Abstract
Background
Polycystic ovary syndrome (PCOS) is a common endocrinopathy affecting women of reproductive age. Common features include menstrual irregularities, hyperandrogenism and polycystic ovarian morphology although the presentation can be heterogeneous. Insulin resistance is thought to be responsible for the hormonal and metabolic derangements observed. PCOS has two phenotypes, overweight/obese and lean, the latter being a much less common presentation of the syndrome.
Aims
The aim of the present review is to summarise cardinal features, and to devise diagnostic and treatment algorithms for lean PCOS based on recent literature.
Methods
We searched PubMed, EBSCOhost and Google Scholar using search terms such as ‘lean polycystic ovary syndrome’ OR ‘lean polycystic ovarian syndrome’ OR ‘lean PCOS’ OR ‘lean polycystic ovary disease’ OR ‘lean polycystic ovarian disease’ OR ‘lean PCOD’ OR ‘hyperandrogenism’ AND ‘low BMI OR ‘low body mass index’ to identify potential articles to be included in the review. Citation searches were subsequently performed in order to find relevant literature.
Results
Hormonal, metabolic and haematological profiles were altered in lean women with PCOS compared to healthy counterparts. However, the derangements were either comparable or less obvious compared to obese women with the syndrome. Insulin resistance seemed inherent in PCOS independent of obesity. Treatment options included weight maintenance, restoration of ovulation with insulin-sensitizers such as metformin, relief of symptoms such as hirsutism, acne and menstrual dysfunction, and assisted reproductive technologies in refractory cases, all of which showed promising results. The literature with evidence on lean PCOS is of low to moderate quality and there are still some uncertainties in the evidence base.
Conclusion
Carefully designed randomised controlled trials are required to confirm findings of previous studies in lean PCOS and to consolidate diagnostic and management algorithms proposed in this review. This paper will aid health professionals to improve their clinical approach in managing lean women with PCOS.
Keywords: Polycystic ovary syndrome, Lean polycystic ovary disease, Insulin resistance, Hyperandrogenism
Introduction
Polycystic ovary syndrome (PCOS) is a heterogeneous endocrinopathy affecting 4–8% of women of reproductive age [1–5]. It is the commonest cause of anovulatory infertility, and around 90–95% of women with anovulatory infertility presenting to infertility clinics are affected by the syndrome [6]. Characteristic features include polycystic ovaries, menstrual disturbance and hyperandrogenism. Up to 70% of women with hyperandrogenism present with hirsutism, or excess body hair. Acne is also a marker of hyperandrogenism, albeit rare and less specific [7, 8].
Although a majority of cases with PCOS are obese/overweight, a small but significant proportion of patients present with normal body mass index (BMI; ≤25 kg/M2) that makes diagnostic work up and therapeutic approach more difficult. These cases are termed as lean PCOS. Other endocrine and genetic disorders with similar clinical picture need to be excluded in such cases before the clinicians can make appropriate management plans. With the aid of up to date scientific literature, we discuss the evidence based diagnostic approach and therapeutic algorithm for patients with lean PCOS in this review.
Methods
We searched EBSCOhost, accessed via the Lancaster University Library website, PubMed and Google Scholar using the terms ‘lean polycystic ovary syndrome’ OR ‘lean polycystic ovarian syndrome’ OR ‘lean PCOS’ OR ‘lean polycystic ovary disease’ OR ‘lean polycystic ovarian disease’ OR ‘lean PCOD’ OR ‘hyperandrogenism’ AND ‘low BMI’ OR low body mass index’. Limits were applied to include only articles published in English between 2003 and 2017. Relevant articles were chosen from the search results and subsequent citation searches of papers were performed.
Pathophysiology of PCOS
PCOS remains an enigmatic condition, despite years of research [8]. The pathophysiology is complex and is thought to be a result of interactions between genetics, epigenetics, ovarian dysfunction, endocrine, neuroendocrine and metabolic alterations, amongst other changes [9].
Ovarian pathology is a major element of PCOS [9]. In a normal fertile female, a single follicle matures and undergoes ovulation from a pool of primordial follicles present in the ovaries since birth. The rate at which primordial follicles are selected for growth is strictly controlled, in order to maintain ovarian reserve and ensure fertility is intact [10]. In PCOS, an imbalance between androgens, anti-Müllerian hormone (AMH) and follicle stimulating hormone (FSH), cause a halt of follicular growth [11]. AMH is produced by ovarian granulosa cells and is important in preventing primordial follicles from transitioning into primary follicles. The characteristic polycystic appearance of ovaries in PCOS is due to large numbers of primordial follicles growing and undergoing subsequent growth arrest [9]. High luteinising hormone (LH) levels are required for androgen synthesis by ovarian theca cells. High LH combined with low FSH levels and decreased oestradiol synthesis through the conversion of androgens results in anovulation due to the absence of a dominant follicle [12].
Insulin resistance (IR) is another important component of PCOS [9]. IR results from inadequate tissue utilisation of insulin for glucose metabolism. Although the exact mechanisms are yet to be elucidated, genetic, intra- and extra uterine factors and adaptations to consumption of high energy food items are considered to be factors causing IR in PCOS [13]. Puberty is also thought to have a major effect on hyperinsulinaemia and IR. During puberty, there may be a temporary increase in insulin levels and IR. Subsequent elevations in insulin-like growth factor-1 (IGF-1) and growth hormone levels mean that more amino acids are available for growth [14]. During puberty, only glucose metabolism is affected by IR while protein metabolism is spared [15]. In PCOS, IR affects the liver, adipose tissue and skeletal muscles. However, the steroidogenic ovaries and adrenal glands remain sensitive to the actions of insulin [16]. Another effect of insulin is to decrease sex hormone binding globulin (SHBG) synthesis in the liver, resulting in elevated levels of free androgens [17]. Women with PCOS often have elevated serum insulin and IR, regardless of androgen concentrations and their levels of ‘adiposity’ [18].
