Abstract
Background
Contemporary incidence estimates of typhoid fever are needed to guide policy decisions and control measures and to improve future epidemiological studies.
Methods
We systematically reviewed 3 databases (Ovid Medline, PubMed, and Scopus) without restriction on age, country, language, or time for studies reporting the incidence of blood culture–confirmed typhoid fever. Outbreak, travel-associated, and passive government surveillance reports were excluded. We performed a meta-analysis using a random-effects model to calculate estimates of pooled incidence, stratifying by studies that reported the incidence of typhoid fever and those that estimated incidence by using multipliers.
Results
Thirty-three studies were included in the analysis. There were 26 study sites from 16 countries reporting typhoid cases from population-based incidence studies, and 17 sites in 9 countries used multipliers to account for underascertainment in sentinel surveillance data. We identified Africa and Asia as regions with studies showing high typhoid incidence while noting considerable variation of typhoid incidence in time and place, including in consecutive years at the same location. Overall, more recent studies reported lower typhoid incidence compared to years prior to 2000. We identified variation in the criteria for collecting a blood culture, and among multiplier studies we identified a lack of a standardization for the types of multipliers being used to estimate incidence.
Conclusions
Typhoid fever incidence remains high at many sites. Additional and more accurate typhoid incidence studies are needed to support country decisions about typhoid conjugate vaccine adoption. Standardization of multiplier types applied in multiplier studies is recommended.
Keywords: incidence studies, meta-analysis, Salmonella enterica serovar Typhi, typhoid fever, systematic review
Salmonella enterica subspecies enterica serovar Typhi (Salmonella Typhi) is the cause of typhoid fever. Typhoid fever is a systemic infection that is an important source of illness and death in low-resource areas [1, 2]. Persons living in areas without access to improved sanitation facilities who are exposed to fecally contaminated water and food are at greatest risk for infection [3–5]. Timely access to effective antimicrobial therapy is central to preventing complications such as intestinal perforation and death. Consequently, the alarming spread of antimicrobial resistance in Salmonella Typhi is likely to contribute further to poor clinical outcomes [6]. Although there has been considerable progress with expanding coverage of access to improved water and sanitation facilities in most regions except Oceania [7] and typhoid incidence has declined in some countries, typhoid remains a major problem worldwide [8]. The emergence and spread of antimicrobial resistance in Salmonella Typhi and the long-term efforts needed to deliver water and sanitation improvements has focused increasing attention on the use of typhoid vaccines.
Two vaccines, Vi polysaccharide (Vi-PS) and live attenuated oral vaccine (Ty21a), have been widely licensed for typhoid prevention [9]. However, their use is restricted to those aged ≥2 years for Vi-PS and ≥6 years for Ty21a [10]. This, combined with waning immunity after 2 years of vaccination [11–13], means that Vi-PS and Ty21a are not widely used in typhoid-endemic areas. Typhoid conjugate vaccines (TCVs) overcome a number of limitations of Vi-PS and Ty21a, having been shown to be safe, immunogenic, and effective in infants and young children [14–16]. In October 2017, the World Health Organization (WHO) Strategic Advisory Group of Experts on Immunization recommended TCV for routine use in children >6 months of age in typhoid-endemic countries. In December 2017, the first TCV was prequalified by WHO [17], enabling typhoid-endemic, low-income countries priority access and funding for the vaccine [18].
Contemporary estimates of the incidence of typhoid fever are helpful in supporting countries’ decision making about typhoid prevention, including vaccine use. While there have been improvements in the sophistication of efforts to model typhoid fever incidence [2, 19, 20], primary data have not always been at the forefront. Recognizing a recent increase in the number of typhoid fever incidence studies [8], we performed a systematic review of the literature and a meta-analysis. Our goal was to describe, summarize, and analyze high-quality primary incidence data on typhoid fever. We focused primarily on prospective studies reporting incidence either through population-based surveillance or studies estimating incidence through sentinel site surveillance with a healthcare utilization survey to establish multipliers to account for underascertainment.
METHODS
Search Strategy
We performed a systematic review of articles published in 3 databases: Ovid, Scopus, and PubMed. We searched Ovid Medline from 1946 to 19 January 2018 with Daily Update, Embase Classic + Embase, and EBM Reviews–Cochrane Central Register of Controlled Trials. Scopus and PubMed databases were searched from inception to 22 January 2018 (search strategies in Supplementary Appendix A). Additionally, reference lists of any article included after the full-text review were searched.
Duplicates from the 3 searches were removed and collated using an online systematic review tool [21]. The resulting list of titles and abstracts were independently screened by 2 authors (J. A. C. and C. Y. H.) for relevance. Any article selected by at least 1 reviewer was moved to full-text review. All subsequent processes of the systematic review were performed in parallel by 2 authors (C. S. M. and J. A. C.), with discrepancies resolved by discussion. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist was used to document the search process [22]. Full texts of the articles were screened for inclusion. After the final list of included articles was established, data for study characteristics and incidence were abstracted.
Inclusion and Exclusion Criteria
Epidemiologic studies of any design reporting the incidence of typhoid fever were included. No restrictions were placed on age of population, country, language, or date. For intervention studies such as controlled field trials, we used only the control and unvaccinated arms. Only cases confirmed through blood or bone marrow culture were included. We excluded studies where we were unable to separate blood or bone marrow culture-confirmed typhoid from the results of serology (eg, Widal test) or other culture sources (eg, stool, urine).
We excluded studies that were outbreak-associated, case reports, or of travel-associated typhoid fever. In addition, editorials, commentaries, and conference presentations or abstracts were excluded. While government surveillance reports (eg, US FoodNet) have valuable data and likely provide very accurate estimates, countries with robust national surveillance systems experience low typhoid incidence, and typhoid fever in these countries is often predominantly travel-associated. The combination of low incidence with the problem of distinguishing travel-associated from non-travel-associated disease underpinned the decision to exclude such reports from this systematic review. Finally, we excluded studies reporting typhoid incidence in special subsets of populations such as only human immunodeficiency virus–infected persons, and healthcare-associated or hospital-acquired infections.
Data Abstraction and Analysis
Study characteristics that were abstracted included PubMed identifier if available, first author, publication year, study design, city or region and country of study, sample size (total population of surveillance or size of control/unvaccinated group), number of cases of fever, number of blood cultures collected, inclusion age and range, and criteria for blood culture. We subsequently classified study sites by their United Nations geographic areas and regions [23].
The currently available tools for assessing the quality and risk of bias did not translate to disease prevalence or incidence reviews [24, 25]. Therefore, we developed criteria specifically for this review, using established quality assessment tools as guidelines (Supplementary Appendix B). Our goal was to evaluate the overall quality of the study in providing an accurate estimate of typhoid incidence in the study setting. We applied greater weight to questions about study design, patient selection, and criteria for collecting a blood culture. Our form evaluated selection and performance and the reference standard, assigning “low,” “moderate,” “high,” “uncertain,” “inapplicable,” “yes,” or “no” to each question. We then assigned an overall score of low, moderate, or high quality to the study.
