Abstract
Hospital-physician integration has substantially grown in the US for the past decade, particularly in certain medical specialties, such as oncology. Yet evidence is scarce on the relation between integration and outpatient specialty care use and spending. We analyzed the impact of oncologist integration on outpatient provider-administered chemotherapy use and spending in Medicare, where prices do not depend on providers’ integration status or negotiating power. We addressed oncologists’ selective integration and patients’ non-random choice of oncologists using an instrumental variables method. We found that integrated oncologists reduced the quantity of outpatient chemotherapy drugs but used more expensive treatments. This led to an increase in chemotherapy drug spending after integration. These findings suggest that changes in treatment patterns – treatment mix and quantity – may be an important mechanism by which integration increases spending. We also found that integration increased spending on chemotherapy administration (the act of injection). This is because integration shifted billing of chemotherapy to hospital outpatient departments, where Medicare payments for chemotherapy administration are higher than those in physician offices. As integration increases, efforts should continue to assess how integration influences patient care and explore policy options to ensure desirable outcomes from integration.
Keywords: Integration, Provider-administered Chemotherapy, Medicare Payments, Outpatient care
I. Background
Ownership of medical practices in the United States has substantially changed over the past decade. Medical practices were traditionally owned by physicians and were financially independent from hospitals. However, they have been increasingly acquired by hospitals (referred to as integration hereafter). Twenty percent of physician practices were hospital-owned in 2002 (Kocher and Sahni, 2011); more than half of physician practices were owned by a hospital or a system in 2016 (Kane, 2017). The growth of integration is observed across the board, but it has been salient in certain specialties, such as oncology and cardiology (Nikpay et al., 2018). About 30 percent of oncology practices were owned by a hospital or a system in 2003, but the corresponding proportion in 2015 was 60 percent (Alpert et al., 2017).
Discussions of the impact of integration on health care delivery are ongoing. A literature review finds that theoretical predictions of integration impacts are inconclusive (Post et al., 2018). Some models predict that integration brings efficiency gains in care delivery and thus lower prices and spending. For example, close relationships among providers can enhance care coordination across settings; and shared information/decision-making leads to a reduction in duplicated services, low transactions costs, and efficient resource allocation. However, economic models focusing on providers’ strategic motives predict that integration increases providers’ market power and restricts market competition, leading to higher prices and spending. This prediction is supported by empirical studies of the relation between integration, service prices, and spending in the private sector (Baker et al., 2014; Carlin et al., 2015; Capps et al., 2018).
However, limited empirical evidence exists on the impact of integration on outpatient service use and spending in Medicare, where prices are administratively determined. A few studies found mixed effects of integration on Medicare inpatient use, measured by the number of procedures performed or length of stay (Madison, 2004; Scott et al., 2017), but none has analyzed the effect of integration on changes in outpatient service utilization.
While Medicare payments do not depend on providers’ market power, a concern has been raised that integration increases spending on outpatient care in Medicare by shifting the location of care from physician offices (Offices) to hospital outpatient departments (HOPDs) (MedPAC, 2012; MedPAC, 2013). Medicare allows practices acquired by hospitals to be re-classified as “off-campus” HOPDs. Thus, services performed by acquired physicians can be billed as HOPD care even when they are provided in Offices. Medicare payments for outpatient care are usually higher when the service is offered in HOPDs than in Offices. These Medicare policies create incentives for integrated providers to change their billing location and practice patterns after integration.
Recently, Koch et al. (2017) provided support for this concern. Analyzing data from 27 large practices acquired by hospital systems, they reported a 70 percent decrease in evaluation and management (E&M) claims in offices of the acquired practices and a 30 percent increase in E&M claims at the acquiring hospital outpatient departments by the acquired physicians, indicating shifts in billing location. However, with these separate estimates at the different levels and no assessment of changes in claims by the acquired physicians at non-acquiring hospitals, they could not obtain the net effect of integration on outpatient claims. Moreover, their findings may apply only to similar large practices. Analyzing net changes in utilization is important because it offers information on whether integration brings efficiency gains that mitigate the spending effects from the payment change.
We examine how oncologist integration influences use and spending for provider-administered outpatient chemotherapy services in Medicare. Despite the recent growth of integration in medical specialties, little is known about the relation between integration and outpatient care use for any specialty. Our study offers the first evidence of the integration impact on outpatient care use and spending for a particular medical specialty.
Oncology is an important medical specialty and a leader in the trend toward integration (Nikpay et al., 2018). We focus on provider-administered chemotherapy1 because chemotherapy is a common cancer treatment modality that is mainly administered by oncologists. Other services are often offered by multiple specialties including primary care providers, making it difficult for researchers to identify who is responsible for those services. By analyzing utilization of chemotherapy services, we assess whether spending changes after integration are driven by payment effects versus changes in utilization.
II. Potential Impacts of Integration
Medicare payments do not depend on providers’ negotiating power. However, Medicare’s site-specific payments, along with the designation of off-campus HOPDs,2 create ways for integration to affect Medicare outpatient care spending. We discuss below the specific Medicare payment policies for provider-administered outpatient chemotherapy (hereafter outpatient chemotherapy), and the potential integration impacts on outpatient chemotherapy use and spending in Medicare.
Medicare payments for outpatient chemotherapy services:
Many chemotherapy agents are available in injectable forms and are administered by providers in outpatient settings (HOPDs or Offices). Medicare Part B covers provider-administered drugs, paying separately for the drug3 and its administration (the act of injection). Medicare pays for the drug with a “buy-and-bill” system: providers purchase drugs and then submit claims to Medicare for reimbursement for the drugs. Medicare pays 106 percent4 of the drug’s Average Sales Price (ASP), which is the average price charged by the manufacturer after any rebates/discounts.
While Medicare’s payment for the drug is the same regardless of the location of service, the payment margin varies among providers because providers have different drug acquisition costs. Hospitals have lower drug acquisition costs than community-based practices because they negotiate prices through purchasing alliances and buy large quantities of drugs (Burns and Lee, 2008; Polite et al, 2015). The purchasing advantage of hospitals is greater for costlier drugs because manufacturers’ discounts usually depend on drug prices; and smaller practices face greater financial risk from providing costlier drugs due to potential failures to recover the acquisition costs (Polite et al., 2015; Koala, 2014).5
Medicare payments for chemotherapy administration differ by care site. When chemotherapy is performed in Offices, Medicare pays the physician based on the physician fee schedule; when performed in HOPDs, Medicare pays the hospital at the Outpatient Prospective Payment System (OPPS) rate.6 OPPS rates for chemotherapy administration are typically higher than the physician fee schedule, although the differences are smaller than for other Part B services (MedPAC, 2013). In 2011, the OPPS rate was $59 higher than the fee schedule for the most commonly used administration code, $12 higher for the second most common code, and $3 higher for the third most common code (The Moran Company, 2013).
Expectations about Integration Impacts:
The Medicare payment policies described above suggest that integration will affect Medicare spending on chemotherapy in three ways.
First, integration will increase spending on chemotherapy administration by shifting chemotherapy billing to HOPDs. When services performed in acquired physicians’ offices are billed as HOPD care after integration, they generate revenues to the acquiring hospital because Medicare payments exceed the cost of service provision in Offices. This creates incentives for integrated providers to select the higher-paid site, increasing spending on chemotherapy administration, when all else is equal.
Second, the buy-and-bill payment system gives providers incentives to change their practice patterns after integration, affecting drug spending. Integrated physicians can access and use drugs bought by the acquiring hospital. This is an opportunity for integrated doctors to offer some expensive drugs that they may have been unable to use when independent due to financial risk. Hospitals may also steer acquired physicians to prescribe costlier drugs, which have larger margins. Kalidindi et al., (2018) showed that Medicare patients visiting HOPDs received higher-cost chemotherapy drugs than those visiting Offices. This suggests that integration can change the treatment mix of chemotherapy to include more expensive drugs. This change will increase spending on chemotherapy drugs, when all else is equal.
