Abstract
Background
A number of studies have shown that consumption of vegetable oils may improve diabetes complications including inflammatory response and oxidative stress, but no study has been done on the effects of canola oil (CO) and olive oil (OO) consumption in patients with type 2 diabetes. This clinical trial was done to compare the effects of CO and OO on insulin resistance, inflammation and oxidative stress in women with type 2 diabetes.
Methods
This randomized controlled clinical trial was done on 77 type 2 diabetic women. 4 weeks before the intervention, lipid-lowering drugs intakes were cut under the supervision of an endocrinologist. The participants were randomly divided into 2 intervention groups (Balanced diet +30 g/day CO or OO) and one control group (Balanced diet +30 g/day of sunflower oil (SFO)). Dietary intakes were assessed using three 24-h food records at baseline and at weeks 4 and 8 of the interventions. At baseline and after 8 weeks, height, weight, waist circumference, fasting blood sugar (FBS), serum insulin, C-reactive protein (CRP) and malondialdehyde (MDA) were measured.
Results
After the intervention in the inter-group analysis, CRP level was reduced significantly in CO and OO groups but no significant changes were observed in other factors. CRP reductions were also significant between all of the groups but not for other factors.
Conclusions
Replacing CO and OO with SFO as part of daily dietary fat in the diet of people with type 2 diabetes is recommended for reducing Inflammation and Oxidative Stress.
Trial registration.
This study is approved by the Ethics Committee of Shiraz University of Medical Sciences (IR.SUMS.REC.1394.27) and is recorded in the Iranian Registry of Clinical Trials (IRCT2015062722818N1).
Keywords: Canola oil, Olive oil, Fasting blood sugar (FBS), Serum insulin, C-reactive protein (CRP), Malondialdehyde (MDA)
Introduction
Prevalence of diabetes mellitus is one of the most challenging and serious health problems in the twenty-first century. The number of people with diabetes is growing more than expected [1].
Insulin resistance plays a major role in the pathogenesis of type 2 diabetes [2]. Previous studies have shown that diets with saturated fatty acids interfere with insulin action [3]. A number of cross-sectional studies have shown that insulin resistance and type 2 diabetes are related with high level of CRP [4]. In addition, it seems that insulin resistance and oxidative stress play important roles in the pathology of type 2 diabetes [5–7].
Oxidative stress can be a pathophysiological link between cardiovascular diseases (CVD) and diabetes [8]. Studies have shown that oxidative stress by reactive oxygen species (ROS) and nitrogen species produced during hyperglycemia have an important role in diabetic damages in different organs [9]. During the diabetes disease, free and active radicals are produced. Increased free radical and reduced antioxidant defense mechanisms damage cellular organelles and enzymes, which lead to the increase of lipid peroxidation and insulin resistance, damage and finally death of beta cells in diabetic patients [10]. Studies have shown that the changes in dietary fatty acids can play an important role in the prevention and treatment of coronary heart disease [11], and inflammatory responses [12].
CO and OO are good sources of monounsaturated fatty acid (MUFA) [13]. CO contains 11% omega-3 polyunsaturated fatty acids (PUFA), 53–59% MUFA, 22% omega-6 PUFAs and 7.1% saturated fatty acids (SFA) [14–16], and its ratio of omega-6 to omega-3 is appropriate [16, 17]. OO contains 1% omega-3 PUFAs, 73.3% oleic acid (a MUFA), 7.9% omega-6 PUFAs and 13.5% SFA [17]. OO phenolic compounds like tyrosol, oleuropein aglycone, hydroxytyrosol and their derivatives have a high antioxidant capacities [18].
By lowering cholesterol level and stimulation of anti-inflammatory effects, high PUFA and alpha-Linolenic acid (ALA) diets have particularly protective effects on the heart [8]. Several studies have reported that CRP level is inversely related with EPA and DHA [8, 19]. The antioxidant activity of oils depends on vitamin E and Phytochemicals value and fatty acid’ compositions and OO consumptions has a protective effect against Oxidative Stress [20–22].
Due to the effects of fatty acids’ composition of diet on insulin resistance, inflammation and oxidative stress and developing of diabetes and CVD, this study was aimed to compare the effects of CO and OO with SFO, which is the most common oil being consumed in Iran, in patients with type 2 diabetes.
