figure 1, B and C, Supplemental Figure 4) and ameliorated the physical condition of Coq6podKO mice (Supplemental Figure 3C), revealing normal appearance of kidneys in Coq6podKO mice after necroscopy (Supplemental Figure 3D). To characterize the glomerular phenotype in Coq6podKO mice we quantified the number of sclerotic glomeruli in the treated and nontreated mutant mice and found that, although nontreated Coq6podKO mice had significantly increased numbers of sclerotic glomeruli (93.87%), treatment with 2,4-diHB effectively mitigated the sclerotic phenotype in Coq6podKO (4.95%) kidneys (Figure 2).
Figure 2.
Development of FSGS is abrogated by treatment with 2,4-diHB in Nphs2.Cre+;Coq6flox/flox mutant mice. Kidney serial sections of 10.5-month-old mice with indicated genotypes were stained with Masson’s trichrome and analyzed to determine the severity of glomerular sclerosis. The Nphs2.Cre+;Coq6flox/flox mutant mice show FSGS, whereas Nphs2.Cre+;Coq6flox/flox mutant mice under treatment with 2,4-diHB displayed virtually no (<10% of glomeruli) abnormal histologic findings (n=3 animals in each group; each graph bar indicates an single animal, numbers inside the bar graphs indicate number of sclerosed glomeruli per total glomeruli counted in one section. Two-way ANOVA P values calculated using Tukey’s multiple comparisons test are shown in the figure. ****P<0.001.
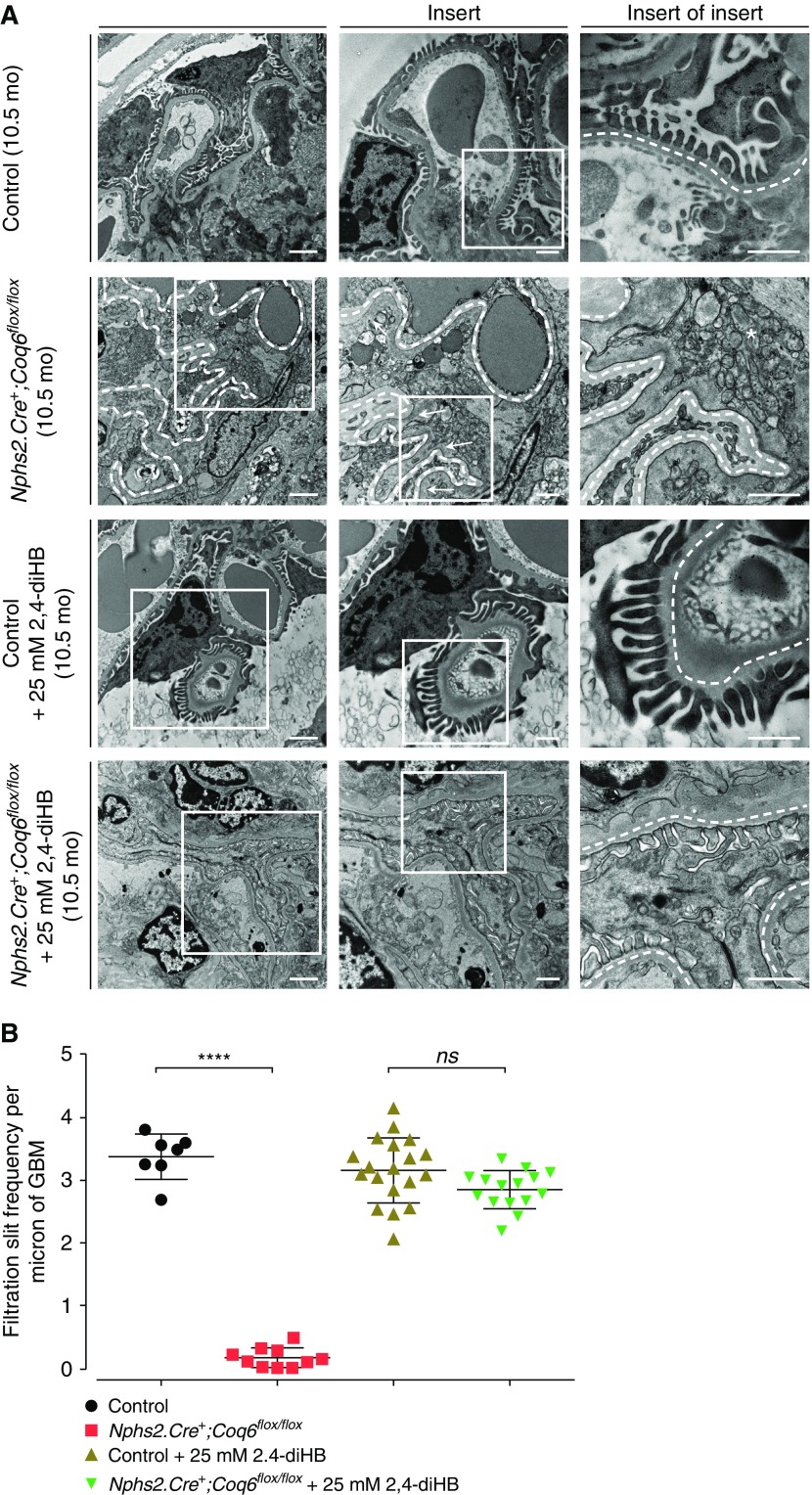
Loss of Coq6 Leads to Podocyte Foot Process Effacement
To characterize the architectural changes in Coq6podKO glomeruli at the ultrastructural level we performed TEM and SEM studies in 5-month-old and 10-month-old Coq6podKO kidneys. Changes in Coq6podKO mouse glomeruli were first observed in 5-month-old mice, with TEM revealing podocyte foot process effacement and SEM demonstrating simplified morphology of the podocytes in Coq6podKO glomeruli (Supplemental Figure 5, A and B). The podocyte architecture became progressively more abnormal in Coq6podKO kidneys by 10 months of age, characterized by lack of primary and foot processes, whereas treatment with 2,4-diHB helped to maintain the normal podocyte configuration in Coq6podKO glomeruli (Figures 3A and 4). To characterize the architectural changes in Coq6podKO glomeruli we compared the number of filtration slit units per micrometer of basement membrane in Coq6podKO and wild-type glomeruli. Whereas filtration slit frequency was significantly diminished in Coq6podKO mice, treatment with 2,4-diHB preserved the normal filtration slit morphology in Coq6podKO glomeruli (Figure 3B). Overall, the glomerular phenotype of podocyte-specific loss of Coq6 recapitulates the human pathology of FSGS of COQ6-deficient individuals.21
Figure 3.