Data for incidence were abstracted in Excel 2016 (Microsoft, Redmond, Washington). We stratified studies into 2 main types. Studies that estimated incidence through sentinel site surveillance with multipliers to account for underascertainment were categorized as multiplier studies [26]. All other studies were categorized as population-based, which included active household or population-based surveillance, prospective observational studies, and randomized vaccine field trials with control arms. We noted the year the observation period began and how many months the surveillance followed participants.
We recorded the number of typhoid cases that occurred during the study period and then divided by the number of months the study conducted surveillance and multiplied by 12 to report incidence as a rate of cases per 100 000 per year. For multiplier studies, we recorded the estimated incidence separately and also noted the multipliers that were used.
Pooled incidence estimates were calculated with MetaXL version 5.3 software (Epigear International) using a random-effects model [27]. As a secondary analysis of published data this study was exempt from institutional review board approval.
RESULTS
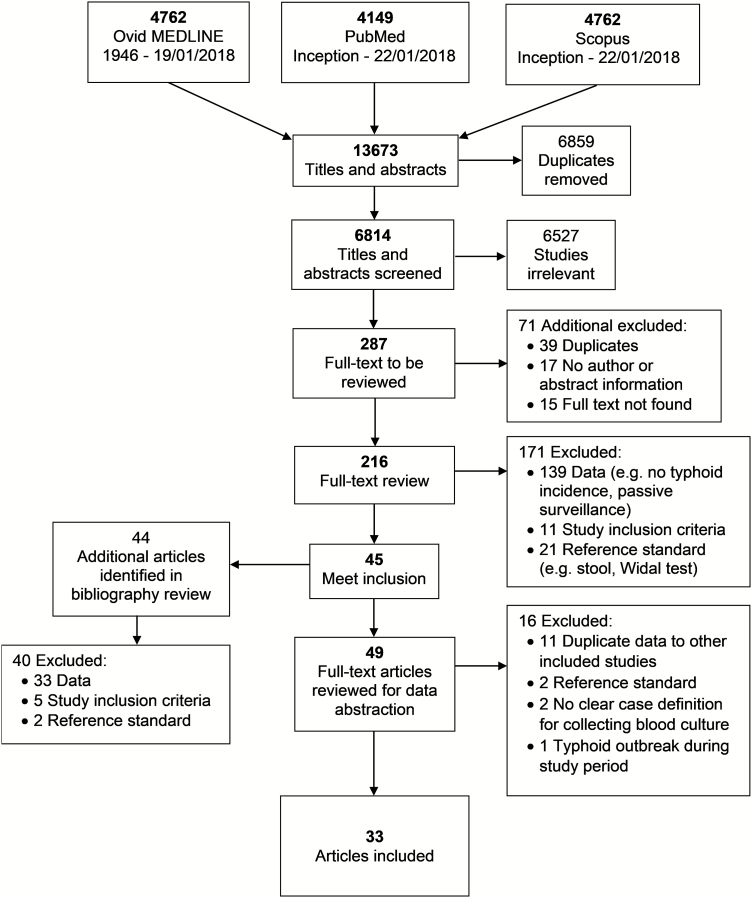
Our search returned 13 673 results; after removing 6859 duplicates, a total of 6814 titles and abstracts were reviewed (Figure 1). We eliminated 6527 studies as irrelevant, 39 as additional duplicates, and 32 that we were unable to locate. Two hundred sixteen full-text articles were reviewed; of those, we included 49. After initial data abstraction of study characteristics, 16 additional studies were excluded: 11 contained duplicate data to other included studies [28–38], 2 used an inappropriate reference standard [39, 40], 2 did not have clear criteria for blood culture collection [41, 42], and 1 was conducted during a typhoid outbreak [43]. A total of 33 articles were included in the analysis [11–13, 15, 44–72].
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of search strategy and selection of articles, systematic review and meta-analysis of global typhoid incidence, 1946–2018.
Study Characteristics and Quality Assessment
Of the 33 included articles, 17 (51.5%) were vaccine field trials, 7 (21.2%) household or population-based active surveillance studies, 4 (12.1%) sentinel surveillance, 3 (9.1%) prospective observational studies, and 2 (6.1%) of mixed study design (Table 1). Eight were categorized as multiplier studies [45, 48, 49, 56–58, 65, 68], estimating incidence with multipliers to account for underascertainment, while the remaining 25 were population based.
Table 1.
Characteristics of Included Studies of Typhoid Fever Incidence by United Nations Classification of Geographic Region, Global, 1954–2018
| United Nations Area, Region | Country [Reference] | Study Design | Years of Data Collection | Population Under Surveillance | Inclusion Age | Criteria for Blood Culture |
|---|---|---|---|---|---|---|
| Africa | 13 sites in 10 African countriesa [56] | Sentinel surveillance with multipliers (12 sites); active surveillance study, household-based (1 site)b,c | 2010–2014 | 7574 to 466 737 | Asante Akim North <15 y; other sites all ages | ≥37.5°C |
| Eastern Africa | Kenya [45] | Active surveillance, population-basedc | 2007–2009 | 28 000 | All ages | ≥38.0°C of any length, respiratory illness |
| Kenya [45] | Active surveillance, population-basedc | 2006–2009 | 25 000 | All ages | ≥38.0°C of any length, respiratory illness, hospitalized | |
| Tanzania [68] | Prospective observationalc | 2009–2010 | 500 600 | >2 mo | ≥37.5°C or history of fever | |
| Middle Africa | … | … | … | … | … | … |
| Northern Africa | Egypt [69] | Vaccine trialb | 1978–1981 | 15 902 | 6–7 y | Fever (undefined) >3 d |
| Egypt [48] | Sentinel surveillance with multipliersc | 2001 | 664 000 | ≥6 mo | Fever (undefined) ≥3 d | |
| Egypt [65] | Sentinel surveillance with multipliersc | 2002 | 2 238 590 | ≥1 y | ≥38.0°C for >2 d or clinical diagnosis of typhoid | |
| Southern Africa | South Africa [52] | Vaccine trialb | 1985–1987 | 11 691 | 5–16 y | Children missing school for ≥3 d with ≥37.5°C |
| South Africa [53] | Vaccine trialb | 1985–1988 | Not provided | 5–16 y | Children missing school for ≥3 d with ≥37.5°C | |
| Western Africa | Burkina Faso [49] | Sentinel surveillance with multipliersc | 2013–2014 | Not provided | 2 mo–15 y | ≥37.5°C for 2 d; ≤35.5°C, suspected severe local infection |
| Ghana [58] | Sentinel surveillance with multipliersc | 2007–2009 | 5333 | <5 y | All admissions to pediatric ward | |
| Asia | ||||||
| Indonesia, Vietnam, China, Pakistan, India [59] | Prospective observational (Indonesia, Vietnam, China); Active surveillance, household-based (Pakistan, India)b | 2001–2004 | 41 845 to 97 928 | China: 5–60 y; India, Jakarta, Indonesia: all ages; Pakistan 2–15 y; Vietnam: 5–18 y | Fever (undefined) ≥3 d | |
| Eastern Asia | China [70] | Vaccine trialb | 1994–1995 | 40 388 | 5–55 y | Suspected cases |
