Significance Statement
CKD of various etiologies manifests with declining kidney function in the setting of progressive interstitial inflammation, with increased myofibroblasts and collagen deposition. In this study, the authors developed a novel genetic model, demonstrating that impairment of protein homeostatic mechanisms in the endoplasmic reticulum of collecting duct cells is sufficient to produce interstitial inflammation, fibrosis, and impaired kidney function. In this model, genes encoding both Sec63, a resident protein in the endoplasmic reticulum membrane, and Xbp1, a transcription factor that is central to the unfolded protein response, were inactivated in the collecting ducts of neonatal mice. The result was progressive macrophage and myofibroblast expansion within 5 weeks and significant progressive kidney dysfunction thereafter. The Sec63-Xbp1 collecting duct knockout mouse offers a novel genetic model for studying chronic tubulointerstitial kidney injury.
Keywords: genetic renal disease, chronic kidney disease, renal tubular epithelial cells, renal fibrosis, endoplasmic reticulum
Abstract
Background
SEC63 encodes a resident protein in the endoplasmic reticulum membrane that, when mutated, causes human autosomal dominant polycystic liver disease. Selective inactivation of Sec63 in all distal nephron segments in embryonic mouse kidney results in polycystin-1–mediated polycystic kidney disease (PKD). It also activates the Ire1α-Xbp1 branch of the unfolded protein response, producing Xbp1s, the active transcription factor promoting expression of specific genes to alleviate endoplasmic reticulum stress. Simultaneous inactivation of Xbp1 and Sec63 worsens PKD in this model.
Methods
We explored the renal effects of postnatal inactivation of Sec63 alone or with concomitant inactivation of Xbp1 or Ire1α, specifically in the collecting ducts of neonatal mice.
Results
The later onset of inactivation of Sec63 restricted to the collecting duct does not result in overt activation of the Ire1α-Xbp1 pathway or cause polycystin-1–dependent PKD. Inactivating Sec63 along with either Xbp1 or Ire1α in this model causes interstitial inflammation and associated fibrosis with decline in kidney function over several months. Re-expression of XBP1s in vivo completely rescues the chronic kidney injury observed after inactivation of Sec63 with either Xbp1 or Ire1α.
Conclusions
In the absence of Sec63, basal levels of Xbp1s activity in collecting ducts is both necessary and sufficient to maintain proteostasis (protein homeostasis) and protect against inflammation, myofibroblast activation, and kidney functional decline. The Sec63-Xbp1 double knockout mouse offers a novel genetic model of chronic tubulointerstitial kidney injury, using collecting duct proteostasis defects as a platform for discovery of signals that may underlie CKD of disparate etiologies.
CKD is a highly prevalent global public health problem affecting over 10% of the United States population, with similar rates worldwide. It has, to date, largely eluded effective prevention and treatment and therefore remains a major cause of morbidity and mortality.1,2 CKD results from pleiotropic causes that can be grossly classified as either glomerular or tubulointerstitial diseases that are affected by varying combinations of genetic, systemic, and environmental factors. The majority of human CKD is thought to have glomerular etiologies and glomerular proteinuric disease is often compounded with attendant tubulointerstitial lesions characterized by inflammation and fibrosis.3,4 It is often considered that although tubulointerstitial disease correlates closely with progression of CKD, it is the secondary result from injuries extrinsic to the tubules, such as excessive proteinuria, primary inflammatory processes, ischemia, or other tubulotoxic injuries.5 Mouse models of isolated tubulointerstitial renal disease outside the spectrum of fibrocystic disorders 6 often rely on acute exogenous injuries from toxins or surgical interventions, often coupled with sensitized genetic backgrounds or, in some instances, transgenic overexpression of pathogenic genes.7
As a consequence, many CKD mouse models indirectly disrupt endoplasmic reticulum (ER) function in kidney tubules and result in ER stress.8 The effects of ER stress are normally mitigated by a housekeeping mechanism termed the unfolded protein response (UPR). In mammalian cells, UPR is mediated by three major stress sensors: inositol-requiring enzyme 1α (Ire1α encoded by Ern1), proteinase kinase R-like ER kinase (Perk), and activating transcription factor 6 (Atf6).9–11 IRE1α is a protein kinase and endoribonuclease that catalyzes unconventional splicing of mRNA encoding X-box binding protein 1 (Xbp1) by removing a 26-base intron to produce transcriptionally active spliced Xbp1 (Xbp1s). Xbp1s drives expression of target genes encoding chaperone and other proteins, including BiP (Grp78), ERdj4, Sec61α, and Herp.12,13 Perk is a transmembrane serine/threonine kinase that phosphorylates and inactivates eukaryotic initiation factor 2α (eIF2α) to reduce protein translation while selectively turning on activating transcription factor 4 (Atf4)10, which in turn induces proapoptotic factor, CCAAT/enhancer-binding homologous protein (Chop).14 In response to ER stress, ATF6 is processed at the Golgi via cleavage by S1P/S2P to release its N terminus, which translocates to the nucleus and activates transcription of ER chaperone and protein-folding enzyme target genes.15–17
We previously found that inactivation of a BiP interacting protein, Sec63, results in selective activation of the Ire1α-Xbp1 branch of UPR.18,19 Sec63 works in concert with the Sec61 protein translocation pore and BiP (GRP78) to mediate cotranslational protein translocation processes across the ER membrane.20 Sec63 is a causative gene for autosomal dominant polycystic liver disease (PCLD).21 Initiation of liver cysts in dominantly inherited PCLD occurs by a recessive mechanism following somatic “second hit” mutations at the cellular level.22,23 The resulting loss of Sec63 adversely affects posttranslational maturation of a large number of integral membrane and secreted client proteins, but cyst formation occurs specifically because of impaired biogenesis of one protein, polycystin-1.22,24 Polycystin-1 is the protein product of the more prevalent and severe polycystic kidney disease gene, PKD1. In the absence of Sec63, activation of Ire1α-Xbp1 and its transcriptional targets has an adaptive role in supporting maturation of polycystin-1 and, by inference, other Sec63 client proteins.18,25,26
In this study, we set out to examine the effects of collecting tubule deletion of Sec63 and the Ire1α-Xbp1 pathway on kidney homeostasis and function. Surprisingly, we found that Sec63 inactivation in collecting ducts did not result in activation of the Ire1α-Xbp1 pathway or a discernible phenotype, but that concomitant inactivation of Ire1α or Xbp1 along with Sec63 caused a severe, progressive inflammatory and fibrotic response leading to kidney dysfunction. This process did not require any renal injury beyond inactivation of Sec63 and the Ire1α-Xbp1 pathway in collecting duct cells, showing that deranged proteostasis in this nephron segment was sufficient to initiate tubulointerstitial inflammatory and consequent fibrotic kidney disease. This study establishes a novel genetic model of slowly progressive tubulointerstitial kidney injury via collecting duct proteostasis defects, and defines a protective role for homeostatic Sec63 and Ire1α-Xbp1 function in maintaining normal kidney function.
Methods
Mouse Strains and Treatment
All animal studies were conducted in accordance with Yale University Institutional Animal Care and Use Committee guidelines and procedures. The mouse strains used in this study have previously been described: Sec63fl,22 Xbp1fl,27 Pkhd1-cre,28 Pkd1F/H-BAC,22,29 Ire1fl,30 Nlrp3‒/‒,31 and ROSA26-XBP1s.18 RosamT/mG mice were used for Cre reporter studies.32 All strains were backcrossed at least four generations on a C57BL6 background (>93.75% C57BL6 congenic). Mice of both sexes were used in this study. Mice were euthanized and tissues processed for histology, immunocytochemistry, RNA extraction, and immunoblotting. Blood was collected for BUN and serum creatinine measurements, which were performed by George M. O’Brien Kidney Center at Yale University.
Immunofluorescence Staining
One kidney from euthanized mice was snap-frozen, and the other kidney was perfusion-fixed with 4% paraformaldehyde (PBS). Sections (5–7 µm) were prepared for immunocytochemical studies according to standard procedures.33 Images were obtained using a Nikon Eclipse TE2000-U microscope under the control of MetaMorph software (Universal Imaging).33 The following antibodies and lectins were used at the indicated dilutions: polyclonal antiserum raised to the COOH terminus of anti-megalin (anti-MC220; 1:300)34; anti-Tamm–Horsfall protein (AB733, 1:200; Millipore); anti-aquaporin 2 (AQP2; sc-9882, 1:100; Santa Cruz Biotechnology); fluorescein-labeled Lotus tetragonolobus lectin (LTL; FL-1321, 1:200; Vector Laboratories); fluorescein-labeled Dolichos biflorus agglutinin (FL-1031, 1:50; Vector Laboratories); anti-mouse F4/80 purified antigen (14–4801–81, 1:200; eBiosience); anti–α−smooth muscle actin antibody (ab5694, 1:300; Abcam); anti-S100A4 antibody (fibroblast-specific protein 1 [FSP1], ab27957, 1:500; Abcam); anti- PDGF receptor-β (PDGFRβ, ab32570, 1:400; Abcam), anti–H+-ATPase (1:100; gift from SueAnn Mentone, Yale University), and anti-pendrin (1:100; gift from Peter Aronson, Yale University). Rat anti-L1CAM (L1; clone S10.33, 5 μg/ml; gift from Ugo Cavallaro, Instituto Europeo di Oncologia)35 was conjugated using Alexa Fluor 647 antibody labeling kit (Invitrogen). Hoechst 33342 (H-3570, 1:2000; Molecular Probes) was used for nuclear staining.
