Abstract
Background.
The tumor microenvironment after treatment with ipilimumab is not well described. Furthermore, the safety of surgery for patients being treated with ipilimumab for metastatic melanoma has not been well reported. This study analyzed the safety of surgery and the immune phenotype of tumors resected while on ipilimumab.
Methods.
From our prospective melanoma database, we identified patients undergoing surgery for any indication within 30 days of receiving a dose of induction ipilimumab or while on maintenance ipilimumab therapy. Surgical toxicity was graded 1–5 by the Clavien classification. Tumor-infiltrating lymphocytes were classified by flow cytometry and compared with peripheral blood.
Results.
23 patients were identified who underwent 34 operations a median of 27 weeks after initiation of ipilimumab (1–123 weeks). Subcutaneous resections were the most frequent, followed by intra-abdominal and nodal procedures. Grade 1 or 2 wound complications were seen in 22 % of patients. No Grade 3–5 complications were seen. Analysis of the T cell infiltrate and matched peripheral blood from ten patients showed an elevated % of CD4+FOXP3+ T-regulatory cells and a 2.8-fold lower ratio of CD8+/CD4+FOXP3+ in the tumor compared with blood (p = 0.02). In addition, all CD8+ T cells had a higher expression of PD-1 in the tumor, compared with peripheral blood.
Conclusions.
Surgery for patients on ipilimumab is safe. This study highlights the immunosuppressive phenotype in tumors not responding to immunotherapy. The high percentage of T-regulatory cells and low T-effector cells in progressive tumors suggests a possible mechanism of immune escape.
The landscape in the treatment of metastatic melanoma is being redefined with the approval of immunotherapies and targeted therapies that improve survival outcomes. Ipilimumab is a fully human IgG1 monoclonal antibody that blocks the critical immunologic checkpoint molecule cytotoxic T lymphocyte antigen 4 (CTLA-4). Ipilimumab has demonstrated improved survival in two phase 3, randomized, controlled trials in patients with metastatic melanoma.1,2 A subset of 20–30 % of patients achieves disease control (complete or partial response or stable disease) with ipilimumab.1,2 Many of the responses are sustained.3 Programmed death 1 (PD-1) is another checkpoint molecule that inhibits the immune response. Specific therapies aimed at this molecule and its ligand (PD-L1) have shown promising results in phase 1 trials.4 Preclinical models have suggested that combining CTLA-4 and PD-1 blockade has synergistic antitumor responses.5
As the field moves forward, aiming to improve response rates and integrate immunotherapy with other combinations of targeted therapies, it is important to understand the characteristics of tumors that do not respond to treatment. Human data suggest a response to ipilimumab correlates with tumor necrosis and an increased CD8+ (T effector cell)/FoxP3+ (regulatory T cell) ratio.6 Preclinical models also have suggested that a response to CTLA-4 blockade is related to the intratumoral balance of effector T cells (T eff) and regulatory T cells (T reg).7 T regs function to inhibit the immune system, and increased numbers of T regs have been found in patients with melanoma, suggesting an important role in preventing antitumor responses.8
This paper describes the safety profile of patients undergoing surgery whilst receiving ipilimumab for melanoma at a single center. In addition, the resected specimens were phenotyped to gain an insight into the immunologic infiltrate of tumors not otherwise responding to ipilimumab.
METHODS
We used a prospectively maintained institutional melanoma database to identify patients receiving ipilimumab who subsequently had surgery at our institution between October 2007 and August 2011. Consent was given for collection and use of patient data and tissue, and the project was approved by the institutional review board.
Patient Cohort
The patients in the cohort received ipilimumab (1) as part of a clinical trial, before FDA approval, or (2) as routine treatment after FDA approval. Some patients were on maintenance therapy after successful induction as part of the initial experience with ipilimumab. Only patients who had surgery within 30 days of receiving a dose of ipilimumab during the induction phase or any patient who had surgery while on maintenance ipilimumab were included in the analysis. Surgery was defined as any procedure performed in the operating room requiring general anesthesia or involving the complete excision of a metastatic lesion. Patients having diagnostic biopsies were excluded. All pathology samples were reviewed by a dermatopathologist.
