Abstract
Recent advances and emerging technologies for metabolic pathway engineering and synthetic biology have transformed the field of natural product discovery, production, and engineering. Despite these advancements, there remain many challenges in understanding how biosynthetic gene clusters are silenced or activated, including changes in the transcription of key biosynthetic and regulatory genes. This knowledge gap is highlighted by the success and failed attempts of manipulating regulatory genes within biosynthetic gene clusters in both native producers and heterologous hosts. These complexities make the choice of native producers versus heterologous hosts, fermentation medium, and supply of precursors crucial factors in achieving the production of the target natural products and engineering designer analogues. Nature continues to serve as inspiration for filling the knowledge gaps and developing new research strategies. By exploiting the evolutionary power of Nature, alternative producers, with the desired genetic amenability and higher titers of the target natural products, and new strains, harboring gene clusters that encode evolutionary optimized congeners of the targeted natural product scaffolds, can be discovered. These newly identified strains can serve as an outstanding biotechnology platform for the engineered production of sufficient quantities of the target natural products and their analogues, enabling biosynthetic studies and potential therapeutic applications. These challenges and opportunities are showcased herein using fredericamycin, iso-migrastatin, platencin and platensimycin, the enediynes of C-1027, tiancimycin, and yangpumicin, and the leinamycin family of natural products.
Keywords: Natural products, heterologous expression, transcriptional regulators, bioinformatics, genome mining
Introduction
Natural products have been a source of inspiration for both chemists and biologists due to their unique, complex structures and vast biological activities. More than 70% of the antibacterial or anticancer drugs currently on the market are natural products and their analogues or derived from and inspired by natural products [30]. Despite this outstanding track record, since the end of the 20th century, natural product isolation programs have significantly decreased with the rise of combinatorial synthesis and high throughput screening (HTS) [36]. Moreover, the discovery of natural products is often hindered by low quantities, poor growth characteristics of the producers, or the inability to cultivate the bacterial strains of interest within a laboratory setting [49, 58]. Advancements in microbial genomics have shown that, in Actinobacteria, approximately 90% of biosynthetic capacity has not been realized [36]. Continued advancement and emerging technologies in bioinformatics, microbial genomics, metabolic pathway engineering, and synthetic biology are poised to place us at the forefront of a new age of natural product discovery and drug development [36, 41].
Common challenges in the production of microbial natural products
Genome mining efforts, enabled by advancements in DNA sequencing and bioinformatics, have allowed for the identification and prioritization of bacterial strains, harboring biosynthetic gene clusters that encode privileged natural product scaffolds and/or novel chemistries, for discovery. The challenges lie in activating the identified biosynthetic gene clusters for natural product discovery. Two complementary approaches have been commonly considered – (i) activating the gene cluster in the native producer, which may require genetic technologies that are not readily available, or (ii) expressing the gene cluster in a heterologous model host, which can take advantage of the expedient genetic tools developed. While applications of the common engineering technologies to manipulate gene expression are generally easier in heterologous hosts, the natural regulatory pathways between the native producers and the gene cluster are severed upon transfer of the gene cluster out of its native producer. Conceptually, one could imagine developing a “universal” system for producing natural products, in which, any biosynthetic gene cluster could be introduced into a genetically tractable and high producing chassis tailored to a desired natural product of interest. However, despite great effort and access to many of the emerging engineering tools, many challenges remain.
Even with enabling technologies to sequence DNA, and clone and express biosynthetic gene clusters in various heterologous hosts (Figure 1), the feasibility and practicality of a universal system is currently unattainable due to a gap in knowledge of natural product production between native producers and heterologous hosts. Recent studies, such as a so-called “pressure test” to produce 10 natural products in 90 days [3], highlight this knowledge gap and demonstrates how little we truly know about interactions between biosynthetic gene clusters and host regulatory systems. Successful examples of heterologous production of natural products are dominated by small, low-complexity gene clusters, with few operons, with exceptions seen only on a case-by-case basis. Therefore, the notion that heterologous expression of biosynthetic gene clusters can systematically facilitate natural product production is far from reality.
Fig. 1.
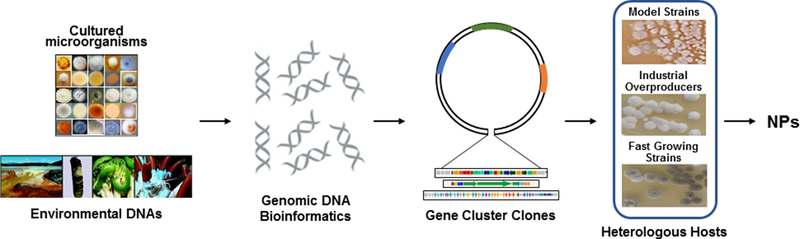
Schematic representation of natural product discovery utilizing genomic DNA from cultured microorganisms and the environment for production in heterologous hosts.
There are two types of strategies that one could implement to activate biosynthetic pathways – (i) epigenetic-related and (ii) genomics-based. Epigenetic approaches rely on modifying culture conditions, interspecies cross talk, stressors, external cues, environmental, and nutritional factors, while genomic-based approaches utilize genome mining, genetic manipulation, and heterologous expression [41, 49, 58]. One genomics-based strategy, discussed herein, to coax production from a biosynthetic gene cluster is overexpression and deletion of regulatory genes. There are three types of regulators within Streptomyces – (i) global regulators that are responsible for morphological differentiation and secondary metabolite production, (ii) pleiotropic regulators that control the production of multiple downstream pathways, and (iii) pathway-specific regulators that govern the titers of a particular compound or set of related compounds from the same gene cluster [4, 33]. These regulators compose large networks and signal transduction pathways that are unique to each producer and govern their own set of biosynthetic gene clusters. This hierarchical nature of the regulatory networks that govern secondary metabolism introduces complexities for activation of biosynthetic gene clusters in heterologous hosts. Upon transfer of a biosynthetic gene cluster from a native producer to a heterologous host, these regulatory networks may be severed and disrupted. Comparing the gene expression for key biosynthetic or regulatory genes in native producers and heterologous hosts therefore could shed light on these intricate networks. The following three examples, using natural products fredericamycin A (FDM A), iso-migrastatin (iso-MGS), and platensimycin (PTM) and platencin (PTN), highlight the important roles played by regulatory networks in engineered production of natural products.
FdmR1 as a pathway specific positive regulator controlling FDM A production
FDM A, first isolated in 1981 from Streptomyces griseus ATCC 49344 [32], features a novel spiro carbon center joining two peri-hydroxy tricyclic aromatic moieties [28, 42] (Figure 2a); the polyketide origin of the pentadecaketide skeleton of FDM A was subsequently established by isotope labeling experiments [2]. The novel structural features and potent antitumor activity of FDM A inspired efforts to study its biosynthesis, improve production, and generate analogues by metabolic pathway engineering. The 25-kb fdm gene cluster, consisting of 28 genes, was identified and cloned from S. griseus ATCC 49344 (Figure 2b) and introduced into two heterologous hosts, Streptomyces albus J1074 and Streptomyces lividans K4–114 [48]. While no FDM A production was detected in S. lividans K4–114, expression of the fdm cluster in S. albus J1074 afforded FDM A in a titer of 130 mg/L, in comparison to 170 mg/L in the native producer, serving as a starting point for engineered production of FDM A [4, 48].
