Abstract
Background:
Alcohol and marijuana use expectancies are presumed to be drug-specific, but prospective study of this assumption is lacking. In addition, these associations may operate differently for adults with ADHD histories, as expectancies have been found to be less associated with alcohol and marijuana use among this population.
Objectives:
The first aim of the present study was to investigate whether associations between alcohol and marijuana expectancies and substance use were specific to the substances they assess. The second aim was to determine whether these associations differed as a function of ADHD history.
Method:
Participants (N = 491; 281 ADHD, 210 nonADHD) were young adults followed longitudinally between ages 21–23 and 29 as part of the Pittsburgh ADHD Longitudinal Study (PALS). Autoregressive models were estimated separately for positive and negative expectancies for frequency of alcohol and marijuana use and compared between ADHD groups.
Results:
Although there were exceptions, results generally support the specificity of associations between outcome expectancies and respective substance use both concurrently and prospectively, but this specificity was primarily present for those without a history of ADHD.
Conclusions/Importance:
These findings suggest that young adults perceive and respond distinctly to the effects of alcohol and marijuana, but a history of ADHD may interfere with this process. These findings also extend our prior cross-sectional findings that expectancies are less associated with alcohol and marijuana use for individuals with ADHD histories. Additional research examining implicit cognitions is needed to further examine risk for substance use among those with ADHD histories.
Keywords: ADHD, alcohol expectancies, alcohol use, marijuana expectancies, marijuana use
Attention-deficit/hyperactivity disorder (ADHD) is one of the most common behavioral health diagnoses of childhood and consists of symptoms of inattention, impulsivity, hyperactivity, and impairment in multiple domains that continue into adolescence and adulthood (American Psychiatric Association, 2013; Barkley, Murphy, & Fischer, 2008; Hechtman et al., 2016). Among these impairments, children with ADHD are at increased risk for problematic alcohol and marijuana use outcomes in adulthood compared to peers without a childhood diagnosis of ADHD (Charach et al., 2011; Groenman et al., 2017; Lee et al., 2011; Molina et al., 2007; 2018).
Substance use expectancies are cognitions that refer to an individual’s beliefs about the positive and negative effects that occur from using a substance (Donovan et al., 2009; Jones et al., 2001; Wardell & Read, 2013). Higher positive substance use expectancies are associated with increased substance use and include beliefs such as enhanced sociability or tension reduction (Dunn & Goldman, 1998; 2000). Higher negative substance use expectancies, such as cognitive and behavioral impairment, are associated with decreased substance use. However, these associations are often weaker than those for positive expectancies. Although substance use expectancies have consistently been established as an influence on substance use, almost no research has examined whether expectancies contribute to substance use more generally as opposed to the specific substance they purport to assess. In addition, it is unclear whether expectancies have the same prospective associations with substance use for all individuals. Specifically, substance use expectancies may operate differently among those with heightened impulsivity and cognitive deficits, such as young adults with a childhood diagnosis of ADHD. The purpose of the current study was to prospectively examine the specificity of substance use expectancies to the substances they directly assess and determine whether these associations differed due to ADHD history among a sample of young adults.
Specificity of Substance Use Expectancies
Co-use of alcohol and marijuana is common among young adults (Barrett, Darredeau, & Pihl, 2006; Collins, Ellickson, & Bell, 1998; Pape, Rossow, & Strovoll, 2009; Yurasek, Aston, & Matrik, 2017). Approximately 11% of drinkers report also using marijuana in the past year (Subbaraman & Kerr, 2015), and almost half of young adults who report marijuana use in the past thirty days report simultaneous alcohol use (Haas et al., 2015). Use of alcohol and marijuana within the same period of time is not only associated with an increased frequency and quantity of substance use but also increases the likelihood of experiencing consequences related to substance use (Hayaki, Anderson, & Stein, 2016; Subbaraman & Kerr, 2015). As such, it is important to understand the factors that may influence use of both substances.
Previous studies of substance use expectancies have tended to examine individual substances in isolation. For example, studies have utilized separate measures of expectancies for alcohol and marijuana and examined the associations between expectancies and use separately for each substance (Anderson et al., 2014; D’Amico et al., 2015; Stoddard & Pierce, 2016; Tucker et al., 2014). Substance use expectancies are assumed to operate specific to the substance they assess (e.g., alcohol expectancies relate only to alcohol use and marijuana expectancies relate only to marijuana use) due to the distinct pharmacologic effects that various substances exert (e.g., sedation and stimulation for alcohol; euphoria and anxiety or anti-anxiety for marijuana; Ashton, 2001; Bushman & Cooper, 1990; Levenson et al., 1980; Williams et al., 1981). There is some evidence that expectancies may contribute to substances other than what they directly measure (McCarthy & Thompsen, 2006), which may partly reflect generalized activation of reward circuitry by all drugs or be associated with a higher frequency of the co-use of the substances examined (Di Chiara & Bassareo, 2007). However, McCarthy and Thompsen (2006), the only study of which we are aware that examined specificity of expectancy-substance use associations, measured expectancies only in relation to alcohol and smoking. Examination of the specificity of substance use expectancies is lacking for alcohol and marijuana use, some of the most common forms of substance use, including co-use, among young adults, in the current literature. Establishing the specificity, or lack thereof, of the associations between substance use expectancies and these frequently used substances may provide important information about why these substances are often used by the same people and can increase understanding about common and unique cognitions across substances.
ADHD and Substance Use Expectancies
Recent research has indicated that substance use expectancies do not always operate equivalently for individuals with, and without, ADHD diagnoses or symptoms. Specifically, research on adolescents and young adults with a history of diagnosed childhood ADHD found that measures of substance use expectancies were less associated with the substances they assess for the ADHD versus the nonADHD groups (Harty et al., 2015; Pedersen et al., 2014). These findings suggest that individuals with ADHD histories may utilize expectancies differently from same-age peers without childhood ADHD. For example, those with childhood ADHD, compared to those without, may rely less on explicit cognitions when making decisions about substance use due to the cognitive difficulties that characterize the disorder (Willcutt et al., 2005). It is also possible that poor inhibitory control might strengthen expectancy-substance use associations among young adults with ADHD histories, but our prior findings have generally not supported this alternative hypothesis (Harty et al., 2015; Pedersen et al., 2014).
