Abstract
Background
On a global scale, type II diabetes mellitus (T2DM) remain a major health problem and it is the driver for chronic kidney disease (CKD). Despite this association, we still do not have sufficient biomarkers to anticipate better outcomes. N-glycosylation profiles are robust biomarkers and can be used for early monitoring of the progression of T2DM towards CKD.
Methods
In this cross-sectional study, we recruited 241 T2DM patients from January to May 2016. Demographic and anthropometric data were collected, following which fasting blood samples were collected for clinical analyses. Renal function decline was determined by estimation of glomerular filtration rate (eGFR) and N-glycosylation profiles were analysed by Ultra-performance liquid chromatography (UPLC).
Results
The prevalence of undiagnosed CKD was 31.53%. Compared to men, women had a statistically significantly higher HbA1c (p = 0.031), TG (p = 0.015), HDL-c (p < 0.0001), creatinine (<0.0001), urea (p < 0.028) and uric acid (p < 0.0001). T2DM patients with undiagnosed CKD had higher serum creatinine (145.75 ± 50.83 vs 88.59 ± 19.46, p < 0.0001), higher uric acid (361.10 ± 115.37 vs 294.54 ± 97.75; p < 0.0001) and higher urea (5.17 ± 2.35 vs 3.58 ± 1.19; p < 0.0001). After performing logistic regression and adjusting for age, sex and BMI, three N-glycan peaks [OR (95%CI): (GP12 (0.05(0.01–0.54), p = 0.013)); GP16 (0.61(0.43–0.87), p = 0.006)); GP22 (0.60(0.39–0.92), p = 0.018)) were associated with renal function.
Conclusion
There was an increased prevalence of undiagnosed CKD among T2DM patients. This prevalence is the consequence of uncontrolled modifiable risk factors, which collectively may lead to end stage renal disease (ESRD). Although, the identified N-glycans could not adequately predict incident CKD, our investigation indicates the potential role of N-glycosylation in renal function and that their inclusion may improve risk stratification for CKD.
Electronic supplementary material
The online version of this article (10.1007/s40200-018-0365-3) contains supplementary material, which is available to authorized users.
Keywords: Chronic kidney disease, Diabetes mellitus, Hypertension, Metabolic risk factors
Introduction
Chronic kidney disease (CKD) is a life-threatening condition responsible for many morbidities and mortalities worldwide [1–4]. According to a systematic analysis on the global burden of diseases, it is the 18th cause of premature deaths [5]. However, apart from premature deaths being the worst outcome, those who survive it are prone to lifelong consequences including frequent hospitalisation [6, 7], cognitive impairment [8], poor quality of life [9] and overwhelming healthcare costs [7].
CKD is established based on kidney damage or decline in function over a 3-month period [10]. For many years, both the National Institute for Health Excellence (NICE) [11] and the Kidney Outcomes Quality Initiative (KDOQI) [12] have recognised the estimates of glomerular filtration rate (eGFR) as a proxy measure of kidney function. Usually, eGFR estimates are evaluated using different equations or formulae such as the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) [13], Modification of Diet in Renal Disease (MDRD) [14], Cockcroft-Gault [15] and cystatin based formula [16]. Based on the eGFR estimates derived from these equations, CKD is classified as follows: Stage 1 (> 90 mL/min/1.73 m2); stage 2 (60–89 mL/min/1.73 m2); stage 3 (30–59 mL/min/1.73 m2); stage 4 (15–29 mL/min/1.73 m2) and stage 5 (< 15 mL/min/1.73 m2) [1, 10, 17]. These evaluations have thus far, enabled disease labelling, risk stratification, intervention, drug dosing and prognostication [18].
The main risk factors for CKD are proteinuria [19], glomerulonephritis [20], nephrolithiasis [21], hypertension [22] and type II diabetes (T2DM) but amongst them, T2DM has been suggested to be the main driver of CKD [18]. Indeed, the projected trajectory is nearly half of all patients with T2DM may suffer from kidney dysfunction at some stage in their life [23]. This is because T2DM leads to oxidative stress and chronic inflammation that in turn fuels many abnormalities including endothelial dysfunction, mesangial-cell contraction, glomerular fibrosis and mesangial expansion. When untreated, these complications then advance into end-stage renal disease (ESRD) [18, 24, 25]. At ESRD, patients can only survive under kidney replacement therapy (i.e. dialysis or kidney transplantation).
Ghana, like many other countries in sub-Saharan Africa (SSA) and worldwide, has large numbers of people with T2DM and if the current trend persists, it will not be surprising to realise an explosion of T2DM and its associated CKD complications in the future [26, 27]. Early recognition of risk factors will promote better treatment and improve survival [26, 27]. However, CKD awareness among T2DM sufferers is generally low in this region [28]. In part, this can be attributed to limited health care resources which in turn, has slowed the commitment to research, health screening and surveillance. In fact, only a few studies have reported the prevalence of CKD in Ghana [28, 29], and these studies were restricted to only some risk factors and failed to adequately explore other potential risk factors. Moreover, several studies have indicated the role of genetic and environmental factors to the pathophysiology of CKD but the contribution of epigenetic factors or posttranslational modifications is scarcely documented.
N-glycosylation is a widely recognised process where complex oligonucleotides (glycans) are pinned to asparagine residues of proteins [30]. When bound to proteins, glycans affect their trafficking, turnover, and other physiochemical properties including solubility and stability [31–34]. Glycans are stable in normal conditions but are aberrant in abnormal or environmental perturbations [35]. Thus, glyco-profiling is a unique approach to deciphering the complexities in both healthy and pathophysiological conditions. For example, N-glycans are defective in inflammatory diseases such as rheumatoid arthritis [36] andsystematic lupus erythematosus (SLE) [37]. However, the role of N-glycans in renal function remains scarce.
Therefore, in a hospital based clinical study, we have used the CDK-EPI equation in conjunction with multiple metabolic risk factors to determine CKD risk among T2DM patients in Ghana. In addition, this study profiles N-glycans in T2DM with or without CKD.
Study design and methods
This cross-sectional study was conducted from January 2016 to May 2016. Of the 260 T2DM participants recruited for the study, analyses were performed on 241 participants because of missing biochemical data. Recruitment for the study was based on a purposive sampling approach where T2DM patients who reported at the Diabetic Centre, Komfo Anokye Teaching Hospital (KATH) were invited to participate. KATH is a referral hospital with over 1200 beds with not less than 100 diabetic/hypertensive patients attending the hospital every week [38].
Inclusion and exclusion criteria
This study was conducted in consultation with clinicians and qualified health professionals. T2DM was diagnosed by clinicians at KATH and it was established based on the international classification of disease (ICD-10-CM Diagnosis Code E11.9). Each patient was carefully examined and their medical records thoroughly reviewed. As a result, we excluded all those individuals who were suffering from cancer, arthritis, infectious diseases, cardiovascular disease, thyroid disorders, pituitary disorders and adrenal disorders. The study did not include pregnant and lactating mothers. Since T2DM is largely a disease of ageing, the study recruited only individuals who were 30 years and above. Furthermore, to limit potential confounding and the likelihood of recruiting participants with type 1 diabetes, we excluded participants on insulin injections.
Demographic and anthropometric examination
Previous and current history of disease, family history of T2DM and hypertension were collected. In addition, information on health status and history of smoking and alcohol consumption as well as current physical activity were also collected using a structured questionnaire. Weight (kg) and height (cm) were measured with a standard stadiometer (SECA, Hamburg, Germany). These data were used to determine the body mass index (BMI); calculated as BMI = weight (kg)/ [height (m)]2. Waist and hip circumference were measured in cm using a tape measure and waist-to-hip ratio (WHR) was calculated as WHR = waist (cm)/hip (cm). Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured using a standard sphygmomanometer (Omron HEM711DLX, UK).
Clinical data
After an overnight fast, blood samples were collected from each participant. Samples were collected into tubes containing ethylenediaminetetraacetic acid (EDTA) anticoagulant, gel separator and fluoride oxalate. Samples were centrifuged (Mendelssohn, USA) at 3000 g at 4 °C for 10 mins (centrifuge Eppendorf 5702R, Germany) to separate the whole blood. Plasma glucose levels were measured using a glucose oxidase method (Roche Diagnostics, COBAS INTEGRA 400 Plus, USA). Serum levels of total cholesterol (TC), triglycerides (TG) and high density lipoprotein (HDL) cholesterol were determined enzymatically with commercially available reagents (Elitech Clinical Systems Elitech Group; Roche Diagnostics, COBAS INTEGRA 400 Plus, USA). Serum lipid levels were quantified based on the National Cholesterol Education Program, Adult Treatment Panel (NCEP-ATP) III guidelines. Low-density lipoprotein (LDL) cholesterol was calculated by the Friedewald formula using the equation LDL = TC−[HDL + TG/5] [38]. In addition, serum creatinine, uric acid and urea were measured using commercially available reagents on the automated chemistry analyser (Elitech Clinical Systems Elitech Group; Roche Diagnostics, COBAS INTEGRA 400 Plus, USA). Quality controls were applied throughout all these assays.
