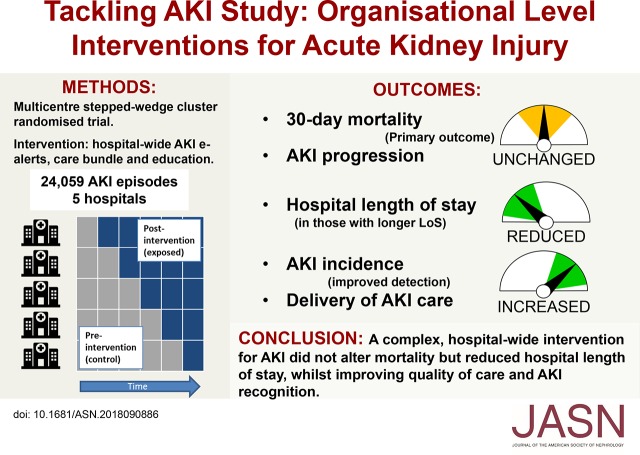
Significance Statement
National and international guidelines recommend supportive approaches to AKI management, but organizational strategies to improve delivery of AKI care have not previously been tested in multicenter randomized studies. The authors describe a pragmatic, multicenter, cluster randomized trial across five United Kingdom hospitals of an intervention comprising an AKI detection and e-alert system, an AKI care bundle, and an educational program for health care workers. Although the intervention did not alter the primary outcome of 30-day mortality, it was associated with reductions in length of hospital stay as well as an increase in AKI incidence that likely reflected improved recognition. These results combined with previous evidence show that strategies to improve the systematic delivery of supportive AKI care can lead to improvements in patient outcomes.
Keywords: acute renal failure, clinical nephrology, e-alert, care bundle, education, AKI
Visual Abstract
Abstract
Background
Variable standards of care may contribute to poor outcomes associated with AKI. We evaluated whether a multifaceted intervention (AKI e-alerts, an AKI care bundle, and an education program) would improve delivery of care and patient outcomes at an organizational level.
Methods
A multicenter, pragmatic, stepped-wedge cluster randomized trial was performed in five UK hospitals, involving patients with AKI aged ≥18 years. The intervention was introduced sequentially across fixed three-month periods according to a randomly determined schedule until all hospitals were exposed. The primary outcome was 30-day mortality, with pre-specified secondary endpoints and a nested evaluation of care process delivery. The nature of the intervention precluded blinding, but data collection and analysis were independent of project delivery teams.
Results
We studied 24,059 AKI episodes, finding an overall 30-day mortality of 24.5%, with no difference between control and intervention periods. Hospital length of stay was reduced with the intervention (decreases of 0.7, 1.1, and 1.3 days at the 0.5, 0.6, and 0.7 quantiles, respectively). AKI incidence increased and was mirrored by an increase in the proportion of patients with a coded diagnosis of AKI. Our assessment of process measures in 1048 patients showed improvements in several metrics including AKI recognition, medication optimization, and fluid assessment.
Conclusions
A complex, hospital-wide intervention to reduce harm associated with AKI did not reduce 30-day AKI mortality but did result in reductions in hospital length of stay, accompanied by improvements in in quality of care. An increase in AKI incidence likely reflected improved recognition.
AKI is common, and it is associated with markedly elevated short-term morbidity and mortality, subsequent risk of CKD, and large increases in health care resource utilization.1 AKI occurs in 5%–22% of hospital admissions, and mortality rates exceed 20%, rising to >50% in those most severely affected.2 In the absence of specific therapies, AKI management requires methodical delivery of basic elements of care.3 Despite universal recommendation of this approach in national and international guidelines,4–6 successive reports have described variation in the quality of clinical care for AKI, with poor standards of care associated with worse outcomes.7–10 Although there are no proven interventions for AKI, the evidence base to support organizational-level interventions to address variations in AKI care is also lacking. In the only previous randomized trial, a text message alert for AKI did not change physician behavior or patient outcomes, possibly because the alert was introduced without recommendations for care or other interventions.11 Conversely, several nonrandomized studies testing broader interventions, generally using before-after comparisons, have shown more positive results, including reductions in mortality, although methodological concerns prevent firm conclusions from being made.12–15 Additionally, all but one of these studies are single center, and therefore, they do not inform on whether successful interventions retain effectiveness if scaled to other organizations. We, therefore, sought to address some of these knowledge gaps by performing a multicenter, randomized trial to test the hypothesis that a complex intervention for AKI (comprising AKI e-alerts, an AKI care bundle, and a program of AKI education) would improve standards of care delivery and lead to better patient outcomes.
Methods
Study Design and Participants
Over a 27-month period, we conducted a multicenter, pragmatic, stepped wedge cluster randomized trial (SWCRT). The study was conducted using a published protocol,16 which was consistent with the extension to cluster randomized trials of the Consolidated Standards Of Reporting Trials 2010 document17 and recommendations for SWCRTs.18 The protocol and statistical analysis plan were published on the National Health Service (NHS) England Think Kidneys Program website19 and are included in Supplemental Material.
The SWCRT design allowed for differentiation between the effect of the intervention and independent time-related factors while avoiding ethical concerns around withholding treatment in line with minimum care standards, with all sites exposed to the intervention by study end. Cluster randomization avoided contamination of the control group that would likely occur with randomization at a patient level.
The intervention, designed to reduce avoidable harm associated with AKI, was introduced across five NHS hospital sites representing academic and nonacademic centers as well as those with and without onsite nephology services. Data collection and analysis were conducted independently by researchers not involved in the delivery of the intervention at the participating hospitals.
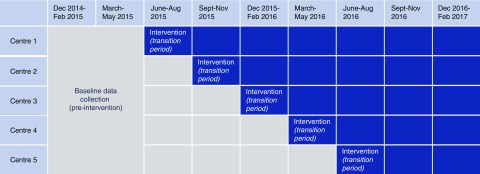
The SWCRT design involved delivery of the intervention sequentially to one hospital at a time across fixed 3-month periods until all five hospitals were exposed to the intervention (Figure 1). A 6-month baseline period before any of the sites introduced the intervention was followed by five 3-month implementation steps (one hospital per step). The 3-month time period during which a site introduced the intervention, when it was expected not to have reached full effect, was considered a transition period and excluded from analyses. All sites had a minimum of one 3-month period of exposure to the intervention after the transition period.