RNA Isolation, RT-PCR, and Quantitative RT-PCR
Total RNA was isolated from kidney tissue using QIAzol reagent (Qiagen), and used for complementary DNA synthesis using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). RT-PCR primers encompassing the spliced sequence in Xbp1 were used, as described previously.36 Quantitative RT-PCR was performed using SYBR green fluorescence reagent, and analyzed by an Mx3000 PCR System (Stratagene). The following primers were used: MCP1 forward, 5′- TTAAAAACCTGGATCGAACCAA-3′ and reverse, 5′- GCATTAGCTTCAGATTTACGGGT-3′; F4/80 forward, 5′-AGTACGATGTGGGGCTTTTG-3′ and reverse, 5′-ACTCCTGGGCCTTGAAAGTT-3′; NLRP3 forward, 5′-AGCCTACAGTTGGGTGAAATG-3′ and reverse, 5′-CCTACCAGGAAATCTCGAAGAC-3′; megalin forward, 5′-ACAGCAAAATTGCCCTGGGA-3′ and reverse, 5′-TGGTCACACCTGTACTCGATG-3′; α−subunit epithelial Na+ channel (ENaC) forward, 5′-TTCCAACTGTGCAACCAGAA-3′ and reverse, 5′-CCTGGTTGAAACGACAGGTAA-3′; Snail1 forward, 5′-CACACTGGTGAGAAGCCATT-3′ and reverse, 5′-GCCAGACTCTTGGTGCTTGT-3′; and GAPDH forward, 5′-GTCCCGTAGACAAAATGGTG-3′ and reverse, 5′-ATTCTCGGCCTTGACTGTG-3′. The design of iNOS,37 Arg1,37 FSP1,38 XBP1s,27 ERdj4,25 AQP2,39 Krüppel-like factor–5 (KLF5),40 and P2ry1441 primers and analysis were described previously.
Protein Preparation and Immunoblot Analysis
Frozen whole kidneys were homogenized using a 2-ml Dounce homogenizer (Wheaton) in ice-cold NET buffer (150 mM NaCl, 50 mM Tris-HCl, pH 7.4, 1 mM EDTA, and 0.1% Triton X-100) containing Complete EDTA-free Protease Inhibitor cocktail tablets (Roche) and PhosSTOP phosphatase inhibitor cocktail tablets. EDTA-free RIPA buffer (150 mM NaCl, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris-HCl, pH 8.0%, and 1% Triton X-100) was used to generate lysates for detecting phosphorylated IRE1α. Protein concentrations were measured with Protein Assay Dye Reagent Concentrate (Bio-Rad). For the IRE1α Western blotting, protein was loaded on a 4.5% SDS-PAGE gel containing Phos-Tag Acrylamide (Wako Pure Chemical Industries), and transferred to PVDF membrane (PerkinElmer). Membranes were sequentially incubated with primary antibodies overnight at 4°C after 1 hour of blocking with 5% powdered milk. The following primary antibodies were used: monoclonal anti–α-smooth muscle actin antibody (A2547, 1:3000; Sigma-Aldrich); anti-S100A4 (FSP1, Ab27957; 1:2000); anti-PDGFRβ (ab32570; 1:5000); anti-IRE1α (#3294, 1:1000; Cell Signaling Technology); rabbit polyclonal anti-ATF6α (1:2000; gift from A.-H. L., Cornell University); anti–phospho-PERK (Thr980) (16F8) rabbit mAb (#3179, 1:2000; Cell Signaling Technology); anti-PERK (C33E10) rabbit mAb (#3192; 1:2000); anti–phospho-NF-κB p65 (Ser536) (#3033, 1:1000; Cell Signaling Technology); anti–NF-κB p65 (#8242, 1:2000; Cell Signaling Technology); phospho-SAPK/JNK (Thr183/Tyr185) (#9251, 1:1000; Cell Signaling Technology); anti-SAPK/JNK (#9258, 1:2000; Cell Signaling Technology); anti-histone H3 (#9715, 1:5000; Cell Signaling Technology); anti-Hsp90 (sc-7947, 1:5000; Santa Cruz Biotechnology); rabbit anti-Sec63 (1:3000; a gift from Richard Zimmermann, Saarland University, Homburg, Germany). Secondary antibodies included anti-mouse/rabbit/rat HRP-conjugated antibodies (1:10,000) (Jackson ImmunoResearch Laboratories), which were incubated with the membrane for 1 hour at room temperature. Western Lightning Plus-ECL (PerkinElmer) or SuperSignal West Femto Chemiluminescent Substrate (Thermo Scientific) was used for chemiluminescence detection. The volume of individual immunoblot bands, in pixels, was determined by optical densitometry using Image Studio v.5.0 (LI-COR).
Affinity Purification and Mass Spectrometry Analysis of IRE1α -Interacting Protein
Ire1α−/− mouse embryonic fibroblast (MEF) cells kindly provided by Dr. David Ron were transduced with MSCVhygro-IRE1-HA or empty retrovirus and selected with 200 µg/ml hygromycin. Ire1α−/−;IRE1-HA cells were lysed in a lysis buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, and 1% digitonin) supplemented with cOmplete Mini Protease Inhibitor (Roche). After clearing the lysates by centrifugation at 14,000 × g, the supernatants were incubated with anti-HA high-affinity matrix (Roche) at 4°C for 4 hours under gentle rotation. The HA beads were washed four times with washing buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, and 0.1% digitonin), resuspended in PreScission protease cleavage buffer (50 mM Tris, pH 7.0, 150 mM NaCl, 1 mM EDTA, and 1 mM dithiothreitol), and incubated with PreScission protease (GE Healthcare) at 4°C for 4 hours. After centrifugation, the supernatant was resolved on an SDS-PAGE gel. The purified proteins were visualized by silver staining (Bio-Rad) or GelCode Blue staining (Pierce). Protein bands were excised from GelCode Blue–stained gel and subjected to mass spectrometry analysis after trypsin digestion.
Kidney Nuclear Fractionation
Kidneys were rinsed with ice-cold PBS and were homogenized in 1 ml of hypotonic buffer (10 mM HEPES, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, and 1 mM DTT) containing Complete protease inhibitor and PhosSTOP phosphatase inhibitor (Roche Diagnostics). The kidney suspension were slowly drawn into the syringe using a 27G needle, and then ejected with a single rapid stroke until >90% of cells were broken and nuclei were visualized under the microscope. Homogenates were centrifuged at 10,000 × g at 4°C for 20 minutes. The supernatants contained non-nuclear extracts, and the pellets contained nuclear extracts. Nuclear pellets were resuspended in extraction buffer (20 mM HEPES, pH 7.9, 1.5 mM MgCl2, 0.42 M NaCl, 0.2 mM EDTA, 25% glycerol, and 1 mM dithiothreitol) under slow rotation for 30 minutes at 4°C. Nuclear suspensions were clarified by centrifugation for 30 minutes at 12,000 × g, and supernatants containing solubilized nuclear proteins were processed for immunoblotting.
FACS Sorting
Kidneys were harvested, minced, homogenized, and a single-cell suspension was made by incubating with Liberase (Roche Diagnostics) containing DNase-I (Sigma Aldrich). Cells were stained with the following antibodies: rat anti-L135 conjugated with Alexa Fluor 647 antibody labeling kit and fluorescein-labeled LTL (1:200). Cells were then analyzed using MoFlo (Beckman Coulter) cell sorters.
Statistical Analyses
Comparison of groups was performed by either t test (two groups) or one-way ANOVA followed by Tukey multiple group comparison post-test (three or more groups), using GraphPad Prism6 software. A value of P<0.05 was considered significant. Data are presented as mean ± SEM.
Results
Defective Proteostasis in the Collecting Duct Is an Intrinsic Model of CKD
We evaluated the effects of Sec63 inactivation in the postnatal collecting duct using the Sec63fl/fl; Pkhd1-cre (single knockout; SKO) mouse model. Wild-type (WT) and SKO mouse kidneys exhibit normal kidney histology and function at postnatal day (P) 70 (Figure 1, A–C). SKO kidneys at P120 began to display mild tubulointerstitial inflammatory changes but still no kidney cysts (Supplemental Figure 1). The absence of kidney cysts was an unexpected observation because we had previously found that Sec63fl/fl; Cdh16(Ksp)-cre mice develop polycystic kidneys by P21 from impaired biogenesis of polycystin-1.22 The only variables that can account for the disparate cystic phenotypes between these models are the differences in the pattern and timing of Cre expression. Pkhd1-cre is active only in the collecting duct, including principal cells and type A and B intercalated cells (Supplemental Figure 2), whereas Cdh16(Ksp)-cre is expressed in medullary thick ascending limb of the loop of Henle (mTAL), distal convoluted tubule (DCT), and collecting duct.33,42 Cdh16(Ksp)-cre is active by embryonic day (E) 15.5,42 whereas Pkdh1-cre becomes completely active in cortical and medullary collecting duct cells by P7, showing minimal activity at E14.5 and only approximately 20% activity at P1.43 Because we had previously found that dual inactivation of Sec63 and Xbp1 in Sec63fl/fl; Xbp1fl/fl; Cdh16(Ksp)-cre mice resulted in more severe impairment of polycystin-1 function than inactivation of Sec63 alone, we next examined mouse kidneys with collecting duct–specific inactivation of both Sec63 and Xbp1 using Pkhd1-cre (double knockout; DKO). Surprisingly, Sec63fl/fl; Xbp1fl/fl; Pkhd1-cre DKO mice had extensive renal interstitial inflammation and fibrosis with mild cystic disease in the cortex and medulla without kidney enlargement at P70 (Figure 1). This was accompanied by kidney functional impairment indicated by rising BUN and serum creatinine (Figure 1C). We evaluated the temporally correlated progression of kidney dysfunction and inflammation (Figure 1C, Supplemental Figure 3). At P16, there was no histologic evidence of inflammation and both creatinine and BUN were normal. At P35, focal areas of inflammation were present in the DKO and BUN was beginning to rise. By P49, the areas of inflammation increased and became more diffuse with the beginnings of cyst formation, and BUN and serum creatinine were significantly increased in DKO mice. This kidney injury and dysfunction progressed further by P70 (Figure 1, B and C, Supplemental Figure 3). These findings show that initial acute inflammation in DKO kidneys begins at P35, before cyst formation, and progresses to more chronic inflammation with impaired kidney function reminiscent of CKD models.
Figure 1.
Inactivation of Sec63 and Xbp1 results in renal interstitial inflammation. (A) Hematoxylin and eosin staining and (B) Masson trichrome staining of renal cortex and medulla of the indicated genotypes at P70. WT and Sec63 mutant (SKO) kidneys do not show interstitial inflammation or fibrosis. Sec63 and Xbp1 double mutants (DKO) have extensive inflammatory infiltrates and areas of collagen deposition. Scale bar, 500 µm. (C) Quantitative assessment of body weight (body wt), percent two-kidney weight to body weight ratio (%KW/body wt), BUN, and serum creatinine (s-Cr). Kidney function indicated by BUN and s-Cr begins to decline by P49 in DKO kidneys. The colors correspond to the genotypes in (A). Results are shown as mean ± SEM (ANOVA among groups at each time point). ***P<0.001; **P<0.01; *P<0.05.