It is also important to note that previous work examining ADHD symptoms and expectancies has predominantly consisted of cross-sectional studies of community samples that primarily relied on self-reported symptoms of young adults who did not meet diagnostic thresholds for ADHD (Dattilo et al., 2013; Elmore, Nikolas, & Canu, 2017). This means that previous samples may not have included young adults with limited insight into their symptoms and impairment, as has been documented among those with childhood ADHD followed into adulthood (Barkley et al., 2002; Sibley et al., 2017). Due to differences in memory, encoding, and executive function, the development of substance specific cognitions may be diminished for individuals with a history of ADHD.
Primary Aims
The purpose of the current study was to assess the specificity of the prospective associations between positive and negative alcohol and marijuana expectancies and alcohol and marijuana use throughout young adulthood for individuals with and without a history of ADHD. We hypothesized that the associations between substance use expectancies and substance use would generally be substance-specific, not only concurrently, but also prospectively. However, due to diminished associations between expectancies and substance use previously found among those with childhood ADHD (Harty et al., 2015; Pedersen et al., 2014), we also hypothesized that these drug specific associations would be stronger over time for participants without a history of ADHD compared to those with a history of ADHD.
Method
Procedure
The participants were from a longitudinal study of individuals with and without childhood ADHD (Pittsburgh ADHD Longitudinal Study: PALS). Initial enrollment into this study occurred from 1999–2003; annual interviews occurred until 2008; assessments have since been age-based (annually to 23, and then at ages 25, 27, and 29; older interviews are ongoing). Interviews were conducted by post-baccalaureate research staff following informed consent. Participants were assured confidentiality of all disclosed material except in cases of impending danger to self or others (reinforced with a DHHS Certificate of Confidentiality). Additional study details may be found in Molina and colleagues (2007).
Sample
ADHD sample.
Participants were selected for the PALS study due to their diagnosis of ADHD and participation in a summer treatment program (STP) for children with ADHD (Pelham & Hoza, 1996). As part of participating in the STP, childhood ADHD was diagnosed with DSM-III-R or DSM-IV ADHD criteria at the ADD Clinic, Western Psychiatric Institute and Clinic, in Pittsburgh, PA between 1987 and 1996. Participants were initially referred for treatment by schools, physicians, mental health workers, or their parents. Average age at this diagnostic evaluation was 9.40 years old (SD = 2.27, range = 5.0–16.92, 90% were ages 5–12).
Diagnostic information for the participants with ADHD was collected using standardized parent and teacher DSM-III-R and DSM-IV symptom rating scales (DBD; Pelham et al., 1992) and a standardized semi-structured diagnostic interview administered to parents by a Ph.D. level clinician. It also included queries about other comorbidities (instrument available through co-author W.E.P.). Two Ph.D. level clinicians independently reviewed all ratings and interviews to confirm DSM diagnoses and when disagreement occurred, a third clinician reviewed the file and the majority decision was used. Exclusion criteria for participation in PALS included a full-scale IQ < 80, a history of seizures or other neurological problems, and/or a history of pervasive developmental disorder, schizophrenia, or other psychotic or organic mental disorders.
Of those eligible for enrollment into PALS (n = 516), 70.5% participated (n = 364; MAge = 17.75, SDAge = 3.39, age range 11 – 25). Enrollment into PALS was an average of 8.35 (SD = 2.79) years after childhood diagnosis and STP participation. While there was some variability in the duration of stimulant medication use following childhood treatment, the majority of the PALS sample (89.9%) used stimulant medication at some point, which prevents meaningful assessment of differences in results based on medication treatment. Enrolled PALS participants were different from non-enrolled on 1 out of 14 childhood demographic and diagnostic variable comparisons. Parent/teacher ratings of conduct disorder symptoms were lower (t = 3.09, p < .01) for participants (M = 2.13; SD = 1.93) versus nonparticipants (M = 2.74; SD = 2.19; Cohen’s d = .30).
NonADHD sample
Participants without ADHD were recruited from the greater Pittsburgh area from several sources (e.g., pediatric practices, university hospital staff newsletter, local schools). A telephone screening with parents gathered basic demographics, history of diagnosis and treatment for ADHD and other behavior problems, including conduct disorder, presence of exclusionary criteria as previously listed for the ADHD participants, and a checklist of ADHD symptoms. Young adults (18+) also provided self-report of the same information. In addition to the exclusion criteria listed for the ADHD sample, individuals who met DSM-III-R criteria for ADHD (presence of 8 or more symptoms reported by either the parent or young adult), currently or historically, were excluded from the nonADHD sample. Subthreshold ADHD symptoms and other psychiatric disorders were not exclusions. By design, the ADHD and nonADHD samples were demographically similar (Molina et al., 2007).
Subsample for the current study.
Data were selected from individuals who participated in at least one of the annual interviews at ages 21–23, during some of the peak drinking ages nationally (Linden-Carmichael et al., 2017), and at age 29 (N = 491; n = 281 ADHD; n = 210 nonADHD). The sample was 87% male (N = 429; n = 245 ADHD; n = 184 nonADHD) and 85% White (N = 417; n = 237 ADHD; n = 180 nonADHD).
Measures
Alcohol and marijuana use expectancies.
Participants self-reported their expectancies at each age about the effects of drinking alcohol on the Comprehensive Effects of Alcohol scale using a 1 (disagree), 2 (somewhat disagree), 3 (somewhat agree), and 4 (agree) scale (CEOA; Fromme et al., 1993). Participants also self-reported their expectancies about the effects of using marijuana on an adapted version of the CEOA at each age. For the purposes of the current project, averages of the positive expectancy subscales (20 items total) assessing sociability (8 items, e.g., I would be outgoing), tension reduction (3 items, e.g., I would feel calm), courage (5 items, e.g., I would be brave and daring), and sexuality (4 items, e.g., I would feel sexy) and negative expectancy subscales (18 items total) assessing cognitive/behavioral impairment (9 items, e.g., I would feel dizzy), risk/aggression (5 items, e.g., I would act tough), and negative self-perception (4 items, e.g., my problems would seem worse) were created separately for alcohol use and marijuana use for all ages included in the model. The Cronbach’s alphas for the positive and negative domains ranged from .66 to .95, and both scales have been the subject of prior research that did not examine substance specificity (Harty et al., 2015; Pedersen et al., 2014).
Frequency of alcohol and marijuana use.
Participants self-reported their frequency of drinking alcohol in the past 12 months using a 0 (not at all) to 11 (several times a day) scale. A parallel self-report question was used to assess frequency of marijuana use in the past 12 months. Descriptive statistics for substance use at each age can be found in Table 1.
Table 1.
