Abstract
Recently, multiple compounds have been synthesized that target the allosteric binding site(s) of CB1. These CB1 positive allosteric modulators, may capture the benefits of cannabinoid receptor activation without unwanted psychoactive effects, such as sedation. For example, ZCZ011 blocks neuropathic pain, absent the catalepsy, sedation, and hypothermia caused by CB1 orthosteric modulators, including Δ9-tetrahydrocannabinol (THC). The primary goal of the present study was to evaluate the potential of ZCZ011 to attenuate somatic signs of cannabinoid withdrawal in mice. Mice were repeatedly administered THC (10 mg/kg, s.c.) or vehicle, and withdrawal was either precipitated using the CB1 antagonist rimonabant (3 mg/kg, ip) or elicited spontaneously via THC abstinence. ZCZ011 (≥10 mg/kg, i.p.) significantly attenuated somatic signs of withdrawal, including head twitches and paw tremors, but had no effect on locomotor activity or conditioned place preference. We next tested the antiulcerogenic properties of CB1 positive allosteric modulation. Mice were fasted for 22 h, administered ZCZ011, and gastric hemorrhages were induced with the nonsteroidal anti-inflammatory drug diclofenac sodium (100 mg/kg, p.o.). ZCZ011 alone had no effect on gastric ulceration, but ZCZ011 (≥10 mg/kg) blocked ulcer formation when combined with a subthreshold MAGL inhibitor (JZL184; 1 mg/kg, i.p.). Thus, CB1 positive allosteric modulation is a novel approach to treat cannabinoid dependence and gastric inflammation.
Keywords: Cannabis use disorder, cannabinoid dependence, NSAID induced gastritis, CB1 allosteric modulation, drug abuse
1. Introduction
Cannabis has been valued for thousands of years for its anti-inflammatory, anti-emetic and analgesic properties (Mechoulam and Parker, 2012). In more recent years, however, the use of cannabinoids as therapeutic agents has been restricted due to abuse and dependence potential (Aceto et al., 1995; Lichtman et al., 1998). Thus, much research has shifted to explore the endocannabinoid system as an alternative route to harness the therapeutic potential of cannabinoids while limiting abuse liability (Kinsey et al., 2011; Piomelli et al., 2014; Schlosburg et al., 2010). The endocannabinoid system modulates many physiological systems and offers multiple druggable targets for therapeutic development. For example, the CB1 cannabinoid receptor subtype is primarily responsible for the psychoactive effects of cannabis. The endogenous orthosteric ligands for CB1 are anandamide and 2-arachidonoyl glycerol (2-AG), which are tightly regulated by synthetic and catabolic enzymes. Chemical inhibition of monoacylglycerol lipase (MAGL) increases tissue levels of 2-AG, resulting in reduced nociceptive pain, inflammation, gastric hemorrhage development, and somatic signs of cannabinoid withdrawal in mice (Crowe and Kinsey, 2017; Kinsey et al., 2009; Schlosburg et al., 2009). MAGL inhibitors present a few challenges, however, as at high doses they induce behavioral tolerance and CB1 downregulation (Schlosburg et al., 2014, 2010).
Positive allosteric modulators (PAMs) bind to distinct allosteric receptor sites, where they increase the efficacy and/or affinity of orthosteric ligands (Kenakin, 2013). Because of their allosteric action, cannabinoid receptor PAMs represent a promising alternative in cannabinoid-based therapies (Ross, 2007). Specifically, PAMs may retain the therapeutic properties of endogenous cannabinoid action (e.g., gastroprotective effects) without cannabimimetic side effects (e.g., high-dose sedation). Several CB1 PAMs show therapeutic potential. For example, lipoxin A4 is an endogenous CB1 PAM that potentiates the action of anandamide both in vitro and in vivo and protects against β-amyloid-induced memory impairment (Pamplona et al., 2012). However, lipoxin A4 also induces classic cannabinoid effects in the tetrad test (Pamplona et al., 2012). Another synthetic CB1 PAM, GAT211, is not only antinociceptive in acute mechanical and neuropathic pain models, but does not elicit cannabimimetic effects in mice (Slivicki et al., 2017). Similarly, the synthetic CB1 PAM ZCZ011, potentiates anandamide-induced cannabimimetic effects, but has no cannabimimetic effects alone, suggesting that ZCZ011 works via a CB1 allosteric-specific mechanism (Ignatowska-Jankowska et al., 2015). As with GAT211, ZCZ011 attenuates neuropathic pain induced by chronic constriction injury of the sciatic nerve in mice (Ignatowska-Jankowska et al., 2015).
Repeated exposure to CB1 agonists, such as the phytocannabinoid Δ9-tetrahydrocannabinol (THC) results in tolerance and dependence, as demonstrated by withdrawal behaviors (Lichtman et al., 2001). THC withdrawal signs such as head twitches and paw tremors are attenuated directly by administration of additional THC, or indirectly by MAGL inhibition (Schlosburg et al., 2009; Trexler et al., 2018). Either approach increases available ligand for the CB1 orthosteric binding site. The present studies aimed to evaluate the efficacy of CB1 positive allosteric modulation in attenuating signs of cannabinoid withdrawal. To this end, we used both rimonabant-precipitated and spontaneous THC withdrawal paradigms.