Methods
Patients
This study was held from July 2015 to November 2015. 81 females over 50 years old with type 2 diabetes and an average body mass index (BMI) of 28 kg/m2 were recruited. Participants were selected from Motahhari clinic in Shiraz, according to these inclusion criteria:
Female gender, records of type 2 diabetes of at least 6 months, and the routine use of SFO. Patients who need insulin and/or lipid-lowering drugs; patients with thyroid disorders, kidney and liver diseases, CVD; participating in other studies in the past 6 months; taking non-steroidal immunosuppressant, cyclosporine and warfarin; smokers, alcohol consumers. People who have triglycerides (TG) > 400 (mg/dL) and/or low-density lipoprotein cholesterol (LDL-C) > 200 (mg/dL) were not included in the study.
Study design
This study was in a single-centered, parallel group, and randomized controlled clinical trial. It was approved by the Ethics Committee of Shiraz University of Medical Sciences (IR.SUMS.REC.1394.27) and was recorded in the Iranian Registry of Clinical Trials.
All study protocols were introduced to the patients and then the written consents were taken. The sample size was estimated based on a previous study by POWER SSC software [23] and with considering the mean difference between independent groups by assuming the probability of Type I error (α) equal to 0.05, the power of (β-1) equal to 80%, the mean difference (μ1-μ2) equal to 0.35 and standard deviation (σ) equal to 0.40. After adding 25% drop out rate, 25 persons waere considered in every group.
Four weeks before the intervention, intakes of lipid-lowering drugs were discontinued under the supervision of an endocrinologist. Then, by using balanced block method, patients were randomly divided into 3 groups.
Weight maintenance (55% carbohydrate, 18% protein and 27% fat) diet was designated for each participant by Estimated Energy Requirement (EER) equation, each diet contained 30 g per day of vegetable oils (SFO, CO and OO) and patients were asked to add it to their salads or their boiled foods by using a small measuring cup. Fatty acids’ compositions of consumed oils are extracted in Shiraz Azmoon Gostar laboratory and they are shown in Table 1.
Table 1.
Fatty acids composition of consumed oils
| Fatty acids | Olive oil | Canola oil | Sunflower oil |
|---|---|---|---|
| C16 | 11.2 | 6.5 | 7.8 |
| C18 | 2.9 | 2.5 | 4.9 |
| C20 | 0 | 0.2 | 0.4 |
| C22 | 0 | 0 | 0.9 |
| C18:1 | 72.5 | 59.4 | 27.6 |
| C18:2 | 11 | 21.3 | 58 |
| C18:3 | 1 | 9.9 | 0 |
| C20:1 | 0 | 0.2 | 0.4 |
| SFA∑ | 14.1 | 9.2 | 14 |
| MUFA∑ | 72.5 | 59.6 | 28 |
| PUFA∑ | 12 | 31.2 | 58 |
All values are % of total fatty acids, SFA: Saturated fatty acid,
MUFA Monounsaturated fatty acid, PUFA Polyunsaturated fatty acid
Anthropometric measurements and assessment of dietary intake
At the baseline and at the end of the intervention, anthropometric indices, including height, weight, and waist circumference were measured.
Patients’ weights were measured in light clothes, and without shoes with an accuracy of 100 g by a digital balance (BF11 OMRON made in France). Height was measured with an accuracy of 0.5 cm by a non-stretchable tape measure. Then, BMI was calculated as Weight (kg)/ (Height (m)* Height (m)).
At baseline, week 4 and week 8 of the intervention, 3 days 24-h record and physical activity record were filled by participants. Participants were asked not to change the recommended diet, medications and daily physical activity during the intervention.
Biochemical evaluation of blood
Five milliliters of blood samples were taken after 12 to 14 h fasting from all patients and were held for 15 to 20 min at room temperature, and then, they were centrifuged for 5 min at 300 rpm. Serums were kept on −76 °C for further analyses. MDA level was measured by spectrophotometry, serum insulin and CRP were measured by ELISA (IBL kit), and insulin resistance was calculated by the following formula:
| 1 |
Statistical analysis
24-h food records were analyzed by Nutritionist IV software. Data were analyzed by SPSS 19. P values less than 0.05 were considered significant.
Normal distribution of variables was assessed by using Kolmogorov-Smirnov test. Paired-Samples T-test was used to compare the anthropometric measurements, energy, dietary intakes, CRP, FBS, Insulin and MDA before and after intervention. One-way ANOVA was used to compare the mean changes of CRP, FBS, Insulin, HOMA-IR and MDA among the three groups and then, Post-Hoc test was used for further analyses.
Data availability
Please contact author for data requests.