Non-HDL was calculated as Non-HDL = total cholesterol-HDL. We then calculated eGFR using the CKD-EPI equation [39];
N-glycan release and labelling
The release of glycans from glycoproteins on a 96-well plate is a unique method for N-glycan analysis. Prior to the analyses, samples were randomised on multiple plates to avoid bias, experimental errors and make data comparable. Briefly, plasma samples (10 μl) were aliquoted in a 96-well plate and denatured with 20 μl 2% (w/v) sodium dodecyl sulphate (SDS; Invitrogen, USA), incubated at 65 °C for 10 mins and cooled to room temperature for 30 mins. Following this, 10 μl of 4% (v/v) Igepal CA-630 (Sigma-Aldrich, USA) was added and mixed. N-glycans were then detached from glycoproteins after the addition of 1.2 U of peptide N-glycosidase F (PNGase F; Promega, USA) in 10 μl 5x PBS and 18 h incubation at 37 °C. The released N-glycans were labelled with 2-amino benzamide (2-AB, Sigma-Aldrich) solution but prior to this, a labelling mixture of 2-AB (19.2 mg/ml) and 2-picoline borane (2-PB, 44.8 mg/ml; Sigma Aldrich) in dimethylsulfoxide (DMSO, Sigma Aldrich) and glacial acetic acid (Merck, Germany) mixture (70:30 v/v) was prepared. Subsequently, 25 μl of the labelling mixture was added to each glycan sample in the plate, sealed, shaken and incubated for 2 h at 65 °C. Shortly thereafter, excess label and reducing agents in samples were removed by hydrophilic interaction liquid chromatography solid phase extraction (HILIC-SPE) on a hydrophilic 0.2 μm AcroPrep GHP filter plate (Pall Corporation, USA) using vacuum manifold (Millipore Corporation, USA). Samples were first cooled for 30 mins after which 700 μl of cold (4 °C) acetonitrile (ACN) was added to each sample. Prior to loading samples in the wells, the wells of GHP filter plate were washed with 200 μl of 70% (v/v) ethanol, 200 μl of ultra-pure water and equilibrated using 200 μl of cold (4 °C) 96% (v/v) ACN. Samples were then loaded into the equilibrated GHP filter plate, incubated briefly and washed with 5 × 200 μl of cold (4 °C) 96% ACN. Two times 90 μl of ultra-pure water was added whilst shaking, followed by centrifugation at 164 g for 5 mins (centrifuge 5804, rotor A-2-DWP, Eppendorf, Germany) in each step, to elute N-glycans from the GHP filter plate. Eluted glycans in a total volume of 180 μl were stored at −20 °C until further analysis.
Ultra-performance liquid chromatography
Separation of fluorescently labelled N-glycans was achieved on a HILIC on an Acquity UPLC instrument (Waters, USA) which comprised of a sample manager, quaternary solvent manager and fluorescence (FLR) detector set with excitation wavelength of 250 nm and an emission wavelength of 428 nm. Using a Waters BEH Glycan chromatography column with dimensions 150 × 2.1 mm i.d. and 1.7 μm BEH particles, labelled N-glycans were then separated in the presence of 100 mM ammonium formate as solvent A (pH 4.4) and ACN as solvent B. A linear gradient of 30–47% solvent A at a flow rate of 0.56 ml/min was applied while separation temperature was maintained at 25 °C and sample temperature at 10 °C. Calibration of the system was done using external standards of hydrolysed and 2-AB labelled glucose oligomers following which the data was automatically processed and integrated. Automatically integrated chromatograms were manually corrected. With the same intervals of integration, the total plasma glycome was separated into 39 glycan peaks and the relative abundance of each N-glycan peak expressed as a percentage of the total integrated area. From these 39 directly measured N-glycan peaks, an additional 21 derived glycan traits were calculated. These are high branching (HB), low branching (LB), neutral or no sialylation (S0), monosialylated (S1), disialylated (S2), trisialylated (S3), tetrasialylated (S4), agalactosylated (G0), monogalactosylated (G1), digalactosylated (G2), trigalactosylated (G3), tetragalactosylated (G4), antennary fucosylated (FUC_A), core fucosylated (FUC_C), biantennary (BA), biantennary agalactosylated (A2), biantennary galactosylated (A2G), monosialylated biantennary (BAMS), disialylated biantennary (BADS), triantennary (TRIA) and tetraantennary (TA) traits. (Supplementary Table 1) [40].
Statistical analysis
All continuous data was recorded as mean ± standard deviation and percentages for categorical variables. Association between CKD and metabolic risk factors were performed using linear regression and multiple logistic regression models. Odds ratios (ORs) at 95% confidence intervals (95% CI) were recorded for logistic regression analysis. Normalisation and batch correction on the UPLC data were performed in order to control for non-biological variability. Normality distribution of data was checked by the Kolmogov Smirnoff test as well as visualisation of QQ plots. However, because of the skewed nature of N-glycan data, interquartile ranges (IQRs) were used to describe the data. Depending on the normality distribution, between groups comparisons for continuous variables were performed using Mann-Whitney U-tests or Student-t tests and intergroup comparisons of categorical variables were performed using Chi-square tests. The Spearman correlation method was used to calculate the correlation coefficients (rho) between biochemical parameters and N-glycans. A p < 0.05 was considered statistically significant.
Results
All participants were physically examined, and their biochemical and clinical data collected. The mean ages of participants were 58.95 ± 10.98 years and 57.04 ± 10.77 years for men and women, respectively. There was female dominance in this study and the ratio of males to females was 99/142. This ratio was not surprising since the registered data from KATH shows that there are more women with T2DM than men. Also, compared to men, women were more willing and consented to participate in the study. Albeit, the age ranges for men and women were similar.
After stratifying by gender, women were generally obese compared to men when BMI (23.9% vs. 10.4%; p = 0.001) was used as an obesity index. A higher proportion of men compared to women had a history of smoking (28.3% vs. 3.5%; p < 0.001) and alcohol consumption (58.6% vs. 31.7%; p < 0.0001). There was a significantly higher HbA1c (p = 0.031), TC (p = 0.015), HDL-c (p < 0.0001), creatinine (<0.0001), urea (p < 0.028) and uric acid levels (p < 0.0001) among women compared to men. Levels of SBP, DBP, FPG, TG, VLDL-c, eGFR and coronary risk ratio among females were not significantly different between males and females (p > 0.05). Generally, men engaged in more moderate physical activity compared to women (p < 0.035) (Table 1).
Table 1.
Characteristics of study participants
| Characteristics | Total | Men (n = 99) | Women (n = 142) | p value |
|---|---|---|---|---|
| Age (years) | 57.99 ± 10.88 | 58.95 ± 10.98 | 57.04 ± 10.77 | 0.18 |
| Body mass index (kg/m2) | ||||
| Underweight | 9(3.8) | 5(5.1) | 4(2.8) | 0.015 |
| Normal weight | 107(44.6) | 53(54.1) | 54(38.0) | |
| Overweight | 80(33.3) | 30(30.6) | 50(35.2) | |
| Obese | 44(18.3) | 10(10.4) | 34(23.9) | |
| Education | 0.0001 | |||
| Tertiary | 36(14.9) | 21(21.2) | 15(10.6) | |
| Senior high school | 57(23.7) | 33(33.3) | 24(16.9) | |
| Junior high school | 78(32.4) | 30(30.3) | 48(33.8) | |
| Lower primary | 28(11.6) | 8(8.1) | 20(14.1) | |
| No formal education | 42(17.4) | 7(7.1) | 35(24.6) | |
| Marital status | ||||
| Married | 164(68.0) | 91(91.9) | 73(51.4) | 0.0001 |
| Never married | 4(1.7) | 1(1.0) | 3(2.1) | |
| Divorced | 25(10.4) | 5(5.0) | 20(14.1) | |
| Widowed | 48(19.9) | 2(2.0) | 46(32.4) | |
| Occupation | ||||
| Employed | 133(55.2) | 67(67.7) | 66(46.5) | 0.0001 |
| Retired | 35(14.5) | 18(18.2) | 17(12.0) | |
| Unemployed | 51(21.1) | 8(8.1) | 43(30.3) | |
| Informal employment | 22(9.1) | 6(6.1) | 16(11.3) | |
| Systolic blood pressure (mmHg) | 139.60 ± 24.32 | 140.73 ± 24.01 | 138.48 ± 24.64 | 0.483 |
| Diastolic blood pressure (mmHg) | 81.61 ± 13.75 | 81.74 ± 13.75 | 81.47 ± 13.74 | 0.878 |
| Fasting plasma glucose (mmol/l) | 9.11 ± 4.42 | 8.75 ± 4.51 | 9.48 ± 4.34 | 0.213 |
| Glycated haemoglobin (%) | 8.22 ± 2.06 | 7.93 ± 1.97 | 8.51 ± 2.15 | 0.031 |
| TC (mmol/l) | 4.69 ± 1.22 | 4.49 ± 1.09 | 4.89 ± 1.36 | 0.015 |
| TG (mmol/l) | 1.26 ± 0.56 | 1.21 ± 0.55 | 1.32 ± 0.57 | 0.13 |
| HDL-c (mmol/l) | 1.34 ± 0.31 | 1.24 ± 0.28 | 1.44 ± 0.33 | 0.0001 |
| NonHDL-c (mmol/l) | 3.35 ± 1.19 | 3.25 ± 1.03 | 3.45 ± 1.36 | 0.208 |
| VLDL-c (mmol/l) | 2.78 ± 1.13 | 2.70 ± 0.99 | 2.86 ± 1.27 | 0.145 |
| LDL-c (mmol/l) | 0.58 ± 0.26 | 0.55 ± 0.25 | 0.60 ± 0.26 | 0.301 |
| Coronary risk | 4.98 ± 1.48 | 5.09 ± 1.33 | 4.88 ± 1.64 | 0.286 |
| Creatinine (mmol/l) | 108.42 ± 40.2 | 119.81 ± 38.64 | 97.03 ± 41.76 | 0.0001 |
| Urea (mmol/l) | 4.13 ± 1.80 | 4.38 ± 1.95 | 3.87 ± 1.65 | 0.028 |
| Uric acid (mmol/l) | 324.24 ± 96.61 | 374.52 ± 100.73 | 273.95 ± 92.49 | 0.0001 |
| eGFR (per mL/min/1.73m2) | 73.35 ± 25.15 | 73.04 ± 24.96 | 73.66 ± 25.33 | 0.852 |
| Family history and activity | ||||
| Diabetes family history (yes) | 184(76.3) | 68(68.7) | 116(81.7) | 0.053 |
| Smoking (yes) | 33(13.7) | 28(28.3) | 5(3.5) | 0.0001 |
| Drinking (Yes) | 103(42.7) | 58(58.6) | 45(31.7) | 0.0001 |
| Physical activity | ||||
| Primarily sedentary | 80(32.8) | 32(31.7) | 48(33.8) | 0.035 |
| Moderate activity | 161(66.8) | 67(67.7) | 94(66.2) | |
Data is expressed as mean ± standard deviation or (n %). Statistically significant differences (p < 0.05) are bold
CKD was more prevalent in females than males. Age (p < 0.0001), education (p = 0.023), occupation (p < 0.035) and physical activity (p = 0.038) were significantly associated with CKD. Meanwhile, being elderly [aOR = 28.86 (3.26–225.9); p = 0.0002], retired [aOR = 3.21 (1.48–6.94) p = 0.0036)], or primarily sedentary [aOR = 2.28 (1.29–4.01); p = 0.005], were significant independent risk factors for CKD after adjusting for age and gender. Surprisingly, T2DM patients who had significant CKD (eGFR <60 ml/min/1.73m2) had lower plasma glycaemic levels (HbA1c 7.88% vs 8.45%; p< 0.049) but had higher serum creatinine (145.75 ± 50.83 vs 88.59 ± 19.46, p < 0.0001), high uric acid (361.10 ± 115.37 vs 294.54 ± 97.75, p < 0.0001) and high urea (5.17 ± 2.35 vs 3.58 ± 1.19; p < 0.0001). However, there were no significant differences between the mean lipid profile and FPG among participants with CKD compared to those without CKD (p > 0.05) (Table 2).