Third, the payment effects discussed above can change the quantity of chemotherapy services, which in turn will influence spending. The direction of the quantity change is not clear. If integrated physicians respond to higher payments or payment margins in HOPDs, the quantity of chemotherapy services will increase. This supply response is likely if physicians arrange volume-based compensation with the acquiring hospital. However, this supply effect will be offset if the integrated physician changes his/her practice patterns through care coordination. Enhanced communication among providers may reduce duplicated services. If integration has this anticipated effect, the quantity of services can decline. In addition, integrated physicians may be less incentivized to increase service provision than before integration because they are no longer under financial pressure to run their own practices. Recently, Kalidindi et al. (2018) reported that patients receiving chemotherapy in HOPDs had fewer chemotherapy claims than those in Offices. Shared information systems may facilitate the spread of such practice styles at hospitals to acquired physicians (Post et al., 2017). Thus, the direction of the quantity effect depends on which of these possibilities dominates. It will be negative if integrated providers’ incentives to reduce service provision exceed the positive supply response.
The discussion above suggests that integration influences spending through several competing effects from payments and integrated providers’ other incentives. Thus, the net effect of integration on outpatient chemotherapy spending is an empirical question, and analyzing utilization – quantity and treatment mix – is critical to identify mechanisms through which integration affects spending.
III. Methods
A. Study Population and Data
The study population is a 10 percent random sample of Medicare fee-for-service (FFS) beneficiaries who had cancer and received Part B chemotherapy between 2009 and 2013. We received claims from these cancer patients from the Centers for Medicare and Medicaid Services (CMS). We required patients to have Part A and Part B coverage for 12 months in a given year.7 We identified patients who used Part B chemotherapy by the Healthcare Common Procedure Coding System Level II codes (J-codes) in claims. Appendix A1 describes details of the population selection process.
The primary data were Medicare Outpatient files, which include records of services in HOPDs, and Carrier files, which have claims for services by non-institutional providers. Outpatient and Carrier claims include information on diagnosis, service date and type, payments, and the provider’s National Provider Identifier (NPI). We supplemented the claims data with beneficiaries’ demographic and health-risk factors from Medicare Beneficiary Summary Files. The American Community Survey supplied ZIP-level income, education, and unemployment rates.
We obtained the information on oncologist integration from the 2009-2013 Quantile/IMS office-based physician data (known as “SK&A data”). The SK&A data identify the practice location and all physicians in each practice by name, NPI, and hospital ownership, listing the name of the owning hospital if the practice is hospital-owned. The SK&A data have been a key source of physician integration status in recent research (Koch et al., 2017; Wagner, 2016). Alpert et al. (2017) reported that the number and integration trends for oncologists in the SK&A data are consistent with those from the American Medical Association Physician Masterfile and CMS’s Physician Compare. Appendix A2 describes the SK&A data.
Most oncologists in our data were aligned with one practice. For oncologists in multiple practices, we selected only those in practices with the same integration status, and excluded those in practices with different integration status (<1% of the oncologists).
B. Outcomes
We measured five outcomes at the patient-year level. First, we constructed two quantity measures: 1) the frequency of chemotherapy drug claims; and 2) the frequency of chemotherapy administration claims. We then created two spending measures: 3) chemotherapy drug spending; and 4) chemotherapy administration spending. Finally, we created a “treatment mix” variable to examine whether integrated doctors shifted to more expensive drugs: 5) spending per chemotherapy drug claim.
We constructed the quantity variables accounting for duplicates in Carrier and Outpatient files (the same code on the same day) to avoid double counting.8 We obtained the spending variables as the sum of the allowed payments, which include Medicare reimbursements and patient out-of-pocket spending, across all claims of the patient in Carrier and Outpatient files.9 We adjusted the spending measures to 2013 dollars based on the Consumer Price Index for medical services.
C. Integration Variable
Our integration variable is an indicator for whether the patient’s oncologist is integrated with a hospital in a given year.10 This variable is constructed in three steps. First, we identified oncologists in the Medicare claims using NPIs. Second, we aligned the patient with the oncologist with whom she had the most outpatient cancer-related visits, defined as any outpatient visits with cancer diagnosis codes including E&M visits and lab tests.11 Third, we added the information on the integration status of the oncologist from the SK&A data. About 28.5 percent of oncologists in our data were integrated with a hospital in 2009, but 47.3 percent were integrated in 2013.
D. Analysis
We began by estimating a difference-in-differences (DD) model:
| (1) |
where Yijt is an outcome for beneficiary i seeing an oncologist j in year t. INTEGjt is an indicator of an integrated oncologist. Its coefficient (β) represents the integration effect as changes in outcomes between pre- and post-integration between oncologists who integrated during the study period and those who were independent. Jj is a vector of oncologist fixed effects, which control for all time-constant doctor-specific factors. YEARt is year fixed effects that apply to all observations. Xijt is a vector of time-varying patient characteristics: age, gender, race, dual eligibility, state buy-in status (an indicator of Medicaid paying the patient’s Part B premium), indicators of chronic conditions, cancer type, metastatic status, income, college education, and unemployment. The last three Xijt variables were measured at the ZIP level because they were unavailable in claims. εijt is an error term.
Instrumental Variables (IV) Analysis:
The DD model above accounts for selective integration by oncologists but its estimates are biased if patients selectively sort into integrated oncologists. For example, sicker patients may choose integrated doctors expecting high levels of referrals or practice patterns resembling hospital care. To address this possibility, we estimated an instrumental variable (IV) model with oncologist fixed effects. We followed Capps et al. (2018) and Wagner (2016) by limiting the sample to patients who saw a non-integrated oncologist in the first year. Then we instrumented the integration variable with the current integration status of the patient’s original oncologist – the oncologist in the first year when the patient was included in the sample.
This approach relies on the fact that cancer patients usually do not switch their doctors. Suppose a patient chooses an oncologist for reasons other than integration and stays with that oncologist. Then, she receives the “integration treatment” when the oncologist is integrated. This would mimic random assignment of patients to integrated oncologists. Our data supported inertia in oncologist choice: about 80 percent of patients who were assigned to an oncologist stayed with their original oncologist in the second year, 71 percent in the third year and 68 percent in the fifth year.
The instrument would perfectly predict integration if no patient changes an oncologist once she chooses a non-integrated oncologist in the first year. In reality, some patients do switch their oncologists. We use the current integration status of the original doctor regardless of switching. Thus, our approach is an “intent-to-treat” analysis. With relatively high inertia in the data, the instrument strongly predicted current integration: the F-statistic in the first-stage estimation was 3,861 (Appendix Table A1).
The validity of the instrument could be challenged if oncologists’ integration decisions were based on health shocks that affect their patients’ chemotherapy use and spending. No formal test of this possibility exists, but we think it is unlikely because integration is a costly and time-consuming process. Providers would not spend resources to integrate (and dis-integrate) on the basis of time-varying health shocks. The postulated health shocks also would have to be correlated across patients to make integration viable.
To further ensure the validity of our approach, we compared pre-integration trends in outcomes between oncologists who integrated and those who did not. Both descriptive and regression analyses showed similar pre-integration trends between those two groups (Appendix Figure A1 and Table A4).12
We estimated all models using linear regressions on the logarithms of outcomes, where inclusion of oncologist fixed effects is straightforward and the issue of outliers can be addressed. 13 Standard errors were clustered within practices.14
IV. Results
Table 1 presents descriptive data for selected variables (Appendix Table A2 reports data for all variables). We compared patient characteristics by the integration status of the patient’s current oncologist as well as her first-year oncologist. Integrated and non-integrated groups have similar demographic factors in a given year (left panel of Table 1). The share of patients with metastasis was higher in the integrated group than the non-integrated group (40.7 versus 32.1 percent). The share of patients with leukemia was also higher (9.8 versus 7.1 percent), but the share of patients with lung cancer was smaller in the integrated group than in the non-integrated group (16.8 versus 21.3 percent).
Table 1.