Figure 1.
Schematic of the stepped wedge study design. After a 6-month period of baseline data collection, the intervention (hospital-wide AKI e-alert, care bundle, and education program) was sequentially introduced to participating sites across fixed 3-month periods of time until all sites were exposed to the intervention. Data collection occurred at each step of the wedge, including in the postintervention period. The 3-month time period during which a site introduced the intervention, when it was expected not to have reached full effect on outcomes, was considered a transition period and excluded from analyses. All sites had a minimum of one 1-month period of exposure to the intervention after the transition period. The sequence was determined by random number generation, and the order of the hospitals was as follows: (1) Frimley, (2) Bradford, (3) Ashford and St. Peters, (4) Leeds General Infirmary, and (5) Leeds St. James.
We included all patients ages ≥18 years old who were hospitalized for at least one night during the study period and sustained AKI during that admission. Patients were defined as having AKI if they had an inpatient serum creatinine result consistent with a modified Kidney Disease Improving Global Outcomes (KDIGO) definition of AKI as identified by the NHS England algorithm. A full description of the algorithm has been published previously,20 but in brief, the algorithm applies the KDIGO criteria to an individual’s current serum creatinine value using a baseline value defined as either the lowest in the last 7 days or a median of values from the preceding 8 to 365 days depending on availability of previous results. Urine output was not used to define AKI for pragmatic reasons. The only exclusion criterion was chronic dialysis for ESRD. The Derbyshire Research Ethics Committee designated the study as service improvement and waived the requirement for individual patient consent. Transfer and collation of patient data by the United Kingdom Renal Registry (UKRR) were approved by the Health Research Authority under section 251 of the NHS Act 2006.
Randomization and Blinding
The unit of randomization (the cluster) was the participating hospital. Randomization was performed by the UKRR and took place on the 11th of May 2015 using random number generation (SAS-9.3, RANUNI function). The first hospital commenced implementation in June 2015. There were no delays to the SWCRT sequence. Because of the nature of the intervention, blinding was not possible.
Intervention
The intervention had three components designed to improve AKI recognition and the delivery of basic elements of AKI care as recommended by National Institute for Health and Care Excellence Clinical Guideline CG169 and other national and international guidelines.4–6,21 The components of the intervention were
An AKI electronic detection and alerting system
An AKI care bundle containing individual elements pertaining to assessment, investigation, and basic management of AKI (summarized in Table 1)22
An educational program to raise awareness and knowledge of AKI in health care workers (summarized in Table 2)
Introduction of the intervention was supported by a structured approach to change management described elsewhere.16 This included permissive tailoring of the elements of the intervention to fit each hospital’s local context, but the same basic elements were present across all sites. The electronic AKI detection system was uniform across all sites, conforming with a nationally mandated specification.20 Audit during the setup phase ensured that the algorithm was running correctly in each laboratory. The detection algorithm ran at all sites throughout the study period, with alerts being released to clinicians at the point when the hospital was randomized to introduce the intervention. The alert message notified the health care professionals that the patient had sustained AKI and the stage of AKI, and it included an advice message advising a clinical response/review of the patient and sign posting of local AKI resources (guidelines and care bundles). All sites also adopted an active element to the alert in that the duty biochemist would telephone AKI stage 2 and 3 results to the clinical areas from which the blood tests were sent (as opposed to a purely “passive” alert within the results reporting system that relies on clinicians seeing the result autonomously).
Table 1.
Core elements that were included in care bundles at each of the Tacking AKI study sites
| Core Elements of the AKI Care Bundle Common across All Sites |
|---|
| Assess volume status and optimize BP |
| Treat sepsis |
| Review medications and stop those contributing to AKI |
| Perform urinalysis |
| Referral (to nephrology or critical care outreach) for AKI stage 3, AKI with complications |
Sites were permitted to tailor the appearance of care bundles, and some sites included additional elements (e.g., additional investigations into cause of AKI, management of hyperkalemia, and informing patients of the presence of AKI).
Table 2.
Description of educational program activities that were delivered across sites
| Type of Education Session | No. of Sessions per Center | Target Audience | Audience Size | Duration |
|---|---|---|---|---|
| Launch event | 1 | All members of staff welcome; hospital chief executive, medical director, chief nurse attended | 30–50 | 1 h |
| Hospital grand roundsa | 2 | All grades of physicians, doctors in training and open to those in other specialties who wish to attend | 40–80 | 1 h |
| Departmental educational or clinical governance meetings | 3–8 | Departmental teaching to a range of specialties (e.g., emergency medicine, acute medicine, surgery, urology, rheumatology, elderly care) | 10–20 | 1 h |
| Postgraduate teaching for doctors in traininga | 3/yr (1 for each grade of doctor) | AKI teaching as part of curriculum (essential teaching) for doctors in training, attendance often mandatory | 20–40 | 1–2 h |
| Induction teaching for new staffa | 1–3 | Shorter sessions, more focused on process rather than education per se | 20–40 | 15 min |
| Nursing, pharmacy, and advanced practitioner teaching | 2–3 | Varied between centers from small group teaching to formal AKI study day for large groups | 5–70 | 1 h to whole day |
| Ward-based teaching sessions | 5–10 | Formal teaching sessions at ward level | 1–10 | 5–30 min |
| Ad hoc teaching sessions | 20+ | Informal teaching delivered by various members of the AKI team, included reminders of resources, patient-based teaching | 1–3 | Varied, usually only minutes |
Other activities included publicity activities and e-learning/use of online teaching videos.
Activities that were already in place before the study.
The care bundles at each site all contained the same core elements (Table 1), although initial care bundle content and design were refined in response to end user feedback during the first 3 months of use. This led to a degree of variation in the number of actionable items in the care bundles between hospitals. Care bundles were delivered in paper form, and they were integrated into patients’ hospital notes apart from one center, where the care bundle was in electronic form.