In addition to well-known behavioral effects of cannabinoids, CB1 orthosteric agonists are gastroprotective. For example, cannabis extract blocks the development of gastric ulceration caused by pyloric ligation in rats (Sofia et al., 1978). In other experimental rat models, CB1 orthosteric agonists attenuate gastric ulceration induced by restraint stress (Germanò et al., 2001), ethanol gavage (Shujaa et al., 2009), or nonsteroidal anti-inflammatory drugs (NSAIDs; (Rutkowska and Fereniec-Golebiewska, 2006). Similarly in mice, CB1 orthosteric agonists (Kinsey and Cole, 2013) or endocannabinoid catabolic enzyme inhibitors (Kinsey et al., 2011; Naidu et al., 2009) prevent the formation of NSAID-induced gastric hemorrhages. Furthermore, these gastroprotective effects can be blocked by the selective CB1 antagonist rimonabant (Kinsey et al., 2011) or genetic deletion of CB1 (Kinsey et al., 2011), suggesting endocannabinoid mediation occurs by CB1 orthosteric binding. Accordingly, the monoacylglycerol lipase (MAGL) inhibitor JZL184 or KML29 block diclofenac-induced gastric hemorrhages by increasing 2-AG tone (Crowe and Kinsey, 2017; Ignatowska-Jankowska et al., 2014). The present studies expanded on these previous studies by testing the gastroprotective effects of CB1 positive allosteric modulation per se, as well as in the presence of increased 2-AG tone, via a subthreshold dose of JZL184.
2. Methods
2.1. Animals
Male C57BL/6J mice (Jackson Laboratories; Bar Harbor, ME) were used in withdrawal and locomotor experiments. Male and female C57BL/6J (Jackson Laboratories; Bar Harbor, ME) mice were used for the conditioned place preference experiment. Male ICR mice (Envigo, Indianapolis, IN) were used in gastric hemorrhage experiments. All mice were group housed (4–5 per cage) in Polysulfone plastic cages on corncob bedding with food and water available ad libitum and housed in a single temperature (20–22°C) and humidity (50 ± 5%) controlled room on a 12:12 h light/dark cycle. Mice aged approximately 10 weeks were randomly assigned to each treatment group such that each cage contained mice from at least two different treatment groups (e.g., no cage contained only THC-treated mice). All experiments were carried out by trained technicians who were blinded to treatment conditions. The Animal Care and Use Committee at West Virginia University approved all experimental protocols.
2.2. Drugs
Δ9-tetrahydrocannabinol (THC) and rimonabant (SR141716A) were generously provided by the National Institute on Drug Abuse Drug Supply Program (Bethesda, MD). ZCZ011 was purchased from Axon Medchem (Reston, VA). JZL184 and diclofenac sodium salt were purchased from Cayman Chemical (Ann Arbor, MI). All drugs were dissolved in a vehicle composed of 5% ethanol, 5% Cremophor (Sigma-Aldrich, St. Louis, MO), and 90% normal saline (Kinsey and Cole, 2013). ZCZ011 was administered 75 min prior to testing in spontaneous withdrawal experiments, final THC injection in precipitated withdrawal experiments, or diclofenac gavage in ulcer experiments (Ignatowska-Jankowska et al., 2015). JZL184 was administered 120 min prior to diclofenac gavage (Crowe and Kinsey, 2017; Long et al., 2009). Doses were based on pilot data as well as published reports for ZCZ011 (Ignatowska-Jankowska et al., 2015) and JZL184 (Schlosburg et al., 2014). All solutions were warmed to room temperature before administration at a volume of 10 μl/g body mass.
2.2.1. Precipitated withdrawal paradigm:
Withdrawal was induced as described previously (Falenski et al., 2010; Schlosburg et al., 2009; Trexler et al., 2018). Mice were weighed daily and injected subcutaneously (s.c.) with THC (10 mg/kg) or vehicle every 12 h for 6 days. On the sixth day, all mice received a final injection of THC or vehicle. After 30 min, mice received an intraperitoneal (i.p.) injection of rimonabant (3 mg/kg) to precipitate withdrawal. Mice were assessed in somatic signs of withdrawal, as detailed below, by an experimenter who was blinded to drug treatment conditions.
2.2.2. Spontaneous withdrawal paradigm:
Spontaneous withdrawal was induced as described previously (Trexler et al., 2018). Mice were weighed daily and injected subcutaneously (s.c.) with either THC (10 mg/kg) or vehicle every 12 h for 6 days (Falenski et al., 2010; Schlosburg et al., 2009). Behavioral assessments were conducted 36h after the final THC or vehicle injection, coincident with peak spontaneous withdrawal-related behaviors (Trexler et al., 2018). Mice were assessed in somatic signs of withdrawal, as detailed below, by an experimenter who was blinded to drug treatment conditions.
2.3. Behavioral Assessments
2.3.1. Somatic withdrawal signs testing:
Somatic signs of withdrawal were measured as previously described (Schlosburg et al., 2009; Trexler et al., 2018). Briefly, each mouse was placed into an empty, plastic test chamber (20 cm W × 20 cm L × 15 cm H) inside a sound-attenuating chamber outfitted with a fan and white LED lighting. The box had three clear sides and one mirrored side that faced a camera to allow for observation of behavior when the mouse faced away from the camera. Mice were habituated to the test apparatus following final THC or vehicle injection for 30 min and were then removed and injected with rimonabant or vehicle. The boxes were cleaned using a paper towel moistened with distilled water. The mice were then placed back into the boxes and video recorded for 60 min. Mice used to evaluate spontaneous withdrawal were tested 36h after final THC injection.
Videos were renamed to remove any identifying information and scored by a trained observer. A subset of nine videos was scored by a second observer to ensure inter-rater reliability (r=.92). The dependent variables were incidences of paw tremors and head twitches (i.e., an incidence was scored for ‘paw tremor’ when the behavior was observed, not for each individual motion). Incidences were considered separate when either (1) another behavior occurred between the incidences, or (2) there was at least 1 s between incidences.