Substance use descriptives by age and ADHD status
| Total | ADHD | nonADHD | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Percentage Any Use | Mean | SD | Percentage Any Use | Mean | SD | Percentage Any Use | Mean | SD | |
| Frequency of Alcohol Use | |||||||||
| Age 21 | 88.70 | 4.80 | 2.67 | 85.40 | 4.50 | 2.86 | 93.00 | 5.19 | 2.35 |
| Age 22 | 86.30 | 4.82 | 2.62 | 82.20 | 4.41 | 2.76 | 91.90 | 5.40 | 2.30 |
| Age 23 | 87.30 | 4.84 | 2.55 | 83.50 | 4.42 | 2.73 | 92.30 | 5.38 | 2.21 |
| Age 29 | 92.00 | 4.84 | 2.47 | 88.10 | 4.42 | 2.60 | 97.00 | 5.38 | 2.20 |
| Frequency of Marijuana Use | |||||||||
| Age 21 | 44.30 | 2.38 | 3.48 | 39.70 | 2.26 | 3.58 | 50.50 | 2.54 | 3.35 |
| Age 22 | 42.20 | 2.48 | 3.64 | 40.40 | 2.48 | 3.70 | 44.70 | 2.47 | 3.56 |
| Age 23 | 37.90 | 2.16 | 3.48 | 36.60 | 2.27 | 3.69 | 39.70 | 2.02 | 3.21 |
| Age 29 | 47.10 | 2.84 | 3.94 | 43.30 | 2.95 | 4.23 | 52.40 | 2.70 | 3.53 |
Notes: Percentage any use describes the percentage of the sample reporting use of the substance at least once in the past year, and frequency of use reflects use in the past 12 months.
Covariates.
In addition to gender (1 = Male, 2 = Female) and race (0 = NonWhite, 1 = White), childhood socioeconomic advantage was calculated from parents’ education and marital status as measured in childhood (ADHD) and at recruitment (nonADHD), with 0 = Low (single parent with high school or less education), 1 = Medium (single parent with more than high school or less education or married parent with high school or less education), and 2 = High (married parent with more than high school education or less education). Parental psychopathology was calculated from parent-reported alcohol use disorder (AUD), antisocial personality disorder (ASPD), and depression (major depressive disorder, dysthymia, or depressive disorder not otherwise specified). Diagnoses were based on the Structured Clinical Interview for the DSM-IV Axis I and Axis II disorders, and diagnoses of AUD were supplemented with the Short Michigan Alcoholism Screening Test (First et al., 1997; Selzer, Vinokur, & van Rooijen, 1975). Each parent was scored as being positive (1) or negative (0) for each diagnosis, and a sum score was calculated that combined scores across parents. See Molina and colleagues (2012; 2014) for previous use of these covariates.
Analytic Overview
Autoregressive models (Figures 1–4) were estimated separately for positive and negative substance use expectancies for frequency of alcohol and marijuana use in the past year. Autoregressive models allow for the examination of cross-sectional and longitudinal associations between multiple constructs while also considering the stability of an individual construct over time (Box, Jenkins, & Reinsel, 2008). Cross-sectional associations are modelled as correlations among constructs measured at the same time point, and longitudinal associations, including stability within a construct, are examined using regression of constructs measured at later time points on constructs measured at earlier time points. Because construct stability is included in the model, cross-lagged associations among different constructs can be interpreted as above and beyond variability explained by stability within a construct. Models were estimated in Mplus 7.0 (Muthen & Muthen, 2012), and maximum likelihood estimation was used to address missing data. ADHD group differences in the associations were tested using chi-square difference tests and successive model estimations (Maruyama, 1998). The base model freely estimated all of the associations between substance use expectancies and substance use across groups. In subsequent models, relations were constrained to be equal across groups one at a time, which was followed by testing whether the model fit significantly changed when an association was constrained. A group difference due to childhood ADHD status was identified when there was a statistically significant change in the chi-square statistic. Once identified as different, subsequent model iterations allowed the parameter to be freely estimated, rather than constrained to be equal, across groups.
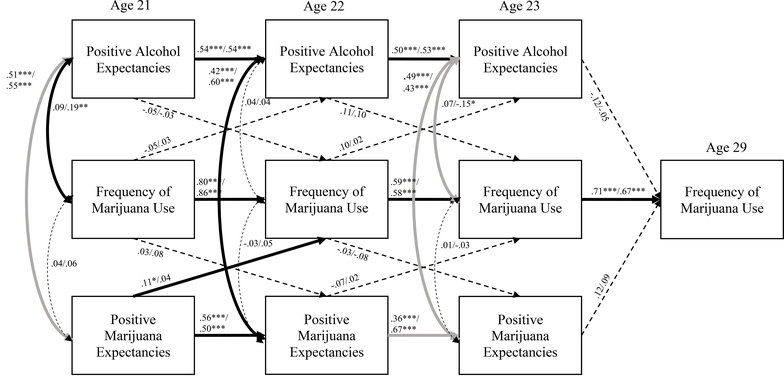
Figure 1.
Frequency of alcohol use with positive substance use expectancies.
*p < .05, **p < .01, ***p < .001
All reported coefficients are standardized and reported as ADHD/nonADHD
Bold paths indicate a significant association for at least one group, and paths in gray indicate a path that was significantly different between the groups
Figure 4.
Frequency of marijuana use with negative substance use expectancies.
*p < .05, **p < .01, ***p < .001
All reported coefficients are standardized and reported as ADHD/nonADHD
Bold paths indicate a significant association for at least one group, and paths in gray indicate a path that was significantly different between the groups
Results
Expectancy Levels
Although our hypotheses pertained to moderation of expectancy-substance use associations by ADHD history, we first explored mean differences in expectancies to inform interpretation of our results by ADHD group. Participants with ADHD histories reported significantly lower levels of positive substance use expectancies (ADHD: M = 2.17 – 2.44; nonADHD: 2.39 – 2.76), t(249) = 2.09 – 4.62, all p < .05), for all ages except age 23 marijuana expectancies, t(249) = 1.44, p = .15. However, for the negative expectancies (ADHD: M = 2.15 – 2.50; nonADHD: 2.29 – 2.57), only age 21 marijuana expectancies differed significantly, t(249) = 2.52, p < .05, with ADHD participants (M = 2.15) reporting lower negative marijuana expectancies at age 21 compared to nonADHD participants (M = 2.36). All remaining negative expectancy ADHD group comparisons were not significant, t(249) = .94 – 1.70, all p > .05. In the autoregressive models, the cross-sectional correlations between alcohol and marijuana positive expectancies (r = .42 - .60, all p < .001) and alcohol and marijuana negative expectancies (r = .35 - .52, all p < .001) were significant and positive at each age and for both groups (ADHD and nonADHD).
