Abstract
The rapid pace of genomic discovery has raised public expectation and concerns about the utility of new discoveries and their potential to exacerbate health disparities. Improving literacy concerning gene and environmental (GxE) contributors to disease is needed to avoid commonly observed deterministic misconceptions about genomics. Mental models approaches that incorporate community engagement processes could be used to inform GxE literacy-building interventions. We used a mental models approach to describe and systematically compare expert and lay understanding of GxE interactions, using the example of podoconiosis, a non-infectious lymphedema endemic in highland Ethiopia. Methods included: (1) specifying elicitation questions for a literature review, (2) eliciting an expert model, (3) eliciting a lay model, and (4) comparing the two models. We used a coding scheme to identify lay participants’ knowledge gaps, misunderstandings and extra knowledge relative to the expert standard. Results indicated that lay participants’ viewed poverty as an important susceptibility factor and considered heredity and contagion to have a joint causal influence. Experts did not endorse either of these viewpoints. Conventional expert-based interventions aimed to correct misconceptions about behaviors important for prevention may be stymied by lay views that social environmental factors have more important influences on health outcomes. GxE literacy interventions that consider multiple levels of influence including social determinants of health and personal resilience to augment health education strategies are needed in diverse settings. Novel communication approaches will be needed to help target audiences disentangle long-held conceptions of heredity and contagion.
Keywords: genomics, mental model, health literacy, low and middle income countries, gene-environment, podoconiosis, expert and lay knowledge, Ethiopia
Introduction
Over the past two decades the pace of genomic discovery has steadily increased (Manolio 2013; Khoury et al. 2007) (see Table 1 for glossary of terms used throughout this report). Consequently, genomic scientists have raised expectations that this knowledge will have broad benefits for the treatment and prevention of diseases worldwide (Condit 2010; Condit and Shen 2011; McBride et al. 2010; Walter et al. 2004). However, unlike the circumstances of many rare health conditions that follow Mendelian patterns of inheritance, genetic variation plays a less predictive role in most health conditions (Bookman et al. 2011). Indeed, many genetic variants only influence disease risk in the presence of environmental exposures, including health behaviors. Scientists will continue to gain insights about how genetic susceptibility interacts with environmental exposures to influence health outcomes (Manuck and McCaffery 2014).
Table 1.
Glossary of Terms
|
Gene by environment (GxE) interaction: different effect of an environmental exposure on disease risk in persons with different genotypes or a different effect of a genotype on disease risk in persons with different environmental exposures.a |
| Genetics: the study of heredity.b |
| Genomics: the study of genes and their functions, and related techniques.b |
|
Genomic health literacy: the capacity to obtain, process, understand, and use genomic information for health-related decision making.c |
|
Genomic literacy: the working knowledge of genomic science and its role in society, including personal decision-making, participation in civic and cultural affairs, and economic productivity.c |
|
Genomic science literacy: the knowledge of basic genetics and genomics concepts and the processes needed to build conceptual understanding, and the necessary mathematical knowledge to support this comprehension.c |
|
Health literacy: the degree to which individuals have the capacity to obtain, process, and understand basic health information and services needed to make appropriate health decisions.d |
|
Mental model: an explanation or representation of an individual’s thoughts about a topic and resulting consequences.e |
Ottman, R. 1996. “Gene-environment interaction: definitions and study designs.” Preventive Medicine 25 (6):764-70.
World Health Organization. 2002. “Genomics and World Health Report of the Advisory Committee on Health Research.” Geneva.
Hurle, B., Cirtin, T., Jenkins, J., Kapingst, K., Lamb, N., Roseman, J.E., Bonhman, V.L. 2013. “What does it mean to be genomically literate?: National Human Genome Research Institute Meeting Report.” Genetics in Medicine 15 (8):658-53.
Institute of Medicine. 2004. “Health Literacy: A Prescription to End Confusion.” Washington, DC: The National Academies Press.
Johnson-Laird, P.N. 2010. “Mental models and human reasoning.” Proceedings of the National Academy of Sciences of the United States of America 107 (43):18243-18250.
Experts agree that there is a pressing need to improve public literacy regarding gene by environment (GxE) influences on health (Bookman et al. 2011; McBride, Wade and Kaphingst 2010; Hurle et al. 2013; Daar et al. 2007). Accordingly, our team used a mixed method approach to encourage risk reduction for podoconiosis, a nonfilarial elephantiasis endemic to highland Ethiopia. Evidence to date supports the notion that the combination of inherited genetic susceptibility and barefoot exposure to soil rich in irritant mineral particles causes podoconiosis (Davey and Newport 2007; Tekola, Mariam and Davey 2006). Despite this condition being highly preventable among those with heightened susceptibility, there has been poor adoption of primary prevention practices (Tora, Davey and Tadele 2011; Tora et al. 2016; Tora et al. 2017).
We have conducted extensive focus groups and structured interviews with individuals in several communities who were affected and unaffected by podoconosis (Ayode et al. 2012; Ayode et al. 2016). Using these strategies, we enumerated a number of barriers to adoption of foot protection. We also found widespread beliefs that podoconiosis is spread via contagion, which is not supported by scientific evidence (Ayode et al. 2012; Ayode et al. 2016). Guided by these results, we developed a metaphor image and oral description that likened tolerance for sun exposure to inherited susceptibility to soil exposure. The metaphor image compared an umbrella to block the sun (tolerance for sun exposure) to the benefits of wearing shoes (inherited susceptibility) to prevent soil exposure (Tora et al. 2016). We evaluated this communication strategy as part of a household-based intervention supported by educational modules that emphasized skills building and peer support. Results of the intervention trial indicated no significant improvement in accuracy of understanding of inherited susceptibility, confidence to prevent podoconiosis, or ability to explain the causes of the condition among those who received these materials. Moreover, there were no improvements in shoe wearing among susceptible families (McBride et al. 2015).
While our intervention development strategies were consistent with widely-used qualitative methods, the trial findings raised questions about whether we had adequately characterized the target audiences’ causal perceptions and their linkage to preventive behaviors. This awareness prompted a renewed effort to consider additional qualitative methods aimed to explore GxE literacy building as means to improve intervention effectiveness. This involved using a mental models approach to describe and systematically compare expert and lay understanding of GxE interactions to provide insight into how to develop a more appropriate, tailored multi-dimensional intervention. It is worth noting that the expectation is not that improvement in GxE literacy would increase preventive behaviors, but such gains would be a necessary element of a broader intervention. In this report, we have the following overarching aims, to: (1) revisit the challenges of improving genomic literacy in low and middle income countries (LMICs) using podoconiosis in Ethiopia as our case study, (2) describe the mental model approach for mapping and systematic comparison of expert and lay understandings of GxE interaction, and (3) show how mental model maps of causal linkages can yield insights to increase GxE literacy.
Improving GxE literacy in LMICs: current challenges
To date, conceptualization and related evaluation of genetic literacy have focused narrowly on familiarity with genetic terminology (Hurle et al. 2013; Abrams et al. 2016). Yet the concept of health literacy encompasses a broader array of competencies including “the capacity to obtain, process, and understand basic health information and services needed to make appropriate health decisions” (Abrams et al. 2016; Nielsen-Bohlman et al. 2004). Studies consistently show that while individuals are generally familiar with genetic terms, they would not be considered to have adequate literacy by these standards (Hurle et al. 2013; Abrams et al. 2016) (see Table 1).