Glomerular structure is disrupted in Nphs2.Cre+;Coq6flox/flox mutant mice and is preserved by 2,4-diHB treatment. (A) TEM representative images at age 10.5 months. Nphs2.Cre+;Coq6flox/flox mutant mice reveal podocyte foot process effacement (arrows) and an increased number of mitochondria (asterisks). Glomerular basement membrane is highlighted by a dotted line. In contrast, Nphs2.Cre+;Coq6flox/flox mutant 2,4-diHB–treated mice display normal foot process morphology. Scale bars, 2 μm left panel, 1 μm middle and right panels. (B) TEM images of 10.5-month-old mice with indicated genotypes (see A) were analyzed to determine the severity of podocyte foot process effacement. The Nphs2.Cre+;Coq6flox/flox mutant mice show significantly reduced frequency of filtration slits per micron of glomerular basement membrane, whereas Nphs2.Cre+;Coq6flox/flox mutant mice under treatment with 2,4-diHB display normal ultrastructural findings compared with littermate controls under treatment. n=2 animals in each group; two glomeruli per animal were analyzed. One-way ANOVA P values calculated using Tukey’s multiple comparisons test are shown in the figure. ****P<0.001; error bars represent mean±SD. GBM, glomerular basement membrane.
Figure 4.
Severely impaired podocyte foot process (FP) structure of Nphs2.Cre+;Coq6flox/flox mutant mice can be preserved by 2,4-diHB administration. SEM representative images in 10.5-month-old mice. Nphs2.Cre+;Coq6flox/flox mutant mice reveal severely impaired podocytic morphology with coarsened cell bodies (CBs) and major processes (MPs; arrow heads) as well as effaced FPs (asterisks). In contrast, Nphs2.Cre+;Coq6flox/flox mutant mice under treatment with 2,4-diHB display normal three-dimensional podocyte morphology, slightly enlarged MPs, and FPs retaining their normal morphology. Scale bars, 4 μm left panel, 2 μm middle and right panels.
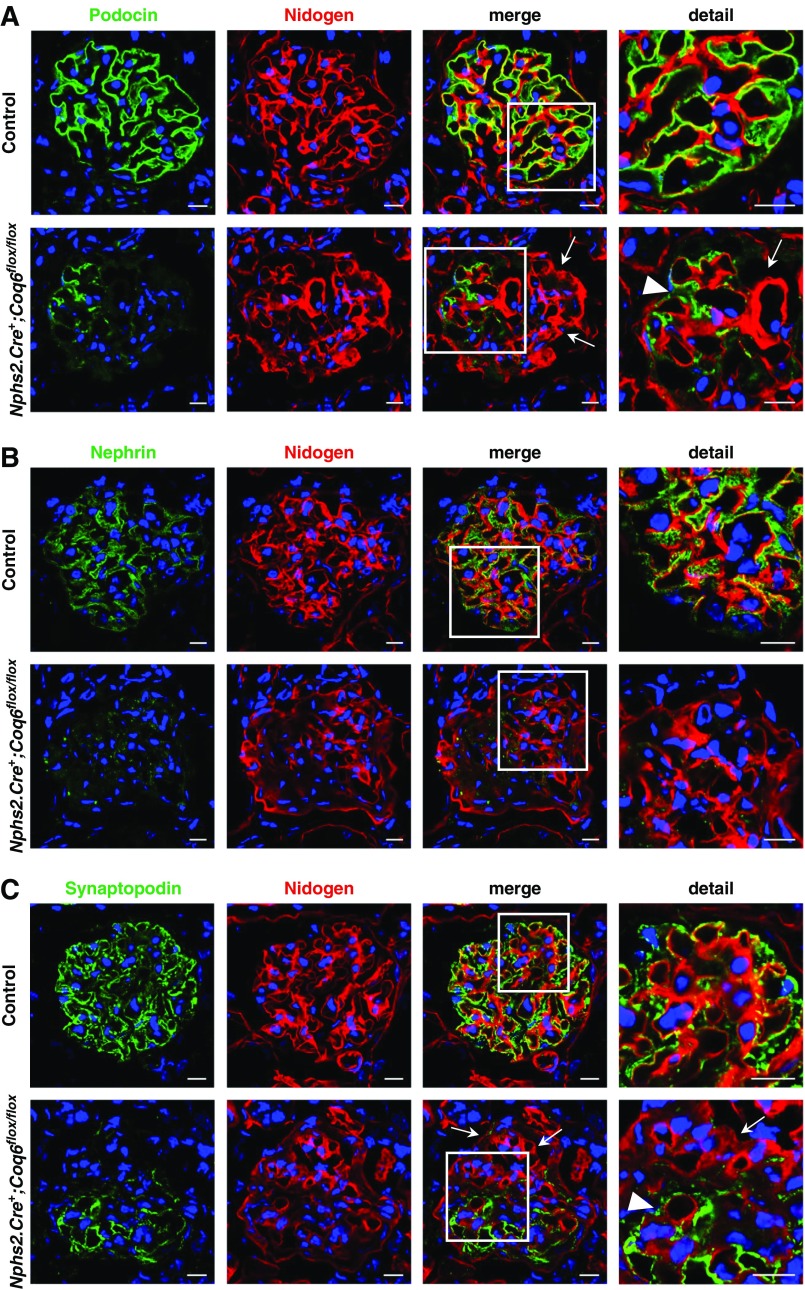
Abrogation of Coq6 Expression Leads to Podocytopathy
To characterize the molecular abnormalities in Coq6podKO glomeruli, we next analyzed the expression patterns of the slit diaphragm proteins nephrin and podocin, the basement membrane marker nidogen, and the primary process marker synaptopodin by confocal microscopy in 10.5-month-old kidneys (Figure 5). A staining pattern for various podocyte markers