Fig. 2.
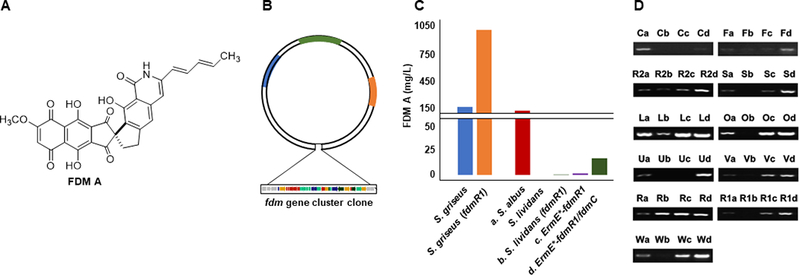
Engineered production of FDM A in native producer and heterologous hosts. (A) The structure of FDM A. (B) The construct for expressing the fdm biosynthetic gene cluster in heterologous hosts. (C) Engineered production of FDM A by overexpressing of fdmR1 in the native producer S. griseus and heterologous hosts S. albus and S. lividans, resulting varying levels of FDM A titer improvement of FDM A titers. (D) RT-PCR analysis showed increased levels of transcription for key genes encoding FDM A biosynthesis upon fdmR1 and fdmC overexpression, correlating to improved FDM A titers: a, S. lividans; b, S. lividans (fdmR1); c, S. lividans (ErmE*-fdmR1); and d, S. lividans (ErmE*-fdmR1/fdmC).
To increase FDM A production, either in the native producer or heterologous hosts, three putative regulatory genes, fdmR, fdmR1, and fdmR2, were identified within the fdm cluster. FdmR1 belongs to the Streptomyces antibiotic regulatory protein (SARP) family of regulators consisting of both pathway-specific and pleiotropic regulators [4]. Inactivation of fdmR1 in S. griseus ATCC 49344 abolished production of FDM A, which could be restored upon genetic complementation. Real time-polymerase chain reaction (RT-PCR) analysis of the effects of ΔfdmR1 inactivation revealed the silencing of five biosynthetic genes (fdmF, fdmL, fdmO, fdmS, and fdmV) and significantly reduced the transcription of four other pathway-related genes (fdmC, fdmR2, fdmU, and fdmW). These findings established FdmR1 as a pathway-specific positive regulator for FDM A biosynthesis in S. griseus ATCC 49344 and encouraged the overexpression of fdmR1 for FDM A titer improvement and structural diversity in both the native and heterologous hosts [4].
Overexpression of fdmR1 indeed resulted in an increased production of FDM A in the native producer or activation of the fdm gene cluster in the heterologous hosts, albeit at significantly different levels between the native producer and the heterologous hosts [48]. In the native producer S. griseus ATCC 49344, fdmR1 overexpression resulted in a 6-fold titer improvement for FDM A to ~1 g/L (Figure 2c). However, activation of FDM A production in S. lividans K4–114, in which the fdm gene cluster was apparently silent, resulted in a low FDM A titer (0.5 mg/L); the titer was improved upon placing fdmR1 under the control of the constitutive ErmE* promoter, increasing the FDM A titer 3-fold to 1.4 mg/L (Figure 2c) [4]. Although FDM A biosynthesis was activated in S. lividans K4–114, the resulting titers were much lower than that in the native producer S. griseus ATCC 49344 or the heterologous host S. albus J1074 (Figure 2c). Subsequent comparison by RT-PCR of the transcription levels of the fdm genes between the native producer and the heterologous hosts identified fdmC, a ketoreductase, as the bottleneck in FDM A production; co-overexpression of fdmC and fdmR1 in S. lividans ultimately resulted in a 12-fold increase in FDM A titer to 17 mg/L (Figure 2d) [4]. Overall, manipulation of regulatory genes can greatly assist in gene cluster activation and increasing the production of natural products. However, this example, and many other examples not mentioned here, highlight how regulatory system alteration, upon gene cluster transfer into heterologous hosts, can result in diminished production or differences in gene transcription levels. This authenticates the notion that regulatory genes that govern secondary metabolism, whether global or pathway-specific, are part of a complex network that complicates rational metabolic pathway engineering and refactoring in both native and heterologous hosts.
Variations of regulation of iso-MGS production between native producer and heterologous hosts
Dorrigocins A and B, first isolated in 1994 from Streptomyces platensis subsp. rosaceus strain AB1981F-75 to inhibit a carboxyl methyltransferase involved in cellular signal transduction via processing of Ras-related proteins [18, 22], belong to the glutarimide-containing polyketide family of natural products. Other members of this family of natural products include migrastatin (MGS), isolated from Streptomyces sp. MK929–43F1 in 2000 and shown to inhibit cell migration [29], and iso-migrastatin (iso-MGS), isolated from S. platensis NRRL18993 in 2002 [50], which was also known as a MGS producer. Subsequent investigation of MGS and iso-MGS biosynthesis in S. platensis NRRL18993 established iso-MGS as the nascent natural product encoded by the mgs biosynthetic gene cluster, with dorrigocin A and B, as well as MGS, as shunt metabolites of iso-MGS formed during isolation (Figure 3a) [19–21, 25].
Fig. 3.
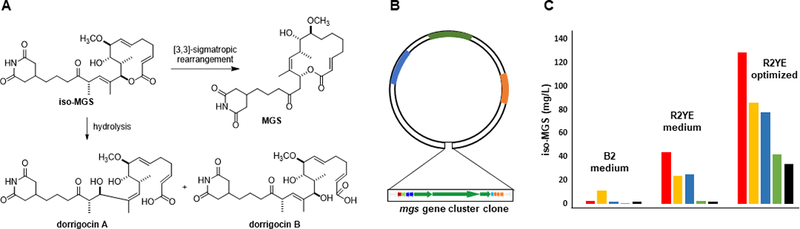
Optimization of iso-MGS production in heterologous hosts. (A) Structures of iso-MGS and its shunt metabolites dorrigocin A, B, and MGS. (B) The construct for expressing the mgs biosynthetic gene cluster in heterologous hosts. (C) iso-MGS titters in five heterologous hosts upon fermentation in three different media: S. albus J1074 (■); S. lividans K4–114 (■); S. coelicolor M512 (■); S. avermitilis SUKA4 (■); and S. avermitilis SUKA5 (■).
The 65-kb mgs gene cluster, consisting of 11 genes, was cloned from S. platensis NRRL 18993 and introduced into five heterologous hosts, i.e., S. albus J1074, S. lividans K4–114, S. coelicolor M512, S. avermitilis SUKA4, and S. avermitilis SUKA5, for iso-MGS production, fermented in three different media, i.e., B2, R2YE, and R2YE (Figure 3b) [11]. iso-MGS production was observed in all five hosts under the three media examined albeit with varying titers [51, 55] (Figure 3c). Differences in the production of iso-MGS among the five heterologous hosts, in the three different media, suggest that in addition to severing regulatory networks, the choice of media could also play an important role in activation of the mgs gene cluster in the heterologous hosts. This is further highlighted by the increased expression of 10 of the 11 genes in the mgs gene cluster, as revealed by RT-PCR, in S. albus J1074 when fermented in R2YE medium compared to the other media [51, 55]. There are many reasons as to why this may occur, including competing biosynthetic pathways, precursor availability, and the roles the promoters and regulators may play, demonstrating the importance in the choice of heterologous host and medium. Currently, there are no general guidelines to predict which combinations will be most fruitful for natural product production.
