Abstract
Aldo-keto reductases (AKRs) are monomeric NAD(P)(H)-dependent oxidoreductases that play pivotal roles in the biosynthesis and metabolism of steroids in humans. AKR1C enzymes acting as 3-ketosteroid, 17-ketosteroid, and 20-ketosteroid reductases are involved in the prereceptor regulation of ligands for the androgen, estrogen, and progesterone receptors and are considered drug targets to treat steroid hormone–dependent malignancies and endocrine disorders. In contrast, AKR1D1 is the only known steroid 5β-reductase and is essential for bile-acid biosynthesis, the generation of ligands for the farnesoid X receptor, and the 5β-dihydrosteroids that have their own biological activity. In this review we discuss the crystal structures of these AKRs, their kinetic and catalytic mechanisms, AKR genomics (gene expression, splice variants, polymorphic variants, and inherited genetic deficiencies), distribution in steroid target tissues, roles in steroid hormone action and disease, and inhibitor design.
Essential Points
There are six human steroid-transforming AKRs (AKR1B15, AKR1C1, AKR1C2, AKR1C3, AKR1C4, and AKR1D1), and exquisite details exist on their structural biology and kinetic and catalytic mechanisms
AKR1C1, AKR1C2, and AKR1C3 have varying ratios of 3-keto-, 17-keto-, and 20-keto-steroid reductase activity and are expressed in peripheral tissues and regulate ligand occupancy of steroid hormone receptors
AKR1D1 is the only human steroid 5β-reductase and is essential for bile acid synthesis
Mutations in AKR1C2 and AKR1C4 are associated with undervirilized male genitalia in the neonate and play roles in the backdoor pathway to 5α-dihydrotestosterone
Mutations in AKR1D1 are the cause of bile acid deficiency
Overexpression of AKR1C3 is a source of androgens in breast cancer, castration-resistant prostate cancer, and polycystic ovarian syndrome
AKR1C inhibitors are warranted for the treatment of hormone-dependent malignancies and hormone-dependent disease syndromes
Hydroxysteroid dehydrogenases (HSDs) are involved in the prereceptor regulation of steroid hormone action and play an important role in the local production of steroids in target tissues (1–3). The concept of target tissues synthesizing their own hormones (intracrine formation) was pioneered by Labrie (4). Pairs of HSDs interconvert potent steroid hormones with their inactive metabolites, regulating the quantity of ligand available to bind and transactivate nuclear receptors (2). This is accomplished when HSDs catalyze positional and stereospecific reactions on keto- or hydroxyl- substituents on the steroid nucleus and side chain, and they function as either NADPH (reduced form of NAD+ phosphate)- dependent ketosteroid reductases or as NAD+-dependent hydroxysteroid oxidases. Pairs of HSDs regulate the activation of human steroid receptors, such as the androgen receptor (AR) (3, 5), the mineralocorticoid receptor (1), the glucocorticoid receptor (6, 7), the estrogen receptor (ER) (8), and the progesterone receptor (PR) (9), affecting the ligand occupancy of the cognate receptor (Table 1). Targeting these HSDs with specific inhibitors represents a promising treatment of endocrine-dependent cancers and endocrine disorders. Compounds that inhibit the function of HSDs involved in the intracrine regulation of steroid hormone action will act as selective intracrine modulators, controlling the levels of tissue-specific steroid hormones and tissue-specific steroid hormone response. Selective intracrine modulators are expected to have a similar pharmacological profile as selective steroid receptor modulators but a different mode of action (see Fig. 1).
Table 1.
HSDs That Control Ligand Occupancy of Steroid Receptors

|
Figure 1.
Regulation of ligand occupancy of nuclear receptors by HSDs that interconvert active and inactive receptor ligands. HSDs belong to two protein superfamilies, the SDRs and the AKRs. The respective roles of individual enzymes are summarized in Table 1. SIM, selective intracrine modulator; SSRM, selective steroid receptor modulator. Orange circle is steroid hormone receptor. [Reproduced with permission from Penning TM. Hydroxysteroid dehydrogenases and prereceptor regulation of steroid hormone action. Human Reproductive Update. 2003;9(3):193–205.]