Table 2.
Characteristics of study participants with or without CKD risk
| Variables | Total | CKD | No CKD | ×2 | p | aOR (95%CI) | p |
|---|---|---|---|---|---|---|---|
| Gender | 0.38 | 0.315 | |||||
| Male | 99(41.1) | 33(44.0) | 66(39.8) | 1# | |||
| Female | 142(58.9) | 42(56.0) | 100(60.2) | 0.84(0.48–1.45) | 0.573 | ||
| Age (years) | 40.53 | 0.0001 | |||||
| 31–40 | 14 (5.8) | 1(0.0) | 13 (8.4) | 1# | |||
| 41–50 | 49(20.3) | 7(9.3) | 42(25.3) | 2.16 (0.24–19.29) | 0.671 | ||
| 51–60 | 81(33.6) | 18(24.0) | 63(38.0) | 3.71 (0.45–30.36) | 0.288 | ||
| 61–70 | 68(28.2) | 30(40.0) | 38(22.9) | 10.26(1.27–82.98) | 0.013 | ||
| 71–80 | 29(12.0) | 20(26.7) | 9(5.4) | 28.86(3.26–225.9) | 0.0002 | ||
| Education | 11.32 | 0.023 | |||||
| Tertiary | 36(14.9) | 11(14.7) | 25(15.1) | 1.0# | |||
| Senior high school | 57(23.7) | 14(18.7) | 43(25.9) | 0.74(0.29–1.88) | 0.632 | ||
| Junior high school | 78(32.4) | 28(37.3) | 50(30.1) | 1.27(0.54–2.96) | 0.673 | ||
| Lower primary | 28(11.6) | 3(4.0) | 25(15.1) | 0.27(0.06–1.09) | 0.072 | ||
| No formal education | 42(17.4) | 19(25.3) | 23(13.9) | 1.88(0.74–4.77) | 0.244 | ||
| Marital Status | 2.2 | 0.699 | |||||
| Married | 164(68.0) | 47(62.7) | 117(70.5) | 1# | |||
| Never married | 4(1.7) | 1(1.3) | 3(1.8) | 0.82(0.08–8.18) | 0.998 | ||
| Divorced/separated | 25(10.4) | 8(10.7) | 17(10.2) | 1.17(0.47–2.89) | 0.814 | ||
| Widowed | 48(19.9) | 19(25.3) | 29(17.5) | 1.63(0.83–3.19) | 0.159 | ||
| Occupation | 10.35 | 0.035 | |||||
| Employed | 133(55.2) | 33(44.0) | 100(60.2) | 1# | |||
| Retired | 35(14.5) | 18(24.0) | 17(10.2) | 3.21(1.48–6.94) | 0.0036 | ||
| Unemployed | 51(21.1) | 17(22.7) | 34(20.4) | 1.51(0.75–3.06) | 0.269 | ||
| Informal employment | 22(9.1) | 7(9.3) | 15(9.0) | 1.41(0.53–3.76) | 0.599 | ||
| Physical activity | 8.4 | 0.038 | |||||
| Primarily sedentary | 79(32.8) | 35(45.9) | 45(27.1) | 2.28(1.29–4.01) | 0.0051 | ||
| Sedentary with activity | 161(66.8) | 41(54.7) | 120(72.3) | 1# | |||
| Biochemical data | |||||||
| FPG (mmol/l) | 9.05 ± 4.49 | 8.71 ± 4.72 | 9.39 ± 4.27 | 0.27 | |||
| HbA1c (%) | 8.16 ± 2.08 | 7.88 ± 2.07 | 8.45 ± 2.08 | 0.049 | |||
| TC (mmol/l) | 4.72 ± 1.28 | 4.71 ± 1.29 | 4.73 ± 1.27 | 0.94 | |||
| TG (mmol/l) | 1.29 ± 0.55 | 1.37 ± 0.54 | 1.22 ± 0.57 | 0.71 | |||
| HDL-c (mmol/l) | 1.34 ± 0.33 | 1.33 ± 0.37 | 1.36 ± 0.30 | 0.612 | |||
| NonHDL-c (mmol/l) | 3.37 ± 1.25 | 3.37 ± 1.27 | 3.37 ± 1.23 | 0.979 | |||
| LDL-c (mmol/l) | 2.78 ± 1.17 | 2.75 ± 1.18 | 2.80 ± 1.16 | 0.74 | |||
| Creatinine (μmol/l) | 117.17 ± 35.14 | 145.75 ± 50.83 | 88.59 ± 19.46 | <0.0001 | |||
| Urea (mmol/l) | 4.38 ± 1.77 | 5.17 ± 2.35 | 3.58 ± 1.19 | <0.0001 | |||
| Uric acid (μmol/l) | 327.82 ± 106.56 | 361.10 ± 115.37 | 294.54 ± 97.75 | <0.0001 | |||
| eGFR | 66.02 ± 15.39 | 46.48 ± 11.19 | 85.57 ± 19.60 | <0.0001 | |||
| (per mL/min/1.73 m2) | |||||||
aOR: adjusted odds ratio, CI confidence interval. Multivariate regression model was adjusted for age and gender; #: reference, Statistically significant differences (p < 0.05) are bold
After adjusting for age and gender, high SBP [aOR = 1.81 (1.08–3.26); p = 0.024)], HbA1c [aOR = 0.5 (1.28–0.89) p = 0.017], and high TG [aOR = 2.38 (1.21–4.70); p = 0.024)] were significant independent risk factors for CKD (Table 3).
Table 3.
Association between CKD and metabolic risk factors
| Variables | Total (n %) | CKD | No CKD | x2 | p value | aOR (95%CI) | p value |
|---|---|---|---|---|---|---|---|
| Body mass index | |||||||
| Underweight | 9(3.8) | 6(8.0) | 3(1.8) | 8.47 | 0.037 | 4.48(1.06–19.04) | 0.059 |
| Normal weight | 107(44.6) | 33(44.0) | 74(44.8) | 1.0# | |||
| Overweight | 80(33.3) | 19(25.3) | 61(37.0) | 0.69(0.36–1.34) | 0.324 | ||
| Obese | 44(18.3) | 17(22.7) | 27(16.4) | 1.41(0.67–2.93) | 0.446 | ||
| Systolic blood pressure | 5.1 | 0.017 | |||||
| Normal systolicBP | 132(54.8) | 33(44.0) | 99(59.6) | 1.0# | |||
| High systolicBP | 109(45.2) | 42(56.0) | 67(40.4) | 1.81(1.08–3.26) | 0.024 | ||
| Diastolic blood pressure | |||||||
| Normal diastolicBP | 177(74.1) | 53(72.6) | 124(74.7) | 0.11 | 0.43 | 1.0# | 0.74 |
| High diastolicBP | 62(25.9) | 20(27.4) | 42(25.3) | 1.11(0.59–2.08) | |||
| Fasting blood glucose | 1.83 | 0.11 | 0.18 | ||||
| Normal | 94(39.0) | 34(45.3) | 60(36.1) | 1.0# | |||
| High | 147(61.0) | 41(54.7) | 106(63.9) | 0.68 (0.39–1.18) | |||
| Glycated Haemoglobin | 5.6 | 0.014 | 0.017 | ||||
| Normal | 74(30.8) | 31(41.3) | 43(26.1) | 1.0# | |||
| High | 166(69.2) | 44(58.7) | 122(73.9) | 0.5(0.28–0.89) | |||
| Cholesterol | 1.05 | 0.19 | 0.307 | ||||
| Normal | 154(64.2) | 51(68.9) | 103(62.0) | 1.0# | |||
| High | 86(35.8) | 23(31.1) | 63(38.0) | 0.74(0.41–1.32) | |||
| Triglycerides | 6.46 | 0.01 | 0.011 | ||||
| Normal | 199(82.6) | 55(73.3) | 144(86.7) | 1.0# | |||
| High | 42(17.4) | 20(26.7) | 22(13.3) | 2.38(1.21–4.70) | |||
| HDL-c | 2.66 | 0.074 | 0.104 | ||||
| Normal | 189(78.4) | 54(72.0) | 135(81.3) | 1.0# | |||
| Low | 52(21.6) | 21(28.0) | 31(18.7) | 1.69(0.89–3.20) | |||
| Non-HDL-c | 0.29 | 0.345 | 0.592 | ||||
| Normal | 117(48.8) | 38(51.4) | 79(47.6) | 1.0# | |||
| High | 123(51.3) | 36(48.6) | 87(52.4) | 0.86(0.49–1.49) | |||
| LDL-c | 0.43 | 0.304 | |||||
| Normal | 106(44.2) | 35(47.3) | 71(42.8) | 1.0# | 0.516 | ||
| High | 134(55.8) | 39(52.7) | 95(57.2) | 0.83(0.48–1.44) | |||
aOR: adjusted odds ratio, CI confidence interval. Multivariate regression model was adjusted for age and gender; #: reference. Statistically significant differences (p < 0.05) are bold
In the bivariate analysis, there was a significant negative relationship between urea (β = −2.62; p < 0.0001) and uric acid (β = −0.02; p = 0.0161) with CKD. The r2 indicated that the cause of CKD was influenced by 30.6% of urea and 4.2% of uric acid. Inverse and non-significant relationships were observed between CKD and age, SBP, FPG, TC, TG, HDL-c, non-HDL-c, LDL-c, coronary risk ratio, and VLDL-c (Table 4).
Table 4.