Descriptive data of selected study variables
| Current oncologist† | First-year oncologist ‡ | |||
|---|---|---|---|---|
| Non-integrated (N=77,159) |
Integrated (N=4,740) |
Remained non-integrated (N=49,124) |
Integrated in later years (N=3,216) |
|
| Mean (Standard Deviation) or % | Mean (Standard Deviation) or % | |||
| Variable | ||||
| Patient characteristics | ||||
| Age | 73.59(8.65) | 73.79(8.64) | 73.08(8.62) | 72.77(8.53) |
| Female (%) | 51.73% | 51.86% | 51.71% | 52.21% |
| White (%) | 87.57% | 87.85% | 87.43% | 88.06% |
| Dual eligibility (%) | 15.10% | 15.17% | 15.73% | 14.33% |
| Number of chronic conditions | 4.60(2.61) | 4.60(2.62) | 4.68(2.59) | 4.39(2.51) |
| Metastasis (%) | 32.13% | 40.70% | 30.29% | 33.05% |
| Cancer type: | ||||
| Breast (%) | 22.05% | 24.30% | 21.73% | 23.54% |
| Prostate (%) | 22.20% | 26.03% | 20.44% | 24.84% |
| Lung (%) | 21.27% | 16.79% | 24.29% | 16.73% |
| Colon (%) | 15.11% | 14.07% | 15.20% | 14.09% |
| Leukemia (%) | 7.08% | 9.77% | 6.76% | 10.07% |
| Lymphoma (%) | 19.26% | 20.19% | 17.77% | 21.05% |
| Measured at the ZIP level | ||||
| Median income($) | 57,042(23,181) | 58,206(23,310) | 56,521(22,876) | 57,897(23,653) |
| Percent college educated among 65 years and over | 21.10(13.30) | 22.01(14.01) | 20.76(13.19) | 21.15(13.70) |
Note:
Comparison of patient characteristics by the integration status of the patient’s oncologist in a given year;
Comparison of patient characteristics only in the first year when the patient was included in the sample based on the whether the patient’s first-year oncologist integrated with a hospital in later years.
The right panel of Table 1 compares data by the first-year oncologist’s integration status. We examined patient characteristics only in the first year when the patient was included in the sample (i.e., when all oncologists were non-integrated). The share of patients with metastasis in the first year was about 30 percent in the never-integrated group and 33 percent among oncologists who integrated in later years. The distribution of cancer types shows a similar pattern to the left panel.
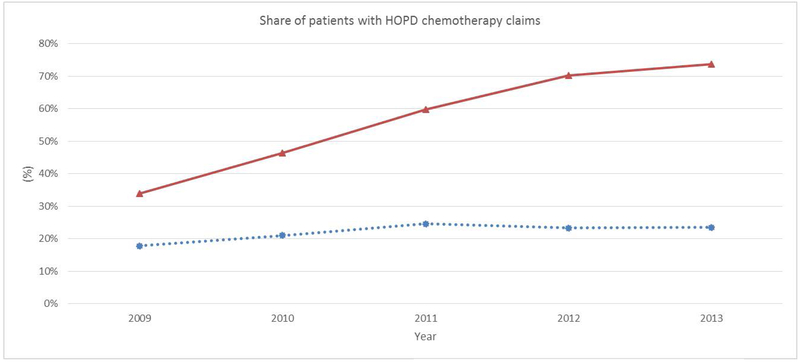
Figure 1 depicts the trend in chemotherapy treatments billed as HOPD care. The share of patients with HOPD chemotherapy bills ranged from 19 to 22 percent among oncologists who remained independent between 2009 and 2013.15 This share was 34 percent in 2009 among oncologists who became integrated in later years, but it jumped to 72 percent in 2013. This is consistent with expectations from Medicare’s site-specific payments: as more doctors are integrated, more chemotherapy treatments are billed as HOPD care.
Figure 1.
Share of patients with chemotherapy bills as HOPD services
Note:  Oncologists who became integrated during the study period;
Oncologists who became integrated during the study period;  Oncologists who remained independent during the study period.
Oncologists who remained independent during the study period.
Table 2 describes outcomes by integration status. We obtained data for patients of integrated oncologists separately before and after integration. Prior to integration, these patients had fewer chemotherapy services but a high-cost treatment mix compared with patients of never-integrated oncologists. This difference suggests that oncologists’ integration is selective, which we address with oncologist fixed effects. Within the integrated group, the quantities of chemotherapy services decreased after integration while treatment mix increased. These changes may represent integration impacts or patients’ non-random choice of integrated oncologists, which we address by the IV approach.16
Table 2.
Descriptive data of outcomes by integration status
| Mean (Standard Deviation) | |||
|---|---|---|---|
| Non-integrated oncologists† | Integrated oncologists ‡ | ||
| Outcome | (N=73,184) | Before integration (N=3,569) |
After integration (N=4,527) |
| Utilization | |||
| Frequency of chemotherapy drug claims | 11.82(11.68) | 10.20(10.72) | 9.15(9.49) |
| Frequency of chemotherapy administration claims | 15.64(16.79) | 13.56(15.47) | 12.30(13.71) |
| Spending | |||
| Chemotherapy drug spending ($) | 18,559(25,225) | 20,047(25,865) | 19,159(26,687) |
| Chemotherapy administration spending ($) | 1,904(2,091) | 1,976(2,568) | 2,037(2,840) |
| Treatment mix | |||
| Chemotherapy drug spending per claim ($) | 1,800(2,484) | 2,167(2,614) | 2,516(3,705) |
Note:
This group consists of patients who were always treated by a non-integrated oncologist during the entire study period;
This group consists of patients who saw an integrated oncologist sometime during the study period (after seeing a non-integrated oncologist in their first year).
Table 3 reports the regression estimates of integration effects. The DD estimates on the quantity of chemotherapy services were smaller and less precise than the IV estimates. Given the negative integration effect on quantity, the bias toward the null in the DD estimates implies that patients using more chemotherapy drugs sorted into integrated oncologists. The DD estimate was also smaller for treatment mix, where the integration effect was positive. This implies patients using less expensive drugs selected integrated oncologists. These findings suggest that patients with a specific risk requiring more drugs but less expensive drugs chose integrated oncologists. We explored risk types presenting this pattern and found that patients with metastasis had more drug claims than those without (14.5 versus 9.7) but lower treatment mix ($1,680 versus $1,890) (data not reported in the table). Patients whose unobserved risk involved treatment profiles similar to metastasis may have self-selected into the integrated group, leading to a bias in the DD estimates.
Table 3.
Estimates of the integration effect
| N=81,899 | Difference-in-differences (DD) Analysis |
Instrumental Variables (IV) Analysis |
|---|---|---|
| Outcome | Estimate (Robust Standard Error) | Estimate (Robust Standard Error) |
| Quantity | ||
| Log(Frequency of chemotherapy drug claims) | −0.04(0.02)** | −0.07(0.03)*** |
| Log(Frequency of chemotherapy administration claims) | −0.03(0.02)* | −0.06(0.03)** |
| Spending ($) | ||
| Log(Chemotherapy drug spending) | 0.15(0.03)*** | 0.18(0.05)*** |
| Log(Chemotherapy administration spending) | 0.09(0.03)*** | 0.08(0.04)* |
| Treatment mix ($) | ||
| Log(Chemotherapy drug spending per claim) | 0.19(0.03)*** | 0.25(0.05)*** |
| First-Stage Statistics | ||
| Estimate on instrument (Robust Standard Error) | 0.71(0.01)*** | |
| F-Statistic | 3,861 |
Notes: Oncologist fixed effects are included in all models; Covariates controlled for in all models are year dummies, patient demographic characteristics, chronic condition indicators, cancer type, metastasis indicator, and ZIP-level socio-economic variables; Standard errors account for clustering within practices; Spending measures are adjusted to 2013 dollars; The estimates are the coefficients on the integration indicator;
p < 0.10,
p < 0.05,
p < 0.01.
The IV results are thus our preferred estimates. Integration decreased the quantity of drugs by 6.8 percent, but it led to an approximately 28.4 percent increase in spending on chemotherapy drugs per claim. Total drug spending per user increased by 19.7 percent, driven by the change in treatment mix (note that payments for drugs are not site-specific). These findings indicate that integrated providers switched to more expensive drugs from less expensive drugs, instead of adding more drugs
Integration decreased the quantity of chemotherapy administration by 5.9 percent. But it increased administration spending by 8.3 percent, although the effect was marginally significant (p<0.1). This corresponds to an about $160 increase per user, which is within a range that we expect from the payment difference between HOPDs and Offices. Between 2009 and 2011, the average payment for commonly used chemotherapy administration codes was about $15-$20 higher in HOPDs than in Offices, and each patient in integrated practices had 11 administration claims.