| China [71] | Vaccine trialb | 1995–1996 | 65 984 | 3–50 y | >38°C for >1 d | |
| Central Asia | … | … | … | … | … | … |
| South-Eastern Asia | Indonesia [63] | Vaccine trialb | 1986–1989 | 10 268 | 3–44 y | Fever (undefined) ≥3 d |
| Indonesia [61] | Prospective observationalb | 2001–2003 | 160 261 | All ages | Fever (undefined) ≥3 d | |
| Vietnam [55] | Prospective observationalb | 1995–1996 | 28 329 | All ages | ≥38.5°C for ≥3 d | |
| Vietnam [54] | Vaccine trialb | 1998–2000 | 5566 | 2–5 y | ≥37.5°C for ≥3 d | |
| Southern Asia | Bangladesh [46] | Active surveillance, population-basedb | 2000–2001 | 889 | All ages | Age ≥5 y: ≥37.8°Cfor ≥3 d; age <5 y: ≥37.8°C any length |
| Bangladesh [57] | Active surveillance, population-basedc | 2003–2004 | 24 893 | All ages | Age ≥5 y: ≥37.8°Cfor ≥3 d; age <5 y: ≥37.8°C any length | |
| India [47] | Vaccine trialb | 1974 | 7292 | 6–17 y | Fever (undefined) ≥3 d | |
| India [64] | Active surveillance, household-basedb | 1995–1996 | 7159 | <40 y | Age >5 y: ≥38°Cfor ≥3 d; age ≤5 y: ≥38°C | |
| India [67] | Active surveillance, household-basedb | 2004 | 60 452 | All ages | Fever (undefined) ≥3 d | |
| India [66] | Vaccine trialb | 2004–2006 | 18 804 | ≥2 y | Fever (undefined) ≥3 d | |
| India [15] | Vaccine trialb | 2012–2013 | 860 | 6 mo–12 y | Fever (undefined) >3 d | |
| Nepal [44] | Vaccine trialb | 1986–1987 | 3450 | 5–44 y | ≥37.8°C for ≥3 d | |
| Pakistan [62] | Active surveillance, household-basedb | 1999–2001 | 11 668 | <16 y | Fever (undefined) ≥5 d then ≥3 d | |
| Pakistan [60] | Active surveillance, household-basedb | 2007–2008 | 5570 | <5 y | ≥38.0°C | |
| Pakistan [11] | Vaccine trialb | 2002–2007 | 13 993 | 2–16 y | Fever (undefined) ≥3 d | |
| Western Asia | … | … | … | … | … | … |
| Europe | ||||||
| Eastern Europe | Russia [50] | Vaccine trialb | 1961–1964 | 91 425 | ≥7 y | Feverish illness lasting >3 d |
| Russia [51] | Vaccine trialb | 1966–1967 | 70 855 | 7–20 y | Feverish illness lasting >3 d | |
| Northern Europe | … | … | … | … | … | … |
| Southern Europe | Yugoslavia [72] | Vaccine trialb | 1954–1960 | 11 988 | 5–50 y | Fever (undefined) |
| Western Europe | … | … | … | … | … | … |
| Americas | ||||||
| Caribbean | … | … | … | … | … | … |
| Central America | … | … | … | … | … | … |
| South America | Chile [13] | Vaccine trialb | 1982–1987 | 27 305 | 5–22 y | Suspected cases |
| Chile [12] | Vaccine trialb | 1983–1986 | 21 906 | 5–19 y | Suspected cases | |
| Chile [12] | Vaccine trialb | 1986–1991 | 10 302 | 5–19 y | Suspected cases | |
| Northern America | … | … | … | … | … | … |
| Oceania | … | … | … | … | … | … |
Abbreviations: y, years; mo, months; d, days; C, Celsius. aSites and countries included: Nioko and Polesgo, Burkina Faso; Bandim, Guinea-Bissau; Pikine, Senegal; Asante Akim North, Ghana; East Wad Medani, Sudan; Butajira, Ethiopia; Imerintsiatosika and Isotry, Madagascar; Pietermaritzburg, South Africa; Moshi Urban and Moshi Rural Districts, Tanzania; Kibera, Kenya.
bPopulation-based study.
cMultiplier study.
Among included studies, data collection was conducted from 1954 through 2014, with 17 (51.5%) of the studies collecting data from 2000 through 2014. With respect to location, studies were from 21 countries, including 6 (18.2%) from India, 4 (12.1%) from Pakistan, 3 (9.1%) from Egypt, and 3 (9.1%) from Indonesia. United Nations regions lacking any study data were Middle Africa, Central and Western Asia, Northern and Western Europe, the Caribbean, Central America, Northern America, and Oceania. The participant population age ranged down to 2 months. The median (range) size of the total population under surveillance or size of the control or unvaccinated group in vaccine trials by study was 28822 (860–2 238 590).
Through our quality assessment, we determined that 5 of the included studies were of high quality [44, 45, 55, 57, 64]. These studies did not place any restrictions on inclusion age, gave clear and explicit temperature and duration for collecting blood culture, and actively searched the community for cases. We rated 12 studies as moderate quality [46, 48, 49, 56, 59–61, 65–68, 71], and the remaining 16 as lower quality. Multiplier studies were discounted compared with population-based surveillance studies on the basis of lack of validation of the “multiplier method,” and assumed shortcomings of accuracy.
Typhoid Incidence Among Population-based Studies
Population-based typhoid incidence studies were available in 26 sites from 16 countries (Supplementary Table 1). There were a total of 50 separate estimates of typhoid incidence; 7 studies provided the number of cases for each separate year of surveillance during the study period [12, 13, 50, 51, 53, 69, 72]. The majority of estimates of typhoid incidence at all sites were older; data for 34 (68.0%) were collected prior to the year 2000. The overall pooled estimate of incidence was 154.0 (95% confidence interval [CI], 115.1–198.6) typhoid cases per 100 000 per year.