Bivariate relationship between predictors and CKD < 60 ml/min/1.73m2
| Factors | β(95%CI) | SE | r2 | p value |
|---|---|---|---|---|
| Age (years) | −0.20(−0.43 to 0.03) | 0.11 | 0.041 | 0.0820 |
| BMI (kg/m2) | 0.19(−0.04 to 0.42) | 0.11 | 0.037 | 0.0968 |
| SBP (mmHg) | −0.05(−0.28 to 0.19) | 0.12 | 0.002 | 0.7004 |
| DBP (mmHg) | 0.14(−0.09 to 0.38) | 0.12 | 0.021 | 0.2150 |
| FPG (mmol/l) | −0.19(−0.75 to 0.36) | 0.28 | 0.007 | 0.4831 |
| HbA1c (%) | 0.46(−0.80 to 1.71) | 0.63 | 0.007 | 0.4720 |
| Urea (mmol/l) | −2.62(−3.55 to −1.70) | 0.46 | 0.306 | < 0.0001 |
| TC (mmol/l) | −0.64(−2.67 to 1.40) | 1.02 | 0.005 | 0.5348 |
| TG (mmol/l) | −3.81(−8.55 to 0.92) | 2.38 | 0.034 | 0.1128 |
| HDL-c (mmol/l) | −0.64(−7.61 to 6.33) | 3.50 | 0.000 | 0.8551 |
| Non-HDL-c (mmol/l) | −0.63(−2.69 to 1.43) | 1.04 | 0.005 | 0.5452 |
| LDL-c (mmol/l) | −0.43(−2.65 to 1.80) | 1.12 | 0.002 | 0.7036 |
| Coronary risk | −0.69(−2.24 to 0.85) | 0.77 | 0.011 | 0.3752 |
| VLDL-c (mmol/l) | −8.16(−18.68 to 2.36) | 5.28 | 0.032 | 0.1263 |
| Uric Acid (mmol/l) | −0.02(−0.04 to 0.00) | 0.01 | 0.042 | 0.0161 |
In the bivariate analysis, it was shown that urea and uric acids were independently associated with CKD. After including uric acid, TC, SBP, DBP, Urea, FPG, BMI, TG, age, HDL-c, HbA1c, coronary risk, VLDL-c and LDL-c in the multivariate linear regression model, they influenced CKD by 55.7% (r2 = 0.557). When the significant predictors, urea and uric acid were included in the model, CKD was influenced by 37.21% (r2 = 0.3721). The predictive equation for this model was CKD =72.07–3.08*Urea-7.30*Uric acid (Table 5).
Table 5.
Multivariate relationship between predictors and CKD < 60 ml/min/1.73m2
| Predictors | Standardized β | p value |
|---|---|---|
| Model 1 | ||
| Age (years) | −0.045 | 0.719 |
| BMI (Kg/m2) | 0.148 | 0.167 |
| SBP (mmHg) | −0.183 | 0.137 |
| DBP (mmHg) | 0.228 | 0.082 |
| FPG (mmol/l) | −0.068 | 0.591 |
| HbA1c (mmol/l) | 0.113 | 0.383 |
| Urea*(mmol/l) | −0.647 | <0.0001 |
| TC (mmol/l) | −0.678 | 0.869 |
| TG (mmol/l) | 5.061 | 0.139 |
| HDL-c (mmol/l) | −0.038 | 0.973 |
| Non-HDL-c (mmol/l) | 0.598 | 0.877 |
| LDL-c (mmol/l) | 0.041 | 0.788 |
| Coronary risk | 0.14 | 0.685 |
| VLDL-c (mmol/l) | −5.157 | 0.109 |
| Uric Acid*(mmol/l) | −0.195 | 0.014 |
| r2 | 0.557 | |
| Adjusted r2 | 0.441 | |
| (Constant) | 71.969 | |
| p value | <0.0001 | |
| Model 2 | ||
| Urea (mmol/l) | −0.6536 | < 0.0001 |
| Uric Acid (mmol/l) | −0.2446 | 0.0178 |
| r2 | 0.372 | |
| Adjusted r2 | 0.354 | |
| (Constant) | 72.069 | |
| p value | <0.0001 | |
Standardized β for predictors in the model 1: (Constant), Uric Acid, TC, DBP, SBP, urea, FPG, HbA1c, BMI, TG. Age, HDL-c, coronary risk, VLDL-c, LDL-c
Standardized β for best predictors in model 2: (Constant), urea, uric acid. Statistical significance (p < 0.05) are bold
*Significant predictors in model 1
Differential plasma N-glycan patterns in T2DM with CKD and those without CKD
The IQRsof all measured N-glycans are shown in Table 6 and there were distinct levels of N-glycans between T2DM with CKD and those without it. Generally, GP10 (FA2G2), GP16 (FA2G2S[6]1) and GP22 (FA2G2S[3,6]2) were higher among in T2DM without CKD compared to T2DM with CKD. In contrast, GP14 (A2G2S[6]1, T2DM with CKD than those without it (p < 0.05). However, in Supplementary Table 2, there were no statistical significance differences between N-glycan traits in T2DM + CKD and those without CKD.
Table 6.
N-glycan traits in normal and CKD
| Normal | CKD | ||||||
|---|---|---|---|---|---|---|---|
| N-glycan | Structure | Median(IQR) | Range | Median(IQR) | Range | W | p |
| GP1 | FA2 | 6.36(2.79) | (5.02–7.81) | 6.61(2.90) | (5.39–8.29) | 18,846 | 0.260 |
| GP2 | FA2B | 2.37(0.85) | (2.02–2.87) | 2.47(0.72) | (2.08–2.81) | 19,007 | 0.425 |
| GP3 | A2[6]BG1 | 0.08(0.05) | (0.07–0.12) | 0.09(0.03) | (0.07–0.11) | 8801 | 0.992 |
| GP4 | FA2[6]G1 | 4.39(1.20) | (3.98–5.19) | 4.77(1.70) | (3.68–5.39) | 8731 | 0.878 |
| GP5 | FA2[3]G1 | 1.83(0.64) | (1.57–2.21) | 1.84(0.66) | (1.48–2.15) | 8411 | 0.419 |
| GP6 | FA2[6]BG1 | 1.18(0.31) | (1.04–1.36) | 1.31(0.57) | (1.00–1.58) | 18,446 | 0.052 |
| GP7 | M6D1-D2 | 0.99(0.18) | (0.92–1.09) | 0.95(0.23) | (0.83–1.07) | 7897 | 0.063 |
| GP8 | A2G2 | 1.12(0.31) | (0.99–1.30) | 1.10(0.37) | (0.97–1.34) | 8650 | 0.750 |
| GP9 | A2BG2 | 0.09(0.04) | (0.09–0.12) | 0.09(0.03) | (0.09–0.12) | 8603 | 0.678 |
| GP10 | FA2G2 | 3.70(1.50) | (3.14–4.64) | 3.46(1.39) | (2.89–4.28) | 7688 | 0.022* |
| GP11 | FA2BG2 | 0.73(0.21) | (0.62–0.83) | 0.71(0.19) | (0.62–0.81) | 8677 | 0.792 |
| GP12 | A2[3]BG1S[3]1 | 1.00(0.16) | (0.93–1.09) | 0.97(0.18) | (0.89–1.06) | 8077 | 0.136 |
| GP13 | FA2[3]G1S[3]1 | 0.81(0.19) | (0.72–0.91) | 0.81(0.32) | (0.66–0.99) | 19,213 | 0.707 |
| GP14 | A2G2S[6]1 | 10.75(1.48) | (9.97–11.46) | 11.19(1.64) | (10.46–12.11) | 18,049 | 0.006* |
| GP15 | A2BG2S[6]1 | 0.38(0.11) | (0.34–0.45) | 0.38(0.10) | (0.34–0.44) | 8690 | 0.813 |
| GP16 | FA2G2S[6]1 | 5.82(1.35) | (5.16–6.52) | 5.28(1.17) | (4.67–5.84) | 7158 | 0.001* |
| GP17 | FA2BG2S[3]1 | 1.56(0.56) | (1.33–1.90) | 1.58(0.48) | (1.32–1.81) | 8780 | 0.958 |
| GP18 | A2G2S[3,6]2 | 3.40(0.76) | (3.01–3.77) | 3.35(0.63) | (3.11–3.74) | 19,338 | 0.904 |
| GP19 | M9 | 1.12(0.22) | (1.00–1.22) | 1.08(0.22) | (0.97–1.20) | 8136 | 0.171 |
| GP20 | A2G2S[3,6]2 | 25.47(3.68) | (23.55–27.23) | 25.65(4.67) | (23.38–28.06) | 19,094 | 0.536 |
| GP21 | A2BG2S[3,6]2 | 0.51(0.17) | (0.44–0.61) | 0.51(0.17) | (0.44–0.61) | 8780 | 0.958 |
| GP22 | FA2G2S[3,6]2 | 4.38(0.96) | (4.01–4.97) | 4.12(1.17) | (3.76–4.93) | 7531 | 0.009* |
| GP23 | FA2BG2S[3,6]2 | 1.89(0.68) | (1.61–2.29) | 1.99(0.66) | (1.66–2.32) | 19,036 | 0.460 |
| GP24 | A3G3S[3,6]2 | 1.73(0.62) | (1.41–2.03) | 1.84(0.73) | (1.38–2.11) | 19,217 | 0.713 |
| GP25 | A3BG3S[3,6]2 | 0.15(0.06) | (0.12–0.19) | 0.16(0.06) | (0.14–0.19) | 18,544 | 0.081 |
| GP26 | A3G3S[3,3]2 | 1.63(0.48) | (1.38–1.87) | 1.68(0.59) | (1.36–1.95) | 19,095 | 0.537 |
| GP27 | A3G3S[3,3,3]3 | 0.48(0.34) | (0.30–0.64) | 0.50(0.37) | (0.34–0.72) | 18,936 | 0.346 |
| GP28 | A3G3S[3,3,6]3 | 0.80(0.32) | (0.64–0.96) | 0.82(0.29) | (0.63–0.92) | 8612 | 0.692 |
| GP29 | FA3G3S[3,3,3]3 | 0.19(0.06) | (0.16–0.22) | 0.18(0.05) | (0.16–0.21) | 8498 | 0.529 |
| GP30 | A3G3S[3,3,6]3 | 5.89(2.01) | (4.86–6.87) | 5.94(2.19) | (4.64–6.84) | 8598 | 0.671 |
| GP31 | FA3G3S[3,3,6]3 | 0.52(0.29) | (0.42–0.72) | 0.50(0.21) | (0.42–0.63) | 8218 | 0.229 |
| GP32 | A3F1G3S[3,3,3]3 | 1.46(0.57) | (1.24–1.82) | 1.56(0.55) | (1.25–1.80) | 19,136 | 0.594 |
| GP33 | A4G4S[3,3,3]3 | 1.83(1.44) | (1.17–2.61) | 2.03(1.51) | (1.34–2.85) | 18,932 | 0.342 |
| GP34 | A4G4S[3,3,6]3 | 0.40(0.15) | (0.33–0.48) | 0.41(0.10) | (0.34–0.45) | 8706 | 0.838 |
| GP35 | A4F1G3S[3,3,3]3 | 0.26(0.15) | (0.19–0.35) | 0.29(0.16) | (0.21–0.36) | 18,959 | 0.371 |
| GP36 | A4G4S[3,3,3,3]4 | 0.44(0.11) | (0.40–0.51) | 0.45(0.13) | (0.39–0.53) | 19,149 | 0.612 |
| GP37 | A4G4S[3,3,3,3]4 | 0.49(0.21) | (0.39–0.60) | 0.48(0.19) | (0.38–0.58) | 8512 | 0.548 |
| GP38 | A4G4S[3,3,3,6]4 | 0.90(0.27) | (0.78–1.06) | 0.91(0.24) | (0.77–1.01) | 8632 | 0.722 |
| GP39 | A4F1G4S[3,3,3,6]4 | 0.51(0.25) | (0.41–0.67) | 0.55(0.23) | (0.45–0.67) | 18,916 | 0.325 |
Data presented as median interquartile range (IQR). W-Wilcoxon statistic, Tests of significance were two tailed (*p < 0.05) and are bold
After performing logistic regression, GP6 (FA2[6]BG1), GP 7 (M6D1-D2), GP10 (FA2G2), GP14 (A2G2S[6]1), GP16 (FA2G2S[6]1) and GP22 (FA2G2S[3,6]2) were significant in the crude (unadjusted) models (p < 0.05) (Table 7). However, after adjusting for Age, gender and BMI, only GP 12, GP16 and GP 22 were significant. There were no significant associations in the derived traits after performing logistic regression (Supplementary Table 3).