Diagnostic criteria
Three different groups, the Rotterdam European Society for Human Reproduction and Embryology/American Society for Reproductive Medicine (ESHRE/ASRM) [19], the National Institutes of Health/National Institute of Child Health and Human Disease (NIH/NICHD) [6] and the Androgen Excess and PCOS Society [20], have proposed diagnostic criteria for PCOS (Table 1) [6, 19, 20]. There is unanimous agreement that PCOS is a diagnosis of exclusion. The syndrome should be diagnosed once conditions such as Cushing’s syndrome, thyroid disorders, idiopathic hirsutism and hyperprolactinaemia have been ruled out. Neither group regards polycystic ovaries without other clinical features as sufficient for diagnosing PCOS [6, 19, 20]. According to the Androgen Excess Society and NIH/NICHD, the clinical presentation of hyperandrogenism, including hyperandrogenaemia, is a prerequisite for PCOS diagnosis [20]. Hyperandrogenaemia is the presence of elevated free testosterone, reduced sex hormone-binding globulin (SHBG) and elevated free testosterone index, dehydroepiandrostene sulphate and usually manifests clinically by hirsutism [6, 20]. The Rotterdam criteria do not require hyperandrogenism or hyperandrogenaemia alone for diagnosis, but can confirm diagnosis when these are present alongside polycystic ovaries, even in the absence of ovulatory or menstrual dysfunction [21]. PCOS cannot be diagnosed by the NIH/NICHD criteria in the absence of menstrual or ovulatory dysfunction [6]. In order for the Rotterdam criteria to consider an ovary as being polycystic, at least 12 follicles each with a diameter between 2 and 9 mm with or without an increase in the volume of the ovary greater than 10 mm3 is required [19]. There have been calls to revise criteria to describe ‘polycystic ovarian morphology’ due to improvements in ultrasound technology, which have enabled smaller follicles to be identified since the Rotterdam criteria were first devised [21].
Table 1.
Summary of diagnostic criteria for Polycystic Ovary Syndrome according to three different groups
| Rotterdam | NIH/NICHD | Androgen Excess Society |
|---|---|---|
| PCOS is considered a diagnosis of exclusion. | PCOS is considered a diagnosis of exclusion. | PCOS is considered a diagnosis of exclusion. |
| Diagnosis requires two of: | Diagnosis requires all of: | Diagnosis requires all of: |
| Hyperandrogenism | Hyperandrogenism | Hyperandrogenism |
| Polycystic ovaries | Menstrual disturbance | Polycystic ovaries and/or ‘ovarian dysfunction’ |
| Anovulation/oligo-ovulation |
Adapted from ‘Epidemiology, diagnosis, and management of polycystic ovary syndrome’ (2014) [6]
NIH/NICHD National Institute of Health/National Institute of Child Health and Human Disease, PCOS polycystic ovary syndrome
The emerging role of Anti-Müllerian hormone in PCOS diagnosis
Anti-Müllerian hormone (AMH) is synthesised by ovarian granulosa cells from around week 25 of intrauterine life until the menopause [22]. Production begins during the menstrual cycle with the recruitment of primordial follicles for development into small antral follicles [23]. Once the dominant follicle is selected, production decreases [24, 25], and early follicular recruitment and premature depletion of oocytes or follicles is avoided [26].
Biochemically plasma AMH can be used to assess the number of growing follicles within the ovaries [27, 28]. Serum AMH is predominantly synthesised by small antral follicles ranging from 2 to 9 mm in diameter. These follicles are counted when antral follicular count (AFC) in the ovary is established via ultrasonography. The detection of serum AMH levels is more sensitive and specific than obtaining AFC, as pre-antral and small antral follicles with diameters of less than 2 mm are detected, unlike with the use of ultrasonography [29]. Serum AMH can be detected at any point in the menstrual cycle, and levels do not vary considerably between menstrual cycles or during a single cycle, as the hormone is not produced by the corpus luteum or dominant follicle [30, 31].
Serum AMH levels can be useful in the diagnosis of PCOS as levels are 2–4 times greater in women with the condition compared with controls [32, 33]. This holds true for all populations of individuals with PCOS and reflects the presence of pre- and small antral follicles [27, 28]. It is not yet possible to define a serum AMH threshold to diagnose PCOS but a value of 35 pmol/L or 4.9 ng/ml using the enzyme immunoassay (AMH-EIA) has been proposed. It has been suggested that other differential diagnoses must be excluded and oligo- or anovulation and hyperandrogenism is established first when diagnosing PCOS. If one of the aforementioned criteria are missing, then AMH or AFC values can be used to aid diagnosis. Until an AMH threshold value has been agreed upon universally, it is advised that each healthcare centre use its own assay-specific reference intervals for diagnosis [23].
Lean polycystic ovary syndrome
Approximately 80% of individuals with PCOS have BMI values above normal or high, and present with typical features such as hyperandrogenism, polycystic ovaries and IR. These individuals often remain undiagnosed until they encounter fertility problems as adults. A smaller but distinct proportion of women with PCOS have a normal or low BMI and may or may not have symptoms such as irregular menstrual cycles and acne [17, 34].
Clinical signs are similar between lean and overweight phenotypes of PCOS
There is some consensus suggesting that clinical manifestations in lean and overweight women with PCOS are comparable [35–37]. Similar prevalence of acanthosis nigricans (darkening of skin creases), menstrual dysfunction, hirsutism and endometrial hyperplasia (thickness of endometrium greater than 4 mm) was demonstrated in both lean and overweight women with the syndrome who presented with infertility. Hormonal profiles were also similar in both phenotypes of PCOS, which included LH:FSH ratio, serum LH and progesterone as well as testosterone levels. Both groups were insulin resistant (prevalence of 83.3% and 93.1% in lean and overweight groups, respectively in one previous report) and it was concluded that IR is inherent in women with PCOS and hyperinsulinaemia ensues as a result [35]. It has proven difficult to quantify insulin resistance in PCOS due to limitations of methods used. Prevalence rates of insulin resistance ranging from 44 to 70% have been reported [36]. Doh et al. hypothesised that PCOS and obesity act synergistically and independently resulting in IR, in a study of women in Cameroon with PCOS [37]. Both phenotypes of women with PCOS were insulin resistant compared to controls, indicating that other factors are involved in IR seen in PCOS, even though obesity could have some contribution to IR. However, IR was not significantly different amongst patients with positive or negative family histories of T2DM in a similar trial [38]. There were also no differences in clinical manifestations of PCOS between the lean and overweight subgroups, such as hirsutism, hyperandrogenism and findings from pelvic ultrasonography. Recent studies therefore indicate that hormonal profiles and insulin resistance with resultant hyperinsulinaemia are similar in both phenotypes of PCOS.
