Compliance of the large arteries (e.g. the aorta and carotid artery) serve to dampen fluctuations in pressure and flow generated during a cardiac cycle, facilitating continuous target organ perfusion. Large artery stiffness increases transmission of hemodynamic pulsatility to the brain which causes cerebrovascular damage, cognitive decline and stroke later in life [1–3]. To date, studies have primarily examined associations between large artery stiffness and cerebral pulsatility in adults.
Arterial stiffness increases from childhood to adolescence with some modification occurring through typical growth/development and some through subclinical atherosclerotic cardiovascular disease [4]. As such, cerebral hemodynamic pulsatility may be influenced by the structural and/or functional status of large arteries at a much younger age than previously appreciated. The purpose of this study was to examine associations between large extracranial artery stiffness (aortic and carotid stiffness), extracranial hemodynamic pulsatility (carotid pulse pressure, flow pulsatility and pressure-flow wave intensity) and intracranial hemodynamic pulsatility (middle cerebral artery flow pulsatility) in children.
Methods
59 healthy children between 9–12 years of age (57% African American; 37% female) from the Syracuse City community participated in this study as part of the Environmental Exposures and Child Health Outcomes (EECHO) study. Detailed inclusion/exclusion criteria have been previously reported [5]. This study was approved by the institutional review board of Syracuse University and SUNY Upstate University. All guardians and participants gave written consent and assent, respectively, prior to enrollment.
Applanation tonometry (Sphygmocor, AtCor Medical, Sydney, Australia) was used to acquire pressure waves at the common carotid and femoral pulse sites and used to calculate a pulse wave velocity (PWV) [4]. Brachial blood pressure was acquired using an oscillometric cuff (Mobil-o-graph, IEM, Germany). Carotid pressure waveforms were calibrated against brachial mean and diastolic blood pressure (BP) and used to estimate carotid systolic pressure, pulse pressure and augmentation index. The common carotid artery was imaged below the carotid bulb using Doppler-ultrasound (ProSound α7, Aloka, Tokyo, Japan) and a 7.5–10.0 MHz linear-array probe. Distension and flow velocity waveforms were used to estimate a single-point carotid PWV and carotid flow velocity pulsatility index (PI) [6]. Wave intensity analysis was used to derive a forward compression wave (W1) and backward travelling compression waves (negative area, NA) [6]. Middle cerebral artery (MCA) blood flow velocity was assessed using a 2-mHz transcranial Doppler (TCD) ultrasound probe (DWL Doppler Box-X, Compumedics, Germany) and used to derive a flow velocity PI [6].