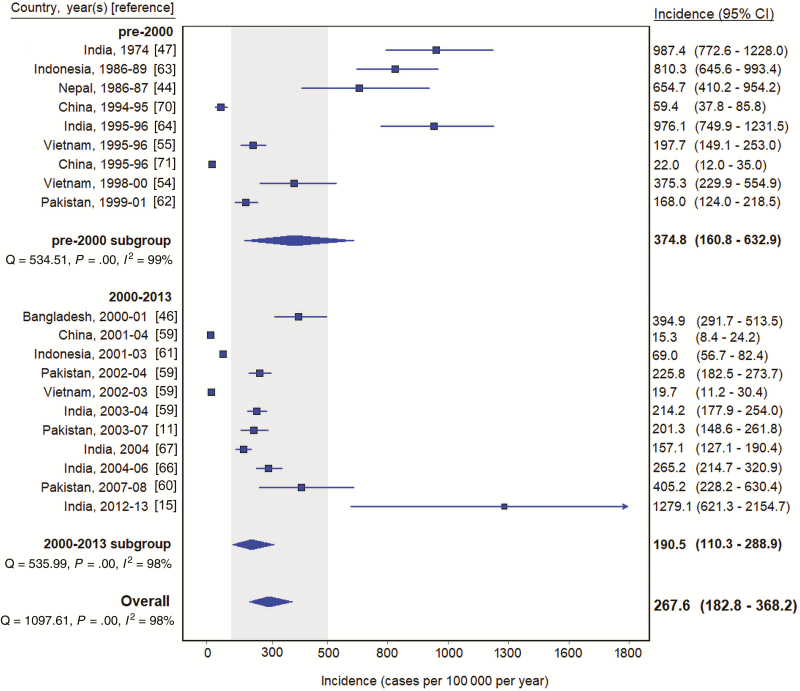
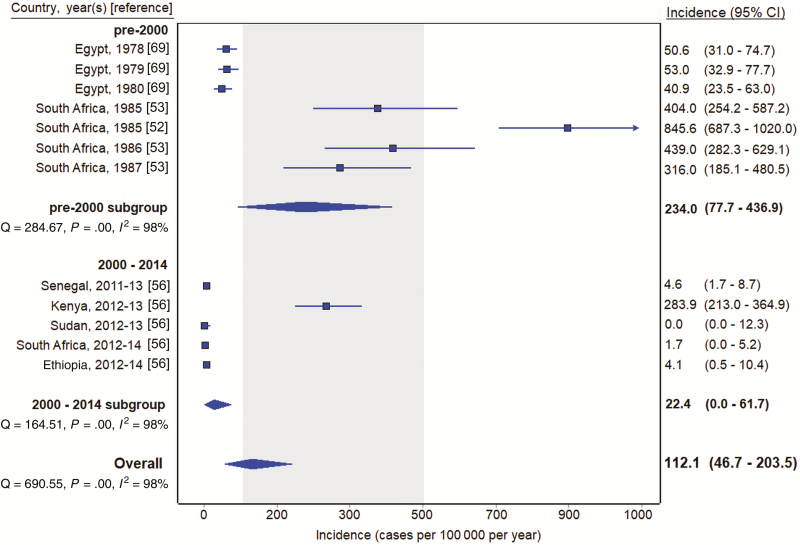
Estimates from South America (9 [18.0%]) and Eastern and Southern Europe (9 [18.0%]) had data collection periods from 1982 through 1991 and 1954 through 1967, respectively. The remaining 32 (64.0%) estimates were from the Asia or Africa geographical region. In study sites located only in Asia, the pooled incidence estimate was 267.6 (95% CI, 182.8–368.2) typhoid cases per 100 000 per year (Figure 2). Six (30.0%) of the sites in the Asia region were from India. The pooled incidence among sites located only in India was 497.2 (95% CI, 291.9–754.8) typhoid cases per 100 000 per year. Two of the Indian studies were from 1974 and 1995 [47, 64], and data were collected from the remaining 4 from 2003 through 2012 [15, 59, 66, 67]. For estimates located only in Africa, the pooled incidence estimate was 112.1 (95% CI, 46.7–203.5) typhoid cases per 100 000 per year (Figure 3). All pooled estimates had significant heterogeneity (I2 > 97%; P < .001, Cochran Q test).
Figure 2.
Typhoid incidence estimates among population-based studies in Asia, 1954–2018. Gray shading indicates 100–500 per 100 000 per year. Abbreviation: CI, confidence interval.
Figure 3.
Typhoid incidence estimates among population-based studies in Africa, 1954–2018. Gray shading indicates 100–500 per 100 000 per year. Abbreviation: CI, confidence interval.
Typhoid Incidence Among Multiplier Studies
Studies that used a multiplier to estimate typhoid incidence were reported in 17 sites in 9 countries (Table 2). Among these studies, 4 types of multipliers were implemented:
Table 2.
Characteristics of Multiplier Studies of Typhoid Fever Incidence, Global, 1954–2018
| United Nations Area, Region | Site, Country | Year Observation Began | Adjusted Incidence Cases/100 000/Year | Age-Specific Adjusted Incidence Cases/100 000/Year | Multipliersa |
|---|---|---|---|---|---|
| Africa | |||||
| Eastern Africa | Lwak, Kenya [45] | 2006 | 445.0b | 0–1 y: 345.7b 2–4 y: 742.6b 5–9 y: 215.5b 10–17 y: 260.4b 18–34 y: 815.7b 35–49 y: 0.0b ≥50 y: 565.8b |
1, 2 |
| Kibera, Kenya [45] | 2007 | 822.0b | 0–1 y: 821.5b 2–4 y: 2242.6b 5–9 y: 1788.0b 10–17 y: 869.9b 18–34 y: 312.1b 35–49 y: 100.0b ≥50 y: 66.6b |
1, 2 | |
| Pemba Island, Tanzania [68] | 2010 | 110 | ≤5 y: 84 5–15 y: 101 >15 y: 128 |
1, 2, 3 | |
| Imerintsiatosika, Madagascar [56] | 2011 | 58b | 0–1 y: 0 2–4 y: 0 5–14 y: 171 <15 y: 95 ≥15 y: 20 All: 58 |
1, 2 | |
| Moshi Urban District, Tanzania [56] | 2011 | 168b | 0–1 y: 0 2–4 y: 1028 5–14 y: 103 <15 y: 155 ≥15 y: 201 All: 168 |
1, 2 | |
| Moshi Rural District, Tanzania [56] | 2011 | 20b | 0–1 y: 0 2–4 y: 0 5–14 y: 18 <15 y: 18 ≥15 y: 28 All: 20 |
1, 2 | |
| Isotry, Madagascar [56] | 2012 | 42b | 0–1 y: 0 2–4 y: 0 5–14 y: 62 <15 y: 42 ≥15 y: 42 All: 42 |
1, 2 | |
| Northern Africa | Bilbeis, Egypt [48] | 2001 | 12.60 | … | 1, 3, 4 |
| Fayoum, Egypt [65] | 2002 | 59 | 0–4 y: 6 5–9 y: 143 10–14 y: 160 ≥15 y: 34 |
1, 3 | |
| East Wad Medani, Sudan [56] | 2012 | 0 | 0–1 y: 0 2–4 y: 0 5–14 y: 0 <15 y: 0 ≥15 y: 0 All: 0 |
1, 2 | |
| Ashanti, Ghana [58] | 2007 | 330 | … | 1, 2 | |
| Asante Akim North, Ghana [56] | 2010 | 389b | 0–1 y: 120 2–4 y: 1079 5–14 y: 314 <15 y: 389 ≥15 y: NA All: NA |
1, 2 | |
| Bandim, Guinea-Bissau [56] | 2011 | 10b | 0–1 y: 0 2–4 y: 53 5–14 y: 18 <15 y: 20 ≥15 y: 4 All: 10 |
1, 2 | |
| Nioko, Burkina Faso [56] | 2012 | 104b | 0–1 y: 0 2–4 y: 251 5–14 y: 315 <15 y: 227 ≥15 y: 0 All: 104 |
1, 2 | |
| Polesgo, Burkina Faso [56] | 2012 | 383b | 0–1 y: 0 2–4 y: 1890 5–14 y: 485 <15 y: 719 ≥15 y: 107 All: 383 |
1, 2 | |
| Nanoro, Burkina Faso [49] | 2013 | <5 y: 224b 5–15 y: 273b |
1, 2, 3, 4 | ||
| Asia | |||||
| Southern Asia | Dhaka, Bangladesh [57] | 2003 | 280b | <5 y: 1600b ≥5 y: 100b |
2 |
Abbreviation: NA, not applicable; y, years.