To increase iso-MGS production, the msgA regulator was identified within the mgs gene cluster from the native producer S. platensis NRRL 18993 [25, 51]. Bioinformatics analysis revealed that MgsA belonged to the family of “PimR-like” SARP regulators [1], inactivation of which in S. platensis NRRL 18993 completely abolished iso-MGS production, establishing its essential role in regulating iso-MGS biosynthesis [25]. However, overexpression of mgsA, alone or under the constitutive ErmE* promoter, in either the native producer or the five heterologous hosts, showed no titer improvement for iso-MGS, suggesting that MgsA is not the rate-limiting factor controlling iso-MGS biosynthesis. More interestingly, transcription of mgsA could not be detected by RT-PCR from all five heterologous hosts, despite the fact that mgsA was essential for iso-MGS production in the native producer S. platensis NRRL 18993 [55]. Of note, when the mgs gene cluster was compared with two other glutarimide-containing polyketide gene clusters, lactimidomycin (LTM) from Streptomyces amphibiosporus ATCC 53964 and cycloheximide (CHX) from Streptomyces sp. YIM 56141, it was realized that the chx gene cluster, consisting of 10 genes, also contained a MgsA homologue, ChxA, while the ltm gene cluster, consisting of nine genes, lacked a MgsA homologue or any apparent regulators [57]. This raises the question as to why only some gene clusters have pathway-specific regulators and why activation or inactivation of positive or negative regulators, respectively, does not always correlate with the titers of the target natural products. Taken together, this implies there are differences in the regulatory networks between the native producers and heterologous hosts, as well as between heterologous hosts, thus adding another layer of complexity when deciding which heterologous host to use. This knowledge gap in understanding how biosynthetic gene clusters interact with the rest of the genomes, either in the native producers or heterologous hosts, limits our ability to activate gene clusters by rational metabolic pathway engineering for natural product production in general.
PtnR1 and PtmR1 as pathway specific repressors controlling PTN and PTM production in both native producers and heterologous hosts
Platensimycin (PTM), first discovered in 2006 from S. platensis MA7327, is a hybrid natural product containing a substituted benzoic acid and diterpenoid-derived ketolide connected via an amide bond (Figure 4a) [15, 46]. PTM was shown to target bacterial type II fatty-acid synthesis (FASII) by inhibiting the FabF/B subunits [46]. Platencin (PTN) was discovered a year late from S. platensis MA7339, which differs from PTM only in the ketolide portion of the molecule but targets FASII by dual inhibition of the FabF/B and FabH subunits (Figure 4a) [45]. Despite their structural novelty, potent activity, and the unprecedented mode of action, the low titers of PTM (~2–4 mg/L) and PTN (~1 mg/L) in the native producers hindered their further biosynthetic studies and biological evaluation.
Fig. 4.
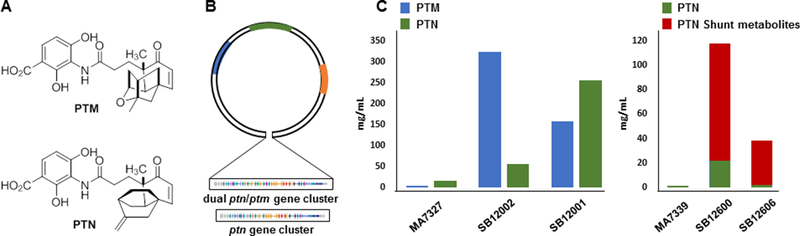
Engineered production of PTM and PTN in native producers and heterologous hosts. (A) Structures of PTM and PTN. (B) The constructs for expressing the ptm and ptn biosynthetic gene clusters in five heterologous hosts. (C) PTM and PTN titters in the native producers, heterologous hosts, as well as accumulation of PTN shunt metabolites. S. platensis MA7327, native PTM-PTN dual producer; S. platensis MA7339, native PTN producer; SB12001 and SB12002, ΔptmR1 mutants of S platensis MA7327) that overproduced PTM and PTN; SB12600, ΔptnR1 mutant of S platensis MA7339 that overproduced PTN and shunt metabolites; and SB12606, expression of the ptn cluster, with ΔptnR1, in S. lividans that produced PTN and shunt metabolites.
To improve PTM and PTN production, both the 47-kb ptm gene cluster, consisting of 36 genes, and the 41-kb ptn gene cluster, consisting of 31 genes, were identified and cloned from S. platensis MA7327 and S. platensis MA7339, respectively, remarkably confirming that the ptm gene cluster from S. platensis MA7327 in fact encoded the biosynthesis of both PTM and PTN, i.e., S. platensis MA7327 as a PTM and PTN dual producer [40]. Within the ptm and ptn clusters were identified ptmR1 and ptnR1, respectively, encoding members of the GntR family of pathway specific repressors. Inactivation of ptmR1 in S. platensis MA7327 afforded two ΔptmR1 mutant strains SB12001 and SB12002 that overproduced PTM and PTN, respectively, in titers of 323 mg/L and 255 mg/L, which represented more than 100-fold increase over the native producer [39]. Similar inactivation of ptnR1 in S. platensis MA7339 afforded the ΔptnR1 mutant strain SB12600 that overproduced PTN in a titer of 22 mg/L, which also represented a major improvement over the native producer but was significantly lower than the PTN titer in SB12002 [56]. Subsequent examination of the metabolite profile revealed that SB12600 accumulated many PTN biosynthetic intermediates, which apparently cannot be converted to PTN, accounting for the reduced overall titer of PTN in SB12600 [56]. Unfortunately, both the ΔptmR and ΔptnR overproducers failed to sporulate, forfeiting additional efforts to engineer the PTM and PTN biosynthetic machinery in the native producers for further titer improvement and structural diversity [39, 40, 56].
To circumvent the difficulty encountered in engineering PMT and PTN biosynthesis in their native producers and shed light into the regulatory network controlling the metabolic pathway flux for PTN biosynthesis, the 41-kb ptn cluster was cloned from S. platensis MA7339 [40] and introduced into five Streptomyces hosts, i.e., S. coelicolor CH999, S. coelicolor M1146, S. coelicolor M1154, S. lividans K4–114, and S. albus J1074, however, resulting in no production of PTN in any of the heterologous hosts (Figure 4b) [38]. PTN production was only observed in S. lividans K4–114 upon inactivation of ptnR1. The resultant ΔptnR mutant strain SB12606 suffered from very low PTN titer of ~1 mg/L with the concomitant accumulation of larger quantities of biosynthetic intermediates and shunt metabolites, an observation reminiscent to the stalled biosynthesis of PTN in the ΔptnR mutant of S. platensis MA7339 (Figure 4c) [38]. While accumulated biosynthetic intermediates and shunt metabolites provided insight into the PTN biosynthetic pathway, they highlight how inactivation of pathway specific repressors may lead to pathway activation and natural product production but also could adversely disrupt metabolic pathway flux and balance, thereby stalling biosynthesis and accumulating side products and shunt metabolites instead of the target natural products. Comparison by RT-PCR of the transcription profiles of the two ΔptnR1 mutant strains, S. platensis SB12600 and S. lividans SB12606, which produces PTN with titers of 22 mg/L and 1 mg/L, respectively, revealed different gene expression profiles [38, 56]. The fact that removal of a repressor can lead to differences in transcription levels of each gene, in a host-specific manner, gives rise to another level of complexity that remains poorly understood currently.