Obese PCOS patients suffer from more severe derangements than lean counterparts
Obese individuals with PCOS suffer from more severe hormonal and metabolic derangements compared to their lean counterparts [39, 40]. Indian women with PCOS did not have significantly different levels of hypertension amongst lean and overweight subtypes. Hyperandrogenism (74.2% overweight and 50.6% lean, P = 0.000, 95% confidence interval CI: 0.14–0.32) and menstrual dysfunction (79.2% overweight and 44% lean, P = 0.000 95% CI: 0.26–0.44) were significantly higher in the overweight group of women. The prevalence of T2DM was higher amongst the overweight group as was impaired glucose tolerance. The relative risk of endometrial hyperplasia was higher (RR = 2.8) in overweight subjects although this was not statistically significant (p = 0.055). The greater prevalence of T2DM, impaired glucose tolerance and endometrial hyperplasia in the obese cohort mean that they are at a greater risk of morbidity at a younger age than lean PCOS patients and need to be treated using a ‘more rigorous’ approach [39].
Metabolic changes in lean women with PCOS relative to their overweight counterparts, as well as the association of such alterations to levels of peptide hormones adiponectin and ghrelin have been investigated [40]. Adiponectin is secreted by adipocytes and is involved in lipid and glucose homeostasis [41], insulin sensitisation and possesses antidiabetic properties [42, 43]. Ghrelin is produced predominantly by neuroendocrine cells in the gastrointestinal tract. This orexigenic hormone is thought to be involved in the regulation of energy homeostasis, glucose metabolism and ‘insulin signalling’ [41, 44–46]. It augments fat tissue and the release of growth hormone, which results in increased food consumption and body mass [47]. High levels of adiponectin and ghrelin appear to be essential for glucose metabolism. In obese women without PCOS, adiponectin levels were significantly reduced compared to levels in lean women without the syndrome. Plasma ghrelin levels were also significantly lower in obese women compared with lean women, regardless of PCOS status. Adiponectin levels were comparable in lean women whether or not they had PCOS [40]. Findings suggest that obesity is associated with lower levels of adiponectin and ghrelin, but fail to demonstrate that PCOS has a deleterious effect on levels of the two peptides. Lean women with PCOS had significantly greater insulin resistance compared with their BMI-matched non-PCOS counterparts. However, the extent of IR was greater still in obese women with the syndrome [40]. Recent studies suggest that although hormonal and metabolic derangements exist in lean women with PCOS, these alterations are more severe in obese individuals with the endocrinopathy.
Lean patients with PCOS and metabolic and haematological derangements
Lean women with PCOS suffer from various pathophysiological abnormalities compared to BMI-matched healthy controls [48, 49]. Investigations of metabolic abnormalities in patients with PCOS revealed significantly higher plasma insulin levels two hours after glucose was given to women in the lean subgroup compared with lean controls. Low density lipoprotein (LDL) and total cholesterol were also significantly raised in women with lean PCOS versus controls. Plasma gamma glutamyl transferase (GGT) levels, which are good predictors of cardiovascular disease, were significantly higher in both lean and overweight PCOS phenotypes relative to controls, as were haemoglobin levels. High GGT levels are correlated with an increased incidence of cardiovascular disease and death, the exact mechanisms of which remain unclear. GGT is thought to be involved in atherogenesis due to its role in oxidative stress, localisation to LDL-containing plaques and its pro-inflammatory nature [50]. Testosterone is thought to stimulate erythropoiesis in a dose-dependent manner [51, 52], which may explain why PCOS groups had higher haemoglobin levels [48]. Pancreatic beta cell function, involved in insulin synthesis and secretion, was greater in all PCOS women compared with controls [9]. However, differences diminished when only lean PCOS women were compared to lean controls. Insulin resistance was increased in women with PCOS compared with controls and this included lean PCOS women and their control counterparts. Researchers proposed that IR needs to be ascertained at an early stage in PCOS, even in women with low BMI and normal glucose tolerance [49].
The haemorheological profile, or study of blood constituents and flow, of lean young women with PCOS was found to be deranged in this cohort of women. Blood viscosity was significantly increased in the lean PCOS group at lower shear rates compared to controls [53]. This is considered to increase the risk of endothelial dysfunction [54], coronary artery calcification [55], and venous thromboembolism [56] among other health risks. It was revealed that erythrocyte aggregation was also significantly elevated in the PCOS group, which may lead to increased resistance to blood flow. This can result in tissue ischaemia in those with dysregulated blood vessel regulatory mechanisms such as in cardiovascular disease and T2DM. The antioxidant defence system was found to be weakened in lean, young PCOS women relative to weight-matched controls [57] and erythrocyte deformability was unequivocal in PCOS and control groups [53]. Numerous aspects of normal physiology appear to be dysregulated in lean individuals with PCOS relative to BMI-matched controls, such as lipid, hormonal and haemorheological profiles.
Differential diagnoses and the investigations to exclude them
Multiple clinical conditions can mimic lean PCOS clinically and need exclusion by appropriate laboratory work up before planning management. These conditions include congenital adrenal hyperplasia (CAH), androgen secreting adrenal or ovarian tumours, Cushing’s syndrome, acromegaly, hyperprolactinemia, thyroid disorders, and medications that can cause hirsutism (e.g., testosterone preparations, anabolic steroids, and corticosteroids). A meticulous history coupled with a thorough clinical examination followed by biochemical investigations narrated below help to exclude these conditions.
Nonclassical/ late-onset CAH presents with hirsutism, and amenorrhoea, and may mimic PCOS. Fasting serum cortisol, 17-hydroxyprogesterone and adrenocorticotrophin (ACTH) followed by stimulation with 250 μg of ACTH and measurement of cortisol and 17-hydroxyprogesterone at 30 and 60 min later form initial screening tests for CAH [58]. Increased levels of basal and post-ACTH 17-hydroxyprogesterone levels (>43 nmol/L) would suggest CAH. Diagnostic confirmation of CAH is with genetic testing for mutations of the genes coding for the enzymes involved in adrenal steroidogenesis [58].