Concurrently, concerns have been raised that populations with low socioeconomic status will not have access to genomics-informed health applications. The reduced reach of these applications will be due, in part, to resistance borne of poor understanding and skepticism about genomics (Condit 2010; Delaney et al. 2016). In turn, this limited reach could result in unintended increases in health disparities. Globally, these considerations are especially germane for LMICs where a confluence of challenges relating to literacy, rurality, and limited access to health services have been well documented.
Improving understanding of GxE influences on health will likely face additional challenges (McBride et al. 2010; Walter et al. 2004; McBride, Wade and Kaphingst 2010; Hurle et al. 2013; Kaphingst et al. 2009). Qualitative studies have consistently shown that lay audiences’ tend to view genetic and environmental factors as having equal and parallel contributions to disease risk (Condit and Shen 2011). Lay audiences find it difficult to conceptualize how GxE influences might work together to impact health outcomes (Condit et al. 2009; Waters, Ball and Gehlert 2017). Indeed, much of the expert information conveyed to the public regarding genetic risk has reified these understandings by separately depicting GxE numeric risks in health communications (Wang et al. 20014). Thus, literacy-building efforts to shift this public narrative and improve understanding of how environmental exposures can be more (or less) harmful for some and not others have been slow to emerge (McBride et al. 2010). Prior research has suggested that lay audiences tend to believe that genes play deterministic roles in disease (Smerecnik et al. 2008; Syurina et al. 2011). However, only a few interventions have aimed to improve GxE literacy in these contexts (McBride and Leppard 2002; McBride et al. 2015).
Mental models as an alternative approach to building GxE literacy: considering common sense understandings of causality
Many conceptual models of health promotion suggest that when faced with a health threat, individuals develop explanations and attributions concerning the causal underpinnings of the threat (Leventhal, Fleming and Glynn 1988). These explanations or “mental models” inform the actions that individuals perceive to be important to take in reducing risk (Bandura 2007; Leventhal, Pillips and Burns 2016). Causal explanations generally come from personal experience and observing occurrences that appear to be connected to health events (Fischhoff, Bostrom and Quadrel 1993; Leventhal, Pillips and Burns 2016; Bandura 1991). Regardless of education level, lay mental models frequently diverge from the scientific and medical evidence that inform expert explanations (Leventhal, Pillips and Burns 2016; Fischhoff, Bostrom and Quadrel 1993).
Thus, it has been suggested that genetic education interventions will be most impactful when they begin by characterizing lay audiences’ mental models for health conditions (Condit 2010; Condit et al. 2009). Behavior change strategies often specifically aim to reinforce the “coherence” between these lay explanations of disease causation and recommended preventive actions (Cameron et al. 2012). However, such efforts have been associated with increased likelihood of behavior change and adherence to health promotion recommendations in some but not all studies (Roter et al. 1981; Michie et al. 2009; Bhuyan 2004). Additionally, few studies have described the systematic efforts taken to develop the mental models that have informed intervention development (e.g., (McBride and Leppard 2002)).
With few exceptions, mental models of GxE contributors have not been considered in the context of health promotion (Bakermans-Kranenburg and van IJzendoorn 2015). This may be particularly important in LMICs where low levels of general literacy are commonplace. Like most technological advances, genomic discovery has been be directed by developed countries and evaluation of genomics-informed applications (e.g., cancer screening) has largely focused on a narrow subgroup of early adopters (Conti et al. 2010). Efforts to reduce the likelihood that genomic discovery widens health disparities must be guided by translation research in LMICs (Solar and Irwin 2010).
In this report we use podoconiosis as a context for applying the mental models approach. The latter approach differs from social cognitive theories in its theoretical foundations. Social cognitive theories give emphasis to identifying barriers and facilitators to preventive actions (Bandura 2007); whereas, the mental models approach gives emphasis to identifying explanatory narratives and logic flows of causality that influence preventive actions taken (Owiti et al. 2015). In turn, the different emphases of these approaches may suggest different interventional solutions.
Constructing mental models
Constructing mental models involves an iterative process. Experts and lay individuals are separately engaged to articulate their understanding about causal factors (or nodes), the interconnectedness of these factors, and how they influence a specific health condition (Morgan, Fischhoff and Bostrom 2001). Expert and lay models are then compared to qualitatively characterize differences in understanding. The thinking is that health promotion interventions can be designed to build on areas of shared understanding, and accordingly, intervention activities can be targeted to gaps in understanding. In these comparisons, the expert model is regarded as the knowledge standard (Binder and Scholl 2010).
Mental models approaches have been used in a number of behavior change contexts to understand lay explanations for sexually transmitted diseases, vaccination risks, natural disasters, workplace safety, and injury prevention (Austin and Fischhoff 2012; Carriger and Barron 2011; Cox et al. 2003; Downs et al. 2010; Ivey, LeJeune and Miller 2012). While these studies have used a variety of techniques to develop mental models, most have included four steps: (1) specify an elicitation question that guides the expert evidence review, (2) identify and engage experts in developing an “expert” model, (3) identify and engage a lay target audience to specify a “lay” model, and (4) systematic comparison of the lay model against the expert model (Cox et al. 2003; Austin and Fischhoff 2012; Schoell and Binder 2008; Binder and Scholl 2010).
Step one: specifying an elicitation question to guide the expert evidence review
Specifying an expert model typically begins with a process of clarifying and bounding the knowledge area to be addressed (Forrester 1991). This is accomplished by identifying an elicitation question to guide the evidence review (Binder and Scholl 2010). A comprehensive list of factors (or nodes) is drawn from the evidence review that can be arranged to illustrate their role in the causal pathway to the health condition in question (Austin and Fischhoff 2012). Thus, the evidence review will aim for breadth of potential causes to gain with input from related disciplines and to identify as many plausible nodes for consideration (Downs et al. 2010). The review also can be used to identify disciplinary perspectives that should be represented in development of the expert model. The review is guided by keywords and a designated time frame for querying the literature.
Step two: identify and engage experts to create the expert model
The next step involves identifying experts who represent the disciplinary perspectives relevant to the elicitation question (Binder and Scholl 2010; Austin and Fischhoff 2012; Schoell and Binder 2008; Otto-Banaszak et al. 2011). The specifics for identifying and engaging these experts varies in the literature and often is not described. However, in the realm of health promotion, this is likely to involve those with expertise in epidemiology, social and behavioral sciences, basic science, and medicine. Ideal experts are those who can confidently represent the scientific perspective they have been assigned. Additionally, and consistent with characteristics important for transdisciplinary team science, each expert should be competent and willing to discuss their mental model with those derived from other scientific perspectives (Falk-Krzesinski et al. 2011).
Experts engage in individual elicitation sessions followed by a group discussion to arrive at an agreed upon model. During the initial individual elicitation session, experts are given the option to use the provided nodes and to suggest additional nodes. Experts use these nodes to sketch a path diagram that illustrates the connections and directionality between nodes. After creating individual expert models, the experts are brought together to consider ways to amalgamate their multiple conceptualizations and come to consensus (Schoell and Binder 2008; Morgan, Fischhoff and Bostrom 2001). The compiled expert model includes constructs that experts can agree are in the influence pathways for the specified health problem (Austin and Fischhoff 2012; Downs et al. 2010). The final model is depicted as a figure that becomes the framework against which the lay model will be compared (Binder and Scholl 2010).