V. Exploration of the Treatment Mix Effect
We performed several analyses to explore the treatment mix effect we found above. First, we analyzed the models adjusting for drug dosage17 to examine how much of the treatment mix effect was due to dosage changes. We found that an increase in drug dosage explained a very small part of the treatment mix effect (Table 4): treatment mix increased by 28.4 percent without dosage adjustment, versus a 25.8 percent increase after dosage adjustment.18
Table 4.
Exploring treatment mix effect
| Instrumental Variables (IV) Analysis | ||||
|---|---|---|---|---|
| Dependent Variable | Estimate (Robust Standard Error) | |||
| Dosage adjustment | ||||
| Log (Frequency of dosage-adjusted drug claims) | −0.06(0.03)** | |||
| Log (Drug spending per dosage-adjusted claim) | 0.23(0.04)*** | |||
| Changes in drug composition among lung cancer patients | ||||
| Use of high-cost drugs | 0.14(0.05)*** | |||
| By practice size | Size <= 2 | 2 < Size <= 6 | Size > 6 | |
| Log(Chemotherapy drug spending per claim) | 0.35(0.11)*** | 0.27(0.08)*** | 0.19(0.14)† | |
| By type of acquiring hospitals | Teaching | Non-teaching | 340B | Non-340B |
| Log(Chemotherapy drug spending per claim) | 0.27(0.07)*** | 0.33(0.08)*** | 0.32(0.07)*** | 0.28(0.07)*** |
Notes: Oncologist fixed effects are included; Covariates controlled for in all models are year dummies, patient demographic characteristics, chronic condition indicators, cancer type, metastasis indicator, and ZIP-level socio-economic variables; Standard errors account for clustering within practices; Spending measures are adjusted to 2013 dollars;
p=0.1
p < 0.10,
p < 0.05,
p < 0.01.
Second, we tested whether use of more expensive drugs increased after integration. Following Jacobson et al. (2006), we identified substitutable lung cancer treatments. Those drugs had different reimbursement amounts with the average payment per claim ranging from $14 to $1,703. The distribution of those drugs among lung cancer patients shifted toward more expensive drugs after integration (Appendix Table A7). To test this, we created an indicator for using an expensive drug (the average per-claim payment > $100) among these substitutable drugs. The regression using this indicator as the dependent variable showed that the probability of using an expensive drug increased by 14 percentage points after integration (Table 4).
Third, we compared the composition of drugs used by leukemia patients before and after integration. We found that expensive drugs moved up in the frequency ranks (Appendix Table A8). We also found that integrated oncologists started using some high-cost drugs they did not use before integration (Appendix Table A9).
Fourth, we estimated the model separately by practice size. We found larger treatment mix effects among oncologists in smaller practices (Table 4). The effect was largest in very small practices, where using expensive drugs may have been most financially risky. This supports our expectation that integrated oncologists use more expensive drugs with higher payment margins because they can access those drugs through the integrating hospitals.
Fifth, we examined differences in the treatment effect by type of integrating hospitals. Teaching hospitals may have different treatment patterns than non-teaching hospitals due to new knowledge acquired from clinical trials at their sites. Hospitals participating in the federal 340B program, which mandates manufactures to give deep discounts for outpatient drugs, may have different prescribing patterns than non-340B hospitals due to lower drug acquisition costs and larger payment margins (Health Policy Brief, 2014; Jung et al., 2018). We stratified the sample by the type of hospital that the current oncologist was integrated with and constructed an IV as the initial oncologist’s current integration with the same type of hospitals. We found a 30.9 percent increase in treatment mix for oncologists integrated with teaching hospitals and a 39.1 percent increase for those with non-teaching hospitals (Table 4). We did not expect this finding, but it suggests that non-teaching hospitals may have treatment patterns that favor high-cost drugs. The treatment mix effect was larger for doctors integrated with 340B hospitals than those with non-340B hospitals (37.7 versus 32.3 percent), which is consistent with our expectation (Table 4).
Finally, we explored implications of the treatment mix effect for patient care. Ideally, one would examine whether oncologists prescribe more appropriate or better quality chemotherapy drugs after integration. This analysis would require detailed clinical information, such as cancer stage, which is not included in claims. We thus used a health outcome measure, patient mortality, and found that integration increased patient mortality by 4 percentage points (a 20 percent increase in average mortality) (Table 5).19 This finding represents the overall integration effect on patient health because mortality is affected by all cancer care given to patients. While increased mortality is unlikely to be a direct consequence of using more expensive chemotherapy drugs, it is discouraging because mortality is an important outcome that we intend to improve through cancer care. Prior research showed mixed effects of integration on patient outcomes (Madison, 2004; Wagner, 2016). More research is needed to assess consequences of oncologist integration on patient health outcomes.
Table 5.
Analysis of Mortality
| Instrumental Variables (IV) analysis | |
|---|---|
| Outcome | Estimate (Robust Standard Errors) |
| Mortality† | 0.04(0.01)*** |
| First-Stage Statistics | |
| Estimate on instrument (Robust Standard Error) | 0.70(0.01)*** |
| F-Statistic | 4,070 |
| N | 101,622 |
Notes: Oncologist fixed effects are included. Covariates controlled for are year dummies, patient demographic characteristics, chronic condition indicators, cancer type, metastasis indicator, and ZIP-level socio-economic variables; Standard errors account for clustering by practice;
The average mortality in the study population was about 20%,. Thus, the estimated effect – 4 percentage point change – corresponds to an about 4% change in the average mortality;
p < 0.10,
p < 0.05,
p < 0.01;
VI. Discussion
We analyzed the impact of oncologists’ integration on outpatient chemotherapy use and spending in Medicare. We summarize three main findings. First, integration decreased the frequency of chemotherapy service use. Second, integration increased spending on chemotherapy drugs through changes in treatment mix. Third, spending on chemotherapy administration increased after integration due to different Medicare payments by site.
Previous studies focused on prices as the main mechanism by which integration increases spending. The results consistently show that integration raises prices (Carlin et al., 2017; Capps et al., 2018; Baker et al., 2014). Our analysis offers evidence of another potentially important mechanism – changes in treatment mix and quantity. Changes in treatment patterns after integration are not surprising because integrated physicians have different financial incentives and organizational structure. In fact, changing providers’ practice patterns is an anticipated outcome of integration, yet limited information exists on whether or what changes occur. Focusing on chemotherapy services, we document that integrated oncologists use fewer services but more expensive treatments.
We lacked the clinical information (e.g., cancer stage) needed to assess whether integrated providers reduced only inappropriate chemotherapy or whether the increased treatment mix included more appropriate and better chemotherapy. However, the finding of increased patient mortality suggests that some changes in chemotherapy practice patterns after integration did not improve patients’ health or maintain quality. Unfortunately, we could not examine whether changes in chemotherapy services contributed to mortality or what types of services contributed the most to mortality. Future studies of use and spending on other cancer-related services can help draw a complete picture of integration impacts on patient care.
In addition, it is important for future research to identify incentives for integrated providers to change their practice patterns – particularly treatment mix. Prior work showed that employed physicians tended to perform intensive inpatient procedures, probably because they had a stake in their hospitals’ financial stability (Madison, 2004). Similarly, we expected that acquired physicians would have financial incentives to prescribe costlier drugs under the Medicare payment policy that gives better payment margins to hospitals. If this is a dominant explanation for the treatment mix change, it is a concern because such incentives exist for other expensive outpatient services, such as discretionary imaging services, and those incentives weaken potential efficiency gains from integration or coordinated care (Colla et al., 2014).
We found that integration had a modest impact on chemotherapy administration spending. Our estimate is within the range of prior work on the difference in administration spending between patients receiving chemotherapy in HOPDs and Offices (Kalidindi et al., 2018). The impact on chemotherapy administration spending is modest because the difference in administration payments by site is relatively small. Integration might have large spending impacts for services with larger payment differences between the sites, such as cardiac imaging (Song et al., 2015). Future research should extend our analysis to those services to fully assess the effect of the Medicare payment policy on spending after integration.