was significantly reduced in Coq6podKO glomeruli (Figure 5, A and C, Supplemental Figure 6), whereas the basement membrane marker nidogen showed a wider expression than normal, demonstrating that Coq6 function is required for podocyte maintenance and homeostasis. Because glomerular sclerosis is associated with increased expression of fibrotic markers, we next analyzed the expression of fibrotic markers αSMA, collagen IV, and Desmin in Coq6podKO kidneys by confocal microscopy. Indeed, Coq6podKO kidneys had significantly increased expression of αSMA, Desmin, and collagen IV in the glomeruli, characteristic of mesangial fibrosis (Supplemental Figures 7–9). We also studied the expression of WT1, a podocyte-specific transcription factor, which is critical for maintaining podocyte differentiation and maturation.36,37 In contrast to control glomeruli, the number of WT1+ podocytes was reduced in Coq6podKO glomeruli, suggesting that loss of Coq6 expression either leads to progressive depletion of podocytes (Supplemental Figure 10) or causes their dedifferentiation. Together, our data show that treatment of Coq6podKO mice with 2,4-diHB protects from disease progression and ameliorates the outcome regarding renal histology.
Figure 5.
Nphs2.Cre+;Coq6flox/flox mutant mice show reduced expression of podocyte-specific proteins. (A and B) Immunofluorescence staining of frozen kidney sections and representative images in 10.5-month-old mice for the slit diaphragm proteins (green) (A) podocin and (B) nephrin and the basement membrane marker nidogen (red). A normal expression pattern of podocin and nephrin is seen in wild-type littermate control mice. Nphs2.Cre+;Coq6flox/flox mutant mice show (A) reduced podocin staining (arrows) appearing only on a few capillary loops (arrow head), whereas (B) nephrin expression is globally reduced. Scale bars, 10 μm. (C) Staining of frozen kidney sections and representative images in 10.5-month-old mice for the podocytic foot process marker synaptopodin (green) and the basement membrane marker nidogen (red). A normal expression pattern of synaptopodin is seen in wild-type littermate control mice. Nphs2.Cre+;Coq6flox/flox mutant mice show reduced synaptopodin (green) staining (arrows) with a signal appearing only on a few capillary loops (arrow head). Scale bars, 10 μm.