Step three: identify and engage a lay target audience in activities to specify a lay model
Generally, methods for identifying lay target audiences vary across studies. Lay participants are selected based on the researchers’ perception that the topic is especially salient or that the audience has adequate experience upon which to base a mental model of causation (Schoell and Binder 2008). For example, Cox and colleagues (2003) identified individual users of chemicals, who were all either workers or supervisors to elicit their mental models regarding the risks of using the selected chemicals in the workplace. Newspapers advertisement were used to recruit volunteers who were current smokers or lived in a household with other smokers to consider the effects of radon exposure (Cox et al. 2003). Similarly, parents with a child aged 1–2 were targeted to identify beliefs relevant to vaccination (Downs, de Bruin and Fischhoff 2008).
The optimal number of lay participants also varies. The aim is to ensure the fullest elicitation of beliefs (Austin and Fischhoff 2012). A sample of 20 individuals from a homogeneous population affords approximately a 50% chance of observing each belief held by at least 5% of a population (Guest, Bunce and Johnson 2006). A sample of 20 to 30 should reveal all beliefs that are at least somewhat common. This modest sample size increases the feasibility of the in-depth interviews and analysis required for constructing mental models in LMICs (Austin and Fischhoff 2012).
Elicitation of the lay model can occur in parallel or after the creation of an expert model. The elicitation process similarly involves qualitative data collection approaches using structured interviews. Individuals and groups are asked to form representations of the specified health topic using nodes provided and suggest additional nodes. After individual and group models are created, these renditions are re-reviewed to arrive at consensus on the key nodal influences and the directionality of connections between nodes. Similar to the expert model, the goal is to arrive at one final lay mental model to characterize prevalent beliefs about the factors that cause the health condition.
Step four: comparing mental models to identify gaps
Methods used to identify gaps between expert and lay models are not well-documented in the literature. However, the aim is to characterize lay causal explanations that do not align with the expert standard of knowledge, lay competencies (areas of agreement with the experts) on which new information can be layered, and important misconceptions (areas where lay audiences disagree with experts) that can be characterized and corrected (Austin and Fischhoff 2012).
Coders independently review expert and lay mental models to compare identified nodes. Some have used tables and tabulated expert and lay identified nodes in rows and columns to visualize cross-over (Skarlatidou, Cheng and Haklay 2012; Cox et al. 2003; Zaksek and Aravi 2004). Using the expert model as the standard, there are a number of patterns that may be observed: (1) node included in the expert model but not the lay model (“gap”), (2) node not included in the expert model but included in the lay model (“extra knowledge”), (3) both experts and lay models acknowledge the node but describe the causal path differently (“misunderstanding”), and (4) node included in both and path described similarly (“shared knowledge”) (Carriger and Barron 2011; Cox et al. 2003). The subjectivity of deciding upon areas of difference and agreement between the two models that are most important is benefitted by dual coding and a process of reaching acceptable levels of interrater agreement (Austin and Fischhoff 2012; Lombard, Snyder-Duch and Bracken 2002). Ultimately, the goal is to identify differences between the mental models of causation that can be linked to motivation for preventive actions.
Methods: Applying mental models approaches to the case of podoconiosis in Ethiopia
Step one: specifying elicitation question for the evidence review and expert selection
Podoconiosis, a non-infectious lymphedema caused by extended barefoot exposure to volcanic red clay soil, affects at least four million people across ten countries. Ethiopia is home to the largest podoconiosis disease burden in the world (Davey et al. 2007; Davey and Newport 2007). Susceptibility to this inflammatory response has been shown to be inheritable; siblings of those with the condition are five times more likely to contract the disease (Tekola et al. 2012). The condition is entirely preventable if those with heightened susceptibility consistently practice foot hygiene and wear shoes starting early in life. Adoption of these preventive practices is relatively low (Yakob, Deribe and Davey 2008). Estimates suggest that up to 50% of those most susceptible to the condition do not routinely wear shoes. To date, health promotion interventions to encourage primary prevention have been intermittent and only marginally successful (McBride et al. 2015).
The elicitation question we specified was: “how would you explain podoconiosis to an adolescent living in a podoconiosis endemic area to encourage them to take preventative actions?” Specification of this question involved an iterative process. Initial versions of the elicitation question did not specify the target audience (adolescent youth) but instead specified heredity and environment. We added “adolescent” to the question as we agreed that this could influence how experts and lay participants viewed the appropriate explanation of causes. We omitted mention of heredity and environment to avoid constraining or unduly influencing causal explanations.
A total of 88 manuscripts were found using the search terms “podoconiosis” and “Ethiopia” in PubMed. Of these, 18 were excluded because they were published prior to 1980 and two were excluded because they were not written in English. Two independent coders (CA, CM) reviewed all the articles to identify key themes (i.e., nodes). A total of 49 potential causal nodes were identified; the two coders discussed and came to agreement on 24 final nodes.
Step two: developing an expert model
Four experts were selected to represent knowledge domains in the etiology of podoconiosis: (1) human genetics, (2) clinical- and population-epidemiology, (3) health promotion interventions, and (4) statistical models of gene-environment interaction. Each of the four experts were provided with an overview of the project, summary of mental model techniques, the specified question for consideration, a review article about podoconiosis, a list of the 24 nodes, and a set of questions to be covered in the semi-structured interview.
Initial interviews with the experts took place via Skype and lasted 90–120 minutes. The objective for the interview was to construct a visual representation of each expert’s views on how the provided nodes (or their recommended additional nodes) were linked and their directional connections. The interviewer (CA) worked with the expert to sketch an initial rendition of their mental model. After completing the interview, each expert reviewed their mental model as a figure and revised it as needed.
After completing all four interviews, two coders (CA, CM) reviewed each of the expert models and discussed key nodes, links and directionality to characterize a compiled expert model. Experts reviewed and discussed the compiled model in a group Skype meeting. After considerable discussion and multiple rounds of review of draft models, the group reached consensus on a composite explanatory mental model of podoconiosis (Figure 1).
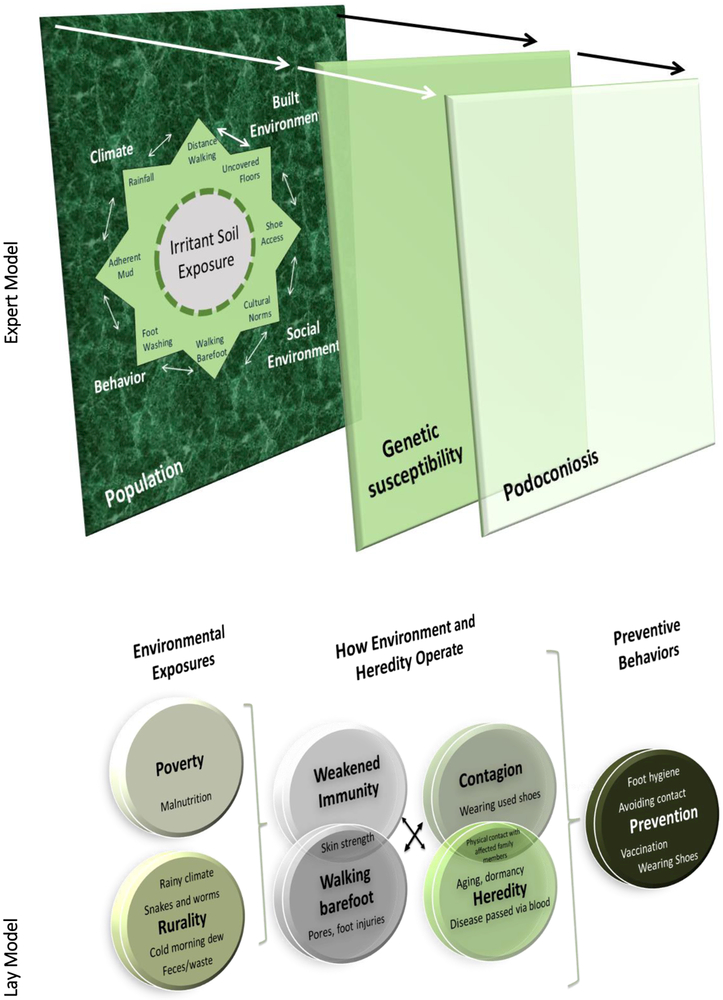
Figure 1. Final Expert and Lay Model.