Table 7.
Logistic regression analysis of N-glycans crude in and adjusted models
| Crude | Age + Gender + BMI | |||||||
|---|---|---|---|---|---|---|---|---|
| B | S.E. | OR(95%CI) | p | B | S.E. | OR(95%CI) | p | |
| GP1 | 0.064 | 0.047 | 1.07(0.97–1.17) | 0.176 | 0.061 | 0.051 | 1.06(0.96–1.18) | 0.232 |
| GP2 | 0.241 | 0.213 | 1.27(0.84–1.93) | 0.257 | 0.045 | 0.240 | 1.05(0.65–1.67) | 0.851 |
| GP3 | −2.153 | 3.384 | 0.12(0.00–88.22) | 0.525 | −5.967 | 4.221 | 0.00(0.00–10.04) | 0.157 |
| GP4 | −0.029 | 0.130 | 0.97(0.75–1.25) | 0.821 | 0.021 | 0.147 | 1.02(0.77–1.36) | 0.886 |
| GP5 | −0.167 | 0.287 | 0.85(0.48–1.48) | 0.559 | −0.204 | 0.323 | 0.82(0.43–1.54) | 0.527 |
| GP6 | 1.062 | 0.474 | 2.89(1.14–7.33) | 0.025* | 0.751 | 0.518 | 2.12(0.77–5.85) | 0.147 |
| GP7 | −1.851 | 0.908 | 0.16(0.03–0.93) | 0.041* | −1.826 | 0.960 | 0.16(0.03–1.06) | 0.057 |
| GP8 | −0.217 | 0.428 | 0.81(0.35–1.86) | 0.612 | −0.501 | 0.533 | 0.61(0.21–1.72) | 0.347 |
| GP9 | −3.519 | 3.145 | 0.03(0.00–14.07) | 0.263 | −4.957 | 3.629 | 0.01(0.00–8.64) | 0.172 |
| GP10 | −0.375 | 0.150 | 0.69(0.51–0.92) | 0.012* | −0.291 | 0.183 | 0.75(0.52–1.07) | 0.112 |
| GP11 | −0.203 | 0.785 | 0.82(0.18–3.80) | 0.796 | −0.249 | 0.845 | 0.78(0.15–4.09) | 0.768 |
| GP12 | −2.043 | 1.028 | 0.13(0.02–0.97) | 0.047* | −2.915 | 1.175 | 0.05(0.01–0.54) | 0.013* |
| GP13 | 0.830 | 0.632 | 2.29(0.67–7.90) | 0.189 | 0.336 | 0.728 | 1.40(0.34–5.82) | 0.645 |
| GP14 | 0.262 | 0.118 | 1.30(1.03–1.64) | 0.026* | 0.132 | 0.130 | 1.14(0.88–1.47) | 0.313 |
| GP15 | −0.811 | 0.795 | 0.44(0.09–2.11) | 0.308 | −1.039 | 0.880 | 0.35(0.06–1.99) | 0.238 |
| GP16 | −0.491 | 0.154 | 0.61(0.45–0.83) | 0.001* | −0.494 | 0.180 | 0.61(0.43–0.87) | 0.006* |
| GP17 | 0.062 | 0.301 | 1.06(0.59–1.92) | 0.836 | −0.012 | 0.327 | 0.99(0.52–1.88) | 0.971 |
| GP18 | 0.071 | 0.260 | 1.07(0.65–1.79) | 0.784 | −0.100 | 0.296 | 0.91(0.51–1.62) | 0.736 |
| GP19 | −1.445 | 0.894 | 0.24(0.04–1.36) | 0.106 | −1.410 | 1.014 | 0.24(0.03–1.78) | 0.164 |
| GP20 | 0.012 | 0.052 | 1.01(0.92–1.12) | 0.816 | 0.011 | 0.058 | 1.01(0.90–1.13) | 0.854 |
| GP21 | −0.612 | 1.062 | 0.54(0.07–4.35) | 0.564 | −1.227 | 1.145 | 0.29(0.03–2.77) | 0.284 |
| GP22 | −0.493 | 0.196 | 0.61(0.42–0.90) | 0.012* | −0.514 | 0.218 | 0.60(0.39–0.92) | 0.018* |
| GP23 | 0.189 | 0.204 | 1.21(0.81–1.80) | 0.353 | −0.019 | 0.233 | 0.98(0.62–1.55) | 0.935 |
| GP24 | 0.099 | 0.290 | 1.10(0.63–1.95) | 0.733 | 0.310 | 0.323 | 1.36(0.73–2.57) | 0.336 |
| GP25 | 5.190 | 3.072 | 179.53(0.44–746.43) | 0.091 | 3.261 | 3.489 | 26.07(0.03–28.75) | 0.350 |
| GP26 | 0.209 | 0.368 | 1.23(0.60–2.53) | 0.571 | 0.656 | 0.447 | 1.93(0.80–4.63) | 0.142 |
| GP27 | 0.531 | 0.521 | 1.70(0.61–4.73) | 0.308 | 0.160 | 0.596 | 1.17(0.37–3.78) | 0.788 |
| GP28 | −0.349 | 0.650 | 0.71(0.20–2.52) | 0.591 | 0.110 | 0.721 | 1.12(0.27–4.58) | 0.879 |
| GP29 | −1.254 | 3.278 | 0.29(0.00–176.23) | 0.702 | −2.296 | 3.580 | 0.10(0.00–112.27) | 0.521 |
| GP30 | −0.051 | 0.090 | 0.95(0.80–1.13) | 0.569 | 0.061 | 0.104 | 1.06(0.87–1.30) | 0.560 |
| GP31 | −0.897 | 0.738 | 0.41(0.10–1.73) | 0.224 | −0.333 | 0.875 | 0.72(0.13–3.99) | 0.704 |
| GP32 | 0.155 | 0.336 | 1.17(0.61–2.25) | 0.645 | 0.657 | 0.419 | 1.93(0.85–4.39) | 0.118 |
| GP33 | 0.135 | 0.130 | 1.14(0.89–1.48) | 0.299 | 0.061 | 0.148 | 1.06(0.80–1.42) | 0.681 |
| GP34 | −0.826 | 1.408 | 0.44(0.03–6.91) | 0.557 | 0.314 | 1.790 | 1.37(0.04–45.73) | 0.861 |
| GP35 | 0.960 | 1.091 | 2.61(0.31–22.16) | 0.379 | 0.686 | 1.231 | 1.99(0.18–22.18) | 0.577 |
| GP36 | 0.736 | 1.676 | 2.09(0.08–55.80) | 0.660 | 1.495 | 1.971 | 4.46(0.09–212.20) | 0.448 |
| GP37 | −0.796 | 0.868 | 0.45(0.08–2.47) | 0.359 | −0.198 | 0.966 | 0.82(0.12–5.45) | 0.838 |
| GP38 | −0.396 | 0.671 | 0.67(0.18–2.51) | 0.555 | 0.110 | 0.776 | 1.12(0.24–5.11) | 0.888 |
| GP39 | 0.241 | 0.632 | 1.27(0.37–4.39) | 0.703 | −0.113 | 0.715 | 0.89(0.22–3.63) | 0.875 |
OR: odds ratio, logistic regression model was adjusted for age, gender and BMI. Two tailed *p < 0.05 is significant and are bold
Discussion
Prevalence and metabolic risk factors that characterise CKD among 241 T2DM patients were evaluated. Here, 31.53% of T2DM patients had CKD as defined by eGFR <60 ml/min/1.73 m2. This prevalence rate is comparable to a previous study conducted among 280 T2DM patients in an urban community in Ghana [28] with the difference being 1.53%. As the determination of prevalence of CKD is based on the CKD-EPI Creatinine Equation in both studies, the slight increase in CKD in our study can be attributed to the proportion of the aged population in our study and the likelihood that these aged individuals may have been suffering from other clinical conditions (e.g. nephrosclerosis and undiagnosed ischaemic kidney disease) which we could not diagnose at the time of our investigation.