To address the payment inequality between the sites, Medicare recently reduced payments for outpatient services offered in “new” off-campus HOPDs (practices acquired after November 2015) to 50 percent of OPPS rates plus the non-facility physician fee schedule (CMS, 2016). However, half of the practices in the nation were already owned by a hospital by 2015. Applying the new payment reduction to practices acquired before 2015 would be a policy option to mitigate the spending impact of integration.
Further, public release of the list of office-based practices owned by each hospital could help patients make informed choices of providers for outpatient care. Patients visiting “off-campus” HOPDs do not usually know those practices are hospital-owned until they get a hospital outpatient bill. This leads to increased patient out-of-pocket spending, particularly for cancer care where more than half of spending goes to outpatient care (American Cancer Society, 2018). As more and more patients use HOPDs with the growth of integration, efforts are needed to realize desirable outcomes from integration. Increased consumer information about which clinics are designated as HOPDs could help with that aim.
Our study has several limitations. First, we could explore only outcomes that can be constructed from claims. We could not assess whether use of more expensive drugs implies more appropriate treatments, and how that change directly benefited patients. However, our study offers evidence that treatment mix change is an important mechanism (other than price) by which integration increases care spending. Second, we did not analyze the impact of integration on overall cancer care spending because that requires information on the integration status of all primary and specialty care physicians involved in caring for cancer patients.
Third, we did not analyze the effect of integration on the probability of using chemotherapy among cancer patients because detailed clinical information would be required to identify patients who are at risk of receiving chemotherapy. These are important topics for future research given our finding of increased deaths after integration.
Fourth, we did not analyze oral cancer drugs covered by Medicare Part D because physicians do not generate profit or revenue from prescribing oral agents. Jung et al. (2017) reported that Part B and Part D cancer drugs are not substitutes. However, substitution by integrated doctors would imply that the integration effect in our analysis is underestimated.
Fifth, we could not distinguish between integrated doctors changing their billing location only and integrated doctors sending patients to an on-campus HOPD. Thus, we could not determine whether the findings were driven by a specific type of integration or by both types.
Finally, our findings may not be generalizable to other specialty services, more recent or longer-term effects, and practices acquired after 2015. Future research should examine how the Medicare payment reduction for new off-campus HOPDs mitigates spending increases after integration.
In summary, we showed that integration increased chemotherapy spending by shifting prescriptions to more expensive drugs and by Medicare’s location-specific administration payments. As integration increases, efforts should continue to assess how integration influences patient care and explore policy options to ensure desirable outcomes from integration.
Acknowledgements:
This work is supported by NIH/NIA grant number 1R01AG047934-01, and NIH grant number R24 HD041025. No conflicts of interest exist.
Appendix A1. Study Sample Selection
The study population is a random sample of Medicare beneficiaries who had cancer and received Part B chemotherapy services between 2009 and 2013. We created the sample in three steps. First, the Centers for Medicare and Medicaid Services (CMS) identified all patients with cancer from 100% of Medicare claims based on the standard algorithm it uses to create cancer indicators in the Chronic Condition Warehouse files: having ≥ 1 inpatient or skilled nursing facility claim with a cancer diagnosis or ≥ 2 outpatient claims of cancer in a given year. Two outpatient claims are required to confirm a cancer diagnosis through a follow-up visit. Second, CMS randomly selected about a half million unique cancer patients, and we received the data from that random sample. Our data contained about 10% of patients across cancer types. Third, we identified patients who used Part B chemotherapy using the Healthcare Common Procedure Coding System (HCPCS) Level II codes (J-codes) in Medicare claims (All J codes used for the study are listed below). We selected claims with both a cancer diagnosis and a cancer drug J-code to exclude patients using cancer drugs for other conditions. We excluded enrollees in Medicare Advantage plans (because their claims data are not available to researchers) and those who did not have Part A and Part B coverage for the full year.
Chemotherapy J-codes used in the study: J9000-J9999, J8521, J8560, J8520, and J8530
Appendix A2. SK&A data
Quantile/IMS office-based physician data (known as “SK&A data”) supplied information on oncologist integration. Quantile/IMS surveys more than 90 percent of office-based physician practices in US by telephone every six months, and archives the data each December. We obtained SK&A data for physicians with a specialty in oncology/hematology, radiation oncology, or gynecologic oncology.
The SK&A data do not have information on hospital-based physicians, which account for 20% of all physicians in the nation. This does not affect our analysis because our focus is on integration between hospitals and office based physician practices. About 15% of the beneficiaries in our data were assigned to an oncologist whose record is not included in SK&A data probably because she/he is a hospital-employed physician.
When a practice is integrated, the SK&A data indicate the name of the acquiring hospital. A practice with no hospital name is “not integrated.” The SK&A data showed that in general, once a practice integrated with a hospital, it remained integrated in the following years. But 11 percent of the practices in the SK&A data were integrated (identified by a hospital name) in a given year but not in any following year(s). Following Wagner (2017) and Capps et al. (2018), we coded those once-integrated practices as integrated in the following years.
Appendix A3. Creating the teaching and 340B hospital indicators
We manually matched the SK&A hospital list with the hospital list released by the Centers for Medicare and Medicaid Services (CMS) by name to assign a Medicare ID to each hospital in the SK&A data. The CMS hospital list is available at https://data.medicare.gov/Hospital-Compare/Hospital-General-Information/xubh-q36u/data. The CMS hospital data contain Medicare Provider ID, hospital name, location, and hospital ownership. We were able to assign a Medicare provider ID to more than 85% of the hospitals in the SK&A data. About one-quarter of the hospitals that could not be matched with the CMS data were Veterans Administration hospitals.
To identify 340B hospitals, we used information available from the database maintained by the Health Resources and Services Administration (HRSA) Office of Pharmacy Affairs. This database includes Medicare provider IDs and 340B hospital indicators. For teaching status, we used the Open Payments List of Teaching Hospitals maintained by the CMS. This list also includes a Medicare Provider ID, by which we assigned the teaching hospital indicator to the hospitals in the SKA data acquiring oncologist practices.
Appendix Table A1.
Results from the first stage of the IV analysis
| All Study Sample (N=81,899) | |
|---|---|
| Variable | Regression estimates (robust SE†) |
| Current integration status of patient’s original oncologist (Instrument) |
0.712(0.011)*** |
| Age | 0.000(0.000) |
| Female | −0.002(0.001) |
| White | 0.000(0.002) |
| Having diabetes | 0.001(0.001) |
| Having hypertension | −0.001(0.001) |
| Having ischemic heart disease | 0.000(0.001) |
| Having hyperlipidemia | −0.000(0.001) |
| Having depression | 0.001(0.001) |
| Having congestive heart failure | −0.001(0.001) |
| Having cataract | 0.002(0.001)* |
| Having COPD‡ | 0.002(0.001)* |
| Number of chronic conditions | −0.000(0.000) |
| Indicator of metastasis | 0.003(0.001)** |
| Breast cancer | 0.001(0.002) |
| Prostate cancer | −0.006(0.002)*** |
| Lung cancer | −0.002(0.002) |
| Colon cancer | 0.003(0.002)* |
| Leukemia | 0.005(0.002)*** |
| Lymphoma | 0.001(0.002) |
| Other cancers | −0.002(0.002) |
| Dual Eligible | −0.000(0.001) |
| Percent Unemployed | −0.000(0.000) |
| Median income | −0.000(0.000)** |
| Percent with bachelor's degree - 65 years and over | 0.000(0.000) |
| Size of practice | 0.000(0.000) |
| 2010 | 0.003(0.001)*** |
| 2011 | −0.003(0.001)*** |
| 2012 | 0.030(0.001)*** |
| 2013 | 0.052(0.003)*** |
| F Statistic of the instrument =3,861 |
Notes: Oncologist fixed effects are included in the model;
Standard errors; Standard errors account for clustering by practice;
Chronic obstructive pulmonary disease;
p < 0.10,
p < 0.05,
p < 0.01.
Appendix Table A2.