Result
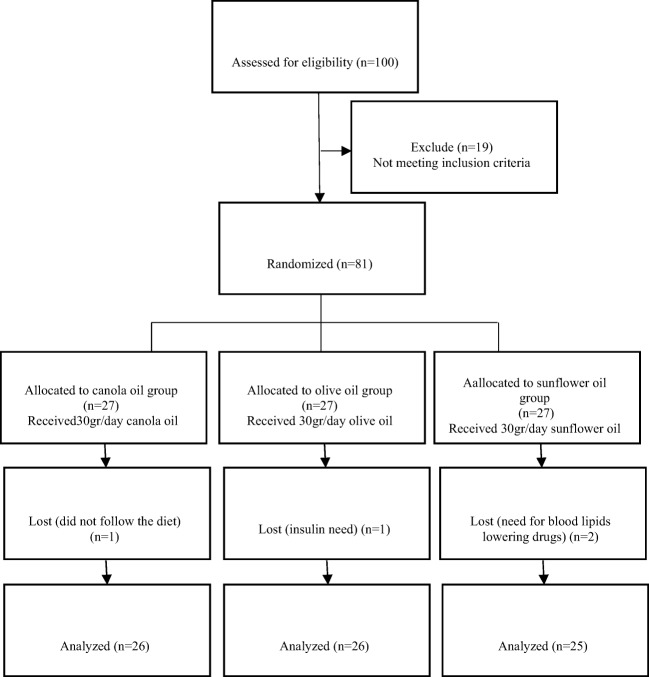
Out of 81 participants, one in the OO group (not following the dietary regimen), one in the CO group (need of insulin) and two in the SFO group (need for blood lipids lowering drugs) were excluded, and 77 of them completed the study (Fig. 1). Participants reported no side effects associated with the consumption of the oils.
Fig. 1.
Participants flow diagram throughout the study
General characteristics, anthropometric status, and the dietary intake of participants at baseline are shown in Table 2. No significant differences in energy and fatty acid intakes, macronutrient distributions, weights, waist circumferences, BMIs and physical activities were observed in the control and the intervention groups.
Table 2.
General characteristics, anthropometric status, physical activity and dietary intake of participants at baseline
| Variables | Olive oil | Canola oil | Sunflower oil | p-Value* |
|---|---|---|---|---|
| Age (Year) | 59 ± 7 | 58 ± 6 | 57 ± 5 | 0.63 |
| Height (cm) | 155 ± 5 | 156 ± 4 | 155 ± 4 | 0.67 |
| Weight (kg) | 69.8 ± 14 | 70.7 ± 7.8 | 68 ± 9.9 | 0.68 |
| BMI (kg/m2) | 28.7 ± 4.8 | 28.9 ± 3.6 | 28.1 ± 3.8 | 0.76 |
| Waist circumference (cm) | 95.2 ± 10.5 | 98.8 ± 6.8 | 95.6 ± 9.7 | 0.31 |
| Physical activity (METh/day) | 27.7 ± 2 | 27.5 ± 2 | 28.6 ± 2.2 | 0.20 |
| Fat (%Energy) | 29.4 ± 3.6 | 27.7 ± 4.5 | 28.9 ± 5.4 | 0.39 |
| Protein (%Energy) | 16.8 ± 2.4 | 17.5 ± 2.9 | 17 ± 2.1 | 0.58 |
| Carbohydrate (%Energy) | 55.7 ± 5.4 | 56.1 ± 6.5 | 55.2 ± 7.1 | 0.89 |
| Energy (kcal/day) | 1586.6 ± 337.3 | 1642.3 ± 299.0 | 1542.6 ± 262.4 | 0.50 |
| Cholesterol (mg/day) | 211.2 ± 71.0 | 206.3 ± 72.1 | 231.6 ± 83.7 | 0.46 |
| SFA (% of total energy) | 13.6 ± 3.6 | 13.4 ± 3.1 | 13.0 ± 4.2 | 0.85 |
| MUFA (% of total energy) | 9.6 ± 3.8 | 10.0 ± 3.8 | 12.1 ± 2.6 | 0.08 |
| PUFA (% of total energy) | 22.7 ± 3.9 | 22.4 ± 8.5 | 18.3 ± 1.5 | 0.06 |
| Dietary fiber (% of total energy) | 14.9 ± 5.6 | 18.0 ± 5.8 | 13.99 ± 3.9 | 0.07 |
| Soluble fiber (% of total energy) | 0.4 ± 0.3 | 0.4 ± 0.2 | 0.39 ± 0.3 | 0.54 |
BMI body mass index, SFAs saturated fatty acids, MUFAs monounsaturated fatty acids, PUFAs polyunsaturated fatty acids. All values are mean ± Standard deviation. * One way ANOVA
Dietary intakes of participants during the intervention are given in Table 3. No significant differences were observed in energy and fiber intakes, macronutrient distributions and physical activities in the three groups. MUFA and PUFA intakes had significant differences among the three groups (P < 0.01 for both).
