Abstract
Purpose
Diabetic neuropathy is a prolonged symptom of diabetes mellitus that affect a number of diabetes mellitus patients. So far, the variants of diabetic neuropathy, either painful (PDN) or non-painful (or painless, non-PDN) response have distinctive clinical entities. This study aims to determine the effects of oxidative stress parameters and pro-inflammatory factors at spinal cord level of streptozotocin-induced diabetic neuropathy rat model.
Methods
Thirty Sprague-Dawley rats were randomly assigned to control (non-diabetic), PDN and non-PDN groups (n = 10). The rats were induced with diabetes by streptozotocin injection (60 mg/kg). Tactile allodynia and thermal hyperalgesia were assessed on day 0, 14 (week 2) and 21 (week 3) in the rats. The rats were sacrificed and the spinal cord tissue was collected for the measurement of oxidative stress (malondialdehyde (MDA), superoxide dismutase (SOD) and catalase) and pro-inflammatory markers (interleukin-1β (IL-1β) and tumour necrosis factor-α (TNF-α)).
Results
PDN rats demonstrated a marked tactile allodynia with no thermal hyperalgesia whilst non-PDN rats exhibited a prominent hypo-responsiveness towards non-noxious stimuli and hypoalgesia towards thermal input. The MDA level and pro-inflammatory TNF-α was significantly increased in PDN rats whilst catalase was reduced in these rats. Meanwhile, non-PDN rats demonstrated reduced SOD enzyme activity and TNF-α level and increased MDA and catalase activity.
Conclusion
The changes in oxidative stress parameters and pro-inflammatory factors may contribute to the changes in behavioural responses in both PDN and non-PDN rats.
Keywords: Painful diabetic neuropathy, Non-painful diabetic neuropathy, Tactile allodynia, Thermal hyperalgesia, Oxidative stress markers, Pro-inflammatory markers
Introduction
Diabetic peripheral neuropathy is a type of neuropathic pain which results from long term effects of diabetes mellitus (DM) of either type I or II. It is reported that 66% of type I DM patients and 59% of type II patients would develop diabetic peripheral neuropathy [22]. Diabetic peripheral neuropathy, as defined by Toronto Expert Panel, is a symmetrical, length-dependent sensorimotor polyneuropathy attributable to metabolic and microvessel modifications as an outcome of chronic hyperglycaemia exposure (diabetes) and cardiovascular risk co-variates [26]. Diabetic neuropathy may arise from multiple causes. The putative mechanisms leading to development of diabetic peripheral neuropathy is believed to derive from glycosylation of neural proteins, microangiopathy, development of neural autoantibodies and ischaemia from the basement membrane thickening of nerve capillaries [25]. The nerves of diabetic peripheral neuropathy patients are damaged and as this disease progresses, symptoms may worsen over a number of years. Generally the development of diabetic neuropathy can be found as painful response as diabetic patients experience tingling, burning and pain sensation. This condition is known as PDN. However, the development of diabetic neuropathy may also result in gradual decrease of peripheral sensation with eventual complete loss of sensations to heat, cold, pressure or pain, and this condition is termed as non-PDN. In rats, short-term diabetes may give rise to development of PDN while longer term diabetes may lead to both presentations of PDN and non-PDN, or non-PDN only [18].
Painful diabetic neuropathy (PDN) is a variant of diabetic peripheral neuropathy, which is considered as one of the most devastating complications of DM [17]. PDN is reported to affect approximately 18% of adult diabetic patients compared with a minimum of 30% of patients with overall diabetic peripheral neuropathy [23]. Vinik [31] defines PDN as “multifactorial and can occur at different stages, beginning at the peripheral sympathetic nervous system in skin (C fibres) and propagating to involve Aβ and Aδ fibres to yield allodynia, and lastly, within central nervous system, spontaneously as opposed to provoked pain”. Sleep disturbance, depression, anxiety and poor quality of life are frequently complained by PDN patients that highly influence productivity at work and increased consumption of health care resource [8, 23]. As aforementioned, patients with PDN normally complain bilateral tingling or burning sensations (paresthesia) that spontaneously increases or decreases in intensity over a length of time. Other than that, they may also experience heightened sensitivity to stimuli such as touch (allodynia), temperature changes or pressure application that results in excruciating pain with the slightest changes [13]. Medication to treat PDN is challenging and not fully satisfying as PDN is not easy to cure. PDN may be progressive and leads to a continual decrease in peripheral sensation. In severe condition, PDN may lead to complete loss of sensation.
Non-painful or painless diabetic neuropathy (non-PDN) is considered as another variant of diabetic neuropathy which is often overlooked or forgotten. It is commonly associated with increased vibration and thermal perception thresholds that progress to loss of sensory perception and deterioration of all fibre types in peripheral nerve [18]. Patients with non-PDN frequently appear asymptomatic or may detect numbness, cold feet (from autonomic shunting of blood from the skin) and/or dry skin (from denervation of sweat glands) [24]. Non-PDN is actually dangerous as patients are incapable of detecting an injury to foot and at a higher risk of getting foot ulcer, infections and even amputation [25].