aMultipliers used: (1) healthcare facility (eligible participants not seeking care at study clinic); (2) enrollment (eligible participants did not have a blood culture collected); (3) test sensitivity; (4) seasonality (surveillance period).
bPer 100 000 person-years observed.
Healthcare facility: eligible participants not seeking care at study clinic (16 [94.1%]);
Enrollment: eligible participants did not have a blood culture collected (15 [88.2%]);
Test sensitivity: an adjustment factor for the sensitivity of blood cultures, most often 2 times or 50% sensitivity (4 [23.5%]);
Seasonality: length of surveillance period (2 [11.8%]).
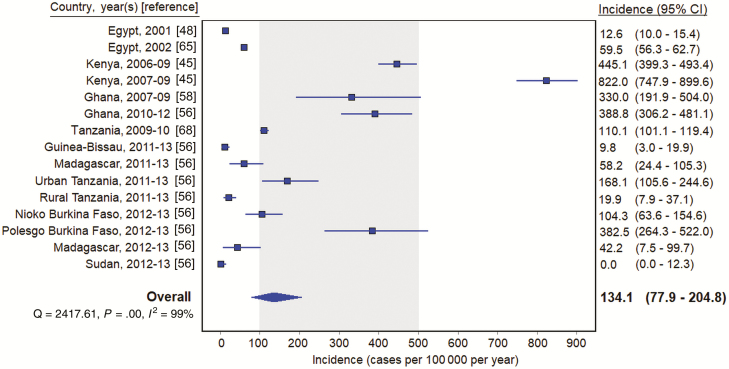
In contrast to incidence studies without multipliers, the majority (11 [64.7%]) reporting an estimated incidence with a multiplier were recent, collecting data from 2010 through 2014. The overall pooled incidence estimate from multiplier studies was 141.8 (95% CI, 85.3–212.2) typhoid cases per 100 000 per year. All but 1 of the multiplier study sites were located in Africa [57]. Removing the site located in Asia, the pooled estimate of multiplier studies located in Africa was 134.1 (95% CI, 77.9–204.8) per 100 000 per year (Figure 4). Typhoid incidence varied between sites and countries, with no cases reported in East Wad Medani, Sudan [56], and 882.0 typhoid cases per 100 000 per year in Kibera, Kenya [45]. One study was excluded from the multiplier pooled estimate calculations because it did not report the population under surveillance, only the incidence by age group [49]. Heterogeneity was significant in each pooled estimate (I2 > 98%; P < .001, Cochran Q test).
Figure 4.
Typhoid incidence estimates among multiplier studies in Africa, 1954–2018. Gray shading indicates 100–500 per 100 000 per year. Abbreviation: CI, confidence interval.
DISCUSSION
We performed a systematic review and meta-analysis of blood culture–confirmed typhoid fever incidence. Although an encouraging number of typhoid incidence studies meeting criteria for inclusion have been published since 2000, a substantial proportion had data collection periods much earlier and as long ago as 1954. When examined by time and region, there appears to be a secular trend of lower incidence in more recent studies. This is consistent with other work suggesting declining typhoid fever incidence worldwide [8].
A number of studies included in this review collected typhoid incidence data from the same location over multiple years of surveillance. While some locations such as Alexandria, Egypt [69], recorded stable typhoid incidence between years of surveillance, significant changes in incidence were recorded at others such as Eastern Transvaal, South Africa [52, 53], and Kolkata, India [66, 67]. As none of the studies included in our systematic review were done during recognized typhoid fever epidemics, the multiyear studies illustrate the considerable variation in typhoid incidence that may occur between years at the same site considered to have endemic disease.
In addition to variation of endemic typhoid fever in time at the same location, our systematic review illustrates the considerable variation in endemic typhoid incidence that may occur between sites within the same country, between countries within a region, and between regions. This serves as a reminder that limited typhoid surveillance data can be misleading and that decisions for typhoid vaccine introduction should be based not only on surveillance data but also be informed by the occurrence of risk factors for typhoid transmission such as unsafe water and unimproved sanitation facilities.
Sites in the Asia area have long been recognized as having high typhoid incidence [73]. However, some considered typhoid fever to be uncommon in the Africa area as recently as 2008 [74]. The substantial number of new typhoid fever incidence estimates from sites in Africa since earlier reviews [2, 8, 19] confirms that some African sites record high typhoid fever incidence [56] and provides evidence that TCV use should be considered beyond Asia.
We demonstrate that eligible contemporary typhoid fever incidence studies were not available for some areas and regions. Notably, no eligible studies were identified from Oceania despite the major typhoid fever problem that is recognized in some Pacific islands [75]. Studies were also lacking from high-income countries, that rely on robust national surveillance systems to monitor typhoid fever. However, typhoid fever incidence is almost universally low in high-income countries, is often travel-associated, and likely contributes little to total global cases [76]. While increasingly sophisticated approaches to modeling typhoid fever incidence provide a means of estimating typhoid fever incidence in locations where data are lacking [2, 19, 20], we sought to focus on systematic presentation of primary data and summary values across eligible studies.