Table 3.
Anthropometric status, physical activity and dietary intake of participants during the intervention
| Variables | Olive oil | Canola oil | Sunflower oil | p-Value* |
|---|---|---|---|---|
| Weight (kg) | 69.5 ± 14.1 | 70.7 ± 8 | 67.8 ± 9.8 | 0.63 |
| BMI (kg/m2) | 28.6 ± 4.9 | 28.9 ± 3.7 | 28 ± 3.7 | 0.71 |
| Waist circumference (cm) | 94.9 ± 10.6 | 98.9 ± 7.3 | 95.2 ± 9.3 | 0.23 |
| Physical activity (METh/day) | 27.7 ± 2.1 | 27.6 ± 1.8 | 28.2 ± 1.9 | 0.67 |
| Fat (%Energy) | 28.3 ± 3.9 | 27.8 ± 2.9 | 28 ± 4.5 | 0.91 |
| Protein (%Energy) | 16.8 ± 2.1 | 16.5 ± 1.4 | 16.8 ± 1.7 | 0.82 |
| Carbohydrate (%Energy) | 56.9 ± 5.6 | 57.3 ± 4.2 | 56.6 ± 5.6 | 0.89 |
| Energy (kcal/day) | 1614.8 ± 267.0 | 1620.8 ± 252.0 | 1614.9 ± 316.7 | 1.00 |
| Cholesterol (mg/day)) | 197.3 ± 76.4 | 183.1 ± 49.8 | 178.5 ± 65.5 | 0.56 |
| SFA (% of total energy) | 13.4 ± 2.9 | 12.0 ± 2.3 | 12.9 ± 3.1 | 0.20 |
| MUFA (% of total energy) | 24.57 ± 2.6 | 21.7 ± 3.0 | 12.3 ± 2.3 | 0.00 |
| PUFA (% of total energy) | 6.8 ± 2.1 | 11.3 ± 2.0 | 19.0 ± 1.4 | 0.00 |
| Dietary fiber (% of total energy) | 18.5 ± 5.5 | 17.7 ± 4.7 | 16.3 ± 4.2 | 0.28 |
| Soluble fiber (% of total energy) | 0.5 ± 0.2 | 0.53 ± 0.2 | 0.4 ± 0.2 | 0.28 |
BMI body mass index, SFAs saturated fatty acids, MUFAs monounsaturated fatty acids, PUFAs polyunsaturated fatty acids. All values are mean ± Standard deviation. * One way ANOVA
Comparisons of the mean changes in FBS, MDA, CRP, Insulin and HOMA-IR levels among the three groups are illustrated in Table 4. There were no significant differences in FBS, insulin and HOMA-IR levels in the three groups. In the inter-group analysis CRP decreased significantly in OO (from 12 to 9.5, P < 0.01) and CO (from 15 to 11.8, P < 0.01) after the intervention. No changes were observed in SFO group. There were significant differences in CRP levels (P = 0.02) among the three groups. Turkey Post Hoc test showed significant reduction of CRP in CO (P = 0.02) in comparison to the SFO group (Table 5).
Table 4.
Comparison of the changes in hematological parameters among the three groups before and after intervention
| Olive Oil | Canola oil | Sunflower oil | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Before | After | Changes | Before | After | Changes | Before | After | Changes | |
| FBS (mg/dL) | 135 ± 30.3 | 134.1 ± 40.3 | −0.9 | 127.85 ± 45.73 | 128.00 ± 42.83 | 0.1538 | 145.44 ± 58.28 | 141.20 ± 48.55 | −4.24 |
| MDA (mg/dL) | 5.4 ± 1.5 | 5 ± 1.4 | −0.4 | 5.21 ± 1.08 | 4.92 ± 1.13 | −0.2869 | 4.84 ± 1.09 | 4.69 ± 1.05 | −0.14 |
| Insulin (mg/dL) | 9.3 ± 8.3 | 8 ± 6.8 | −1.3 | 8.70 ± 6.07 | 10.47 ± 6.09 | 1.7726 | 8.97 ± 7.69 | 9.55 ± 5.95 | 0.58 |
| Homa-IR | 3.3 ± 3.6 | 2.7 ± 2.7 | −0.6 | 2.57 ± 1.79 | 3.13 ± 1.78 | 0.5564 | 3.41 ± 4.29 | 3.11 ± 1.82 | −0.30 |
| CRP (mg/dL) | 12 ± 3.8 | 9.5 ± 2.8 | −2.5 | 15.05 ± 6.28 | 11.77 ± 3.67 | −3.2779 | 11.06 ± 4.35 | 10.74 ± 3.50 | −0.32 |
FBS fast blood sugar, MDA malondialdehyde, CRP C-reactive Protein
Table 5.