There are inconsistent reported clinical manifestation of PDN and non-PDN in diabetic neuropathy patients. Tsigos et al. [27] discovered that PDN was related with small somatic fibre malfunction i.e. cooling and warming thresholds and preserved sympathetic nerve activity (plasma noradrenaline) with various types of abnormalities in large somatic fibres. In contrast, Krämer et al. [14] did not find any distinguishable difference in thermal threshold between PDN and non-PDN. In fact, pathogenesis of diabetic neuropathy is poorly investigated even though impaired cutaneous endothelium-associated vasodilation and C-fibre-mediated vasoconstriction as well as elevated sural nerve epineurial blood flow in diabetic PDN patients are detected, compared to non-PDN subjects [18]. In specific, the existing studies on animal models of PDN and non-PDN have serious limitations [3]. Therefore, in a diabetic rat model induced with streptozotocin that represent early development to diabetic neuropathy, this study aimed to understand mechanisms involving oxidative stress parameters and pro-inflammatory factors on tactile allodynia and thermal hyperalgesia in both painful and non-painful (painless) variants of diabetic neuropathy. In addition, we also aimed to uncover the association of oxidative stress parameters and pro-inflammatory factors levels in spinal dorsal horn with tactile allodynia and thermal hyperalgesia in PDN and non-PDN rats model. We hypothesized that there are distinctive changes in oxidant-antioxidant and pro-inflammatory factors levels between both variants at early development of diabetic neuropathy in spinal cord of streptozotocin-induced diabetic rat model.
Materials and methods
Animals
Thirty Sprague-Dawley (SD) male rats (8–10 weeks old, 200–230 g) with initial weight ranged from 200 to 230 g were used. Animals were housed individually under standard laboratory environment and allowed free access to water and food sources. The animals were acclimatized to experimental environment for four days in Animal Research and Service Centre, Universiti Sains Malaysia, Malaysia before beginning the experimentation. Procedures in the present methodology were approved by Animal Ethics Committee, Universiti Sains Malaysia, Malaysia [USM/Animal Ethics Approval/2014 (91) (560)]. The rats were randomly assigned into three different groups (n = 10): (1) non-diabetic rat (Control), (2) diabetic rats with painful response (PDN) and (3) diabetic rats with non-painful response (non-PDN).
Induction of diabetes
Thirty-eight SD rats were induced with diabetes mellitus (DM) by intraperitoneal injection of streptozotocin (STZ) at 60 mg/kg (Sigma Aldrich, Germany) dissolved in ice-cold citrate buffer pH 4.5 as a vehicle. Ten SD control rats (non-diabetic, age-matched) were administered with vehicle intraperitoneally. Meanwhile, other 28 rats were subjected to STZ injection, i.p. The rats were given 10% glucose solution for 24 h to prevent hypoglycaemia [9]. Diabetes was confirmed at 72 h after the STZ injection by measuring blood glucose level with pin-prick at rat’s tail using a glucometer (Accu-Chek Performa, Roche Diagnostics, Paris, France). Only animals with a final blood glucose ≥15 mmol/L were included in the study. The blood glucose level was measured on day 0 (baseline; before diabetic induction), day 3, day 14 (week 2) and day 21 (week 3) throughout the experimental period.
Behavioural tests paradigm
Von-Frey and hot-plate tests were conducted before the diabetic induction (day 0), day 14 (week 2) and day 21 (week 3).
Tactile stimulus: von-Frey test
Rats were placed on a wire mesh floor separated by compartments individually. They were allowed for adaptation to room environment for 15 min before starting the assessment. Nociceptive withdrawal threshold, expressed in grams, was measured using a von-Frey test (Bioseb, USA) by applying increasing pressure of a semi-flexible microfilament (tip diameter of probe: 0.01 mm, weight: 50 g) to both right and left hind paws. The pressure was given until either an obvious retraction, licking or jumping was detected. The pressure applied by the filament which induced hind paw withdrawal represented a maximal threshold. Five measurements were taken with three of precise readings were selected and averaged [16]. The stimulation of nociception was employed to both hind paws separated by 10 min interval from the previous tactile stimulation of the paw [32].
On day 14 (pre-intervention day), the classification of either painful (PDN) or non-painful diabetic neuropathy (non-PDN) was performed followed a method by Daulhac et al. [6]. Diabetic rats were considered as having hyperalgesia if the reduction in the noxious withdrawal threshold assessed with von-Frey test was more than 15% of baseline value before diabetic induction (day 0). This rat was regarded as having painful response of diabetic neuropathy (PDN). Meanwhile, the diabetic rats that have reduction in noxious withdrawal threshold by von-Frey test of less than 15% from baseline value before the diabetic induction were regarded as having non-painful response of diabetic neuropathy (non-PDN), therefore, were assigned into non-PDN group.
Thermal stimulus: hot-plate test
Hot-plate test was applied to evaluate effects of the intervention on thermal pain threshold following Hunskaar et al. [10]. The rats were placed on the hot-plate at constant temperature of 52.5 °C. Duration from the onset of heat stimulation to the responses produced either paw licking, flicking or jumping was recorded, in which represents as reaction time. Cut off value was 30 s and each animal was tested only once to prevent adaptation.
