Abstract
Smokers attempting to quit often attribute smoking relapse to negative affect, craving, and other nicotine withdrawal symptoms. Additionally, there is evidence that smoking relapse can increase these symptoms, particularly negative affect. To address this issue, we analyzed data from an 11-week smoking cessation clinical trial in which smokers (n=1246) were randomized to receive either nicotine replacement therapy (NRT), varenicline, or placebo, combined with behavioral counseling. Using cross-lagged analyses, we examined the temporal bidirectional relationships between self-reported measures of affect, craving, and composite withdrawal symptoms and biochemically verified smoking abstinence. The relative strength of these temporal relationships was examined by comparing the explained variances of the models. The results showed that higher negative affect, craving, and composite withdrawal symptoms increased the likelihood of subsequent smoking relapse, and that smoking relapse led to subsequent increases in these same symptoms. A comparison of the explained variances found symptom predicting subsequent relapse models to be stronger than those where relapse predicted subsequent symptoms. While the explained variance findings generally support a negative reinforcement conceptualization of nicotine dependence, the bidirectional relationship between symptoms and smoking relapse suggests that struggling with quitting smoking leads to significant negative affect, craving, and other withdrawal symptoms that do not quickly resolve. These findings highlight the important of addressing specific symptoms within the context of smoking cessation.
Keywords: smoking, nicotine withdrawal, abstinence, negative affect, craving
Introduction
Nicotine withdrawal is characterized by many symptoms, including negative affect (e.g., depression, irritability), difficulty concentrating, increased appetite, restlessness, sleep disturbance, and weight gain (Shiffman, West, & Gilbert, 2004). Additionally, craving (or urge) to smoke has been conceptualized as a central component of abstinence from tobacco (American Psychiatric Association, 2013; Tiffany, Warthen, & Goedeker, 2009). Smokers' concerns about experiencing withdrawal symptoms and craving have been associated with decreased intention to quit (Orleans, Rimer, Cristinzio, Keintz, & Fleisher, 1991) and increased smoking relapse (McKee, O'Malley, Salovey, Krishnan-Sarin, & Mazure, 2005). While much research has investigated the relationship between individual and composite nicotine withdrawal symptoms and subsequent smoking relapse, the findings have been mixed (Kassel, Stroud, & Paronis, 2003; Patten & Martin, 1996; Wray, Gass, & Tiffany, 2013). Additionally, there is evidence that smoking relapse can increase acute negative affect (e.g., Piasecki, Jorenby, Smith, Fiore, & Baker, 2003) and craving symptoms (e.g., Blalock, Robinson, Wetter, Schreindorfer, & Cinciripini, 2008), suggesting that these symptoms are not simply the result of withdrawal (Robinson et al., 2011). Thus, the relationship between some withdrawal-associated symptoms and relapse is potentially multidetermined and bidirectional.
Early findings concerning the impact of individual and composite nicotine withdrawal symptoms on smoking relapse were equivocal (see Patten & Martin, 1996, for review). Many of the early studies examining the impact of withdrawal symptoms on relapse used withdrawal obtained at a single post-quit session (Gunn, 1986), only examined symptoms early in the quit attempt (Hughes, Gust, Skoog, Keenan, & Fenwick, 1991; Hughes & Hatsukami, 1986), or conducted analyses using only means of repeated measures (Gritz, Carr, & Marcus, 1991; Norregaard, Tonnesen, & Petersen, 1993). These methodological approaches likely obscured any relationship between withdrawal and relapse by focusing on early and limited time windows. In contrast, daily diary and ecological momentary assessment (EMA) studies, which provide a more proximal measure of withdrawal over time (Stone & Shiffman, 1994) and reduces recall errors (Shiffman et al., 1997; Stone & Shiffman, 1994), have more consistently found a link between withdrawal symptoms and subsequent smoking relapse (Shiffman et al., 2007).
Negative affect (i.e., unpleasant emotional states) has been one of the most frequently studied withdrawal-associated predictors of smoking relapse. Negative affect has been associated with subsequent smoking relapse in many retrospective (Borland, 1990; Cummings, Jaen, & Giovino, 1985; Swan et al., 1988) and prospective (Kenford et al., 2002; Piper et al., 2011) studies, including those involving EMA (Shiffman & Gwaltney, 2008; Shiffman, Paty, Gnys, Kassel, & Hickcox, 1996). However, in other studies, negative affect failed to predict relapse among quitting smokers (Hall, Havassy, & Wasserman, 1990; Javitz, Lerman, & Swan, 2012) or smoking behavior among a non-quitting sample (Shiffman et al., 2002), or varied by gender (Nakajima & al'Absi, 2012). Thus, despite being cited as the main cause of relapse by many smokers (Shiffman, 1982), the evidence for a causal relationship between negative affect and relapse has been mixed.
The impact of craving, or urge, to smoke on subsequent smoking relapse has been widely studied, but the evidence for such a relationship is mixed. For example, only around half of the studies reviewed by Wray and colleagues (2013) found a significant association between higher craving and subsequent smoking relapse. However, studies using EMA assessments typically find a relationship between craving and subsequent relapse (see Serre, Fatseas, Swendsen, & Auriacombe, 2015, for review), which suggests that these discrepancies may be due to study design and measurement method differences.
While not considered a symptom of nicotine withdrawal, positive affect (e.g., pleasant emotional states) has been conceptualized as having a role in relapse. There is evidence that smoking increases positive affect (Shiffman & Kirchner, 2009), and that the amount of positive affect experienced during smoking predicts relapse after quitting (Shiffman & Kirchner, 2009; Strong et al., 2011). Additionally, some studies that used retrospective recall found that relapses can occur during positive affect situations (Brandon, Tiffany, Obremski, & Baker, 1990; Shiffman, 1986).
The relationships between relapse and withdrawal-associated symptoms is further complicated because relapse itself has been associated with subsequent acute negative affect (Blalock et al., 2008; Cinciripini et al., 2013; Piasecki et al., 2003; Robinson et al., 2011) and craving (Blalock et al., 2008; Robinson et al., 2011). This suggests that negative affect and craving are multi-determined during the smoking cessation process, and are not just the result of withdrawal. For example, the negative affect experienced by relapsers might be due to attributions of self-blame that result in feelings of guilt, a phenomenon termed the abstinence violation effect (AVE; Curry, Marlatt, & Gordon, 1987). Another potential reason for why relapsers might experience withdrawal-associated symptoms is suggested by findings that relapsers often do not return to their baseline level of cigarettes consumption (Lam et al., 2012), even up to a year later (Conklin et al., 2005) and, thus, may experience prolonged withdrawal symptoms due to being partially abstinent (Shiffman, 1979).
To examine the potentially bidirectional relationship between relapse and the experience of withdrawal-associated symptoms, including composite withdrawal, negative affect, positive affect, and craving, we conducted a series of cross-lagged analyses on clinical trial data collected by the Pharmacogenomics Research Network: Pharmacogenetics of Nicotine Addiction Treatment (PGRN-PNAT) research group. The aims of this secondary data analysis were to establish whether nicotine withdrawal-associated symptoms, both as composite measures and as specific symptoms (i.e., craving and negative affect), and smoking relapse influence each other in a bidirectional manner, and to measure the relative strengths of these temporal relationships.
Method
Design
Participants were randomized to receive nicotine replacement therapy (NRT), varenicline, or placebo during an 11-week smoking cessation clinical trial (NCT01314001; Lerman et al., 2015); all received behavioral counseling. Randomization was prospectively stratified by the plasma nicotine metabolite ratio (NMR), a phenotypic marker of the rate of nicotine metabolism and clearance. Participants were followed for 12 months after the target quit date (TQD), with smoking abstinence self-report collected at weeks 1, 4, 8, 11, 24, and 52, and biochemically verified by expired CO (≤ 8 ppm) at weeks 1, 4, 11, 24, and 52. Measures of composite and specific (negative affect and craving) nicotine withdrawal symptoms were captured at weeks 1, 4, 8, 11, 24, and 52 post-TQD.
Participants
Participants were 1246 treatment-seeking smokers recruited from the community by four North American academic medical centers. As outlined in detail previously (Lerman et al., 2015), to be eligible, participants must have been 18–65 years old, reported smoking at least 10 cigarettes per day (CPD) over the previous 6 months, and produced an expired carbon monoxide (CO) reading greater than 10 ppm. Individuals were considered ineligible if they met criteria for: (1) current use of tobacco products other than cigarettes; (2) history of substance abuse treatment (other than nicotine); (3) current alcohol consumption greater than 25 standard drinks/week; (4) history of a DSM-IV Axis 1 psychiatric disorder (except history of major depression, in remission, for greater than 6 months); (5) suicide risk score on the MINI greater than 1; (6) recent diagnosis of cancer or kidney or liver failure; (7) abnormal heart rhythms, pulmonary disease, stroke, angina, heart attack, or uncontrolled hypertension within the past 6 months; (8) current use or recent discontinuation (within the last 14 days) of smoking cessation medication; (9) current use of anti-psychotic medications, prescription stimulants, and certain antidepressants (bupropion, monoamine oxidase inhibitors, and tricyclic antidepressants) or opiate-containing medications; (10) current use of anticoagulants, rescue inhalers, or heart medications; and (11) current pregnancy or lactation. The protocol was approved by the Institutional Review Boards of the University of Pennsylvania, the University of Toronto, the Centre for Addiction and Mental Health, the State University of New York at Buffalo, and The University of Texas MD Anderson Cancer Center. Participants could earn up to $315 for completing all study requirements.