We confirmed the genotype dependence of this inflammatory kidney disease. Xbp1fl/fl; Pkhd1-cre mice, which have normal Sec63 expression, and Sec63fl/fl;Xbp1fl/+;Pkhd1-cre mice, which have a single functional copy of Xbp1 in the absence of Sec63, both had normal kidney structure and function at P70 (Supplemental Figure 4). To further exclude the possibility that the kidney disease, we observed in Sec63fl/fl; Xbp1fl/fl; Pkhd1-cre DKO model is related to polycystin-1 function, we intercrossed the DKO mice with the Pkd1F/H-BAC transgenic mouse line that expresses three additional copies of Pkd1.29 Our previous studies showed that Sec63fl/fl;Cdh16(Ksp)-cre and Sec63fl/fl;Xbp1fl/fl;Cdh16(Ksp)-cre mice get kidney cysts because of reduced functional dosage of polycystin-1, and this is prevented by transgenic overexpression of Pkd1 using this same Pkd1F/H-BAC transgenic line.18,22 The Pkd1F/H-BAC transgene did not rescue the inflammatory and fibrotic CKD phenotype in DKO mice, indicating that kidney injury in the DKO genetic model is independent of polycystin-1 dosage (Supplemental Figure 5). Together, these findings show that the observed inflammatory changes in the DKO model are not related to a polycystin-1–dependent polycystic kidney disease. To determine whether the inflammatory response is exclusive to the loss of Sec63 and Xbp1 in the kidney collecting duct cells, we examined extrarenal expression of Pkhd1-cre (Supplemental Figure 6). We found that in addition to collecting duct, Pkhd1-cre is expressed in periportal areas in the liver and the transitional epithelium in the ureter and bladder. Neither of these tissues showed evidence of inflammation in DKO mice (Supplemental Figure 7). We conclude that the observed inflammatory response and subsequent progressive kidney injury in DKO mice is intrinsic to the collecting duct specific inactivation in the kidney. Cell autonomous loss of Sec63 and Xbp1 in collecting ducts initiates a process of renal interstitial inflammation and fibrosis that is likely to be related to defective protein biogenetic homeostasis, i.e., defective proteostasis.
Sec63 and Xbp1 Inactivation Leads to Inflammation and Myofibroblast Activation in the Kidney
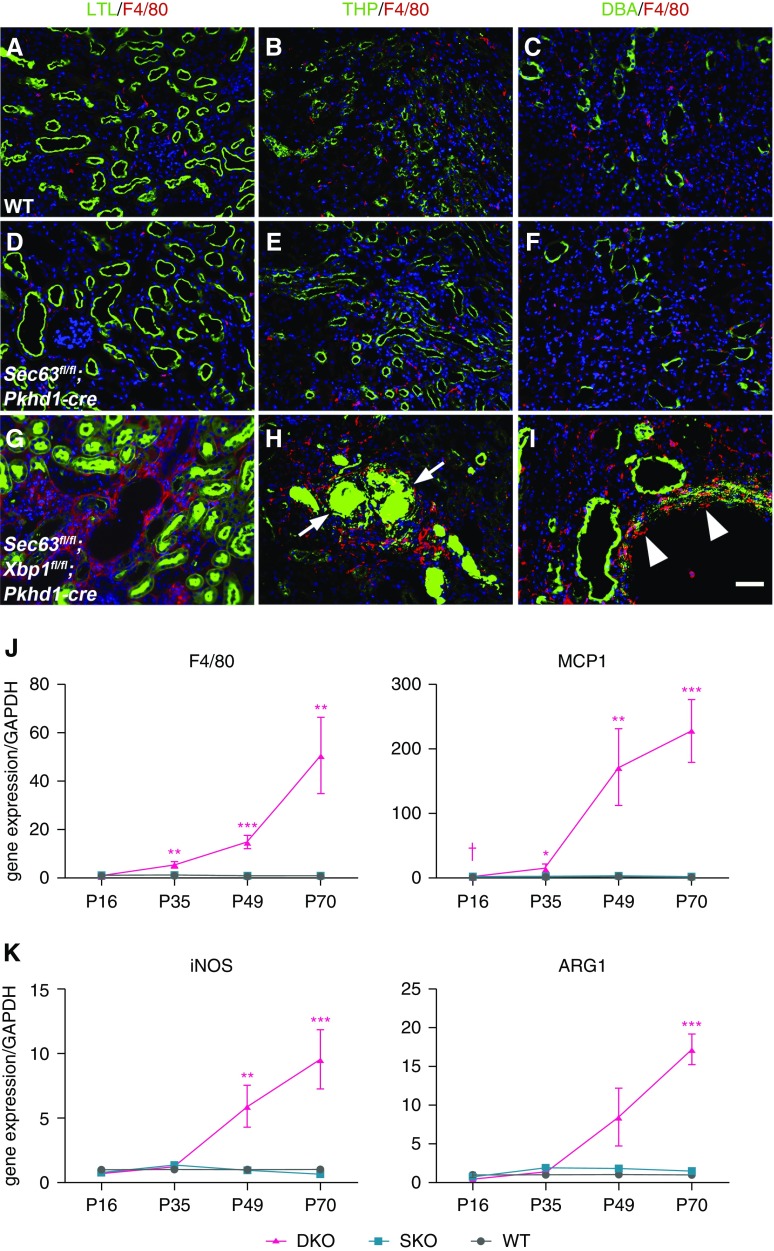
Macrophages play a pivotal role in the onset of renal inflammation, interstitial fibrosis, and tubular and vascular atrophy,44 with macrophage recruitment to the injured kidney being a hallmark of initiation of these processes.45,46 We initially investigated the inflammatory response using the mouse macrophage-monocyte-specific cell surface marker F4/80 in WT, SKO, and DKO kidneys. Immunocytochemistry in WT and SKO kidneys at P35 showed that a few macrophages were present around proximal tubules, mTAL, and collecting duct (Figure 2, A–F, Supplemental Figure 8). In contrast, DKO kidneys showed an abundance of macrophages infiltrating throughout the kidney (Figure 2,G–I, Supplemental Figure 8). Quantitative RT-PCR showed that expression of F4/80 and Mcp1, a chemokine that recruits macrophages,47 were steadily increasing in DKO kidneys compared with SKO and WT at P35, P49, and P70 (Figure 2J). The expression of iNOS, a marker of proinflammatory macrophages, was increased in DKO kidneys compared with SKO and WT at P49 and P70, and Arg1 expressed by alternatively activated macrophages was significantly increased in DKO kidneys at P70 (Figure 2K). In ischemia-reperfusion injury models, macrophages undergo a switch from a proinflammatory to a proreparative phenotype with time.37 In the DKO model, inflammation due to loss of collecting duct Sec63 and Xbp1 results in progressive increase in expression of both iNOS and Arg1 over time, suggesting a persistent and progressive injury response as may occur in CKD.
Figure 2.
Macrophage infiltrates in kidneys with dual inactivation of Sec63 and Xbp1. (A–I) Immunocytochemical analysis of (A–C) WT, (D–F) SKO, and (G–I) DKO kidneys at P35. (A, D, and G) show macrophages (F4/80; red) and proximal tubules (LTL; green); (B, E, and H) macrophages (red) and thick ascending limbs of the loop of Henle (Tamm–Horsfall protein [THP]; green); and (C, F, and I) macrophages (red) and collecting duct (Dolichos biflorus agglutinin [DBA]; green) in the outer medulla. (G–I) The macrophage infiltrates are much more prominent in DKO kidneys. (H) The inflamed DKO mTAL contains urinary THP casts (arrows). (I) Macrophages are found infiltrating around the collecting duct tubules (“tubulitis”; arrowheads). Nuclei are labeled with Hoechst, blue. Scale bar, 50 µm. (J) Gene transcript expression by quantitative RT-PCR for F4/80 and monocyte chemoattractant protein 1 (MCP1) indicates significantly increased mRNA levels in DKO kidneys compared with WT and SKO beginning at P35 (F4/80, n=3 and MCP1, n=6 at each time point for each genotype). Results are shown as mean ± SEM (ANOVA). ***P<0.001; **P<0.01; *P<0.05. (K) mRNA expression of iNOS is significantly increased in DKO at P49 and P70. Gene expression of Arg1 begins to increase at P49 and is significantly increased at P70. n=3 for each time point; results are shown as mean ± SEM (ANOVA). ***P<0.001; **P<0.01 compared with both WT and SKO.
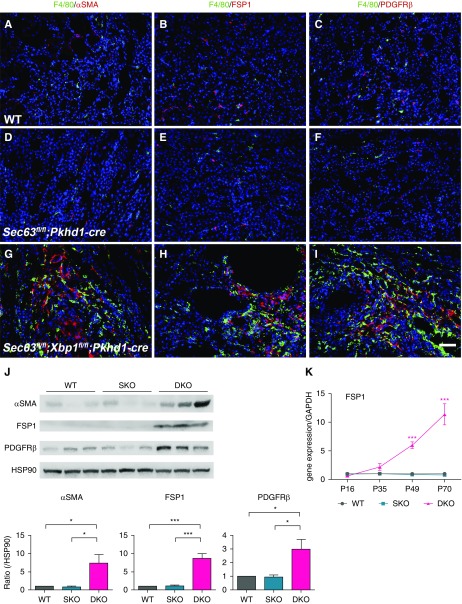
Compared with WT and SKO kidneys, regions of DKO kidneys with enhanced macrophage infiltration indicated by F4/80 staining also showed markedly increased presence of myofibroblasts positive for α-smooth muscle actin (Figure 3, A, D, and G), FSP1 (Figure 3, B, E, and H), and PDGFRβ (Figure 3, C, F, and I) at P49. These results were corroborated by immunoblotting of P49 whole kidney lysates, which showed higher levels of the respective proteins in DKO kidneys compared with WT and SKO kidneys (Figure 3J). We examined the time course of FSP1 mRNA expression and found a progressively significant increase in DKO kidneys after P35 (Figure 3K). These data support the conclusion that dual inactivation of Sec63 and Xbp1 in mouse kidney collecting ducts show a genotype-dependent initial inflammatory response that progresses and is associated with an expanded myofibroblast population mimicking key elements of chronic progressive kidney disease.