Oxidative stress markers
Malondialdehyde
Malondialdehyde (MDA) level was assessed in spinal cord tissue sample by using an available commercial enzyme-linked immunoabsorbent assay (ELISA) kit (Northwest Life Sciences, USA). Spinal cord sample was initially added into a vial containing butylated hydroxytoluene (BHT) followed by the addition of Acid reagent and thiobarbituric acid (TBA) reagent respectively. The mixture was vigorously vortex to ensure the complete reactions of chemicals with the sample. The vial was then incubated at 60 °C for an hour before being centrifuged at 10000 x g for 3 min. Absorbance of the mixture was measured at 400–700 nm wavelengths using a spectrophotometer and the highest peak of the sample was recorded (i.e 532 nm). A third derivative was performed by measuring reaction in the sample mixture at 532 nm wavelength with an ELISA reader (PerkinElmer, UK). The obtained results were presented as mmol/mg protein.
Catalase
Catalase enzyme activity in spinal cord tissue samples was measured using an available commercial kit (EnzyChrom™ Bioassay Systems, USA). The samples were initially added into a 96-wells flat-bottom microplate followed by addition of hydrogen peroxide (H2O2) Substrate. The mixture was incubated for 30 min at room temperature. After the incubation, Detection reagent was added into the wells and re-incubated for 10 min at room temperature before the absorbance of H2O2 reaction was measured at 570 nm by an ELISA reader. The obtained result was presented as U/mg protein.
Superoxide dismutase
Superoxide dismutase (SOD) enzyme activity in spinal cord tissue samples was measured using an available commercial kit (Abnova, Taiwan). Working reagent which is comprised of Assay Buffer, Xanthine and WST-1 was added into the samples in 96-wells flat-bottom microplate. The plate was tapped gently before xanthine oxidase reagent was added into the wells. The plate was immediately measured at 440 nm for absorbance at 0 min (OD0) and incubated for an hour at room temperature in dark. The absorbance was re-measured after the incubation at the similar wavelength (OD60). Calculation of the sample was done by using the formula ΔOD = OD60 – OD0 and presented as U per mg protein.
Protein concentration
Principle of protein concentration measurement is based on a modified Lowry method using bicinchoninic acid and protein concentration in the samples was measured using an available commercial kit (Fisher Scientific, USA). Working solution was added into a 96-wells flat-bottom microplate containing samples. The mixture was incubated at 37 °C for 30 min before being measured at 562 nm within 10 min after the incubation.
Pro-inflammatory cytokines
Tumour necrosis factor-α
Tumour necrosis factor-α (TNF-α) level in samples was determined by using an available commercial ELISA kit (RayBiotech®, USA). The samples were initially added into TNF-α-coated wells and incubated for 2.5 h at room temperature with a gentle shaking. The plate was washed and biotinylated antibody was added into each well and re-incubated for an hour at room temperature. Streptavidin solution was then added after the washing of the plate and re-incubated at room temperature for 45 min. It is followed by addition of One-Step Substrate reagent after the washing of the plate and re-incubated at room temperature in dark. Finally, the reaction was halted by adding Stop Solution and the reaction was immediately measured at 450 nm using an automated ELISA reader.
Interleukin-1β
Interleukin-1β (IL-1β) level in the samples was determined by using an available commercial ELISA kit (Cusabio, China). The samples were initially added into IL-1β-coated microplate wells and incubated for two hours at 37 °C. The liquid was then completely removed before Biotin-antibody was added into the wells and re-incubated for an hour at 37 °C. The plate was then washed with Wash Buffer and HRP-avidin was added into the wells and re-incubated at 37 °C for an hour. The plate was again washed and 3,3′,5,5’-Tetramethylbenzidine (TMB) substrate was added into the wells and re-incubated for 15 min at 37 °C in dark. Finally, Stop Solution was added into the wells and the absorbance was read at 450 nm within 5 min with an ELISA reader.
Statistical analysis
Data were analyzed using Statistical Package for Social Sciences (SPSS) version 22 (IBM, USA). Distribution of data was initially determined before any statistical tests were conducted. The data for MDA level, catalase and SOD enzymes activities, IL-1β and TNF-α levels were analyzed by one-way ANOVA with post-hoc LSD or Dunnett’s T3 test. Meanwhile, the data for paw withdrawal threshold by von-Frey test and thermal threshold by hot-plate test were analyzed by one-way repeated measures ANOVA with post-hoc LSD test. Pearson coefficient correlation was carried out to explore the relationships between paw withdrawal thresholds and thermal hyperalgesia with oxidative stress markers and pro-inflammatory factors. The data were expressed as mean ± standard error of mean (SEM) or standard deviation (SD) and the p value of less than 0.05 was considered as significant.