Alcohol Use
For positive substance use expectancies and alcohol use (Figure 1; χ2(86) = 217.51, p < .001; RMSEA = .08; CFI = .93), there was evidence of expectancy specificity due to the presence of significant prospective associations between positive alcohol expectancies and alcohol use (β = .13 - .22, all p < .05) but no significant associations between positive marijuana expectancies and alcohol use. The significant associations between alcohol expectancies and alcohol use over time were primarily present for those without a history of ADHD, including cross-lagged associations between age 21 positive alcohol expectancies/age 22 alcohol use (β = .22, p < .01) and age 22 alcohol use/age 23 positive alcohol expectancies (β = .20, p < .01). Based on the consistent statistical significance of the coefficients, moderate stability for positive substance use expectancies and alcohol use over time was observed for both groups (β = .35 - .66, all p < .001). Only some of the cross-sectional correlations between positive expectancies and alcohol use were significant (i.e., positive alcohol expectancies and alcohol use at age 21; ADHD: r = .22, p < .01; nonADHD: r = .27, p < .01, and age 22 for nonADHD: r = .16, p < .05). The model accounted for a significant amount of variance in alcohol use at age 29 (ADHD R2 = .22, p < .001; nonADHD R2 = .41, p < .001), but neither alcohol, nor marijuana, expectancies predicted alcohol use six years later at age 29.
For negative substance use expectancies and alcohol use (Figure 2; χ2(86) = 194.04, p < .001; RMSEA = .07; CFI = .93), there were two prospective associations but, overall, a lack of evidence of expectancy specificity. One significant prospective association between negative alcohol expectancies at age 21 and alcohol use at age 22 (β = .19, p < .01) was opposite in direction to the expected (more expectation of negative consequences predicting more frequent alcohol use). The other significant prospective association was between negative marijuana expectancies at age 22 and alcohol use at age 23 (β = −.17, p < .01). Both of these associations were present for the nonADHD group but not for the ADHD group, and the association was significantly stronger for the nonADHD group compared to the ADHD group. Similar to the positive expectancy and frequency of alcohol use model, both groups demonstrated stability in the statistical significance of the coefficients for negative substance use expectancies and alcohol use over time (β = .33 - .71, all p < .001). Only some cross-sectional correlations between negative expectancies and alcohol use were significant (i.e., negative marijuana expectancies and alcohol use at age 21; ADHD: r = −.18, p < .01; nonADHD: r = −.27, p < .001, and negative alcohol expectancies and alcohol use at age 23 for nonADHD: r = −.18, p < .01). The model accounted for a significant amount of variance in alcohol use at age 29 (ADHD R2 = .22, p < .001; nonADHD R2 = .43, p < .001), but age 29 alcohol use was not predicted by prior alcohol or marijuana expectancies.
Figure 2.
Frequency of alcohol use with negative substance use expectancies.
*p < .05, **p < .01, ***p < .001
All reported coefficients are standardized and reported as ADHD/nonADHD
Bold paths indicate a significant association for at least one group, and paths in gray indicate a path that was significantly different between the groups
Marijuana Use
For positive substance use expectancies and marijuana use (Figure 3; χ2(86) = 192.17, p < .001; RMSEA = .07; CFI = .95), there was some evidence of specificity, with one significant prospective association between positive marijuana expectancies at age 21 and marijuana use at age 22 for those with a history of ADHD (β = .11, p < .05). While this path was only significant for the ADHD group, the association did not differ significantly between groups (χ2(1) = .84, p = .36). Both those with and without childhood ADHD had statistically significant coefficients for positive substance use expectancies and marijuana use over time (β = .36 - .86, all p < .001). There were only some significant cross-sectional correlations between positive expectancies and marijuana use (i.e., positive alcohol expectancies and marijuana use at age 21 for nonADHD: r = .19, p < .01, and age 23 for nonADHD: r = −.15, p < .05). The model accounted for a significant amount of variance in marijuana use at age 29 (ADHD R2 = .50, p < .001; nonADHD R2 = .46, p < .001), but age 29 marijuana use was not predicted by prior alcohol or marijuana expectancies.
Figure 3.
Frequency of marijuana use with positive substance use expectancies.
*p < .05, **p < .01, ***p < .001
All reported coefficients are standardized and reported as ADHD/nonADHD
Bold paths indicate a significant association for at least one group, and paths in gray indicate a path that was significantly different between the groups
For negative substance use expectancies and marijuana use (Figure 4; χ2(86) = 165.24, p < .001; RMSEA = .06; CFI = .96), there was evidence of specificity, with significant prospective associations between negative marijuana expectancies and marijuana use (β = −.13 - −.20, all p < .05) and no significant prospective associations between negative alcohol expectancies and marijuana use. The significant associations between negative marijuana expectancies and marijuana use over time included associations between age 21 marijuana use/age 22 negative marijuana expectancies (β = −.20, p < .01), age 22 negative marijuana expectancies/age 23 marijuana use (β = −.13, p < .05), and age 23 negative marijuana expectancies/ age 29 marijuana use (β = −.24, p < .05) for those without a history of ADHD. Even though the associations between marijuana expectancies and marijuana use were only significant for those without a history of ADHD, the associations did not differ significantly between groups (χ2(1) = .004 – 1.05, p = .31 - .95). Similar to the positive expectancy and frequency of marijuana use model, both groups demonstrated stability in the statistical significance of the coefficients for negative substance use expectancies and marijuana use over time (β = .34 - .83, all p < .001). Some cross-sectional correlations between negative expectancies and marijuana use were significant (i.e., negative marijuana expectancies and marijuana use at age 21; ADHD: r = −.26, p < .001; nonADHD: r = −.44, p < .001, and negative alcohol expectancies and marijuana use at age 23 for nonADHD: r = −.19, p < .01). The model accounted for a significant amount of variance in marijuana use at age 29 (ADHD R2 = .49, p < .001; nonADHD R2 = .47, p < .001).
Discussion
These findings partially support the specificity of both concurrent and prospective associations between substance use expectancies and the substances they assess. There was evidence of specificity for both positive and negative expectancies, and this included one prospective association between negative marijuana expectancies at age 23 and marijuana use at age 29. These associations are particularly noteworthy given the significant correlations between alcohol and marijuana expectancies at each age. While many of the associations between expectancies and substance use did not differ significantly between the groups, associations between expectancies and substance use were primarily present for young adults without childhood ADHD. These results extend our prior findings that have shown substance use expectancies to be less associated with substance use for those with childhood ADHD compared to same age peers without a history of ADHD (Harty et al., 2015, Pedersen et al., 2014). These findings also have implications for expectancy-related interventions. Namely, expectancy interventions could be more beneficial if they are focused solely on a drug of choice rather than addressing other expectancies and substance of abuse.