Procedures
Eligibility Assessment
Participant demographics, nicotine product use, alcohol/drug use, medication use, and medical/psychiatric history were collected during a telephone screening to determine initial eligibility. Participants who were initially eligible and interested were then scheduled and completed an intake screening session where the remainder of the eligibility criteria were assessed and a physical exam was conducted. Expired CO was used to assess smoking status. The MINI International Neuropsychiatric Interview (MINI) was used to assess for history of DSM-IV Axis I psychiatric disorders (Sheehan et al., 1998). Nicotine dependence was measured using the Fagerstrom Test of Cigarette Dependence (FTCD, formerly the FTND; Fagerstrom, 2012; Heatherton, Kozlowski, Frecker, & Fagerström, 1991).
Treatment randomization and study medication
After completing the intake screening session, participants were randomly assigned to one of three treatment groups, stratified on NMR group. Participants then attended a pre-quit session for counseling, completed study questionnaires, and received study medication. Varenicline (or placebo pills) were taken for 12 weeks, starting the day after the pre-quit visit. NRT (or placebo patches) was taken for 11 weeks, starting on the morning of the TQD. Participants in the varenicline group (n=420; 52.4% slow metabolizers) received active medication, with dose titration at days 1–3 (0.5 mg once daily), days 4–7 (0.5 mg twice daily), and days 8–84 (1.0 mg twice daily), and placebo patches. Participants in the NRT group (n=418; 54.3% slow metabolizers) received placebo pills and active NRT, with dose titration at weeks 1-6 (21 mg), weeks 7-8 (14 mg) and weeks 9-11 (7 mg). Participants in the placebo group (n=408; 52.7% slow metabolizers) received placebo pills and patches. Participants and study staff were blinded to NMR group and treatment assignment.
Behavioral counseling
All participants received the National Cancer Institute's "Clearing the Air" smoking cessation self-help manual (US Department of Health and Human Services, 2008) and counseling, starting with a 1-hour in-person pre-quit session. A target quit date was set for week 1, at which time a second counseling session occurred. Subsequent brief (15-min) counseling sessions were conducted over the telephone by two counselors from the University of Pennsylvania at weeks 4 and 8 post-TQD, and included collection of medication use, withdrawal, and side-effect data. The counseling and manual both took a cognitive-behavioral approach to smoking cessation, including coping with triggers and withdrawal-related symptoms.
Study measures collected over time
Three questionnaires assessing negative affect, withdrawal, and craving to smoke were measured at the pre-quit session and at the end of weeks 1, 4, 8, 11 (end of treatment), 24, and 52 post-TQD. The Positive and Negative Affect Schedule (PANAS; Watson, Clark, & Tellegen, 1988) was used to measure negative (PANAS NA subscale) and positive (PANAS PA subscale) affect. The Minnesota Nicotine Withdrawal Scale-Revised (MNWS-R) assessed nicotine withdrawal (Hughes, 2012). The Questionnaire on Smoking Urges – Brief (QSU-B) was used to measure craving to smoke (Cox, Tiffany, & Christen, 2001). The PANAS and MNWS assessed symptoms "during the last week," and the QSU-B assessed craving "right now."
Daily smoking was assessed at each study time point using the timeline follow-back method (TLFB; Brown et al., 1998). Repeated-measures abstinence status was calculated for weeks 1-4, weeks 5-8, weeks 9-11, weeks 12-24, and weeks 25-52 by examining all TLFB data within each time period. Participants were classified at each time period as a nonabstainer (smoking at least one cigarette during each week during the preceding time period), a partial abstainer (smoking at least one cigarette in some but not all of the weeks during the preceding time period), or a complete abstainer (never smoking during the time period). We included the category of partial abstinence because we wanted to model the extent to which degree of abstinence related to symptom scores over time.
Data Analyses
We performed a series of cross-lagged analyses in which we used prior symptom scores (PANAS NA, PANAS PA, MNWS-R, QSU-B) to predict subsequent abstinence status (nonabstainer, partial abstainer, complete abstainer), and prior abstinence status to predict subsequent symptom scores (see Figure 1) in separate regression models at different time points. The symptom scores were treated as continuous variables, and analyzed using linear regression, while abstinence status was treated as an ordinal variable, and analyzed using ordinal logistic regression models. The log regression coefficients from the ordinal logistic models quantified the relationship between the predictor (i.e., negative affect) and a corresponding change in abstinence outcome (nonabstinent; partially abstinent; completely abstinent), such that each single unit increase in the predictor was associated with the likelihood of being in a more restrictive abstinence category (i.e., from nonabstinence to partial abstinence, and from partial abstinence to complete abstinence). Neither treatment group nor NMR level predicted the outcomes of interest. However, all models included treatment group and NMR level (slow vs. fast metabolizers) as covariates.
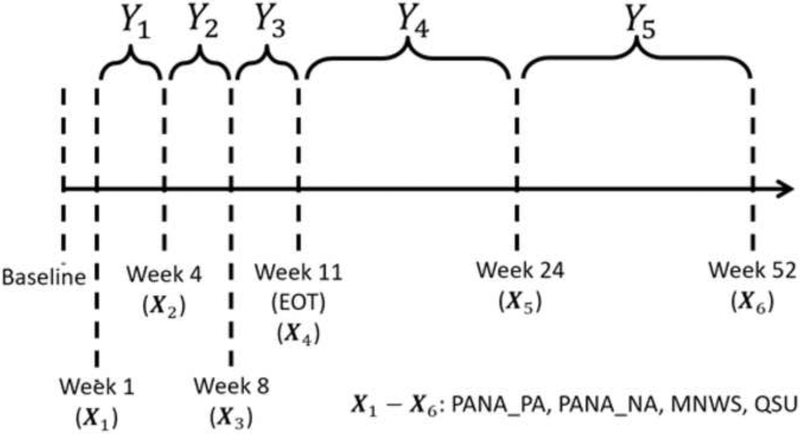
Figure 1.
A graphical illustration of the longitudinal structure of the cross-lagged analyses. The four nicotine withdrawal symptom scores (PANAS NA, PANAS PA, MNWS-R, QSU-B) were measured at the end of weeks 1, 4, 8, 11, 24, and 52, and are denoted by X1-X6. Abstinence status (non-, partial, and complete abstinence) during each of the five time periods (between weeks 1 and 4, between weeks 5 and 8, between weeks 9 and 11, between weeks 12 and 24, and between weeks 25 and 52) are denoted by Y1 through Y5. The temporal order of the data was: X1, Y1, X2, Y2, X3, Y3, X4, Y4, X5, Y5, X6.
We first examined the bidirectional relationships between symptom and abstinence using a series of univariate models. Next, we evaluated a series of multivariate models to determine whether the effects observed in the univariate models remained when we included either the symptom score or the abstinence status from the previous time point as a covariate. Both the univariate and multivariate analyses were conducted separately for each symptom score.
To determine which of the directional relationships was stronger, we compared the increase in predictive power when adding a covariate of interest by the percent increase in McFadden’s pseudo r-squared (McFadden, 1974) and r-squared statistics for the logistic regression (i.e., the impact of symptoms on subsequent abstinence) and linear regression (i.e., the impact of abstinence status on subsequent symptoms) models, respectively. The r-squared statistic in linear model has an interpretation as being a measure of the percent of variation in the outcome explained by the covariates. The pseudo r-squared statistic has been advocated as having an analogous interpretation in the context of logistic regression, but they quantify different aspects of the association between covariates and outcome that may not be reflected in the regression coefficients, such as odds ratio (Allison, 2014; Hu, Shao, & Palta, 2006).
Results
Participant characteristics
Enrolled participants were 55.6% White (37.1% Black), 56.4% men, and 45.7 years of age on average. They smoked 18.3 CPD on average and reported moderate nicotine dependence (mean FTCD = 5.3) at baseline. Participant characteristics are further detailed elsewhere (Lerman et al., 2015).
Descriptive statistics of the study sample
The means and standard deviations of the four symptom scores (PANAS NA, PANAS PA, MNWS-R, QSU-B) at each time point (weeks 1, 4, 8, 11, 24, and 52) are presented in Table 1. Abstinence status within each of the five observational periods are summarized in Table 2. The percentage of nonabstainers and partial abstainers who met the CO cutoff during, and their mean CPD, for each of the five time periods is reported in Table 3. Correlations between the four symptoms scores and abstinence at each time point are presented in Table 4. Overall, the proportion of abstinent participants remained stable during the treatment period (through week 11) but declined notably after that. This decline in abstinence also coincided with an increase in symptom scores starting at week 24 (X5).
Table 1.