Our quality assessment identified a number of concerns with published typhoid fever incidence studies. We rated 5 studies as high quality, thus classifying the majority as of moderate or low quality. Our data were also limited by the significant heterogeneity, but given the variation in location, inclusion ages, study design, and dates, this was to be expected. Our search strategy may not have identified negative studies from sites with low typhoid incidence.
A number of factors drove downgrading of study quality. Multiplier studies have been increasingly used to estimate typhoid fever incidence for cost and logistical reasons in recent years [48]. Such studies are less expensive and less logistically complex than population-based active surveillance studies and underpin considerable recent progress in available typhoid fever incidence data. However, the multiplier method has not been validated in the context of population-based surveillance and is associated with greater uncertainty introduced at each stage of correction for underascertainment. Furthermore, we identified considerable variation in the types of multipliers that were used. We recommend that consensus be reached on standardization of multiplier selection for future work to ensure that incidence estimates derived from multiplier studies are directly comparable.
We also found that patient selection may have introduced bias in a large number of studies. The control arms of vaccine trials remain an important source of data on typhoid fever incidence. However, it must be recognized that vaccine trials are likely to be preferentially done in areas of high typhoid incidence to increase statistical power. Furthermore, a number of studies were done in specific age groups or communities, and resulting incidence estimates may not reflect disease in all age groups or the wider population. We also identified variation in who received blood or bone marrow cultures in studies, which may have introduced bias, and blood culture volume adequacy and the proportion of blood cultures contaminated were rarely reported.
While there are concerns for bias, we describe an overall pattern of declining incidence among included studies, with variation in typhoid incidence between surveillance years at the same site and also variation in place within countries and regions. These observations support the recognition of heterogeneity in endemic typhoid fever in both time and place. They also underscore the importance of considering both surveillance data and typhoid fever risk factors or “receptivity” when making vaccine introduction decisions.
We encourage ongoing efforts to generate more contemporary estimates of typhoid incidence, focusing on areas and regions where data are lacking, including Oceania and many parts of Africa. We also identify the need to develop a consensus standard on the types of adjustments that should be made in multiplier studies. This systematic review serves as a resource for site selection for future incidence studies, as a data source for typhoid fever modeling efforts, and in policy decisions for typhoid fever control.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. J. A. C. conceived the study. J. A. C. and C. Y. H. developed the protocol and search strategy, and performed the title and abstract review. J. A. C. and C. S. M. reviewed full-text articles and abstracted the data. C. S. M. performed the analysis, created the tables and figures, and wrote the first draft of the manuscript. J. A. C. provided major revisions and comments to the manuscript. All authors reviewed the manuscript for final revisions before submission.
Acknowledgments. The authors thank Dr Richard German, Divisional Librarian, Division of Health Sciences Library, University of Otago, for assistance with development of the search strategy.
Financial support. This publication is based on research funded in part by a grant from the Bill & Melinda Gates Foundation (OPP1151153). J. A. C., C. Y. H., and C. S. M. received support from BMGF grant OPP1151153. J. A. C. also received support from BMGF (grant numbers OPP1125993 and OPP1158210), the US National Institutes of Health (grant number R01AI121378), and the New Zealand Health Research Council through the e-ASIA Joint Research Program (grant number 16/697).
Supplement sponsorship. This supplement is sponsored by the Center for Vaccine Development and Global Health (CVD) at the University of Maryland School of Medicine.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018; 392:1736–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories. Lancet 2018; 392:1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Soper G. The work of a chronic typhoid germ distributor. J Am Med Assoc 1907; XLVIII:2019–22. [Google Scholar]
- 4. Sedgwick WT, Macnutt JS. On the Mills-Reincke phenomenon and Hazen’s theorem concerning the decrease in mortality from diseases other than typhoid fever following the purification of public water-supplies. J Infect Dis 1910; 7:489–564. [Google Scholar]
- 5. Budd W. Typhoid fever, its nature, mode of spreading and prevention. London: Longmans Green and Co, 1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Klemm EJ, Shakoor S, Page AJ, et al. Emergence of an extensively drug-resistant Salmonella enterica serovar Typhi clone harboring a promiscuous plasmid encoding resistance to fluoroquinolones and third-generation cephalosporins. mBio 2018; 9. doi: 10.1128/mBio.00105-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.UNICEF. Progress on drinking-water and sanitation: 2015 update and MDG assessment. Geneva, Switzerland; UNICEF, 2015. [Google Scholar]
- 8. Crump JA. Progress in typhoid fever epidemiology. Clin Infect Dis 2019; 68(Suppl 2):S4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Anwar E, Goldberg E, Fraser A, Acosta CJ, Paul M, Leibovici L. Vaccines for preventing typhoid fever. Cochrane Database Syst Rev 2014; CD001261. [DOI] [PubMed] [Google Scholar]
- 10. World Health Organization. Typhoid vaccines: WHO position paper. March 2018 - Recommendations. Vaccine 2019; 37:214–6. [DOI] [PubMed] [Google Scholar]
- 11. Khan MI, Soofi SB, Ochiai RL, et al. ; DOMI Typhoid Karachi Vi Effectiveness Study Group. Effectiveness of Vi capsular polysaccharide typhoid vaccine among children: a cluster randomized trial in Karachi, Pakistan. Vaccine 2012; 30:5389–95. [DOI] [PubMed] [Google Scholar]
- 12. Levine MM, Ferreccio C, Abrego P, Martin OS, Ortiz E, Cryz S. Duration of efficacy of Ty21a, attenuated Salmonella Typhi live oral vaccine. Vaccine 1999; 17(Suppl 2):S22–7. [DOI] [PubMed] [Google Scholar]
- 13. Black RE, Levine MM, Ferreccio C, et al. Efficacy of one or two doses of Ty21a Salmonella Typhi vaccine in enteric-coated capsules in a controlled field trial. Chilean Typhoid Committee. Vaccine 1990; 8:81–4. [DOI] [PubMed] [Google Scholar]