The expert model includes an eight-point star with summative nodes. Each of these nodes has a moderating influence on soil exposure and interact with each other. The largest box depicts the population of highland Ethiopia and the smaller transparent box depicts genetic susceptibility, which is a smaller subset of the population. A subset of those who are genetically susceptible get podoconiosis, as depicted by the final box. The lay model describes exposures to include poverty as it relates to malnutrition and inability to buy shoes. Lay individual viewed exposures through weakened immunity because of poverty or malnutrition, walking barefoot, contagion, and heredity. In turn, they suggested preventive behaviors.
Step three: eliciting the lay mental model
The Ethiopian field team (KE, DA) employed qualitative methods to engage Ethiopian affected and unaffected youth ages 15–24 to address the elicitation question. Informants were selected purposely with the help of Mossy Foot International NGO. Youth in this targeted age range were the study population because of the large literature suggesting their effectiveness as peer encouragers of preventive behaviors (Peters et al. 2009). Thus, our intent was to first gain understanding of the accuracy of youth mental models and identify areas in need of correction on the way to considering the feasibility of engaging them to serve as peer encouragers. Methods closely mirrored those used to elicit the expert model. Thirty in-depth interviews (15 from families affected by podoconiosis and 15 from unaffected families) were conducted in two small communities in an area of endemic podoconiosis in Southern Ethiopia. Our prior work has shown striking differences in the beliefs of affected and unaffected adults (Ayode et al. 2016; Ayode et al. 2012). Interviews were conducted in Amharic and recorded. Each interview lasted between 30 to 60 minutes. All interviews were transcribed and translated verbatim into English. Data were then coded in Nvivo 11.
The Ethiopian research team used line-by-line coding to identify the spectrum of beliefs that could serve as nodes. The team then organized the nodes in a path diagram to illustrate what they heard in the lay interviews. The causal pathways were then shared and discussed with different participants in the community setting. The mental model amalgamated across participant’s individual models was created and shared with US researchers. US and Ethiopian collaborators continued to discuss and refine the lay model based on US investigator’s review of the original lay transcripts.
Step four: comparing mental models to identify gaps
In phase four, the goal was to systematically compare the expert and lay mental models. Coders (CA, CM) independently reviewed both mental models and created a list of expert and lay nodes. The nodes were summarized in a table where expert model nodes and lay model nodes were compared side-by-side. Cells in the table were coded to indicate four domains of differences: (1) expert noted, lay did not (“gap”), (2) expert did not note, lay did (“extra knowledge”), (3) noted by both expert and lay but described in different causal role (“misunderstanding”), (4) noted by both and described similarly (“shared knowledge”) (Table 2). The two coders met for consensus about these differences.
Table 2.
Example of Side-by-Side Comparison of Lay and Expert Model
| Expert Model Node | Lay Model Node(s) | Domain Difference |
|---|---|---|
| Silica particles in soil | Cold morning dew Snake and worms Bacteria |
Misunderstanding |
| Built environment | Poverty | Extra Knowledge |
| Cultural norms | Gap | |
| Inherited susceptibility to inflammation from soil exposure |
Heredity (i.e., disease passed by blood) Contagion |
Extra Knowledge |
| Malnutrition | Extra Knowledge | |
| Shoe access | Wearing shoes | Misunderstanding |
| Weakened immunity Vaccination |
Extra Knowledge | |
| Walking barefoot | Walking barefoot | Shared Knowledge |
Cells in the table were coded to indicate four domain differences: (1) expert noted, lay did not (gap); (2) expert did not note, lay did (extra knowledge); and (3) noted by both expert and lay but described in different causal role (misunderstanding); (4) node included in both and path described similarly (shared knowledge).
Results
Expert mental model: knowledge standard
The experts’ mental model was depicted as a three dimensional configuration of exposure, inherited susceptibility and their joint role in prevention (Figure 1). The first level of the figure shows an eight-point star characterizing the multifactorial complexity of exposures. The exposures align with four themes: climate, behavior, built environment, and social environment. Each point depicted an aspect of one theme or the intersection of a theme. For example, foot washing is listed under the behavior theme and cultural norms is listed under the social environment theme. Between behavior and social environment is walking barefoot, which could be considered part of both behavior and social environment. Each of these junctures was considered to influence the extent of soil exposure regarded by the experts as an essential causal factor for the condition.
These exposures were contained in an encompassing box to depict the environmental context that is shared by the population. Heredity is depicted as a smaller semi-transparent box to depict genetic susceptibility that operates as a filter through which a smaller subset of the population is at heightened risk. Further, among those at heightened risk due to genetic susceptibility, a subset of those individuals get podoconosis, which is depicted by the final box. As described by the experts, the three dimensional knowledge suggests conceptualization of exposures, exposure and susceptibility operating jointly, and their downstream influence on prevention.
Construction of the lay mental model
The three dimensional expert model was used as the reference for guiding the arrangement of nodes identified by lay individuals (Figure 1). Lay participants described exposures to include poverty as it related to malnutrition and inability to buy shoes. Also included was exposure to feces in the soil, cold morning dew, snakes, insects and bacteria in the soil and contact with the fluids of individuals with the disease. Lay participants viewed these exposures operating through weakened immunity because of poverty or malnutrition, walking barefoot, contagion and heredity. In turn, they suggested preventive behaviors to include vaccination to boost immunity, wearing shoes/foot hygiene, and avoiding contact with affected individuals.
Expert and lay conceptualizations of exposure
Barefoot exposure was a common theme in both expert and lay mental models. Experts emphasized soil exposure and specifically silica particles as necessary to cause the condition. Experts also described soil exposure being compounded by the confluence of rainy conditions, dense mud that adheres to the bare foot, and limited availability of water for cleaning feet.
In contrast, there were only one or two mentions of silica particles among the 30 lay participants. Instead, the majority of lay individuals had extensive narratives about exposure to cold dew, areas of open defecation, insects and snakes as the factors that made it risky to walk barefoot. Two examples include: ‘Based on my personal observation, people develop the disease while walking barefoot early in the morning…due to their exposure to dew…’ (16-year-old girl, unaffected) and ‘There are tiny worms in the soil which discharge their poison into the feet that gradually cause the disease’ (15-year-old boy, affected).