2.3.2. Spontaneous locomotor activity:
A single plastic test chamber (30 cm W × 40 cm L × 16 cm H) was placed inside a sound-attenuating chamber outfitted with a fan and LED lighting. Each mouse was placed individually into the chamber and allowed to freely explore for 90 min. Locomotor activity was recorded for the duration of the test by a camera mounted on the top of the test chamber. The video data were analyzed in real time using ANY-maze™ (Stoelting, Wood Dale, IL) video tracking software. Immobility was operationalized as time immobile of the centroid (calculated by 90% of the mouse image pixels) for greater than 1.2 s and was analyzed in 5 min bins.
2.3.3. Conditioned place preference:
The conditioned place preference test was performed as previously described (Curry et al., 2018). In this paradigm, Pavlovian conditioning was used to associate an environmental stimulus (e.g., spotted or plain walls) with an unconditioned stimulus (e.g., drug) to induce a response when exposed to the environmental stimulus. The test apparatus (Stoelting, Wood Dale, IL) consisted of two equally sized chambers (18 cm W × 20 cm L × 35 cm H) and a small connecting chamber (20 cm W × 10 cm L × 35 cm H). To distinguish the two equally sized chambers, one chamber had black walls decorated with white circles and the other chamber had only black walls. During the 3-day habituation phase, mice freely explored the apparatus for 15 min per day. On the third day, innate chamber preference was assessed, and mice that preferred one chamber were counter-balanced across treatment groups and drug-chamber pairings. The purpose of this block design is to evenly distribute the potential effects of bias across all treatment groups (Manzanedo et al., 2001). After the biased mice were counterbalanced, the remaining mice were randomly assigned across treatment groups. To ensure that baselines were equal across all groups, a 2 × 4 ANOVA (drug paired side × drug treatment group) was conducted and revealed baselines were not significantly different across groups [p=.77]. During the training phase, mice were injected with either ZCZ011 or vehicle, and placed into one of the chambers 60 min later for 30 min (the connecting chamber was closed during training). Pairing for ZCZ011 and vehicle happened on alternating days, with each treatment having 5 pairing days total. During the test phase, the connecting chamber was opened allowing each mouse to freely explore the apparatus for 15 min. Movement was recorded using an overhead camera (Logitech HD Pro Webcam C920) and tracked and analyzed in real time using ANY-maze™ software. Time spent in the drug-paired and vehicle-paired chambers were analyzed.
2.4. Gastric hemorrhage scoring
Gastric hemorrhages were induced as previously described (Crowe and Kinsey, 2017; Kinsey et al., 2011). Mice were weighed and then food deprived for 24 h with free access to water. To minimize the incidence of coprophagia, thus reducing stomach contents, a wire grid was placed on the floor of each cage and all bedding was removed. On the day of testing, mice were administered diclofenac or vehicle via oral gavage and returned to their home cage for 6 h. Mice were euthanized via CO2 asphyxiation, and stomachs were carefully harvested, cut along the greater curvature, rinsed with distilled water, placed on a lighted tracing table (Artograph light pad 1920), and photographed (Canon T3 Rebel digital camera with 10× close up lens). Image files were renamed and an experimenter blinded to treatment conditions quantified the gastric hemorrhages relative to a 1 mm reference in each photo using Adobe Photoshop (San Jose, CA).
2.6. Statistical analyses
All data are reported as mean ± SEM. Precipitated and spontaneous withdrawal data were analyzed using a 2×3 factorial analysis of variance (ANOVA). Locomotor data were analyzed using repeated-measure ANOVA with time as the within-subjects variable. Conditioned place preference data were analyzed using a repeated measure ANOVA with time spent in each side as the within-subjects variable. Gastric hemorrhage data were analyzed using a one-way ANOVA. Main or interaction effects were followed by Bonferonni post hoc tests, as appropriate. Differences were considered statistically significant if p < 0.05.
3. Results
3.1. Experiment 1: ZCZ011 attenuates classic somatic signs of THC withdrawal.
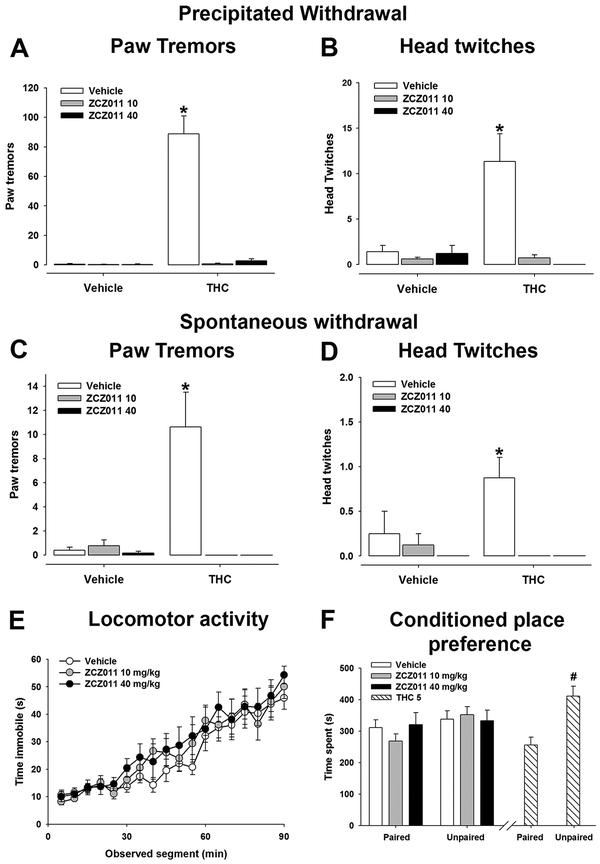
To test how well CB1 positive allosteric modulation attenuates cannabinoid withdrawal, mice were treated with either THC (10 mg/kg) or vehicle for 6 days (Trexler et al., 2018). On day 6, withdrawal was precipitated using rimonabant (3 mg/kg, i.p.) (Lichtman et al., 2001; Trexler et al., 2018). ZCZ011 (10 or 40 mg/kg, i.p.) was administered 75 min prior to final THC or vehicle injection. As reported previously, precipitated THC withdrawal induced both paw tremors [F(2,40)= 45.1, p<.05; Fig. 1A] and head twitches [F(2,40)= 9.8, p<.05; Fig. 1B]. ZCZ011 significantly decreased precipitated THC withdrawal-induced paw tremors and head twitches.