- 14. Mohan VK, Varanasi V, Singh A, et al. Safety and immunogenicity of a Vi polysaccharide-tetanus toxoid conjugate vaccine (Typbar-TCV) in healthy infants, children, and adults in typhoid endemic areas: a multicenter, 2-cohort, open-label, double-blind, randomized controlled phase 3 study. Clin Infect Dis 2015; 61:393–402. [DOI] [PubMed] [Google Scholar]
- 15. Mitra M, Shah N, Ghosh A, et al. Efficacy and safety of Vi-tetanus toxoid conjugated typhoid vaccine (PedaTyph) in Indian children: school based cluster randomized study. Hum Vaccin Immunother 2016; 12:939–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jin C, Gibani MM, Moore M, et al. Efficacy and immunogenicity of a Vi-tetanus toxoid conjugate vaccine in the prevention of typhoid fever using a controlled human infection model of Salmonella Typhi: a randomised controlled, phase 2b trial. Lancet 2017; 390:2472–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. Typhoid vaccines: WHO position paper, March 2018—recommendations. Vaccine 2018. Available at: https://doi.org/10.1016/j.vaccine.2018.04.022. Accessed 19 August 2018. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. Typhoid vaccine prequalified. Available at: http://www.who.int/medicines/news/2018/WHOprequalifies-breakthrough-typhoid-vaccine/en/. Accessed 30 July 2018. [Google Scholar]
- 19. Mogasale V, Mogasale VV, Ramani E, et al. Revisiting typhoid fever surveillance in low and middle income countries: lessons from systematic literature review of population-based longitudinal studies. BMC Infect Dis 2016; 16:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Antillón M, Warren JL, Crawford FW, et al. The burden of typhoid fever in low- and middle-income countries: a meta-regression approach. PLoS Negl Trop Dis 2017; 11:e0005376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veritas Health Innovation. Covidence systematic review software. Available at: https://www.covidence.org/home. Accessed 16 August 2018. [Google Scholar]
- 22. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339 Available at: http://www.bmj.com/content/bmj/339/bmj.b2535.full.pdf. Accessed 15 August 2018. [PMC free article] [PubMed] [Google Scholar]
- 23.United Nations Statistics Division. Standard country or area codes for statistical use. Available at: https://unstats.un.org/unsd/methodology/m49/. Accessed 19 August 2018. [Google Scholar]
- 24. Higgins JP, Altman DG. Assessing risk of bias in included studies. In: Cochrane handbook for systematic reviews of interventions. West Sussex, UK: Wiley-Blackwell, 2008:187–241. Available at: https://onlinelibrary.wiley.com/doi/abs/10.1002/9780470712184.ch8. Accessed 30 July 2018. [Google Scholar]
- 25. Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011; 155:529–36. [DOI] [PubMed] [Google Scholar]
- 26. Crump JA, Kirk MD. Estimating the burden of febrile illnesses. PLoS Negl Trop Dis 2015; 9:e0004040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.EpiGear International Pty Ltd. MetaXL. 2016. Available at: https://www.epigear.com/index_files/metaxl.html. Accessed 13 August 2018. [Google Scholar]
- 28. Tabu C, Breiman RF, Ochieng B, et al. Differing burden and epidemiology of non-Typhi Salmonella bacteremia in rural and urban Kenya, 2006–2009. PLoS One 2012; 7:e31237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sur D, Ali M, von Seidlein L, et al. Comparisons of predictors for typhoid and paratyphoid fever in Kolkata, India. BMC Public Health 2007; 7:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ochiai RL, Wang X, von Seidlein L, et al. Salmonella Paratyphi A rates, Asia. Emerg Infect Dis 2005; 11:1764–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marks F, Adu-Sarkodie Y, Hünger F, et al. Typhoid fever among children, Ghana. Emerg Infect Dis 2010; 16:1796–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Levine MM, Ferreccio C, Black RE, Germanier R. Large-scale field trial of Ty21a live oral typhoid vaccine in enteric-coated capsule formulation. Lancet 1987; 1:1049–52. [DOI] [PubMed] [Google Scholar]
- 33. Levine MM, Ferreccio C, Cryz S, Ortiz E. Comparison of enteric-coated capsules and liquid formulation of Ty21a typhoid vaccine in randomised controlled field trial. Lancet 1990; 336:891–4. [DOI] [PubMed] [Google Scholar]
- 34. Khan MI, Soofi SB, Ochiai RL, et al. Epidemiology, clinical presentation, and patterns of drug resistance of Salmonella Typhi in Karachi, Pakistan. J Infect Dev Ctries 2012; 6:704–14. [DOI] [PubMed] [Google Scholar]
- 35. Khan MI, Ochiai RL, Soofi SB, et al. Risk factors associated with typhoid fever in children aged 2–16 years in Karachi, Pakistan. Epidemiol Infect 2012; 140:665–72. [DOI] [PubMed] [Google Scholar]
- 36. Khan MI, Sahito SM, Khan MJ, et al. Enhanced disease surveillance through private health care sector cooperation in Karachi, Pakistan: experience from a vaccine trial. Bull World Health Organ 2006; 84:72–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hejfec LB, Salmin LV, Lejtman MZ, et al. A controlled field trial and laboratory study of five typhoid vaccines in the USSR. Bull World Health Organ 1966; 34:321–39. [PMC free article] [PubMed] [Google Scholar]
- 38. Bahl R, Sinha A, Poulos C, et al. Costs of illness due to typhoid fever in an Indian urban slum community: implications for vaccination policy. J Health Popul Nutr 2004; 22:304–10. [PubMed] [Google Scholar]
- 39. Dong B-Q, Yang J, Wang X-Y, et al. Trends and disease burden of enteric fever in Guangxi province, China, 1994–2004. Bull World Health Organ 2010; 88:689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ashcroft MT, Singh B, Nicholson CC, Ritchie JM, Sorryan E, Williams F. A seven-year field trial of two typhoid vaccines in Guyana. Lancet 1967; 2:1056–9. [DOI] [PubMed] [Google Scholar]
- 41. Khasanov MI, Kheifets LB, Salmin LV. A controlled field trial of the typhoid component of polyvalent enteric vaccine (NIISI polyvaccine). Bull World Health Organ 1962; 26:371–9. [PMC free article] [PubMed] [Google Scholar]
- 42. Ashcroft MT, Ritchie JM, Nicholson CC. Controlled field trial in British Guiana school children of heat-killed-phenolized and acetone-killed lyophilized typhoid vaccines. Am J Hyg 1964; 79:196–206. [DOI] [PubMed] [Google Scholar]
- 43. Stuhl L, Benda R, Frey N. A controled field trial of the effectiveness of acetone-dried and inactivated and heat-phenol-inactivated typhoid vaccines in Yugoslavia. Bull World Health Organ 1964; 30:623–30. [PMC free article] [PubMed] [Google Scholar]
- 44. Acharya IL, Lowe CU, Thapa R, et al. Prevention of typhoid fever in Nepal with the Vi capsular polysaccharide of Salmonella Typhi. A preliminary report. N Engl J Med 1987; 317:1101–4. [DOI] [PubMed] [Google Scholar]