Exploiting the evolutionary power of Nature for natural product titer improvement and structural diversity
Although there is a lack of knowledge on how biosynthetic gene clusters, regulatory genes, and hosts interact to enhance or silence production of natural products, there are ways in which we can overcome these challenges by exploiting Nature’s power of evolution. Nature has incorporated numerous gene clusters, into multiple strains, allowing for the identification of alternative producing strains to afford the target natural products, as well as evolutionarily optimized structural analogues, in higher titers. Genome mining of a diverse strain collection could facilitate the targeted discovery of novel scaffolds and analogues for natural product discovery and structural diversity. Comparative studies of gene clusters, encoding the biosynthesis of a family of natural products, sharing a common scaffold, promise to teach us how Nature does combinatorial biosynthesis at its best. Three examples, PTM, the enediyne natural products of C-1027, tiancimycin A (TNM A), and yangpumicin A (YPM A), and the leinamycin (LNM) family of natural products, are discussed herein to highlight how to exploit the evolutionary power of Nature for the discovery of alternative producers of known natural products, with the desired growth characteristics, genetic amenability, and higher titers, and new producers encoding the designer analogues of targeted natural product scaffolds.
Genome mining for genetically amenable alternative producers and engineering of PTM and PTN overproducers
Metabolic pathway engineering and combinatorial biosynthesis rely on the ability to genetically manipulate the producers and metabolite titers efficiently for isolation [36]. As exemplified by PTM and PTN, the native producers S. platensis MA7339 and S. platensis MA7327 suffered from the low titers, and PTM and PTN, as well as selected biosynthetic intermediates and congeners with titers as low as 3 μg/L, were isolated from fermentations up to 3,400 L [15, 45, 46]. The ΔptnR1 and ΔptmR1 mutant strains of S. platensis MA7339 and S. platensis MA7327 discussed above significantly improved PTM and PTN titers but suffered from poor sporulation, thereby limiting their genetic amenability for further metabolic pathway engineering efforts. To overcome these challenges, genome mining and strain prioritization by a high-throughput RTPCR method identified six alterative producers that were genetically amenable [16]. The six alternative producers, with varying growth characteristics, all contained the ptm gene cluster and were confirmed to be PTM and PTN dual producer that produced PTM and PTN in titers similar to the S. platensis MA7327 and S. platensis MA7339 strains [16]. Inactivation of the pathway specific repressor, ptmR1, in the six alternative producers led to significantly higher PTM and PTN titers (up to 310-fold over the wild-type strains), with three of the ΔptmR1 mutant strains exhibiting excellent sporulation.
The ability of Nature to produce a targeted natural product across multiple strains can be exploited, using genome mining, to identify alternative producers that are amenable to genetic manipulation, as exemplified by PTM and PTN. This now provides an excellent biotechnology platform to apply the metabolic pathway engineering and synthetic biology principles to the PTM and PTN biosynthetic machinery for further titer improvement and structural diversity. Indeed, upon medium and fermentation optimization, PTM titer by the ΔptmR1 mutant strain of S. sp. SB12026 was enhanced to ~1.6 g/L, allowing for the isolation of 45 g of PTM from a 60 L fermentation [34, 37]. Access to a genetically amenable producer for metabolic pathway engineering and combinatorial biosynthesis allowed the generation of additional mutant strains in the ΔptmR1 background, including the ΔptmB1, ΔptmB2, and ΔptmO4 double mutants, which together resulted in the production and isolation of 28 PTM and PTN congeners [9, 34]. This demonstrates how genome mining can be judicially applied to exploit Nature’s evolutionary power for alternative producers to overcome challenges related to natural product discovery and production. Moreover, identification of alternative producers that are genetically amenable allow for the engineered production of designer structural analogues within the targeted natural product scaffolds.
Genome mining for alternative C-1027 producers with high titers and the discovery of new enediyne natural products TNM A and YPM A
The enediyne family of natural products, the first structure of which was elucidated in 1985 [10], is best known for their unprecedented molecular architecture and phenomenal anticancer activity. As of 2015, 11 enediyne natural products had been discovered by the traditional natural product discovery program (i.e., in contrast to the contemporary genomic-based natural product discovery program) [24, 44]. Basic and translational studies of this class of natural products and development of them into clinic drugs have been impeded by the small number of producing strains, lack of genetic amenability of the producers, low titers, instability of the enediynes, or a combination thereof [35].
Genome mining for alternative producers and new producers, encoding the biosynthesis of new members of the enediyne family of natural products, provided an outstanding opportunity to overcome these pitfalls. Bioinformatics analysis of the known enediyne biosynthetic gene clusters revealed a minimum of five genes that were common among all 11 enediynes known to date. These five genes, E10/E/E5/E4/E3, encoded the so-called enediyne polyketide synthase (PKS) cassette, were used for strain prioritization and targeted discovery of new enediyne natural products [35, 53]. A high-throughput RT-PCR method was used to screen 3,400 actinomycetes for the enediyne PKS gene cassette, resulting in identification of 81 hits that could be further grouped into 28 clades on the basis of the sequence similarity of the enediyne PKS cassettes [53]. Upon examination of the 28 clades, it was realized that four producers grouped together with Streptomyces globisporus, the known producer of C-1027 (Figure 5a). Sequencing analysis of representative strains confirmed that they harbored the C-1027 biosynthetic gene clusters, and fermentation optimization of all four strains resulted in the production of C-1027 in titers of up to 67 mg/L of the C-1027 chromophore or 900 mg/L as the C-1027 chromoprotein complex, representing an 11-fold increase over the C-1027 titers from the original producer S. globisporus (Figure 5b) [54]. On the basis of the successful C-1027 titer improvement by manipulating regulation of C-1027 biosynthesis in S. globisporus [5, 6], applications of the similar strategies by manipulating the pathway-specific regulators sgcR and sgcR1 in these newly discovered alternative producers promise to further improve C-1027 titers. Since the C-1027 biosynthetic gene clusters all reside on extrachromosomal linear plasmids and possess high similarity among the alternative producers [27, 47, 54], comparative analysis of C-1027 biosynthesis and production among the alternative producers provides an outstanding platform to study the regulatory networks between the biosynthetic gene clusters and the genomic background of the hosts. This may shed light on the different regulation systems in each host and how these systems interact with the same biosynthetic gene cluster, thereby guiding further efforts in selecting heterologous hosts and optimizing medium and fermentation conditions for natural product discovery and production.
Fig. 5.