Results
Reaction to pain stimuli
Tactile stimuli
Before STZ or citrate buffer injection, paw withdrawal thresholds were not significantly different between the groups on both left and right hind paws. The paw withdrawal thresholds in both left and right hind paws in control group were remain unchanged throughout three weeks of experimentation. A progressive reduction in paw withdrawal threshold was seen in diabetic PDN group whilst a progressive increased in paw withdrawal threshold was detected in diabetic non-PDN group compared to control group for both left and right hind paws (p < 0.001). There was a significant difference in the paw withdrawal threshold between diabetic PDN and non-PDN groups at Day 14 and 21 after STZ injection in both hind paws (p < 0.001) (Fig. 1a and b).
Fig. 1.
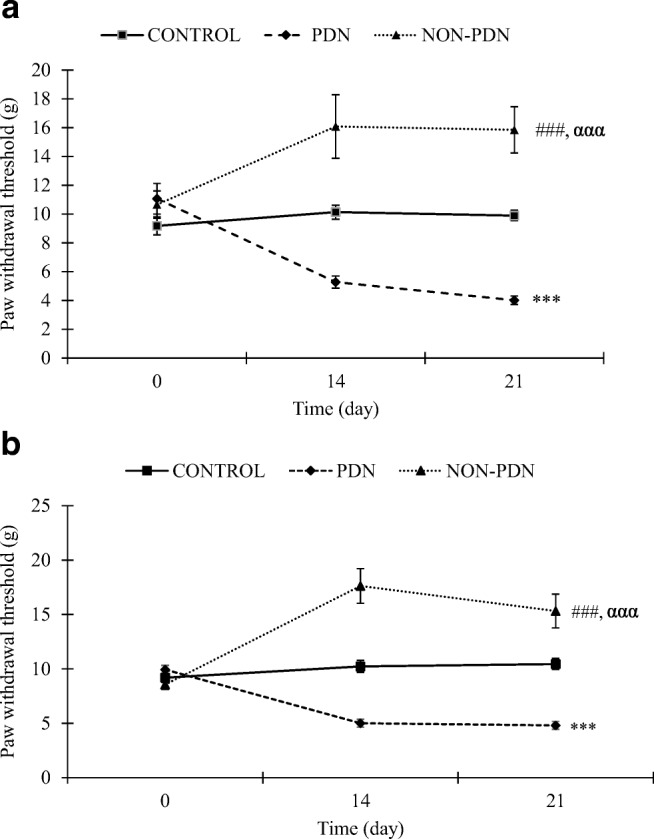
Time course of paw withdrawal threshold in diabetic PDN and non-PDN and control groups in a left hind paw and b right hind paw. Values are expressed as mean ± SEM (n = 10). ***p < 0.001 comparison between PDN and control groups, ###p < 0.001 significant between non-PDN and control groups and ααα p < 0.001 significant between non-PDN and PDN groups
Thermal stimuli
Before STZ injection, reaction time for thermal threshold did not differ between the groups. However, two weeks after diabetic induction, the reaction time was significantly increased in diabetic non-PDN group compared to control and diabetic PDN groups (p < 0.001). The reaction time in diabetic non-PDN group was maintained increase in third week (Day 21) compared to control and diabetic PDN groups (p < 0.001). However, there was no significant change in the reaction time for thermal threshold in second week (Day 14) of diabetic PDN group compared to control group. The reaction time showed a trend of reduction in diabetic PDN group which was not statistically significant compared to control group in the third week (Day 21) (Fig. 2).
Fig. 2.
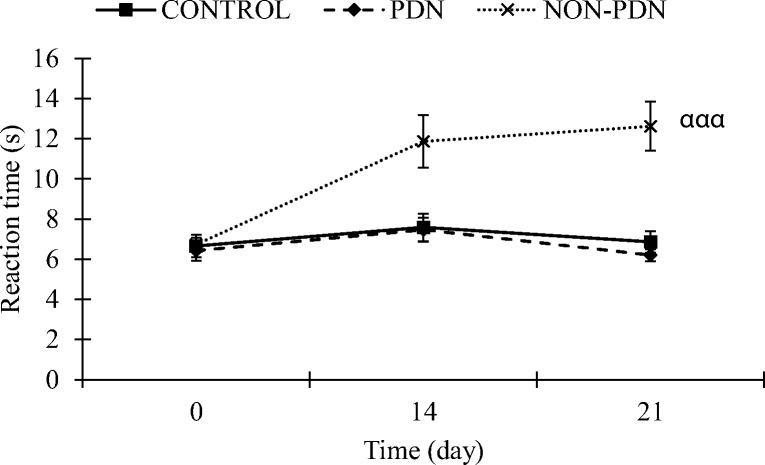
Thermal threshold values of diabetic PDN, non-PDN and control groups submitted to hot-plate test on Day 0, 14 and 21. The values represent mean ± SEM (n = 10). ααα p < 0.001 comparison between non-PDN to control and PDN groups
Oxidative stress markers
Analysis by one-way ANOVA of oxidative stress markers demonstrated significant effect in MDA level (p < 0.001), catalase (p < 0.05) and SOD (p < 0.05) enzymes activities between the groups. In specific, post-hoc Dunnett’s T3 test revealed that MDA level in PDN group was significantly increased compared to control group (p < 0.05). Meanwhile, catalase enzyme activity was significantly reduced in PDN group compared to control group (p < 0.05). However, no changes was detected in SOD enzyme activity in PDN group compared to control group (Table 1).