Summary statistics of the four nicotine withdrawal symptom scores at baseline and at each of the six time points (at the end of weeks 1, 4, 8, 11, 24, and 52) for the overall sample.
| Withdrawal Symptom Measurement Time Point | |||||||
|---|---|---|---|---|---|---|---|
| Covariate | Baseline | Week 1 (X1) | Week 4 (X2) | Week 8 (X3) | Week 11 (X4) | Week 24 (X5) | Week 52 (X6) |
| PANAS NA | |||||||
| Mean (SD) | 12.94 (4.02) | 14.11 (5.55) | 13.63 (5.28) | 13.26 (5.23) | 13.32 (5.53) | 16.24 (7.07) | 16.99 (7.59) |
| N | 1245 | 1136 | 991 | 875 | 958 | 879 | 823 |
| PANAS PA | |||||||
| Mean (SD) | 32.46 (8.89) | 33.12 (9.93) | 33.76 (10.37) | 33.65 (10.61) | 34.18 (10.84) | 35.87 (9.21) | 36.09 (8.88) |
| N | 1245 | 1136 | 991 | 875 | 958 | 879 | 823 |
| MNWS-R | |||||||
| Mean (SD) | 7.09 (4.66) | 8.91 (6.49) | 8.09 (6.27) | 7.45 (6.17) | 7.15 (6.36) | 10.41 (7.50) | 11.25 (8.01) |
| N | 1245 | 1135 | 991 | 875 | 957 | 879 | 822 |
| QSU-B | |||||||
| Mean (SD) | 29.79 (14.72) | 20.49 (12.82) | 18.47 (12.31) | 17.12 (11.70) | 17.33 (12.09) | 20.37 (13.94) | 21.76 (15.23) |
| N | 1244 | 1135 | 991 | 874 | 956 | 879 | 822 |
Note. PANAS = Positive and Negative Affect Scale; NA = PANAS Negative Affect Scale; PA = PANAS Positive Affect Scale; MNWS-R = Minnesota Nicotine Withdrawal Scale-Revised; QSU-B = Questionnaire on Smoking Urges – Brief.
Table 2.
Smoking abstinence status, in N and percentiles, during each of the five time periods for the overall sample.
| Abstinence Status Time Period | |||||
|---|---|---|---|---|---|
| Covariate | Weeks 1-4 (Y1) | Weeks 5-8 (Y2) | Weeks 9-11 (Y3) | Weeks 12-24 (Y4) | Weeks 25-52 (Y5) |
| Nonabstainer | 567 (45.5%) | 625 (50.2%) | 658 (52.8%) | 833 (66.9%) | 974 (78.2%) |
| Partial Abstainer | 237 (19.0%) | 162 (13.0%) | 135 (10.8%) | 56 (4.5%) | 57 (4.6%) |
| Complete Abstainer | 442 (35.5%) | 459 (36.8%) | 453 (36.4%) | 357 (28.7%) | 215 (17.3%) |
Table 3.
The percentage of nonabstainers and partial abstainers who met the CO cutoff during, and their mean CPD, for each of the five time periods.
| Time Period | Abstinence Status | Meet CO Cutoff (%)* | Mean CPD |
|---|---|---|---|
| Weeks 1-4 | Non-abstainers Partial abstainers |
97.7% 90.8% |
6.0 0.6 |
| Weeks 5-8 | Non-abstainers Partial abstainers |
99.6% 74.6% |
7.2 1.0 |
| Weeks 9-11 | Non-abstainers Partial abstainers |
99.5% 67.9% |
7.9 0.9 |
| Weeks 12-24 | Non-abstainers Partial abstainers |
99.9% 99.1% |
9.8 2.5 |
| Weeks 25-52 | Non-abstainers Partial abstainers |
99.9% 92.0% |
11.3 2.5 |
Note.
: CO verified at 8ppm.
Table 4.
Correlations between the four symptom scores and abstinence at each of the 5 time points.
| Weeks 1-4 (Y1) | |||||
| Abstinence | PANAS NA | PANAS PA | MNWS-R | QSU-B | |
| Abstinence | 1.00 | ||||
| PANAS NA | −0.14 | 1.00 | |||
| PANAS PA | 0.072 | −0.16 | 1.00 | ||
| MNWS-R | −0.15 | 0.68 | −0.15 | 1.00 | |
| QSU-B | −0.23 | 0.31 | −0.16 | 0.41 | 1.00 |
| Weeks 5-8 (Y2) | |||||
| Abstinence | PANAS NA | PANAS PA | MNWS-R | QSU-B | |
| Abstinence | 1.0000 | ||||
| PANAS NA | −0.20 | 1.00 | |||
| PANAS PA | 0.065 | −0.086 | 1.00 | ||
| MNWS-R | −0.23 | 0.68 | −0.11 | 1.00 | |
| QSU-B | −0.33 | 0.26 | −0.11 | 0.37 | 1.00 |
| Weeks 9-11 (Y3) | |||||
| Abstinence | PANAS NA | PANAS PA | MNWS-R | QSU-B | |
| Abstinence | 1.00 | ||||
| PANAS NA | −0.24 | 1.00 | |||
| PANAS PA | 0.094 | −0.16 | 1.00 | ||
| MNWS-R | −0.27 | 0.68 | −0.18 | 1.00 | |
| QSU-B | −0.39 | 0.30 | −0.16 | 0.38 | 1.00 |
| Weeks 12-24 (Y4) | |||||
| Abstinence | PANAS NA | PANAS PA | MNWS-R | QSU-B | |
| Abstinence | 1.00 | ||||
| PANAS NA | −0.18 | 1.00 | |||
| PANAS PA | 0.099 | −0.16 | 1.00 | ||
| MNWS-R | −0.24 | 0.69 | −0.22 | 1.00 | |
| QSU-B | −0.28 | 0.34 | −0.17 | 0.43 | 1.00 |
| Weeks 25-52 (Y5) | |||||
| Abstinence | PANAS NA | PANAS PA | MNWS-R | QSU-B | |
| Abstinence | 1.00 | ||||
| PANAS NA | −0.18 | 1.00 | |||
| PANAS PA | 0.14 | −0.29 | 1.00 | ||
| MNWS-R | −0.23 | 0.72 | −0.30 | 1.00 | |
| QSU-B | −0.30 | 0.33 | −0.17 | 0.42 | 1.00 |
Note. PANAS = Positive and Negative Affect Scale; NA = PANAS Negative Affect Scale; PA = PANAS Positive Affect Scale; MNWS-R = Minnesota Nicotine Withdrawal Scale-Revised; QSU-B = Questionnaire on Smoking Urges – Brief.
Univariate analyses of the relationships between symptoms and abstinence
The impact of symptoms on subsequent abstinence.
To examine the impact of symptom scale scores on subsequent abstinence status, we fit separate ordinal logistic regression models to each of the five observational periods for each symptom scale, including treatment and NMR as covariates. In the notation of Figure 1, the temporal relationships evaluated were X1 → Y1, X2 → Y2, X3 → Y3, X4 → Y4, X5 → Y5, where X refers to the symptom and Y to the abstinence status at the respective time points. All four symptom scores were significantly associated with the log probability odds of complete or partial abstinence in the subsequent observational period (see Table 5). Specifically, higher PANAS NA, MNWS-R, QSU-B scores predicted lower probability of complete or partial abstinence out to one-year post-quit. Higher PANAS PA scores predicted increased probability of complete or partial abstinence, but only for abstinence during weeks 25-52.
Table 5.
Ordinal logistic regression models predicting abstinence status from previous withdrawal symptom score. In the notation of Figure 1, the temporal relationships evaluated were X1 → Y1, X2 → Y2, X3 → Y3, X4 → Y4, X5 → Y5. Coefficients in the cell indicate the probability change being partial or complete abstinence, for one standard deviation increase from prior symptom mean score.
| Abstinence Status Time Period | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Covariate | Weeks 1-4 (Y1) | Weeks 5-8 (Y2) | Weeks 9-11 (Y3) | Weeks 12-24 (Y4) | Weeks 25-52 (Y5) | |||||
| Partial | Complete | Partial | Complete | Partial | Complete | Partial | Complete | Partial | Complete | |
| PANAS NA | −.021 | −.043 | −.015 | −.086 | −.011 | −.117 | −.042 | −.082 | −.072 | −.057 |
| (−.033, −.009)** | (−.062, −.024)*** | (−.024, −.007)*** | (−.112, −.059)*** | (−.019, −.004)** | (−.15, −.083)*** | (−.059, −.025)*** | (−.103, −.061)*** | (−.095, −.049)*** | (−.071, −.043)*** | |
| PANAS PA | .012 | .036 | .002 | .027 | <0.001 | .042 | .007 | .026 | .037 | .052 |
| (.005, .018) | (.014, .058) | (0, .004)* | (−.001, .055) | (−.002, .001) | (.01, .074)* | (.001, .014) | (.001, .05)* | (.023, .05) | (.03, .074)* | |
| MNWS-R | −.025 | −.048 | −.02 | −.099 | −.013 | −.127 | −.053 | −.093 | −.091 | −.064 |
| (−.037, −.013)*** | (−.065, −.03)*** | (−.029, −.01)*** | (−.124, −.074)*** | (−.021, −.005)** | (−.156, −.098)*** | (−.07, −.036)*** | (−.111, −.075)*** | (−.115, −.068)*** | (−.076, −.052)*** | |
| QSU-B | −.044 | −.072 | −.042 | −.145 | −.046 | −.21 | −.096 | −.124 | −.183 | −.077 |
| (−.059, −.028)*** | (−.089, −.055)*** | (−.058, −.026)*** | (−.17, −.121)*** | (−.064, −.027)*** | −.239, −.181)*** | (−.121, −.07)*** | (−.141, −.106)*** | (−.21, −.156)*** | (−.09, −.065)*** | |
Note. PANAS = Positive and Negative Affect Scale; NA = PANAS Negative Affect Scale; PA = PANAS Positive Affect Scale; MNWS-R = Minnesota Nicotine Withdrawal Scale-Revised; QSU-B = Questionnaire on Smoking Urges – Brief.
p < .05;
p < .01;
p < .001.