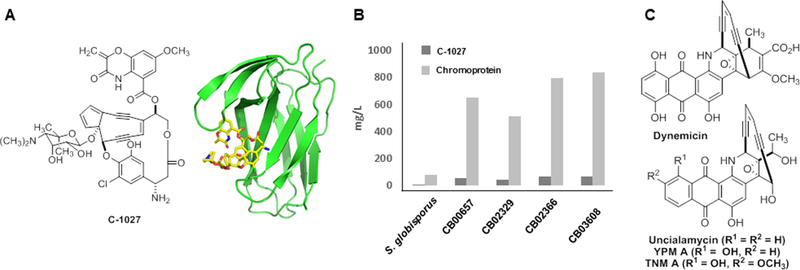
Identification of alternative C-1027 producers and new enediyne natural products. (A) Structures of C-1027 and the chromoprotein complex. (B) Four alternative C-1027 producers, CB00657, CB02329, CB02366, and CB03608, that produce C-1027 with significantly higher titers than the original C-1027 producer S. globisporus. (C) Discovery of the TNM A and YPM A expanding the family of the anthraquinone-fused enediynes which include dynemicin and uncialamycin.
Further analysis of the 28 clades also led to the identification of Streptomyces sp. CB03234, which was predicted to encode the production of a new 10-membered enediyne [53]. Genome sequencing of S. sp. CB03234 indeed unveiled a distinct enediyne biosynthetic gene cluster, and fermentation optimization of S. sp. CB03234 ultimately resulted in the discovery, production, isolation, and structural elucidation of TNM A, a new member of the anthraquinone-fused enediyne family (Figure 5c). Two members of the anthaquinone-fused enediynes are known, dynemicin, isolated from Micromonospora chersina [23] and uncialamycin isolated from Streptomyces uncialis [8]. Manipulation of anthraquinone-fused enediyne biosynthetic machinery, however, has been challenging due to the lack of an expedient genetic system, low titers, or inability to produce the enediyne by submerged fermentation [53]. In contrast, S. sp. CB03234 is genetically amenably, as exemplified by the ΔtnmH mutant strain that accumulated two new TNM congeners [53]. S. sp. CB03234 therefore provides an excellent biotechnology platform to study anthraquinone-fused enediyne biosynthesis and engineer the TNM biosynthetic machinery to access other members of the anthraquinone-fused enediyne family of natural products and generate designer analogues.
The enediyne PKS gene cassettes were applied to mine the microbial genomes available at Joint Genomics Institute (JGI) and NBCI for enediyne gene clusters, leading to the identification of Micromonospora yangpuensis DSM 45577 that harbored yet another distinct enediyne biosynthetic gene cluster predicted to encode a new member of the anthraquinone-fused enediynes [52]. Fermentation optimization of M. yangpuensis DSM 45577 resulted in the discovery, production, isolation, and structural elucidation of YPM A, thus further expanding this family of natural products and allowing for structure activity relationship (SAR) comparisons (Figure 5c). Taken together, these examples highlight the power of modern bioinformatics tools, which, together with the ever-increasing available genomic data and fundamental knowledge governing natural product biosynthesis, allow the exploitation of the diversity generated by Nature to discover alternative producers with favorable growth characteristics and new strains enabling the targeted discovery of natural products with a desired scaffold.
Genome mining for pathway-defining biosynthetic feature leading to the discovery of the LNM family of natural products
LNM, first discovered in 1989 from Streptomyces atroolivacceus S-140 [12, 14] is a hybrid peptide-polyketide natural product featuring an usual 1,3-dioxo-1,2-dithiolane moiety (Figure 6a) [17]. LNM has shown to be active against tumors that are resistant to cisplatin, cyclophosphamide, doxorubicin, and mitomycin [13]. Inspired by its unprecedented structural features, the potent antitumor activity, and the novel mode of action, the 61-kb lnm gene cluster, consisting of 27 genes, was identified and cloned from S. atroolivaceus S-140 and sequenced [7, 43]. Extensive genetic and biochemical characterizations of the LNM biosynthetic machinery led to the discovery of two domains, an unknown function (DUF) and a cysteine lyase (SH) within the last PKS module of LnmJ, which were responsible for sulfur incorporation into the 1,3-dioxo-1,2-dithiolane moiety of LNM [26, 43]. Strikingly, since the discovery of LNM nearly 30 years ago [11], no other member of the LNM family of natural products has been reported. The latter motivated us to exploit the diversity Nature may have already generated, by genome mining for pathway-defining features, to discover the LNM family of natural products.
Fig. 6.
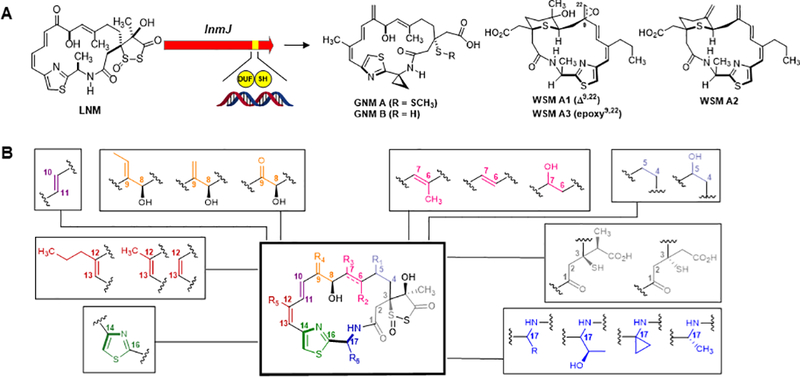
Nature as a combinatorial chemist for the LNM family of natural products. (A) Structure of LNM and the key LnmJ-DUF-SH didomain for sulfur incorporation, genome mining of which led to the discovery of GNM A, B and WSM A1, A2, and A3. (B) Combinatorial approach used by Nature to construct the LNM family of natural products.
The LnmJ-DUF-SH didomain was used to mine over 50,000 bacterial genomes available at JGI, NBCI, and The Scripps Research Institute for producers of the LNM family of natural products, resulting in the identification of 49 strains, all of which harbored a LNM-like biosynthetic gene cluster, featuring a LnmJ-DUF-SH didomain homologue (Figure 6a) [26, 31]. The 49 strains could be further prioritized based on the sequence similarity of the LnmJ-DUF-SH didomain homologues, affording 18 groups [31]. Fermentation optimization of representative strains from each of the 18 groups lead to the discovery, production, isolation, and structural elucidation of guangnanmycin (GNM) A and B from S. sp. CB01883 and weishanmycin (WSM) A1, A2, and A3 from of Streptomyces sp. CB01883, respectively [31]. The GNMs and WSMs are new members of the LNM family of natural products that apparently have been hidden in plain sight without being uncovered by traditional natural product discovery programs (Figure 6a) [31]. Comparative analysis of the LNM, GNM, WSM biosynthetic machineries, together with biochemical characterizations of substrate specificity of other representative LNM-like biosynthetic gene clusters from the 18 groups, enabled the mapping of the 18-membered hybrid peptide-polyketide macrolactam scaffolds for the LNM family of natural products, showcasing once again Nature as the best combinatorial biosynthetic chemist to generate natural product structural diversity. The modularity seen in Nature could certainly be exploited, where one could imagine mixing and matching the different modules from known LNM scaffolds to further expand the structural diversity of the LNM family of natural products (Figure 6b) [31].