Significant value of changes in hematological parameters among the three groups before and after intervention
| Inter-group analysis P-Valuea | Intra-group analysis P-Valueb | |||
|---|---|---|---|---|
| Olive oil | Canola oil | Sunflower oil | Between groups | |
| FBS (mg/dL) | 0.88 | 0.98 | 0.56 | 0.92 |
| MDA (mg/dL) | 0.13 | 0.23 | 0.51 | 0.89 |
| Insulin (mg/dL) | 0.35 | 0.14 | 0.74 | c0.10 |
| Homa-IR | 0.36 | 0.13 | 0.73 | c*0.05 |
| CRP (mg/dL) | *0.001 | *0.003 | 0.47 | 0.02 |
aThe value of paired-samples t-test, bThe value of ANOVA test, cNonparametric test value, *significant changes
FBS fast blood sugar, MDA malondialdehyde, CRP C-reactive Protein
Discussion
The results of our study showed that there were no significant differences among the effects of OO, CO and SFO consumption on MDA in women with diabetes. These changes were also not significant in each group. Based on previous studies, the effects of different kinds of oils on MDA are controversial and they are as follow.
OO with high content of MUFA, low content of PUFA and also with antioxidants like polyphenol and tocopherol causes strong protective effects against Oxidative Stress [20–22]. Positive results for CO consumption are also reported in some studies while this effect did not observe with SFO consumption in which MUFA content is lower and PUFA content is higher with respect to CO and OO (Table 1). [24].
It is shown that omega-3 fatty acids reduce oxidative stress in fat mass [3], and also, Ebrahimi et al. have reported that MUFA-enriched diet increased the resistance to oxidative damage compared to PUFA (omega-6 type) [24]. In regard to other study results on MDA, it seems that CO and OO consumptions have positive effects on oxidative stress, but it seems that the short period of the intervention in this study causes non-significant results.
We found no significant variations in the effects of OO, CO and SFO consumption on FBS and insulin resistance in women with type 2 diabetes. Also, no significant improvement was seen in glycemic status in each group.
On previous studies, controversial results were derived. In the Nigam study, consumption of 20 g OO, CO or SFO for 6 months showed significant reductions in fasting insulin and HOMA-IR in OO group. Results of other studies indicated that CO and OO have positive effects on insulin resistance [25, 26]. MUFs reduce insulin resistance by the oxidation of fatty acids which causes the activation and proliferation of alpha receptor. This leads to the reductions in activation of sterol regularity element-binding proteins and lipogenesis inhibition [25]. OO with high content of MUFA and PUFA may stimulate insulin secretion from beta cells [27]. In Gillingham study, SFA substitution with MUFA led to modulation of insulin sensitivity and glycemic control [28]. Lotfi and Zhao showed that MUFA in CO and OO compared to SFA, may improve glucose intolerance and insulin resistance but there were no significant differences on the peripheral insulin sensitivity [19, 29]. In Soaser study, significant increases in insulin, insulin resistance and FBS were observed [30].
In this study a significant reduction of CRP was found in CO in regard to SFO. Based on previous studies with dietary enrichments in omega-3 such as CO, ALA with high content PUFA diet, fish oil with weight loss diet, and Mediterranean diet, significant reductions of CRP were observed [19, 31–33], while opposite results were also reported [3, 8, 24]. But, no study has been done on the efficiency of CO and OO on the CRP of patients with type 2 diabetes. It seems that the causes and mechanisms of reducing CRP by compounds in CO and OO are as follow:
ALA and omega-3 content of CO and OO have anti-inflammatory effects. By formation of prostaglandin I3, ALA prevents formation of thromboxane A2 which decreases cardiovascular risks [24]. Omega-3 fatty acids in OO inhibit the inflammation process of omega-6 fatty acids and decrease concentrations of arachidonic acid [34]. Anti-inflammatory effects of omega-3 are shown by reducing the formation of leukocytes [8]. Also, OO has phenolic compounds which are anti-inflammatory [35].
Conclusion
Prevalence of diabetes mellitus is one of the most challenging and serious health problems and the consumption of vegetable oils may improve diabetes complications. In this study, a clinical trial considered the effects of CO and OO on insulin resistance, inflammation and oxidative stress in women with type 2 diabetes. Limitations include short duration of intervention and dietary examination based on individual reports. The strengths part include cutting lipid lowering drugs to demonstrate the effect of diet on health improvement, gender, age, menopause and hormonal status of participants.