All data are reported as mean ± standard deviation. Associations of interest were explored using Pearson Correlation Coefficients. Path analysis was used to generate a model linking key vascular-hemodynamic constructs. Model fit was assessed using the normal fit index, the comparative fit index, the Chi-square minimum statistic, and the root mean square error of approximation. All analyses were performed using Statistical Package for the Social Sciences (SPSS, version 23, Chicago, IL) with significance set a priori as p<0.05.
Results
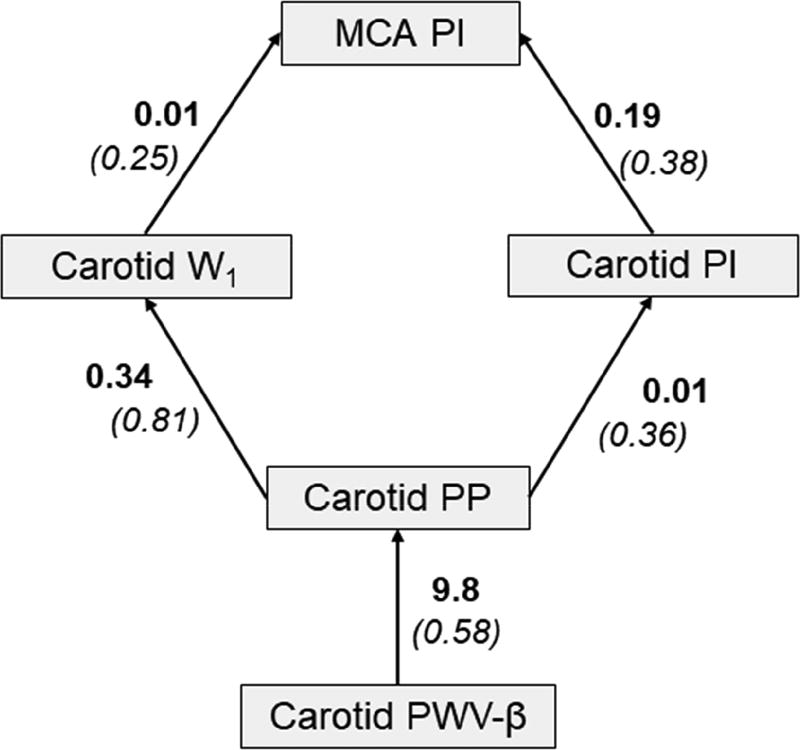
Group characteristics are presented in Table 1. Systemic vascular-hemodynamic properties are shown in Table 2. Table 3 presents a correlation matrix of all vascular-hemodynamic properties examined in this study. There were significant inter-associations between carotid PWB-β, carotid PP, W1, NA, carotid PI, and MCA PI (p<0.05). Our path analysis confirmed relationships between vascular-hemodynamic outcomes and suggests that carotid PWB-β is associated with carotid PP, W1 and PI which in turn is associated with MCA PI (Fit metrics: NFI = 0.92; CFI = 0.96; CMIN = 8.95, p = 0.11; RMSEA = 0.11).
Table 1.
Descriptive characteristics (mean ± SD).
| Boys (n=37) | Girls (n=22) | p value | |
|---|---|---|---|
| Race (African American/White) | 22/15 | 11/11 | 0.33 |
| Age (yrs) | 10.1 ± 0.96 | 10.2 ± 0.94 | 0.72 |
| SES (z-score) | −0.04 ± 0.81 | −0.04 ± 0.65 | 0.89 |
| Height (m) | 1.39 ± 0.09 | 1.45 ± 0.11 | 0.04 |
| Weight (kg) | 38.0 ± 11.8 | 47.1 ± 14.9 | 0.01 |
| BMI (kg/m2) | 19.3 ± 4.5 | 21.8 ± 6.9 | 0.09 |
| BMI percentile | 62.4 ± 31.6 | 69.2 ± 33.6 | 0.44 |
| Self-reported maturation (n, %) | |||
| Pre-pubertal | 6 (16.2) | 2 (9.1) | 0.13 |
| Early puberty | 16 (43.2) | 4 (18.2) | |
| Midpuberty | 12 (32.4) | 13 (59.1) | |
| Late puberty | 3 (8.1) | 2 (9.1) | |
| Postpubertal | 0 (0.0) | 1 (4.5) |
SES, socioeconomic status; BMI, body mass index.
Table 2.
Extra- and intra-cranial hemodynamics (mean ± SD).
| Boys (n=37) | Girls (n=22) | p value | |
|---|---|---|---|
| Brachial | |||
| Systolic BP (mmHg) | 111 ± 8 | 115 ± 8 | 0.03 |
| Diastolic BP (mmHg) | 67 ± 7 | 70 ± 4 | 0.05 |
| Mean pressure (mmHg) | 87 ± 6 | 91 ± 5 | 0.02 |
| Heart rate (b/min) | 73 ± 13 | 83 ± 11 | 0.02 |
| Aorta | |||
| cf PWV (m/s) | 4.5 ± 0.8 | 4.8 ± 0.6 | 0.10 |
| Carotid | |||
| Augmentation index (%) | −16 ± 14 | −20 ± 10 | 0.26 |
| Pulse pressure (mmHg) | 45 ± 7 | 45 ± 6 | 0.46 |
| IMT (mm) | 0.38 ± 0.05 | 0.38 ± 0.05 | 0.98 |
| PWV-β (m/s) | 3.6 ± 0.5 | 3.8 ± 0.4 | 0.33 |
| Pulsatility index | 1.57 ± 0.26 | 1.58 ± 0.19 | 0.59 |
| W1 (mmHg/m/s3) | 8.0 ± 3.6 | 8.1 ± 2.3 | 0.46 |
| NA (mmHg/m/s2) | −61.81 ± 28.19 | −55.94 ± 25.44 | 0.38 |
| Mean diameter (mm) | 5.15 ± 0.38 | 4.99 ± 0.44 | 0.20 |
| Middle cerebral | |||
| Mean velocity (cm/s) | 79 ± 17 | 90 ± 17 | 0.03 |
| Pulsatility index | 0.80 ± 0.12 | 0.79 ± 0.1 | 0.98 |
| Resistance (mmHg/cm/s) | 1.09 ± 0.29 | 0.99 ± 0.21 | 0.18 |
BP, blood pressure; cf, carotid-femoral; PWV, pulse wave velocity; IMT, intima-media thickness; W1, forward wave intensity; NA, reflected wave intensity.