Figure 3.
Myofibroblasts coexist with macrophages in the kidneys of mice of double inactivation with Sec63 and Xbp1. (A–I) Immunocytochemical analysis of F4/80 (green) and (A, D, and G) α-smooth muscle actin (αSMA; red), (B, E, and H) FSP1 (red), and (C, F, and I) PDGFRβ (red) in kidneys with the indicated genotypes at P49. (G) αSMA-positive, (H) FSP1-positive, and (I) PDGFRβ-positive fibroblasts coexist with macrophages in DKO kidneys. Nuclei are labeled with Hoechst, blue. Scale bar, 50 µm. (J) Representative immunoblots (top) and aggregate densitometric quantification from kidney lysates (bottom) show that levels of αSMA, FSP1, and PDGFRβ are significantly increased in DKO compared with WT and SKO kidneys at P49. Densitometry is normalized to HSP90, which serves as loading control. n=3 per genotype; results are shown as mean ± SEM (ANOVA). ***P<0.001; *P<0.05. (K) Time course for mRNA expression level by quantitative RT-PCR of FSP1 shows significantly increased levels of transcripts in DKO kidneys at P49 and P70. n=3 at each time point for each genotype; results are shown as mean ± SEM (ANOVA). ***P<0.001.
Sec63 Inactivation in Collecting Duct Does Not Activate UPR
We had previously shown that inactivation of Sec63 by Cdh16(Ksp)-cre results in selective activation of the Ire1α-Xbp1 branch of UPR, but not the Perk/eIF2α and Atf6α branches of UPR.18,19 Given the discordant kidney phenotypes between the Sec63fl/fl;Xbp1fl/fl;Cdh16(Ksp)-cre models that produce polycystic kidneys and Sec63fl/fl;Xbp1fl/fl;Pkhd1-cre models in this study that result in an inflammatory and fibrotic kidney injury without overt polycystin-1–dependent polycystic kidney disease, we sought to determine whether Sec63fl/fl;Pkhd1-cre SKO kidneys also showed selective activation of the Ire1α-Xbp1 branch of UPR. Consistent with the Cdh16(Ksp)-cre models, there was no activation of either the Perk/eIF2α or Atf6α branches of UPR in WT, SKO, and DKO kidneys at P35 (data not shown). Surprisingly, there was also no significant expression of Xbp1s indicative of Ire1α-Xbp1 activation in whole kidney lysates from SKO mice as determined by RT-PCR, whereas the tunicamycin-treated control mice showed robust Xbp1 splicing (Figure 4A). This was confirmed by Xbp1s quantitative PCR (Figure 4B). Active XBP1s promotes transcription of genes encoding chaperones and other proteins involved in UPR, including BiP, ERdj4, Sec61α, and Herp.12,13 We looked for indirect evidence of XBP1s activity by measuring transcript levels of the canonical Xbp1s target ERdj4 by quantitative PCR and found that there was no difference between WT, SKO, and DKO (Figure 4C). ER stress induces Ire1α hyperphosphorylation, which activates its endoribonuclease function, mediating splicing of Xbp1 mRNA to the active Xbp1s form. We examined Ire1α hyperphosphorylation by Phos-Tag gel electrophoresis. We were unable to detect activation of Ire1α in SKO whole kidney lysates, although there was hyperphosphorylation of Ire1α in DKO kidneys (Figure 4D). Loss of Xbp1 is known to result in Ire1α hyperphosphorylation.18 Because we used whole kidney lysates at P35, a stage at which inflammation markers are active in DKO, the observed activation of Ire1α in DKO kidneys may represent activation in collecting duct cells, inflammatory cells, and activated myofibroblasts.
Figure 4.
Absence of activation of the Ire1α/Xbp1 branch of UPR after collecting duct selective inactivation of Sec63. (A) Xbp1 mRNA splicing (Xbp1s) is absent in WT, Sec63fl/fl;Pkhd1-cre (SKO), and Sec63fl/fl;Xbp1fl/fl;Pkhd1-cre (DKO) whole kidney mRNA by RT-PCR. Tunicamycin (Tun) serves as positive control. (B) mRNA expression of Xbp1s by quantitative RT-PCR indicates no difference among WT, SKO, and DKO kidneys at P35 (n=5 samples per genotype). Results are shown as mean ± SEM (ANOVA). (C) Gene expression of the canonical Xbp1 target ERdj4 is also not different among WT, SKO, and DKO kidneys at P35 (n=3 samples per genotype). Results are shown as mean ± SEM (ANOVA). (D) Ire1α activation examined by immunoblotting analysis using Phos-Tag electrophoresis to separate phosphorylated Ire1α (p-Ire1α) from the unphosphorylated form in P35 kidney lysates increased p-Ire1α in DKO kidneys, but not in WT and SKO lysates. Tun serves as positive control. (E) Immunocytochemical analysis of WT kidney at P35. L1CAM recognizes Tamm–Horsfall protein (THP)- and AQP2-positive cells but not LTL-positive cells. Scale bar, 25 µm. (F) Gating for selecting L1(+), LTL(−) cells and LTL(+), L1(−) cells from dissociated SKO kidneys for RNA isolation. (G) Quantitative RT-PCR showing relative expression of megalin and AQP2, and of megalin and ENaC from WT P35 whole kidneys. Expression of AQP2 and ENaC arbitrarily set at 1. Results are shown as mean ± SEM (two-tailed t test). n=3; **P<0.01; ***P<0.001. (H) Quantitative RT-PCR of megalin and AQP2, and of megalin and ENaC in L1(+), LTL(−) cells and LTL(+), L1(−) cells of dissociated WT kidneys at P35. Expression of megalin in L1(+), LTL(−) cells is arbitrarily set at 1; expression of AQP2 and ENaC in LTL(+), L1(−) cells is arbitrarily set at 1. Results are shown as mean ± SEM (two-tailed t test). n=3; ***P<0.001; **P<0.01; *P<0.05. (I) Quantitative RT-PCR showing Xbp1s and ERdj4 in L1(+), LTL(−) cells from WT, SKO, and DKO kidneys at P35. Xbp1s is unchanged among genotypes. ERdj4 is increased in LTL(+), L1(−) cells from SKO kidneys. Results are shown as mean ± SEM (ANOVA). n=3 per genotype; **P<0.01; *P<0.05.
Given the clear Xbp1 genotype dependence of the inflammatory and fibrotic phenotype in the DKO kidneys, we considered the possibility that our inability to detect activated Xbp1s in the SKO whole kidney lysates was due to inadequate sensitivity because Pkhd1-cre only deleted Sec63 in collecting duct cells. To test this possibility, we enriched for collecting duct cells by FACS to improve sensitivity of detection of Xbp1s or its targets in SKO kidneys. We used L1, a transmembrane glycoprotein belonging to the Ig superfamily of cell adhesion molecules, for enrichment by FACS of dissociated kidneys.48 In the mouse kidney, L1 is expressed in Tamm–Horsfall protein–positive mTAL and Aqp2-positive collecting duct cells but is absent from LTL-positive proximal tubule cells (Figure 4E). We selected L1+/LTL‒ cells from freshly dissociated kidney tissues (Figure 4F). Expression of the proximal tubule marker megalin is approximately three- to four-fold higher than AQP2 and ENaC in whole kidney mRNA (Figure 4G), whereas the L1+/LTL‒ cells showed approximately three-fold higher expression of Aqp2 and ENaC relative to megalin (Figure 4H), indicating an approximately ten-fold relative enrichment of collecting duct cells by FACS compared with whole kidney lysates. There was a commensurate 8- to 12-fold depletion of collecting duct cells in the LTL+/L1‒ cell population (Figure 4H). There were no genotype dependent differences in expression of Xbp1s in the L1+/LTL‒ populations enriched for collecting duct cells from WT, SKO, and DKO mouse kidneys (Figure 4I). There was a modest but significant increase in ERdj4 levels in SKO kidneys (with the caveat of a potentially underpowered comparison) compared with control and DKO (Figure 4I), although another Xbp1s target gene, EDEM, did not show similarly increased expression in SKO (data not shown). In aggregate, an increase in XBP1s activity in the absence of Sec63 in this model is likely very modest and rapidly degraded.49 These data indicate that in the absence of Sec63, loss of collecting duct cell autonomous homeostatic levels of Xbp1, without overt activation of UPR or significant increased steady-state levels of Xbp1s, disrupts normal proteostasis and is sufficient to cause CKD, beginning with proinflammatory injury signals from collecting duct cells and leading to a progressive inflammatory and fibrotic phenotype.