Additionally, the lay model regarded exposure to used shoes and the bacteria they contained as important. A 19-year-old girl who was unaffected stated, ‘I sometimes buy pre-used shoes from the market. If those shoes are used by an affected individual, I might get it. There was a girl who used to wear second hand shoes. Due to that, she developed the disease.’
Experts did not mention poverty as an exposure. In contrast, lay individuals consistently alluded to poverty as an exposure factor. For example, ‘…life in the rural setting is very hard and people don’t feed on variety of food stuffs unlike urban people do. We use unclean water and live in poor hygienic conditions that facilitate the spread of disease’ (17-year-old boy, affected).
Expert and lay conceptualization of how exposure and susceptibility operate jointly
Experts described inherited susceptibility as a ubiquitous filter; only a subset of those exposed to the soil would develop the condition. Lay views of this filtering effect were more complex. In the lay model, the filter was whether the disease was inherited or acquired through contagion or some combination of the two. Lay participants believed that podoconiosis could be inherited through the blood of their family. They also viewed contagion to operate hand-in-hand with heredity. Family members not only shared blood but also lived in close proximity to other affected family members. These close living situations exposed family members to fluid from oozing wounds on infected legs and feet, and via sharing shoes and bathing water. For example, a 18-year-old unaffected female stated, ‘It is inevitable in affected families because they are highly susceptible to the disease having contact with their parents. Children may sleep with their parents in the same bed, on the same bench and inherit the disease.’
Additionally, lay participants perceived that living in conditions of poverty operated as a filter because it weakened individual’s immunity to health threats. This weakened immunity combined with walking barefoot increased risk for the disease, ‘the patient family most likely developed the disease for he is poor and cannot afford to buy enough food stuffs that are rich with nutrients to build the body. His children are more likely to be susceptible to podoconiosis. Whereas the healthy person is rich and his children can get balanced food and are less likely to develop the disease’ (15-year-old boy, affected).
Expert and lay conceptualization of exposure and susceptibility in prevention
Experts talked solely about shoe wearing and foot hygiene as preventative actions. Experts noted that wearing shoes that provided full coverage of the foot was critically important. Lay participants perceived that podoconiosis could be prevented but with considerable equivocating. In the lay model, wearing shoes was perceived to be preventive for some exposures. However, this was countered by their beliefs that shoes were also a source of bacteria that caused the disease, and so only new shoes were considered safe. Lay individuals who identified the disease being caused by environmental factors (e.g., soil exposure) perceived that shoe wearing was a preventative action. For example, a 23-year-old unaffected boy stated, ‘We can’t prevent the podoconiosis that run in families. Nothing can be done in that situation. But, we can prevent the one that comes due to walking on barefoot, by wearing shoes consistently.’
Some lay individuals agreed with expert notions that even if susceptibility was inherited, the condition could be prevented by wearing shoes. For example, a 22-year-old unaffected boy stated, ‘If someone is affected in the family, it is an indication that the disease could be inherited and therefore, all family members should regularly wear shoes and wash their feet in order to prevent the disease.’
Additionally, perceptions that weakened immunity was a cause suggested that vaccination also could be an appropriate preventive action. Experts did not mention vaccination as a means of prevention.
Discussion
This report provides an overview of using mental models methods to systematically identify causal conceptualizations of risk and prevention among lay audiences at risk for developing podoconiosis. Mental models approaches asserts that GxE health education strategies can be optimized by finding gaps in expert and lay individual’s understandings of the causes of podoconiosis, identifying misconceptions, and accurate beliefs that align with expert viewpoints (Zaksek and Aravi 2004).
Applying mental models: a critical discussion of methods
It was noteworthy that experts were slow to reach consensus about the nodes and the arrangement that was most important for promoting accurate understanding the causes of podoconiosis among youth. This was particularly true for how to depict exposure and susceptibility. We needed several iterations after a first composite model was developed to refine the arrangement of the nodes and secure consensus among the experts. Our panel represented medical, social behavioral, gene x environment, and human genetic expertise, one of whom has lived in Ethiopia and worked with the communities for over a decade. Other attempts at using this approach have also identified the issue of inconsistency among experts. Most health outcomes arise from a complex interplay of influences and as such, expert models are best derived with input from diverse disciplines. Previous researchers have described similar challenges when attempting to merge expert models from diverse backgrounds, noting that experts with culturally similar backgrounds have more homogenous viewpoints, thus, making it easier to develop a final expert model (Schoell and Binder 2008; Binder and Scholl 2010; Pretty and Shah 1997). Consolidation of more heterogeneous viewpoints such as genomics, social sciences, and behavior change will inevitably lose some of complexity of explanatory pathways. Moreover, in the case of GxE influences, there is considerable uncertainty about when, how, and for whom these interactions influence health. Since the expert model is regarded as the ‘accurate’ interpretation or standard that serves as the basis for analyzing the lay model, it is important to consider this inherent subjectivity and its influence on health education targets (Otto-Banaszak et al. 2011). Further exploration of the politics of knowledge as they relate to the distinctions between expert knowledge about this topic may offer additional insight into the complexities and nuances of this topic (Baert and Rubio 2012).
Addressing differences in perceptions of social determinants over proximal causes of podoconiosis
Consistent with prior health education research, our lay target audience had their own explanations for causal factors that influenced podoconiosis. Some of these beliefs aligned in synchrony with the expert knowledge standard (e.g., susceptibility can be inherited). However, even in these areas of agreement, lay perceptions included other important contributing factors that disagreed with the expert knowledge standard.
One area of extra knowledge (poverty), and one area of misunderstanding (contagion) are compelling and worth further discussion. Poverty was considered to be a critical factor that affected susceptibility by the lay participants; not mentioned explicitly by the experts, this viewpoint constituted extra knowledge on the part of the lay participants. The chronic and debilitating effects of poverty on health were mentioned frequently in lay narratives. Poverty was viewed to weaken immune response and leave some individuals at heightened risk for podoconiosis. Experts acknowledged that built environment, rurality, and walking long distances, all influenced by poverty, but did not directly acknowledge poverty. Experts focused more on proximal causes that they perceived to be addressable.
Lay participants’ greater emphasis on poverty presents challenges for the mental model approach. Once differences in expert and lay beliefs are identified, they can be validated or corrected via traditional and widely used health education strategies (Nutbeam 2006). However, traditional health education strategies provide little to populations that consider macro-level social influences as primary causes of the health outcome in question (Binder and Scholl 2010; Bostrom 1997; Murphy and Gardoni 2006). Indeed, lay participants often described poverty influencing access to shoes without specifying walking barefoot or exposure to minerals in the soil (Austin and Fischhoff 2012). Interventions based on these methods are unlikely to be effective if they overlook broader system influences suggested by social ecological models of health promotion.
Fundamental causes like poverty have been increasingly acknowledged to be important to consider in health promotion interventions (Thorton et al. 2016). However, the majority of health risk messages derive from basic and medical sciences and give more focus to specific and proximal influences on disease causation (Marmot 2007). Bringing these two perspective together to broaden expert models by including “place” characteristics in risk assessment is beginning to gain traction (Hartmann, Marshall and Goldenberg 2015). Interventions aimed at fostering resilience, the ability to cope with everyday challenges, among those living in impoverished LMIC settings or stressful circumstances have shown positive influences on empowerment (Langsford and Griffiths 2015; Wong et al. 2009). These approaches make children aware of emotions linked to their personal story and show how these emotions impede or facilitate adaptive decision-making, empowerment, and empathy.