A baseline testosterone level > 5.0 nmol/L usually indicate an androgen secreting adrenal or ovarian tumour that needs further hormonal and imaging work up [59]. Cushing’s syndrome is excluded by screening for glucocorticoid excess with at least two of the four following tests: 24-h urine free cortisol, overnight dexamethasone suppression test, late night salivary cortisol assay or low dose dexamethasone suppression test [60]. Screening for acromegaly involves testing for elevated serum levels of insulin-like growth factor 1 (IGF-1) and the oral glucose tolerance test [61]. Raised serum prolactin level indicates hyperprolactinemia and warrants further evaluation for the causes. Alterations in the serum thyrotrophin (TSH) usually indicate thyroid dysfunction. A meticulous history on drug intake including topical creams will help to exclude a drug-induced aetiology that simulates PCOS.
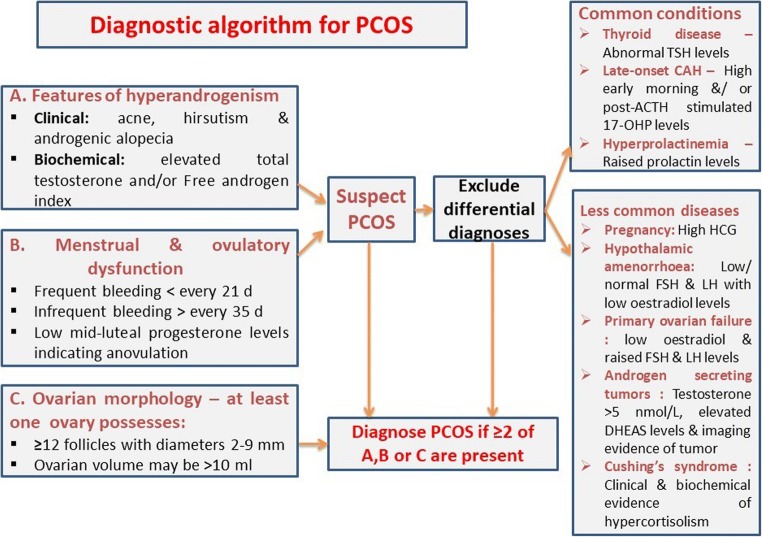
A diagnostic algorithm for work up of suspected cases of PCOS is shown in Fig. 1.
Fig. 1.
A diagnostic algorithm for polycystic ovary syndrome (PCOS). TSH – thyrotrophin, ACTH – adrenocorticotrophin, 17-OHP – 17-hydroxyprogesterone, HCG – human chorionic gonadotrophin, FSH – follicle stimulating hormone, LH – luteinizing hormone, DHEAS – dehydroepiandrosterone sulphate
Management options for lean PCOS
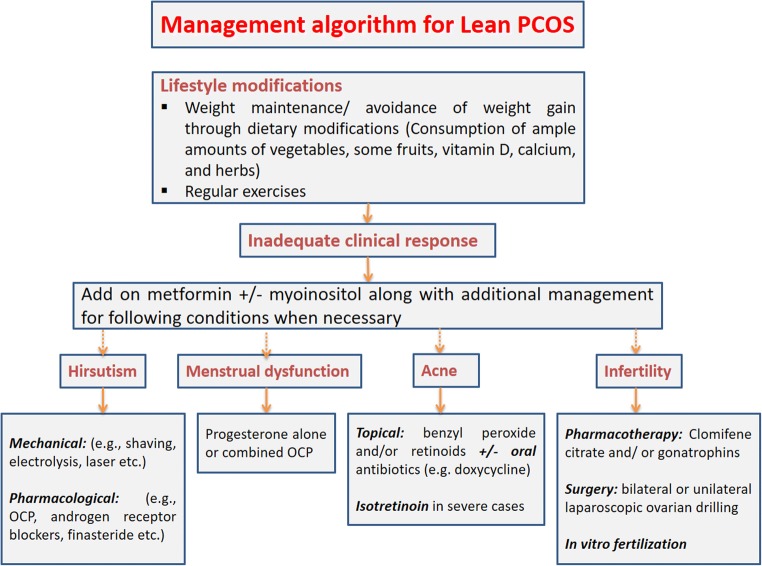
Various management options are available for lean PCOS as summarised in Fig. 2. Pharmacological measures could ameliorate IR, decrease elevated androgen levels in blood and downregulate androgen synthesis by the ovaries [17].
Fig. 2.
A management algorithm for patients with lean polycystic ovary syndrome (PCOS). OCP – oral contraceptive pills
Diet & lifestyle modifications
Weight loss is considered first-line treatment in women exhibiting the obese phenotype of PCOS, but this is not considered in lean women with the syndrome [62]. Caloric restrictions are unnecessary as lean women may not necessarily be required to lose weight. Instead lean PCOS women should aim to maintain their weight. Lifestyle modifications by dietary interventions and regular physical activity have demonstrated improved insulin resistance and ameliorated hyperandrogenism amongst other beneficial effects on PCOS symptoms [62–64] Lean individuals with PCOS must be encouraged to consume vegetables and fruit to ensure they are having an adequate supply of various minerals, vitamins and nutrients.
Restoring ovulation
Anovulation is a more probable occurrence in obese rather than thin individuals with PCOS. Some authors believe that overweight women have a higher chance of being less responsive to pharmacological measures to induce ovulation [62]. However, there is inadequate data to justify this.
Metformin therapy
Metformin is an insulin sensitising agent which reduces serum glucose levels by improving glucose uptake and use in the periphery and reduction of hepatic glucose output [13]. Metformin use was more successful in restoring menstruation (55%) and ovulation (45%) in lean women with PCOS compared with their obese counterparts. Only lean phenotypes exhibited significant reductions in fasting glucose, testosterone and insulin resistance [65]. Metformin treatment was associated with improvement of disordered menstrual cycles even in underweight patients with PCOS [66]. It is important to remember that there is a low risk of hypoglycaemia in thin anovulatory women undergoing metformin therapy [67].
3 g/day of myoinositol
appeared to have a positive effect on lean women with PCOS. Treatment led to reductions in luteinising hormone, androgen levels, ‘high-sensitivity C-reactive protein’ with a role in inflammation and facilitated insulin tolerance [68]. The drug is thought to improve insulin resistance by acting as a messenger for insulin signalling, subsequently increasing the uptake of glucose in the periphery. The hormonal profile and ovulation were restored in PCOS women [62, 68]. It may be unsuitable in lean women with PCOS, particularly if insulin resistance is not present. It has also been shown to have deleterious effects on the quality of oocytes at high doses [62].