Mitochondrial Deficiency Underlies the Glomerular Abnormalities in Coq6podKO Mice
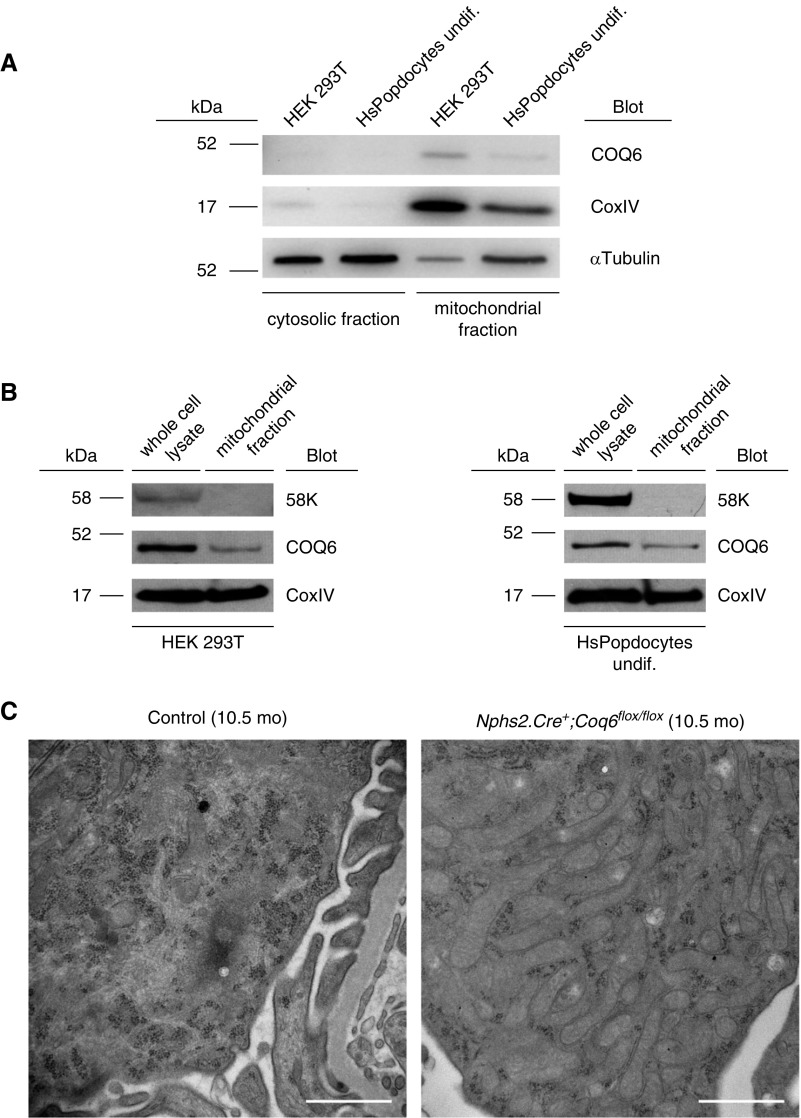
Although Coq6 function is associated with the mitochondrial CoQ10 biosynthesis pathway, the subcellular localization of Coq6 has remained controversial.21 Using cellular fractionation of HEK293 and human podocyte cell lysates, we now demonstrate that endogenous Coq6 localizes to the mitochondria (Figure 6, A and B). Consistent with Coq6's localization and function in the mitochondria, the podocytes in Coq6podKO glomeruli appeared to contain abnormal mitochondria (characterized by hyperproliferation and increased size), presumably to compensate for defective energy metabolism (Figure 6C, Supplemental Figure 11).
Figure 6.
COQ6 localizes to mitochondria and its loss from glomerular podocytes causes mitochondrial intumescence. (A) Subcellular fractions of wild-type HEK293T cell line and of wild-type undifferentiated human podocyte cell line were subjected to western blot analysis. COQ6 was detected in both cell lines, predominantly in the mitochondrial fraction. CoxIV and α-Tubulin were used as markers for mitochondrial and cytosolic subcellular fractions, respectively. Representative image of two independent experiments. (B) Mitochondrial fraction does not contain Golgi complex proteins. Whole cell lysates and the mitochondrial fraction from HEK293T and human podocytes were analyzed using antibodies against the Golgi complex protein 58K, mitochondrial protein CoxIV, and COQ6. 58K was not detected in the mitochondrial fraction, confirming that COQ6 is a mitochondrial protein. (C) TEM of podocytes in 10.5-month-old mice. Compared with the number and morphology of mitochondria in wild-type podocytes, the mitochondria in Nphs2.Cre+;Coq6flox/flox podocytes are enlarged and increased in number indicating impairment of mitochondrial function. Scale bars, 1 μm. Undif, undifferentiated.
To further characterize the role of COQ6 in podocyte function, we employed COQ6 knockdown human podocytes in a cellular migration assay that we recently established to identify compounds that modulate podocyte mobility.38 Using transient, siRNA-based COQ6 knockdown in human podocytes (Supplemental Figure 12), we observed reduced migration rate in COQ6-depleted cells compared with control podocytes (Supplemental Figure 12B), emphasizing the role of COQ6 in normal podocyte function. Supplementing 2,4-diHB to the culture medium mitigated the migration defect (Supplemental Figure 12B). Having shown that the defect in podocyte migration rate is a functional and specific readout of COQ6 deficiency, we decided to use this cellular model to test the effects of two other 4-dihydroxybenzoic acid (4-HB) analogs, 4-Hydroxy-3-methoxybenzoic acid (vanillic acid) and 3,4-Dihydroxybenzoic acid (3,4-diHB), in rescuing COQ6 deficiency. Similar to 2,4-diHB, these compounds are all highly similar to 4-HB, a precursor molecule of CoQ10 biosynthesis. Previously, it has been demonstrated that treatment with vanillic acid or 3,4-diHB improved the biosynthesis of COQ6 in a yeast model.39,40 Indeed, using vanillic acid we saw a partial reversal of the migratory defect in COQ6 knockdown cells (Supplemental Figure 12C); however, treatment with 3,4-diHB showed no positive effect to the siCOQ6 podocyte migration phenotype (Supplemental Figure 12D). Together, these data demonstrate that loss of COQ6 function in podocytes can be partially reversed by vanillic acid and fully reversed by 2,4-diHB of the tested CoQ10 intermediate precursors, at least under in vitro conditions.
Discussion
The CoQ10 biosynthesis pathway is thought to consist of sequential enzymatic steps.41 Moreover, studies in yeast have demonstrated that CoQ biosynthesis pathway enzymes assemble into a high molecular mass protein complexes, whose stability is dependent on the presence of individual peptides.42,43
There is a wide spectrum of clinical symptoms, including neurologic disorders, myopathy, and SRNS, in patients caused by deficiency of CoQ10 in consequence of mutations in a variety of genes involved in the CoQ10 biosynthesis process.18,20–23,29,44–50 On the basis of these data, it is very important to accurately identify these treatable patients as early as possible.