The impact of abstinence on subsequent symptoms.
To evaluate the impact of abstinence on subsequent symptoms, we fit separate linear regression models to each of the five observational periods for each symptom scale, including treatment and NMR as covariates. In the notation of Figure 1, the temporal relationships evaluated were Y1 → X2, Y2 → X3, Y3 → X4, Y4 → X5, Y5 → X6. Smoking abstinence, particularly complete abstinence, was significantly associated with subsequent symptom scores in most models (see Table 6). Overall, being a complete abstainer, compared to a nonabstainer, resulted in lower PANAS NA, MNWS-R, and QSU-B scores, and higher PANAS PA scores, across the four time periods. A similar but smaller effect was generally observed for partial abstinence vs. nonabstinence at most time points, except for PANAS PA, which only significantly differed at the final time period (week 52; X6). Overall, the results suggest that the impact of complete and partial abstinence on subsequent symptom score tended to increase over time and was greatest at the final time period, weeks 52 (X6).
Table 6.
Linear regression models predicting withdrawal symptoms from prior abstinence status, with treatment and NMR as covariates. The coefficient quantifies the mean difference in the outcome symptom score for each unit increase in the covariate. In the notation of Figure 1, the temporal relationships evaluated were Y1 → X2, Y2 → X3, Y3 → X4, Y4 → X5, Y5 → X6.
| Withdrawal Symptom Measurement Time Point | |||||
|---|---|---|---|---|---|
| Covariate | Week 4 (X2) | Week 8 (X3) | Week 11 (X4) | Week 24 (X5) | Week 52 (X6) |
| PANAS NA | |||||
| Partial | −0.94 (−1.72, −0.16)* | 0.07 (−0.89, 1.03) | −0.73 (−1.84, 0.38) | −1.74 (−2.89, −0.659)** | −1.22 (−2.4, −.04)* |
| Complete | −2.12 (−2.93, −1.32)*** | −2.25 (−3.01, −1.48)*** | −2.66 (−3.41, −1.91)*** | −4.28 (−5.37, −3.19)*** | −5.05 (−6.42, −3.69)*** |
| PANAS PA | |||||
| Partial | 0.65 (−0.90, 2.21) | 1.95 (−0.03, 3.94) | −1.02 (−3.25, 1.20) | 0.78 (−0.76, 2.32) | 2.62 (1.22, 4.03)*** |
| Complete | 1.75 (0.15, 3.36)* | 1.72 (0.14, 3.29)* | 2.09 (0.60, 3.58)** | 3.33 (1.87, 4.78)*** | 3.74 (2.12, 5.36)*** |
| MNWS-R | |||||
| Partial | −1.89 (−2.81, −0.97)*** | −0.67 (−1.79, 0.45) | −1.36 (−2.61, −0.10)* | −2.41 (−3.61, −1.21)*** | −3.28 (−4.48, −2.08)*** |
| Complete | −2.83 (−3.78, −1.88)*** | −3.26 (−4.15, −2.37)*** | −3.97 (−4.81, −3.13)*** | −5.55 (−6.68, −4.42)*** | −7.53 (−8.92, −6.15)*** |
| QSU-B | |||||
| Partial | −7.33 (−9.06, −5.61)*** | −4.67 (−6.73, −2.61)*** | −5.88 (−8.20, −3.56)*** | −6.00 (−8.15, −3.85)*** | −10.64 (−12.83, −8.45)*** |
| Complete | −9.16 (−10.94, −7.38)*** | −8.70 (−10.34, −7.06)*** | −9.62 (−11.17, −8.07)*** | −13.22 (−15.25, −11.19)*** | −16.64 (−19.19, −14.14)*** |
Note. The reference group was the nonabstainers. PANAS = Positive and Negative Affect Scale; NA = PANAS Negative Affect Scale; PA = PANAS Positive Affect Scale; MNWS-R = Minnesota Nicotine Withdrawal Scale-Revised; QSU-B = Questionnaire on Smoking Urges – Brief; Partial = partial abstinence; Complete = complete abstinence.
p < .05;
p < .01;
p < .001.
Multivariate analyses of the relationships between symptoms and abstinence
The impact of symptoms on subsequent abstinence, adjusting for prior abstinence.
As with our univariate models, we fit separate ordinal logistic regression models to each of the five observational periods for each symptom scale, but with the addition of abstinence status from the prior observational period as a covariate. In the notation of Figure 1, the temporal relationships evaluated were Y1 + X2 → Y2, Y2 + X3 → Y3, Y3 + X4 → Y4, Y4 + X5 → Y5. In these multivariate analyses, higher PANAS NA, MNWS-R, QSU-B scores significantly predicted lower probability of complete or partial abstinence out to one-year post-quit (see Table 7), consistent with the univariate analyses. However, the association between PANAS PA and subsequent abstinence was nonsignificant at all but the last time point (weeks 25-52; Y5).
Table 7.
Ordinal logistic regression models predicting abstinence status from previous withdrawal symptom score, with abstinence status from the prior observational period, treatment, and NMR as covariates. In the notation of Figure 1, the temporal relationships evaluated were Y1 + X2 → Y2, Y2 + X3 → Y3, Y3 + X4 → Y4, Y4 + X5 → Y5. Positive log odds coefficients indicate an increase in the likelihood of being in either complete or partial abstinence status versus nonabstinent, while negative log odds coefficients indicate a decrease in the likelihood of being a complete abstainer versus either a partial or nonabstainer.
| Abstinence Status Time Point | ||||
|---|---|---|---|---|
| Covariate | Weeks 5-8 (Y2) | Weeks 9-11 (Y3) | Weeks 12-24 (Y4) | Weeks 25-52 (Y5) |
| PANAS NA | −0.06 (−0.09, −0.02)** | −0.08 (−0.12, −0.04)*** | −0.03 (−0.07, 0.00)* | −0.03 (−0.05, 0.00)* |
| Partial | 2.88 (2.51, 3.25)*** | 2.99 (2.52, 3.47)*** | 2.39 (1.91, 2.88)*** | 2.48 (2.06, 2.91)*** |
| Complete | 5.12 (4.63, 5.61)*** | 5.70 (5.15, 6.25)*** | 4.80 (4.34, 5.27)*** | 5.82 (5.18, 6.46)*** |
| PANAS PA | 0.00 (−0.01, 0.02) | 0.01 (0.00, 0.03) | 0.00 (−0.01, 0.02) | 0.02 (0.00, 0.04)* |
| Partial | 2.88 (2.51, 3.25)*** | 2.89 (2.43, 3.35)*** | 2.40 (1.92, 2.88)*** | 2.51 (2.08, 2.93)*** |
| Complete | 5.16 (4.67, 5.65)*** | 5.73 (5.18, 6.28)*** | 4.86 (4.4, 5.33)*** | 5.87 (5.23, 6.51)*** |
| MNWS-R | −0.06 (−0.08, −0.03)*** | −0.06 (−0.09, −0.02)*** | −0.04 (−0.06, −0.01)** | −0.04 (−0.06, −0.01)** |
| Partial | 2.85 (2.47, 3.22)*** | 2.93 (2.46, 3.39)*** | 2.37 (1.89, 2.86)*** | 2.46 (2.03, 2.88)*** |
| Complete | 5.12 (4.63, 5.61)*** | 5.66 (5.11, 6.21)*** | 4.76 (4.29, 5.23)*** | 5.77 (5.13, 6.41)*** |
| QSU-B | −0.03 (−0.04, −0.01)*** | −0.05 (−0.07, −0.03)*** | −0.02 (−0.04, 0.00)* | −0.06 (−0.08, −0.04)*** |
| Partial | 2.75 (2.37, 3.12)*** | 2.83 (2.36, 3.30)*** | 2.26 (1.77, 2.75)*** | 2.43 (1.99, 2.87)*** |
| Complete | 5.00 (4.5, 5.49)*** | 5.57 (5.01, 6.12)*** | 4.71 (4.23, 5.18)*** | 5.55 (4.90, 6.20)*** |
Note. The reference group was the nonabstainers. PANAS = Positive and Negative Affect Scale; NA = PANAS Negative Affect Scale; PA = PANAS Positive Affect Scale; MNWS-R = Minnesota Nicotine Withdrawal Scale-Revised; QSU-B = Questionnaire on Smoking Urges – Brief.
p < .05;
p < .01;
p < .001.