Assisted reproductive technologies
Lean women with PCOS exhibited a significantly greater rate of fertilisation compared with overweight counterparts (BMI ≥25 kg/M2) [69]. BMI appears to have a marked effect on in vitro fertilisation (IVF) outcomes. There was a significantly elevated fertilisation rate with ovarian stimulation, with both gonadotrophin releasing hormone (GnRH)-agonists and antagonists among lean women with PCOS compared to overweight women with the condition (p < 0.02 and p < 0.01 respectively). Higher pregnancy rates were observed in women undergoing ovarian stimulation using mid-luteal long GnRH-agonist suppressive regimens. This may be because high LH levels can be reduced which consequently reduces damaging effects to the quality and ‘implantation potential’ of an individual oocyte [70]. Surgical techniques such as unilateral or bilateral laparoscopic ovarian drilling was found to be associated with comparable rates in successful pregnancies in a recent meta-analysis [71].
Management of hirsutism
Hirsutism can be quite worrisome in some patients with lean PCOS and may need cosmetic treatment (hair removal by electrolysis or laser therapy) along with pharmacological interventions to slow down the hair growth. Suppression of ovarian androgen production using oral contraceptive preparations is useful [71]. Androgen receptor blockers (cyproterone acetate, flutamide, spironolactone, and 5 α-reductase inhibitors [finasteride]) are useful to reduce the hair growth [72].
Management of acne
Acne can be severe in some cases of PCOS that may prove to be difficult to manage. Mild and moderate cases of acne may respond to topical preparations such as benzylperoxide and/ or clindamycin or retinoids creams [73]. However, severe cases may need additional oral treatment with tetracycline group of antibiotics and in some cases oral isotretinoin. Because of the potential toxicity or systemic retinoid preparations, close supervision of treatment under a dermatologist is recommended in such cases [73].
Limitations of currently available evidence and areas of uncertainty
Results from recent literature should be interpreted with caution as various limitations were apparent with regard to study designs. Among methodological limitations, sample sizes were often too small to report significant results [13, 37, 38, 48, 49, 53]. Rotterdam criteria were most often used to diagnose PCOS [13, 37–40, 49, 53, 70] but this was not always the case [48]. Findings from studies on women of certain ethnicities could not be generalised for the entire population of women with PCOS, further complicating findings [37, 39, 40, 49]. Furthermore, PCOS characteristics overlap with those that arise during puberty such as acne, irregular menstruation and polycystic ovarian morphology [74] rendering PCOS diagnosis more difficult in adolescents [48]. In the future, large-scale randomised controlled trials should be conducted to ascertain the significance of these differences. Specific age and ethnic groups with the syndrome should be investigated and the same diagnostic criteria used to ensure robust conclusions are generated.
Conclusions
From recent research it is apparent that metabolic, hormonal and haematological derangements exist in lean PCOS, although these are equal or less obvious compared to those affecting the obese phenotype. Insulin resistance is inherent in PCOS regardless of BMI and should be managed early on. Results are promising in terms of managing the lean phenotype. Lifestyle modifications, restoration of ovulation with the use of pharmacological interventions and even IVF in refractory cases, can provide symptomatic relief and increase chances of successful pregnancy. Well-designed RCTs with women from specific ethnic and age demographics are required to devise treatment algorithms for the lean subgroup of women with this enigmatic syndrome.
Compliance with ethical standards
Conflicts of interest
There no competing interests among authors of this work.
References
- 1.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–2749. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 2.Diamanti-Kandarakis E, Kouli CR, Bergiele AT, Filandra FA, Tsianateli TC, Spina GG, Zapanti ED, Bartzis MI. A survey of the polycystic ovary syndrome in the Greek Island of Lesbos: hormonal and metabolic profile. J Clin Endocrinol Metab. 1999;84:4006–4011. doi: 10.1210/jcem.84.11.6148. [DOI] [PubMed] [Google Scholar]
- 3.Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab. 1998;83:3078–3082. doi: 10.1210/jcem.83.9.5090. [DOI] [PubMed] [Google Scholar]
- 4.Asunción M, Calvo RM, San Millán JL, Sancho J, Avila S, Escobar-Morreale HF. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metab. 2000;85:2434–2438. doi: 10.1210/jcem.85.7.6682. [DOI] [PubMed] [Google Scholar]
- 5.Michelmore K, Balen A, Dunger D, Vessey M. Polycystic ovaries and associated clinical and biochemical features in young women. Clin Endocrinol. 1999;51:779–786. doi: 10.1046/j.1365-2265.1999.00886.x. [DOI] [PubMed] [Google Scholar]
- 6.Sirmans SM, Pate KA. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol. 2014;6:1–13. doi: 10.2147/CLEP.S37559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fauser B, Tarlatzis B, Rebar R, Legro R, Balen A, Lobo R, et al. Consensus on women's health aspects of polycystic ovary syndrome (PCOS) Hum Reprod. 2012;27:14–24. doi: 10.1093/humrep/der396. [DOI] [PubMed] [Google Scholar]
- 8.Azziz R, Sanchez LA, Knochenhauer ES, Moran C, Lazenby J, Stephens KC, Taylor K, Boots LR. Androgen excess in women: experience with over 1000 consecutive patients. J Clin Endocrinol Metab. 2004;89:453–462. doi: 10.1210/jc.2003-031122. [DOI] [PubMed] [Google Scholar]