Chart review was performed to identify details of surgery. The indication for surgery was separated into patients with isolated disease (a solitary residual focus of disease or a new focus after a complete response at all other sites), progressive disease (a solitary site of progressive disease in the setting of stable or responding disease elsewhere), and symptomatic disease (surgery was required irrespective of disease status). Surgical toxicity was graded 1–5 by the Clavien classification.9
Correlative Immunology
Ten of the patients included in the series had matched blood and tumor-infiltrating lymphocytes (TILs) harvested at the time of surgery and available in our tumor bank. Peripheral blood mononuclear cells were isolated from the whole blood, and TILs were extracted as previously described.10 The phenotype of T cell subsets in the TIL population was analyzed using flow cytometry on a FACSCalibur. Specific antibodies and fluorochromes are detailed in supplementary material. Staining for PD-L1 expression on tumors was not performed.
Statistical Analysis
Calculations were performed by expressing each T cell subset as a percentage of the total T cell population (CD3+). Statistical analysis was performed using Graph-Pad Prism v5. Statistical significance was calculated with unpaired t test.
RESULTS
Of the 428 patients who were started on ipilimumab at the author’s institution between October 2007 and August 2011, 46 had tissue samples collected (10.6 %) of which 23 patients were excluded. Patients were excluded either because they were part of an ongoing clinical trial (n = 2) or because the tissue was collected as part of a diagnostic test (i.e., not a surgical resection, n = 12) or more than 30 days after an induction dose of ipilimumab (n = 9). The remaining 23 patients (5.4 %) make up the study population. This cohort of patients was heavily pretreated: 83 % had prior chemotherapy, 57 % had prior radiotherapy, and 52 % had prior surgery for metastatic disease (Table 1).
TABLE 1.
Patient demographics and prior treatment details for the total cohort and patients with immune correlative data
| Total cohort | Patients with immune data | |||
|---|---|---|---|---|
| n (23) | % | n (10) | % | |
| Male | 16 | 70 | 7 | 70 |
| Female | 7 | 30 | 3 | 30 |
| Median age at start of ipilimumab | 55 | (38–71) | 55 | (38–71) |
| Stage at diagnosis | ||||
| I | 3 | 13 | 1 | 10 |
| II | 2 | 9 | 2 | 20 |
| III | 11 | 48 | 5 | 50 |
| IV | 3 | 13 | 1 | 10 |
| U/K | 4 | 17 | 1 | 10 |
| Stage at start of ipilimumab | ||||
| IIIB | 1 | 4 | 0 | 0 |
| IIIC | 1 | 4 | 0 | 0 |
| IV | 21 | 91 | 10 | 100 |
| Prior therapy | ||||
| Prior metastasectomy | 12 | 52 | 7 | 70 |
| Prior chemotherapy | 19 | 83 | 9 | 90 |
| Prior radiotherapy | 13 | 57 | 5 | 50 |
The median age at the time of starting ipilimumab was 55 (range 38–71; Table 1). 21 (91 %) patients had stage IV disease, and two patients had unresectable, recurrent stage IIIB and stage IIIC disease.
24 weeks after starting ipilimumab, disease control was seen in 16 patients (76 %), the majority of whom had disease stabilization (Table 2). Grades 1, 2, and 3 ipilimumab-related toxicities were seen in 52, 39, and 4 % of patients respectively. The most common side effects were pruritus (56 %) and colitis (13 %).
TABLE 2.