Descriptive data for all study variables
| Current oncologist† | First-year oncologist ‡ | |||
|---|---|---|---|---|
| Non-integrated (N=77,159) |
Integrated (N=4,740) |
Remained non-integrated (N=49,124) |
Integrated in later years (N=3,216) |
|
| Mean (Standard Deviation) or % | Mean (Standard Deviation) or % | |||
| Variable | ||||
| Patient characteristics | ||||
| Age | 73.59(8.65) | 73.79(8.64) | 73.08(8.62) | 72.77(8.53) |
| Female (%) | 51.73% | 51.86% | 51.71% | 52.21% |
| White (%) | 87.57% | 87.85% | 87.43% | 88.06% |
| Having diabetes (%) | 15.10% | 15.17% | 15.73% | 14.33% |
| Having hypertension (%) | 30.44% | 30.72% | 30.91% | 29.63% |
| Having ischemic heart disease (%) | 68.23% | 66.22% | 69.98% | 67.26% |
| Having hyperlipidemia (%) | 39.61% | 38.92% | 39.99% | 37.25% |
| Having depression (%) | 50.69% | 50.53% | 52.24% | 52.64% |
| Having congestive heart failure (%) | 16.75% | 19.60% | 17.35% | 17.07% |
| Having cataract (%) | 20.28% | 19.28% | 20.30% | 16.48% |
| Having COPD‡ (%) | 21.73% | 23.71% | 20.98% | 22.48% |
| Number of chronic conditions | 23.36% | 20.57% | 25.74% | 19.34% |
| Metastasis (%) | 4.60(2.61) | 4.60(2.62) | 4.68(2.59) | 4.39(2.51) |
| Cancer type | ||||
| Breast (%) | 32.13% | 40.70% | 30.29% | 33.05% |
| Prostate (%) | 22.05% | 24.30% | 21.73% | 23.54% |
| Lung (%) | 22.20% | 26.03% | 20.44% | 24.84% |
| Colon (%) | 21.27% | 16.79% | 24.29% | 16.73% |
| Leukemia (%) | 15.11% | 14.07% | 15.20% | 14.09% |
| Lymphoma (%) | 7.08% | 9.77% | 6.76% | 10.07% |
| Measured at the Zip level | ||||
| Percent Unemployed | 8.88(4.06) | 9.08(4.25) | 8.85(4.08) | 8.69(4.13) |
| Median income($) | 57,042(23,181) | 58,206(23,310) | 56,521(22,876) | 57,897(23,653) |
| Percent college educated among 65 years and over | 21.10(13.30) | 22.01(14.01) | 20.76(13.19) | 21.15(13.70) |
| Chemotherapy outcomes | ||||
| Frequency of chemotherapy drug claims | 11.73(11.63) | 9.14(9.51) | 11.48(10.95) | 9.39(9.77) |
| Frequency of chemotherapy administration claims | 15.52(16.72) | 12.24(13.63) | 14.97(15.69) | 12.32(13.78) |
| Chemotherapy drug spending ($) | 18,626(25,256) | 19,228(26,729) | 16,413(23,168) | 18,313(26,705) |
| Chemotherapy administration spending ($) | 1,906(2,118) | 2,030(2,843) | 1,871(2,021) | 1,884(2,486) |
| Chemotherapy drug spending per claim ($) | 1,819(2,489) | 2,507(3,655) | 1,613(2,208) | 2,325(6,265) |
| Dosage-adjusted chemotherapy outcomes | ||||
| Frequency of dosage-adjusted chemotherapy drug claims | 12.0(15.32) | 10.97(48.48) | 12.13(14.41) | 10.79(15.45) |
| Chemotherapy drug spending per dosage-adjusted claim ($) | 1,964(8,898) | 2,685(6,034) | 1,783(9,948) | 2,313(4,677) |
Note:
Comparison of patient characteristics by the integration status of the patient’s oncologist in a given year;
Comparison of patient characteristics only in the first year when the patient was included in the sample based on the whether the patient’s first-year oncologist integrated with a hospital in later years.
Appendix Table A3.
Results from the sensitivity checks
| Instrumental Variables (IV) analysis | ||
|---|---|---|
| Estimate (Robust Standard Error) | ||
| Outcomes | % of visits integrated oncologists† |
Including patients who died during the year‡ |
| Utilization | ||
| Log(Frequency of chemotherapy drug claims) | −0.08(0.03)*** | −0.07(0.03)** |
| Log (Frequency of chemotherapy administration claims) | −0.06(0.03)** | −0.06(0.03)** |
| Spending ($) | ||
| Log Chemotherapy drug spending) | 0.19(0.06)*** | 0.19(0.05)*** |
| Log(Chemotherapy administration spending) | 0.09(0.05)* | 0.07(0.04)* |
| Treatment mix | ||
| Log(Chemotherapy drug spending per claim) | 0.27(0.05)*** | 0.26(0.04)*** |
| First-Stage Statistics | ||
| Estimate on instrument (Robust Standard Error) | 0.66(0.01)*** | 0.70(0.01)*** |
| F-Statistic | 3,993 | 4,067 |
| N | 81,899 | 101,622 |
Notes: Oncologist fixed effects are included. Covariates controlled for in all models are patient demographic characteristics, chronic condition indicators, cancer type, metastasis indicator, and ZIP-level socio-economic variables; Spending measures are adjusted to 2013 dollars; Standard errors account for clustering by practice in all models;
Integration is measured by the share of total visits to an integrated oncologist;
This sample is different from the primary analysis, which requires the patients to have full-year enrollment in both Part A and Part B;
p < 0.10,
p < 0.05,
p < 0.01.
Appendix Figure A1. Pre-integration trends in outcomes
Note:  Oncologists who were never integrated;
Oncologists who were never integrated;  Pre-integration among oncologists who integrated during the study period
Pre-integration among oncologists who integrated during the study period
Appendix A4.
Results on select variables from analyses testing pre-integration trends in outcomes
| Estimate (Robust Standard Errors) | |||
|---|---|---|---|
| Outcome | Integrated group*year10† | Integrated group*year11† | Integrated group*year12† |
| Quantity | |||
| Log(Frequency of chemotherapy drug claims) | −0.02(0.03) | −0.03(0.03) | −0.07(0.04) |
| Log(Frequency of chemotherapy administration claims)‡ | −0.02(0.03) | −0.04(0.03) | −0.12(0.05)** |
| Spending ($) | |||
| Log(Chemotherapy drug spending) | −0.03(0.06) | 0.02(0.05) | 0.00(0.08) |
| Log(Chemotherapy administration spending) | 0.03(0.05) | 0.06(0.05) | −0.01(0.07) |
| Treatment mix | |||
| Log(Chemotherapy drug spending per claim) | −0.00(0.05) | 0.05(0.05) | 0.08(0.07) |
Notes:
These variables are the interaction terms between the integrated group indicator and year dummy. The integrated group includes oncologists who became integrated during the study period. Years include only pre-integration periods;
The interaction between the integration group and year 2012 indicators was significant for this analysis. This appears to be due to a deviation in the trend in the non-integrated group in 2012: the number of administration claims in this group was 2.36 from 2009 through 2012, 2.38 in 2012, and 2.32 in 2013. In the integration group, it was 2.29 in 2009, 2.23 in 2010, 2.22 in 2011, and 2.21 in 2012 (pre-integration years). It dropped to 2.11 in 2013 when all doctors in this group were integrated.
Appendix A5.
Comparison of results between using original and log-transformed outcomes
| Estimate (Robust Error) | ||||
|---|---|---|---|---|
| Difference-in-differences (DD) Analysis | Instrumental Variables (IV) analysis | |||
| Outcome | Original outcomes | Log outcomes | Original outcomes | Log outcomes |
| Quantity | ||||
| Frequency of chemotherapy drug claims | −0.33(0.18)* | −0.04(0.02)** | −0.65(0.29)** | −0.07(0.03)*** |
| Frequency chemotherapy administration claims | −0.47(0.26)* | −0.03(0.02)* | −0.74(0.35)** | −0.06(0.03)** |
| Spending ($) | ||||
| Chemotherapy drug spending | 1,224.51(469.19)** | 0.15(0.05)*** | 1,258.08(777.49) | 0.18(0.05)*** |
| Chemotherapy administration spending | 235.91(47.69)*** | 0.09(0.03)*** | 227.30(59.59)*** | 0.08(0.04)* |
| Treatment mix | ||||
| Chemotherapy drug spending per claim | 295.90(78.73)*** | 0.19(0.03)*** | 387.30(136.67)*** | 0.25(0.05)*** |
Notes: Oncology fixed effects are included in all models; Covariates controlled for in all models are year dummies, patient demographic characteristics, chronic condition indicators, cancer type, metastasis indicator, and ZIP-level socio-economic variables; Standard errors account for clustering within practices; Spending measures are adjusted to 2013 dollars;
p < 0.10,
p < 0.05,
p < 0.01.