The impact of abstinence status on subsequent symptoms, adjusting for prior withdrawal symptoms.
As with the univariate models, we fit separate linear regression models to each of the five observational periods for each symptom scale, but with the addition of prior symptom score as an additional covariate. In the notation of Figure 1, the temporal relationships evaluated were X1 + Y1 → X2, X2 + Y2 → X3, X3 + Y3 → X4, X4 + Y4 → X5, X5 + Y5 → X6. Overall, being a complete abstainer, compared to a nonabstainer, resulted in lower PANAS NA, MNWS-R, and QSU-B scores, across the four time periods (see Table 8), consistent with the univariate results, but the associations appear weaker after adjusting for prior symptom scores. Additionally, the association between abstinence (complete vs. nonabstinence) and subsequent PANAS PA was nonsignificant in the multivariate analyses, when controlling for previous PANAS PA, except for at the 24-week (X5) and 52-week (X6) time points. The multivariate results were consistent with the univariate results and showed that the impact of complete and partial abstinence on subsequent symptom score tended to be greatest at the final time periods, weeks 24 (X5) and 52 (X6).
Table 8.
Linear regression models predicting withdrawal symptoms from prior abstinence status, with prior withdrawal symptoms scores, treatment, and NMR as covariates. The coefficient quantifies the mean difference in the outcome symptom score for each unit increase in the covariate. In the notation of Figure 1, the temporal relationships evaluated were X1 + Y1 → X2, X2 + Y2 → X3, X3 + Y3 → X4, X4 + Y4 → X5, X5 + Y5 → X6.
| Symptom Score Measurement Time Point | |||||
|---|---|---|---|---|---|
| Covariate | Week 4 (X2) | Week 8 (X3) | Week 11 (X4) | Week 24 (X5) | Week 52 (X6) |
| PANAS NA | 0.51 (0.46, 0.56)*** | 0.51 (0.45, 0.57)*** | 0.46 (0.40, 0.52)*** | 0.46 (0.38, 0.55)*** | 0.47 (0.40, 0.54)*** |
| Partial | −0.31 (−0.96, 0.35) | 0.30 (−0.54, 1.14) | 0.30 (−0.72, 1.32) | −0.71 (−1.83, 0.42) | −0.14 (−1.27, 0.98) |
| Complete | −1.26 (−1.94, −0.659)*** | −1.07 (−1.75, −0.39)** | −1.18 (−1.89, −0.47)** | −2.99 (−4.07, −1.92)*** | −2.52 (−3.84, −1.20)*** |
| PANAS PA | 0.76 (0.71, 0.80)*** | 0.76 (0.71, 0.81)*** | 0.78 (0.73, 0.82)*** | 0.49 (0.44, 0.53)*** | 0.51 (0.45, 0.57)*** |
| Partial | −0.12 (−1.20, 0.95) | 1.35 (−0.01, 2.71) | −1.14 (−2.69, 0.41) | 0.48 (−0.81, 1.78) | 1.06 (−0.21, 2.34) |
| Complete | 0.11 (−1.01, 1.22) | 0.38 (−.71, 1.47) | 0.45 (−0.61, 1.51) | 1.94 (0.71, 3.17)** | 2.19 (0.74, 3.64)** |
| MNWS-R | 0.54 (0.49, 0.59)*** | 0.64 (0.58, 0.69)*** | 0.60 (0.55, 0.66)*** | 0.51 (0.43, 0.58)*** | 0.54 (0.47, 0.60)*** |
| Partial | −0.86 (−1.63, −0.10)* | 0.24 (−0.64, 1.13) | 0.12 (−0.93, 1.17) | −1.22 (−2.35, −0.10)* | −1.53 (−2.64, −0.42)** |
| Complete | −1.56 (−2.35, −0.76)*** | −1.29 (−2.02, −0.57)** | −1.39 (−2.12, −0.65)*** | −3.61 (−4.70, −2.51)*** | −3.82 (−5.14, −2.51)*** |
| QSU-B | 0.48 (0.43, 0.54)*** | 0.53 (0.47, 0.58)*** | 0.59 (0.53, 0.64)*** | 0.48 (0.41, 0.55)*** | 0.59 (0.52, 0.66)*** |
| Partial | −5.50 (−7.00, −3.99)*** | −2.22 (−3.92, −0.52)** | −0.66 (−2.52, 1.21) | −2.36 (−4.439, −0.32)*** | −4.28 (−6.32, −2.25)*** |
| Complete | −6.04 (−7.61, −4.46)*** | −3.77 (−5.19, −2.34)*** | −2.76 (−4.09, −1.43)*** | −8.36 (−10.21, −6.25)*** | −7.88 (−10.27, −5.49)*** |
Note. The reference group was the nonabstainers. PANAS = Positive and Negative Affect Scale; NA = PANAS Negative Affect Scale; PA = PANAS Positive Affect Scale; MNWS-R = Minnesota Nicotine Withdrawal Scale-Revised; QSU-B = Questionnaire on Smoking Urges – Brief; Partial = partial abstinence; Complete = complete abstinence.
p < .05;
p < .01;
p < .001.
Comparing the strengths of the bidirectional relationships
To compare the strength of the directionality of the relationship between symptom scores and abstinence status over time, we next estimated the predictive power of covariates of interest through either the r-squared or the pseudo r-squared. Table 9 reports the Pseudo R-Squared statistics for the ordinal logistic regression models (i.e., the impact of treatment, NMR, and previous symptoms on subsequent abstinence), and Table 10 reports the R-Squared statistics for the linear regression models (i.e., the impact of treatment, NMR, and previous abstinence status on subsequent symptoms). We focused on the percent increase in R-Squared or Pseudo R-Squared statistics as a measure of the incremental predictive power gained by adding the specified covariates to the models. Overall, our results suggest that symptom scores add more predictive power to the model for subsequent abstinence (Table 9) than abstinence status adds to the model for subsequent symptom scores (Table 10). In other words, the temporal bidirectional relationships between symptom scores and abstinence are not symmetric, as the predictive relationship from an earlier symptom to later partial or complete abstinence is stronger than the predictive relationship from earlier abstinence to the later symptom.
Table 9.
The amount of variance explained, beyond that of prior abstinence status, by treatment, NMR, and symptom scores when predicting subsequent abstinence status. Variance explained was calculated using the pseudo r-squared statistic, while the percent increase captured the amount of additional variance captured when symptom scores from the prior time point is included in the model.
| Abstinence Status Time Point | ||||
|---|---|---|---|---|
| Covariate | Weeks 5-8 (Y2) | Weeks 9-11 (Y3) | Weeks 12-24 (Y4) |
Weeks 25-52 (Y5) |
| Prior Abstinence Only | 0.41 | 0.53 | 0.45 | 0.44 |
| With Treatment & NMR | 0.42 | 0.53 | 0.45 | 0.44 |
| Percent increase | 0.6% | 0.6% | 0.14% | 0.7% |
| With PANAS NA | 0.47 | 0.61 | 0.49 | 0.54 |
| Percent increase | 13.84% | 14.43% | 9.4% | 21.14% |
| With PANAS PA | 0.47 | 0.61 | 0.49 | 0.54 |
| Percent increase | 12.69% | 13.39% | 8.98% | 21.18% |
| With MNWS-R | 0.48 | 0.61 | 0.49 | 0.54 |
| Percent increase | 14.52% | 14.25% | 9.7% | 21.71% |
| With QSU-B | 0.47 | 0.62 | 0.49 | 0.56 |
| Percent increase | 14.01% | 16.14% | 10.06% | 25.32% |
Note. PANAS = Positive and Negative Affect Scale; NA = PANAS Negative Affect Scale; PA = PANAS Positive Affect Scale; MNWS-R = Minnesota Nicotine Withdrawal Scale-Revised; QSU-B = Questionnaire on Smoking Urges – Brief.
Table 10.