Surprisingly, compared with T2DM patients without CKD, those with CKD in our present study had lower HbA1c levels (Table 2), however, the clinical implications of this is negligible, given the difference was only 0.57% and a p value of 0.049. Besides, as shown in Table 2, the levels of FPG in T2DM with CKD and T2DM without CKD were not statistically significantly different. Other plausible explanation is the fewer number of T2DM participants with CKD compared to those without it.
The use of BMI as an obesity index has been criticised, hence the association between BMI and CKD has been conflicting in many studies. For example, whereas one study showed that the risk of developing advanced kidney malfunction is threefold higher in patients with BMI > 30, another study showed that the association between BMI and kidney dysfunction was insignificant [41, 42]. The present study agrees with the latter in that BMI was not an independent risk factor for CKD. Perhaps, complementing BMI with other fat indicators or measurements such as visceral fat and fat mass index (FMI) would have yielded a better association.
Consistent with other studies [43, 44], our findings show that T2DM patients with high systolic BP had increased odds for CKD (Table 3). Although it remains controversial whether elevated blood pressure is the cause or the consequence of kidney dysfunction, it has been suggested that elevated blood pressure promotes arterial stiffening and intimal thickening in the kidney parenchyma and subsequently results in glomerulosclerosis [45, 46]. Similarly, T2DM patients with high TG levels had increased odds for developing CKD. This confirms the findings by other studies [41, 47]. Although the exact mechanism is not fully understood, it has been suggested that the correlation between high TG and CKD is related to regulation of lipoprotein lipase activity during TG catabolism. This enzyme is regulated by two proteins: apolipoprotein C-II and apolipoprotein C-III that act antagonistically. That is, whilst apolipoprotein C-II activates lipoprotein lipase, apolipoprotein C-III inhibits it. During CKD, there is an increase in apolipoprotein C-III (i.e. decrease apolipoprotein C-II/C-III ratio) and this results in lipase inactivation and subsequent TG accumulation [48–50].
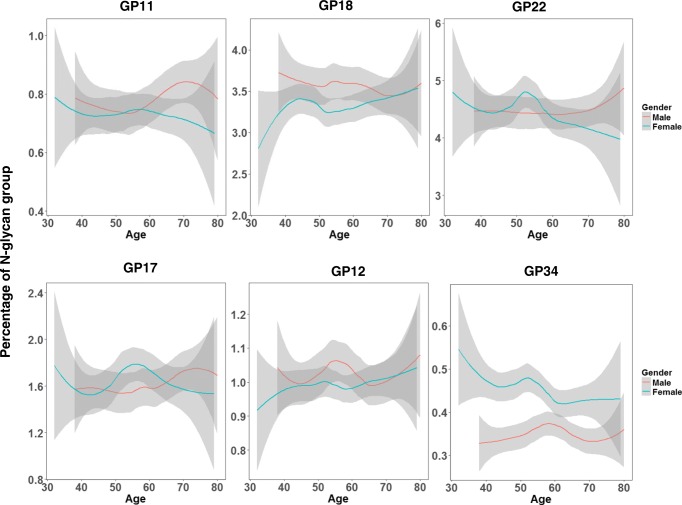
Thus far, we have used routine biochemical markers to establish the presence of CKD amongst T2DM, however, it is worth investigating the potential role of post-translational modification in kidney function. From the results, it was evident that N-glycosylation was associated with age and gender as previously reported [51–59] and this is attributable to hormonal differences (Fig. 1). The results of this present study showed that core fucosylated N-glycans without bisection [GP 10 (FA2G2), GP 16 (FA2G2S[6]1) and GP22 (FA2G2S[3,6]2) was significantly higher in T2DM patients with normal renal function (Table 6). Our findings agree with that of Barrios et al. [59] who showed that core fucosylated glycans that lack bisecting GlcNAc was associated with decreased risk of CKD. Core fucosylated N-glycan is an important molecule in notch signalling, growth factor receptor expression and adhesion molecule activity. Moreover, bisecting N-acetyl glucosamine on IgG facilitates antibody dependent cellular cytotoxicity (ADCC) or a pro-inflammatory state since the presence of bisecting N-acetyl glucosamine on N-glycans inhibits core fucosylation and indirectly promotes the binding of IgG molecules to Fcγ receptor III [54, 58]. Further, after performing a logistic regression and adjusting for covariates, CKD was associated with an increased levels of complex N-glycans (GP12, GP16 and GP22) (Table 7). These identified N-glycans have crucial role in kidney function. For example, GP12, a monogalactosylated, monosialylated biantennary N-glycan with bisecting GlcNAc, has previously been associated with increased HbA1c levels among CKD patients with type I diabetes mellitus (T1DM). Also, GP16 has been associated with albumin-to-creatinine ratio and eGFR slope in T1DM [55]. Plausible explanation in the context of renal function are that increased plasma glycemia triggers the flux of glucose in the hexosamine pathway. In turn, this leads to the production of uridine-diphosphate N-acetylgucosamine, which acts as a substrate in N-glycosylation and increase the production of complex N-glycans. In parallel, this events indirectly upregulate epidermal growth receptor; a molecule which as been suggested to be central in renal function. Over activation of EGF receptor may underlie diabetic kidney disease [54, 55]. Further, since the identified N-glycans (GP12 and GP16) have been implicated in renal function in both T1DM and T2DM, this result suggests a possible mechanistic similarity between the two main types of diabetes. However, further investigation is warranted to gain additional insights.
Fig. 1.
Loiss plots of the relationship between N-glycans and Age among cases. Blue and red curves are fitted linear regression models. The shaded region is the 95% confidence intervals on the fitted values
A previous study has shown that IgG galactosylation is associated with complement activation and renal damage [56] and that decreased IgG galactosylation is linked with CKD. On the contrary, the present study showed that galactosylated N-glycan GP14 (A2G2S[6]1), was higher in T2DM patients with CKD compared to those without it. Also, after performing logistic regression in the crude model, agalactosylated N-glycans GP7 (M6D1-D2), core fucosylated monogalactosylated N-glycans GP6 (FA2[6]BG1) and digalactosylated N-glycans (GP14) were found to be associated with increased risk of CKD. These discrepancies may be attributed to the glycoprotein under investigation. In this study, total N-glycome was measured, exploring the whole plasma proteins whereas the previous study focused on IgG N-glycan. Exploring total plasma N-glycome is more beneficial as it reflects N-glycosylation and the relative abundance of proteins in circulation.
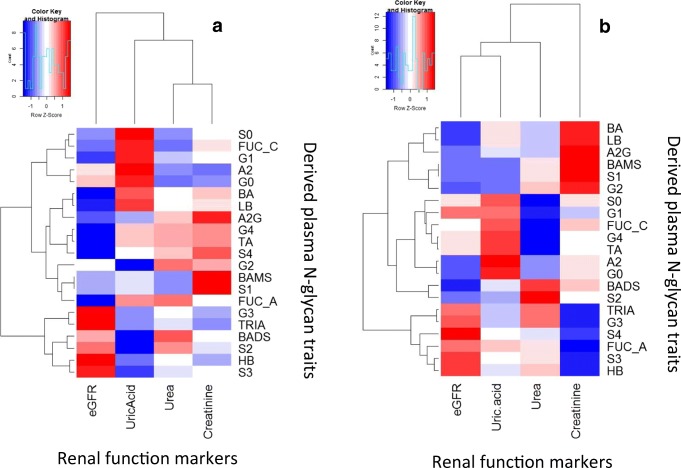
The present study has revealed the independent association between elevated uric acid, urea and creatinine and renal dysfunction as previously reported [60–65] (Tables 4 and 5). Subsequently, we sought to investigate how N-glycans correlate with these renal function markers. Some important highlights were that trigalactosylated [(GP26, GP30, GP31, GP32) and tetragalactosylated structures (GP 34 and GP36) were negatively correlated with creatinine whereas core fucosylated (GP4, GP5, GP20) and trigalactosylated N-glycans (GP30) significantly correlated with uric acid amongst T2DM patients with CKD (Fig. 2 and Supplementary Tables 4 & 5). However, these analyses does not adequately distinguish or adequately predict incident CKD. This can be blamed on the fewer number of T2DM patients with CKD and the impact of antidiabetic medications. A recent study has shown that the majority of the participants utilised different medications including diuretics, statins, glucocorticoids and anti-malarial agents [27]. These medications may influence serum uric acid levels and hence, the observed results could be overestimated.
Fig. 2. Correlation between derived plasma N-glycan traits with or without CKD. (a) No CKD (b) CKD-T2DM.
LB (rs = 0.21, p = 0.008), BA (rs = 0.22, p = 0.005), A2G (rs = 0.18, p = 0.025), S1 (rs = 0.19, p = 0.017), and BAMS (rs = 0.19, p = 0.017) were positively correlated with creatinine among T2DM without CKD. However, S3 (rs = −0.19, p = 0.013), G3 (rs = −0.23, p = 0.004), FUC_A (rs = −0.19, p = 0.014) and TRIA (rs = −0.23, p = 0.003) were negatively associated with creatinine. There were no statistically significant correlations between N-glycans and urea and uric acid in this group. On the other hand, TRIA was negatively associated with creatinine among T2DM with CKD. S0 (rs = 0.26, p = 0.023), G1 (rs = 0.27, p = 0.021) and FUC_C (rs = 0.30, p = 0.035) were positively associated with uric acid whereas S2 (rs = −0.30, p = 0.011), G3 (rs = −0.25, p = 0.036), BADS (rs = −0.26, p = 0.028), TRIA (rs = −0.025, p = 0.029) were negatively associated with uric acid in this group.
The present study has limitations that need to be mentioned. First, metabolic risk factors such as blood pressure, blood glucose, lipid profiles and kidney dysfunction markers were limited to only one measurement whereas CKD presence should be established following multiple estimates for over 3 months. Again, because the study was a cross-sectional one, we were unable to determine the direction or the causal relationship between risk factors and CKD.