- 9.Ibáñez L, Oberfield SE, Witchel S, Auchus RJ, Chang RJ, Codner E, Dabadghao P, Darendeliler F, Elbarbary NS, Gambineri A, Garcia Rudaz C, Hoeger KM, López-Bermejo A, Ong K, Peña AS, Reinehr T, Santoro N, Tena-Sempere M, Tao R, Yildiz BO, Alkhayyat H, Deeb A, Joel D, Horikawa R, de Zegher F, Lee PA. An international consortium update: pathophysiology, diagnosis, and treatment of polycystic ovarian syndrome in adolescence. Horm Res Paediatr. 2017;88:371–395. doi: 10.1159/000479371. [DOI] [PubMed] [Google Scholar]
- 10.Hsueh AJW, Kawamura K, Cheng Y, Fauser BCJM. Intraovarian control of early Folliculogenesis. Endocr Rev. 2015;36:1–24. doi: 10.1210/er.2014-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franks S, Stark J, Hardy K. Follicle dynamics and anovulation in polycystic ovary syndrome. Hum Reprod Update. 2008;14:367–378. doi: 10.1093/humupd/dmn015. [DOI] [PubMed] [Google Scholar]
- 12.Lebbe M, Woodruff TK. Involvement of androgens in ovarian health and disease. Mol Hum Reprod. 2013;19:828–837. doi: 10.1093/molehr/gat065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumari AS, Haq A, Jayasundaram R, Abdel-Wareth LO, Al Haija SA, Alvares M. Metformin monotherapy in lean women with polycystic ovary syndrome. Reprod BioMed Online. 2005;10:100–104. doi: 10.1016/S1472-6483(10)60809-7. [DOI] [PubMed] [Google Scholar]
- 14.Amiel SA, Sherwin RS, Simonson DC, Lauritano AA, Tamborlane WV. Impaired insulin action in puberty. A contributing factor to poor glycemic control in adolescents with diabetes. N Engl J Med. 1986;315:215–219. doi: 10.1056/NEJM198607243150402. [DOI] [PubMed] [Google Scholar]
- 15.Saenger P. Metabolic consequences of growth hormone treatment in paediatric practice. Horm Res. 2000;53(Suppl. 1):60–69. doi: 10.1159/000053207. [DOI] [PubMed] [Google Scholar]
- 16.Geffner ME, Golde DW. Selective insulin action on skin, ovary, and heart in insulin-resistant states. Diabetes Care. 1988;11:500–505. doi: 10.2337/diacare.11.6.500. [DOI] [PubMed] [Google Scholar]
- 17.Nestler JE, Jakubowicz DJ. Lean women with polycystic ovary syndrome respond to insulin reduction with decreases in ovarian P450c17α activity and serum androgens. J Clin Endocrinol Metab. 1997;82:4075–4079. doi: 10.1210/jcem.82.12.4431. [DOI] [PubMed] [Google Scholar]
- 18.Dunaif A, Segal KR, Shelley DR, Green G, Dobrjansky A, Licholai T. Evidence for distinctive and intrinsic defects in insulin action in polycystic ovary syndrome. Diabetes. 1992;41:1257–1266. doi: 10.2337/diab.41.10.1257. [DOI] [PubMed] [Google Scholar]
- 19.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE, Witchel SF, Androgen Excess Society Criteria for defining polycystic ovary syndrome as a predominantly Hyperandrogenic syndrome: an androgen excess society guideline. J Clin Endocrinol Metab. 2006;91:4237–4245. doi: 10.1210/jc.2006-0178. [DOI] [PubMed] [Google Scholar]
- 21.Lujan ME, Jarrett BY, Brooks ED, Reines JK, Peppin AK, Muhn N, Haider E, Pierson RA, Chizen DR. Updated ultrasound criteria for polycystic ovary syndrome: reliable thresholds for elevated follicle population and ovarian volume. Hum Reprod. 2013;28:1361–1368. doi: 10.1093/humrep/det062. [DOI] [PubMed] [Google Scholar]
- 22.Rajpert-De Meyts E, Jørgensen N, Græm N, Müller J, Cate RL, Skakkebæk NE. Expression of anti-Müllerian hormone during Normal and pathological gonadal development: association with differentiation of Sertoli and granulosa cells. J Clin Endocrinol Metab. 1999;84:3836–3844. doi: 10.1210/jcem.84.10.6047. [DOI] [PubMed] [Google Scholar]
- 23.Dumont A, Robin G, Catteau-Jonard S, Dewailly D. Role of anti-Müllerian hormone in pathophysiology, diagnosis and treatment of polycystic ovary syndrome: a review. Reprod Biol Endocrinol. 2015;13:137. doi: 10.1186/s12958-015-0134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salmon NA, Handyside AH, Joyce IM. Oocyte regulation of anti-Müllerian hormone expression in granulosa cells during ovarian follicle development in mice. Dev Biol. 2004;266:201–208. doi: 10.1016/j.ydbio.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Durlinger A, Visser JA, Themmen A. Regulation of ovarian function: the role of anti-Mullerian hormone. Reproduction. 2002;124(5):601–609. doi: 10.1530/rep.0.1240601. [DOI] [PubMed] [Google Scholar]
- 26.Durlinger AL, Kramer P, Karels B, de Jong FH, Uilenbroek JTJ, Grootegoed JA, et al. Control of primordial follicle recruitment by anti-Mullerian hormone in the mouse ovary. Endocrinology. 1999;140(12):5789–5796. doi: 10.1210/endo.140.12.7204. [DOI] [PubMed] [Google Scholar]
- 27.Laven JS, Mulders AG, Visser JA, Themmen AP, De Jong FH, Fauser BC. Anti-Mullerian hormone serum concentrations in normoovulatory and anovulatory women of reproductive age. J Clin Endocrinol Metab. 2004;89:318–323. doi: 10.1210/jc.2003-030932. [DOI] [PubMed] [Google Scholar]
- 28.Pigny P, Merlen E, Robert Y, Cortet-Rudelli C, Decanter C, Jonard S, Dewailly D. Elevated serum level of anti-mullerian hormone in patients with polycystic ovary syndrome: relationship to the ovarian follicle excess and to the follicular arrest. J Clin Endocrinol Metab. 2003;88:5957–5962. doi: 10.1210/jc.2003-030727. [DOI] [PubMed] [Google Scholar]
- 29.Dewailly D, Andersen CY, Balen A, Broekmans F, Dilaver N, Fanchin R, Griesinger G, Kelsey TW, la Marca A, Lambalk C, Mason H, Nelson SM, Visser JA, Wallace WH, Anderson RA. The physiology and clinical utility of anti-Müllerian hormone in women. Hum Reprod Update. 2014;20:370–385. doi: 10.1093/humupd/dmt062. [DOI] [PubMed] [Google Scholar]