HSDs belong to two major superfamilies, the short-chain dehydrogenases/reductases (SDRs) (10, 11) and the aldo-keto reductases (AKRs) (12, 13). Human HSDs that are SDRs include 3β-HSD/ketosteroid isomerase, type 1 and 2 (SDR11E1, SDR11E2), 11β-HSD isoforms, type 1 and 2 (SDR26C1, SDR9C3), and 17β-HSD isoforms, type 1 and 3 (SDR28C1, SDR12C2), which catalyze the NADPH-dependent reduction of the 17-ketone group giving rise to potent ligands for the ER and AR and type 2 and 4 (SDR9C2, SDR8C1), which catalyze the NAD+-dependent oxidation of the 17β-hydroxyl group, decreasing ligand availability for the same receptors. This list of 17β-HSDs is not comprehensive and does not include type 5 17β-HSD, which is an AKR (AKR1C3). In addition to these five 17β-HSDs there are up to seven more isoforms in the human genome, making 12 total (14, 15). These seven additional 17β-HSDs are SDRs. However, in some instances it is debatable as to whether they work solely as 17β-HSDs and other functions may predominate. For example, type 6 17β-HSD (SDR9C6) is a retinol dehydrogenase and acts as 3α-hydroxysteroid dehydrogenase/epimerase (16, 17).
SDRs are evolutionarily conserved; in general, they are most often tetrameric (but monomeric and dimeric forms exist) and contain 280 amino acid residues per monomer. Despite their low level of sequence identity (>20%), SDRs show a conserved α/β-folding pattern, where a central β-sheet is flanked by several helices. The central β-sheet represents a typical Rossmann fold for binding cofactor, and enzymes exhibit 4-proS hydride transfer. SDRs can be found in every subcellular compartment (depending on the particular gene product) (18–20). A systematic nomenclature exists for the SDR superfamily (20).
AKRs are a separate superfamily of proteins that catalyze the reduction of carbonyl groups as well as steroid double bonds in the presence of NADPH (21). AKRs exist across all phyla and in general are cytosolic monomeric enzymes, between 34 and 37 kDa in molecular mass, and are 322 amino acids in length (22). They exhibit stereospecificity for 4-proR hydride transfer from NAD(P)H to the acceptor group but exhibit varying degrees of regioselectivity, positional selectivity, and stereoselectivity for their steroid substrates (12). Steroid 5β-reductases (AKR1D1) are also AKRs. Superimposition of the active site of HSDs that are SDRs and AKRs shows conservation of the catalytic residues within the two protein superfamilies (23).
In vitro, AKR enzymes act as 3α/β-HSDs, 17β-HSDs, and 20α-HSDs, catalyzing NAD(P)(H)-dependent oxidoreduction of substituents at the C3, C17, and C20 positions of the steroid nucleus and its side chain (24, 25), but overwhelming evidence indicates that these enzymes work in the reduction direction in mammalian cells (9, 26–28). By converting ketosteroids to their hydroxysteroid counterparts, which are then conjugated by sulfotransferases or by uridine diphosphate glucuronsyl transferases, AKRs play important roles in the phase I metabolism of all steroid hormones (29). Unique among the steroid-transforming AKRs are the steroid 5β-reductases that catalyze the irreversible reduction of Δ4-3-ketosteroids to yield their 5β-reduced counterparts, some of which have biological function (21, 30). In this review we discuss the properties of human AKR genes and proteins, their roles in steroid hormone physiology and disease, and the development of AKR inhibitors.