Outlook and perspectives
This study provides a stimulus for future research. Here, we have established the presence of CKD by estimating GFR with CKD-EPI equation. Apart from the aforementioned biomarkers, imaging techniques should be employed to establish the presence or absence of CKD. For example, ultrasonographic techniques can be used to examine the size and texture of the kidneys and determine possible abnormalities. In addition, spectral doppler and color doppler with ultrasonography as well as computer tomography, elastography and radiography are powerful tools for detecting CKD. Further, these efforts can be complemented with histopathological methods (e.g. renal biopsy) to confirm diagnosis [66–69].
Conclusion
The present study showed that undiagnosed CKD is prevalent among the T2DM patients. This was established based on the CKD-EPI equation. However, reporting incident CKD was not sufficient with this equation alone and therefore we explored the N-glycosylation profiles of the participants. The study revealed specific complex N-glycans that were associated with fucosylated N-glycans. Future investigations may better reveal the role of the identified complex N-glycans and renal function.
Electronic supplementary material
(DOCX 33 kb)
Compliance with ethical standards
Conflict of interest
The authors declared that they have no conflict of interest
References
- 1.Hill NR, Fatoba ST, Oke JL, Hirst JA, O’Callaghan CA, Lasserson DS, Hobbs FDR. Global prevalence of chronic kidney disease–a systematic review and meta-analysis. PLoS One. 2016;11(7):e0158765. doi: 10.1371/journal.pone.0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Initiative KDOQ. Kidney disease outcomes quality initiative (K/DOQI) clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–S266. [PubMed] [Google Scholar]
- 3.Stenvinkel P. Chronic kidney disease: a public health priority and harbinger of premature cardiovascular disease. J Intern Med. 2010;268(5):456–467. doi: 10.1111/j.1365-2796.2010.02269.x. [DOI] [PubMed] [Google Scholar]
- 4.Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, Saran R, Wang AYM, Yang CW. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382(9888):260–272. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 5.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, AlMazroa MA, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Abdulhak AB, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, de Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FGR, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Memish ZA, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KMV, Nasseri K, Norman P, O'Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA, III, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, de León FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJL. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu C-Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. New Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 7.De Nicola L, Zoccali C. Chronic kidney disease prevalence in the general population: heterogeneity and concerns. Nephrol Dial Transplant. 2015;31(3):331–335. doi: 10.1093/ndt/gfv427. [DOI] [PubMed] [Google Scholar]
- 8.Perlman RL, Finkelstein FO, Liu L, Roys E, Kiser M, Eisele G, Burrows-Hudson S, Messana JM, Levin N, Rajagopalan S, Port FK, Wolfe RA, Saran R. Quality of life in chronic kidney disease (CKD): a cross-sectional analysis in the renal research institute-CKD study. Am J Kidney Dis. 2005;45(4):658–666. doi: 10.1053/j.ajkd.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 9.Kimmel PL, Patel SS, editors. Quality of life in patients with chronic kidney disease: focus on end-stage renal disease treated with hemodialysis. Semin Nephrol; 2006: Elsevier. [DOI] [PubMed]
- 10.Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, Zeeuw DDE, Hostetter TH, Lameire N, Eknoyan G. Definition and classification of chronic kidney disease: a position statement from kidney disease: improving global outcomes (KDIGO) Kidney Int. 2005;67(6):2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 11.Crowe E, Halpin D, Stevens P. Guidelines: early identification and management of chronic kidney disease: summary of NICE guidance. BMJ. 2008;337(7673):812–815. doi: 10.1136/bmj.a1530. [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Coresh J, Bolton K, Culleton B, Harvey KS, Ikizler TA, et al. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 SUPPL. 1). [PubMed]
- 13.Stevens LA, Schmid CH, Greene T, Zhang YL, Beck GJ, Froissart M, et al. Comparative performance of the CKD epidemiology collaboration (CKD-EPI) and the modification of diet in renal disease (MDRD) study equations for estimating GFR levels above 60 mL/min/1.73 m 2. Am J Kidney Dis. 2010;56(3):486–495. doi: 10.1053/j.ajkd.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poggio ED, Wang X, Greene T, Van Lente F, Hall PM. Performance of the modification of diet in renal disease and Cockcroft-Gault equations in the estimation of GFR in health and in chronic kidney disease. J Am Soc Nephrol. 2005;16(2):459–466. doi: 10.1681/ASN.2004060447. [DOI] [PubMed] [Google Scholar]
- 15.Stevens LA, Schmid CH, Greene T, Li L, Beck GJ, Joffe MM, Froissart M, Kusek JW, Zhang Y(L), Coresh J, Levey AS. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009;75(6):652–660. doi: 10.1038/ki.2008.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, van Lente F, Zhang YL, Coresh J, Levey AS, CKD-EPI Investigators Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379(9811):165–180. doi: 10.1016/S0140-6736(11)60178-5. [DOI] [PubMed] [Google Scholar]
- 18.Pergola PE, Raskin P, Toto RD, Meyer CJ, Huff JW, Grossman EB, Krauth M, Ruiz S, Audhya P, Christ-Schmidt H, Wittes J, Warnock DG, BEAM Study Investigators Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med. 2011;365(4):327–336. doi: 10.1056/NEJMoa1105351. [DOI] [PubMed] [Google Scholar]
- 19.Yamagata K, Ishida K, Sairenchi T, Takahashi H, Ohba S, Shiigai T, Narita M, Koyama A. Risk factors for chronic kidney disease in a community-based population: a 10-year follow-up study. Kidney Int. 2007;71(2):159–166. doi: 10.1038/sj.ki.5002017. [DOI] [PubMed] [Google Scholar]
- 20.Foley RN, Murray AM, Li S, Herzog CA, McBean AM, Eggers PW, Collins AJ. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol. 2005;16(2):489–495. doi: 10.1681/ASN.2004030203. [DOI] [PubMed] [Google Scholar]
- 21.Rule AD, Bergstralh EJ, Melton LJ, Li X, Weaver AL, Lieske JC. Kidney stones and the risk for chronic kidney disease. Clin J Am Soc Nephrol. 2009;4(4):804–811. doi: 10.2215/CJN.05811108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Appel LJ, Wright JT, Jr, Greene T, Agodoa LY, Astor BC, Bakris GL, et al. Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med. 2010;363(10):918–929. doi: 10.1056/NEJMoa0910975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Câmara NOS, Iseki K, Kramer H, Liu ZH, Sharma K. Kidney disease and obesity: epidemiology, mechanisms and treatment. Nat Rev Nephrol. 2017;13(3):181–190. doi: 10.1038/nrneph.2016.191. [DOI] [PubMed] [Google Scholar]
- 24.Hayashi H, Karasawa R, Inn H, Saitou T, Ueno M, Nishi S, Suzuki Y, Ogino S, Maruyama Y, Kouda Y, Arakawa M. An electron microscopic study of glomeruli in Japanese patients with non-insulin dependent diabetes mellitus. Kidney Int. 1992;41(4):749–757. doi: 10.1038/ki.1992.117. [DOI] [PubMed] [Google Scholar]
- 25.Lemley KV, Abdullah I, Myers BD, Meyer TW, Blouch K, Smith WE, Bennett PH, Nelson RG. Evolution of incipient nephropathy in type 2 diabetes mellitus. Kidney Int. 2000;58(3):1228–1237. doi: 10.1046/j.1523-1755.2000.00223.x. [DOI] [PubMed] [Google Scholar]
- 26.Adua E, Frimpong K, Li X, Wang W. Emerging issues in public health: a perspective on Ghana’s healthcare expenditure, policies and outcomes. EPMA J. 2017:1–10. [DOI] [PMC free article] [PubMed]
- 27.Adua E, Roberts P, Sakyi SA, Yeboah FA, Dompreh A, Frimpong K, Anto EO, Wang W. Profiling of cardio-metabolic risk factors and medication utilisation among type II diabetes patients in Ghana: a prospective cohort study. Clin Transl Med. 2017;6(1):32. doi: 10.1186/s40169-017-0162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ephraim RK, Biekpe S, Sakyi SA, Adoba P, Agbodjakey H, Antoh EO. Prevalence of chronic kidney disease among the high risk population in South-Western Ghana; a cross sectional study. Can J Kidney Health Dis. 2015;2(1):40. doi: 10.1186/s40697-015-0076-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osafo C, Mate-Kole M, Affram K, Adu D. Prevalence of chronic kidney disease in hypertensive patients in Ghana. Ren Fail. 2011;33(4):388–392. doi: 10.3109/0886022X.2011.565140. [DOI] [PubMed] [Google Scholar]
- 30.Lauc G. Precision medicine that transcends genomics: Glycans as integrators of genes and environment. Biochim Biophys Acta. 2016;1860(8):1571–1573. doi: 10.1016/j.bbagen.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Rosnoblet C, Peanne R, Legrand D, Foulquier F. Glycosylation disorders of membrane trafficking. Glycoconj J. 2013;30(1):23–31. doi: 10.1007/s10719-012-9389-y. [DOI] [PubMed] [Google Scholar]
- 32.Adua E, Russell A, Roberts P, Wang Y, Song M, Wang W. Innovation analysis on postgenomic biomarkers: glycomics for chronic diseases. OMICS. 2017;21(4):183–196. doi: 10.1089/omi.2017.0035. [DOI] [PubMed] [Google Scholar]
- 33.Taylor ME, Drickamer K. Introduction to glycobiology. Oxford: Oxford University Press; 2011. [Google Scholar]