Table 3.
Correlation matrix between select demographics, extracranial hemodynamics, and intracranial pulsatility in the full sample (n=59).
| MCA PI |
cf PWV |
CCA PWV-β |
CCA PI |
CCA W1 |
CCA NA |
CCA PP |
CCA AIx |
CCA IMT |
Age | SES | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| cf PWV | 0.14 | ||||||||||
| CCA PWV-β | −0.03 | 0.16 | |||||||||
| CCA PI | 0.47 | 0.05 | 0.18 | ||||||||
| CCA W1 | 0.32 | 0.15 | 0.23 | 0.34 | |||||||
| CCA NA | 0.22 | 0.09 | 0.19 | 0.72 | 0.35 | ||||||
| CCA PP | 0.25 | 0.12 | 0.58 | 0.78 | 0.37 | 0.67 | |||||
| CCA AIx | −0.21 | −0.19 | −0.40 | −0.33 | −0.46 | −0.41 | −0.42 | ||||
| CCA IMT | 0.27 | 0.15 | 0.22 | 0.35 | 0.19 | 0.24 | 0.33 | −0.04 | |||
| Age | 0.05 | 0.26 | 0.04 | 0.01 | 0.10 | 0.07 | 0.01 | −0.16 | 0.24 | ||
| SES | −0.08 | 0.01 | −0.01 | −0.19 | −0.10 | −0.24 | −0.30 | −0.03 | −0.06 | 0.24 | |
| BMI | 0.11 | 0.42 | 0.24 | 0.12 | 0.01 | −0.13 | 0.17 | −0.34 | 0.23 | 0.19 | 0.21 |
MCA, middle cerebral artery; PI, pulsatility index; cf, carotid-femoral; PWV, pulse-wave velocity; CCA, common carotid artery; W1, forward-wave intensity; NA, reflected wave intensity; PP, pulse pressure; AIx, augmentation index; IMT, intima-media thickness; SES, socioeconomic status; BMI, body mass index.
Bold denotes p<0.05.
Discussion
Our primary findings support a relationship between extracranial vascular stiffness and intracranial hemodynamic pulsatility in children. Carotid artery stiffness was associated with carotid pressure pulsatility, which in turn was associated with carotid flow pulsatility and ultimately cerebral flow pulsatility. Wave intensity analysis offered insight into the genesis of extracranial hemodynamic pulsatility. Left ventricular ejection into a stiffer/thicker carotid artery may have increased forward compression wave intensity, resulting in enhanced transmission of flow pulsatility into the cerebrovascular bed.
Additional findings of interest should be noted. Carotid pressure and flow pulsatility were both associated with IMT suggesting a relationship between regional pulsatile hemodynamic load and detrimental vascular remodeling in children [7]. Carotid AIx was inversely associated with NA suggesting that AIx and NA may capture different aspects of wave reflections (i.e. systemic/global vs regional) in children. Aortic stiffness was not associated with carotid stiffness or carotid/cerebral hemodynamic pulsatility in children. Age and BMI were correlates of cfPWV but not carotid PWV in children and this is consistent with previous observations in adults [8]. The aorta and carotid artery “age” at different rates in response to different CVD risk factor exposure [8].
Examination of vascular-hemodynamic function in childhood may offer a window into cardiovascular and cerebrovascular risk in adulthood [9]. Accumulation of CVD risk factors in childhood influences both arterial stiffness/thickness and cognitive function in young adulthood [10–12]. Our findings demonstrate that associations between large artery stiffness and cerebral hemodynamic pulsatility manifest at a young age. Whether targeting arterial stiffness in childhood abrogates subsequent cerebrovascular damage and benefits rates of cognitive decline in adulthood requires further scrutiny [13].