Sec63 Is in a Complex with Ire1α
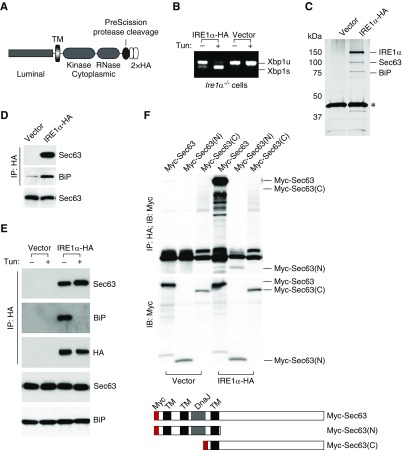
Our previous finding that inhibition of protein synthesis was able to prevent activation of UPR after loss of Sec63 indicated that accumulation of defective proteins in the absence of Sec63 was the cause of UPR activation.18 The absence of significant activation of UPR in the SKO model in this study indicates that the absence of Sec63 in collecting ducts does not result in comparably high levels of generalized ER stress. In light of this, the strict genotype dependence of the chronic kidney injury model on loss of both Sec63 and Xbp1 suggested that there may be an alternative regulatory interaction between Sec63 and Xbp1 proteins. Because Ire1α function is central to Xbp1 activation, we considered the possibility Sec63 regulates Ire1α activity through a direct mechanism rather than indirectly through ER stress. To test this possibility, we performed an IRE1α pull-down in Ire1α−/− knockout MEFs reconstituted with a double HA-tagged human IRE1α (Figure 5A). Re-expressed HA-tagged IRE1α spliced the endogenous Xbp1 mRNA upon tunicamycin treatment, indicating that it is functionally equivalent to the endogenous Ire1α (Figure 5B). IRE1α-HA and its interacting proteins were isolated from the cell lysates using HA-agarose beads. Immunoprecipitation with anti-HA recovered two distinct proteins with apparent molecular mass 78 and 95 kDa in addition to the band at the expected migration of IRE1α (Figure 5C). Mass spectrometry analysis identified the 78 kDa band as BiP, which is a well known IRE1α-interacting protein50 and therefore provided an internal positive control. The 95 kDa band was identified as Sec63, indicating that Sec63 likely exists in a complex with IRE1α and BiP. IRE1α-Sec63 association was confirmed by immunoblotting using a Sec63-specific antiserum after IRE1α immunoprecipitation (Figure 5D). We tested whether IRE1α-Sec63 interaction is altered by IRE1α activation or is dependent on the association with BiP. BiP was dissociated from IRE1α by tunicamycin treatment as previously reported,50 and IRE1α was immunoprecipitated to assess continued presence of Sec63 in the complex. In contrast to BiP, Sec63 binding to IRE1α was unchanged or perhaps even slightly increased by tunicamycin treatment (Figure 5E), suggesting that the dissociation from Sec63 is neither a prerequisite for, nor a consequence of, IRE1α activation. Deletion of the N-terminal ER luminal DnaJ domain in Sec63 abolished the binding of Sec63 to IRE1α, suggesting that Sec63-IRE1α interaction occurs in the ER lumen (Figure 5F). In aggregate, the data suggest two experimentally separable functions of Sec63. Loss of Sec63 can result in protein maturation defects that result in activation of the Ire1α-Xbp1 branch of UPR, but independently of this function, Sec63 interacts with Ire1α in a non-UPR–dependent manner.
Figure 5.
Sec63 and IRE1α are present in a complex. (A) Schematic representation of IRE1α-HA construct. Two copies of HA tags and a PreScission Protease cleavage site were fused to the C-terminal of human IRE1α. (B) Xbp1 mRNA splicing by tunicamycin treatment in Ire1α-deficient MEF cells reconstituted with IRE1α-HA. (C) Lysates of Ire1α−/− MEFs stably transfected with IRE1α-HA or empty vector were subjected to affinity purification using anti-HA (3F10)-agarose matrix. IRE1α was eluted from the matrix using PreScission Protease. IRE1α and two proteins copurified by immunoprecipitation were revealed by silver staining and subsequently identified by mass spectrometry. *PreScission Protease. (D) Immunoblot analysis of anti-HA immunoprecipitate prepared from Ire1α−/− MEF cells stably expressing IRE1α-HA or empty vector showing coimmunoprecipitation of endogenous Sec63 and BiP. Bottom panel shows Sec63 immunoblot of lysates. (E) Activation of UPR by tunicamycin does not dissociate the IRE1α-Sec63 complex. Mock- or IRE1α-HA reconstituted Ire1α−/− MEF cells were treated with tunicamycin (2 µg/ml for 2 hours) and subjected to coimmunoprecipitation analysis. Sec63, but not BiP, remains associated with IRE1α after tunicamycin treatment. Bottom two panels are immunoblots of lysates from the starting material. (F) The DnaJ domain is required for interaction between IRE1α and Sec63. HEK293 cells were transfected with indicated pairs of/plasmids. Immunoprecipitated samples were subjected to immunoblotting (top panel). Only Sec63 constructs that include the DnaJ domain coimmunoprecipitate with IRE1α. Middle panel shows immunoblotting of cell lysates to control for protein expression. Bottom panel shows schematic representation of Sec63 expression plasmids.
CKD Occurs Due to Loss of Xbp1s Homeostatic Activity in the Absence of Sec63
Because there was no increased level of Xbp1s in SKO, we sought to evaluate alternative mechanisms by which expression of Xbp1 may mediate a protective anti-inflammatory state after loss of Sec63. Chronic hyperphosphorylation of Ire1α, as we have shown occurs after loss of Xbp1,18 results in the activation of JNK,51 NF-κβ,52 and the NLRP3 inflammasome.53,54 We examined the activation status of NF-κβ in the nuclear fraction and of JNK in whole kidney lysates of WT, SKO, and DKO kidneys and found no difference in the phosphorylation status of either protein (Supplemental Figure 9, A and B). We also directly tested the in vivo functional role of Nlrp3 in the kidney disease in our DKO mice by intercrossing DKO mice with Nlrp3‒ alleles to generate Sec63fl/fl;Xbp1fl/fl;Nlrp3−/−;Pkhd1-cre mice. Loss of Nlrp3 inflammasome did not rescue the kidney function, inflammation, or fibrosis on the Sec63-Xbp1 double mutant background as determined by histology, BUN, and serum creatinine (Supplemental Figure 9, C and D). These data do not support a role for activation of Ire1α in the proinflammatory phenotype resulting from the absence of Sec63 and Xbp1. We also examined potential inflammatory signals expressed by the collecting duct on the basis of existing evidence.40 We looked at the differential expression of KLF5, P2ry14, and Snail 40,41,55,56 and found no significant difference between SKO and DKO kidneys (Supplemental Figure 10, A–C). It is likely that signals other than the ones evaluated above underlie the chronic kidney injury phenotype observed in the DKO mice.
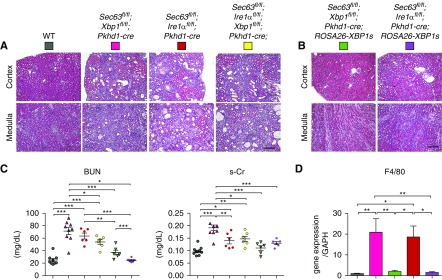
Because the function of Ire1α and Xbp1 are highly interrelated, we sought to define the relative roles of each gene product in the kidney injury phenotype associated with concomitant loss with Sec63. In addition to splicing Xbp1, the endoribonuclease activity of activated Ire1α is responsible for regulated IRE1-dependent decay of mRNA (RIDD), an Xbp1-independent mechanism for maintaining ER homeostasis.57 It is therefore possible that loss of Xbp1 and Sec63 mediates an inflammatory kidney phenotype through hyperactivation of Ire1α and RIDD, perhaps also influenced by disruption of Sec63-Ire1α complex formation. To evaluate a possible role for Ire1α activation and RIDD, we first determined whether loss of Ire1α and Sec63 leads to a similar phenotype as loss of Xbp1 and Sec63. As a control, Ire1αfl/fl;Pkhd1-cre mice do not develop an inflammatory kidney phenotype (Supplemental Figure 11). We generated Sec63fl/fl;Ire1αfl/fl;Pkhd1-cre mice and found that inactivation of Sec63 and Ire1α in the collecting duct caused renal interstitial inflammation and fibrosis similar to loss of Sec63 and Xbp1, as indicated by histologic appearance, measures of kidney function (BUN, creatinine), and whole kidney mRNA expression of F4/80 (Figure 6, A, C, and D). These data indicate that in the absence of Sec63, Ire1α is as important to non-UPR–dependent proteostasis as Xbp1, but neither activation of Ire1α kinase activity nor RIDD in collecting ducts is the cause of the kidney injury phenotype because it still occurs in the absence of Ire1α. To exclude the possibility that Ire1α and Xbp1 inactivation cause similar phenotypes by independent mechanisms, we produced mice with triple knockout Sec63fl/fl;Ire1αfl/fl;Xbp1fl/fl;Pkhd1-cre in the collecting ducts. If Ire1α and Xbp1 produced kidney injury by distinct mechanisms, we would anticipate some degree of additive severity in the clinical presentation of the triple knockout. The triple knockout showed a similar phenotype to either the Sec63-Xbp1 or Sec63-Ire1α models (Figure 6, A and C), indicating the absence of any additive effects (e.g., due to RIDD and unspliced Xbp1), and confirming that chronic kidney injury occurs because of homeostatic interaction between Sec63 and the Ire1α-Xbp1 arm of UPR.
Figure 6.
Xbp1s is essential to suppress renal interstitial inflammation and fibrosis in kidneys with inactivation of Sec63. (A) Hematoxylin and eosin (H&E) staining of renal cortex and medulla of the indicated genotypes at P70 showing chronic kidney injury both DKOs and the triple knockout. Scale bar, 500 µm. (B) H&E staining of renal cortex and medulla of the indicated genotypes at P70 showing rescue of chronic kidney injury in both DKOs with transgenic re-expression of XBP1s. Scale bar, 500 µm. (C) Quantitative assessment of BUN and serum creatinine (s-Cr). The colors of the symbols correspond to the colors indicated for each of the genotype from (A and B). BUN and s-Cr indicate that Sec63-Xbp1 and Sec63-Ire1α as well as the triple knockout have impaired kidney function at P70. This impaired kidney function is resolved by re-expression of XBP1s on either DKO background. Results are shown as mean ± SEM (ANOVA). ***P<0.001; **P<0.01; *P<0.05. (D) Gene expression of F4/80 indicate that both DKO models have elevated levels that are resolved by re-expression of XBP1s. The color of the bars defines the genotypes on the basis of (A and B). Results are shown as mean ± SEM (ANOVA). n=3 mice for group; **P<0.01; *P<0.05.