Education was mainly delivered by face to face teaching across a number of different settings, but also, it included the development of educational materials, e-learning, and awareness raising. Formal teaching sessions were typically delivered using PowerPoint presentations, whereas ad hoc or opportunistic teaching on wards was focused around real time patient examples or sign posting project resources. A summary of educational activities that were delivered in each hospital is shown in Table 2.
The intervention was delivered by an AKI project team at each hospital, which consisted of staff provided by the central team (project managers M.J., N.J., and C.J.), principle investigators (J.S., Y.S., A.J.L., R.R., and N.S.), and hospital staff not funded by the project.
Outcomes
The primary outcome was 30-day mortality after an episode of AKI comparing control and intervention periods. Predefined secondary outcomes included incidence of hospital-acquired AKI, AKI progression to higher stages, incidence of individual AKI stages, and length of hospital stay (LoS).16 We defined hospital-acquired AKI as that with its onset >24 hours after hospital admission, and AKI progression was defined as an increase of one or more AKI stages from time of detection.23 After LoS analysis, a post hoc analysis was undertaken for duration of AKI (calculated as days between first and last serum creatinine results that met the definition of AKI). Technical issues prevented data collection for two prespecified secondary end points (number of critical care bed days and renal recovery).
Outcome data were collected using biochemical results to identify episodes of AKI, which were then linked to data from each hospital’s patient administration system to determine patient identifiers and demographics, dates of admission and discharge, all diagnosis codes from the index admission (as per the International Classification of Diseases, Tenth Revision [ICD-10] and the Charlson comorbidity score),24 and date of death. These data were transferred directly to the UKRR from each site independent of the study teams, and they were analyzed by an independent statistician. NHS tracing was performed by the UKRR at the end of the study to identify any additional out of hospital deaths. Summary data for each hospital were generated for each 3-month period for total number of adult admissions grouped by age, sex, and ethnicity to allow for calculation of AKI incidence. In September 2016, there was an computer systems failure of the laboratory information management system (LIMS) that served three of the participating hospitals. This meant that the AKI detection algorithm was not available, and laboratory data collection was not possible during this period. For this reason, the trial was extended to allow for an extra period of data collection (December 2016 to February 2017) so that the planned number of data collection blocks was achieved; data from the affected period were excluded.
Process outcomes included the proportion of patients receiving elements of basic care (AKI recognition, fluid assessment, medication review, investigation, senior clinician/specialty review, and care bundle usage) as determined by repeated cycles of clinical audit (30 sequential patients per site from each 3-month data collection period, giving a planned sample of 1050 patient notes evenly distributed across AKI stages 1–3). A standard data collection form and a data specification sheet were used; these are included as Supplemental Material.
Sample Size Calculation
An a priori sample size calculation was undertaken.25 The total number of annual hospital admissions across the five sites (434,000) was taken from the Health and Social Care Information Centre (www.hscic.gov.uk; April 2014 to March 2015). The most conservative published rates for assumptions of the proportions of hospital admissions with AKI (2.5%)26 and 30-day mortality (16%)27 were used. Power was set at 80%, α was set at 0.05, and a range of values for intracluster correlation between 0.01 and 0.2 was considered. With a trial study time of 2 years, five participating sites (one per randomization step), one transition period per site, and the design effect of the SWCRT,25 we calculated that, to detect an absolute decrease in 30-day mortality of 3.2%,12,13 10,850 AKI episodes should be studied.
Statistical Analyses
Analysis of 30-day mortality was undertaken using multilevel logistic regression at the individual patient level, with hospital modeled as a random effect and adjusting for time, patients’ covariables (age, sex, and comorbid conditions), and the effect of seasonality. We pooled time into quarterly intervals treated as equally spaced in analytic models. Only first hospitalizations in those patients with multiple AKI episodes were included; results were similar when analyses used last or multiple episodes per patient. The primary outcome response was the estimated mortality odds ratio for the intervention versus the control period.
Secondary analyses were also undertaken at the individual patient level, again adjusting for time, patients’ covariables (age, sex, and comorbid conditions), cluster (hospital), and the effect of seasonality. AKI incidence was calculated using the total number of overnight hospitalization episodes within each time period as the denominator and analyzed using multilevel negative binomial regression. AKI progression was analyzed as a binary outcome for each overnight hospitalization episode using multilevel logistic regression as for the primary outcome (excluding AKI stage 3). The hospital LoS and AKI duration data were highly skewed, and the fit of prespecified Poisson and negative binomial regression models was poor (inadequate correlation between observed versus predicted values). Therefore, quantile regression models were fitted to allow for comparisons at points across the whole distribution (after adjustment for age, sex, comorbid conditions, time, season, and center) in addition to comparison of average values; this approach does not make assumptions about the distribution of the dataset and is robust against the presence of gross outliers.28,29 For LoS analyses, only patients who survived to hospital discharge were included. Statistical analyses were conducted at the UKRR in collaboration with the University of Bristol using Stata MP12 and SAS 9.3.
Results
During the study period, there were a total of 316,413 hospital admissions from which a total of 24,059 AKI episodes occurred in 20,179 patients, giving a crude incidence of 7.6 AKI episodes per 100 admissions. During the control period, there were 14,042 episodes (58.4%), with 10,017 (41.6%) in the intervention period. The distribution across AKI stages was as follows: 62% of episodes were AKI stage 1, 21% were stage 2, and 17% were stage 3; 12,507 episodes (52%) were hospital acquired. Patient demographics in control and intervention periods are shown in Table 3, and data for individual hospitals are shown in Supplemental Table 1. Differences in the populations served by each site and the SWCRT design (meaning that sites contributed different amounts of data to control and exposed periods depending on their place in the randomization sequence) resulted in differences in patient demographics between control and intervention periods. These differences between control and intervention periods were not seen when comparing patient demographics at a hospital level. We also observed a significant effect of season on AKI incidence, with higher AKI rates observed during winter (rate ratio in winter [December to February], 1.08; 95% confidence interval [95% CI], 1.02 to 1.13; P<0.01 compared with spring [March to May]). Outcome analyses were adjusted for these covariables.