Both participants with and without a history of ADHD demonstrated stability in substance use and expectancies during the early twenties. Research on the developmental period of emerging adulthood, which includes ages 18 to 25, has found that substance use, including heavy substance use, is relatively normative during the ages included in the current study. Thus, rates of substance use will often remain consistently high or increase during this time (Arnett, 1992; 2000). As young adults transition to adult roles and encounter new societal expectations, substance use usually decreases (Dawson et al., 2006; Littlefield et al., 2009; Windle, 2003). Similarly, substance use expectancies, which change as substance use knowledge and experience changes, are likely to follow suit (Donovan et al., 2009; Gillmore et al., 1998; Johnson & Johnson, 1995; Miller et al., 1990; Schell et al., 2005), and proximal expectancies may be more strongly associated with substance use than expectancies from the early twenties, which could explain the relative lack of prospective associations to age 29 found in the current study. Because many in their early twenties have high levels of positive expectancies, changes in expectancies, or a lack thereof, may differentiate those who are at risk for higher levels of substance use later in adulthood. In addition, there is evidence of within person variability in expectancies, particularly those that are context dependent, during the late teens and early twenties (Lee et al., 2015). Future research would benefit from examining stability in expectancies and associations with substance use through adulthood across developmental stages and within subjects. The relatively modest number of prospective associations found may also be due to the high statistical bar set in these models, where prospective effects had to be demonstrated above and beyond stabilities and concurrent associations (Allison, 2009). Most prior studies of expectancies and substance use do not use such conservative models.
Our findings that ADHD was associated with lower expectations of positive substance use effects, and decreased associations between substance use expectancies and substance use, suggest that some characteristics of ADHD are interfering with the typical development and utilization of expectancies. Cognitive impairments associated with this disorder, including attention and working memory, may be responsible. These deficits invoke the dual process model of addiction suggested by Stacey and Weirs (2010). Specifically, automatic appetitive cognitive processes may have a greater influence on behavior for those with poor executive control, especially working memory, such as in ADHD (Peeters et al., 2015). However, rational cognitive processes that involve organized forethought and planning may guide behavior more for those with greater executive functioning (Thush et al., 2008). As executive functions, including working memory, are known to be frequently impaired for individuals with ADHD (Willcutt et al., 2005), substance use for this population may be driven less by explicit beliefs about substances and more by automatic processes. Insight into functioning is often lower among adults with ADHD histories (Sibley et al., 2012; 2017), and, although the specific mechanisms are unknown, it further supports the probability that future research on substance use expectancies among those with ADHD histories may benefit from the use of implicit measures. It should be noted that prior research examining implicit cognitions has found support for specificity of substance use cognitions (McCarthy & Thompson, 2006).
Both alcohol and marijuana expectancies demonstrated specificity in the analyses, but this specificity was primarily limited to positive alcohol and negative marijuana expectancies. The role of positive alcohol expectancies as a risk for emerging adult alcohol use compared to negative alcohol expectancies is well-established in the literature (Ham & Hope, 2003; Jones et al., 2001; McBride et al., 2014; Oei & Morawska, 2004), and it is somewhat surprising that positive marijuana expectancies did not operate in a similar manner. However, these results join a growing body of literature that finds a stronger protective role of negative marijuana expectancies in relation to the frequency of marijuana use among emerging adults compared to alcohol expectancies (Hayaki et al., 2010; Vangsness, Bry, & LaBouvie, 2005). This finding may be due to the relatively lower frequency of use of marijuana relative to alcohol among young people and marijuana’s illegal status in Pennsylvania at the time of data collection. Relative to alcohol, expectations of negative consequences may have stronger effects on behavioral choice for marijuana use. In addition, prior research has found that alcohol expectancies become less negative and more positive with drinking experience (Jones & Gordon, 2017). Marijuana expectancies may follow a similar pattern, and these patterns may become more apparent with changes in marijuana policies or in relation to changes in marijuana use with age. Future research should examine changes in marijuana use and expectancies in relation to the changing legal status of marijuana.
While there are many strengths to the current study (e.g., longitudinal data collection for both expectancies and substance use, well-documented history of ADHD), the adaptation of the CEOA for marijuana use may be a potential limitation, as some domains of marijuana expectancies are specific to marijuana use (Kristjansson et al., 2012). This may be particularly true for young adults with ADHD, as qualitative work with adolescents and young adults with a history of ADHD has identified ADHD-specific expectancies related to substance use (Mitchell et al. 2016; 2018). These include symptom management, which would not have been captured in the measure of marijuana expectancies used in the current study. Future studies should include measures of marijuana expectancies that assess domains of symptoms or functioning that are applicable to individuals with ADHD. In addition, the study collapsed across all domains of positive expectancies and all domains of negative expectancies rather than examining associations for specific expectancy subscales. Examination of these specific domains, which was beyond the scope of the current study, may further clarify the associations between expectancies and substance use over time (Friedman et al., 2009; Testa et al., 2006). In addition, expectancies for substances other than alcohol and marijuana were not available for the current analyses, and future studies should examine expectancy specificity for other substances of abuse that are frequently used among those with ADHD histories, such as cigarettes (Rhodes et al., 2016). Finally, ADHD subtype differences, which may be differentially associated with substance use expectancies, could not be examined in the current analyses. Previous research has found differences in expectancy development associated with differences in hyperactive symptoms (Squeglia et al., 2016), and persisting symptomatology due to differences in childhood ADHD subtype could be related to different levels of positive and negative substance use expectancies. The PALS sample was predominantly combined subtype in childhood, which prevented consideration of childhood subtype in the multiple group models. Examination of subtype differences, and, perhaps even more importantly, changing symptoms with age and deficiencies in cognitive functioning (e.g., working memory deficits) in relation to expectancies and substance use is an important consideration for future research.
The findings in the current study suggest that young adult cognition-behavior relations regarding alcohol and marijuana may have some degree of specificity beyond just a general sense of reward and punishment associated with substance use. These findings may partly explain, in addition to other factors such as legality and accessibility, the different rates of alcohol and marijuana use by young adults. However, these associations may be less applicable for special populations, which, in the case of the current study, is adults with histories of ADHD. Additional research examining implicit substance use cognitions, as well as other long-term alcohol and marijuana use outcomes, is needed to fully understand the lasting impact of childhood ADHD on both substance use cognitions and substance use.