The amount of variance explained, beyond that of previous symptom score, by treatment, NMR, and abstinence status (complete or partial vs. nonabstinence) when predicting subsequent symptom scores. Variance explained was calculated using the r-squared statistic, while the percent increase captured the amount of additional variance captured when abstinence status from the prior time period is included in the model.
| Withdrawal Symptom Measurement Time Point | |||||
|---|---|---|---|---|---|
| Covariates | Week 4 (X2) |
Week 8 (X3) |
Week 11 (X4) |
Week 24 (X5) |
Week 52 (X6) |
| PANAS NA Only | 0.32 | 0.26 | 0.22 | 0.14 | 0.22 |
| With Treatment & NMR | 0.32 | 0.25 | 0.22 | 0.15 | 0.22 |
| Percent increase | 0.42% | −0.44% | −0.87% | 3.13% | −0.31% |
| With Treatment, NMR & Abstinence | 0.33 | 0.26 | 0.23 | 0.18 | 0.23 |
| Percent Increase | 2.56% | 3.95% | 5.19% | 19.48% | 6.36% |
| PANAS PA Only | 0.53 | 0.54 | 0.58 | 0.34 | 0.29 |
| With Treatment & NMR | 0.53 | 0.54 | 0.58 | 0.34 | 0.29 |
| Percent increase | −0.26% | −0.15% | −0.02% | −0.65% | −0.95% |
| With Treatment, NMR & Abstinence | 0.53 | 0.54 | 0.58 | 0.35 | 0.30 |
| Percent Increase | −0.17% | 0.18% | 0.19% | 1.83% | 2.40% |
| MNWS-R Only | 0.34 | 0.41 | 0.39 | 0.23 | 0.32 |
| With Treatment & NMR | 0.34 | 0.42 | 0.39 | 0.23 | 0.32 |
| Percent increase | 1.40% | 0.61% | −0.13% | 1.97% | 0.15% |
| With Treatment, NMR & Abstinence | 0.35 | 0.43 | 0.40 | 0.27 | 0.34 |
| Percent Increase | 2.61% | 2.34% | 2.70% | 15.51% | 8.89% |
| QSU-B Only | 0.30 | 0.37 | 0.43 | 0.25 | 0.40 |
| With Treatment & NMR | 0.31 | 0.37 | 0.43 | 0.25 | 0.40 |
| Percent increase | 1.85% | −0.35% | −0.01% | 0.49% | −0.59% |
| With Treatment, NMR & Abstinence | 0.36 | 0.39 | 0.44 | 0.31 | 0.43 |
| Percent Increase | 15.9% | 4.91% | 2.48% | 22.24% | 8.5% |
Note. PANAS = Positive and Negative Affect Scale; NA = PANAS Negative Affect Scale; PA = PANAS Positive Affect Scale; MNWS-R = Minnesota Nicotine Withdrawal Scale-Revised; QSU-B = Questionnaire on Smoking Urges – Brief.
Discussion
Overall, our analyses suggest a cyclic relationship between symptoms of withdrawal and craving and abstinence, with higher symptoms, as measured by the PANAS NA, MNWS-R, and QSU-B, increasing the likelihood of subsequent smoking relapse. Additionally, our analyses showed that abstinence, in particular being a complete abstainer, was associated with lower symptoms, compared to relapse. The multivariate analyses involving the PANAS NA, MNWS-R, and QSU-B were largely consistent with the findings of the univariate models. The associations between positive affect, as measured by the PANAS PA, and abstinence were largely nonsignificant in both directional models, except that complete abstinence was associated with higher PA at the 24- and 52-week time points. Finally, the relationships were stronger for models in which earlier symptoms predicted later abstinence status than models in which abstinence status predicted later symptom, when we compared the amount of variance explained between each set of models.
Our findings suggest that higher negative affect, craving, and composite withdrawal symptoms increased the likelihood of subsequent smoking relapse, independent of previous smoking status. Additionally, this effect appears to be strongest for predicting smoking relapse at weeks 25-52 (Y5), where all symptoms explained more than 20% of the variance beyond that of prior abstinence and treatment (see Table 9). To our knowledge, this is the first study to use a cross-lagged analysis to investigate the bidirectional relations between abstinence and symptoms of withdrawal, craving and affect. These findings support negative reinforcement conceptualizations of nicotine dependence by suggesting that chronic smoking is a behavior motivated by the relief of unpleasant mood and withdrawal states (Baker, Piper, McCarthy, Majeskie, & Fiore, 2004; Koob & Le Moal, 2008). Our findings are consistent with many other studies, particularly those involving EMA (Allen, Bade, Hatsukami, & Center, 2008; Shiffman et al., 1996), with which our study shares the advantage of measuring the relationship between withdrawal and abstinence repeatedly over time. Our study extends previous findings by virtue of its timeframe and its use of multiple measures of withdrawal-related symptoms (PANAS NA, MNWS-R, QSU-B).
Our finding that smoking relapse resulted in higher withdrawal-related symptoms compared to abstinence does not necessarily contradict negative reinforcement conceptualizations of addiction. One explanation is that relapsed smokers have often been found to smoke reduced amounts compared to their baseline amount (see Hughes & Carpenter, 2005, for review), and thus may still experience withdrawal. Indeed, one study found that relapsed smokers who did not return to smoking their baseline amount were more likely to report mood disturbances than those who did (Kahler et al., 2002). Another possibility is the AVE, the phenomenon by which smokers who relapse experience negative affect in the form of self-blame and guilt resulting from failing to achieve a desired goal (i.e., abstinence) (Curry et al., 1987). However, Marlatt and Gordon (1985) postulated that the abstinence violation effect mediates the relationship between lapse and relapse, and not the subsequent impact of relapse. Additionally, more recent evidence found that self-blame reduced the likelihood of a lapse, suggesting that self-blame may not always be maladaptive in this context (Kirchner, Shiffman, Schroeder, & Wileyto, 2012). Thus, it is unclear whether relapse leads to an increase in negative affect due to feelings of guilt.
The design of this study has several features that may have influenced our results and that may reduce the generalizability of our findings. First, the inclusion and exclusion criteria used in this clinical trial likely excluded factors that are over represented among smokers, such as those who abused alcohol or those with a history of a DSM-IV Axis 1 psychiatric disorder (except major depression), along with smokers who used more than one tobacco product. Second, this study supplemented pharmacotherapy with counseling and a treatment manual to assist all participants in quitting. These cognitive-behavioral elements included imparting skills for coping with withdrawal symptoms, which may account for us not finding a drug treatment effect. Additionally, counseling is not a part of most "real-world" quitting attempts, which are largely done unaided (Chapman & MacKenzie, 2010), meaning that most smokers are not going to have the benefit of interventions focused on coping with withdrawal symptoms. In addition to potentially limiting the generalizability of our findings, the results of these design choices likely restricted the amount of variance in withdrawal symptoms experienced by our sample than if we had recruited participants using less strict criteria and had not included a cognitive-behavioral counseling component. However, we were still able to observe relationships between withdrawal symptoms and abstinence despite these limitations, which supports the use of interventions that focus on withdrawal symptoms, particularly negative affect. Third, our definition of smoking abstinence, differed from commonly used abstinence definitions (e.g., Hughes et al., 2003). The reason we did not use one of the established abstinence guidelines is that we were interested in measuring the relationship between partial nicotine exposure (i.e., partial abstinence) and withdrawal, not just the relationship between abstinence/nonabstinence and withdrawal, because there is some evidence that partial smoking abstinence may prolong withdrawal symptoms (Shiffman, 1979). We did find that partial abstinence predicted subsequent withdrawal symptoms out to week 52 (see Table 6), suggesting that partial abstinence does have long-term consequences for symptoms such as negative affect. Fourth, the MNWS and PANAS NA were highly correlated, suggesting that they were not measuring distinct constructs. This finding is not unique to our study, as others have found that the MNWS is similarly highly correlated with other measures of negative affect (Aguirre, Madrid, & Leventhal, 2015), and that it does not provide additional incremental validity to that of negative affect when modeling smoking abstinence (Piasecki, Kenford, Smith, Fiore, & Baker, 1997). Finally, we want to caution that our use of cross-lagged analyses, which consisted of regression models for the purpose of exploring the association among the observed abstinence and withdrawal symptoms at various time points, should not be taken as an implication for causal relationship and likely inflated the likelihood of a Type I error.
In conclusion, our findings showed that withdrawal-related symptoms and smoking relapse are related in a bidirectional manner over time among those trying to quit smoking. Consistent with negative reinforcement conceptualizations, higher negative affect, craving, and composite withdrawal symptoms increased the likelihood of subsequent relapse. Additionally, smokers who relapsed reported higher negative affect, craving, and composite withdrawal symptoms than abstinent smokers, suggesting that withdrawal-related symptoms are multidetermined. Our findings provide further evidence that negative affect and other unpleasant withdrawal symptoms should be a target for intervention within smoking cessation therapy.
Acknowledgments
This project was supported by a grant from the National Institutes of Health (U01DA20830) and by MD Anderson's Cancer Center Support Grant (P30CA016672). Pfizer provided varenicline and placebo pills at no cost. Caryn Lerman received study medication and support for medication packaging from Pfizer; she has also consulted to Gilead, and has been a paid expert witness in litigation against tobacco companies. Rachel Tyndale has acted as a consultant to Apotex and to Quinn Emmanuel. Paul Cinciripini served on the scientific advisory board of Pfizer Pharmaceuticals, did educational talks sponsored by Pfizer on smoking cessation from 2006 to 2008, and has received grant and medication support from Pfizer. Dr. Schnoll receives medication and placebo free of charge from Pfizer for other clinical trials and has provided consultation to Pfizer and GlaxoSmithKline. Dr. Benowitz has been a consultant to pharmaceutical companies that market smoking cessation medications, including Pfizer, and has served as an expert witness in litigation against tobacco companies. Dr. Hawk receives medication and placebo free of charge from Pfizer for an ongoing clinical trial. The remaining authors declare no competing interests.