- 30.Fanchin R, Taieb J, Lozano DHM, Ducot B, Frydman R, Bouyer J. High reproducibility of serum anti-Müllerian hormone measurements suggests a multi-staged follicular secretion and strengthens its role in the assessment of ovarian follicular status. Hum Reprod. 2005;20:923–927. doi: 10.1093/humrep/deh688. [DOI] [PubMed] [Google Scholar]
- 31.van Disseldorp J, Lambalk C, Kwee J, Looman C, Eijkemans M, Fauser B, et al. Comparison of inter-and intra-cycle variability of anti-Müllerian hormone and antral follicle counts. Hum Reprod. 2009;25:221–227. doi: 10.1093/humrep/dep366. [DOI] [PubMed] [Google Scholar]
- 32.Pellatt L, Hanna L, Brincat M, Galea R, Brain H, Whitehead S, Mason H. Granulosa cell production of anti-Mullerian hormone is increased in polycystic ovaries. J Clin Endocrinol Metab. 2007;92:240–245. doi: 10.1210/jc.2006-1582. [DOI] [PubMed] [Google Scholar]
- 33.Villarroel C, Merino P, Lopez P, Eyzaguirre F, Van Velzen A, Iniguez G, et al. Polycystic ovarian morphology in adolescents with regular menstrual cycles is associated with elevated anti-Müllerian hormone. Hum Reprod. 2011;26:2861–2868. doi: 10.1093/humrep/der223. [DOI] [PubMed] [Google Scholar]
- 34.Williams RM, Ong KK, Dunger DB. Polycystic ovarian syndrome during puberty and adolescence. Mol Cell Endocrinol. 2013;373:61–67. doi: 10.1016/j.mce.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 35.Saxena P, Prakash A, Nigam A, Mishra A. Polycystic ovary syndrome: is obesity a sine qua non? A clinical, hormonal, and metabolic assessment in relation to body mass index. Indian J Endocrinol Metab. 2012;16:996–999. doi: 10.4103/2230-8210.103011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012;33:981–1030. doi: 10.1210/er.2011-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doh E, Mbanya A, Kemfang-Ngowa JD, Dohbit S, Tchana-Sinou M, Foumane P, Donfack OT, Doh AS, Mbanya JC, Sobngwi E. The relationship between adiposity and insulin sensitivity in African women living with the polycystic ovarian syndrome: a clamp study. Int J Endocrinol. 2016;2016:9201701. doi: 10.1155/2016/9201701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bozkirli E, Bakiner O, Ertörer E, Anaforoglu I, Tütüncü NB, Demirag NG. Insulin resistance in non-obese polycystic ovary syndrome subjects and relation with family history of diabetes mellitus. Turk J Endocrinol Metab. 2015;19(2):55–59. doi: 10.4274/tjem.2761. [DOI] [Google Scholar]
- 39.Majumdar A, Singh TA. Comparison of clinical features and health manifestations in lean vs. obese Indian women with polycystic ovarian syndrome. J Hum Reprod Sci. 2009;2:12–17. doi: 10.4103/0974-1208.51336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bik W, Baranowska-Bik A, Wolinska-Witort E, Chmielowska M, Martynska L, Baranowska B. The relationship between metabolic status and levels of adiponectin and ghrelin in lean women with polycystic ovary syndrome. Gynecol Endocrinol. 2007;23:325–331. doi: 10.1080/09513590701260169. [DOI] [PubMed] [Google Scholar]
- 41.Meier U, Gressner A. Endocrine regulation of energy metabolism: review of pathobiochemical and clinical chemical aspects of leptin, ghrelin, adiponectin, and resistin. Clin Chem. 2004;50:1511–1525. doi: 10.1373/clinchem.2004.032482. [DOI] [PubMed] [Google Scholar]
- 42.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 43.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 44.Broglio F, Arvat E, Benso A, Gottero C, Muccioli G, Papotti M, Lely AJ, Deghenghi R, Ghigo E. Ghrelin, a natural GH secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. J Clin Endocrinol Metab. 2001;86:5083–5086. doi: 10.1210/jcem.86.10.8098. [DOI] [PubMed] [Google Scholar]
- 45.Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409(6817):194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 46.Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, Kennedy AR, Roberts GH, Morgan DGA, Ghatei MA, Bloom SR. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000;141:4325–4328. doi: 10.1210/endo.141.11.7873. [DOI] [PubMed] [Google Scholar]
- 47.Hosoda H, Kojima M, Kangawa K. Biological, physiological, and pharmacological aspects of ghrelin. J Pharmacol Sci. 2006;100:398–410. doi: 10.1254/jphs.CRJ06002X. [DOI] [PubMed] [Google Scholar]
- 48.Han Y, Kim HS, Lee H-J, Oh J-Y, Sung Y-A. Metabolic effects of polycystic ovary syndrome in adolescents. Ann Pediatr Endocrinol Metab. 2015;20:136–142. doi: 10.6065/apem.2015.20.3.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song DK, Hong YS, Sung Y-A, Lee H. Insulin resistance according to β-cell function in women with polycystic ovary syndrome and normal glucose tolerance. PLoS One. 2017;12:e0178120. doi: 10.1371/journal.pone.0178120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ndrepepa G, Kastrati A. Gamma-glutamyl transferase and cardiovascular disease. Ann Transl Med. 2016;4(24):481. doi: 10.21037/atm.2016.12.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berria R, Gastaldelli A, Lucidi S, Belfort R, De Filippis E, Easton C, et al. Reduction in hematocrit level after pioglitazone treatment is correlated with decreased plasma free testosterone level, not hemodilution, in women with polycystic ovary syndrome. Clin Pharmacol Ther. 2006;80:105–114. doi: 10.1016/j.clpt.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 52.Coviello AD, Kaplan B, Lakshman KM, Chen T, Singh AB, Bhasin S. Effects of graded doses of testosterone on erythropoiesis in healthy young and older men. J Clin Endocrinol Metab. 2008;93:914–919. doi: 10.1210/jc.2007-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simmonds MJ, Milne N, Ong K, Brotherton E, McNamee AP, Horobin J, et al. Physical properties of blood are altered in young and lean women with polycystic ovary syndrome. PLoS One. 2016;11:e0167290. doi: 10.1371/journal.pone.0167290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sprung VS, Atkinson G, Cuthbertson DJ, Pugh CJA, Aziz N, Green DJ, Cable NT, Jones H. Endothelial function measured using flow-mediated dilation in polycystic ovary syndrome: a meta-analysis of the observational studies. Clin Endocrinol. 2013;78:438–446. doi: 10.1111/j.1365-2265.2012.04490.x. [DOI] [PubMed] [Google Scholar]