Table 3.
Patient demographics in control and intervention periods
| Characteristics | Control | Intervention |
|---|---|---|
| No. of AKI episodes | 14,042 | 10,017 |
| Men, % | 50.3 | 48.1 |
| Age group, yr, % | ||
| 18–59 | 23.1 | 20.3 |
| 60–69 | 15.7 | 15.3 |
| 70–79 | 23.7 | 23.5 |
| 80–89 | 27.2 | 29.8 |
| 90+ | 10.3 | 11.1 |
| Median age, yr | 75.4 | 76.6 |
| Charlson comorbidity score, % | ||
| 0 | 16.4 | 18.8 |
| 1 | 20.3 | 21.0 |
| 2 | 20.2 | 19.4 |
| 3+ | 43.1 | 40.8 |
| Individual comorbidities, % | ||
| Previous myocardial infarction | 15.1 | 14.4 |
| Heart failure | 23.0 | 22.6 |
| Previous stroke | 7.0 | 6.9 |
| Diabetes mellitus | 27.3 | 28.1 |
| CKD | 22.0 | 23.5 |
| Chronic liver disease | 8.8 | 7.0 |
| Ethnicity, % | ||
| Afro-Caribbean | 1.4 | 0.8 |
| South Asian | 5.5 | 5.9 |
| Other | 2.8 | 2.8 |
| White | 86.1 | 85.3 |
| Missing | 4.2 | 5.2 |
| Social deprivation score,a % | ||
| 1 (least deprived) | 23.6 | 36.4 |
| 2 | 17.8 | 16.7 |
| 3 | 16.0 | 15.8 |
| 4 | 15.7 | 13.3 |
| 5 (most deprived) | 26.8 | 17.6 |
| Missing | 0.1 | 0.2 |
| Peak AKI stage, % per stage | ||
| 1 | 60.6 | 64.5 |
| 2 | 21.4 | 19.8 |
| 3 | 18.0 | 15.7 |
| Hospital-acquired AKI,b % | 53.8 | 49.4 |
Unadjusted data are shown, and differences between control and intervention populations largely reflect the different amounts of data submitted to control and intervention periods as a result of the stepped wedge cluster randomized trial design. There were no major differences between control and intervention periods (including in AKI severity) when patient demographics were analyzed at a hospital level; these data are available in Supplemental Material.
Social deprivation scores show the proportion of patients in each quintile of the Index of Multiple Deprivation.
Hospital-acquired AKI is defined as AKI onset >24 hours after hospital admission.
The 30-Day Mortality
Crude 30-day mortality across the entire study period was 24.5%; 30-day mortality was not affected by the intervention. In the fully adjusted model (Table 4), the odds ratio for 30-day mortality in the intervention period versus the control period was 1.04 (95% CI, 0.91 to 1.21; P=0.55). Analyses performed for individual AKI stages and for community- and hospital-acquired AKI separately also did not show any difference in 30-day mortality between intervention and control periods.
Table 4.
Results of multilevel logistic regression for mortality
| Parameter | Odds Ratio | 95% CI | P Value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Intervention (reference = control period) | 1.04 | 0.91 | 1.21 | 0.55 |
| Time (linear trend) | 1.00 | 0.91 | 1.10 | 0.97 |
| Season (reference = spring) | ||||
| Summer | 0.88 | 0.79 | 0.98 | 0.02 |
| Autumn | 1.03 | 0.91 | 1.17 | 0.61 |
| Winter | 1.13 | 1.04 | 1.22 | <0.01 |
| Age group (reference =80+), yr | ||||
| 18–34 | 0.15 | 0.11 | 0.20 | <0.001 |
| 35–49 | 0.30 | 0.25 | 0.36 | <0.001 |
| 50–64 | 0.36 | 0.32 | 0.40 | <0.001 |
| 65–79 | 0.56 | 0.52 | 0.60 | <0.001 |
| Sex (reference = men) | 0.86 | 0.80 | 0.92 | <0.001 |
| Charlson comorbidity score (reference =0) | ||||
| 1 | 1.82 | 1.58 | 2.09 | <0.001 |
| 2 | 2.18 | 1.90 | 2.50 | <0.001 |
| 3 | 2.80 | 2.43 | 3.22 | <0.001 |
| 4 | 3.56 | 3.06 | 4.13 | <0.001 |
| 5+ | 5.76 | 5.03 | 6.59 | <0.001 |
| Hospital-acquired AKI (reference = community acquired) | 0.94 | 0.88 | 1.0 | 0.06 |
The period in which the hospitals were exposed to the intervention compared with the control (reference) period is shown in the first row. Effects are seen with season, age, sex, and comorbidity, but there is no time effect on mortality over the study period. Cluster (hospital) was also included in the model. 95% CI, 95% confidence interval.
AKI Incidence
After adjustment for other variables, the incidence of AKI was higher in the intervention period compared with the control period (incidence rate ratio, 1.12; 95% CI, 1.03 to 1.22; P<0.01). The same effect size was observed across each stage of AKI when analyzed separately (Supplemental Table 2). The increase in AKI incidence was mirrored by a large increase in the proportion of patients with a coded diagnosis of AKI (ICD-10 code N17.X) during the intervention period (adjusted incidence rate ratio, 1.27; 95% CI, 1.15 to 1.39; P<0.001), suggesting improved AKI recognition.
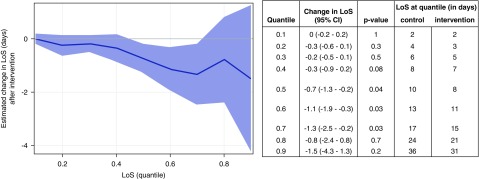
LoS and AKI Duration
A total of 18,887 admissions in which the patient was discharged alive were included in the LoS quantile regression analyses. The median hospital LoS for all AKI admissions was 9 days (interquartile range, 4–19). LoS was reduced in the intervention period as shown in Figure 2. The effect was seen in those with longer LoS (from quantiles 0.5 upward). At the 0.5 quantile, the effect size was a reduced LoS of −0.7 days (95% CI, −1.3 to −0.2; P=0.04), extending to −1.3 days (95% CI, −2.5 to −0.2; P=0.03) at the 0.7 quantile. When the analysis was repeated to include all admissions, regardless of whether the patient was alive at discharge, the same pattern of results was observed (Supplemental Figure 1).