Footnotes
Author Note
Jason D. Robinson, Department of Behavioral Science, The University of Texas MD Anderson Cancer Center; Liang Li, Department of Biostatistics, The University of Texas MD Anderson Cancer Center; Minxing Chen, Department of Biostatistics, The University of Texas MD Anderson Cancer Center; Charles E. Green, Department of Pediatrics & the Center for Clinical Research and Evidence-Based Medicine, The University of Texas Health Science Center at Houston; Caryn Lerman, Department of Psychiatry, Annenberg School for Communication, and Abramson Cancer Center, University of Pennsylvania; Rachel F. Tyndale, Centre for Addiction and Mental Health, Departments of Pharmacology & Toxicology and Psychiatry, University of Toronto. Robert A. Schnoll, Department of Psychiatry and Abramson Cancer Center, University of Pennsylvania, Philadelphia, Pennsylvania; Larry W. Hawk, Jr., Department of Psychology, University at Buffalo, SUNY, Buffalo, NY; Tony P. George; Centre for Addiction and Mental Health and Division of Brain and Therapeutics, Department of Psychiatry, University of Toronto, Toronto, Ontario, Canada; Neal L. Benowitz, Departments of Medicine, and Bioengineering & Therapeutic Sciences, University of California, San Francisco, CA.
Contributor Information
Jason D. Robinson, The University of Texas MD Anderson Cancer Center, Houston, Texas
Liang Li, The University of Texas MD Anderson Cancer Center, Houston, Texas.
Minxing Chen, The University of Texas MD Anderson Cancer Center, Houston, Texas.
Caryn Lerman, University of Pennsylvania, Philadelphia, Pennsylvania.
Rachel F. Tyndale, Centre for Addiction and Mental Health, and University of Toronto, Toronto, Ontario, Canada
Robert A. Schnoll, University of Pennsylvania, Philadelphia, Pennsylvania
Larry W. Hawk, Jr., University at Buffalo, SUNY, Buffalo, NY
Tony P. George, Centre for Addiction and Mental Health and University of Toronto, Toronto, Ontario, Canada
Neal L. Benowitz, University of California, San Francisco, CA
Paul M. Cinciripini, The University of Texas MD Anderson Cancer Center, Houston, Texas
References
- Aguirre CG, Madrid J, & Leventhal AM (2015). Tobacco withdrawal symptoms mediate motivation to reinstate smoking during abstinence. Journal of Abnormal Psychology, 124(3), 623–634. 10.1037/abn0000060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen SS, Bade T, Hatsukami D, & Center B (2008). Craving, withdrawal, and smoking urges on days immediately prior to smoking relapse. Nicotine & Tobacco Research, 10(1), 35–45. 10.1080/14622200701705076 [DOI] [PubMed] [Google Scholar]
- Allison PD (2014). Measures of Fit for Logistic Regression. SUGI Proceedings, 14 Retrieved from https://support.sas.com/resources/papers/proceedings14/1485-2014.pdf [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition Arlington, VA: American Psychiatric Association. [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, & Fiore MC (2004). Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychological Review, 111(1), 33–51. 10.1037/0033-295X.111.1.33 [DOI] [PubMed] [Google Scholar]
- Blalock JA, Robinson JD, Wetter DW, Schreindorfer LS, & Cinciripini PM (2008). Nicotine withdrawal in smokers with current depressive disorders undergoing intensive smoking cessation treatment. Psychology of Addictive Behaviors, 22(1), 122–128. 10.1037/0893-164X.22.1.122 [DOI] [PubMed] [Google Scholar]
- Borland R (1990). Slip-ups and relapse in attempts to quit smoking. Addictive Behaviors, 15, 235–245. 10.1016/0306-4603(90)90066-7 [DOI] [PubMed] [Google Scholar]
- Brandon TH, Tiffany ST, Obremski KM, & Baker TB (1990). Postcessation cigarette use: The process of relapse. Addictive Behaviors, 15, 105–114. 10.1016/0306-4603(90)90013-N [DOI] [PubMed] [Google Scholar]
- Brown RA, Burgess ES, Sales SD, Whiteley JA, Evans DM, & Miller IW (1998). Reliability and validity of a smoking timeline follow-back interview. Psychology of Addictive Behaviors, 12(2), 101–112. 10.1037/0893-164X.12.2.101 [DOI] [Google Scholar]
- Chapman S, & MacKenzie R (2010). The Global Research Neglect of Unassisted Smoking Cessation: Causes and Consequences. PLOS Medicine, 7(2), e1000216 10.1371/journal.pmed.1000216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinciripini PM, Robinson JD, Karam-Hage M, Minnix JA, Lam C, Versace F, … Wetter DW (2013). Effects of Varenicline and Bupropion Sustained-Release Use Plus Intensive Smoking Cessation Counseling on Prolonged Abstinence From Smoking and on Depression, Negative Affect, and Other Symptoms of Nicotine Withdrawal. JAMA Psychiatry, 70(5), 522–533. 10.1001/jamapsychiatry.2013.678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin CA, Perkins KA, Sheidow AJ, Jones BL, Levine MD, & Marcus MD (2005). The Return to Smoking: 1-year Relapse Trajectories Among Female Smokers. Nicotine & Tobacco Research, 7(4), 533–540. 10.1080/14622200500185371 [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, & Christen AG (2001). Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & Tobacco Research, 3(1), 7–16. 10.1080/14622200020032051 [DOI] [PubMed] [Google Scholar]
- Cummings KM, Jaen CR, & Giovino GA (1985). Circumstances surrounding relapse in a group of recent exsmokers. Preventive Medicine, 14, 195–202. 10.1016/0091-7435(85)90035-0 [DOI] [PubMed] [Google Scholar]
- Curry S, Marlatt GA, & Gordon JR (1987). Abstinence violation effect: Validation of an attributional construct with smoking cessation. Journal of Consulting and Clinical Psychology, 55(2), 145–149. 10.1037/0022-006X.55.2.145 [DOI] [PubMed] [Google Scholar]
- Fagerström K (2012). Determinants of tobacco use and renaming the FTND to the Fagerstrom Test for Cigarette Dependence. Nicotine & Tobacco Research, 14(1), 75–78. 10.1093/ntr/ntr137 [DOI] [PubMed] [Google Scholar]
- Gritz ER, Carr CR, & Marcus AC (1991). The tobacco withdrawal syndrome in unaided quitters. British Journal of Addiction, 86, 57–69. 10.1111/j.1360-0443.1991.tb02629.x [DOI] [PubMed] [Google Scholar]
- Gunn RC (1986). Reactions to withdrawal symptoms and success in smoking cessation clinics. Addictive Behaviors, 11, 49–53. 10.1016/0306-4603(86)90008-0 [DOI] [PubMed] [Google Scholar]
- Hall SM, Havassy BE, & Wasserman DA (1990). Commitment to abstinence and acute stress in relapse to alcohol, opiates, and nicotine. Journal of Consulting and Clinical Psychology, 58, 175–181. 10.1037/0022-006X.58.2.175 [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, & Fagerström KO (1991). The Fagerstrom Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction, 86(9), 1119–1127. 10.1111/j.1360-0443.1991.tb01879.x [DOI] [PubMed] [Google Scholar]
- Hu B, Shao J, & Palta M (2006). Pseudo R2 in logistic regression model. Statistica Sinica, 16(847-860). [Google Scholar]
- Hughes JR (2012). Background on the Minnesota Withdrawal Scale-Revised (MNWS-R). Retrieved from http://contentmanager.med.uvm.edu/docs/default-source/behavior-and-health-documents/background_8_2012.pdf?