Response to ipilimumab (total patient cohort and patients with immune correlative data)
| Total cohort Week 12 (n = 23) | Week 24 (n = 21) | Patients with immune data Week 12 (n = 10) | Week 24 (n = 10) | |
|---|---|---|---|---|
| Partial response | 17 % | 5 % | 20 % | 0 % |
| Stable disease | 48 % | 71 % | 50 % | 80 % |
| Progressive disease | 35 % | 24 % | 30 % | 20 % |
The 23 patients underwent 34 surgical procedures a median of 27 weeks after initiation of ipilimumab (1–123 weeks). Surgery was performed a median of 25 days after the last dose (range 3–74 days); five patients had surgery within 1 week of their last dose of ipilimumab. The indication for surgery was symptomatic disease in 50 %, a progressive focus of disease in 41 %, and an isolated focus of residual disease in only 9 % of cases (Table 3). Surgical procedures included: subcutaneous metastasectomy (39 %), laparotomy (35 %) with half of these requiring bowel resections, and lymph node resection (9 %). Five patients (15 %) required craniotomy for symptomatic brain metastases.
TABLE 3.
Indications for surgery stratified by site of disease
| Metastasectomy site | n (%) | Isolated disease | Progressive disease | Symptomatic disease |
|---|---|---|---|---|
| Subcutaneous | 12 (35 %) | 2 | 5 | 5 |
| Intra-abdominala | 11 (32 %) | 0 | 7 | 4 |
| Brain | 5 (15 %) | 0 | 0 | 5 |
| Nodal | 3 (9 %) | 1 | 2 | 0 |
| Otherb | 3 (9 %) | 0 | 0 | 3 |
Five of these cases involved a bowel resection and anastomosis
One patient had surgery as a result of ipilimumab toxicity
Surgical complications were minimal. Grade 1 wound complications were seen in 17 % of patients. Only one patient had a Grade 2 wound complication. No Grade 3–5 complications were seen.
After a median follow-up from starting ipilimumab of 86 weeks, three patients had no evidence of disease (13 %), ten are alive with disease (43 %), and ten have died of disease (43 %; Fig. 1). The median survival from the time of first surgery for the group of patients with isolated progressive disease was 9.5 months compared with 5.3 months for patients having surgery for symptomatic disease (p = 0.2).
FIG. 1.
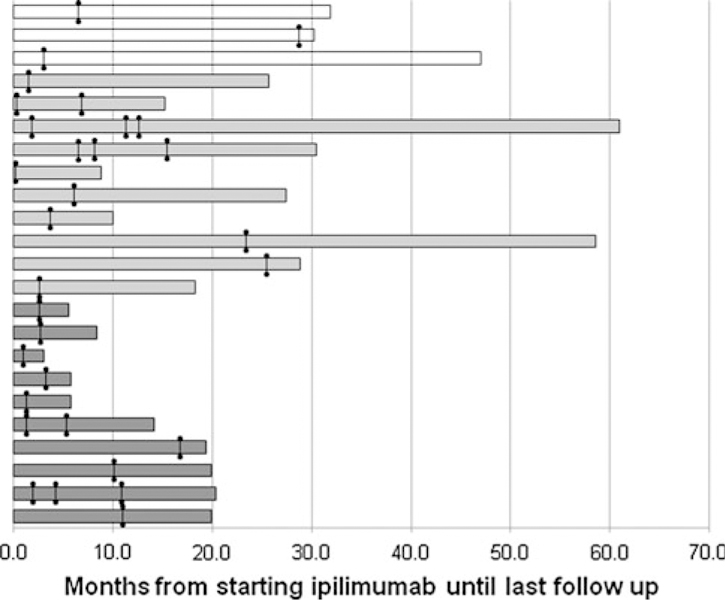
Outcomes of patients after starting ipilimumab. Each line represents an individual patient. Cross marks represent the time of surgery. Status at last follow-up is reflected in the shading (white bars NED, light gray bars AWD, dark gray bars DOD)
Correlative Immunological Data
Ten patients with matched tumor samples and peripheral blood were studied. These patients’ characteristics were representative of the larger cohort (Table 1). They also had similar responses to ipilimumab; 50 % experienced stable disease at 12 weeks and 80 % had stable disease at 24 weeks (Table 2). The indications for surgery in these patients were a progressive focus of disease in 60 % of patients and symptomatic disease in the remaining 40 %. Four of these resections were intra-abdominal, three subcutaneous, two nodal, and one was a spinal metastasis.