Figure 1.
The CB1 positive allosteric modulator ZCZ011 attenuates somatic signs of THC withdrawal. Male mice were administered THC (10 mg/kg, s.c) for 6 days and withdrawal was induced with the CB1 selective antagonist rimonabant (3 mg/kg, i.p.) 30 prior to testing. ZCZ011 (10 or 40 mg/kg, i.p.) was administered 75 min prior to final THC injection. (A,B) ZCZ011 (10 or 40 mg/kg, i.p.) reduced withdrawal-induced paw tremors and head twitches (n=8, except the vehicle/vehicle and THC/ZCZ011 40 groups, which each has n=7). Withdrawal was evaluated at 36 h after the final THC injection following a 75 min pretreatment with ZCZ011 (10 or 40 mg/kg, i.p.). (C,D) Acute ZCZ011 (10 or 40 mg/kg, i.p.) reduced paw tremors and head twitches (n=8, except the vehicle/ZCZ011 40 and THC/ZCZ011 40 groups, which each has n=7). (E) Male mice were injected with ZCZ011 (10 or 40 mg/kg, i.p.) and tested for spontaneous locomotor activity in an open chamber (n=8 per treatment). (F) In a separate group of male and female mice, ZCZ011 had no effect on conditioned place preference, but THC (5 mg/kg, i.p.) induced conditioned place aversion (n= 6M+6F per group, except the ZCZ011 10 treatment, which had n=6M+5F). Data represent mean ± SEM. *p < .05 vs. vehicle; # p<.05 vs. drug-paired side.
Previous research suggests that ZCZ011 decreases binding of rimonabant in vitro (Ignatowska-Jankowska et al., 2015). Thus, it is plausible that the reduced THC withdrawal behaviors were caused by to reduced rimonabant sensitivity. A separate group of mice was evaluated in a spontaneous THC withdrawal model, that is, abstinence-induced withdrawal (Trexler et al., 2018). Mice were treated with THC (10 mg/kg) or vehicle for 6 days. Mice were tested 36h after the final THC or vehicle injection to assess signs of spontaneous withdrawal, which peak at 36h THC abstinence (Trexler et al., 2018). ZCZ011 (10 or 40 mg/kg, i.p.) was administered 75 min prior to testing. As previously reported, THC abstinence induced paw tremors [F(2,38)= 11.5, p<.05; Fig. 1C] and head twitches [F(2,38)= 3.2, p<.05; Fig. 1D]. ZCZ011 (10 or 40 mg/kg) significantly decreased both spontaneous THC withdrawal-induced paw tremors and head twitches.
To determine possible sedative effects of ZCZ011, we quantified locomotor activity following acute ZCZ011 treatment. Male mice were injected with ZCZ011 (10 or 40 mg/kg, i.p.) or vehicle 75 min prior to testing. As shown previously, though time immobile increased over time [F(17,357)= 46.285, p<.05, ZCZ011 had no effect on time immobile in a spontaneous locomotor test [p=.96; Fig. 1E]. Similarly, ZCZ011 had no effect on total distance traveled [p = .92].
Finally, to determine whether ZCZ011 has rewarding or aversive properties, a separate group of male and female mice were injected with ZCZ011 (10 or 40 mg/kg, i.p.) and tested for conditioned place preference/aversion. ZCZ011 had no effect on chamber preference [treatment × chamber time interaction: p=.66; Fig. 1F]. Mice treated with THC (5 mg/kg, i.p.), which served as a positive control, showed a statistically significant conditioned place aversion [t(11)=2.792, p<.05]. No sex differences were observed [p=.86].
3.2. Experiment 2: ZCZ011 potentiates the gastroprotective effects of MAGL inhibition
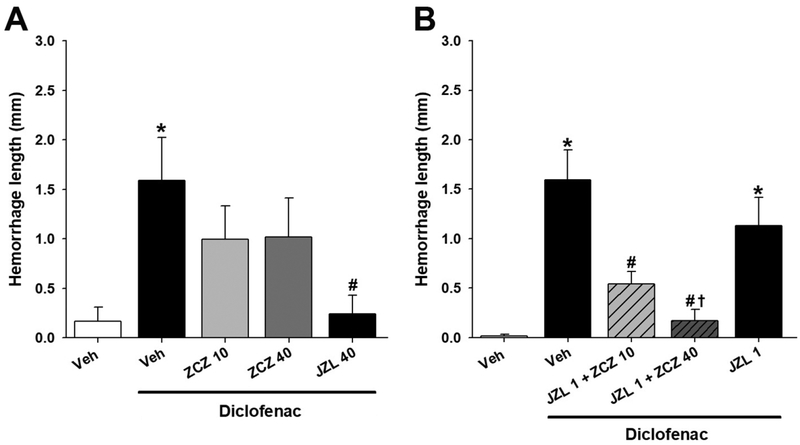
To determine the ability of CB1 allosteric modulation to block NSAID-induced gastric hemorrhages, fasted mice were injected with ZCZ011 (10 or 40 mg/kg, i.p.) vehicle, or JZL184 (40 mg/kg, ip), and gastric hemorrhages were induced with diclofenac sodium (100 mg/kg, po). As shown previously (Kinsey et al., 2011), high-dose JZL184 (40 mg/kg) attenuated gastric hemorrhage formation [F(4,34)=3.84, p<.05; Fig. 2A]. However, ZCZ011 (10 or 40 mg/kg) did not affect ulcer formation [p = .18].