Acknowledgments
This research was supported by grants from the National Institute on Alcohol Abuse and Alcoholism and the National Institute on Drug Abuse (AA011873, DA12414, AA00202, and K01 AA021135). Funds were provided to UPMC by Shire Pharmaceuticals and NEOS Therapeutics in support of a conference directed by Brooke Molina.
References
- Allison PD (2009). Fixed effects regression models. Thousand Oaks, CA: Sage Publications. [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: American Psychiatric Association. [Google Scholar]
- Anderson KG, Brackenbury L, Quackenbush M, Buras M, Brown SA, & Price J (2014). A-SIDE: Video simulation of teen alcohol and marijuana use contexts. Journal of Studies on Alcohol and Drugs, 75, 953–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett JJ (1992). Reckless behavior in adolescence: A developmental perspective. Developmental Review, 12, 339–373. [Google Scholar]
- Arnett JJ (2000). A theory of development from the late teens through the twenties. American Psychologist, 55, 469–480. [PubMed] [Google Scholar]
- Ashton CH (2001). Pharmacology and effects of cannabis: A brief review. British Journal of Psychiatry, 178, 101–106. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Fischer M, Smallish L, & Fletcher K (2002). The persistence of attention-deficit/hyperactivity disorder into young adulthood as a function of reporting source and definition of disorder. Journal of Abnormal Psychology, 111(2), 279–289. [PubMed] [Google Scholar]
- Barkley RA, Murphy KR, & Fischer M (2008). ADHD in adults: What the science says. New York, NY: Guilford Press. [Google Scholar]
- Barnow S, Schultz G, Lucht M, Ulrich I, Preuss UW, & Freyberger HJ (2004). Do alcohol expectancies and peer delinquency/substance use mediate the relationship between impulsivity and drinking behavior in adolescence?. Alcohol and Alcoholism, 39, 213–219. [DOI] [PubMed] [Google Scholar]
- Barrett SP, Darredeau C, & Pihl RO (2006). Patterns of simultaneous polysubstance use in drug using university students. Human Psychopharmacology: Clinical and Experimental, 21, 255–263. [DOI] [PubMed] [Google Scholar]
- Box GEP, Jenkins GM, & Reinsel GC (2008). Time series analysis: Forecasting and control (4th ed.). Hoboken, N.J.: John Wiley & Sons, Inc. [Google Scholar]
- Bushman BJ, & Cooper HM (1990). Effects of alcohol on human aggression: An integrative research review. Psychological Bulletin, 107, 341–354. [DOI] [PubMed] [Google Scholar]
- Charach A, Yeung E, Climans T, & Lillie E (2011). Childhood attention-deficit/hyperactivity disorder and future substance use disorders: Comparative meta-analyses. Journal of the American Academy of Child and Adolescent Psychiatry, 50, 9–21. [DOI] [PubMed] [Google Scholar]
- Collins RL, Ellickson PL, & Bell RM (1998). Simultaneous polydrug use among teens: Prevalence and predictors. Journal of Substance Abuse, 10, 233–253. [DOI] [PubMed] [Google Scholar]
- D’Amico EJ, Houck JM, Hunter SB, Miles JNV, Osilla KC, & Ewing BA (2015). Group motivational interviewing for adolescents: Change talk and alcohol and marijuana outcomes. Journal of Consulting and Clinical Psychology, 83, 68–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dattilo L, Glass Murphy K Van Eck K, & Flory K. (2013). Do ADHD symptoms moderate the relation between positive alcohol expectancies and alcohol-related outcomes?. ADHD Attention Deficit and Hyperactivity Disorders, 5, 93–104. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Grant BF, Stinson FS, & Chou PS (2006). Maturing out of alcohol dependence: The impact of transitional life events. Journal of Studies on Alcohol, 67, 195–203. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, & Bassareo V (2007). Reward system and addiction: What dopamine does and doesn’t do. Current Opinion in Pharmacology, 7, 69–76. [DOI] [PubMed] [Google Scholar]
- Donovan JE, Molina BSG, & Kelly TM (2009). Alcohol outcome expectancies as socially shared and socialized beliefs. Psychology of Addictive Behaviors, 23, 248–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn ME, & Goldman MS (1998). Age and drinking-related differences in the memory organization of alcohol expectancies in 3rd-, 6th-, and 12th-grade children. Journal of Consulting and Clinical Psychology, 66, 579–585. [DOI] [PubMed] [Google Scholar]
- Dunn ME, & Goldman MS (2000). Validation of multidimensional scaling-based modeling of alcohol expectancies in memory: Age and drinking-related differences in expectancies of children assessed as first associates. Alcoholism: Clinical and Experimental Research, 24, 1639–1646. [PubMed] [Google Scholar]
- Elmore A, Nikolas M, & Canu W (2017). Positive alcohol expectancies mediate associations between ADHD behaviors and alcohol-related problems among college students. ADHD Attention Deficit and Hyperactivity Disorders. Advance online publication. doi: 10.1007/s12402-017-0231-z [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW, & Benjamin LS (1997). Structured clinical interview for DSM-IV axis II personality disorders, (SCID-II). Washington, D.C.: American Psychiatric Press. [Google Scholar]
- Friedman RS, McCarthy DM, Pedersen SL, & Hicks JA (2009). Alcohol expectancy priming and drinking behaviors: The role of compatibility between prime and expectancy content. Psychology of Addictive Behaviors, 23, 329–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromme K Stroot EA, & Kaplan D (1993). Comprehensive effects of alcohol: Development and psychometric assessment of a new expectancy questionnaire. Psychological Assessment, 5, 19–26. [Google Scholar]
- Gillmore MR, Wells EA, Simpson EE, Morrison DM, Hoppe MJ, & Wilsdon A (1998). Children’s beliefs about drinking. American Journal of Drug & Alcohol Abuse, 24, 131–151. [DOI] [PubMed] [Google Scholar]
- Groenman AP, Janssen TWP, & Oosterlaan J (2017). Childhood psychiatric disorders as risk factor for subsequent substance abuse: A meta-analysis. Journal of the American Academy of Child & Adolescent Psychiatry, 56, 556–569. [DOI] [PubMed] [Google Scholar]
- Haas AL, Wickham R, Macia K, Shields M, Macher R, & Schulte T (2015). Identifying classes of conjoint alcohol and marijuana use in entering freshmen. Psychology of Addictive Behaviors, 29, 620–626. [DOI] [PubMed] [Google Scholar]