Figure 2.
Reduction in hospital length of stay in the intervention period, as shown by quantile regression analysis. LoS is shown on the y axis at different quantiles of the distribution. The solid line represents the estimated changes in LoS distribution quantiles from before to after the introduction of the intervention across the different quantiles of the distribution after adjustment for time, age, sex, comorbid conditions, cluster (hospital), and seasonality, and the shaded area represents the 95% confidence interval (95% CI). Results show a reduced LoS during the intervention period (from quantiles 0.5 upward; effect size and median LoS at individual quantiles are shown in the table).
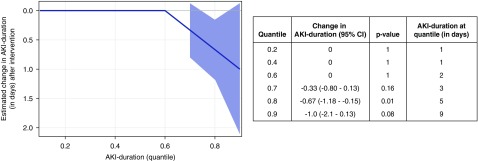
Similarly, we observed a reduction in AKI duration during the intervention period; these data are shown in Figure 3. The median duration of AKI was 2 days (interquartile range, 1–4). The effect of the intervention was seen in those at the 0.7, 0.8, and 0.9 quantiles; at the 0.8 quantile, the reduction in duration of AKI was −0.7 days (95% CI, −1.2 to −0.2; P=0.01).
Figure 3.
Reduction in AKI duration in the intervention period, as shown by quantile regression analysis. AKI duration is shown on the y axis at different quantiles of the distribution. The solid line represents the estimated changes in AKI duration distribution quantiles from before to after the introduction of the intervention across the different quantiles of the distribution after adjustment for time, age, sex, comorbid conditions, cluster (hospital), and seasonality, and the shaded area represents the 95% confidence interval (95% CI). Results show a reduced AKI duration during the intervention period (from quantiles 0.8 onward; effect size and median AKI duration at individual quantiles are shown in the table).
Quantile regression was chosen in place of the prespecified analyses for LoS and AKI duration, because both negative binomial and Poisson regression showed a significant lack of model fit with poor residual plots. However, results from these analyses were consistent with those from quantile regression. With negative binomial regression, LoS was decreased in the intervention period by 6.6% (95% CI, 1.3% to 11.6%; P=0.02). With Poisson regression, LoS was decreased by 6.2% (95% CI, 4.7% to 7.7%; P<0.001). With negative binomial regression, AKI duration decreased by 14.7% (95% CI, 8.8% to 20.3%; P<0.001), and with Poisson regression, AKI duration decreased by 14.0% (95% CI, 11.4% to 16.5%; P<0.001).
AKI Progression
AKI progression was assessed only in patients with AKI stage 1 or 2 at the time of AKI onset (21,672 AKI episodes). There was no significant effect of the intervention on AKI progression in the fully adjusted model (odds ratio, 0.94; 95% CI, 0.8 to 1.1; P=0.40). These data are shown in Table 5. A total of 630 patients (2.6%) were coded as receiving acute RRT; the odds ratio of receiving RRT during the intervention period compared with the control period was 1.1 (95% CI, 0.8 to 1.6).
Table 5.
Results of multilevel logistic regression for AKI progression
| Parameter | Odds Ratio | 95% CI | P Value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Intervention (reference = control period) | 0.94 | 0.80 | 1.10 | 0.41 |
| Time (linear trend) | 1.00 | 0.90 | 1.11 | 0.99 |
| Season (reference = spring) | ||||
| Summer | 0.99 | 0.89 | 1.10 | 0.86 |
| Autumn | 1.00 | 0.87 | 1.14 | 0.95 |
| Winter | 1.03 | 0.94 | 1.13 | 0.58 |
| Age group (reference =80+), yr | ||||
| 18–34 | 0.93 | 0.76 | 1.13 | 0.45 |
| 35–49 | 1.27 | 1.08 | 1.48 | 0.003 |
| 50–64 | 1.20 | 1.08 | 1.34 | 0.001 |
| 65–79 | 1.19 | 1.09 | 1.30 | <0.001 |
| Sex (reference = men) | 0.83 | 0.77 | 0.90 | <0.001 |
| Charlson comorbidity score (reference =0) | ||||
| 1 | 1.18 | 1.03 | 1.36 | 0.02 |
| 2 | 1.58 | 1.38 | 1.81 | <0.001 |
| 3 | 1.85 | 1.60 | 2.14 | <0.001 |
| 4 | 2.30 | 1.98 | 2.68 | <0.001 |
| 5+ | 2.32 | 2.03 | 2.66 | <0.001 |
| Hospital-acquired AKI (reference = community acquired) | 0.96 | 0.89 | 1.03 | 0.26 |
AKI progression was defined as AKI stage 1 or 2 that worsened to a higher stage of AKI. The period in which the hospitals were exposed to the intervention compared with the control (reference) period is shown in the first row. Cluster (hospital) was also included in the model. 95% CI, 95% confidence interval.
Sensitivity Analyses
Because of the effect of season on AKI incidence and outcome, we performed a sensitivity analysis to test the effect of the intervention on mortality during winter compared with other seasons by adding an interaction term to the model. We also explored whether time from a site’s initial exposure to the intervention was important. This tested whether an effect was sustained or diminished over time or if there were differences in the time required to reach maximal effect. Neither interaction showed differences in effect by season or time from exposure.
A sensitivity analysis for AKI progression was also performed that included patients with AKI stage 3 who progressed to RRT as well as those with AKI stages 1 and 2. This produced similar results to those of the primary analysis, with no significant difference between control and intervention periods.