Table 1.
Oxidative stress markers and pro-inflammatory cytokines levels in the spinal cord of control, PDN and non-PDN groups. Values are expressed as mean ± SD (n = 10)
| Group | Oxidant-antioxidant status (per mg protein) | Pro-inflammatory cytokines (× 103 pg/mL) | |||
|---|---|---|---|---|---|
| MDA (mU) | Catalase (mmol) | SOD (U) | TNF-α | IL-1β | |
| Control | 15.952 ± 4.687 | 363.442 ± 5.661 | 8.2 ± 5.661 | 3.261 ± 1.150 | 80.258 ± 48.082 |
| PDN | 22.065 ± 2.337* | 181.038 ± 46.332# | 11.351 ± 3.793 | 40.812 ± 30.15*** | 102.647 ± 19.736 |
| Non-PDN | 29.868 ± 5.261###, ααα | 312.217 ± 12.079*** | 3.581 ± 0.879#, ### | 27.954 ± 17.566# | 83.7 ± 24.747 |
*p < 0.05, ***p < 0.001 significant between PDN and control groups, #p < 0.05, ###p < 0.001 significant between non-PDN with control groups and ααα p < 0.001 significant between non-PDN and PDN groups
Meanwhile, post-hoc Dunnett’s T3 test also showed a dramatic increase in MDA level of non-PDN group compared to control and PDN groups (p < 0.001). Meanwhile, catalase enzyme activity was significantly increased in non-PDN group compared to PDN group (p < 0.001) but insignificant compared to control group. Furthermore, non-PDN also demonstrated a marked reduction in SOD enzyme activity compared to control (p < 0.05) and non-PDN (p < 0.001) groups (Table 1).
Pro-inflammatory factors
There was no significant effect of IL-1β level between the groups (p > 0.05) in spinal cord. Meanwhile, there was a significant difference in TNF-α level between the groups. Post–hoc Dunnett’s T3 test demonstrated a significant increase in TNF-α level in PDN and non-PDN groups compared to control group (p < 0.05) and no significant difference in TNF-α level between PDN and non-PDN groups (Table 1).
Relationships between the parameters investigated
Pearson coefficient correlation revealed no significant correlation between thermal withdrawal threshold with oxidative stress markers and pro-inflammatory factors investigated. However, there was a significant, positive correlation detected between paw withdrawal threshold of both left (r = 0.620 (moderate correlation), p < 0.001) and right (r = 0.362 (weak correlation), p < 0.05) hind paws and catalase enzyme activity. Significant, positive correlations were also detected between paw withdrawal threshold of left (r = 0.568 (strong correlation), p < 0.05) and right (r = 0.482 (weak correlation), p < 0.05) hind paws with SOD enzyme activity. In addition, there was also a significant, negative and moderate correlation between paw withdrawal threshold of left (r = −0.536, p < 0.05) and right (r = −0.563, p < 0.05) hind paws with MDA level (Table 2).
Table 2.
Relations between results of paw withdrawal threshold (left and right hind paws) by von Frey test with malondialdehyde level, catalase and superoxide dismutase enzymes activities
| MDA (mU/mg protein) | Catalase (mmol/mg protein) | SOD (U/mg protein) | ||
|---|---|---|---|---|
| Paw withdrawal threshold (left hind paw) | Pearson correlation Sig. (2-tailed) (r) | −0.536* | 0.620*** | 0.568* |
| Paw withdrawal threshold (right hind paw) | Pearson correlation Sig. (2-tailed) | −0.563* | 0.362* | 0.482* |
*Correlation is significant at the 0.05 level (two-tailed)
***Correlation is significant at the 0.001 level (two-tailed)
Discussion
Tactile allodynia and spontaneous pain has been observed in a number of patients with DM, indicating painful type of diabetic neuropathy, which produces seriously devastating symptoms of pain. Present study has shown that induction of DM (Type I DM) in rats caused disturbances in the responses to nociceptive or non-noxious stimuli. Decreased threshold of noxious tactile stimuli was observed in von Frey test for PDN rats in both right and left hind paws after two weeks of diabetic induction. Paw withdrawal threshold was found to be progressively reduced at the third week of diabetic induction in PDN rats, indicating PDN rats experienced allodynia throughout the study period. However, results of hot-plate test suggested that PDN rats had no evidence of thermal hyperalgesia throughout the experimentation period compared to control group, which was similarly reported by Malcangio and Tomlinson [15]. It is possible that different types of peripheral nerve fibres are selectively affected in diabetic neuropathy rat model as paw withdrawal responses to thermal signals are related to supraspinal sensory processing [20]. It is also well-known that thermal nociceptive activation does not stimulate as much as mechanoreceptor signals like mechanical stimulation do [1]. The development of tactile allodynia in PDN rats has also been reported by Courteix et al. [4]. It is possible that the development of allodynia in PDN rats is not solely related to the hyperglycaemia as persistent increase of blood glucose level was also observed in non-PDN rats in this study. It is possible that degeneration and regeneration processes at nerve endings causes axonal dystrophy at peripheral nerves of PDN rats [11]. Furthermore, Tsigos et al. [27] also agreed that the occurrence of allodynia in PDN is highly related to dysfunction of small somatic fibres with a wide range of large somatic fibres and autonomic reflex abnormalities. Other than that, downregulation of GABAB receptors on nociceptive fibre terminals [15] in spinal cord of PDN rats may contribute to the development of allodynia and spontaneous inflammatory pain in this group.