Figure 2.
ZCZ011 in combination with low-dose JZL184 attenuates gastric hemorrhage formation. Separate groups of male mice were administered JZL184 (40 mg/kg) 2 h prior to diclofenac (100 mg/kg, p.o.) or ZCZ011 (10 or 40 mg/kg) 75 min prior to diclofenac or a combination of JZL184 (1 m/kg, i.p.) and ZCZ011 (10 or 40 mg/kg, i.p.). (A) JZL184 (40mg/kg, i.p.), but not ZCZ011 (10 or 40 mg/kg, i.p.), attenuated ulcer formation when administered alone (n=8, except the JZL 40 group, which has n=7). (B) In combination with JZL184 (1 mg/kg, i.p.), ZCZ011 (10 or 40 mg/kg, i.p.) blocked ulcer formation (n=8, expect the JZL 1 and JZL + ZCZ011 10 groups, which have n=7 and n=9, respectively). Data represent mean ± SEM (n = 7–9/group). *p < .05 vs. vehicle; # p < .05 vs. diclofenac, † p < .05 vs. JZL184.
We next probed whether CB1 positive allosteric modulation elicits gastroprotection when coadministered with a subthreshold dose of the JZL184 (Kinsey et al., 2013). A separate group of fasted mice was pretreated with JZL184 (1 mg/kg, i.p.) and ZCZ011 (0, 10, or 40 mg/kg, i.p.) and gastric hemorrhages were induced. The combination ZCZ011+JZL184 treatment significantly attenuated diclofenac-induced gastric ulcers [F(4,35)=11.47, p< .05; Fig. 2B]. Post hoc analyses revealed that, as previously reported, JZL184 (1 mg/kg) alone was insufficient to affect diclofenac-induced gastric hemorrhages.
4. Discussion
CB1 positive allosteric modulators offer a novel approach to manipulate endocannabinoid signaling, possibly harnessing the benefits without appreciable side effects typically reported with orthosteric modulators. Indeed, the synthetic CB1 PAM ZCZ011 reduces inflammatory and neuropathic pain in mice (Ignatowska-Jankowska et al., 2015). The present experiments determined the ability of ZCZ011 to attenuate THC withdrawal and gastric hemorrhage formation. Most notably, we report that ZCZ011 (≥10 mg/kg) attenuated both rimonabant-precipitated and spontaneous THC withdrawal, using well-established somatic withdrawal signs tests. Of note, the withdrawal-suppressing effects of ZCZ011 were not caused by suppressed locomotor activity and did not require precipitation with the CB1 receptor inverse agonist rimonabant. Further, although ZCZ011 had no overt anti-ulcerogenic effects on its own, ZCZ011 significantly potentiated the gastroprotective effects of a subthreshold dose of the MAGL inhibitor JZL184.
CB1 positive allosteric modulators can alter receptor binding by changing the conformation of the ligand binding pocket to more easily bind an agonist or antagonist (Khurana et al., 2016). For example, ZCZ011 increases CB1 binding of the agonists CP55,940 and WIN55,212–2, but reduces CB1 binding of the antagonist/inverse agonist rimonabant (Ignatowska-Jankowska et al., 2014). In the rimonabant-precipitated THC withdrawal model, ZCZ011-mediated THC withdrawal attenuation may be interpreted as ZCZ011 reducing rimonabant binding to CB1, and preventing rimonabant withdrawal-inducing effects. Thus, we also evaluated the withdrawal-attenuating effects of ZCZ011 in a spontaneous THC withdrawal paradigm. CB1 positive allosteric modulation attenuated withdrawal, independent of rimonabant administration. Although twice daily injections of a relatively high dose THC differs from typical human cannabis smoking behavior, the finding that positive allosteric modulation can attenuate both precipitated and spontaneous THC withdrawal is a promising step forward in using CB1 positive allosteric modulation for therapeutic purposes.
Cannabis Use Disorder (CUD) affects between 2–6% of lifetime cannabis users (Hasin et al., 2016) and is typically characterized by anxiety, depression, irritability and somatic symptoms, such as headache and gastrointestinal issues, following cessation of drug use (American Psychiatric Association, 2013). Currently, there are no FDA approved pharmaceutical treatments for CUD, though more individuals seek treatment for cannabis use than any other federally illicit drug (Substance Use and Mental Health Services Administration, 2015). Medication assisted therapies to treat the symptoms of withdrawal are being developed. For example noradrenergic, serotonergic and GABAergic agents have all been used as anti-depressant and anti-anxiety medication to treat specific aspects of withdrawal (Brezing and Levin, 2017). To treat withdrawal as a whole, several studies have used THC or dronabinol (i.e., synthetically produced Δ9-THC, Marinol), which patients reported reduced anxiety and depression, but were unsuccessful preventing relapse (Haney et al., 2008, 2004). Additionally, combinations of cannabinoid and non-cannabinoid therapies are effective in attenuating some symptoms of withdrawal, but enhance other side effects, such as withdrawal-induced anorexia and sedation (Haney et al., 2008; Levin et al., 2016). Thus, the present finding that CB1 positive allosteric modulation attenuates symptoms of withdrawal at a dose that does not affect locomotor activity highlights its potential utility as a therapeutic approach for CUD.