Several recent in vitro and in vivo studies, including ours, have demonstrated the beneficial effect of CoQ10 in improving the outcome of primary mitochondrial diseases due to CoQ10 deficiency.51,52 Because CoQ10 requires high-dose use and is not water soluble, limiting its use in cell culture systems, we decided to examine the effect of its more soluble and hydrophilic 4-HB analogs—2,4-diHB, 3,4-diHB, and vanillic acid—on podocyte function.
There are controversial data in the literature regarding the efficacy of using oral CoQ10 in patients with primary CoQ10 mitochondriopathy, likely stemming from CoQ10 tissue-specific bioavailability, its limited delivery to mitochondria, and gene-specific mutations. For example, previous studies have demonstrated the beneficial effect of oral CoQ10 for neural improvements, and in some cases partially for renal dysfunction.19,53,54 After we had demonstrated the beneficial effect of CoQ10 precursor compounds for podocyte functional improvement by migration assay we decided to investigate whether the compounds can attenuate proteinuria in Coq6podKO mice. On the basis of our data and data in the literature we selected 2,4-diHB to answer this question.55
Coq6podKO mice appeared to be normal in development and body condition. Upon the onset of significant proteinuria at age 5 months, Coq6podKO mice appeared to deteriorate gradually and became moribund at 10 months of age with advanced decline of renal function. Histologic analysis of 10-month-old Coq6podKO mice revealed progressive FSGS associated with simplified morphology of podocytes and foot process effacement. Immunofluorescence studies showed decreased expression of podocyte markers and increased expression of fibrotic markers. Coq6podKO mice treated with 2,4-diHB were protected from disease progression, and survival, proteinuria, and renal histology improved dramatically. Studies in cultured human podocytes with transient knockdown of COQ6 showed reduction in podocyte migration rate that could be completely reversed to control levels by treating the podocytes with 2,4-diHB.
Together, our data demonstrate that 2,4-diHB, an analog of 4-HB that functions to bypass certain deficiencies of the CoQ10 biosynthesis pathway, efficiently ameliorates proteinuria and prevents FSGS in Coq6podKO mice. There was a sex-specific susceptibility to proteinuria in Coq6podKO mice, with female mice being more resistant, and male mice more susceptible to proteinuria after deletion of Coq6. The reason for this is unclear. However, several aspects of siCOQ6 podocyte physiology were rescued with 4-HB analogs, with 2,4-diHB having the most robust effect. It is surprising that 2,4-diHB was most effective in the Coq6podKO mice, because this 4-HB analog was originally shown to restore CoQ biosynthesis in a coq7 yeast mutant and is designed to bypass the final hydroxylation step in CoQ biosynthesis.56
In conclusion, we report that 2,4-diHB administration markedly improves proteinuria and podocyte morphology in our Coq6podKO model of SRNS caused by primary mitochondrial dysfunction. This effect is elicited without the risk of adverse effects, because several “hydroxybenzoic acid compounds” and especially 2,4-diHB are FDA- (EAF 3045, CAS RN 89–86–1) and EFSA- (Ref. no. 00910) approved food additives. In addition, 2,4-diHB is widely present as a by-product of food processing in a variety of food products, such as snack foods, beverages, and fish products, as well as being used as an additive in some drugs, e.g., Nicardipine (NDC: 0143–9542–01). Moreover, animal studies revealed high safety of use for 2,4-diHB with LD50 of >800 mg/kg body wt,57 and there is one case report of 2,4-diHB administration to a patient with rheumatic fever in a dose of up to 6 g per day without observation of any severe side effects of treatment.58 Because of the apparent safety in preclinical and clinical use, it is very likely that patients would very well tolerate treatment with 2,4-diHB. The translational relevance of this discovery is heightened by the feasibility of therapeutic delivery. Translational studies are warranted to determine whether this type of strategy may be used to promote proteinuria remission. It would be useful to perform animal studies with a human mutation using a knock-in strategy. Growing evidence suggests that impaired mitochondrial function causes podocyte damage and leads to proteinuria. Given the present lack of effective therapies of this disease entity and the apparent safety of CoQ10, clinical trials of these compounds in genetically identified cases of SRNS seem appropriate.
Disclosures
F.H. is a cofounder of Goldfinch-Bio. No other authors have competing financial interests.
Supplementary Material
Acknowledgments
The authors thank Maria Ericsson, Louis Trakimas, Elizabeth Benecchi, and Peg Coughlin from the Electron Microscope Core Facility, Harvard Medical School, for excellent transmission electron microscopy services. We also thank Evelyn Flynn for her outstanding technical assistance and Eliso Nudelman for providing mouse cartoon illustrations. This work was performed in part at the Center for Nanoscale Systems (CNS), a member of the National Nanotechnology Coordinated Infrastructure Network, which is supported by the National Science Foundation (NSF) under NSF award no. 1541959. CNS is part of Harvard University.