Tumor-infiltrating lymphocytes (TILs) and peripheral blood mononuclear cells (PBMCs) were analyzed for subpopulations of effector T cells (T eff; CD3+CD8+), regulatory T cells (T reg; CD3+CD4+FOXP3+), PD-1+ CD4+ cells, and PD-1+CD8+ cells.
Among individual patients, there was no clear difference in the percentage of CD3+CD4+ and CD3+CD8+ T cells between the TILs and PBMCs (Fig. 2a). Subset analysis, however, demonstrated an almost fourfold increase in the fraction of T regs (CD4+FOXP3+) in TILs compared with PBMCs (19.8 vs. 5.2 %, p = 0.002). This difference was seen in all patients analyzed (Fig. 2b). Furthermore, there was 2.8-fold lower ratio of T eff/T reg cells (CD8+/CD4+FOXP3+) in the TILs compared with PBMCs (p = 0.02; Fig. 2c).
FIG. 2.
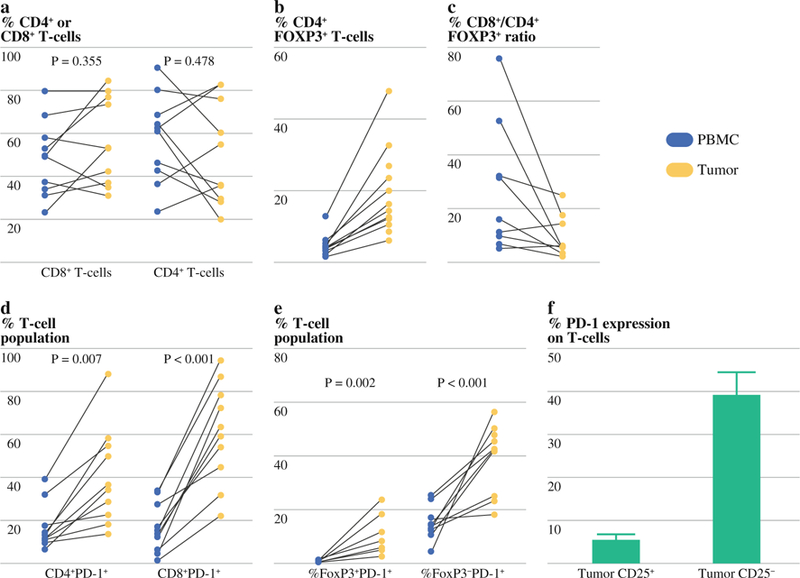
Flow cytometry analysis of subfractions of tumor peripheral blood mononuclear cells (PBMC) and tumor infiltrating lymphocytes (TILs): CD8+ and CD4+ subfractions in PBMC and tumors (a), CD4+FOXP3+ T cell subset as a fraction of the lymphocyte population in PBMCs compared with TILs (b), T eff:T reg ratio in PBMCs compared to TILs (c), PD-1+ subfraction of CD4+ and CD8+ populations in PBMC and TILs (d), FoxP3 expression on PD1+ subfraction of TILs (e), PD1 expression on CD25+ and CD25− subset of TILs (f)
Analysis of resected tumors also revealed a higher frequency of PD-1 expressing TILs compared to peripheral blood. The mean CD4+PD-1+ fraction size was 16.8 % in PBMC and 40.6 % in TILs. In the CD8+PD-1+ fraction, the difference was more striking with a mean of 16.4 % in PBMC and 60.9 % in TILs. Greater than 50 % of TILs were CD8+PD1+ in 70 % of the tumors sampled (Fig. 2d). The majority of the PD-1+ T cells were negative for Foxp3 and CD25 (Fig. 2e, f).