Human AKR genes and proteins
A systematic nomenclature system was introduced for AKRs to overcome the confusion concerning enzyme identity where the same enzyme was given different names based on the substrate assayed (22, 31). For example, AKR1C3 has been referred to as type 2 3α-HSD, type 5 17β-HSD, prostaglandin F synthase, and dihydrodiol dehydrogenase X. The AKR nomenclature was adopted by HUGO and was adapted from that established for the cytochrome P450 superfamily. Naming of AKR enzymes starts with the root symbol of AKR, followed by the Arabic number designating the family (>40% sequence identity), a letter indicating the subfamily (>60% sequence identity), and an Arabic number for the representative protein sequence. For example, AKR1C3 belongs to AKR family 1, subfamily C, and the number corresponds to the unique enzyme described above. To date, there are 16 AKR families identified by sequence alignment using cluster analysis. An important feature of this nomenclature is that each protein and gene has its own unique name. In this system, the human gene that encodes AKR1C3 is italicized and becomes AKR1C3 but avoids naming the murine gene as akr1c3 when in fact no murine protein or gene paralog of AKR1C3 exists (32).
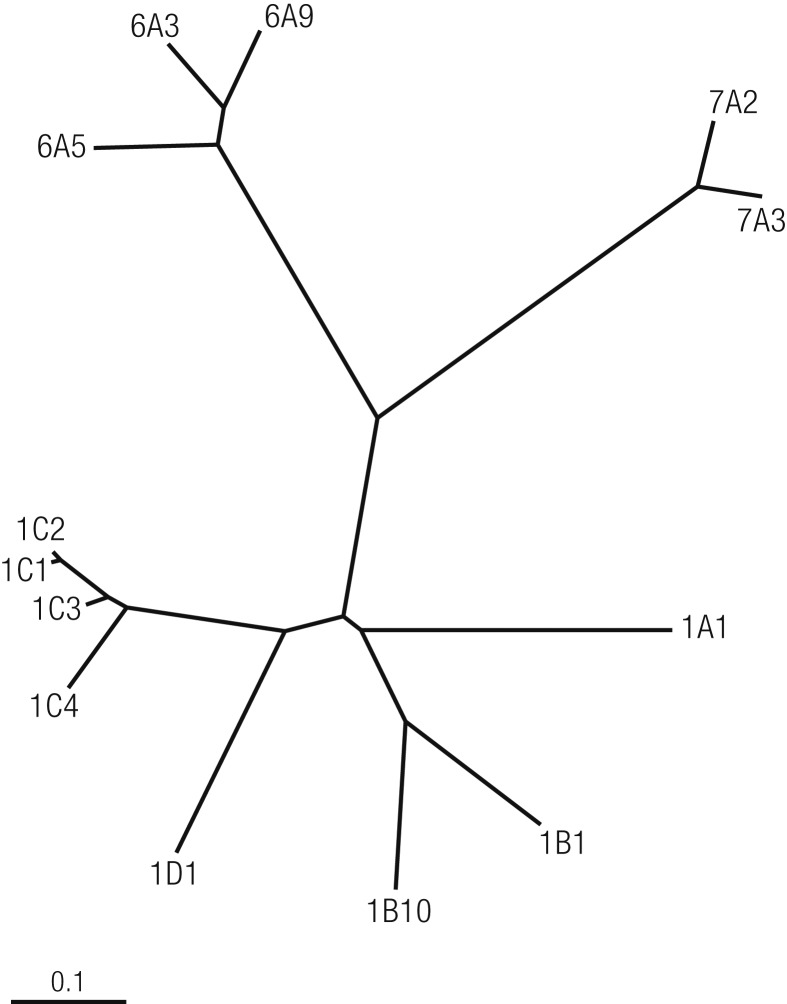
Cluster analysis based on the MultiAlign program generates a cladogram of 13 human AKRs with three distinct major groups (Fig. 2). All members of each group have evolved by descent from a common precursor protein and are more closely related to one another than to other precursor proteins. The human steroid-converting AKRs include AKR1B15, AKR1C1, AKR1C2, AKR1C3, AKR1C4, and Δ4-3-ketosteroid-5β-reductase (AKR1D1). AKR1C1 to AKR1C4 are closely related and share >86% amino acid sequence identity, and AKR1C1 and AKR1C2 differ by only seven amino acids with only one amino acid difference at the active site (Table 2).
Figure 2.
Evolution of the family tree structure of human AKRs. Dendogram adapted from the AKR Superfamily Web site (https://www.med.upenn.edu/akr/) using the multiple sequence alignment program. [Dendogram adapted from the Aldo-Keto Reductase (AKR) Superfamily website (https://www.med.upenn.edu/akr/) using the multiple sequence alignment program. Illustration presentation copyright by the Endocrine Society.]