- Ham LS, & Hope DA (2003). College students and problematic drinking: A review of the literature. Clinical Psychology Review, 23, 719–759. [DOI] [PubMed] [Google Scholar]
- Harty SC, Pedersen SL, Gnagy EM, Pelham WE, & Molina BSG (2015). ADHD and marijuana use expectancies in young adulthood. Substance Use & Misuse, 50, 1470–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayaki J, Anderson BJ, & Stein MD (2016). Dual cannabis and alcohol use disorders in young adults: Problems magnified. Substance Abuse, 37, 579–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayaki J, Hagerty CE, Herman DS, de Dios MA, Anderson BJ, & Stein MD (2010). Expectancies and marijuana use frequency and severity among young females. Addictive Behaviors, 35, 995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayaki J, Herman DS, Hagerty CE, de Dios MA, Anderson BJ, & Stein MD (2011). Expectancies and self-efficacy mediate the effects of impulsivity on marijuana use outcomes: An application of the acquired preparedness model. Addictive Behaviors, 36, 389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hechtman L, Swanson JM, Sibley MH, Stehli A, Owens EB, Mitchell JT, Arnold LE, Molina BS, Hinshaw SP, Jensen PS, Abikoff HB, Perez Algorta G, Howard AL, Hoza B, Etcovitch J, Houssais S, Lakes KD, Nichols JQ, & MTA Cooperative Group (2016). Functional adult outcomes 16 years after childhood diagnosis of attention-deficit/hyperactivity disorder: MTA results. Journal of the American Academy of Child and Adolescent Psychiatry, 55(11), 945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson HL, & Johnson PB (1995). Children’s alcohol-related cognitions: Positive versus negative alcohol effects. Journal of Alcohol & Drug Education, 40, 1–12. [Google Scholar]
- Jones BT, Corbin W, & Fromme K (2001). A review of expectancy theory and alcohol consumption. Addiction, 96, 57–72. [DOI] [PubMed] [Google Scholar]
- Jones SC, & Gordon CS (2017). A systematic review of children’s alcohol-related knowledge, attitudes and expectancies. Preventive Medicine, 105, 19–31. [DOI] [PubMed] [Google Scholar]
- Kristjansson SD, Agrawal A Lynskey MT, & Chassin LA (2012). Marijuana expectancies and relationships with adolescent and adult marijuana use. Drug and Alcohol Dependence, 126, 102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CM, Atkins DC, Cronce JM, Walter T, & Leigh BC (2015). A daily measure of positive and negative alcohol expectancies and evaluations: Documenting a two-factor structure and within- and between-person variability. Journal of Studies on Alcohol and Drugs, 76(2), 326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Humphreys KL, Flory K, Liu R, & Glass K (2011). Prospective association of childhood attention-deficit/hyperactivity disorder (ADHD) and substance use and abuse/dependence: A meta-analytic review. Clinical Psychology Review, 31, 328–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson RW, Sher KJ, Grossman LM, Newman J, & Newlin DB (1980). Alcohol and stress response dampening: Pharmacological effects, expectancy, and tension reduction. Journal of Abnormal Psychology, 89, 528–538. [DOI] [PubMed] [Google Scholar]
- Linden-Carmichael AN, Vasilenko SA, Lanza ST, & Maggs JL (2017). High-intensity drinking versus heavy episodic drinking: Prevalence rates and relative odds of alcohol use disorder across adulthood. Alcoholism: Clinical and Experimental Research, 41, 1754–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlefield AK, Sher KJ, & Wood PK (2009). Is “maturing out” of problematic alcohol involvement related to personality change?. Journal of Abnormal Psychology, 118, 360–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama GM (1998). Basics of structural equation modeling. Thousand Oaks, CA: Sage Publications. [Google Scholar]
- McBride NM, Barrett B, Moore KA, & Schonfeld L (2014). The role of positive alcohol expectancies in underage binge drinking among college students. Journal of American College Health, 62, 370–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DM, & Thompsen DM (2006). Implicit and explicit measures of alcohol and smoking cognitions. Psychology of Addictive Behaviors, 20, 436–444. [DOI] [PubMed] [Google Scholar]
- Miller PM, Smith GT, & Goldman MS (1990). Emergence of alcohol expectancies in childhood: A possible critical period. Journal of Studies on Alcohol, 51, 343–349. [DOI] [PubMed] [Google Scholar]
- Mitchell JT, Sweitzer MM, Tunno AM, Killins SH, & McClernon FJ (2016). “I use weed for my ADHD”: A qualitative analysis of online forum discussion on cannabis and ADHD. Plos ONE, 11, e0156614. doi: 10.1371/journal.pone.0156614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JT, Weisner TS, Jensen PS, Murray DW, Molina BSG, Arnold LE, Hechtman L, Swanson JM, Hinshaw SP, Victor EC, Kollins SH, Wells KC, Belendiuk KA, Blonde A, Nguyen C, Ambriz L, & Nguyen JL (2018). How substance users with ADHD perceive the relationship between substance use and emotional functioning. Journal of Attention Disorders, 22(Suppl. 9), 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BSG, Howard AL, Swanson JM, Stehli A, Mitchell JT, Kennedy TM, Epstein JN, Arnold LE, Hechtman L, Vitiello B, & Hoza B (2018). Substance use through adolescence into early adulthood after childhood‐diagnosed ADHD: Findings from the MTA longitudinal study. Journal of Child Psychology and Psychiatry, 59, 692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BSG, Pelham WE, Cheong J, Marshal M, Gnagy E, & Curran PJ (2012). Childhood ADHD and growth in adolescent alcohol use: The roles of functional impairments, ADHD symptom persistence, and parental knowledge. Journal of Abnormal Psychology, 121, 922–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BS, Pelham WE, Gnagy EM, Thompson AL, & Marshal MP (2007). Attention-deficit/hyperactivity disorder risk for heavy drinking and alcohol use disorder is age specific. Alcoholism: Clinical and Experimental Research, 31, 643–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BSG, Walther CAP, Cheong J, Pedersen S, Gnagy EM, & Pelham WE (2014). Heavy alcohol use in early adulthood as a function of childhood ADHD: Developmentally-specific mediation by social impairment and delinquency. Experimental and Clinical Psychopharmacology, 22, 110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthen LK, & Muthen BO (2012). Mplus: Statistical analysis with latent variables: User’s guide. Los Angeles, CA: Muthen & Muthen. [Google Scholar]