Process Outcomes
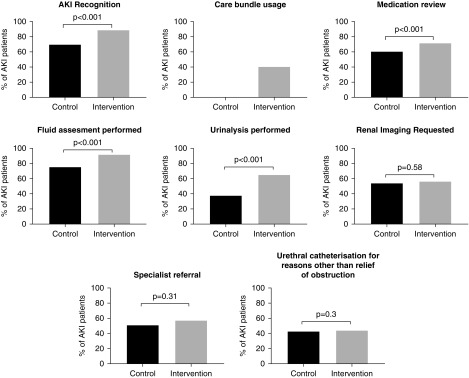
Process measures were assessed in 1048 patients. Comparisons between control and intervention periods are shown in Figure 4. Care bundle usage increased from 0% to 40.2% from control to intervention periods. Increases were also seen in AKI recognition (69.4% versus 88.8%), medication review (60.1% versus 71.3%), fluid assessment (74.4% versus 91.2%), and urinalysis (37.4% versus 64.7%). Changes in rates of specialist referral, renal imaging, and urinary catheterization were not seen. There were differences between sites in the degree of improvement and baseline levels of compliance; these data are included in Supplemental Figure 2.
Figure 4.
Improvements in processes of care with the intervention. Urinary catheterization was included as a balancing measure, and we did not observe an unintended increase in the proportion of patients catheterized for reasons other than relief of urinary obstruction.
Discussion
In this multicenter SWCRT, a complex organizational-level intervention did not alter 30-day AKI mortality, but it did result in shorter duration of AKI episodes, a reduction in LoS, and improved AKI recognition. These findings were consistent across sensitivity and subgroup analyses.
Multiple reports from a variety of care settings consistently show that AKI in hospitalized patients is both common and associated with poor outcomes.30,31 In the absence of specific therapies, efforts to improve outcomes for patients have focused on increasing the consistency and quality of supportive care for AKI, exemplified by national and international campaigns, such as the International Society of Nephrology “0by25” campaign and the “Think Kidneys” national program in England.21,32 In parallel with these initiatives, there is a need to test the effectiveness of potential strategies and how they should be delivered across different health care systems. Our aim was to establish a more rigorous approach to this than previously used and evaluate an intervention aimed at improving AKI care within a multicenter, randomized study design. The pragmatic trial methodology allowed for adequate statistical power, with numbers of patients and event rates exceeding assumptions in the sample size calculation. Adherence to the allocated times for implementation was excellent across all five sites, and use of the UKRR infrastructure allowed the study to be undertaken efficiently and with independent data collection and analysis. The demographics of the study population were consistent with previous epidemiologic studies,27,33 and the higher AKI incidence and mortality in winter, recently described elsewhere,34 were important observations that required adjustment in statistical modeling and have relevance to the design of future studies. The SWCRT is a relatively novel trial design that is increasingly popular, particularly in the evaluation of complex interventions. It is more robust than before-after studies, because it allows for differentiation between the effect of the intervention and independent time-related factors (i.e., changes that would have happened anyway). In our study, because of the nature of the intervention, it overcame the problem of contamination of the control group (health care professionals within individual hospitals exposed to the intervention but treating patients in both control and intervention groups) that would have occurred with randomization at the patient level. SWCRTs are well suited to pragmatic aspects of the rollout of complex interventions, ethical issues are avoided if concerns about withholding an intervention in the control arm exist, and efficient trial design is possible. Disadvantages include the need for more complex statistical approaches (including those to avoid confounding) and biases that may arise if cluster size is too small; additionally, if individual patient data collection is required, that can lead to selection bias.18
We did not observe any change in 30-day mortality, and this held true across a number of subgroup analyses. A previous single-center randomized trial showed that an isolated e-alert for AKI did not result in any change in physician behavior or patient outcomes.11 Our results differ in that we did observe improvements in AKI care delivery, including an increase in care bundle usage from zero during the control period to approximately 40% with the intervention. One interpretation of our results is that better AKI care does not translate into improved mortality, although an alternative explanation is that uptake of the intervention was incomplete across participating sites, whereas outcomes were measured on a hospital-wide basis. This would be supported by the bundle completion rates. Hence, even if an intervention is effective at changing provider behaviors, a challenge remains concerning spread and sustainability across an organization. Previous studies that have reported reductions in patient mortality after complex interventions for AKI have generally used less robust methodology (e.g., before-after comparisons that cannot exclude effects of temporal trends on outcomes or limited statistical analysis); results from single-center studies may also be subject to attenuation of effect size when scaling this type of intervention to a larger number of sites. Our study was adequately powered to detect similar size reductions in mortality, although a recent study with a before-after design is notable for the very large sample size (>64,000 patients) required to show a small but significant reduction in mortality with the introduction of computer decision support for AKI.15 However, our study was more than double the estimated sample size, and we did not observe any trend toward mortality reduction. The primary end point of 30-day mortality was chosen on the basis of previous single-center quality improvement studies that did show improvements in this outcome.12,13 However, mortality associated with AKI is driven by multiple factors, including effects of comorbidity and coexisting acute illness in addition to effects from AKI.35 In view of our findings, it may be advisable for future trials of complex interventions for AKI to consider alternative primary outcomes, particularly those that are organ specific (e.g., AKI duration and recovery of renal function) but that retain importance from a patient’s perspective.
There was a beneficial effect of the intervention on LoS and AKI duration. The effect of the intervention on LoS was only apparent in those with a longer hospital stay. A similar pattern was seen with AKI duration, likely explained by limited potential for improvement in those with very short LoS or AKI duration. The positive effects of the intervention on LoS may be considered relatively modest for the individual patient, but given the very large numbers of patients who sustain AKI, this could translate into a significant health economic benefit; in England alone, it is estimated that there are >800,000 hospital admissions with AKI annually.36 Our post hoc analysis to examine the effect on AKI duration was undertaken to explore plausible reasons of how the intervention could directly reduce LoS. Its inclusion was further justified, because we were unable to study the effect of the intervention on another prespecified secondary end point (critical care bed days). It is possible that the reduction in AKI duration may have a positive benefit on long-term patient outcomes, because AKI duration has been shown to be a very strong independent predictor of both subsequent CKD and long-term mortality.37,38 Unfortunately, reliable data collection to evaluate renal recovery in this study was not possible.