Table 2.
Human AKRs
| Gene | Protein Names (Aliases) | Chromosomal Localization |
|---|---|---|
| AKR1A1 | Aldehyde reductase; dihydrodiol dehydrogenase 1 | 1p33–p32 |
| AKR1B1 | Aldose reductase | 7q35 |
| AKR1B10 | Small intestine–like aldose reductase; 9-cis-retinal reductase | 7q33 |
| AKR1B15 | 3-Keto-acyl CoA reductase | 7q33 |
| AKR1C1 | 3α(20α)-Hydroxysteroid dehydrogenase; dihydrodiol dehydrogenase 1 | 10p15–10p14 |
| AKR1C2 | 3α-Hydroxysteroid dehydrogenase type 3; dihydrodiol dehydrogenase 2; bile acid–binding protein | 10p15–10p14 |
| AKR1C3 | 3α-Hydroxysteroid dehydrogenase type 2; 17β-hydroxysteroid dehydrogenase type 5; prostaglandin F synthase; dihydrodiol dehydrogenase X | 10p15–10p14 |
| AKR1C4 | 3α-Hydroxysteroid dehydrogenase type 1; dihydrodiol dehydrogenase 4; chlordecone reductase | 10p15–10p14 |
| AKR1D1 | Steroid 5β-reductase | 7q32–7q33 |
| AKR1E2 | 1,5-Anhydro-D-fructose reductase | 10p15 |
| AKR6A3 | Potassium voltage gated channel β-subunit-1 | 3q26.1 |
| AKR6A5 | Potassium voltage gated channel β-subunit-2 | 1p36.3 |
| AKR6A9 | Potassium voltage gated channel β-subunit-3 | 17p13.1 |
| AKR7A2 | Aflatoxin aldehyde reductase | 1p35.1–p36.23 |
| AKR7A3 | Aflatoxin aldehyde reductase | 1p35.1–p36.23 |
Role in steroid biosynthesis and metabolism
The human AKR1B15 gene is expressed abundantly in reproductive organs, adipose tissue, and skeletal muscle (Fig. 3). AKR1B15 colocalizes to the mitochondria where it exhibits 17β-HSD activity and catalyzes the reduction of androgens, estrogens, and 3-keto-acyl-CoA thioesters using NADP(H) as cofactor. Whether AKR1B15 plays an important role in regulating intracellular steroid hormone concentrations is still under investigation (33).
Figure 3.
Distribution of human steroid-transforming AKRs in adult male and female target tissues. Expression in adipose tissue is not shown for clarity.
AKR1C1 to AKR1C3 are expressed in many steroidogenic tissues and steroid hormone target tissues (24). AKR1C1 to AKR1C3 regulate active hormone concentrations for nuclear receptors such as the AR, ER, and PR and are responsible for the prereceptor regulation of steroid hormone action (2) (Fig. 3). In contrast, AKR1C4 is liver specific where its major function is in bile acid biosynthesis and steroid hormone metabolism (34, 35). Although AKR1C enzymes display the ability to catalyze both oxidation and reduction in vitro, transient and stable transfection studies of AKR1C cDNAs into mammalian cells determined that these enzymes solely conduct keto-steroid reduction at the intracellular concentration of the cofactor available (9, 26, 27). Transfection studies showed that AKR1C1 acts as a 20-ketosteroid reductase to inactivate progesterone by converting it to 20α-hydroxyprogesterone (9); AKR1C2 serves as a 3-ketosteroid reductase to deactivate 5α-DHT by converting it to 3α-Adiol (5α-androstane-3α,17β-diol) (9); and AKR1C3 acts as a 17-ketosteroid reductase to reduce Δ4-androstene-3,17-dione to testosterone and estrone to 17β-estradiol, respectively (26, 27) (Fig. 4). These AKR1C enzymes might possess additional activities with other steroid substrates, but this possibility has not been exhaustively explored. For example, AKR1C3 also converts 11-deoxycorticosterone to its 20α-hydroxy metabolite in the kidney, which attenuates the activity of 11-deoxycorticosterone to act as a mineralocorticoid (36).