- Hughes JR, Gust SW, Skoog K, Keenan RM, & Fenwick JW (1991). Symptoms of tobacco withdrawal: A replication and extension. Archives of General Psychiatry, 48, 52–59. 10.1001/archpsyc.1991.01810250054007 [DOI] [PubMed] [Google Scholar]
- Hughes JR, & Hatsukami D (1986). Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry, 43, 289–294. 10.1001/archpsyc.1986.01800030107013 [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, & Swan GE (2003). Measures of abstinence in clinical trials: Issues and recommendations. Nicotine & Tobacco Research, 5(1), 13–25. 10.1080/1462220031000070552 [DOI] [PubMed] [Google Scholar]
- Hughes JR, & Carpenter MJ (2005). The feasibility of smoking reduction: an update. Addiction, 100(8), 1074–1089. 10.1111/j.1360-0443.2005.01174.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitz HS, Lerman C, & Swan GE (2012). Comparative dynamics of four smoking withdrawal symptom scales. Addiction, 107(8), 1501–1511. 10.1111/j.1360-0443.2012.03838.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahler CW, Brown RA, Ramsey SE, Niaura R, Abrams DB, Goldstein MG, … Miller IW (2002). Negative mood, depressive symptoms, and major depression after smoking cessation treatment in smokers with a history of major depressive disorder. Journal of Abnormal Psychology, 111(4), 670–675. 10.1037/0021-843X.111.4.670 [DOI] [PubMed] [Google Scholar]
- Kassel JD, Stroud LR, & Paronis CA (2003). Smoking, stress, and negative affect: Correlation, causation, and context across stages of smoking. Psychological Bulletin, 129(2), 270–304. 10.1037/0033-2909.129.2.270 [DOI] [PubMed] [Google Scholar]
- Kenford SL, Smith SS, Wetter DW, Jorenby DE, Fiore MC, & Baker TB (2002). Predicting relapse back to smoking: Contrasting affective and physical models of dependence. Journal of Consulting and Clinical Psychology, 70, 216–227. 10.1037/0022-006X.70.1.216 [DOI] [PubMed] [Google Scholar]
- Kirchner TR, Shiffman S, Schroeder SA, & Wileyto EP (2012). Relapse dynamics during smoking cessation: recurrent abstinence violation effects and lapse-relapse progression. Journal of Abnormal Psychology, 121(1), 187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, & Le Moal M (2008). Addiction and the brain antireward system. Annual Review of Psychology, 59, 29–53. 10.1146/annurev.psych.59.103006.093548 [DOI] [PubMed] [Google Scholar]
- Lam CY, Robinson JD, Versace F, Minnix JA, Cui Y, Carter BL, … Cinciripini PM (2012). Affective reactivity during smoking cessation of never-quitters as compared with that of abstainers, relapsers, and continuing smokers. Experimental and Clinical Psychopharmacology, 20(2), 139–150. 10.1037/a0026109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman C, Schnoll RA, Hawk LW JR, Cinciripini P, George TP, Wileyto EP, … Tyndale RF (2015). Else of the nicotine metabolite ratio as a genetically informed biomarker of response to nicotine patch or varenicline for smoking cessation: A randomised, double-blind placebo-controlled trial. The Lancet Respiratory Medicine, 3(2), 131–138. 10.1016/S2213-2600(14)70294-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt GA (1985). Relapse prevention: Theoretical rationale and overview of the model In Marlatt G & Gordon J (Eds.), Relapse prevention: Maintenance strategies in the treatment of addictive behaviors (pp. 3–70). New York: Guilford Press. [Google Scholar]
- McFadden D (1974). Conditional logit analysis of qualitative choice behavior In Zarembka P (Ed.), Frontiers in Econometrics (pp. 105–142). New York: Academic Press. [Google Scholar]
- McKee SA, O'Malley SS, Salovey P, Krishnan-Sarin S, & Mazure CM (2005). Perceived risks and benefits of smoking cessation: gender-specific predictors of motivation and treatment outcome. Addictive Behaviors, 30(3), 423–435. 10.1016/j.addbeh.2004.05.027 [DOI] [PubMed] [Google Scholar]
- Nakajima M, & al'Absi M (2012). Predictors of risk for smoking relapse in men and women: A prospective examination. Psychology of Addictive Behaviors : Journal of the Society of Psychologists in Addictive Behaviors, 26(3), 633–637. 10.1037/a0027280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norregaard J, Tonnesen P, & Petersen L (1993). Predictors and reasons for relapse in smoking cessation with nicotine and placebo patches. Preventive Medicine, 22(2), 261–271. 10.1006/pmed.1993.1021 [DOI] [PubMed] [Google Scholar]
- Orleans CT, Rimer BK, Cristinzio S, Keintz MK, & Fleisher L (1991). A national survey of older smokers: treatment needs of a growing population. Health Psychology, 10(5), 343–351. 10.1037/0278-6133.10.5.343 [DOI] [PubMed] [Google Scholar]
- Patten CA, & Martin JE (1996). Does nicotine withdrawal affect smoking cessation? Clinical and theoretical issues. Annals of Behavioral Medicine, 18(3), 190–200. 10.1007/BF02883397 [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Jorenby DE, Smith SS, Fiore MC, & Baker TB (2003). Smoking withdrawal dynamics: I Abstinence distress in lapsers and abstainers. Journal of Abnormal Psychology, 112(1), 3–13. 10.1037/0021-843X.112.1.3 [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Kenford SL, Smith SS, Fiore MC, & Baker TB (1997). Listening to nicotine: Negative affect and the smoking withdrawal conundrum. Psychological Science, 8(3), 184–189. [Google Scholar]
- Piper ME, Schlam TR, Cook JW, Sheffer MA, Smith SS, Loh WY, … Baker TB (2011). Tobacco withdrawal components and their relations with cessation success. Psychopharmacology, 216, 569–578. 10.1007/s00213-011-2250-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JD, Lam CY, Carter BL, Minnix JA, Cui Y, Versace F, … Cinciripini PM (2011). A multimodal approach to assessing the impact of nicotine dependence, nicotine abstinence, and craving on negative affect in smokers. Experimental and Clinical Psychopharmacology, 19(1), 40–52. 10.1037/a0022114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serre F, Fatseas M, Swendsen J, & Auriacombe M (2015). Ecological momentary assessment in the investigation of craving and substance use in daily life: A systematic review. Drug and Alcohol Dependence, 148, 1–20. 10.1016/j.drugalcdep.2014.12.024 [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, … Dunbar GC (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry, 59 Suppl 20, 22–33. [PubMed] [Google Scholar]
- Shiffman S (1979). The tobacco withdrawal syndrome InKrasnegor N (Ed.), Cigarette Smoking as a Dependence Process (pp. 158–184). Rockville, Maryland: National Institute on Drug Abuse, United States Department of Health, Education, and Welfare. [Google Scholar]
- Shiffman S (1982). Relapse following smoking cessation: a situational analysis. Journal of Consulting and Clinical Psychology, 50(1), 71–86. 10.1037/0022-006X.50.1.71 [DOI] [PubMed] [Google Scholar]
- Shiffman S (1986). A cluster-analytic classification of smoking relapse episodes. Addictive Behaviors, 11 295–307. 10.1016/0306-4603(86)90057-2 [DOI] [PubMed] [Google Scholar]
- Shiffman S, Balabanis MH, Gwaltney CJ, Paty JA, Gnys M, Kassel JD, … Paton SM (2007). Prediction of lapse from associations between smoking and situational antecedents assessed by ecological momentary assessment. Drug and Alcohol Dependence, 91(2-3), 159–168. 10.1016/j.drugalcdep.2007.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, & Gwaltney CJ (2008). Does heightened affect make smoking cues more salient? Journal of Abnormal Psychology, 117(3), 618–624. 10.1037/0021-843X.117.3.618 [DOI] [PubMed] [Google Scholar]
- Shiffman S, Gwaltney CJ, Balabanis MH, Liu KS, Paty JA, Kassel JD, … Gnys M (2002). Immediate antecedents of cigarette smoking: An analysis from ecological momentary assessment. Journal of Abnormal Psychology, 111(4), 531–545. 10.1037/0021-843X.111.4.531 [DOI] [PubMed] [Google Scholar]
- Shiffman S, Hufford M, Hickcox M, Paty JA, Gnys M, & Kassel JD (1997). Remember that? A comparision of real-time versus retrospective recall of smoking lapses. Journal of Consulting and Clinical Psychology, 65(2), 292–300. 10.1037/0022-006X.65.2.292.a [DOI] [PubMed] [Google Scholar]
- Shiffman S, & Kirchner TR (2009). Cigarette-by-cigarette satisfaction during ad libitum smoking. Journal of Abnormal Psychology, 118(2), 348–359. 10.1037/a0015620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Paty J, Gnys M, Kassel J, & Hickcox M (1996). First lapses to smoking: Within-subjects analysis of real-time reports. Journal of Consulting and Clinical Psychology, 64(2), 366–379. 10.1037/0022-006X.64.2.366 [DOI] [PubMed] [Google Scholar]
- Shiffman S, West RJ, & Gilbert DG (2004). Recommendation for the assessment of tobacco craving and withdrawal in smoking cessation trials. Nicotine & Tobacco Research, 6(4), 599–614. 10.1080/14622200410001734067 [DOI] [PubMed] [Google Scholar]
- Stone AA, & Shiffman S (1994). Ecological momentary assessment (EMA) in behavioral medicine. Annals of Behavioral Medicine, 16(3), 199–202. [Google Scholar]
- Strong DR, Leventhal AM, Evatt DP, Haber S, Greenberg BD, Abrams D, & Niaura R (2011). Positive reactions to tobacco predict relapse after cessation. Journal of Abnormal Psychology, 120(4), 999–1005. 10.1037/a0023666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan GE, Denk CE, Parker SD, Carmelli D, Furze CT, & Rosenman RH (1988). Risk factors for late relapse in male and female ex-smokers. Addictive Behaviors, 13, 253–266. 10.1016/0306-4603(88)90052-4 [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Warthen MW, & Goedeker KC (2009). The functional significance of craving in nicotine dependence In Bevins R & Caggiula A (Eds.), The motivational impact of nicotine and its role in tobacco use, Nebraska symposium on motivation, vol 55 (pp. 171–197). New York: Springer-Verlag. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services. (2008). Clearing the Air (NIH Publication No. 11-1647). Retrieved from https://smokefree.gov/sites/default/files/pdf/clearing-the-air-accessible.pdf
- Watson D, Clark LA, & Tellegen A (1988). Development and validation of brief measures of positive and negative affect: The PANAS Scales. Journal of Personality and Social Psychology, 54, 1063–1070. 10.1037/0022-3514.54.6.1063 [DOI] [PubMed] [Google Scholar]
- Wray JM, Gass JC, & Tiffany ST (2013). A systematic review of the relationships between craving and smoking cessation. Nicotine & Tobacco Research, 15(7), 1167–1182. 10.1093/ntr/nts268 [DOI] [PMC free article] [PubMed] [Google Scholar]