- Oei TPS, & Morawska A (2004). A cognitive model of binge drinking: The influence of alcohol expectancies and drinking refusal self-efficacy. Addictive Behaviors, 29, 159–179. [DOI] [PubMed] [Google Scholar]
- Pape H, Rossow I, & Storvoll EE (2009). Under double influence: Assessment of simultaneous alcohol and cannabis use in general youth populations. Drug and Alcohol Dependence, 101, 69–73. [DOI] [PubMed] [Google Scholar]
- Pedersen SL, Harty SC, Pelham WE, & Molina BSG (2014). Differential associations between alcohol expectancies and adolescent alcohol use as a function of childhood ADHD. Journal of Studies on Alcohol and Drugs, 75, 145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters M, Janssen T, Monshouwer K, Boendermaker W, Pronk T, Wiers R, & Vollebergh W (2015). Weaknesses in executive functioning predict the initiating of adolescents’ alcohol use. Developmental Cognitive Neuroscience, 16, 139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham WE, Gnagy EM, Greenslade KE, & Milich R (1992). Teacher ratings of DSM-III-R symptoms for the disruptive behavior disorders. Journal of the American Academy of Child and Adolescent Psychiatry, 31, 210–218. [DOI] [PubMed] [Google Scholar]
- Pelham WE, & Hoza B (1996). Intensive treatment: A summer treatment program for children with ADHD In Hibbs Euthymia D. & Jensen Peter S. (Eds.), Psychosocial treatments for child and adolescent disorders: Empirically based strategies for clinical practice (pp. 311–340). New York: APA Press. [Google Scholar]
- Rhodes JD, Pelham WE, Gnagy EM, Shiffman S, Derefinko KJ, & Molina BSG (2016). Cigarette smoking and ADHD: An examination of prognostically-relevant smoking behaviors among adolescents and young adults. Psychology of Addictive Behaviors, 30(5), 588–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell TL, Martino SC, Ellickson PL, Collins RL, & McCaffrey D (2005). Measuring developmental changes in alcohol expectancies. Psychology of Addictive Behaviors, 19, 217–220. [DOI] [PubMed] [Google Scholar]
- Selzer ML, Vinokur A, & van Rooijen L (1975). A self-administered Short Michigan Alcoholism Screening Test (SMAST). Journal of Studies on Alcohol, 36, 117–126. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Walitzer KS, Wood PK, & Brent EE (1991). Characteristics of children of alcoholics: Putative risk factors, substance use and abuse, and psychopathology. Journal of Abnormal Psychology, 100, 427–448. [DOI] [PubMed] [Google Scholar]
- Sibley MH, Pelham WE, Molina BSG, Gnagy EM, Waxmonsky JG, Waschbusch DA, Derefinko KJ, Wymbs BT, Garefino AC, Babinski DE, & Kuriyan AB (2012). When diagnosing ADHD in young adults emphasize informant reports, DSM items, and impairment. Journal of Consulting and Clinical Psychology, 80, 1052–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley MH, Swanson JM, Arnold LE, Hechtman LT, Owens EB, Stehli A, Abikoff H, Hinshaw SP, Molina BSG, Mitchell JT, Jensen PS, Howard AL, Lakes KD, Pelham WE, & MTA Cooperative Group (2017). Defining ADHD symptom persistence in adulthood: Optimizing sensitivity and specificity. Journal of Child Psychology and Psychiatry, 58, 655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Brammer WA, Ray LA, & Lee SS (2016). Attention deficit/hyperactivity disorder (ADHD) symptoms predict alcohol expectancy development. Journal of Child & Adolescent Substance Abuse, 25(2), 169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey AW, & Wiers RW (2010). Implicit cognition and addiction: A tool for explaining paradoxical behavior. Annual Review of Clinical Psychology, 6, 551–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard SA, & Pierce J (2016). Alcohol and marijuana use and intentions among adolescents: The role of the reasoned action approach and positive future orientation. Youth & Society. Advance online publication. doi: 0.1177/0044118X16671610. [Google Scholar]
- Subbaraman MS, & Kerr WC (2015). Simultaneous vs. concurrent use of alcohol and cannabis in the National Alcohol Survey. Alcoholism: Clinical and Experimental Research, 39, 872–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa M, Fillmore MT, Norris J, Abbey A, Curtin JJ, Leonard KE, Mariano KA, Thomas MC, Nomensen KJ, George WH, VanZile-Tamsen C, Livingston JA, Saenz C, Buck PO, Zawacki T, Parkhill MR, Jacques AJ, & Hayman LW (2006). Understanding alcohol expectancy effects: Revisiting the placebo condition. Alcoholism: Clinical and Experimental Research, 30, 339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thush C, Wiers RW, Ames SL, Grenards JL, Sussman S, & Stacy AW (2008). Interactions between implicit and explicit cognition and working memory capacity in the prediction of alcohol use in at-risk adolescents. Drug and Alcohol Dependence, 94, 116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker JS, Pedersen ER, Miles JNV, Ewing BA, Shih RA, D’Amico EJ (2014). Alcohol and marijuana use in middle school: Comparing solitary and social-only users. Journal of Adolescent Health, 55, 744–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vangsness L, Bry BH, & LaBouvie EW (2005). Impulsivity, negative expectancies, and marijuana use: A test of the acquired preparedness model. Addictive Behaviors, 30, 1071–1076. [DOI] [PubMed] [Google Scholar]
- Wardell JD, & Read JP (2013). Alcohol expectancies, perceived norms, and drinking behavior among college students: Examining the reciprocal determinism hypothesis. Psychology of Addictive Behaviors, 27, 191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, & Pennington BF (2005). Validity of the executive function theory of Attention-Deficit/Hyperactivity Disorder: A meta-analytic review. Biological Psychiatry, 57, 1336–1346. [DOI] [PubMed] [Google Scholar]
- Williams RM, Goldman MS, & Williams DL (1981). Expectancy and pharmacological effects of alcohol on human cognitive and motor performance: The compensation for alcohol effect. Journal of Abnormal Psychology, 90, 267–270. [DOI] [PubMed] [Google Scholar]
- Windle M (2003). Alcohol use among adolescents and young adults. Alcohol Research & Health, 27, 79–85. [PMC free article] [PubMed] [Google Scholar]
- Yurasek AM, Aston ER, & Matrik J (2017). Co-use of alcohol and cannabis: A review. Current Addiction Reports, 4, 184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]