In these ten tumor samples, analysis of TIL subsets revealed interesting differences according to tumor response. Patients experiencing a partial response to ipilimumab at 24 weeks had a 2.3-fold higher CD8:CD4 fraction in the resected (progressing) tumor compared with those with stable disease (p = 0.1). No difference was seen in FoxP3 expression (p = 0.59) or PD-1 expression (p = 0.63) between the resected tumors of patients responding and progressing on ipilimumab.
DISCUSSION
In this highly selected group of patients, surgery after administration of ipilimumab was not associated with an increase in adverse events. This was the case even in the five patients operated on within 1 week of receiving a dose of ipilimumab, including a bowel resection. Prior studies have demonstrated the safety of neoadjuvant ipilimumab 11; however, in that study, surgery was scheduled 4 weeks after the last dose of antibody. While neoadjuvant studies are currently underway in melanoma and prostate cancer (www.clinicaltrials.gov), some do require a 4-week interval between the administration of ipilimumab and surgery. The data from this small patient cohort suggest that there is no reason to delay surgery from a complication standpoint.
Metastasectomy for stage IV disease in carefully selected patients has been associated with durable clinical response with median survival after complete metastasectomy of approximately 16–39 months.12–14 Best results are seen in patients with oligometastatic disease.15,16 Previously, elective surgery for asymptomatic melanoma metastasis has been limited to patients in whom all sites of metastatic disease can be resected.17,18 In patients undergoing immunotherapy, durable responses can be seen with disease stabilization and not necessarily regression hence the implementation of immune-related response criteria.19 This study demonstrates a median survival of 9.5 months from surgery in patients with resection of foci of progressive disease in the setting of other sites of stable disease. Two patients are still being followed beyond 34 months, one of whom has had an eventual complete response and is currently without evidence of disease. In carefully selected patients, an isolated progressive lesion in the setting of otherwise stable disease should be considered for metastasectomy, because the phenotype of this particular progressing tumor may not allow for a response to immunotherapy.
In the current study, we observed high expression of PD-1 on TILs harvested from tumors that were not otherwise responding to ipilimumab. We did not observe a difference in PD-1 expression in the tumors of patients based on response; however, the resected specimen was progressing or stable in all cases and that was the indication for surgery. Therefore, this resected tumor does not necessarily reflect the immune milieu in the other sites of disease. Furthermore, the number of patients with immune correlative data is small. The high expression of PD-1 on TILs is however an important observation as PD-1 has evolved as a marker for impaired TILs and therapeutic blocking PD-1 antibodies are in clinical use. Tumor infiltrating lymphocytes with high PD-1 expression harvested from renal cell carcinomas have impaired T cell effector function and are associated with an increased risk of cancer-specific mortality.20 In a study of stage IV melanoma,21 the majority of NY-ESO-1–specific PBMC CD8+ T cells express PD-1, and blockade of PD-1/PD-L1 increased the number of proliferating and cytokine-producing activated NY-ESO-1–specific CD8+ T cells. Furthermore, anti-PD-1 and anti-PD-L1 antibodies have been shown to have significant clinical efficacy in patients with melanoma,4,22 and current studies are investigating the role of combination therapy with ipilimumab and anti-PD-1 (www.clinicaltrials.gov).
This study is limited by its small sample size. We have attempted to keep the patient group as homogeneous as possible by only including patients undergoing surgery within a certain time window following ipilimumab therapy (approximately 2 half lives of drug). Furthermore, the immunological correlative data would be strengthened by analyzing the immune phenotype of tumors that have responded to therapy; however, these tumors are not resected outside of a clinical trial and therefore we did not have access to this data.