- 34.Cummings RD, Pierce JM. The challenge and promise of glycomics. Chem Biol. 2014;21(1):1–15. doi: 10.1016/j.chembiol.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gornik O, Wagner J, Pučić M, Knežević A, Redžić I, Lauc G. Stability of N-glycan profiles in human plasma. Glycobiology. 2009;19(12):1547–1553. doi: 10.1093/glycob/cwp134. [DOI] [PubMed] [Google Scholar]
- 36.Gudelj I, Salo PP, Trbojević-Akmačić I, Albers M, Primorac D, Perola M, et al. Low galactosylation of IgG associates with higher risk for future diagnosis of rheumatoid arthritis during 10 years of follow-up. Biochim Biophys Acta. 2018;1864(6 Pt A):2034–2039. doi: 10.1016/j.bbadis.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 37.Vučković F, Krištić J, Gudelj I, Teruel M, Keser T, Pezer M, Pučić-Baković M, Štambuk J, Trbojević-Akmačić I, Barrios C, Pavić T, Menni C, Wang Y, Zhou Y, Cui L, Song H, Zeng Q, Guo X, Pons-Estel BA, McKeigue P, Leslie Patrick A, Gornik O, Spector TD, Harjaček M, Alarcon-Riquelme M, Molokhia M, Wang W, Lauc G. Association of systemic lupus Erythematosus with decreased immunosuppressive potential of the IgG Glycome. Arthritis Rheum. 2015;67(11):2978–2989. doi: 10.1002/art.39273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Danquah I, Bedu-Addo G, Terpe KJ, Micah F, Amoako YA, Awuku YA, et al. Diabetes mellitus type 2 in urban Ghana: characteristics and associated factors. BMC Public Health. 2012;12(210):1–8. doi: 10.1186/1471-2458-12-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dagostino C, De Gregori M, Gieger C, Gudelj I, Lauc G, Divizia L, Wang W, et al. Validation of standard operating procedures in a multicenter retrospective study to identify-omics biomarkers for chronic low back pain. PLoS One. 2017;12:e0176372. doi: 10.1371/journal.pone.0176372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Altemtam N, Russell J, El Nahas M. A study of the natural history of diabetic kidney disease (CKD) Nephrol Dial Transplant. 2011;27(5):1847–1854. doi: 10.1093/ndt/gfr561. [DOI] [PubMed] [Google Scholar]
- 42.Rossing K, Christensen PK, Hovind P, Tarnow L, Rossing P, Parving H-H. Progression of nephropathy in type 2 diabetic patients. Kidney Int. 2004;66(4):1596–1605. doi: 10.1111/j.1523-1755.2004.00925.x. [DOI] [PubMed] [Google Scholar]
- 43.Chiasson J-L, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M, et al. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. 2002;359(9323):2072–2077. doi: 10.1016/S0140-6736(02)08905-5. [DOI] [PubMed] [Google Scholar]
- 44.Ku E, McCulloch CE, Mauer M, Gitelman SE, Grimes BA. Hsu cy. Association between blood pressure and adverse renal events in type 1 diabetes. Diabetes Care. 2016;39(12):2218–2224. doi: 10.2337/dc16-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, Pahor M, Bauer D, Newman A, Health ABC Study Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111(25):3384–3390. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 46.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Atertio Thromb Vasc Biol. 2005;25(5):932–943. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
- 47.Tozawa M, Iseki K, Iseki C, Oshiro S, Ikemiya Y, Takishita S. Triglyceride, but not total cholesterol or low-density lipoprotein cholesterol levels, predict development of proteinuria. Kidney Int. 2002;62(5):1743–1749. doi: 10.1046/j.1523-1755.2002.00626.x. [DOI] [PubMed] [Google Scholar]
- 48.Tsimihodimos V, Mitrogianni Z, Elisaf M. Dyslipidemia associated with chronic kidney disease. Open Cardiovasc Med J. 2011;5:41–48. doi: 10.2174/1874192401105010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chan DT, Dogra GK, Irish AB, Ooi EM, Barrett PH, Chan DC, Watts GF. Chronic kidney disease delays VLDL-apoB-100 particle catabolism: potential role of apolipoprotein C-III. J Lipid Res. 2009;50(12):2524–2531. doi: 10.1194/jlr.P900003-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hirano T, Sakaue T, Misaki A, Murayama S, Takahashi T, Okada K, Takeuchi H, Yoshino G, Adachi M. Very low-density lipoprotein-apoprotein CI is increased in diabetic nephropathy: comparison with apoprotein CIII. Kidney Int. 2003;63(6):2171–2177. doi: 10.1046/j.1523-1755.2003.00019.x. [DOI] [PubMed] [Google Scholar]
- 51.Yu X, Wang Y, Kristic J, Dong J, Chu X, Ge S, Wang H, Fang H, Gao Q, Liu D, Zhao Z, Peng H, Pucic Bakovic M, Wu L, Song M, Rudan I, Campbell H, Lauc G, Wang W. Profiling IgG N-glycans as potential biomarker of chronological and biological ages: a community-based study in a Han Chinese population. Medicine (Baltimore) 2016;95(28):e4112. doi: 10.1097/MD.0000000000004112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Knežević A, Gornik O, Polašek O, Pučić M, Redžić I, Novokmet M, Rudd PM, Wright AF, Campbell H, Rudan I, Lauc G. Effects of aging, body mass index, plasma lipid profiles, and smoking on human plasma N-glycans. Glycobiology. 2010;20(8):959–969. doi: 10.1093/glycob/cwq051. [DOI] [PubMed] [Google Scholar]
- 53.Knezevic A, Polasek O, Gornik O, Rudan I, Campbell H, Hayward C, et al. Variability, heritability and environmental determinants of human plasma N-glycome. J Proteome Res. 2008;8(2):694–701. doi: 10.1021/pr800737u. [DOI] [PubMed] [Google Scholar]
- 54.Lau KS, Partridge EA, Grigorian A, Silvescu CI, Reinhold VN, Demetriou M, Dennis JW. Complex N-glycan number and degree of branching cooperate to regulate cell proliferation and differentiation. Cell. 2007;129(1):123–134. doi: 10.1016/j.cell.2007.01.049. [DOI] [PubMed] [Google Scholar]
- 55.Bermingham ML, Colombo M, McGurnaghan SJ, Blackbourn LA, Vučković F, Baković MP, et al. N-glycan profile and kidney disease in type 1 diabetes. Diabetes Care:dc171042. [DOI] [PubMed]
- 56.Cook HT. Complement and kidney disease. Curr Opin Nephrol Hypertens. 2013;22(3):295–301. doi: 10.1097/MNH.0b013e32835ff9cb. [DOI] [PubMed] [Google Scholar]
- 57.Russell AC, Šimurina M, Garcia MT, Novokmet M, Wang Y, Rudan I, Campbell H, Lauc G, Thomas MG, Wang W. The N-glycosylation of immunoglobulin G as a novel biomarker of Parkinson's disease. Glycobiology. 2017;27(5):501–510. doi: 10.1093/glycob/cwx022. [DOI] [PubMed] [Google Scholar]
- 58.Lemmers RF, Vilaj M, Urda D, Agakov F, Šimurina M, Klaric L, et al. IgG glycan patterns are associated with type 2 diabetes in independent European populations. Biochim Biophys Acta. 2017;1861(9):2240–2249. doi: 10.1016/j.bbagen.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 59.Barrios C, Zierer J, Gudelj I, Stambuk J, Ugrina I, Rodriguez E, et al. Glycosylation profile of IgG in moderate kidney dysfunction. J Am Soc Nephrol. 2016;27(3):933–941. doi: 10.1681/ASN.2015010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johnson RJ, Segal MS, Srinivas T, Ejaz A, Mu W, Roncal C, Sánchez-Lozada LG, Gersch M, Rodriguez-Iturbe B, Kang DH, Acosta JH. Essential hypertension, progressive renal disease, and uric acid: a pathogenetic link? J Am Soc Nephrol. 2005;16(7):1909–1919. doi: 10.1681/ASN.2005010063. [DOI] [PubMed] [Google Scholar]
- 61.Obermayr RP, Temml C, Gutjahr G, Knechtelsdorfer M, Oberbauer R, Klauser-Braun R. Elevated uric acid increases the risk for kidney disease. J Am Soc Nephrol. 2008;19(12):2407–2413. doi: 10.1681/ASN.2008010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yan D, Tu Y, Jiang F, Wang J, Zhang R, Sun X, Wang T, Wang S, Bao Y, Hu C, Jia W. Uric acid is independently associated with diabetic kidney disease: a cross-sectional study in a Chinese population. PLoS One. 2015;10(6):e0129797. doi: 10.1371/journal.pone.0129797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rosolowsky ET, Ficociello LH, Maselli NJ, Niewczas MA, Binns AL, Roshan B, Warram JH, Krolewski AS. High-normal serum uric acid is associated with impaired glomerular filtration rate in nonproteinuric patients with type 1 diabetes. Clin J Am Soc Nephrol. 2008;3(3):706–713. doi: 10.2215/CJN.04271007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nakagawa T, Kang D, Feig D, Sanchez-Lozada L, Srinivas T, Sautin Y, et al. Unearthing uric acid: an ancient factor with recently found significance in renal and cardiovascular disease. Kidney Int. 2006;69(10):1722–1725. doi: 10.1038/sj.ki.5000391. [DOI] [PubMed] [Google Scholar]
- 65.Tomita M, Mizuno S, Yamanaka H, Hosoda Y, Sakuma K, Matuoka Y, Odaka M, Yamaguchi M, Yosida H, Morisawa H, Murayama T. Does hyperuricemia aeffect mortality? A prospective cohort study of japanese male workers. J Epidemiol. 2000;10(6):403–409. doi: 10.2188/jea.10.403. [DOI] [PubMed] [Google Scholar]
- 66.Alaini A, Malhotra D, Rondon-Berrios H, Argyropoulos CP, Khitan ZJ, Raj DS, et al. Establishing the presence or absence of chronic kidney disease: uses and limitations of formulas estimating the glomerular filtration rate. World J Methodol. 2017;7(3):73–92. doi: 10.5662/wjm.v7.i3.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Radermacher J, Chavan A, Schaffer J, Stoess B, Vitzthum A, Kliem V, et al. Detection of significant renal artery stenosis with color Doppler sonography: combining extrarenal and intrarenal approaches to minimize technical failure. Clin Nephrol. 2000;53(5):333–343. [PubMed] [Google Scholar]
- 68.Moe S, Drüeke T, Cunningham J, Goodman W, Martin K, Olgaard K, Ott S, Sprague S, Lameire N, Eknoyan G, Kidney Disease: Improving Global Outcomes (KDIGO) Definition, evaluation, and classification of renal osteodystrophy: a position statement from kidney disease: improving global outcomes (KDIGO) Kidney Int. 2006;69(11):1945–1953. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- 69.Radcliffe NJ, Seah JM, Clarke M, RJ MI, Jerums G, Ekinci EI. Clinical predictive factors in diabetic kidney disease progression. J Diabetes Invest. 2017;8(1):6–18. doi: 10.1111/jdi.12533. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 33 kb)