Other innovative programming has emerged from the strategic communication literature, which highlights strategies to better promote behavior change in light of new insights such as the new knowledge we gained on structural barriers like poverty. For example, UNICEF promotes Communication for Development (C4D), which contextualizes health risk communication in broader community mobilization and implementation strategies that address social and behavioral determinants (e.g., fundamental cause) (Obregon and Hickler 2014). Such approaches could precede or otherwise augment traditional health education approaches. In turn, individual community members could be disseminators of these interventions, thus reinforcing their long-term sustainability.
Addressing lay misconceptions about inherited influences
In contrast to experts, lay participants also viewed contagion and heredity to work jointly in the development of podoconiosis. While podoconiosis is a disease where apparent wound oozing might logically raise these concerns, contagion beliefs about other conditions such as epilepsy and same-sex attraction also are well documented in LMICs (Herrmann et al. 2016; Winskell et al. 2016). Findings suggest that contagion beliefs are difficult to override and may not be responsive to standard health education strategies (Curtis 2011).
Indeed, the recalcitrance of contagion beliefs may be rooted in notions that disgust and related aversion are functional and protect groups from exposures to infectious agents (Oaten, Stevenson and Case 2011). Whether increasing genomic literacy could be a way to counteract contagion beliefs remains untested. In our prior intervention trial, we used a metaphor about sun exposure to counteract perceptions of contagion (McBride et al. 2015). However, our qualitative interviews that informed this initial approach did not illuminate the tight linkage between contagion and hereditary that was uncovered using the mental models approach. Our prior findings suggested that the two were competing and not complementary perceptions. Thus, our prior intervention approach sought to override contagion beliefs with increased understanding of inherited susceptibility. Our results indicated some benefit in reducing stigmatizing behaviors of unaffected families, but contagion beliefs were not completely overridden (McBride et al. 2015).
Going forward, the challenge will be to untwine contagion from heredity. It is understandable that lay perceptions may see the two as working together, sharing blood and sharing daily living. Discussions of family history could be used to reframe contagion by illustrating other exposures that families share such as norms related to wearing shoes, or the conditions of poverty that impede all family members from wearing shoes. This approach could offer opportunity for counter-arguing contagion via bodily fluids and acknowledge the broader influence of poverty.
Links between causal beliefs and preventive actions
As reported in other mental model studies, young adults in our study saw linkages between the causes they endorsed and the preventive actions they perceived to be important (Morgan, Fischhoff and Bostrom 2001). For example, beliefs about poverty and impaired immune function conjured endorsement of vaccination as a mode of prevention. Beliefs about contagion were associated with avoiding re-used shoes as a means of preventing the condition. The more intensive and theory-based application of mental models demonstrate that simply using health education approaches to emphasize the expert knowledge, even in language and using metaphors likely will not be effective (Morgan, Fischhoff and Bostrom 2001).
There were areas of agreement between experts and lay participants related to GxE influences. For example, both acknowledged differential susceptibility as a concept. However, experts noted this to be inherited, whereas lay participants viewed weakened immunity as a susceptibility factor that was not inherited but a result of harsh living conditions. Recently, there have been efforts to engage individuals who have understandings that align with expert knowledge standards in story-writing competitions (Winskell et al. 2016). These stories are then used to augment health education interventions by contextualizing and contrasting with widely accepted lay perceptions.
Conclusions
In conclusion, broad dissemination of genomics-informed health applications is needed to reduce the likelihood of further entrenching health disparities (Sankar, Cho and Condit 2004; Cooper, Hill and Powe 2002; Austin and Fischhoff 2012). In turn, the success of genomic literacy building efforts will rest not only on target audiences’ having the skills to understand and use the information to benefit personal health, but these efforts also must target the broader social context and its influence on health (Solar and Irwin 2010). Novel approaches to engage community organizations including schools and health extension agencies in promoting health- and genomic literacy specifically will be needed to ensure broad reach of health benefits.
Acknowledgements
We would like to thank our study participants for contributing to the development of mental models. This study was funded by the National Institutes of Health Funding Opportunity Announcement, “Human Heredity and Health In Africa (H3Africa): Ethical, Legal, and Societal Issues (ELSI) Research Program (U01).” Grant number: 5U01HG007628 – 03.
This work was supported by National Institutes of Health Funding Opportunity Announcement, “Human Heredity and Health in Africa (H3Africa): Ethical, Legal, and Societal Issues (ELSI) Research Program (U01). Grant number: 5U01HG007628 – 03. The authors report no conflicts of interest.
References
- Abrams LR, McBride CM, Hooker GW, Cappella JN, and Koehly LM (2016). “The many facets of genetic literacy: Assessing the scalability of multiple measures for broad use in survey research.”PLOS ONE 10 (10):e0141532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin LC, and Fischhoff B (2012). “Injury prevention and risk communication: A mental models approach.”Inj Prev 18:124–9. [DOI] [PubMed] [Google Scholar]
- Ayode D, McBride CM, de Heer H, Watanabe E, Gebreyesus T, Tadele G, and Davey G (2012). “He association of beliefs about heredity with preventive and interpersonal behaviors in communities affected by podoconiosis in rural ethiopia.”The American Journal of Tropical Medicine and Hygiene 87 (4):623–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayode D, Tora A, Farrell D, Tadele G, Davey G, and McBride CM (2016). “Association between causal beliefs and shoe wearing to prevent podoconiosis: A baseline study.”Am J Trop Med Hyg 4 (94):1123–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baert Patrick, and Rubio Fernando Domínguez. (2012). The politics of knowledge. Edited by Baert Patrick and Rubio Fernando Domínguez. New York: Routledge. [Google Scholar]
- Bakermans-Kranenburg MJ, and van IJzendoorn MH (2015). “The hidden efficacy of interventions: Gene×environment experiments from a differential susceptibility perspective.”Annual Review of Psychology 66:381–409. [DOI] [PubMed] [Google Scholar]
- Bandura A (1991). “Social cognitive theory of self-regulation.”Organizational Behavior and Human Decision Processes 50 (2):248–87. [Google Scholar]
- ———. (2007). “Health promotion from the perspective of social cognitive theory.”Psychology & Health 13 (4):623–49. [Google Scholar]
- Bhuyan K (2004). “Health promotion through self-care and community participation: Elements of a proposed programme in the developing countries.”BioMed Central Public Health 4 (11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder C, and Scholl R (2010). “Structured mental model approach for analyzing perception of risks to rural livelihood in developing countries.”Sustainability 2 (1):1–29. [Google Scholar]