In conclusion, similar to what has be previously reported in adults [14], extracranial vascularhemodynamic pulsatility may influence intracranial hemodynamic pulsatility in children. Future research is required to scrutinize the clinical implications of these associations.
Supplementary Material
Figure 1.
Path analysis highlighting inter-relation between carotid artery stiffness, carotid hemodynamic pulsatility and cerebral hemodynamic pulsatility in children. Presented as unstandardized (standardized) coefficients.
Highlights.
Carotid artery stiffness was associated with cerebral blood flow pulsatility
Carotid forward/compression wave intensity was associated with cerebral pulatility
Extracranial hemodynamics correlate with intracranial pulsatility in children
Findings have implications for age-associated cerebrovascular disease
Acknowledgments
This study was funding by a grant from the National Institutes of Health NIH NIEHS R01 ES023252 02 (PI Gump)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mitchell GF, et al. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility--Reykjavik study. Brain. 2011;134(Pt 11):3398–407. doi: 10.1093/brain/awr253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pase MP, et al. Association of Aortic Stiffness With Cognition and Brain Aging in Young and Middle-Aged Adults: The Framingham Third Generation Cohort Study. Hypertension. 2016 doi: 10.1161/HYPERTENSIONAHA.115.06610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Webb AJ, et al. Increased cerebral arterial pulsatility in patients with leukoaraiosis: arterial stiffness enhances transmission of aortic pulsatility. Stroke. 2012;43(10):2631–6. doi: 10.1161/STROKEAHA.112.655837. [DOI] [PubMed] [Google Scholar]
- 4.Townsend RR, et al. Recommendations for Improving and Standardizing Vascular Research on Arterial Stiffness: A Scientific Statement From the American Heart Association. Hypertension. 2015;66(3):698–722. doi: 10.1161/HYP.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lefferts WK, et al. Racial Differences in Aortic Stiffness in Children. J Pediatr. 2017;180:62–67. doi: 10.1016/j.jpeds.2016.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heffernan KS, et al. Arterial stiffness and cerebral hemodynamic pulsatility during cognitive engagement in younger and older adults. Exp Gerontol. 2018;101:54–62. doi: 10.1016/j.exger.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Boutouyrie P, et al. Association between local pulse pressure, mean blood pressure, and large-artery remodeling. Circulation. 1999;100(13):1387–93. doi: 10.1161/01.cir.100.13.1387. [DOI] [PubMed] [Google Scholar]
- 8.Paini A, et al. Carotid and aortic stiffness: determinants of discrepancies. Hypertension. 2006;47(3):371–6. doi: 10.1161/01.HYP.0000202052.25238.68. [DOI] [PubMed] [Google Scholar]
- 9.Fernhall B, Agiovlasitis S. Arterial function in youth: window into cardiovascular risk. J Appl Physiol (1985) 2008;105(1):325–33. doi: 10.1152/japplphysiol.00001.2008. [DOI] [PubMed] [Google Scholar]
- 10.Rovio SP, et al. Cardiovascular Risk Factors From Childhood and Midlife Cognitive Performance: The Young Finns Study. J Am Coll Cardiol. 2017;69(18):2279–2289. doi: 10.1016/j.jacc.2017.02.060. [DOI] [PubMed] [Google Scholar]
- 11.Raitakari OT, et al. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. Jama. 2003;290(17):2277–83. doi: 10.1001/jama.290.17.2277. [DOI] [PubMed] [Google Scholar]
- 12.Juonala M, et al. Risk factors identified in childhood and decreased carotid artery elasticity in adulthood: the Cardiovascular Risk in Young Finns Study. Circulation. 2005;112(10):1486–93. doi: 10.1161/CIRCULATIONAHA.104.502161. [DOI] [PubMed] [Google Scholar]
- 13.Magnussen CG, Smith KJ, Juonala M. When to prevent cardiovascular disease? As early as possible: lessons from prospective cohorts beginning in childhood. Curr Opin Cardiol. 2013;28(5):561–8. doi: 10.1097/HCO.0b013e32836428f4. [DOI] [PubMed] [Google Scholar]
- 14.Tarumi T, et al. Cerebral hemodynamics in normal aging: central artery stiffness, wave reflection, and pressure pulsatility. J Cereb Blood Flow Metab. 2014;34(6):971–8. doi: 10.1038/jcbfm.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.