We also observed an increase in the incidence of AKI during the intervention period. This was not an effect of time or season. The most likely explanation is improved testing and recognition resulting from health care staff education. This is supported by the parallel increase in AKI diagnostic coding and the improvement in AKI recognition seen in the nested study of process measures. A similar effect has been reported in other studies.15 Importantly, in terms of interpreting the effect of the intervention on other outcomes, the increase in AKI incidence was seen equally across all stages of AKI, suggesting that improvements in LoS and AKI duration were not an artifact of a disproportionate increase in AKI stage 1 during the intervention.
There are some limitations of this study. The use of an electronic algorithm to identify patients with AKI may result in some misclassification of a small number of patients with AKI (e.g., progressive CKD).39 The inclusion of data from such patients may produce a small bias in favor of the null hypothesis. Using serum creatinine criteria without urine output may result in an underestimation of AKI incidence, but it was the only pragmatic approach for hospital-wide assessment of AKI, because the majority of patients do not have hourly urine output measurements. Results from analyses of secondary and exploratory outcomes were not adjusted for the effects of multiple testing and need to be interpreted in light of this fact. The potential for the change in AKI incidence to affect other outcomes should be noted, although we found no evidence to suggest that there was a shift toward less severe AKI in the intervention period, and we did not see any change in mortality that would be expected if severity of AKI was altered. The audit of process measures was conducted in a subgroup of patients, and therefore, no direct inferences can be drawn regarding these results and outcomes. The LIMS failure interrupted data collection for a short period, although this was successfully mitigated by extending the study duration. Finally, our findings may not be generalizable to other health care systems that differ substantially from the NHS in England.
In conclusion, a strategy to reduce avoidable harm associated with AKI did not alter 30-day AKI mortality. However, it was effective in reducing duration of AKI episodes and LoS, and it resulted in better AKI recognition. These results support a continued focus on improving the delivery of person-centered AKI care across acute specialties.
Disclosures
None.
Supplementary Material
Acknowledgments
We would like to thank and acknowledge the input of Mr. Peter Naish and Dr. Clare Corps who, as patient representatives, provided valuable insights during study design and delivery. Collaborators were Mike Bosomworth, Georgie Duncan, and Ashley Garner of Leeds Teaching Hospitals; Rafaq Azad of Bradford Teaching Hospitals; Bethany Bal, Arlene Batuista, and Razya Hussain of Frimley Health National Health Service Foundation Trust; Erica Heppleston of Ashford and St. Peters; Sally Benton and Craig McKibben of Surrey Pathology Services; and Julie Slevin of United Kingdom Renal Registry.
This study was funded by Health Foundation award 1502-Derby-Selby-SUI.
The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “A Pragmatic Step Forward: AKI and Beyond,” on pages 371–372.
Contributor Information
Collaborators: Mike Bosomworth, Georgie Duncan, Ashley Garner, Rafaq Azad, Bethany Bal, Arlene Batuista, Razya Hussain, Erica Heppleston, Sally Benton, Craig McKibben, and Julie Slevin
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018090886/-/DCSupplemental.
Supplemental Figure 1. Quantile regression for change in hospital LoS (in days) with all patients included.
Supplemental Figure 2. Process measures presented individually per site.
Supplemental Material. Protocol, statistical analysis plan, and supplemental methods.
Supplemental Table 1. Patient demographics in control and intervention periods at each hospital and overall.
Supplemental Table 2. Results of multilevel logistic regression for AKI incidence overall and for each AKI stage.
References
- 1.Lameire NH, Bagga A, Cruz D, De Maeseneer J, Endre Z, Kellum JA, et al.: Acute kidney injury: An increasing global concern. Lancet 382: 170–179, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Selby NM, Fluck RJ, Kolhe NV, Taal MW: International criteria for acute kidney injury: Advantages and remaining challenges. PLoS Med 13: e1002122, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vanmassenhove J, Kielstein J, Jörres A, Biesen WV: Management of patients at risk of acute kidney injury. Lancet 389: 2139–2151, 2017 [DOI] [PubMed] [Google Scholar]
- 4.KDIGO AKI Work Group : Clinical practice guideline for acute kidney injury. Kidney Int Suppl 2[Suppl 1]: 1–141, 2012 [Google Scholar]
- 5.Lewington A, Kanagasundaram S: Renal association clinical practice guidelines on acute kidney injury. Nephron Clin Pract 118[Suppl 1]: c349–c390, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Ftouh S, Thomas M; Acute Kidney Injury Guideline Development Group : Acute kidney injury: Summary of NICE guidance. BMJ 347: f4930, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Stevens PE, Tamimi NA, Al-Hasani MK, Mikhail AI, Kearney E, Lapworth R, et al.: Non-specialist management of acute renal failure. QJM 94: 533–540, 2001 [DOI] [PubMed] [Google Scholar]
- 8.NCEPOD : Acute Kidney Injury: Adding Insult to Injury, London, National Confidential Enquiry into Patient Outcomes and Death, 2009 [Google Scholar]
- 9.Aitken E, Carruthers C, Gall L, Kerr L, Geddes C, Kingsmore D: Acute kidney injury: Outcomes and quality of care. QJM 106: 323–332, 2013 [DOI] [PubMed] [Google Scholar]