We found that ZCZ011 potentiates the gastroprotective effects of the MAGL inhibitor JZL184. Increasing endogenous levels of AEA or 2-AG by inhibiting their catabolic enzymes FAAH and MAGL, respectively, has robust gastroprotective effects (Crowe and Kinsey, 2017; Kinsey et al., 2011; Naidu et al., 2009). These effects are absent in CB1-deficient mice or wild-type mice pretreated with rimonabant, suggesting that cannabinoid-mediated gastroprotection occurs through CB1 (Kinsey et al., 2011; Naidu et al., 2009). Thus, we hypothesized that the CB1 PAM ZCZ011 may also display gastroprotective properties. Interestingly, ZCZ011 did not affect gastric hemorrhage formation when administered alone, but gastric hemorrhages were fully attenuated by a combination of ZCZ011 and an otherwise ineffective dose of JZL184. Thus, the gastroprotective effects of CB1 positive allosteric modulation depend upon elevated endocannabinoid tone.
The antiulcerogenic effects of ZCZ011 reported here may be mediated by CB1 expressed in the central nervous system or in the stomach. In addition to the central nervous system, CB1 is expressed in the gut of mice (Casu et al., 2003) and humans (Pazos et al., 2008). It is plausible that the action of ZCZ011, like MAGL and FAAH catabolic enzyme inhibitors, increases CB1 receptor mediated activity in the stomach (Kinsey et al., 2011; Sasso et al., 2012). However, either systemic or intracerebroventricular administration of the orthosteric CB1 agonists AEA, methanandamide, or WIN55,212–2 attenuated ethanol-induced gastric hemorrhages in rats (Shujaa et al., 2009), indicating central CB1 mediation. Rimonabant blocked these effects, whether given centrally or systemically, further implicating a CB1 gastroprotective mechanism (Shujaa et al., 2009). Additionally, destruction of sensory nerves, via repeated systemic capsaicin treatment, partially blocks the gastroprotective effects of AEA following water/restraint-induced ulcers suggesting at least partial CNS involvement of cannabinoid-dependent ulcer blockade (Warzecha et al., 2011). On the other hand, JZL184 causes a two-fold increase in stomach 2-AG levels, which is correlated with ulcer blockade (Kinsey et al., 2011) and decreased neutrophil infiltration, as measured by myeloperoxidase levels (Crowe and Kinsey, 2017). Thus, it is likely that the gastroprotective effects observed here are mediated by both central and peripheral CB1 activation, as neither pathway appears to have an exclusive role in NSAID-induced ulcer attenuation.
In conclusion, CB1 positive allosteric modulation via ZCZ011 attenuates somatic signs of THC withdrawal and blocks NSAID-induced gastric hemorrhages. Either effect of ZCZ011 occurred at doses that were insufficient to affect locomotor activity or conditioned place preference. While other endpoints have yet to be evaluated (e.g., tolerance to these acute therapeutic outcomes), these data provide support for CB1 positive allosteric modulation as a promising therapeutic approach for reducing cannabis use disorder as well as gastric inflammation.
Highlights.
ZCZ011 attenuates somatic withdrawal signs without locomotor or reinforcing effects
ZCZ011 attenuates gastric hemorrhages when combined with the MAGL inhibitor JZL184
CB1 positive allosteric modulation is a strategy for reducing cannabis use disorder
5. Acknowledgements
The authors thank Tania Nguyen, Annika Naylor, and Selena Engebretson, for technical assistance. This work was financially supported by the National Institutes of Health [DA039335, DA038714, AR066806, GM081741, and GM103434].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- Aceto MD, Scates SM, Lowe JA, Martin BR, 1995. Cannabinoid precipitated withdrawal by the selective cannabinoid receptor antagonist, SR 141716A. Eur. J. Pharmacol 282, 1–2. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, 2013. Diagnostic and Statistical Manual of Mental Health Disorders, 5th ed. Washington, D.C. [Google Scholar]
- Brezing CA, Levin FR, 2017. The Current State of Pharmacological Treatments for Cannabis Use Disorder and Withdrawal. Neuropsychopharmacology 1–71. 10.1038/npp.2017.212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casu MA, Porcella A, Ruiu S, Saba P, Marchese G, Carai MAM, Reali R, Gessa GL, Pani L, 2003. Differential distribution of functional cannabinoid CB1 receptors in the mouse gastroenteric tract. Eur. J. Pharmacol 459, 97–105. 10.1016/S0014-2999(02)02830-3 [DOI] [PubMed] [Google Scholar]
- Crowe MS, Kinsey SG, 2017. MAGL inhibition modulates gastric secretion and motility following NSAID exposure in mice. Eur. J. Pharmacol 807, 198–204. 10.1016/j.ejphar.2017.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry Z, Wilkerson J, Bagdas D, Kyte S, Patel N, Donvito G, Mustafa MA, Poklis J, Niphakis M, Hsu K-L, Cravatt BF, Gewirtz DA, Damaj MI, Lichtman AH, 2018. Monoacylglycerol lipase inhibitors reverse paclitaxel-induced nociceptive behavior and proinflammatory markers in a mouse model of chemotherapy-induced neuropathy. J. Pharmacol. Exp. Ther jpet.117.245704. 10.1124/jpet.117.245704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falenski KW, Thorpe AJ, Schlosburg JE, Cravatt BF, Abdullah RA, Smith TH, Selley DE, Lichtman AH, Sim-Selley LJ, 2010. FAAH−/− Mice Display Differential Tolerance, Dependence, and Cannabinoid Receptor Adaptation After Δ9-Tetrahydrocannabinol and Anandamide Administration. Neuropsychopharmacology 35, 1775–1787. 10.1038/npp.2010.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germanò MP, D’Angelo V, Mondello MR, Pergolizzi S, Capasso F, Capasso R, Izzo AA, Mascolo N, De Pasquale R, 2001. Cannabinoid CB1-mediated inhibition of stress-induced gastric ulcers in rats. Naunyn. Schmiedebergs. Arch. Pharmacol 363, 241–244. 10.1007/s002100000360 [DOI] [PubMed] [Google Scholar]