This study was supported by National Institutes of Health grants to F.H. (DK076683) and R.A. (R00DK099434 and RO1DK115403), and by the National Science Foundation Grant MCB-1330803 (to C.F.C.). F.H. is the William E. Harmon Professor. E.W. is supported by the Leopoldina Fellowship Program, German National Academy of Sciences Leopoldina (LPDS 2015-07)
E.W., M.A., H.H., D.S., J.W., C.C.G., R.S., J.C., and M.S. carried out the animal experiments. E.W., M.A., A.M.A., A.N., C.F.C., and R.A. carried out the cell experiments. E.W., R.A., and F.H. conceived of and directed the project. E.W. and R.A. wrote the paper with help from F.H. The manuscript was critically reviewed by all of the authors.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018060625/-/DCSupplemental.
Supplemental Figure 1. Generation and genotyping strategy for Nphs2.Cre+;Coq6flox/flox mouse model.
Supplemental Figure 2. At 5 months of age Nphs2.Cre+;Coq6flox/flox mice exhibit proteinuria and renal histopathologic changes consistent with FSGS.
Supplemental Figure 3. Coq6 knockout in Nphs2.Cre+;Coq6flox/flox mutant mice leads to reduced physical condition and macroscopic morphologic changes of kidneys.
Supplemental Figure 4. Coq6 knockout in Nphs2.Cre+;Coq6flox/flox mutant mice causes FSGS. Treatment with 2.4-diHB prevents disease progression, resulting in normal histologic findings.
Supplemental Figure 5. Electron microscopy reveals podocyte foot process effacement in Nphs2.Cre+;Coq6flox/flox mutant mice at 5 months of age.
Supplemental Figure 6. Quantitative analysis of the expression of podocyte-specific proteins in Nphs2.Cre+;Coq6flox/flox glomeruli.
Supplemental Figure 7. Nphs2.Cre+;Coq6flox/flox mice show increased glomerular fibrosis and staining for mesangial markers.
Supplemental Figure 8. Quantitative analysis of the expression of the fibrotic markers αSMA and Desmin in Nphs2.Cre+;Coq6flox/flox glomeruli.
Supplemental Figure 9. Nphs2.Cre+;Coq6flox/flox mice develop renal fibrosis.
Supplemental Figure 10. Nphs2.Cre+;Coq6flox/flox mice show a reduced staining of podocyte-specific markers.
Supplemental Figure 11. Deletion of Coq6 leads to morphologic abnormalities in podocyte mitochondria.
Supplemental Figure 12. COQ6 siRNA-mediated transient knockdown reduces podocyte migration rate of cultured human podocytes with full rescue by 2,4-diHB, partial rescue by vanillic acid, and absent rescue by 3,4-diHB.
References
- 1.Smith JM, Stablein DM, Munoz R, Hebert D, McDonald RA: Contributions of the transplant registry: The 2006 annual report of the North American pediatric Renal Trials and collaborative Studies (NAPRTCS). Pediatr Transplant 11: 366–373, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Lovric S, Ashraf S, Tan W, Hildebrandt F: Genetic testing in steroid-resistant nephrotic syndrome: When and how? Nephrol Dial Transplant 31: 1802–1813, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim JS, Bellew CA, Silverstein DM, Aviles DH, Boineau FG, Vehaskari VM: High incidence of initial and late steroid resistance in childhood nephrotic syndrome. Kidney Int 68: 1275–1281, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Benoit G, Machuca E, Antignac C: Hereditary nephrotic syndrome: A systematic approach for genetic testing and a review of associated podocyte gene mutations. Pediatr Nephrol 25: 1621–1632, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trautmann A, Bodria M, Ozaltin F, Gheisari A, Melk A, Azocar M, et al.: PodoNet Consortium : Spectrum of steroid-resistant and congenital nephrotic syndrome in children: The PodoNet registry cohort. Clin J Am Soc Nephrol 10: 592–600, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vivante A, Hildebrandt F: Exploring the genetic basis of early-onset chronic kidney disease. Nat Rev Nephrol 12: 133–146, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Somlo S, Mundel P: Getting a foothold in nephrotic syndrome. Nat Genet 24: 333–335, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Zenker M, Machuca E, Antignac C: Genetics of nephrotic syndrome: New insights into molecules acting at the glomerular filtration barrier. J Mol Med (Berl) 87: 849–857, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Imasawa T, Rossignol R: Podocyte energy metabolism and glomerular diseases. Int J Biochem Cell Biol 45: 2109–2118, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Abe Y, Sakairi T, Kajiyama H, Shrivastav S, Beeson C, Kopp JB: Bioenergetic characterization of mouse podocytes. Am J Physiol Cell Physiol 299: C464–C476, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mele C, Iatropoulos P, Donadelli R, Calabria A, Maranta R, Cassis P, et al.: PodoNet Consortium : MYO1E mutations and childhood familial focal segmental glomerulosclerosis. N Engl J Med 365: 295–306, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown EJ, Schlöndorff JS, Becker DJ, Tsukaguchi H, Tonna SJ, Uscinski AL, et al.: Mutations in the formin gene INF2 cause focal segmental glomerulosclerosis. Nat Genet 42: 72–76, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaplan JM, Kim SH, North KN, Rennke H, Correia LA, Tong HQ, et al.: Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet 24: 251–256, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Heath KE, Campos-Barros A, Toren