Conclusion and future perspectives
Advances in DNA sequencing, bioinformatics, and metabolic engineering have tremendously impacted natural products chemistry. However, their full potential has not been realized due to the many challenges that remain within the field. The lack of knowledge governing the complex biology that regulates secondary metabolism has prevented metabolic engineering from reaching the predictability seen in other branches of engineering. This gap is highlighted in the three case studies provided here using FDM, iso-MGS, and PTM and PTN. These examples demonstrate that (i) regulators within the gene clusters and their interactions with the hosts play key roles in activation and transcription of the gene clusters, (ii) different heterologous hosts and their endogenous regulatory networks may have varying responses to a given biosynthetic gene cluster, hence yielding different levels of gene cluster activation and natural product production, and (iii) the regulatory hierarchy for a given cluster might be more complicated than currently appreciated, forced disruption or activation of which may led to staggering or stalling of the biosynthetic pathway, thereby accumulation of side products or shunt metabolites.
To overcome these challenges, we can look to Nature for inspiration. Advances in DNA sequencing and genome mining approaches have allowed for targeted discovery of natural products. Identification of alternative producers has afforded strains with high titers, addressing the practical supply of valuable natural products, as exemplified by C-1027, and strains with the desired growth characteristics, allowing the application of metabolic pathway engineering principle to improve titer and generate structural diversity, as exemplified by PTM and PTN. Comparative studies of the gene clusters from a given family of natural products have made it possible to exploit Nature’s biosynthetic potential to rapidly expand natural product structural diversity, as exemplified by enediynes. Genome mining for pathway-defining biosynthetic features has demonstrated the feasibility to discover natural products with targeted scaffolds, as exemplified by LNM. These newly identified strains could in turn provide the opportunities to learn from Nature how to optimize the cross-talks between the host and the gene cluster to achieve high titers and how to exploit modularity for combinatorial biosynthesis for natural product structural diversity. Bridging the knowledge gap between the interactions of biosynthetic gene clusters, regulatory genes, and hosts should greatly facilitate the discovery, production, and isolation of natural products and their congeners. Tapping into the evolutionary power of Nature will open new opportunities to further expand natural product structural diversity by metabolic pathway engineering, combinatorial biosynthesis, and synthetic biology. This would ultimately allow the development of a set of guiding principles for engineered production of natural products and their designers in genetically tractable and high producing chassis hosts.
Acknowledgements
Research on natural product discovery, biosynthesis, engineering and drug discovery in the Shen lab is currently supported by NIH grants CA106150, GM114353, GM115575, and the Natural Products Library Initiative at The Scripps Research institute. We thank past and current members of the Shen Lab for their dedication, creativity, and contributions to research on natural product discovery, biosynthesis, engineering, and genome mining, and Guohui Pan, Jeffery Rudolf, and Andrew Steele for their critical reading and valued inputs to this review. This is manuscript no. 29761 from The Scripps Research Institute.
References
- 1.Anton N, Mendes MV, Martin JF, Aparicio JF (2004) Identification of PimR as a positive regulator of pimaricin biosynthesis in Streptomyces natalensis. J Bacteriol 186:2567–2575. 10.1128/JB.186.9.2567-2575.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Byrne KM, Hilton BD, White RJ, Misra R, Pandey RC (1985) Biosynthesis of fredericamycin A, a new antitumor antibiotic. Biochemistry 24:478–486. 10.1021/bi00323a035 [DOI] [PubMed] [Google Scholar]
- 3.Casini A, Chang F-Y, Eluere R, King AM, Young EM, Dudley QM, Karim A, Pratt K, Bristol C, Forget A, Ghodasara A, Warden-Rothman R, Gan R, Cristofaro A, Borujeni AE, Ryu M-H, Li J, Kwon Y-C, Wang H, Tatsis E, Rodriguez-Lopez C, O’Connor S, Medema MH, Fischbach MA, Jewett MC, Voigt C, Gordon DB (2018) A pressure test to make 10 molecules in 90 days: external evaluation of methods to engineer biology. J Am Chem Soc 140:4302–4316. 10.1021/jacs.7b13292 [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Wendt-Pienkowski E, Shen B (2008) Identification and utility of FdmR1 as a Streptomyces antibiotic regulatory protein activator for fredericamycin production in Streptomyces griseus ATCC 49344 and heterologous hosts. J Bacteriol 190:5587–5596 10.1128/JB.00592-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Yin M, Horsman GP, Huang S, Shen B (2010) Manipulation of pathway regulation in Streptomyces globisporus for overproduction of the enediyne antitumor antibiotic C-1027. J Antibiot 63:482–485. 10.1038/ja.2010.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y, Yin M, Horsman GP, Shen B (2011) Improvement of the enediyne antitumor antibiotic C-1027 production by manipulating its biosynthetic pathway regulation in Streptomyces globisporus. J Nat Prod 74:420–424. 10.1021/np100825y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng Y-Q, Tang G-L, Shen B (2002) Identification and localization of the gene cluster encoding biosynthesis of the antitumor macrolactam leinamycin in Streptomyces atroolivaceus S-140. J Bacteriol 184:7013–7024. 10.1128/JB.184.24.70137024.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies J, Wang H, Taylor T, Warabi K, Huang X-H, Andersen RJ (2005) Uncialamycin, a new enediyne antibiotic. Org Lett 7:5233–5236. 10.1021/ol052081f [DOI] [PubMed] [Google Scholar]
- 9.Dong L-B, Rudolf JD, Shen B (2016) A mutasynthetic library of platensimycin and platencin analogues. Org Lett 18:4606–4609. 10.1021/acs.orglett.6b02248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edo K, Mizugaki M, Koide Y, Seto H, Furihata K, Otake N, Ishida N (1985) The structure of neocarzinostatin chromophore possessing a novel bicylco-[7,3,0]dodecadiyne system. Tetrahedron Lett 26:331–334. 10.1016/S0040-4039(01)80810-8 [DOI] [Google Scholar]