After the intervention in the inter-group analysis, results show that CRP levels were reduced significantly in CO and OO groups but no significant changes were observed in other factors. CRP reductions were also significant between all of the groups but not for other factors. Replacing CO and OO with sunflower oil as part of daily dietary fat in the diet of people with type 2 diabetes is recommended to reduce Inflammation and Oxidative Stress. In future studies it is recommended to increase the number of samples, to study with different intervention periods, to study on hypertension or lipid abnormalities.
Acknowledgements
We wish to thank Shiraz University of Medical Sciences for their support. Also, we thank our participants for their cooperation.
Abbreviations
- ALA
Alpha-Linolenic Acid
- BMI
Body Mass Index
- CO
Canola Oil
- CRP
C - reactive protein
- CVD
Cardiovascular Diseases
- EER
Estimated Energy Requirement
- FBS
Fasting Blood Sugar
- LDL-C
Low-density Lipoprotein Cholesterol
- MDA
Malondialdehyde
- MUFA
Monounsaturated Fatty Acid
- OO
Olive Oil
- PUFA
Polyunsaturated Fatty Acid
- ROS
Reactive Oxygen Species
- SFA
Saturated Fatty Acid
- SFO
Sunflower Oil
- TG
Triglycerides
Authors’ contributions
Masoumeh Atefi carried out the all protocols described in the manuscript and wrote it too. Gholam Reza Pishdad list the probable patients for the study and cut lipid-lowering drug of selected patients. Shiva Faghih participated in the design of the study and verified the study and edit the manuscript.
Funding
This article was extracted from an MSc thesis written by Masoumeh Atefi which was funded by Shiraz University of Medical Sciences with the grant number of “94–7493”.
Ethics approval and consent to participate
This study is approved by the Ethics Committee of Shiraz University of Medical Sciences (IR.SUMS.REC.1394.27) and is recorded in the Iranian Registry of Clinical Trials (IRCT2015062722818N1).
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Masoumeh Atefi, Email: atefimasoumeh@gmail.com.
Gholam Reza Pishdad, Email: grpishdad@gmail.com.
Shiva Faghih, Phone: +989126305829, Email: sh_faghih@sums.ac.ir.
References
- 1.Simsek S, Van Den Oever I a M, Raterman HG, Nurmohamed MT. Endothelial dysfunction, inflammation, and apoptosis in diabetes mellitus. Mediat Inflamm. 2010;2010:1–15. doi: 10.1155/2010/792393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahimi GRM, Attarzadeh Hosseini SR. The effect of aerobic training and diet on lipid profile and liver enzymes in obese women with type II diabetes. Daneshvar Med. 2014;21:1–11. [Google Scholar]
- 3.Azizi-Soleiman F, Jazayeri S, Eghtesadi S, Rajab A, Heidari I, Vafa MR, Gohari MR. Effects of pure eicosapentaenoic and docosahexaenoic acids on oxidative stress, inflammation and body fat mass in patients with type 2 diabetes. Int J Prev Med. 2013;4:922–928. [PMC free article] [PubMed] [Google Scholar]
- 4.De Rekeneire N, Peila R, Ding J, Colbert LH, Visser M, Shorr RI, et al. Diabetes, hyperglycemia, and inflammation in older individuals: the health, aging and body composition study. Diabetes Care. 2006;29(8):1902–1908. doi: 10.2337/dc05-2327. [DOI] [PubMed] [Google Scholar]
- 5.Smith A, Patterson C, Yarnell J, Rumley A, Ben-Shlomo Y, Lowe G. Which hemostatic markers add to the predictive value of conventional risk factors for coronary heart disease and ischemic stroke? The Caerphilly study. Circulation. 2005;112:3080–3087. doi: 10.1161/CIRCULATIONAHA.105.557132. [DOI] [PubMed] [Google Scholar]
- 6.Hoy AJ, Brandon AE, Turner N, Watt MJ, Bruce CR, Cooney GJ, Kraegen EW. Lipid and insulin infusion-induced skeletal muscle insulin resistance is likely due to metabolic feedback and not changes in IRS-1, Akt, or AS160 phosphorylation. Am J Physiol Endocrinol Metab. 2009;297:E67–E75. doi: 10.1152/ajpendo.90945.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron R, Kim JK, Cushman SW, Cooney GJ, Atcheson B, White MF, Kraegen EW, Shulman GI. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem. 2002;277:50230–50236. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- 8.Mori TA, Woodman RJ, Burke V, Puddey IB, Croft KD, Beilin LJ. Effect of eicosapentaenoic acid and docosahexaenoic acid on oxidative stress and inflammatory markers in treated-hypertensive type 2 diabetic subjects. Free Radic Biol Med. 2003;35:772–781. doi: 10.1016/S0891-5849(03)00407-6. [DOI] [PubMed] [Google Scholar]
- 9.Cai L, Kang Y. Oxidative stress and diabetic cardiomyopathy. Cardiovasc Toxicol. 2001;1:181–194. doi: 10.1385/CT:1:3:181. [DOI] [PubMed] [Google Scholar]
- 10.Azizi Z, Mansoorpoor S, Sabzehvarifard a. Effect of estradiol valerate on pancreatic beta cells resistance in diabetic female rats by streptozotocin. Teb Jonoub 2014;17:107–119. http://ismj.bpums.ac.ir/browse.php?a_code=A-10-3-434&slc_lang=fa&sid=1. Accessed 13 Apr 2016.