- Bookman EB, McAlister K, Gillanders E, Wanke K, Balshaw D, Rutters J, Reedy J, et al. (2011). “Gene-environment interplay in common complex diseases: Forging an integrative model--recommendations from an nih workshop.”Genetic Epidemiology 35 (4):217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostrom A (1997). “Risk perceptions: “Experts” vs. “Lay people”.”Duke Envtl L Pol’y 8 (1):101–13. [Google Scholar]
- Cameron LD, Marteau TM, Brown PM, Klein WMP, and Sherman KA (2012). “Communication strategies for enhancing understanding of the behavioral implications of genetic and biomarker tests for disease risk: The role of coherence.”J Behav Med 35:286–98. [DOI] [PubMed] [Google Scholar]
- Carriger J, and Barron M (2011). “Minimizing risks from spilled oil to ecosystem services using influence diagrams: The deepwater horizon spill response.”Environmental Science & Technology 45 (18):7631–9. [DOI] [PubMed] [Google Scholar]
- Condit CM (2010). “Public attitudes and beliefs about genetics.”Annual review of Genomic and Human Genetics 11:339–59. [DOI] [PubMed] [Google Scholar]
- Condit CM, Gronnvoll M, Landau J, Shen L, Wright L, and Harris TM (2009). “Believing in both genetic determinism and behavioral action: A materialist framework and implications.”Public Understanding of Science 18 (6):730–46. [Google Scholar]
- Condit CM, and Shen L (2011). “Public understanding of risk from gene-environment interaction in common diseases: Implications for public communications.”Public Health Genomics 14:115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti R, Veenstra D, Armstrong K, Lesko L, and Grosse S (2010). “Personalized medicine and genomics: Challenges and opportunities in assessing effectiveness, cost-effectiveness, and future research priorities.”Med Decision Making 30 (3):328–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper LC, Hill MN, and Powe NR (2002). “Designing and evaluating interventions to eliminate racial and ethnic disparities in health care.”J Gen Intern Med 17 (6):477–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox P, Niewöhmer J, Pidgeon N, Gerrard S, Fischhoff B, and Riley D (2003). “The use of mental models in chemical risk protection: Developing a generic workplace methodology.”Risk Analysis 23 (2):311–24. [DOI] [PubMed] [Google Scholar]
- Curtis V (2011). “Why disgust matters.”Phil Trans R. Soc. B (366):3478–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daar A, Singer P, Persad D, Pramming S, Matthews D, Beaglehole R, and Bell J (2007). “Grand challenges in chronic non-communicable diseases.”Nature 450 (7169):494–6. [DOI] [PubMed] [Google Scholar]
- Davey G, Gebrehanna E, Adeyemo A, Rotimi CN, Newport MJ, and Desta K (2007). “ Podoconiosis: A tropical model for gene-environment interactions?”Transactions of the Royal Society of Tropical Medicine and Hygiene 101 (1):91–6. [DOI] [PubMed] [Google Scholar]
- Davey G, and Newport MJ (2007). “Podoconiosis: Non-infectious geochemical elephantiasis.”Transactions of the Royal Society of Tropical Medicine and Hygiene 101 (12):1175–80. [DOI] [PubMed] [Google Scholar]
- Delaney SK, Hultner ML, Jacob HJ, Ladbetter DH, McCarthy JJ, Ball M, and Beckman KB (2016). “Toward clinical genomics in everyday medicine: Perspectives and recommendations.”Expert Review of Molecular Diagnostics 16 (5):521–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs JS, Arslanian S, de Bruin W, Copeland V, Doswell W, Herman W, and Charron-Prochownik D (2010). “Implications of type 2 diabetes on adolescent reproductive health risk: An expert model.”The Diabetes Educator 36 (6):911–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs JS, de Bruin WB, and Fischhoff B (2008). “Parents’ vaccination comprehension and decisions.”Vaccine 26:1595–607. [DOI] [PubMed] [Google Scholar]
- Falk-Krzesinski HJ, Börner K, Contractor N, Cummings J, Fiore SM, Hall KL, Keyton J, et al. (2011). “Advancing the science of team science.”Clin Triansl Sci 3 (5):263–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischhoff B, Bostrom A, and Quadrel M (1993). “Risk perception and communication.”Annual Review of Public Health 14 (1). [DOI] [PubMed] [Google Scholar]
- Forrester JW 1991. “System dynamics and the lessons of 35 years” In The systemic basis of policy making in the 1990s, edited by De Greene KB. [Google Scholar]
- Guest G, Bunce A, and Johnson L. (2006). “How many interviews are enough? An experiment with data saturation and variability.”Field Meth 18:59–82. [Google Scholar]
- Hartmann CD, Marshall AJ, and Goldenberg AJ (2015). “Is there a space for place in family history assessment? Underserved community views on the impact of neighborhood factors on health and prevention.”The Journal of Primary Prevention 36 (2):119–30. [DOI] [PubMed] [Google Scholar]
- Herrmann LK, Welter E, Berg AT, Perzynski AT, Van Doren JR, and Sajatovic M 5. (2016). “Epilepsy misconceptions and stigma reduction: Current status in western countries.”Epilepsy Behav 60:165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurle B, Cirtin T, Jenkins J, Kapingst K, Lamb N, Roseman JE, and Bonham VL (2013). “What does it mean to be genomically literate?: National human genome research institute meeting report.”Genetics in Medicine 15 (8):658–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivey M, LeJeune J, and Miller S (2012). “Vegetable producers’ perceptions of food safety hazards in the midwestern USA.”Food Control 26 (2):453–65. [Google Scholar]
- Kaphingst K, Lachance CR, Gepp A, Hoyt D’Anna L, and Rios-Ellis B (2009). “Educating underserved latino communities about family health history using lay health advisors.”Public Health Genomics 14:211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury M, Gwinn M, Yoon P, Dowling N, Moore C, and Bradley L (2007). “The continuum of translation research in genomic medicine: How can we accelerate the appropriate integration of human genome discoveries into health care and disease prevention.”Genetics in Medicine 9 (10):665–74. [DOI] [PubMed] [Google Scholar]
- Langsford M, and Griffiths T. (2015). “Learning and teaching emotional logic in zimbabwe: A lifelong learning emotional literacy training package that promotes healthy adjustment in resource-poor settings.”Tropical Doctor 45 (3):158–63. [DOI] [PubMed] [Google Scholar]
- Leventhal H, Fleming R, and Glynn K 1988. “A cognitive-developmental approach to smoking intervention” In Topics in health psychology, edited by Maes S, Spielberger CD, Defares PB and Sarason IG, 79–105. Oxford, England: John Wiley & Sons. [Google Scholar]
- Leventhal H, Pillips A, and Burns E (2016). “The common-sense model of self-reglation (csm): A dynamic framework for understanding illness self-management.”J Behav Med 39:935–46. [DOI] [PubMed] [Google Scholar]
- Lombard M, Snyder-Duch J, and Bracken CC (2002). “Content analysis in mass communication: Assessment and reporting of intercoder reliability.”Human Communication Research 28:587–604. [Google Scholar]
- Manolio TA. (2013). “Bringing genome-wide association findings into clinical use.”Nat Rev Genet 14 (8):549–58. [DOI] [PubMed] [Google Scholar]
- Manuck SB, and McCaffery JM (2014). “Gene-environment interaction.”Annual Review of Psychology 65:41–70. [DOI] [PubMed] [Google Scholar]
- Marmot M (2007). “Achieving health equity: From root causes to fair outcomes.”The Lancet 370 (9593):1153–63. [DOI] [PubMed] [Google Scholar]