- 11.Feng Z, Wang L, Rajski SR, Xu Z, Coeffet-LeGal MF, Shen B (2009) Engineered production of iso-migrastatin in heterologous Streptomyces hosts. Bioorg Med Chem 17:2147–2153. 10.1016/j.bmc.2008.10.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hara M, Asano K, Kawamoto I, Takiguchi T, Katsumata S, Takahashi K, Nakano H (1989) Leinamycin, a new antitumor antibiotic form Streptomyces: producing organism, fermentation, and isolation J Antibiot 42:1768–1774. 10.7164/antibiotics.42.1768 [DOI] [PubMed] [Google Scholar]
- 13.Hara M, Saitoh Y, Nakano H (1990) DNA strand scission by the novel antitumor antibiotic leinamycin. Biochemistry 29:5676–5681. 10.1021/bi0047a005 [DOI] [PubMed] [Google Scholar]
- 14.Hara M Takahashi I, Yoshida M, Asano K, Kawamoto I, Morimoto M, Nakano H (1989) DC 107, a novel antitumor antibiotic produced by a Streptomyces sp. J Antibiot 42:333–335. 10.7164/antibiotics.42.33 [DOI] [PubMed] [Google Scholar]
- 15.Herath KB, Zhang C, Jayasuriya H, Ondeyka JG, Zink DL, Burgess B, Wang J, Singh SB (2008) Structure and semisynthesis of platensimide A produced by Streptomyces platensis. Org Lett 10:1699–1702. 10.1021/ol800251v [DOI] [PubMed] [Google Scholar]
- 16.Hindra, Huang T, Dong Y, Rudolf JD, Xie P, Xie G, Teng Q, Lohman JR, Zhu X, Huang Y, Zhao L-X, Jang Y, Duan Y, Shen B (2014) Strain prioritization for natural product discovery by a high-throughput real-time PCR method. J Nat Prod 77:2296–2303. 10.1021/np5006168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirayama N, Shimizu ME (1993) Molecular structure of a novel antitumor antibiotic leinamycin. Chem Lett 22:1957–1958. 10.1246/cl.1993.1957 [DOI] [Google Scholar]
- 18.Hochlowski JE, Whittern DN, Hill P, McAlpine JB, (1994) Dorrigocins: novel antifungal antibiotics that change the morphology or ras-transformed NIH/3T3 cells to that of normal cells II isolation and elucidation of structures. J Antibiot 47:870–874. 10.7164/antibiotics.47.870 [DOI] [PubMed] [Google Scholar]
- 19.Ju J, Lim S-K, Jiang H, Seo J-W, Her Y, Shen B (2006) Thermolysis of iso-migrastatin and its congeners via [3,3]-sigmatropic rearrangement: a new route to the synthesis of migrastatin and its analogues. Org Lett 8:5868–5868. 10.1021/ol062470p [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ju J, Lim S-K, Jiang H, Seo J-W, Shen B (2005) Iso-migrastatin congeners from Streptomyces platensis and generation of a glutarimide polyketide library featuring the dorrigocin, lactimidomycin, migrastatin, and nk30424 scaffolds. J Am Chem Soc 127:11930–11931. 10.1021/ja053118u [DOI] [PubMed] [Google Scholar]
- 21.Ju J, Lim S-K, Jiang H, Shen B (2005) Migrastatin and dorrigocins are shunt metabolites of iso-migrastatin. J Am Chem Soc 127:1622–1623. https://doi.org/10.1021ja043808i [DOI] [PubMed] [Google Scholar]
- 22.Karwowski JP, Jackson M, Sunga G, Sheldon P, Poddig JB, Kohl WL, Kadan S, (1994) Dorrigocins: novel antifungal antibiotics that change the morphology or ras-transformed NIH/3T3 cells to that of normal cells I taxonomy of the producing organism, fermentation and biological activity. J Antibiot 47:862–869. 10.7164/antibiotics.47.862 [DOI] [PubMed] [Google Scholar]
- 23.Konishi M, Ohkuma H, Matsumoto K, Tsuno T, Kamei H, Miyaki T, Oki T, Kawaguchi H, VanDuyne GD, Clardy J (1989) Dynemicin A, a novel antibiotic with the anthraquinone and 1,5-diyn-3-ene subunit. J Antibiot 42:1449–1452. 10.7164/antibiotics.42.1449 [DOI] [PubMed] [Google Scholar]
- 24.Liang Z-X (2010) Complexity and simplicity in the biosynthesis of enediyne natural products. Nat Prod Rep 27:499–528. 10.1039/B908165H [DOI] [PubMed] [Google Scholar]
- 25.Lim S-K, Ju J, Zazopoulos E, Jiang H, Seo J-W, Chen Y, Feng Z, Rajski SR, Farnet CM, Shen B (2009) iso-Migrastatin, migrastatin, and dorrigocin production in Streptomyces platensis NRRL18993 is governed by a single biosynthetic machinery featuring an acyltransferase-less type I polyketide synthase. J Biol Chem 284:29746–29756. 10.1074/jbc.M109.046805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma M, Lohman JR, Liu T, Shen B (2015) C-S bon cleavage by a polyketide synthase domain. Proc Natl Acad Sci USA 112:10359–10364. 10.1073/pnas.1508437112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medema MH, Trefzer A, Kovalchuk A, Berg MVD, Muller U, Heijne W, Wu L, Alam MT, Ronning CM, Nierman WC, Bovenberg RA, Breitling R, Takano R (2010) The sequence of a 1.8-Mb bacterial linear plasmid reveals a rich evolutionary reservoir of secondary metabolic pathways. Genome Biol Evol 2:212–224. 10.1093/gbe/evq013/570411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Misra R, Pandey RC, Silverton JV (1982) Fredericamycin A, an antitumor antibiotic of novel skeletal type. J Am Chem Soc 104:4478–4479. 10.1021/ja00380a025 [DOI] [PubMed] [Google Scholar]
- 29.Nakae K, Yoshimoto Y, Sawa T, Homma Y, Hamada M, Takeuchi T, Imoto M (2000) Migrastatin, a new inhibitor of tumor cell migration from Streptomyces s. MK929–43F1. Taxonomy, fermentation, isolation and biological activites. J Antibiot 53:1130–1136. 10.7164/antibiotics.53.1130 [DOI] [PubMed] [Google Scholar]
- 30.Newman DJ, Cragg GM (2016) Natural products as sources of new drugs from 1981 to 2014. J Nat Prod 79:629–661. 10.1021/acs.jnatprod.5b01055 [DOI] [PubMed] [Google Scholar]
- 31.Pan G, Xu Z, Guo Z, Hindra, Ma M, Yang D, Zhou H, Gansemans Y, Zhu X, Huang Y, Zhao L-X, Jiang Y, Cheng J, Van Nieuwerburgh F, Suh J-W, Duan Y, Shen B (2017) Discovery of the leinamycin family of natural products by mining actinobacterial genomes. Proc Natl Acad Sci USA 114:E11131–E11140. 10.1073/pnas.1716245115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pandey RC, Toussaint MW, Stroshane RM, Kalita CC, Aszalos AA, Garretson AL, Wei TT, Byrne KM, Geoghegan RF Jr (1981) Fredericamycin A, a new antitumor antibiotic I. production, isolation and physicochemical properties. J Antibiot 34:1389–1401. 10.7164/antibiotics.34.1389 [DOI] [PubMed] [Google Scholar]
- 33.Romero-Rodriguez A, Robledo-Casados I, Sanchez S (2015) An overview on transcriptional regulators in Streptomyces. Biochimica et Biophysica Acta 1849:1017–1039. 10.1016/j.bbagrm.2015.06.007 [DOI] [PubMed] [Google Scholar]