Meanwhile, increased paw withdrawal threshold (reduced tactile allodynia) in non-PDN rats was shown at two weeks after diabetic induction in both right and left hind paws and maintained reduced in paw withdrawal threshold on the third week post-STZ injection, indicating non-PDN rats experiencing hypoalgesia throughout the experimentation. Moreover, thermal hypoalgesia was identified in non-PDN rats started at two weeks after the diabetic induction. Although there are limited studies investigating pathogenesis of diabetic neuropathy, especially non-painful diabetic neuropathy, a number of studies reported manifestation of elevated vibration and mechanical hypoalgesia in the rats with non-PDN [6, 18]. It is postulated that the occurrence of allodynia in PDN rats and the occurrence of hyporesponsiveness to tactile allodynia and thermal hypoalgesia in non-PDN rats could be associated with changes in peroneal motor conduction velocity that tend to distinguish between occurrence of those variants [27]. In a clinical study, Britland et al. [2] suggested that loss of sensation in patients with non-PDN could be associated with worse myelinated fibre loss and less fibre regeneration compared to patients with PDN, as the non-PDN patients were demonstrated to have a greater depletion of myelinated fibre density, fibre area and axon area along with evidence of less-efficient myelinated fibre regeneration. It is highly possible that the elevated sensory thresholds for pain, temperature and touch in non-PDN rats as assessed by von Frey and hot-plate tests possibly due to the severe dysfunction and loss of all nerve fibre populations compared to PDN rats [27]. However, in specific, magnitude of abnormalities in myelinated and unmyelinated nerve fibres between PDN or non-PDN could not be distinguished as discovered by Tsigos et al. [27] in a clinical study. There could be perhaps other impairments that may distinguish between both complications which yet to be explored.
Biochemical alteration that occurs during DM interferes with roles of mitochondria leading to overproduction of ROS and reduced antioxidant defence in tissues and cells of diabetic animals. Oxidant MDA, antioxidant SOD and catalase are the endogenous enzymes strictly intertwined with oxidative stress. In this study, oxidant MDA level was found to increase in PDN group, whilst non-PDN group showed the highest MDA level compared to other groups. This result demonstrates that higher increase of lipid peroxidation (determining the high level of free radicals) in non-PDN group logically explain severe deterioration of nerve fibres leading to numbness and amputation. This effect may also suggest reduced pain behaviour score and elevated withdrawal threshold of non-PDN rats in this study. Apart from that, SOD enzyme activity was reported to be quite erratic and demonstrated an irregular pattern in occurrence of diabetes [7, 12, 19, 29]. As in the present study, SOD enzyme activity was shown to be insignificant compared to control group. Besides that, reduced catalase enzyme activity in PDN group may explain the altered oxidant-antioxidant balance in this group, thereby leading to less protection of PDN rat’s spinal cord from antioxidant enzymes. Meanwhile, non-PDN group exhibited a marked reduction in SOD enzyme activity with a significant increase in catalase enzyme activity compared to PDN group. In fact, it is believed that the increase catalase activity in non-PDN group and the reduced catalase activity in PDN group is highly associated with function of noradrenaline hormone. In a clinical study, it was found that PDN patients have preserved sympathetic nerve activity (plasma noradrenaline) whilst non-PDN patients were found to have more severe and uniformly impaired parasympathetic nerve function [27]. Perovic et al. [21] discovered that exogenous noradrenaline injection to rat may reduce the activity of antioxidant catalase enzyme, therefore explaining the reduced catalase activity in PDN, but not in non-PDN group. The oxidant-antioxidant imbalance is highly associated with hyperglycaemic effect as both of PDN and non-PDN groups demonstrated chronic hyperglycaemia throughout the study period. Hyperglycaemia may promote increased production of oxidative stress markers via both enzymatic and non-enzymatic mechanisms of glucose metabolism [5], causes direct damage to nerve parenchyma, reduced blood flow, vascular damage, formation of advanced glycation end products and others in the development of diabetic neuropathy [30].
The present study also revealed increased level of TNF-α and insignificant level of IL-1β in spinal cord of both PDN and non-PDN rats compared to control group whilst no significant difference in the level of these pro-inflammatory cytokines between PDN and non-PDN groups. The increased release of TNF-α level in the PDN rat’s spinal cord is believed to be associated with increased activated microglial expression via extracellular regulated kinase signaling [28]. The overproduction of TNF-α as clearly demonstrated in PDN group may be implicated in the nerve injury-induced tactile allodynia as non-PDN group showed reduced amount of TNF-α and increased paw withdrawal threshold (no tactile allodynia) although it was not statistically significant compared with PDN group. Compared to Pabreja et al. [20], although IL-1β level was not statistically significant compared to control group, but PDN rats did show a slight increase in the IL-1β level in the spinal cord. This insignificant result could be accounted by some methodological differences, such as injection route and period of harvesting the spinal cord samples.