- McBride CM, Bowen D, Brody LC, Condit CM, Croyle RT, Gwinn M, Khoury M, et al. (2010). “Future health applications of genomics.”Am J Prev Med 38 (5):556–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride CM, Price CS, Ayode D, Tora A, Farrell D, and Davey G (2015). “A cluster randomized intervention trial to promote shoe use by children at high risk for podoconiosis.”IJHSR 5 (6):518–28. [Google Scholar]
- McBride CM, Wade CH, and Kaphingst KA. (2010). “Consumers’ views of direct-to-consumer genetic information.”Annual Review of Genomics and Human Genetics 11:427–46. [DOI] [PubMed] [Google Scholar]
- McBride S, and Leppard B (2002). “Attitudes and beliefs of an albino population toward sun avoidance - advice and services provided by an outreach albino clinic in tanzania.”Arch Dermatol 138:629–32. [DOI] [PubMed] [Google Scholar]
- Michie S, Jochelson K, Markham W, and Bridle C (2009). “Low-income groups and behaviour change interventions: A review of intervention content, effectiveness and theoretical frameworks.”J Epidemiol Community Health 63:610–22. [DOI] [PubMed] [Google Scholar]
- Morgan G, Fischhoff B, and Bostrom A (2001). Risk communication--a mental model approach: Cambridge University Press. [Google Scholar]
- Murphy C, and Gardoni P (2006). “The role of society in engineering risk analysis: A capabilities-based approach.”Risk Analysis 26 (4):1073–83. [DOI] [PubMed] [Google Scholar]
- Nielsen-Bohlman L, Panzer A, Hamlin B, and Kindig D (2004). Health literacy: A perscription to end confusion. Washington, D.C.: The National Academies Press. [PubMed] [Google Scholar]
- Nutbeam D (2006). “Health literacy as a public health goal: A challenge for contemporary health education and communication strategies into the 21st century.”Health Promot Int 15 (3):259–67. [Google Scholar]
- Oaten M, Stevenson RJ, and Case TI (2011). “Disease avoidance as a functional basis for stigmatization.”Phil Trans R. Soc B 366 (1583): 3433–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obregon R, and Hickler B (2014). “Opportunities and challenges for health communication in health disparities settings.”Journal of Communication in Healthcare 7 (2):77–9. [Google Scholar]
- Otto-Banaszak I, Matczak P, Wesseler J, and Wechsung F (2011). “Different perceptions of adaptation to climate change: A mental model approach applied to the evidence from expert interviews.”Regional Environmental Change 11 (2):217–28. [Google Scholar]
- Owiti JA, Greenhalgh T, Sweeney L, Foster GR, and Bhui KS (2015). “Illness perceptions and explanatory models of viral hepatitis b & c among immigrants and refugees: A narrative systematic review.”BMC Public Health 15 (151):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters LW, Wiefferink CH, Hoekstra F, Buijs GJ, Ten Dam GT, and Paulussen TG (2009). “A review of similarities between domain-specific determinants of four health behaviors among adolescents.”Health Educ Res 24 (2):198–223. [DOI] [PubMed] [Google Scholar]
- Pretty JN, and Shah P (1997). “Making soil and water conservation sustainable: From coercion and control to partnerships and participation.”Land Degredation and Development 8 (1):39–58. [Google Scholar]
- Roter D, Rudd R, Frantz S, and Comings J (1981). “Community-produced materials for health education.”Public Health Reports 96 (2):169–72. [PMC free article] [PubMed] [Google Scholar]
- Sankar P, Cho MK, and Condit CM (2004). “Genetic research and health disparities.”JAMA 291 (24):2985–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoell R, and Binder C (2008). “System perspectives of experts and farmers regarding the role of livelihood assets in risk perception: Results from the structured mental model approach.”Risk Analysis 29 (2):205–22. [DOI] [PubMed] [Google Scholar]
- Skarlatidou A, Cheng T, and Haklay M (2012). “What do lay people want to know about the disposal of nuclear waste? A mental model approach to the design and development of an online risk communication.”Risk Analysis 32 (9):1496–511. [DOI] [PubMed] [Google Scholar]
- Smerecnik C, Mesters I, de Vries NK, and de Vries H (2008). “Educating the general public about multifactorial genetic disease: Applying a theory-based framework to understand current public knowledge.”Genet Med 10:251–8. [DOI] [PubMed] [Google Scholar]
- Solar O, and Irwin A 2010. “A conceptual framework for action on the social determinants of health.” In. [Google Scholar]
- Syurina EV, Brankovic I, Probst-Hensch N, and Brand A (2011). “Genome-based health literacy: A new challenge for public health genomics.”Public Health Genomics 14 (4–5):201–10. [DOI] [PubMed] [Google Scholar]
- Tekola F, Adeyemo A, Finan C, Hailu E, Sinnott P, Burlinson ND, Aseffa A, Rotimi CN, Newport MJ, and Davey G (2012). “Hla class ii locus and susceptibility to podoconiosis.”N Engl J Med 366 (13):1200–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekola F, Mariam D, and Davey G (2006). “Economic costs of endemic non-filarial elephantiasis in wolaita zone, ethiopia.”Tropical Medicine & International Health 11 (7):1136–44. [DOI] [PubMed] [Google Scholar]
- Thorton RL, Glover CM, Cene CW, Glik DC, Henderson JA, and Williams DR (2016). “Evaluating strategies for reducing health disparities by addressing the social determinants of health.”Health Affairs 35 (8):1416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tora A, Ayode D, Tadele G, Farrell D, Davey G, and McBride CM (2016). “Interpretations of education about gene-environment influences on health in rural ethiopia: The context of a neglected tropical disease.”International Health 8 (4):253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tora A, Davey G, and Tadele G (2011). “A qualitative study on stigma and coping strategies of patients with podoconiosis in wolaita zone, southern ethiopia.”International Health 3:176–81. [DOI] [PubMed] [Google Scholar]
- Tora A, Tadele G, Aseffa A, McBride CM, and Davey G (2017). “Health beliefs of school-age rural children in podoconiosis-affected families: A qualitative study in southern ethiopia.”PLoS Negl Trop Dis 11 (5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter FM, Emery J, Braithwaite D, and Marteu TM (2004). “Lay understanding of familial risk of common chronic disease: A systematic review and synthesis of qualitative research.”Ann Fam Med 2:583–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Gordon ES, Stack CB, Liu C, Norkunas T, Wawak L, Christman MF, Green RC, and Bowen DJ (20014). “A randomized trial of the clinical utility of genetic testing for obesity: Design and implementation considerations.”Clin Trials 11 (1):102–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters EA, Ball LB, and Gehlert S (2017). ““I don’t believe it.” Acceptance and skepticism of genetic health informationg among african-american and white smokers.”Soc Sci Med 184:153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winskell K, Sabben G, Pruitt KL, Allen K, Findlay T, and Stephenson R (2016). “Young africans’ representations of the origins of same-sex attraction and implications for sexual and mental health.”Culture, Health and Sexuality:1–16. [DOI] [PubMed] [Google Scholar]
- Wong MC, Sun J, Lee A, Stewart D, Cheng FF, Kan W, and Ho M (2009). “The impact of a newly designed resilience-enhancing programme on parent- and teacher-perceived resilience environment among health promoting schools in hong kong.”J Epidemiol Community Health 63 (3):209–14. [DOI] [PubMed] [Google Scholar]
- Yakob B, Deribe K, and Davey G (2008). “High levels of misconceptions and stigma in a community highly endemic for podoconiosis in southern ethiopia.”Transactions of the Royal Society of Tropical Medicine and Hygiene 102 (5):439–44. [DOI] [PubMed] [Google Scholar]
- Zaksek M, and Aravi JL (2004). “Toward improved communication about wildland fire:Mental models research to identify information needsfor natural resource management.”Risk Analysis 24 (6):1503–14. [DOI] [PubMed] [Google Scholar]