- Haney M, Hart CL, Vosburg SK, Comer SD, Reed SC, Foltin RW, 2008. Effects of THC and Lofexidine in a Human Laboratory Model of Marijuana Withdrawal and Relapse. Psychopharmacol 197, 157–168. 10.2217/nnm.12.167.Gene [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Hart CL, Vosburg SK, Nasser J, Bennett A, Zubaran C, Foltin RW, 2004. Marijuana Withdrawal in Humans: Effects of Oral THC or Divalproex. Neuropsychopharmacology 29, 158–170. 10.1038/sj.npp.1300310 [DOI] [PubMed] [Google Scholar]
- Hasin DS, Kerridge BT, Saha TD, Huang B, Pickering R, Smith SM, Jung J, Zhang H, Grant BF, 2016. Prevalence and correlates of DSM-5 cannabis use disorder, 2012–2013: Findings from the national epidemiologic survey on alcohol and related conditions-III. Am. J. Psychiatry 173, 588–599. 10.1176/appi.ajp.2015.15070907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatowska-Jankowska BM, Baillie GL, Kinsey S, Crowe M, Ghosh S, Owens RA, Damaj IM, Poklis J, Wiley JL, Zanda M, Zanato C, Greig IR, Lichtman AH, Ross RA, 2015. A Cannabinoid CB1 Receptor-Positive Allosteric Modulator Reduces Neuropathic Pain in the Mouse with No Psychoactive Effects. Neuropsychopharmacology 40, doi: 10.1038/npp.2015.148. 10.1038/npp.2015.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatowska-Jankowska BM, Ghosh S, Crowe MS, Kinsey SG, Niphakis MJ, Abdullah RA, Tao Q, O’Neal ST, Walentiny DM, Wiley JL, Cravatt BF, Lichtman AH, 2014. In vivo characterization of the highly selective monoacylglycerol lipase inhibitor KML29: Antinociceptive activity without cannabimimetic side effects. Br. J. Pharmacol 171, 1392–1407. 10.1111/bph.12298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T, 2013. Analytical pharmacology and allosterism: The importance of quantifying drug parameters in drug discovery. Drug Discov. Today Technol 10, e229–e235. 10.1016/j.ddtec.2012.07.006 [DOI] [PubMed] [Google Scholar]
- Khurana L, Mackie K, Piomelli D, Kendall DA, 2016. Modulation of CB1 Cannabinoid Receptor by Allosteric Ligands: Pharmacology and Therapeutic Opportunities. Neuropeptides 124, 3–12. 10.1161/CIRCULATIONAHA.114.010270.Hospital [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey SG, Cole EC, 2013. Acute Δ9-tetrahydrocannabinol blocks gastric hemorrhages induced by the nonsteroidal anti-inflammatory drug diclofenac sodium in mice. Eur. J. Pharmacol 715, 111–116. 10.1016/j.ejphar.2013.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey SG, Long JZ, O’Neal ST, Abdullah RA, Poklis JL, Boger DL, Cravatt BF, Lichtman AH, 2009. Blockade of endocannabinoid-degrading enzymes attenuates neuropathic pain. J. Pharmacol. Exp. Ther 330, 902–10. 10.1124/jpet.109.155465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey SG, Nomura DK, O’Neal ST, Long JZ, Mahadevan A, Cravatt BF, Grider JR, Lichtman AH, 2011. Inhibition of Monoacylglycerol Lipase Attenuates Nonsteroidal Anti-Inflammatory Drug-Induced Gastric Hemorrhages in Mice. J. Pharmacol. Exp. Ther 338, 795–802. 10.1124/jpet.110.175778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey SG, Wise LE, Ramesh D, Abdullah R, Selley DE, Cravatt BF, Lichtman AH, 2013. Repeated Low-Dose Administration of the Monoacylglycerol Lipase Inhibitor JZL184 Retains Cannabinoid Receptor Type 1–Mediated Antinociceptive and Gastroprotective Effects. J. Pharmacol. Exp. Ther. J Pharmacol Exp Ther 345, 492–501. 10.1124/jpet.112.201426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin FR, Mariani JJ, Pavlicova M, Brooks D, Glass A, Mahony A, Nunes EV, Bisaga A, Dakwar E, Carpenter KM, Sullivan MA, Choi JC, 2016. Dronabinol and lofexidine for cannabis use disorder : A randomized, 159, 53–60. 10.1016/j.drugalcdep.2015.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH, Fisher J, Martin BR, 2001. Precipitated cannabinoid withdrawal is reversed by Delta(9)-tetrahydrocannabinol or clonidine. Pharmacol. Biochem. Behav 69, 181–8. https://doi.org/S0091-3057(01)00514-7 [pii] [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Wiley JL, Lavecchia KL, Neviaser ST, Arthur DB, Wilson DM, Martin BR, 1998. Effects of SR 141716A after acute or chronic cannabinoid administration in dogs. Eur. J. Pharmacol 357, 139–148. [DOI] [PubMed] [Google Scholar]
- Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, Pavón FJ, Serrano AM, Selley DE, Parsons LH, Lichtman AH, Cravatt BF, 2009. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat. Chem. Biol 5, 37–44. 10.1038/nchembio.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanedo C, Aguilar MA, Rodríguez-Arias M, Miarro J, 2001. Effects of dopamine antagonists with different receptor blockade profiles on morphine-induced place preference in male mice. Behav. Brain Res 121, 189–197. 10.1016/S0166-4328(01)00164-4 [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Parker L. a., 2012. The Endocannabinoid System and the Brain. Annu. Rev. Psychol 64, 120717165617008 10.1146/annurev-psych-113011-143739 [DOI] [PubMed] [Google Scholar]
- Naidu PS, Booker L, Cravatt BF, Lichtman AH, 2009. Synergy between enzyme inhibitors of fatty acid amide hydrolase and cyclooxygenase in visceral nociception. J. Pharmacol. Exp. Ther 329, 48–56. 10.1124/jpet.108.143487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamplona FA, Ferreira J, Lima OM De, Silveira F, 2012. Anti-in fl ammatory lipoxin A 4 is an endogenous allosteric enhancer of CB 1 cannabinoid receptor. Proc. Natl. Acad. Sci. U. S. A 109, 21134–21139. 10.1073/pnas.1202906109/-/DCSupplemental.www.pnas.org/cgi/doi/10.1073/pnas.1202906109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazos MR, Tolón RM, Benito C, Rodríguez CF, Gorgojo JJ, Nevado M, Álvarez M, Arias F, Almodóvar F, Fernández MTP, Lledó JL, González S, Fernández-Ruiz JJ, Romero J, 2008. Cannabinoid CB 1 Receptors Are Expressed by Parietal Cells of the Human Gastric Mucosa. J. Histochem. Cytochem 56, 511–516. 10.1369/jhc.2008.950741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D, Hohmann AG, Seybold V, Hammock BD, 2014. A Lipid Gate for the Peripheral Control of Pain. J. Neurosci 34, 15184–15191. 10.1523/JNEUROSCI.3475-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RA, 2007. Allosterism and cannabinoid CB1receptors: the shape of things to come. Trends Pharmacol. Sci 28, 567–572. 10.1016/j.tips.2007.10.006 [DOI] [PubMed] [Google Scholar]
- Rutkowska M, Fereniec-Golebiewska L, 2006. ACEA (arachidonyl-2-chloroethylamide), the selective cannabinoid CB 1 receptor agonist, protects against aspirin-induced gastric ulceration. Pharmazie 61, 341–342. [PubMed] [Google Scholar]
- Sasso O, Bertorelli R, Bandiera T, Scarpelli R, Colombano G, Armirotti A, Moreno-Sanz G, Reggiani A, Piomelli D, 2012. Peripheral FAAH inhibition causes profound antinociception and protects against indomethacin-induced gastric lesions. Pharmacol. Res 65, 553–563. 10.1021/nl061786n.Core-Shell [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosburg JE, Blankman JL, Long JZ, Nomura DK, Pan B, Kinsey SG, Nguyen PT, Ramesh D, Booker L, Burston JJ, Thomas EA, Selley DE, Sim-Selley LJ, Liu Q, Lichtman AH, Cravatt BF, 2010. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat. Neurosci 13, 1113–1119. 10.1038/nn.2616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosburg JE, Carlson B.L. a, Ramesh D, Abdullah R. a, Long JZ, Cravatt BF, Lichtman AH, 2009. Inhibitors of endocannabinoid-metabolizing enzymes reduce precipitated withdrawal responses in THC-dependent mice. AAPS J 11, 342–352. 10.1208/s12248-009-9110-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosburg JE, Kinsey SG, Ignatowska-Jankowska B, Ramesh D, Abdullah RA, Tao Q, Booker L, Long JZ, Selley DE, Cravatt BF, Lichtman AH, 2014. Prolonged Monoacylglycerol Lipase Blockade Causes Equivalent Cannabinoid Receptor Type 1 Receptor-Mediated Adaptations in Fatty Acid Amide Hydrolase Wild-Type and Knockout Mice. J. Pharmacol. Exp. Ther 350, 196–204. 10.1124/jpet.114.212753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shujaa N, Zadori ZS, Ronai AZ, Mergl BA, Mozes MM, Gyires K, 2009. Analysis of the effect of different neuropeptides in gastric mucosal defense initiated centrally. J. Physiol. Pharmacol 60, 93–100. 10.1159/000338428 [DOI] [PubMed] [Google Scholar]
- Slivicki RA, Xu Z, Kulkarni PM, Pertwee RG, Mackie K, Thakur GA, Hohmann AG, 2017. Positive Allosteric Modulation of Cannabinoid Receptor Type 1 Suppresses Pathological Pain Without Producing Tolerance or Dependence. Biol. Psychiatry 011, 1–12. 10.1016/j.biopsych.2017.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofia DR, Diamantis W, Harrison JE, Melton J, 1978. Evaluation of antiulcer activity of tetrahydrocannabinol in the shay rat test. Pharmacology 17 10.1002/2016WR019535.Received [DOI] [PubMed] [Google Scholar]
- Substance Use and Mental Health Services Administration, 2015. Results from the 2015 National Survey on Drug Use and Health: Detailed Tables,. 2015 Natl. Surv. Drug Use Heal [Google Scholar]
- Trexler KR, Nass SR, Crowe MS, Gross JD, Jones MS, McKitrick AW, Siderovski DP, Kinsey SG, 2018. Novel behavioral assays of spontaneous and precipitated THC withdrawal in mice. Drug Alcohol Depend 10.1016/j.drugalcdep.2018.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warzecha Z, Dembinski A, Ceranowicz P, Dembinski M, Cieszkowski J, Kownacki P, Konturek PC, 2011. Role of sensory nerves in gastroprotective effect of anandamide in rats. J. Physiol. Pharmacol 62, 207–217. [PubMed] [Google Scholar]
- Wiley JL, Marusich JA, Martin BR, Huffman JW, 2012. 1-Pentyl-3-Phenylacetylindoles and JWH-018 Share In Vivo Cannabinoid Profiles in Mice. Drug Alcohol Depend 123, 148–153. 10.1016/j.drugalcdep.2011.11.001.1-Pentyl-3-Phenylacetylindoles [DOI] [PMC free article] [PubMed] [Google Scholar]