In overall, this study gives an insight to differences in oxidant-antioxidant status and pro-inflammatory factors between PDN and non-PDN variants in order to give some understanding to pathogenesis of diabetic neuropathy. Hopefully this study may contributes some ideas to develop new therapeutic drugs to cure both painful and non-painful diabetic neuropathy in future.
Acknowledgements
We thanked Universiti Sains Malaysia and Ministry of Higher Education for funding of Research University Grant (RUI 1001/PPSK/812139) and Fundamental Research Grant Scheme (FRGS 203/PPSK/6171189), respectively. We thanked all Physiology Laboratory, Central Research Laboratory, Maxillo-Craniofacial Laboratory, Biological Molecular Laboratory staffs and Wan Muhammad Hilmi for direct and indirect assistance and technical work.
Compliance with ethical standards
Conflict of Interest
There is no conflict of interest in the process of preparing this article.
References
- 1.Besson JM, Chaouch A. Peripheral and spinal mechanisms of nociception. Physiol Rev. 1987;67:67–186. doi: 10.1152/physrev.1987.67.1.67. [DOI] [PubMed] [Google Scholar]
- 2.Britland ST, Young RJ, Sharma AK, Clarke BF. Association of painful and painless diabetic polyneuropathy with different patterns of nerve fiber degeneration and regeneration. Diabetes. 1990;39:898–908. doi: 10.2337/diab.39.8.898. [DOI] [PubMed] [Google Scholar]
- 3.Calcutt NA. Potential mechanisms of neuropathic pain in diabetes. Int Rev Neurobiol. 2002;50:205–228. doi: 10.1016/S0074-7742(02)50078-7. [DOI] [PubMed] [Google Scholar]
- 4.Courteix C, Eschalier A, Lavarenne J. Streptozocin-induced diabetic rats: behavioural evidence for a model of chronic pain. Pain. 1993;53:81–88. doi: 10.1016/0304-3959(93)90059-X. [DOI] [PubMed] [Google Scholar]
- 5.Cui XP, Li BY, Gao HQ, Wei N, Wang WL, Lu M. Effects of grape seed proanthocyanidin extracts on peripheral nerves in streptozocin-induced diabetic rats. J Nutr Sci Vitaminol. 2008;54:321–328. doi: 10.3177/jnsv.54.321. [DOI] [PubMed] [Google Scholar]
- 6.Daulhac L, Maffre V, Mallet C, Etienne M, Privat A, Kowalski-Chavuvel A, Seva C, Fialip J, Eschalier A. Phosphorylation of spinal N-methyl-D-aspartate receptor NR1 subunits by extracellular signal-regulated kinase in dorsal horn neurons and microglia contributes to diabetes-induced paiful neuropathy. Eur J Pain. 2011;15:169. doi: 10.1016/j.ejpain.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 7.El-Khatib AS, Moustafa AM, Abdel-Aziz AAH, Al-Shabanah OA, El-Kashef HA. Effects of aminoguanidine and desferrioxamine on some vascular and biochemical changes associated with streptozotocin-induced hyperglycaemia in rats. Pharmacol Res. 2001;43:233–240. doi: 10.1006/phrs.2000.0772. [DOI] [PubMed] [Google Scholar]
- 8.Fishbain DA, Cole B, Lewis JE, Gao J. What is the evidence for chronic pain being etiologically associated with the DSM-IV category of sleep disorder due to a general medical condition? A structured evidence-based review. Pain Med. 2010;11:158–179. doi: 10.1111/j.1526-4637.2009.00706.x. [DOI] [PubMed] [Google Scholar]
- 9.Fröde T, Medeiros Y. Animal models to test drugs with potential antidiabetic activity. J Ethnopharmacol. 2008;115:173–183. doi: 10.1016/j.jep.2007.10.038. [DOI] [PubMed] [Google Scholar]
- 10.Hunskaar S, Berge OG, Hole K. A modified hot-plate test sensitive to mild analgesics. Behav Brain Res. 1986;21:101–108. doi: 10.1016/0166-4328(86)90088-4. [DOI] [PubMed] [Google Scholar]
- 11.Jirmanova I. Giant axonopathy in streptozotocin diabetes of rats. Acta Neuropathol. 1993;86:42–48. doi: 10.1007/BF00454897. [DOI] [PubMed] [Google Scholar]
- 12.Kȩdziora-Kornatowska KZ, Luciak M, Błaszczyk J, Pawlak W. Effect of Aminoguanidine on Erythrocyte Lipid Peroxiclation and Activities of Antioxidant Enzymes in Experimental Diabetes. Clin Chem Lab Med. 1998;36:771–775. doi: 10.1515/CCLM.1998.137. [DOI] [PubMed] [Google Scholar]
- 13.Kline KM, Caroll DG, Malnar KF. Painful diabetic peripheral neuropathy relieved with use of oral topiramate. South Med J. 2003;96 [DOI] [PubMed]
- 14.Krämer HH, Rolke R, Bickel A, Birklein F. Thermal thresholds predict painfulness of diabetic neuropathies. Diabetes Care. 2004;27:2386–2391. doi: 10.2337/diacare.27.10.2386. [DOI] [PubMed] [Google Scholar]