A, Rozenfeld-Granot G, Carlsson LE, Savige J, et al.: Nonmuscle myosin heavy chain IIA mutations define a spectrum of autosomal dominant macrothrombocytopenias: May-Hegglin anomaly and Fechtner, Sebastian, Epstein, and Alport-like syndromes. Am J Hum Genet 69: 1033–1045, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zenker M, Aigner T, Wendler O, Tralau T, Müntefering H, Fenski R, et al.: Human laminin beta2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Hum Mol Genet 13: 2625–2632, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Has C, Spartà G, Kiritsi D, Weibel L, Moeller A, Vega-Warner V, et al.: Integrin α3 mutations with kidney, lung, and skin disease. N Engl J Med 366: 1508–1514, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kambham N, Tanji N, Seigle RL, Markowitz GS, Pulkkinen L, Uitto J, et al.: Congenital focal segmental glomerulosclerosis associated with beta4 integrin mutation and epidermolysis bullosa. Am J Kidney Dis 36: 190–196, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Rötig A, Appelkvist EL, Geromel V, Chretien D, Kadhom N, Edery P, et al.: Quinone-responsive multiple respiratory-chain dysfunction due to widespread coenzyme Q10 deficiency. Lancet 356: 391–395, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Salviati L, Sacconi S, Murer L, Zacchello G, Franceschini L, Laverda AM, et al.: Infantile encephalomyopathy and nephropathy with CoQ10 deficiency: A CoQ10-responsive condition. Neurology 65: 606–608, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Quinzii C, Naini A, Salviati L, Trevisson E, Navas P, Dimauro S, et al.: A mutation in para-hydroxybenzoate-polyprenyl transferase (COQ2) causes primary coenzyme Q10 deficiency. Am J Hum Genet 78: 345–349, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heeringa SF, Chernin G, Chaki M, Zhou W, Sloan AJ, Ji Z, et al.: COQ6 mutations in human patients produce nephrotic syndrome with sensorineural deafness. J Clin Invest 121: 2013–2024, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diomedi-Camassei F, Di Giandomenico S, Santorelli FM, Caridi G, Piemonte F, Montini G, et al.: COQ2 nephropathy: A newly described inherited mitochondriopathy with primary renal involvement. J Am Soc Nephrol 18: 2773–2780, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Ashraf S, Gee HY, Woerner S, Xie LX, Vega-Warner V, Lovric S, et al.: ADCK4 mutations promote steroid-resistant nephrotic syndrome through CoQ10 biosynthesis disruption. J Clin Invest 123: 5179–5189, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peng M, Falk MJ, Haase VH, King R, Polyak E, Selak M, et al.: Primary coenzyme Q deficiency in Pdss2 mutant mice causes isolated renal disease. PLoS Genet 4: e1000061, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ernster L, Dallner G: Biochemical, physiological and medical aspects of ubiquinone function. Biochim Biophys Acta 1271: 195–204, 1995 [DOI] [PubMed] [Google Scholar]
- 26.Lenaz G, Genova ML: Mobility and function of coenzyme Q (ubiquinone) in the mitochondrial respiratory chain. Biochim Biophys Acta 1787: 563–573, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Quinzii CM, López LC, Gilkerson RW, Dorado B, Coku J, Naini AB, et al.: Reactive oxygen species, oxidative stress, and cell death correlate with level of CoQ10 deficiency. FASEB J 24: 3733–3743, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bentinger M, Brismar K, Dallner G: The antioxidant role of coenzyme Q. Mitochondrion 7[Suppl]: S41–S50, 2007 [DOI] [PubMed] [Google Scholar]
- 29.López LC, Schuelke M, Quinzii CM, Kanki T, Rodenburg RJ, Naini A, et al.: Leigh syndrome with nephropathy and CoQ10 deficiency due to decaprenyl diphosphate synthase subunit 2 (PDSS2) mutations. Am J Hum Genet 79: 1125–1129, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moeller MJ, Sanden SK, Soofi A, Wiggins RC, Holzman LB: Podocyte-specific expression of cre recombinase in transgenic mice. Genesis 35: 39–42, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Takemoto M, Asker N, Gerhardt H, Lundkvist A, Johansson BR, Saito Y, et al.: A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol 161: 799–805, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saleem MA, O’Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, et al.: A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol 13: 630–638, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Gee HY, Saisawat P, Ashraf S, Hurd TW, Vega-Warner V, Fang H, et al.: ARHGDIA mutations cause nephrotic syndrome via defective RHO GTPase signaling. J Clin Invest 123: 3243–3253, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luna-Sánchez M, Díaz-Casado E, Barca E, Tejada MA, Montilla-García Á, Cobos EJ, et al.: The clinical heterogeneity of coenzyme Q10 deficiency results from genotypic differences in the Coq9 gene. EMBO Mol Med 7: 670–687, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, Oxer D, Hekimi S: Mitochondrial function and lifespan of mice with controlled ubiquinone biosynthesis. Nat Commun 6: 6393, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mundlos S, Pelletier J, Darveau A, Bachmann M, Winterpacht A, Zabel B: Nuclear localization of the protein encoded by the Wilms’ tumorgene WT1 in embryonic and adult tissues. Development 119: 1329–1341, 1993 [DOI] [PubMed] [Google Scholar]