It is not known whether the high PD-1 and Foxp3 expression within the tumors of patients undergoing resection represents a change from pretreatment levels or a sustained immunosuppressive phenotype. Future studies analyzing tumors from responding and nonresponding lesions in a prospective fashion will provide insight into the changes after therapy. This study supports the fact that the TIL CD8:CD4 ratio is critical for tumor control. Furthermore, these data support the combination of treatments with anti-CTLA-4 and anti-PD-1 antibodies that are currently underway in clinical trials.
Footnotes
Poster presented at American Society of Clinical Oncology Annual Meeting, Chicago, 2012.
DISCLOSURES CA and JW serve on advisory board for BMS; JPA is inventor of intellectual property licensed to BMS.
REFERENCES
- 1.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363(8):711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011;364(26):2517–26. [DOI] [PubMed] [Google Scholar]
- 3.Prieto PA, Yang JC, Sherry RM, et al. CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res 2012;18(7):2039–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366(26):2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci USA 107(9):4275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hodi FS, Butler M, Oble DA, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci USA 2008;105(8):3005–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest 2006;116(7):1935–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobs JF, Nierkens S, Figdor CG, de Vries IJ, Adema GJ. Regulatory T cells in melanoma: the final hurdle towards effective immunotherapy? Lancet Oncol 2012;13(1):e32–42. [DOI] [PubMed] [Google Scholar]
- 9.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240(2):205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan J, Page DB, Ku GY, et al. Correlation of clinical and immunological data in a metastatic melanoma patient with heterogeneous tumor responses to ipilimumab therapy. Cancer Immun 2010;10:1. [PMC free article] [PubMed] [Google Scholar]
- 11.Carthon BC, Wolchok JD, Yuan J, et al. Preoperative CTLA-4 blockade: tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin Cancer Res 2010;16(10):2861–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002;8(8):793–800. [DOI] [PubMed] [Google Scholar]
- 13.Morton DLM,N; Kashani-Sabet M, Thompson JF, Kelley MC, et al. Long-term cure after complete resection and adjuvant immunotherapy for distant melanoma metastases (Suppl abstr 8534) vol 30 Chicago: American Society of Clinical Oncology (ASCO); 2012. [Google Scholar]
- 14.Howard JH, Thompson JF, Mozzillo N, et al. Metastasectomy for distant metastatic melanoma: analysis of data from the first Multicenter Selective Lymphadenectomy Trial (MSLT-I). Ann Surg Oncol 2012;19(8):2547–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neuman HB, Patel A, Hanlon C, Wolchok JD, Houghton AN, Coit DG. Stage IV melanoma and pulmonary metastases: factors predictive of survival. Ann Surg Oncol 2007;14(10):2847–53. [DOI] [PubMed] [Google Scholar]
- 16.Tafra L, Dale PS, Wanek LA, Ramming KP, Morton DL. Resection and adjuvant immunotherapy for melanoma metastatic to the lung and thorax. J Thorac Cardiovasc Surg 1995;110(1): 119–28, discussion 129. [DOI] [PubMed] [Google Scholar]
- 17.Leung AM, Hari DM, Morton DL. Surgery for distant melanoma metastasis. Cancer J 2012;18(2):176–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong JH, Skinner KA, Kim KA, Foshag LJ, Morton DL. The role of surgery in the treatment of nonregionally recurrent melanoma. Surgery 1993;113(4):389–94. [PubMed] [Google Scholar]
- 19.Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 2009;15(23):7412–20. [DOI] [PubMed] [Google Scholar]
- 20.Thompson RH, Dong H, Lohse CM, et al. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin Cancer Res 2007;13(6):1757–61. [DOI] [PubMed] [Google Scholar]
- 21.Fourcade J, Kudela P, Sun Z, et al. PD-1 is a regulator of NY-ESO-1-specific CD8 + T cell expansion in melanoma patients. J Immunol 2009;182(9):5240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366(26):2455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]