- 15.Malcangio M, Tomlinson DR. A pharmacologic analysis of mechanical hyperalgesia in streptozotocin-diabetic rats. Pain. 1998;76:151–157. doi: 10.1016/S0304-3959(98)00037-2. [DOI] [PubMed] [Google Scholar]
- 16.Martinov T, Mack M, Sykes A, Chatterjea D. Measuring changes in tactile sensitivity in the Hind paw of mice using an electronic von Frey apparatus. J Vis Exp. 2013;82:5212. doi: 10.3791/51212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Obrosova IG. Diabetes and the peripheral nerve. Biochim Biophys Acta. 1792;2009:931–940. doi: 10.1016/j.bbadis.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Obrosova IG. Diabetic painful and insensate neuropathy: pathogenesis and potential treatments. Neurotherapeutics. 2009;6:638–647. doi: 10.1016/j.nurt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Obrosova IG, Fathallah L, Greene D. Early changes in lipid peroxidation and antioxidative defense in diabetic rat retina: effect of DL-α-lipoic acid. Eur J Pharmacol. 2000;398:139–146. doi: 10.1016/S0014-2999(00)00286-7. [DOI] [PubMed] [Google Scholar]
- 20.Pabreja K, Dua K, Sharma S, Padi SS, Kulkarni SK. Minocycline attenuates the development of diabetic neuropathic pain: possible anti-inflammatory and anti-oxidant mechanisms. Eur J Pharmacol. 2011;661:15–21. doi: 10.1016/j.ejphar.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 21.Perovic A, Vuckovic T, Cvijic G, Djordjevic J, Davidovic V. Involvement of nitric oxide in noradrenaline-induced changes in the activity of antioxidant enzymes and lipid peroxidation in rat brown adipose tissue and heart. Physiol Res. 2008;57:95. doi: 10.33549/physiolres.931026. [DOI] [PubMed] [Google Scholar]
- 22.Sadosky A, McDermott AM, Brandenburg NA, Strauss M. A review of the epidemiology of painful diabetic peripheral neuropathy, postherpetic neuralgia, and less commonly studied neuropathic pain conditions. Pain Pract. 2008;8:45–56. doi: 10.1111/j.1533-2500.2007.00164.x. [DOI] [PubMed] [Google Scholar]
- 23.Spallone V, Greco C. Painful and painless diabetic neuropathy: one disease or two? Current Diabetes Report. 2013;13:533–549. doi: 10.1007/s11892-013-0387-7. [DOI] [PubMed] [Google Scholar]
- 24.Tanenberg R. Painless diabetic peripheral neuropathy: the forgotten complication. Journal of Diabetes, Metabolic Disorders & Control. 2016;3:1–2. [Google Scholar]
- 25.Tanenberg RJ. Diabetic peripheral neuropathy: painful or painless. Hospital Physician. 2009;45:1–8. [Google Scholar]
- 26.Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, Lauria G, Malik RA, Spallone V, Vinik A. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33:2285–2293. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsigos C, White A, Young R. Discrimination between painful and painless diabetic neuropathy based on testing of large somatic nerve and sympathetic nerve function. Diabet Med. 1992;9:359–365. doi: 10.1111/j.1464-5491.1992.tb01797.x. [DOI] [PubMed] [Google Scholar]
- 28.Tsuda M, Ueno H, Kataoka A, Tozaki-Saitoh H, Inoue K. Activation of dorsal horn microglia contributes to diabetes-induced tactile allodynia via extracellular signal-regulated protein kinase signaling. Glia. 2008;56:378–386. doi: 10.1002/glia.20623. [DOI] [PubMed] [Google Scholar]
- 29.Van Dam PS, Van Asbeck BS, Bravenboer B, Van Oirschot JF, Gispen WH, Marx JJ. Nerve function and oxidative stress in diabetic and vitamin E-deficient rats. Free Radic Biol Med. 1998;24:18–26. doi: 10.1016/S0891-5849(97)00122-6. [DOI] [PubMed] [Google Scholar]
- 30.Vincent AM, Russel JW, Low P, Feldman EL. Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr Rev. 2004;25:612–628. doi: 10.1210/er.2003-0019. [DOI] [PubMed] [Google Scholar]
- 31.Vinik AI. Advances in diabetes for the millennium: new treatments for diabetic neuropathies. Medscape Gen Med. 2004;6:13. [PMC free article] [PubMed] [Google Scholar]
- 32.Zulazmi NA, Gopalsamy B, Farouk AAO, Sulaiman MR, Bharatham BH, Perimal EK. Antiallodynic and antihyperalgesic effects of zerumbone on a mouse model of chronic constriction injury-induced neuropathic pain. Fitoterapia. 2015;105:215–221. doi: 10.1016/j.fitote.2015.07.011. [DOI] [PubMed] [Google Scholar]