Appendix A6.
Results from the sensitivity analysis with clustering by county
| Instrumental Variables (IV) analysis | |
|---|---|
| Outcome | Estimate (Robust Standard Error) |
| Utilization | |
| Log(Frequency of chemotherapy drug claims) | −0.07(0.03)* |
| Log(Frequency of chemotherapy administration claims) | −0.06(0.02)** |
| Spending ($) | |
| Log(Chemotherapy drug spending) | 0.18(0.05)*** |
| Log(Chemotherapy administration spending) | 0.08(0.04)** |
| Treatment mix | |
| Log(Chemotherapy drug spending per claim) | 0.25(0.04)*** |
| First-Stage Statistics | |
| Estimate on instrument (Robust Standard Error) | 0.71(0.01)*** |
| F-Statistic | 5,442.43 |
| N | 81,899 |
Notes: Oncologist fixed effects are included in all models. Covariates controlled for in all models are year dummies, patient demographic characteristics, chronic condition indicators, cancer type, metastasis indicator, and ZIP-level socio-economic variables; Spending measures are adjusted to 2013 dollars;
p < 0.10,
p < 0.05,
p < 0.01.
Appendix Table A7.
Composition of substitutable lung cancer drugs†
| Lung cancer treatments |
Average per-claim payment ($) |
Before integration | After integration |
|---|---|---|---|
| Nab-paclitaxel | $1,704 | 0.90% | 4.26% |
| Gemcitabine | $385 | 8.78% | 14.45% |
| Docetaxel | $119 | 9.01% | 12.90% |
| Carboplatin | $26 | 59.68% | 43.10% |
| Etoposide | $14 | 15.32% | 10.45% |
| Paclitaxel | $32 | 26.58% | 21.29% |
Notes:
The distribution of the drugs among lung cancer patients.
Appendix Table A8.
Distribution of drugs used to treat leukemia†
| Rank by frequency |
Before integration | After integration | ||
|---|---|---|---|---|
| HCPCS_CD‡ | Average payment ($) | HCPCS_CD‡ | Average payment ($) | |
| 1 | J9310 | $4,453 | J9310 | $4,453 |
| 2 | J9025 | $883 | J9025 | $883 |
| 3 | J9033 | $2,797 | J9033 | $2,797 |
| 4 | J9185 | $114 | J9185 | $114 |
| 5 | J9070 | $167 | J9070 | $167 |
| 6 | J9017 | $522 | J9010 | $1,648 |
| 7 | J9370 | $4 | J9017 | $522 |
| 8 | J9217 | $742 | J9302 | $7,628 |
| 9 | J9209 | $69 | J9370 | $4 |
| 10 | J9302 | $7,628 | J9041 | $1,282 |
| 11 | J9208 | $85 | J9217 | $742 |
| 12 | J9181 | $4 | J9201 | $583 |
| 13 | J9355 | $2,891 | J9250 | $0 |
| 14 | J9999 | $3,216 | J9000 | $10 |
| 15 | J9000 | $10 | J9035 | $6,079 |
| 16 | J9045 | $18 | J9181 | $4 |
| 17 | J9091 | $44 | J9268 | $1,050 |
| 18 | J9201 | $583 | J9045 | $18 |
| 19 | J9390 | $21 | J9100 | $10 |
| 20 | J9035 | $6,079 | J9190 | $15 |
Notes:
The distribution of the drugs (ranked by frequency) among leukemia patients;
HCPCS_CD: Healthcare Common Procedure Coding System (HCPCS) Level II (J-code) for a chemotherapy agent.
Appendix Table A9.
Drugs that oncologists started using after integration
| Drugs used by leukemia patients | Drugs used by lung cancer patients | ||||
|---|---|---|---|---|---|
| HCPCS_CD† | Average payment ($) |
Approval year | HCPCS_CD† | Average payment ($) |
Approval year |
| J9041 | $1,282 | 2003 | J9025 | $630 | 2002 |
| J9190 | $15 | 1962 | J9185 | $78 | 1991 |
| J9263 | $272 | 2002 | J9041 | $1,477 | 2003 |
| J9264 | $1,895 | 2005 | J9350 | $2,088 | 1996 |
| J9305 | $4,254 | 2004 | J9033 | $3,204 | 2008 |
| J9098 | $2,664 | 1999 | J9202 | $433 | 1989 |
| J9293 | $136 | 2000 | J9280 | $150 | 1998 |
| J9155 | $689 | 2008 | J9207 | $5,872 | 2007 |
| J9266 | $5,895 | 1994 | J9002 | $2,137 | 1995 |
Note:
HCPCS_CD: Healthcare Common Procedure Coding System (HCPCS) Level II (J-code)
Appendix A10.
Results from IV analyses testing outliers in dosage-adjusted claims
| IV Estimate (Robust Standard Error) | ||||
|---|---|---|---|---|
| Including all sample | Excluding top and bottom 0.1 percentiles | |||
| Original outcomes | Log outcomes | Original outcomes | Log outcomes | |
| Dosage adjustment | ||||
| Frequency of dosage-adjusted drug claims | −0.02(0.90) | −0.06(0.03)** | −0.63(0.34)* | −0.06(0.03)** |
| Drug spending per dosage-adjusted claim | 378.41(288.80) | 0.23(0.04)*** | 424.65(106.37)*** | 0.24(0.05)*** |
Notes: Oncologist fixed effects are included in all models; Covariates controlled for in all models are year dummies patient demographic characteristics, chronic condition indicators, cancer type, metastasis indicator, and ZIP-level socio-economic variables; Standard errors account for clustering within practices; Spending measures are adjusted to 2013 dollars;
p < 0.10,
p < 0.05,
p < 0.01.
Footnotes
We use the term chemotherapy to include all anti-cancer drugs: immuno-, hormonal, and target therapy.
Medicare recently lowered payments for services provided at “new” off-campus HOPDs (practices acquired after November 2015). We analyzed data prior to 2015.
OPPS payments for drugs that cost less than $80 (in 2013) are bundled into the payment for administration. Most chemotherapy agents cost more than $80, so they are separately payable when administered in HOPDs.
The 2013 budget sequestration reduced payments received by providers to 104.3 percent of ASP (MedPAC, 2016).
Polite et al. (2015; page 4) comment that “Many small- to medium-size practices recognize that they cannot risk providing new, expensive therapeutics in the office. An entire clinic can be jeopardized by a failure to be wholly or partially reimbursed in a reasonable time frame for a given dose or cycle of an expensive drug.” Koala (2014) introduces an oncologist’s quote on overhead costs of running an independent oncology practice: “If a patient gets too sick to receive a drug or dies, the doctor takes the loss.”
Most other Part B services generate two bills in HOPDs: one for the hospital by OPPS; and another for the physician service by the physician fee schedule.
This requirement is standard for studying service use/spending because it ensures all services used by patients are measured. It removes patients who died in a given year and thus had partial-year coverage. We constructed a separate population including those who died. In this population, we estimated models that included a death indicator as a covariate to account for partial-year coverage. This analysis produced very similar results to the primary analysis (Appendix Table A3). The separate population also was used in the mortality analysis in Section V.
Chemotherapy generates either an Outpatient or Carrier claim (MedPAC, 2017). Thus, duplicates are a minor issue. Less than 4 percent of chemotherapy claims in our data were present in both files.
It is not necessary to identify same-service claims for the spending variables aggregated at the patient-year level because the Outpatient claim records the hospital facility fee and the Carrier claims records the physician fee.
We found similar results when we measured integration by the share of total visits to integrated oncologists (Appendix Table A3).
About 60 percent visited only one oncologist, 22 percent visited two oncologists, and 9 percent visited three oncologists. This suggests limited “fragmentation” in care for cancer patients. We used patients with at least three visits to an oncologist to identify regular oncology patients.
The interaction between the integrated group and year 2012 indicators in the analysis of administration frequency was significant, possibly due to a deviation in the trend in the non-integrated group in 2012 (Appendix Table A4).