Finally, we sought direct evidence that loss of Xbp1s was the predominant determinant of kidney injury in Sec63 knockouts. The majority of previously described Xbp1 functions are focused on the spliced form. However, some studies have examined the function of unspliced Xbp1, which was shown to reduce autophagy via the degradation of FoxO1 by the 20S proteasome in cancer cells,58 to protect endothelial cells from oxidative stress via upregulation of heme-oxygenase-1,59 and to regulate NF-κβ activity via the estrogen receptor α.60 To determine the role of XBP1s specifically in the chronic kidney injury models, we re-expressed XBP1s in Sec63-Xbp1 and Sec63-Ire1α knockout kidneys in vivo using the previously described ROSA-XBP1s transgenic line that has a loxP-flanked transcriptional stop sequence coupled to human XBP1s complementary DNA inserted into the ROSA26 locus.18 Expression of Pkhd1-cre removes the stop sequence and turns on the expression of XBP1s only in collecting duct cells that express Cre. Sec63fl/fl;Xbp1fl/fl;Pkhd1-cre;ROSA-XBP1s mice show that expression of XBP1s is sufficient to fully rescue the inflammation, fibrosis, and kidney function of Sec63-Xbp1 DKO mutant mice as determined by histology, BUN, serum creatinine, and expression of F4/80 (Figure 6, B–D). The absolute dependence of the chronic kidney injury phenotype on XBP1s was further confirmed by showing that ROSA-XBP1s transgene also rescued the kidney function and inflammation of Sec63 and Ire1α double mutant mice, as determined by histology, and reduction in BUN, creatinine, and gene expression of F4/80 (Figure 6, B–D). Expression of XBP1s also rescued renal inflammation of Sec63 and Ire1α and Xbp1 triple mutant mouse (Supplemental Figure 12). The aggregate data from this study show that basal levels of Xbp1s activity in collecting ducts is both necessary and sufficient to maintain proteostasis and protect against renal inflammation and functional decline in the absence of Sec63. The Sec63-Xbp1 DKO mouse offers a novel genetic model of chronic kidney injury that can serve as a platform for discovery of signals the may underlie CKD of disparate etiologies.
Discussion
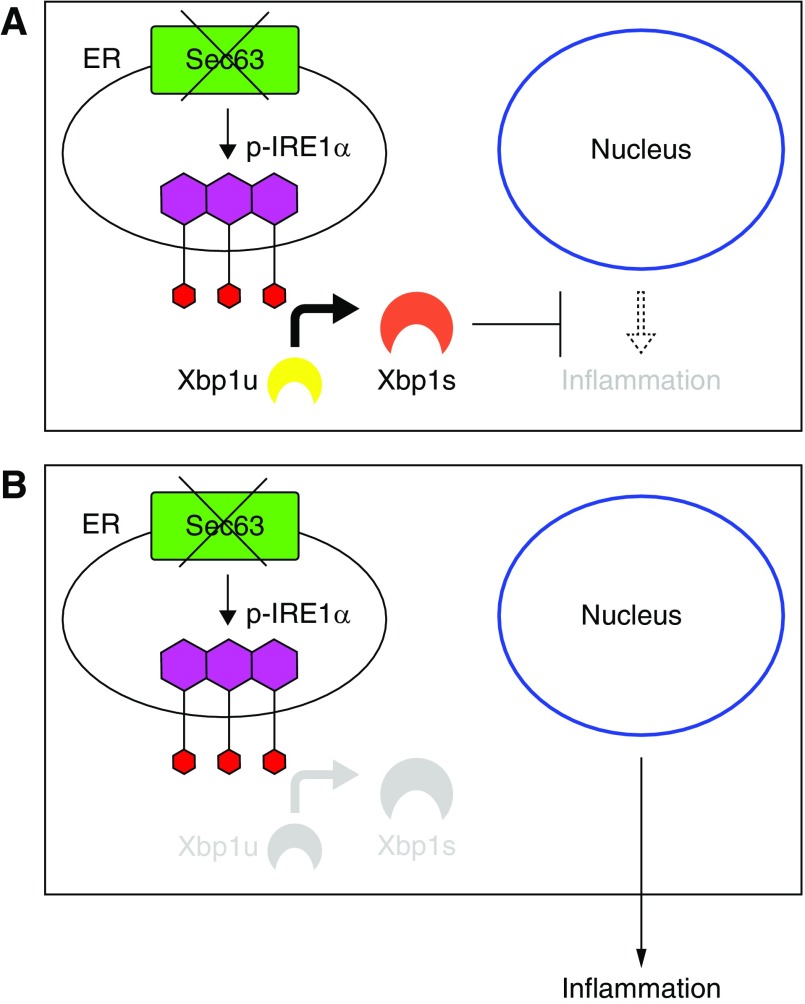
We have developed a genetic model of tubulointerstitial kidney disease on the basis of postnatal inactivation of Sec63 and either Xbp1 or Ire1α in collecting ducts. Like other models of tubulointerstitial kidney disease, this model manifests by interstitial inflammation and increased myofibroblasts and collagen deposition complicated by progressive kidney dysfunction. Inactivation of Sec63 in the collecting duct provides a sensitized background upon which intact Xbp1-Ire1α are required to maintain a homeostatic state that prevents dysregulated signals from initiating a proinflammatory cascade (Figure 7A). This proteostasis depends specifically on basal levels of the active, Xbp1s, the formation of which is dependent on the endoribonuclease activity of Ire1α. When Xbp1s function is inactivated by targeting either Xbp1 or Ire1α in addition to Sec63 in the collecting duct, proteostasis is disrupted triggering a cell-autonomous process that promotes a whole kidney inflammatory response with increased myofibroblasts and collagen deposition that progresses to tubulointerstitial CKD (Figure 7B).
Figure 7.
Schematic diagram depicting the interaction among Sec63, Ire1alpha, and Xbp1. (A) Xbp1 compensates for loss of Sec63 and prevents inflammation. (B) Dual loss of Sec63 and Xbp1 promotes renal interstitial inflammation.
Genetic tubulointerstitial kidney diseases in man that affect proteostasis and present with inflammation and fibrosis as key manifestations include autosomal dominant tubulointerstitial kidney diseases (ADTKD). The known causative genes for human ADTKD to date are UMOD,61 MUC1,62,63 REN,64 HNF1β,65 and SEC61A1.66 Among these, ADTKD due to mutations in the UMOD, MUC1, and REN affect the structure of the respective secreted proteins, which consequently accumulate in cells and act in a dominant manner to trigger UPR. The primary site of the initial cellular damage is the thick ascending limb of the loop of Henle in the UMOD mutants and the renin secreting cells in the case of REN mutants. In this regard, the collecting duct origin of the kidney disease in our DKO model is notable. Recent studies have shown that collecting duct cells in particular play an important role in the response to renal injury. For example, the transcription factor KLF5 expressed in collecting duct cells is a pivotal regulator of the renal injury response in the unilateral ureteral obstruction model.40 Collecting duct cells are also responsible for upregulation of apoptosis-associated speck-like protein containing a caspase recruitment domain, a component of the inflammasome assembly complex, which plays a role in renal inflammatory and fibrotic responses after unilateral ureteral obstruction.67 Perhaps the role of the collecting duct in the antimicrobial defense of the kidney68,69 also predisposes this segment to eliciting inflammatory responses when proteostasis is perturbed. Furthermore, changes in the microenvironment of tubule cells due to altered electrolyte metabolism may affect the chemokine and inflammatory milieu.70
Another causal gene for ADTKD, SEC61A1, encodes the key component of the protein translocation channel in the ER membrane that associates with SEC63. Homozygous partial loss of function missense mutation in Sec61a1 in mice, although not studied in the kidney, does result in activation of UPR in pancreatic β cells with consequent cell death and dysregulation of glucose homeostasis due to insulin deficiency.71 Despite the fact that Sec63 associates and functions closely with the Sec61a1 translocon, we found no discernible activation of the UPR in the Sec63-only SKO collecting duct inactivation model. Although there was evidence of mild interstitial inflammation after 4 months in SKO mice, much earlier onset progressive tubulointerstitial kidney disease was elicited by simultaneous inactivation of the Ire1α-Xbp1 UPR pathway components with Sec63 in collecting ducts. This suggests that the DKO model of kidney disease results from dysregulated basal protein homeostasis (i.e., proteostasis) rather than loss of adaptive protection conferred by activation of UPR. This appears to be a unique feature of collecting ducts because we had previously found that inactivating Sec63 in podocytes resulted in activation of at least the Ire1α-Xbp1 branch of UPR, which served as a compensatory mechanism to prevent podocyte dysfunction and loss.25 Similarly, inactivation of Sec63 using Cdh16(Ksp)-Cre, which targets mTAL, DCT, and the collecting duct, resulted in activation of the Ire1α-Xbp1 pathway detectable in whole kidney lysates. Concomitant inactivation of Xbp1 resulted in worsening kidney cyst formation, corroborating the adaptive value of UPR activation in this model as well. Although it remains possible that loss of Sec63 in collecting duct alone does activate very low levels of UPR as perhaps suggested by the approximately 25% increase in expression of the canonical Xbp1s transcriptional target Erdj4 that we observed, our inability to detect Xbp1s even in cell populations enriched for collecting duct cells suggests that activation of this branch of UPR is not the critical determinant of the kidney disease observed in DKO animals. Nonetheless, a key aspect of our DKO model is the clear involvement of renal tubule cell-autonomous, Xbp1-dependent pathways without any Ire1-dependent/Xbp1-independent contributions. This conclusion is supported by our finding that Xbp1s re-expression rescued both the Sec63-Xbp1 and Sec63-Ire1α dual inactivation models. Coupled with the findings of a complex formation between Sec63 and Ire1α20 and the strict genotype dependence on Xbp1s expression of the tubulointerstitial kidney disease after loss of Sec63, this model suggests a possible novel homeostatic functional relationship of the Sec63-Ire1α/Xbp1 complex.
Another novel feature of this model is the absence of overt polycystin-1–dependent polycystic kidney disease with collecting duct–only inactivation of Sec63. This again appears to be a feature unique to collecting ducts and possibly the timing of gene inactivation. Whereas inactivation of Sec63 by Cdh16(Ksp)-cre in mTAL, DCT, and the collecting duct before E15.5 causes cyst formation,18,22 the collecting duct–specific inactivation occurring largely after birth does not result in cyst formation. Cyst formation in the Cdh16(Ksp)-Cre model is truly a hypomorphic manifestation of autosomal dominant polycystic kidney disease (ADPKD) due to ineffective biogenesis of polycystin-1 that is rescued by transgenic overexpression of additional copies of the Pkd1 gene.22 Dual inactivation of Sec63 and Xbp1 using Cdh16(Ksp)-Cre worsened the polycystic kidney disease, indicating that, as in other models, UPR is a protective adaptation. This worsening polycystic kidney disease in the Sec63-Xbp1 knockout was also partially reversed by compensatory increases in Pkd1 dosage.18 It is therefore striking that SKO and DKO early gene inactivation in all distal nephron segments gives rise to genotype-dependent severity of polycystic kidney disease, but later-onset gene inactivation confined to the collecting duct in our DKO model gives rise to isolated tubulointerstitial kidney disease with very mild cystic disease that is not responsive to Pkd1 dosage and therefore represents a distinct pathophysiologic process.