- 34.Rudolf JD, Dong L-B, Huang T, Shen B (2015) A genetically amenable platensimycin- and platencin-overproducer as a platform for biosynthetic explorations: a showcase of PtmO4. A long-chain acyl-CoA dehydrogenase. Mol Biosyst 11:2717–2726 10.1039/c5mb00303b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rudolf JD, Yan X, Shen B (2016) Genome neighborhood network reveals insights into enediyne biosynthesis and facilitates prediction and prioritization for discovery. J Ind Microbiol Biotechnol 43:261–276. 10.1007/s10295-015-1671-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen B (2015) A new golden age of natural products drug discovery. Cell 163:1297–1300. 10.1016/j.cell.2015.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi J, Pan J, Yang D, Lu S, Zhu X, Shen B, Duan Y, Huang Y (2016) Titer improvement and pilot-scale production of platensimycin from Streptomyces platensis SB12026. J Ind Microbiol Biotechnol 43:1027–1035 10.1007/s10295-016-1769-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smanski MJ, Casper J, Peterson RM, Yu Z, Rajski SR, Shen B (2012) Expression of the platencin biosynthetic gene cluster in heterologous hosts yielding new platencin congeners. J Nat Prod 75:2158–2167. 10.1021/np3005985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smanski MJ, Peterson RM, Rajski SR, Shen B (2009) Engineered Streptomyces platensis strains that overproduce antibiotics platensimycin and platencin. Antimicrob Agents Chemother 53:1299–1304. 10.1128/AAC.01358-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smanski MJ, Yu Z, Casper J, Lin S, Peterson RM, Chen Y, Wendt-Pienkowski E, Rajski SR, Shen B (2011) Dedicated ent-kaurene and ent-atiserene synthases for platensimycin and platencin biosynthesis. Proc Natl Acad Sci USA 108:13498–13503. 10.1073/pnas.1106919108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smanski MJ, Zhou H, Claesen J, Shen B, Fishbach MA, Voigt CA (2016) Synthetic biology to access and expand nature’s chemical diversity. Nat Rev Micobiol 14:135–149. 10.1038/nrmicro.2015.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stontag B, Muller JG, Hansske FG (2004) Fredericamycin B and C from Streptomyces griseus: structure elucidation after 23 years. J Antibiot 57:823–828. 10.7164/antibiotics.57.823 [DOI] [PubMed] [Google Scholar]
- 43.Tang G-L, Cheng Y-Q, Shen B (2004) Leinamycin biosynthesis revealing unprecedented architectural complexity for a hybrid polyketide synthase and nonribosomal peptide synthetase. Chem Biol 11:33–45. 10.1016/j.chembiol.2003.12.014 [DOI] [PubMed] [Google Scholar]
- 44.Van Lanen SG, Shen B (2008) Biosynthesis of enediyne antitumor antibiotics. Curr Top Med Chem 8:448–459. 10.2174/156802608783955656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang J, Kodali S, Lee SH, Galgoci A, Painter R, Dorso K, Racine F, Motyl M, Hernandez L, Tinney E, Colletti SL, Herath K, Cummings R, Salazar O, Gonzalez I, Basillo A, Vicente F, Genilloud O, Pelaez F, Jayasuriya H, Young K, Cully DF, Singh SB (2007) Discovery of platencin, a dual FabF and FabH inhibitor with in vivo antibiotic properties. Proc Natl Acad Sci USA 104:7612–7616. 10.1073/pnas.0700746104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang J, Soisson SM, Young K, Shoop W, Kodali S, Galgoci A, Painter R, Parthasarathy G, Tang YS, Cummings R, Ha S, Dorso K, Motyl M, Jayasuriya H, Ondeyka J, Herath K, Zhang C, Hernandez L, Allocco J, Basilio A, Tormo JR, Genilloud O, Vicente F, Pelaez F, Colwell L, Lee SH, Michael B, Felcetto T, Gill C, Silver LL, Hermes JD, Bartizal K, Barrett J, Schmatz D, Becker JW, Cully D, Singh SB (2006) Platensimycin is a selective FabF inhibitor with potent antibiotic properties. Nature 441:358–361. 10.1038/nature04784 [DOI] [PubMed] [Google Scholar]
- 47.Wang L, Wang S, He Q, Yu T, Li Q, Hong B (2012) Draft genome sequence of Streptomyces globisporus C-1027, which produces an antitumor antibiotic consisting of a nine-membered enediyne with a chromoprotein. J Bacteriol 194:4144 10.1128/jb.00797-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wendt-Pienkowski E, Huang Y, Zhang J, Li B, Jiang H, Kwon H, Hutchinson R, Shen B (2005) Cloning, sequencing, analysis, and heterologous expression of the fredericamycin biosynthetic gene cluster from Streptomyces griseus. J Am Chem Soc 127:16442–16452. 10.1021/ja054376u [DOI] [PubMed] [Google Scholar]
- 49.Wenzel SC, Müller R (2005) Recent developments towards the heterologous expression of complex bacterial natural product biosynthetic pathways. Curr Opin Biotechnol 16:594–606. 10.1016/j.copbio.2005.10.001 [DOI] [PubMed] [Google Scholar]
- 50.Woo EJ, Starks CM, Carney JR, Arslanin R, Cadapan L, Zavala S, Licari P (2002) Migrastatin and a new compound, isomigrastatin, from Streptomyces platensis. J Antibiot 55:141–146. 10.7164/antibiotics.55.141 [DOI] [PubMed] [Google Scholar]
- 51.Wu X, Yang D, Zhu X, Feng Z, Lv Z, Zhang Y, Shen B, Xu Z (2010) Iso-migrastatin titer improvement in the engineered Streptomyces lividans SB11002 strain by optimization of fermentation conditions. Biotechnol Bioprocess Eng 15:664–669. 10.1007/s12257-009-3129-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yan X, Chen J-J, Adhikari A, Yang D, Crnovic I, Wang N, Chang C-Y, Rader C, Shen B (2017) Genome mining of Micromonospora yangpuensis DSM 45577 as a producer of an anthraquinone-fused enediyne. Org Lett 19:6192–6196. 10.1021/acs.orglett.7b03120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yan X, Ge H, Huang T, Hindra, Yang D, Teng Q, Crnovcic I, Li, Rudolf JD, Lohman JR, Gansemans Y, Zhu X, Huang Y, Zhao L-X, Jiang Y, Van Nieuwerburgh F, Rader C, Duan Y, Shen B (2016) Strain prioritization and genome mining for enediyne natural products. mBio 7:e02104–16. 10.1128/mbio.02104-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yan X, Hindra, Ge H, Yang D, Huang T, Crnovcic I, Chang C-Y, Fang S-M, Annaval T, Zhu X, Huang Y, Zhao L-X, Jiang Y, Duan Y, Shen B (2018) Discovery of alternative producers of the enediyne antitumor antibiotic C-1027 with high titers. J Nat Prod 81:594–599. 10.1021/acs.jnatprod.7b01013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang D, Zhu X, Wu X, Fend Z, Huang L, Shen B, Xu Z (2011) Titer improvement of iso-migrastatin in selected heterologous Streptomyces hosts and related analysis of mRNA expression by quantitative RT-PCR. Appl Microbial Biotechnol 89:1709–1719. 10.1007/s00253-010-3025-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu Z, Smanski MJ, Peterson RM, Marchillo K, Andes D, Rajski SR, Shen B (2011) Engineering of Streptomyces platensis MA7339 for overproduction of platencin and congeners. Org Lett 12:1744–1747. 10.1021/ol100342m [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang B, Yang D, Yan Y, Pan G, Xiang W, Shen B (2016) Overproduction of lactimidomycin by cross-overexpression of genes encoding Streptomyces antibiotic regulatory proteins. Appl Microbiol Biotechnol 100:2267–2277. 10.1007/s00253-015-7119-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang H, Wang Y, Pfeifer BA (2008) Bacterial hosts for natural product production. Mol Pharm 5:212–225. 10.1021/mp70013 [DOI] [PubMed] [Google Scholar]