- 11.De Caterina R. n− 3 Fatty acids and cardiovascular disease. N Engl J Med. 2011;364:2439–2450. doi: 10.1056/NEJMra1008153. [DOI] [PubMed] [Google Scholar]
- 12.Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med. 2008;233:674–688. doi: 10.3181/0711-MR-311. [DOI] [PubMed] [Google Scholar]
- 13.Tarrago-Trani MT, Phillips KM, Lemar LE, Holden JM. New and existing oils and fats used in products with reduced trans-fatty acid content. J Am Diet Assoc. 2006;106:867–880. doi: 10.1016/j.jada.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 14.Patade A, Devareddy L, Lucas EA, Korlagunta K, Daggy BP, Arjmandi BH. Flaxseed reduces total and LDL cholesterol concentrations in native American postmenopausal women. J Women's Health (Larchmt) 2008;17:355–366. doi: 10.1089/jwh.2007.0359. [DOI] [PubMed] [Google Scholar]
- 15.Stark KD, Park EJ, Maines VA, Holub BJ. Effect of a fish-oil concentrate on serum lipids in postmenopausal women receiving and not receiving hormone replacement therapy in a placebo-controlled, double-blind trial. Am J Clin Nutr. 2000;72:389–394. doi: 10.1093/ajcn/72.2.389. [DOI] [PubMed] [Google Scholar]
- 16.Gaziano JM, Hennekens CH, O’Donnell CJ, Breslow JL, Buring JE. Fasting triglycerides, high-density lipoprotein, and risk of myocardial infarction. Circulation. 1997;96:2520–2525. doi: 10.1161/01.CIR.96.8.2520. [DOI] [PubMed] [Google Scholar]
- 17.Quiles JL, Ramírez-Tortosa MC, Yaqoob P. Olive oil and health. Wallingford: CABI; 2006. [Google Scholar]
- 18.Mozaffarian D, Wu JHY. Omega-3 fatty acids and cardiovascular disease: Effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58:2047–2067. doi: 10.1016/j.jacc.2011.06.063. [DOI] [PubMed] [Google Scholar]
- 19.Zhao G, Etherton TD, Martin KR, West SG, Gillies PJ, Kris-Etherton PM. Dietary alpha-linolenic acid reduces inflammatory and lipid cardiovascular risk factors in hypercholesterolemic men and women. J Nutr. 2004;134:2991–2997. doi: 10.1093/jn/134.11.2991. [DOI] [PubMed] [Google Scholar]
- 20.Nakbi A, Tayeb W, Grissa A, Issaoui M, Dabbou S, Chargui I, Ellouz M, Miled A, Hammami M. Effects of olive oil and its fractions on oxidative stress and the liver’s fatty acid composition in 2,4-Dichlorophenoxyacetic acid-treated rats. Nutr Metab (Lond) 2010;7:80. doi: 10.1186/1743-7075-7-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Covas MI. Bioactive effects of olive oil phenolic compounds in humans: reduction of heart disease factors and oxidative damage. Inflammopharmacology. 2008;16:216–218. doi: 10.1007/s10787-008-8019-6. [DOI] [PubMed] [Google Scholar]
- 22.Perona J, Montero E, Sánchez-Domínguez J, Cañizares J, García M, Ruiz-Gutiérrez V. Evaluation of the effect of dietary virgin oil on blood pressure and lipid composition of serum and low-density lipoprotein in elderly type 2 diabetic subjects. Chem J Agric Food. 2009;57:11427–11433. doi: 10.1021/jf902321x. [DOI] [PubMed] [Google Scholar]
- 23.Kuriyan R, Gopinath N, Vaz M, Kurpad A V. Use of rice brain oil in patients with hyperlipidaemia. Natl Med J India 2005;18:292–296. http://www.nmji.in/archives/Volume_18-6_November_December2005/Original_articles/Use_of_rice_bran.htm. Accessed 14 Apr 2016. [PubMed]
- 24.Seied-Ebrahimi S, Shidfar F, Heydari I, Haghighi L, Gohari M, Hoseini H. Comparison of the effects of canola oil with sunflower oil on blood pressure, lipid profile, apoproteins, lipoprotein(a), total antioxidant capacity, and CRP in hyperlipidemic postmenopausal women. Iran J Nutr Sci Food Technol. 2011;6. http://nsft.sbmu.ac.ir/browse.php?a_code=A-10-1-210&slc_lang=en&sid=1. Accessed 14 Apr 2016.