Although the mechanism underlying this distinction can only be speculated at present, it does pose the interesting hypothesis that the ADPKD/PCLD genes involved in general protein biogenesis pathways can cause a spectrum of renal manifestations ranging from generalized polycystic kidney disease to tubulointerstitial kidney disease, depending on the timing and affected cell type of the particular molecular “injury.” Under this formulation, mild disruption of ER protein biogenesis that nonetheless affects effective biogenesis of the low abundance complex polypeptide chain of polycystin-1 will manifest with polycystic kidney disease, whereas disruption of ER biogenesis with more significant consequences for a broad spectrum of proteins (e.g., Sec61a1 mutations) may engender damage-related proinflammatory signals giving rise to primarily inflammatory kidney disease, which may lead to mild cystic disease as a secondary manifestation. Variation along this spectrum is determined both by the nature of the affected gene as well as properties of the affected cell type. For example, cells with polycystin-1 expression levels far above a critical threshold may be more resistant to the polycystic phenotype despite ER proteostasis defects, and cells whose normal state is critically dependent on a broad array of proteins synthesized in the ER may be more susceptible to expressing damage responses leading to inflammation in the setting of disrupted protein homeostasis. In support of this hypothesis, a recent study has identified dominant mutations in DNAJB11, encoding another DnaJ domain containing BiP cochaperone similar to Sec63, as responsible for a phenotypic hybrid manifesting features of both ADPKD/PCLD and ADTKD.72 Patients with DNAJB11 mutations had varying presentations that included PCLD and cystic kidneys without enlargement that progressed to atrophy with significant tubulointerstitial fibrosis and progressive loss of kidney function.
To our knowledge, our genetic model is the first one that points to a direct role of homeostatic Xbp1s in modulating kidney inflammation and fibrosis, and suggests that discovery of the proinflammatory damage signals resulting from loss of this homeostatic function in the collecting duct will enable novel directions for investigation of causes of chronic tubulointerstitial nephritis. Furthermore, these findings suggest that avenues that can affect baseline Xbp1s signaling in the kidney may represent a viable therapeutic strategy for patients with chronic tubulointerstitial kidney diseases.
Disclosures
S.S. is a founder, shareholder, and consultant for Goldfinch Bio.
Supplementary Material
Acknowledgments
We thank Dr. Ugo Cavallaro for providing rat anti-L1CAM antibody. We also thank Dr. Tomokazu Sumida for technical assistance with FACS sorting.
Y.I., S.F., A.M., C.Z., A.-R.G., and A.-H.L. performed experiments. S.F. and S.S. designed the study. Y.I., S.F., and S.S. wrote the manuscript.
This work was supported by grants DK54053 and DK100592 to S.S., and by the George M. O’Brien Kidney Center at Yale University (P30 DK079310 to Dr. Peter Aronson).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018060614/-/DCSupplemental.
Supplemental Figure 1. Mild inflammation in Sec63 single mutant kidneys at P120.
Supplemental Figure 2. Pkhd1-cre expression examined with the RosamT/mG, double-fluorescent Cre reporter.
Supplemental Figure 3. Progression of inflammatory infiltrates in Sec63fl/fl; Xbp1fl/fl; Pkhd1-cre kidneys.
Supplemental Figure 4. Collecting duct inactivation Xbp1 does not result in inflammation or kidney dysfunction.
Supplemental Figure 5. Pkd1F/H-BAC does not rescue the inflammatory phenotype in DKO mouse kidneys.
Supplemental Figure 6. Extrarenal activity of Pkhd1-cre.
Supplemental Figure 7. Hematoxylin and eosin (H&E) staining of liver, ureter, and bladder of WT and Sec63-Xbp1 mutants (DKO) at P70.
Supplemental Figure 8. High magnification view of extensive macrophage infiltration indicated by F4/80 staining in Sec63fl/fl; Xbp1fl/fl; Pkhd1-cre (DKO) kidneys.
Supplemental Figure 9. NF-κβ, JNK, and Nlrp3 are not the inflammatory signals in DKO kidneys.
Supplemental Figure 10. KLF5, P2ry14, and Snail1 are unchanged between SKO and DKO kidneys at P35.
Supplemental Figure 11. Five-month-old Ire1αfl/fl; Pkhd1-cre mice do not display signs of inflammation or fibrosis.
Supplemental Figure 12. XBP1s transgene rescues kidney injury in Sec63, Ire1α, Xbp1 triple knockout kidneys.
References
- 1.Radhakrishnan J, Remuzzi G, Saran R, Williams DE, Rios-Burrows N, Powe N, et al. CDC-CKD Surveillance Team; European CKD Burden Consortium; CKD.QLD group : Taming the chronic kidney disease epidemic: A global view of surveillance efforts. Kidney Int 86: 246–250, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mills KT, Xu Y, Zhang W, Bundy JD, Chen CS, Kelly TN, et al.: A systematic analysis of worldwide population-based data on the global burden of chronic kidney disease in 2010. Kidney Int 88: 950–957, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El Karoui K, Viau A, Dellis O, Bagattin A, Nguyen C, Baron W, et al.: Endoplasmic reticulum stress drives proteinuria-induced kidney lesions via Lipocalin 2. Nat Commun 7: 10330, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim TW, Kim YJ, Seo CS, Kim HT, Park SR, Lee MY, et al.: Elsholtzia ciliata (Thunb.) Hylander attenuates renal inflammation and interstitial fibrosis via regulation of TGF-ß and Smad3 expression on unilateral ureteral obstruction rat model. Phytomedicine 23: 331–339, 2016 [DOI] [PubMed] [Google Scholar]
- 5.Cybulsky AV: Endoplasmic reticulum stress, the unfolded protein response and autophagy in kidney diseases. Nat Rev Nephrol 13: 681–696, 2017 [DOI] [PubMed] [Google Scholar]
- 6.Song CJ, Zimmerman KA, Henke SJ, Yoder BK: Inflammation and fibrosis in polycystic kidney disease. Results Probl Cell Differ 60: 323–344, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang HY, Kwon J, Cho EJ, Choi HI, Park C, Park HR, et al.: Proteomic analysis of protein expression affected by peroxiredoxin V knock-down in hypoxic kidney. J Proteome Res 9: 4003–4015, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Inagi R, Ishimoto Y, Nangaku M: Proteostasis in endoplasmic reticulum--new mechanisms in kidney disease. Nat Rev Nephrol 10: 369–378, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Tirasophon W, Welihinda AA, Kaufman RJ: A stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein kinase/endoribonuclease (Ire1p) in mammalian cells. Genes Dev 12: 1812–1824, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harding HP, Zhang Y, Ron D: Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397: 271–274, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Yoshida H, Haze K, Yanagi H, Yura T, Mori K: Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins. Involvement of basic leucine zipper transcription factors. J Biol Chem 273: 33741–33749, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Shaffer AL, Shapiro-Shelef M, Iwakoshi NN, Lee AH, Qian SB, Zhao H, et al.: XBP1, downstream of Blimp-1, expands the secretory apparatus and other organelles, and increases protein synthesis in plasma cell differentiation. Immunity 21: 81–93, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Lee AH, Iwakoshi NN, Glimcher LH: XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol 23: 7448–7459, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim B, Koo H, Yang S, Bang S, Jung Y, Kim Y, et al.: TC1(C8orf4) correlates with Wnt/beta-catenin target genes and aggressive biological behavior in gastric cancer. Clin Cancer Res 12: 3541–3548, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Ye J, Rawson RB, Komuro R, Chen X, Davé UP, Prywes R, et al.: ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol Cell 6: 1355–1364, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto K, Sato T, Matsui T, Sato M, Okada T, Yoshida H, et al.: Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev Cell 13: 365–376, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Adachi Y, Yamamoto K, Okada T, Yoshida H, Harada A, Mori K: ATF6 is a transcription factor specializing in the regulation of quality control proteins in the endoplasmic reticulum. Cell Struct Funct 33: 75–89, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Fedeles SV, So JS, Shrikhande A, Lee SH, Gallagher AR, Barkauskas CE, et al.: Sec63 and Xbp1 regulate IRE1α activity and polycystic disease severity. J Clin Invest 125: 1955–1967, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monk KR, Voas MG, Franzini-Armstrong C, Hakkinen IS, Talbot WS: Mutation of sec63 in zebrafish causes defects in myelinated axons and liver pathology. Dis Model Mech 6: 135–145, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plumb R, Zhang ZR, Appathurai S, Mariappan M: A functional link between the co-translational protein translocation pathway and the UPR. ELife 4, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davila S, Furu L, Gharavi AG, Tian X, Onoe T, Qian Q, et al.: Mutations in SEC63 cause autosomal dominant polycystic liver disease. Nat Genet 36: 575–577, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Fedeles SV, Tian X, Gallagher AR, Mitobe M, Nishio S, Lee SH, et al.: A genetic interaction network of five genes for human polycystic kidney and liver diseases defines polycystin-1 as the central determinant of cyst formation. Nat Genet 43: 639–647, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janssen MJ, Waanders E, Te Morsche RH, Xing R, Dijkman HB, Woudenberg J, Drenth JP: Secondary, somatic mutations might promote cyst formation in patients with autosomal dominant polycystic liver disease. Gastroenterology 141: 2056–2063 e2, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Fedeles SV, Gallagher AR, Somlo S: Polycystin-1: A master regulator of intersecting cystic pathways. Trends Mol Med 20: 251–260, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hassan H, Tian X, Inoue K, Chai N, Liu C, Soda K, et al.: Essential role of X-box binding protein-1 during endoplasmic reticulum stress in podocytes. J Am Soc Nephrol 27: 1055–1065, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hetz C, Glimcher LH: Fine-tuning of the unfolded protein response: Assembling the