We performed regressions with the original outcomes (without log-transformation) and found consistent results (Appendix Table A5). For log outcomes, we calculated percent changes in outcomes as (eβ – 1) X100% where β is the coefficient of interest.
We found similar results in a model with clustering at the county level to account for potential market-wide integration impacts (Appendix Table A6).
The non-integrated group includes these records with HOPD bills. Our analysis thus estimates the impact of integration status – not the impact of service provision/billing in HOPDs.
The number of administration claims in all groups is higher than the number of drug claims because an administration code is often for up to one hour of chemotherapy infusion. A separate administration code exists for an additional hour of infusion. Thus, more than one administration claim can be submitted for one drug claim.
We obtained the average dosage for each chemotherapy drug across all the claims for the drug from the entire sample. We then divided each drug claim’s dosage and spending by the average dosage of the same drug.
The standard deviations of the dosage-adjusted variables were much larger than those without dosage adjustment (Appendix Table A2). This suggests the presence of outliers. We compared the results from the analyses with and without observations in the top and bottom 0.1 percentiles. We found similar results from the models of log-transformed outcomes (Appendix Table A10).
This analysis includes those who died in a given year. This is different from the primary sample, which required 12-month Part A/B coverage (Section III.A and footnote 7).
Contributor Information
Jeah Jung, Department of Health Policy and Administration, The Pennsylvania State University, 604 Ford Building, University Park, PA 16802, USA, Phone: 814-863-8129, Fax: 814-863-2905, kuj11@psu.edu
Roger Feldman, Division of Health Policy and Management, University of Minnesota, 420 Delaware Street SE, Minneapolis, MN 55455, USA feldm002@umn.edu
Yamini Kalidindi, Department of Health Policy and Administration, The Pennsylvania State University, 604 Ford Building, University Park, PA 16802, USA, ypk100@psu.edu
References
- Alpert A, Hsi H & Jacobson M (2017). Evaluating the role of payment policy in driving vertical integration in the oncology market. Health Affairs, 36(4), 680–688. [DOI] [PubMed] [Google Scholar]
- American Cancer Society. (2018). Economic impact of cancer. Retrieved from https://www.cancer.org/cancer/cancer-basics/economic-impact-of-cancer.html
- Baker LC, Bundorf MK & Kessler DP (2014). Vertical integration: Hospital ownership of physician practices is associated with higher prices and spending. Health Affairs, 33(5), 756–763. [DOI] [PubMed] [Google Scholar]
- Burns LR, & Lee JA (2008). Hospital purchasing alliances: Utilization, services, and performance. Health Care Management Review, 33(3), 203–215 [DOI] [PubMed] [Google Scholar]
- Capps C, Dranove D & Ody C (2018). The effect of hospital acquisitions of physician practices on prices and spending. Journal of Health Economics, 59, 139–152 [DOI] [PubMed] [Google Scholar]
- Carlin C, Feldman R, and Dowd B (2017). The impact of provider consolidation on physician prices. Health Economics, 26(12), 1789–1806 [DOI] [PubMed] [Google Scholar]
- Centers for Medicare and Medicaid Services. (2016, November 1). CMS finalizes hospital outpatient prospective payment changes for 2017. Retrieved from https://www.cms.gov/newsroom/fact-sheets/cms-finalizes-hospital-outpatient-prospective-payment-changes-2017
- Colla CH, Goodney PP, Lewis VA, Nallamothu BK, Gottlieb DJ, and Meara ER (2014). Implementation of a pilot ACO payment model and the use of discretionary and non-discretionary cardiovascular care. Circulation, 130(22), 1954–1961. doi: 10.1161/CIRCULATIONAHA.114.011470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Policy Brief. (2014). “The 340B Drug Discount Program” Health Affairs. Retrieved from https://www.healthaffairs.org/do/10.1377/hpb20141117.14335/full/healthpolicybrief_130.pdf [Google Scholar]
- Jacobson M, Earle CC, Price M & Newhouse JP (2010). Patterns of Treatment How Medicare’s Payment Cuts For Cancer Chemotherapy Drugs Changed. Health Affairs. 29:7. 1391–1399 [DOI] [PubMed] [Google Scholar]
- Jung J, Feldman R, & McBean M (2017) “The price elasticity of specialty drug use: Evidence from Medicare Part D enrollees with cancer” Forum for Health Economics and Policy, 20(2). pii: 20160007. doi: 10.1515/fhep-2016-0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J, Xu W, & Kalidindi Y (2018) “The Impact of the 340B drug pricing program on care site and spending in Medicare” Health Services Research, 53(5), 3528–3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalidindi Y, Jung J & Feldman R (2018). Differences in spending on provider-administered chemotherapy by site of care in Medicare. American Journal of Managed Care, 24(7), 328–333. [PMC free article] [PubMed] [Google Scholar]
- Kane C (2017). Updated data on physician practice arrangements. American Medical Association Policy Research Perspectives. Retrieved from https://www.ama-assn.org/sites/default/files/media-browser/public/health-policy/PRP-2016-physician-benchmark-survey.pdf [Google Scholar]
- Koch TG, Wendling BW, & Wilson NE (2017). How vertical integration affects the quantity and cost of care for Medicare beneficiaries. Journal of Health Economics, 52, 19–32. [DOI] [PubMed] [Google Scholar]
- Kocher R, & Sahni NR (2011). Hospitals’ race to employ physicians: The logic behind a money-losing proposition. New England Journal of Medicine, 364, 1790–1793. doi: 10.1056/NEJMp1101959 [DOI] [PubMed] [Google Scholar]
- Kolata G (2014, November 3). Private oncologists being forced out, leaving patients to face higher bills. The New York Times. Retrieved from https://www.nytimes.com/2014/11/24/health/private-oncologists-being-forced-out-leaving-patients-to-face-higher-bills.html [Google Scholar]
- Madison K (2004). Hospital-Physician affiliations and patient treatments, expenditures, and outcomes. Health Services Research, 39(2), 257–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medicare Payment Advisory Commission. (2012). Report to Congress: Medicare payment policy. Washington DC. [Google Scholar]
- Medicare Payment Advisory Commission. (2013). Report to Congress: Medicare and health care delivery system. Washington DC. [Google Scholar]
- Medicare Payment Advisory Commission. (2016). Report to Congress: Medicare part B drug and oncology payment policy issues. Washington DC. [Google Scholar]
- Medicare Payment Advisory Commission. (2017). Report to Congress: Medicare and health care delivery system. Washington DC. [Google Scholar]
- Nikpay SS, Richards MR & Penson D (2018). Hospital-physician consolidation accelerated in the past decade in cardiology, oncology. Health Affairs, 37(7), 1123–1127. [DOI] [PubMed] [Google Scholar]
- Polite B, Conti R & Ward J (2015). Reform of the buy-and-bill system for outpatient chemotherapy care is inevitable: Perspectives from an economist, a realpolitik, and an oncologist. American Society of Clinical Oncology Education Book, e75–e80. doi: 10.14694/EdBook_AM.2015.35.e75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post B, Buchmueller T & Ryan A (2018). Vertical integration of hospitals and physicians: Economic theory and empirical evidence on spending and quality. Medical Care Research and Review, 75(4), 399–433. [DOI] [PubMed] [Google Scholar]
- Scott KW, Orav J, Culter DM, & Jha A (2017). Changes in hospital–physician affiliations in u.s. hospitals and their effect on quality of care. Annals of Internal Medicine, 166(1), 1–8. doi: 10.7326/M16-0125. [DOI] [PubMed] [Google Scholar]
- Song Z, Wallace J, Neprash HT, McKellar MR, Chernew ME, & McWilliams JM (2015). Medicare fee-cuts and cardiologist-hospital integration, 175(7), 1229–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Moran Company. (2013). Cost differences in cancer care across settings. Retrieved from https://www.communityoncology.org/UserFiles/Moran_Cost_Site_Differences_Study_P2.pdf
- Wagner A (2016). Effect of physician-hospital financial integration on health outcomes and spending (Job Market Paper). Retrieved from https://cpb-us-e1.wpmucdn.com/sites.northwestern.edu/dist/0/1428/files/2016/10/WagnerAR_JobPaper_161113-26qkl9f.pdf