- 25.Nigam P, Bhatt S, Misra A, Chadha DS, Vaidya M, Dasgupta J, Pasha QMA. Effect of a 6-month intervention with cooking oils containing a high concentration of monounsaturated fatty acids (olive and canola oils) compared with control oil in male Asian Indians with nonalcoholic fatty liver disease. Diabetes Technol Ther. 2014;16:255–261. doi: 10.1089/dia.2013.0178. [DOI] [PubMed] [Google Scholar]
- 26.Nydahl M, Gustafsson IB, Ohrvall M, Vessby B. Similar effects of rapeseed oil (canola oil) and olive oil in a lipid-lowering diet for patients with hyperlipoproteinemia. J Am Coll Nutr. 1995;14:643–651. doi: 10.1080/07315724.1995.10718554. [DOI] [PubMed] [Google Scholar]
- 27.López-Miranda J, Pérez-Jiménez F, Ros E, De Caterina R, Badimón L, Covas MI, et al. Olive oil and health: summary of the II international conference on olive oil and health consensus report, Jaén and Córdoba (Spain) 2008. Nutr Metab Cardiovasc Dis. 2010;20:284–294. doi: 10.1016/j.numecd.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Lin L, Allemekinders H, Dansby A, Campbell L, Durance-Tod S, Berger A, Jones PJH. Evidence of health benefits of canola oil. Nutr Rev. 2013;71:370–385. doi: 10.1111/nure.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lotfi MH, Saadati H, Afzali M. Prevalence of diabetes in people aged ≥30 years: the results of screening program of Yazd province, Iran, in 2012. J Res Health Sci 2014;14:88–92. http://www.ncbi.nlm.nih.gov/pubmed/24402857. Accessed 13 Apr 2016. [PubMed]
- 30.Soaser da Costa CA, Carlos AS, de Sousa dos Santos A, Vieira Monteiro AM, de Moura EG, Nascimento-Saba CCA. Abdominal adiposity, insulin and bone quality in young male rats fed a high-fat diet containing soybean or canola oil. Clinics. 2011;66:1811–1816. doi: 10.1590/S1807-59322011001000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plat J, Jellema A, Ramakers J, Mensink RP. Weight loss, but not fish oil consumption, improves fasting and postprandial serum lipids, markers of endothelial function, and inflammatory signatures in moderately obese men. J Nutr. 2007;137:2635–2640. doi: 10.1093/jn/137.12.2635. [DOI] [PubMed] [Google Scholar]
- 32.Krummel D. Medical nutrition therapy for cardiovascular disease, dalam Krause’s food nutrition and diet therapy. 2008. https://scholar.google.com/scholar?q=Krummel+DA+%282008%29.+Medical+nutrition+therapy+for+cardiovascular+disease&btnG=&hl=en&as_sdt=0%2C5#0. Accessed 14 Apr 2016.
- 33.Corona GS. Extra virgin olive oil phenolics: absorption, metabolism, and biological activities in the GI tract. Toxicol Ind Health. 2009;25:285–293. doi: 10.1177/0748233709102951. [DOI] [PubMed] [Google Scholar]
- 34.Wardhana R, Surachmanto ES, Datau EA. The role of omega-3 fatty acids contained in olive oil on chronic inflammation. Acta Med Indones 2011;43:138–143. http://www.inaactamedica.org/archives/2011/21785178.pdf. Accessed 13 Apr 2016. [PubMed]
- 35.Lucas L, Russell A, Keast R. Molecular mechanisms of inflammation. Anti-inflammatory benefits of virgin olive oil and the phenolic compound oleocanthal. Curr Pharm Des. 2011;17:754–768. doi: 10.2174/138161211795428911. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Please contact author for data requests.