- 37.Kreidberg JA, Sariola H, Loring JM, Maeda M, Pelletier J, Housman D, et al.: WT-1 is required for early kidney development. Cell 74: 679–691, 1993 [DOI] [PubMed] [Google Scholar]
- 38.Widmeier E, Tan W, Airik M, Hildebrandt F: A small molecule screening to detect potential therapeutic targets in human podocytes. Am J Physiol Renal Physiol 312: F157–F171, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doimo M, Trevisson E, Airik R, Bergdoll M, Santos-Ocaña C, Hildebrandt F, et al.: Effect of vanillic acid on COQ6 mutants identified in patients with coenzyme Q10 deficiency. Biochim Biophys Acta 1842: 1–6, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ozeir M, Mühlenhoff U, Webert H, Lill R, Fontecave M, Pierrel F: Coenzyme Q biosynthesis: Coq6 is required for the C5-hydroxylation reaction and substrate analogs rescue Coq6 deficiency. Chem Biol 18: 1134–1142, 2011 [DOI] [PubMed] [Google Scholar]
- 41.Yen HC, Liu YC, Kan CC, Wei HJ, Lee SH, Wei YH, et al.: Disruption of the human COQ5-containing protein complex is associated with diminished coenzyme Q10 levels under two different conditions of mitochondrial energy deficiency. Biochim Biophys Acta 1860: 1864–1876, 2016 [DOI] [PubMed] [Google Scholar]
- 42.Tran UC, Clarke CF: Endogenous synthesis of coenzyme Q in eukaryotes. Mitochondrion 7[Suppl]: S62–S71, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hsieh EJ, Gin P, Gulmezian M, Tran UC, Saiki R, Marbois BN, et al.: Saccharomyces cerevisiae Coq9 polypeptide is a subunit of the mitochondrial coenzyme Q biosynthetic complex. Arch Biochem Biophys 463: 19–26, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Hekimi S: Molecular genetics of ubiquinone biosynthesis in animals. Crit Rev Biochem Mol Biol 48: 69–88, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salviati L, Trevisson E, Rodriguez Hernandez MA, Casarin A, Pertegato V, Doimo M, et al.: Haploinsufficiency of COQ4 causes coenzyme Q10 deficiency. J Med Genet 49: 187–191, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mollet J, Giurgea I, Schlemmer D, Dallner G, Chretien D, Delahodde A, et al.: Prenyldiphosphate synthase, subunit 1 (PDSS1) and OH-benzoate polyprenyltransferase (COQ2) mutations in ubiquinone deficiency and oxidative phosphorylation disorders. J Clin Invest 117: 765–772, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mollet J, Delahodde A, Serre V, Chretien D, Schlemmer D, Lombes A, et al.: CABC1 gene mutations cause ubiquinone deficiency with cerebellar ataxia and seizures. Am J Hum Genet 82: 623–630, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lagier-Tourenne C, Tazir M, López LC, Quinzii CM, Assoum M, Drouot N, et al.: ADCK3, an ancestral kinase, is mutated in a form of recessive ataxia associated with coenzyme Q10 deficiency. Am J Hum Genet 82: 661–672, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duncan AJ, Bitner-Glindzicz M, Meunier B, Costello H, Hargreaves IP, López LC, et al.: A nonsense mutation in COQ9 causes autosomal-recessive neonatal-onset primary coenzyme Q10 deficiency: A potentially treatable form of mitochondrial disease. Am J Hum Genet 84: 558–566, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hirano M, Garone C, Quinzii CM: CoQ(10) deficiencies and MNGIE: Two treatable mitochondrial disorders. Biochim Biophys Acta 1820: 625–631, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saiki R, Lunceford AL, Shi Y, Marbois B, King R, Pachuski J, et al.: Coenzyme Q10 supplementation rescues renal disease in Pdss2kd/kd mice with mutations in prenyl diphosphate synthase subunit 2. Am J Physiol Renal Physiol 295: F1535–F1544, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vazquez Fonseca L, Doimo M, Calderan C, Desbats MA, Acosta MJ, Cerqua C, et al.: Mutations in COQ8B (ADCK4) found in patients with steroid-resistant nephrotic syndrome alter COQ8B function. Hum Mutat 39: 406–414, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Montini G, Malaventura C, Salviati L: Early coenzyme Q10 supplementation in primary coenzyme Q10 deficiency. N Engl J Med 358: 2849–2850, 2008 [DOI] [PubMed] [Google Scholar]
- 54.Atmaca M, Gulhan B, Korkmaz E, Inozu M, Soylemezoglu O, Candan C, et al.: Follow-up results of patients with ADCK4 mutations and the efficacy of CoQ10 treatment. Pediatr Nephrol 32: 1369–1375, 2017 [DOI] [PubMed] [Google Scholar]
- 55.Freyer C, Stranneheim H, Naess K, Mourier A, Felser A, Maffezzini C, et al.: Rescue of primary ubiquinone deficiency due to a novel COQ7 defect using 2,4-dihydroxybensoic acid. J Med Genet 52: 779–783, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xie LX, Ozeir M, Tang JY, Chen JY, Jaquinod SK, Fontecave M, et al. : Overexpression of the Coq8 kinase in Saccharomyces cerevisiae coq null mutants allows for accumulation of diagnostic intermediates of the coenzyme Q6 biosynthetic pathway. J Biol Chem 287: 23571–23581, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grady RW, Graziano JH, Akers HA, Cerami A: The development of new iron-chelating drugs. J Pharmacol Exp Ther 196: 478–485, 1976 [PubMed] [Google Scholar]
- 58.Clarke NE, Clarke CN, Mosher RE: Phenolic compounds in chemotherapy of rheumatic fever. Am J Med Sci 235: 7–22, 1958 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.