- 10.James MT, Wald R, Bell CM, Tonelli M, Hemmelgarn BR, Waikar SS, et al.: Weekend hospital admission, acute kidney injury, and mortality. J Am Soc Nephrol 21: 845–851, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson FP, Shashaty M, Testani J, Aqeel I, Borovskiy Y, Ellenberg SS, et al.: Automated, electronic alerts for acute kidney injury: A single-blind, parallel-group, randomised controlled trial. Lancet 385: 1966–1974, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ebah L, Hanumapura P, Waring D, Challiner R, Hayden K, Alexander J, et al.: A multifaceted quality improvement programme to improve acute kidney injury care and outcomes in a large teaching hospital. BMJ Qual Improv Rep 6: pii:u219176.w7476, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chandrasekar T, Sharma A, Tennent L, Wong C, Chamberlain P, Abraham KA: A whole system approach to improving mortality associated with acute kidney injury. QJM 110: 657–666, 2017 [DOI] [PubMed] [Google Scholar]
- 14.Kolhe NV, Reilly T, Leung J, Fluck RJ, Swinscoe KE, Selby NM, et al.: A simple care bundle for use in acute kidney injury: A propensity score-matched cohort study. Nephrol Dial Transplant 31: 1846–1854, 2016 [DOI] [PubMed] [Google Scholar]
- 15.Al-Jaghbeer M, Dealmeida D, Bilderback A, Ambrosino R, Kellum JA: Clinical decision support for in-hospital AKI. J Am Soc Nephrol 29: 654–660, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selby NM, Casula A, Lamming L, Mohammed M, Caskey F; Tackling AKI Investigators : Design and rationale of ‘tackling acute kidney injury,’ a multicentre quality improvement study. Nephron 134: 200–204, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Campbell MK, Piaggio G, Elbourne DR, Altman DG; CONSORT Group : Consort 2010 statement: Extension to cluster randomised trials. BMJ 345: e5661, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Hemming K, Haines TP, Chilton PJ, Girling AJ, Lilford RJ: The stepped wedge cluster randomised trial: Rationale, design, analysis, and reporting. BMJ 350: h391, 2015 [DOI] [PubMed] [Google Scholar]
- 19.NHS England : Kidney Quality Improvement Partnership National Projects: Tackling AKI. 2018. Available at: http://www.thinkkidneys.nhs.uk/kquip/hub/tackling-aki/. Accessed February 2, 2019
- 20.Selby NM, Hill R, Fluck RJ; NHS England ‘Think Kidneys’ AKI Programme : Standardizing the early identification of acute kidney injury: The NHS England national patient safety alert. Nephron 131: 113–117, 2015 [DOI] [PubMed] [Google Scholar]
- 21.NHS England : Acute Kidney Injury (AKI) Programme, 2014. Available at: www.thinkkidneys.nhs.uk. Accessed February 2, 2019
- 22.Resar R, Griffin FA, Haradan C, Nolan TW: Using Care Bundles to Improve Health Care Quality. IHI Innovation Series White Paper, Cambridge, MA, Institute for Healthcare Improvement, 2012 [Google Scholar]
- 23.KDIGO AKI Work Group : Section 2: AKI definition. Kidney Int Suppl (2011) 2: 19–36, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charlson ME, Pompei P, Ales KL, MacKenzie CR: A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 40: 373–383, 1987 [DOI] [PubMed] [Google Scholar]
- 25.Hemming K, Girling A: A menu-driven facility for power and detectable-difference calculations in stepped-wedge cluster-randomized trials. Stata J 14: 363–380, 2014 [Google Scholar]
- 26.Xu X, Nie S, Liu Z, Chen C, Xu G, Zha Y, et al.: Epidemiology and clinical correlates of AKI in Chinese hospitalized adults. Clin J Am Soc Nephrol 10: 1510–1518, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selby NM, Crowley L, Fluck RJ, McIntyre CW, Monaghan J, Lawson N, et al.: Use of electronic results reporting to diagnose and monitor AKI in hospitalized patients. Clin J Am Soc Nephrol 7: 533–540, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Olsen CS, Clark AE, Thomas AM, Cook LJ: Comparing least-squares and quantile regression approaches to analyzing median hospital charges. Acad Emerg Med 19: 866–875, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Wei Y, Carroll RJ: Quantile regression with measurement error. J Am Stat Assoc 104: 1129–1143, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Susantitaphong P, Cruz DN, Cerda J, Abulfaraj M, Alqahtani F, Koulouridis I, et al.: Acute Kidney Injury Advisory Group of the American Society of Nephrology : World incidence of AKI: A meta-analysis. Clin J Am Soc Nephrol 8: 1482–1493, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR: Long-term risk of mortality and other adverse outcomes after acute kidney injury: A systematic review and meta-analysis. Am J Kidney Dis 53: 961–973, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mehta RL, Cerdá J, Burdmann EA, Tonelli M, García-García G, Jha V, et al.: International society of nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): A human rights case for nephrology. Lancet 385: 2616–2643, 2015 [DOI] [PubMed] [Google Scholar]
- 33.Holmes J, Rainer T, Geen J, Roberts G, May K, Wilson N, et al.: Welsh AKI Steering Group : Acute kidney injury in the era of the AKI E-alert. Clin J Am Soc Nephrol 11: 2123–2131, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phillips D, Young O, Holmes J, Allen LA, Roberts G, Geen J, et al.: Welsh AKI steering group : Seasonal pattern of incidence and outcome of Acute Kidney Injury: A national study of Welsh AKI electronic alerts [published online ahead of print September 4, 2017]. Int J Clin Pract doi:10.1111/ijcp.13000 [DOI] [PubMed] [Google Scholar]
- 35.Selby NM, Kolhe NV, McIntyre CW, Monaghan J, Lawson N, Elliott D, et al.: Defining the cause of death in hospitalised patients with acute kidney injury. PLoS One 7: e48580, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kerr M, Bedford M, Matthews B, O’Donoghue D: The economic impact of acute kidney injury in England. Nephrol Dial Transplant 29: 1362–1368, 2014 [DOI] [PubMed] [Google Scholar]
- 37.Palomba H, Castro I, Yu L, Burdmann EA: The duration of acute kidney injury after cardiac surgery increases the risk of long-term chronic kidney disease. J Nephrol 30: 567–572, 2017 [DOI] [PubMed] [Google Scholar]
- 38.Coca SG, King JT Jr, Rosenthal RA, Perkal MF, Parikh CR: The duration of postoperative acute kidney injury is an additional parameter predicting long-term survival in diabetic veterans. Kidney Int 78: 926–933, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sawhney S, Marks A, Ali T, Clark L, Fluck N, Prescott GJ, et al.: Maximising acute kidney injury alerts--A cross-sectional comparison with the clinical diagnosis. PLoS One 10: e0131909, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.