- 45. Breiman RF, Cosmas L, Njuguna H, et al. Population-based incidence of typhoid fever in an urban informal settlement and a rural area in Kenya: implications for typhoid vaccine use in Africa. PLoS One 2012; 7:e29119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brooks WA, Hossain A, Goswami D, et al. Bacteremic typhoid fever in children in an urban slum, Bangladesh. Emerg Infect Dis 2005; 11:326–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chuttani CS, Prakash K, Gupta P, Grover V, Kumar A. Controlled field trial of a high-dose oral killed typhoid vaccine in India. Bull World Health Organ 1977; 55:643–4. [PMC free article] [PubMed] [Google Scholar]
- 48. Crump JA, Youssef FG, Luby SP, et al. Estimating the incidence of typhoid fever and other febrile illnesses in developing countries. Emerg Infect Dis 2003; 9:539–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Guiraud I, Post A, Diallo SN, et al. Population-based incidence, seasonality and serotype distribution of invasive salmonellosis among children in Nanoro, rural Burkina Faso. PLoS One 2017; 12:e0178577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hejfec LB, Levina LA, Kuz’minova ML, Salmin LV, Slavina AM, Vasil’eva AV. Controlled field trials of paratyphoid B vaccine and evaluation of the effectiveness of a single administration of typhoid vaccine. Bull World Health Organ 1968; 38:907–15. [PMC free article] [PubMed] [Google Scholar]
- 51. Hejfec LB, Levina LA, Kuz’minova ML, et al. A controlled field trial to evaluate the protective capacity of a single dose of acetone-killed agar-grown and heat-killed broth-grown typhoid vaccines. Bull World Health Organ 1969; 40:903–7. [PMC free article] [PubMed] [Google Scholar]
- 52. Klugman KP, Gilbertson IT, Koornhof HJ, et al. Protective activity of Vi capsular polysaccharide vaccine against typhoid fever. Lancet 1987; 2:1165–9. [DOI] [PubMed] [Google Scholar]
- 53. Klugman KP, Koornhof HJ, Robbins JB, Le Cam NN. Immunogenicity, efficacy and serological correlate of protection of Salmonella Typhi Vi capsular polysaccharide vaccine three years after immunization. Vaccine 1996; 14:435–8. [DOI] [PubMed] [Google Scholar]
- 54. Lin FY, Ho VA, Khiem HB, et al. The efficacy of a Salmonella Typhi Vi conjugate vaccine in two-to-five-year-old children. N Engl J Med 2001; 344:1263–9. [DOI] [PubMed] [Google Scholar]
- 55. Lin FY, Vo AH, Phan VB, et al. The epidemiology of typhoid fever in the Dong Thap Province, Mekong Delta region of Vietnam. Am J Trop Med Hyg 2000; 62:644–8. [DOI] [PubMed] [Google Scholar]
- 56. Marks F, von Kalckreuth V, Aaby P, et al. Incidence of invasive Salmonella disease in sub-Saharan Africa: a multicentre population-based surveillance study. Lancet Glob Health 2017; 5:e310–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Naheed A, Ram PK, Brooks WA, et al. Burden of typhoid and paratyphoid fever in a densely populated urban community, Dhaka, Bangladesh. Int J Infect Dis 2010; 14(Suppl 3):e93–9. [DOI] [PubMed] [Google Scholar]
- 58. Nielsen MV, Sarpong N, Krumkamp R, et al. Incidence and characteristics of bacteremia among children in rural Ghana. PLoS One 2012; 7:e44063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ochiai RL, Acosta CJ, Danovaro-Holliday MC, et al. Domi Typhoid Study Group. A study of typhoid fever in five Asian countries: disease burden and implications for controls. Bull World Health Organ 2008; 86:260–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Owais A, Sultana S, Zaman U, Rizvi A, Zaidi AK. Incidence of typhoid bacteremia in infants and young children in southern coastal Pakistan. Pediatr Infect Dis J 2010; 29:1035–9. [PMC free article] [PubMed] [Google Scholar]
- 61. Punjabi NH, Agtini MD, Ochiai RL, et al. Enteric fever burden in North Jakarta, Indonesia: a prospective, community-based study. J Infect Dev Ctries 2013; 7:781–7. [DOI] [PubMed] [Google Scholar]
- 62. Siddiqui FJ, Rabbani F, Hasan R, Nizami SQ, Bhutta ZA. Typhoid fever in children: some epidemiological considerations from Karachi, Pakistan. Int J Infect Dis 2006; 10:215–22. [DOI] [PubMed] [Google Scholar]
- 63. Simanjuntak CH, Paleologo FP, Punjabi NH, et al. Oral immunisation against typhoid fever in Indonesia with Ty21a vaccine. Lancet 1991; 338:1055–9. [DOI] [PubMed] [Google Scholar]
- 64. Sinha A, Sazawal S, Kumar R, et al. Typhoid fever in children aged less than 5 years. Lancet 1999; 354:734–7. [DOI] [PubMed] [Google Scholar]
- 65. Srikantiah P, Girgis FY, Luby SP, et al. Population-based surveillance of typhoid fever in Egypt. Am J Trop Med Hyg 2006; 74:114–9. [PubMed] [Google Scholar]
- 66. Sur D, Ochiai RL, Bhattacharya SK, et al. A cluster-randomized effectiveness trial of Vi typhoid vaccine in India. N Engl J Med 2009; 361:335–44. [DOI] [PubMed] [Google Scholar]
- 67. Sur D, von Seidlein L, Manna B, et al. The malaria and typhoid fever burden in the slums of Kolkata, India: data from a prospective community-based study. Trans R Soc Trop Med Hyg 2006; 100:725–33. [DOI] [PubMed] [Google Scholar]
- 68. Thriemer K, Ley B, Ame S, et al. The burden of invasive bacterial infections in Pemba, Zanzibar. PLoS One 2012; 7:e30350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wahdan MH, Sérié C, Cerisier Y, Sallam S, Germanier R. A controlled field trial of live Salmonella Typhi strain Ty 21a oral vaccine against typhoid: three-year results. J Infect Dis 1982; 145:292–5. [DOI] [PubMed] [Google Scholar]
- 70. Wang ZG, Zhou WZ, Shi J. Efficacy and side effects following immunization with Salmonella Typhi Vi capsular polysaccharide vaccine. Zhonghua Liu Xing Bing Xue Za Zhi 1997; 18:26–9. [PubMed] [Google Scholar]
- 71. Yang HH, Wu CG, Xie GZ, et al. Efficacy trial of Vi polysaccharide vaccine against typhoid fever in south-western China. Bull World Health Organ 2001; 79:625–31. [PMC free article] [PubMed] [Google Scholar]
- 72.Yugoslav Typhoid Commission. A controlled field trial of the effectiveness of phenol and alcohol typhoid vaccines: final report. Bull World Health Organ 1962; 26:357–69. [PMC free article] [PubMed] [Google Scholar]
- 73. Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ 2004; 82:346–53. [PMC free article] [PubMed] [Google Scholar]
- 74. Mweu E, English M. Typhoid fever in children in Africa. Trop Med Int Health 2008; 13:532–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Prasad N, Jenkins AP, Naucukidi L, et al. Epidemiology and risk factors for typhoid fever in Central Division, Fiji, 2014–2017: a case-control study. PLoS Negl Trop Dis 2018; 12:e0006571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lynch MF, Blanton EM, Bulens S, et al. Typhoid fever in the United States, 1999–2006. JAMA 2009; 302:859–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.