- 55.Christian RC, Dumesic DA, Behrenbeck T, Oberg AL, Sheedy PF, Fitzpatrick LA. Prevalence and predictors of coronary artery calcification in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:2562–2568. doi: 10.1210/jc.2003-030334. [DOI] [PubMed] [Google Scholar]
- 56.Okoroh EM, Hooper WC, Atrash HK, Yusuf HR, Boulet SL. Is polycystic ovary syndrome another risk factor for venous thromboembolism? United States, 2003–2008. Am J Obstet Gynecol. 2012;207:377-e1-8. doi: 10.1016/j.ajog.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blair SA, Kyaw-Tun T, Young IS, Phelan NA, Gibney J, McEneny J. Oxidative stress and inflammation in lean and obese subjects with polycystic ovary syndrome. J Reprod Med. 2013;58(3–4):107–114. [PubMed] [Google Scholar]
- 58.Bourdeau I, El Ghorayeb N, Gagnon N, Lacroix A. MANAGEMENT OF ENDOCRINE DISEASE: Differential diagnosis, investigation and therapy of Bilateral Adrenal Incidentalomas. Eur J Endocrinol. 2018;179(2):R57–R67. [DOI] [PubMed]
- 59.Pugeat M, Plotton I, de la Perrière AB, Raverot G, Déchaud H, Raverot V. MANAGEMENT OF ENDOCRINE DISEASE Hyperandrogenic states in women: pitfalls in laboratory diagnosis. Eur J Endocrinol. 2018;178:R141–R154. doi: 10.1530/EJE-17-0776. [DOI] [PubMed] [Google Scholar]
- 60.Pappachan JM, Hariman C, Edavalath M, Waldron J, Hanna FW. Cushing's syndrome: a practical approach to diagnosis and differential diagnoses. J Clin Pathol. 2017;70:350–359. doi: 10.1136/jclinpath-2016-203933. [DOI] [PubMed] [Google Scholar]
- 61.Katznelson L, Laws ER, Jr, Melmed S, Molitch ME, Murad MH, Utz A, Wass JA, Endocrine Society Acromegaly: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99:3933–3951. doi: 10.1210/jc.2014-2700. [DOI] [PubMed] [Google Scholar]
- 62.Goyal M, Dawood AS. Debates regarding lean patients with polycystic ovary syndrome: a narrative review. J Hum Reprod Sci. 2017;10:154–161. doi: 10.4103/jhrs.JHRS_77_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arentz S, Abbott JA, Smith CA, Bensoussan A. Herbal medicine for the management of polycystic ovary syndrome (PCOS) and associated oligo/amenorrhoea and hyperandrogenism; a review of the laboratory evidence for effects with corroborative clinical findings. BMC Complement Altern Med. 2014;14:511. doi: 10.1186/1472-6882-14-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wright CE, Zborowski JV, Talbott EO, McHugh-Pemu K, Youk A. Dietary intake, physical activity, and obesity in women with polycystic ovary syndrome. Int J Obes Relat Metab Disord. 2004;28:1026–1032. doi: 10.1038/sj.ijo.0802661. [DOI] [PubMed] [Google Scholar]
- 65.Popova P, Ivanova L, Karonova T, Grineva E. Ovulation induction by metformin in lean and obese women with polycystic ovary syndrome. Endocr Abstr 2011;26(P90).
- 66.Anastasiou OE, Canbay A, Fuhrer D, Reger-Tan S. Metabolic and androgen profile in underweight women with polycystic ovary syndrome. Arch Gynecol Obstet. 2017;296:363–371. doi: 10.1007/s00404-017-4422-9. [DOI] [PubMed] [Google Scholar]
- 67.Atiomo W, Sinha A. The role of metformin in the treatment of infertile women with polycystic ovary syndrome. Obstet Gynaecol. 2004;6:145–51. 10.1576/toag.6.3.145.26996.
- 68.Genazzani AD, Santagni S, Ricchieri F, Campedelli A, Rattighieri E, Chierchia E, Marini G, Despini G, Prati A, Simoncini T. Myo-inositol modulates insulin and luteinizing hormone secretion in normal weight patients with polycystic ovary syndrome. J Obstet Gynaecol Res. 2014;40:1353–1360. doi: 10.1111/jog.12319. [DOI] [PubMed] [Google Scholar]
- 69.Kar S. Anthropometric, clinical, and metabolic comparisons of the four Rotterdam PCOS phenotypes: a prospective study of PCOS women. J Hum Reprod Sci. 2013;6:194–200. doi: 10.4103/0974-1208.121422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Orvieto R, Nahum R, Meltcer S, Homburg R, Rabinson J, Anteby EY, Ashkenazi J. Ovarian stimulation in polycystic ovary syndrome patients: the role of body mass index. Reprod BioMed Online. 2009;18:333–336. doi: 10.1016/S1472-6483(10)60090-9. [DOI] [PubMed] [Google Scholar]
- 71.Abu Hashim H, Foda O, El Rakhawy M. Unilateral or bilateral laparoscopic ovarian drilling in polycystic ovary syndrome: a meta-analysis of randomized trials. Arch Gynecol Obstet. 2018;297:859–870. doi: 10.1007/s00404-018-4680-1. [DOI] [PubMed] [Google Scholar]
- 72.Mihailidis J, Dermesropian R, Taxel P, Luthra P, Grant-Kels JM. Endocrine evaluation of hirsutism. Int J Womens Dermatol. 2017;3(1 Suppl):S6–S10. doi: 10.1016/j.ijwd.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Asai Y, Baibergenova A, Dutil M, Humphrey S, Hull P, Lynde C, Poulin Y, Shear NH, Tan J, Toole J, Zip C. Management of acne: Canadian clinical practice guideline. CMAJ. 2016;188:118–126. doi: 10.1503/cmaj.140665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, Welt CK, Endocrine Society Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565–4592. doi: 10.1210/jc.2013-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]