IRE1alpha interactome. Mol Cell 35: 551–561, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee AH, Scapa EF, Cohen DE, Glimcher LH: Regulation of hepatic lipogenesis by the transcription factor XBP1. Science 320: 1492–1496, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel V, Li L, Cobo-Stark P, Shao X, Somlo S, Lin F, et al.: Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia. Hum Mol Genet 17: 1578–1590, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cai Y, Fedeles SV, Dong K, Anyatonwu G, Onoe T, Mitobe M, et al.: Altered trafficking and stability of polycystins underlie polycystic kidney disease. J Clin Invest 124: 5129–5144, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwawaki T, Akai R, Yamanaka S, Kohno K: Function of IRE1 alpha in the placenta is essential for placental development and embryonic viability. Proc Natl Acad Sci U S A 106: 16657–16662, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sutterwala FS, Ogura Y, Szczepanik M, Lara-Tejero M, Lichtenberger GS, Grant EP, et al.: Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity 24: 317–327, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L: A global double-fluorescent Cre reporter mouse. Genesis 45: 593–605, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Shibazaki S, Yu Z, Nishio S, Tian X, Thomson RB, Mitobe M, et al.: Cyst formation and activation of the extracellular regulated kinase pathway after kidney specific inactivation of Pkd1. Hum Mol Genet 17: 1505–1516, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zou Z, Chung B, Nguyen T, Mentone S, Thomson B, Biemesderfer D: Linking receptor-mediated endocytosis and cell signaling: Evidence for regulated intramembrane proteolysis of megalin in proximal tubule. J Biol Chem 279: 34302–34310, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Magrini E, Villa A, Angiolini F, Doni A, Mazzarol G, Rudini N, et al.: Endothelial deficiency of L1 reduces tumor angiogenesis and promotes vessel normalization. J Clin Invest 124: 4335–4350, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iwakoshi NN, Lee AH, Glimcher LH: The X-box binding protein-1 transcription factor is required for plasma cell differentiation and the unfolded protein response. Immunol Rev 194: 29–38, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, et al.: Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol 22: 317–326, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Copple BL: Hypoxia stimulates hepatocyte epithelial to mesenchymal transition by hypoxia-inducible factor and transforming growth factor-beta-dependent mechanisms. Liver Int 30: 669–682, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nilsson L, Madsen K, Topcu SO, Jensen BL, Frøkiær J, Nørregaard R: Disruption of cyclooxygenase-2 prevents downregulation of cortical AQP2 and AQP3 in response to bilateral ureteral obstruction in the mouse. Am J Physiol Renal Physiol 302: F1430–F1439, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Fujiu K, Manabe I, Nagai R: Renal collecting duct epithelial cells regulate inflammation in tubulointerstitial damage in mice. J Clin Invest 121: 3425–3441, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Azroyan A, Cortez-Retamozo V, Bouley R, Liberman R, Ruan YC, Kiselev E, et al.: Renal intercalated cells sense and mediate inflammation via the P2Y14 receptor. PLoS One 10: e0121419, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shao X, Somlo S, Igarashi P: Epithelial-specific Cre/lox recombination in the developing kidney and genitourinary tract. J Am Soc Nephrol 13: 1837–1846, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Ma M, Tian X, Igarashi P, Pazour GJ, Somlo S: Loss of cilia suppresses cyst growth in genetic models of autosomal dominant polycystic kidney disease. Nat Genet 45: 1004–1012, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anders HJ, Ryu M: Renal microenvironments and macrophage phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney Int 80: 915–925, 2011 [DOI] [PubMed] [Google Scholar]
- 45.Alikhan MA, Ricardo SD: Mononuclear phagocyte system in kidney disease and repair. Nephrology (Carlton) 18: 81–91, 2013 [DOI] [PubMed] [Google Scholar]
- 46.Kinsey GR: Macrophage dynamics in AKI to CKD progression. J Am Soc Nephrol 25: 209–211, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu B, Rutledge BJ, Gu L, Fiorillo J, Lukacs NW, Kunkel SL, et al.: Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J Exp Med 187: 601–608, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Helbert M, Bodger S, Cavenagh J, D’Cruz D, Thomas JM, MacCallum P: Optimising testing for phospholipid antibodies. J Clin Pathol 54: 693–698, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Majumder M, Huang C, Snider MD, Komar AA, Tanaka J, Kaufman RJ, et al.: A novel feedback loop regulates the response to endoplasmic reticulum stress via the cooperation of cytoplasmic splicing and mRNA translation. Mol Cell Biol 32: 992–1003, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D: Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol 2: 326–332, 2000 [DOI] [PubMed] [Google Scholar]
- 51.Rincón M, Flavell RA, Davis RA: The JNK and P38 MAP kinase signaling pathways in T cell-mediated immune responses. Free Radic Biol Med 28: 1328–1337, 2000 [DOI] [PubMed] [Google Scholar]
- 52.Dan HC, Sun M, Kaneko S, Feldman RI, Nicosia SV, Wang HG, et al.: Akt phosphorylation and stabilization of X-linked inhibitor of apoptosis protein (XIAP). J Biol Chem 279: 5405–5412, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Lerner AG, Upton JP, Praveen PV, Ghosh R, Nakagawa Y, Igbaria A, et al.: IRE1α induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell Metab 16: 250–264, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bronner DN, Abuaita BH, Chen X, Fitzgerald KA, Nuñez G, He Y, et al.: Endoplasmic reticulum stress activates the inflammasome via NLRP3- and caspase-2-driven mitochondrial damage. Immunity 43: 451–462, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grande MT, Sánchez-Laorden B, López-Blau C, De Frutos CA, Boutet A, Arévalo M, et al.: Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nat Med 21: 989–997, 2015 [DOI] [PubMed] [Google Scholar]
- 56.Simon-Tillaux N, Hertig A: Snail and kidney fibrosis. Nephrol Dial Transplant 32: 224–233, 2017 [DOI] [PubMed] [Google Scholar]
- 57.Maurel M, Chevet E, Tavernier J, Gerlo S: Getting RIDD of RNA: IRE1 in cell fate regulation. Trends Biochem Sci 39: 245–254, 2014 [DOI] [PubMed] [Google Scholar]
- 58.Zhao Y, Li X, Ma K, Yang J, Zhou J, Fu W, et al.: The axis of MAPK1/3-XBP1u-FOXO1 controls autophagic dynamics in cancer cells. Autophagy 9: 794–796, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martin D, Li Y, Yang J, Wang G, Margariti A, Jiang Z, et al.: Unspliced X-box-binding protein 1 (XBP1) protects endothelial cells from oxidative stress through interaction with histone deacetylase 3. J Biol Chem 289: 30625–30634, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu R, Warri A, Jin L, Zwart A, Riggins RB, Fang HB, et al.: NF-κB signaling is required for XBP1 (unspliced and spliced)-mediated effects on antiestrogen responsiveness and cell fate decisions in breast cancer. Mol Cell Biol 35: 379–390, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hart TC, Gorry MC, Hart PS, Woodard AS, Shihabi Z, Sandhu J, et al.: Mutations of the UMOD gene are responsible for medullary cystic kidney disease 2 and familial juvenile hyperuricaemic nephropathy. J Med Genet 39: 882–892, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kirby A, Gnirke A, Jaffe DB, Barešová V, Pochet N, Blumenstiel B, et al.: Mutations causing medullary cystic kidney disease type 1 lie in a large VNTR in MUC1 missed by massively parallel sequencing. Nat Genet 45: 299–303, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu SM, Bleyer AJ, Anis K, Herlitz L, Živná M, Hůlková H, et al.: Autosomal dominant tubulointerstitial kidney disease due to MUC1 mutation. Am J Kidney Dis 71: 495–500, 2018 [DOI] [PubMed] [Google Scholar]
- 64.Zivná M, Hůlková H, Matignon M, Hodanová K, Vylet’al P, Kalbácová M, et al.: Dominant renin gene mutations associated with early-onset hyperuricemia, anemia, and chronic kidney failure. Am J Hum Genet 85: 204–213, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lindner TH, Njolstad PR, Horikawa Y, Bostad L, Bell GI, Sovik O: A novel syndrome of diabetes mellitus, renal dysfunction and genital malformation associated with a partial deletion of the pseudo-POU domain of hepatocyte nuclear factor-1beta. Hum Mol Genet 8: 2001–2008, 1999 [DOI] [PubMed] [Google Scholar]
- 66.Bolar NA, Golzio C, Živná M, Hayot G, Van Hemelrijk C, Schepers D, et al.: Heterozygous loss-of-function SEC61A1 mutations cause autosomal-dominant tubulo-interstitial and glomerulocystic kidney disease with anemia. Am J Hum Genet 99: 174–187, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Komada T, Usui F, Shirasuna K, Kawashima A, Kimura H, Karasawa T, et al.: ASC in renal collecting duct epithelial cells contributes to inflammation and injury after unilateral ureteral obstruction. Am J Pathol 184: 1287–1298, 2014 [DOI] [PubMed] [Google Scholar]
- 68.Zasloff M: The antibacterial shield of the human urinary tract. Kidney Int 83: 548–550, 2013 [DOI] [PubMed] [Google Scholar]
- 69.Chassin C, Tourneur E, Bens M, Vandewalle A: A role for collecting duct epithelial cells in renal antibacterial defences. Cell Microbiol 13: 1107–1113, 2011 [DOI] [PubMed] [Google Scholar]
- 70.Berry MR, Mathews RJ, Ferdinand JR, Jing C, Loudon KW, Wlodek E, et al. : Renal sodium gradient orchestrates a dynamic antibacterial defense zone. Cell 170: 860–874.e19, 2017 [DOI] [PubMed] [Google Scholar]
- 71.Lloyd DJ, Wheeler MC, Gekakis N: A point mutation in Sec61alpha1 leads to diabetes and hepatosteatosis in mice. Diabetes 59: 460–470, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cornec-Le Gall E, Olson RJ, Besse W, Heyer CM, Gainullin VG, Smith JM, et al. Genkyst Study Group; HALT Progression of Polycystic Kidney Disease Group; Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease : Monoallelic mutations to DNAJB11 cause atypical autosomal-dominant polycystic kidney disease. Am J Hum Genet 102: 832–844, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.