Figure 4.
Regulation of steroid receptor ligands by human AKRs. AKR1D1 generates 5β-reduced cholestanes that are precursors to the cholanic bile acids, which then bind and activate the FXR.
AKR1D1 is the only human steroid 5β-reductase and reduces a variety of C18, C19, C21, C24, and C27 steroids, which contain the Δ4-3-ketone functionality in the A ring to yield 5β-dihydrosteroids (37). AKR1D1 is essential for bile acid biosynthesis to produce 5β-dihydrocholestanes and acts one step upstream from AKR1C4, and together these enzymes produce the 3α5β-tetrahydrosteroid configuration present in all bile acids (35).
Most steroid hormones contain the Δ4-3-ketone functionality, which can be sequentially reduced to yield four stereoisomeric tetrahydrosteroids (Fig. 5) (38). Cytosolic formation of these tetrahydrosteroids is conducted by the sequential actions of AKR1D1, AKR1C isoforms, SRD5A, and HSD3B1. Importantly, AKR1C1 reduces 5α-DHT to yield 3β-Adiol (5α-androstane-3β,17β-diol) whereas AKR1C2 reduces 5α-DHT to yield 3α-Adiol (25).
Figure 5.
Production of tetrahydrosteroids (THS) by the sequential action of AKRs.
Crystal Structures of Human AKRs
Apoenzyme
As of December 2017, there are ~77 structures of human steroid-transforming AKRs deposited in the RCSB protein data bank as follows: AKR1C1 (n = 4), AKR1C2 (n = 15), AKR1C3 (n = 43), AKR1C4 (n = 1), and AKR1D1 (n = 14). AKRs adopt an (α/β)8 or triose phosphate isomerase barrel motif that consists of an alternating arrangement of α-helix and β-strand that repeats itself eight times. The β-strands coalesce in the center of the structure to make up the staves of the barrel. The (α/β)8 barrel also contains two additional helices, and at the back of the barrel three large loops exist (A-, B-, and C-) that act as antennae and determine substrate specificity (Fig. 6) (21). Crystal structures show that the NAD(P)(H) cofactor occupies the edge of the barrel, whereas the steroid substrate binds to the loops so that it lies perpendicular to the cofactor. Catalysis occurs at the active site located at the base of the barrel where the substrate acceptor group and the nicotinamide head group are in close proximity to each other.
Figure 6.
(a–c) Typical crystal structures of human AKR1C enzymes adapted from the crystal structure of AKR1C3 in complex with NADP+ and 3′-[(4-nitronaphthalen-1-yl)amino]benzoic acid-BMT4-158 (PDB ID: 4DBS). (a) Ribbon drawing displays the common (α/β)8-barrel motif of the AKRs. The α-helices (in cyan) and β-sheets (in red) of the barrel are indicated. The two helices that are not in the barrel are labeled as H1 and H2. (b) Exhibits the same spatial relationship of conserved active-site residues Asp50, Tyr55, Lys84, and His117 commonly found in human AKR1C isozymes. (c) Positions of the A-loop, B-loop, and C-terminal loop.
Cofactor binding
The AKR cofactor binding site lacks the Rossmann fold for binding pyridine nucleotide cofactors observed in SDRs. The AKRs retain high affinity for NADPH to act as ketosteroid reductases (39). The NADP(H)-binding residues are highly conserved and include T24, D50, S166, N167, Q190, Y216, L219, S221, R270, S271, F272, R276, E279, and N280, which contribute toward the binding affinity and specificity of the cofactor [Fig. 7, where the numbering is from AKR1C9 (rat liver 3α-HSD)]. These amino acids bind NADP(H) in an extended anti-conformation (with respect to the N-glycosidic bond that links the ribose moiety to the nicotinamide head group) and ensures 4-proR-hydride transfer (40). The nicotinamide head group interacts with Y216 by π-π stacking whereas the carboxamide side chain is held in place by S166, N167, and Q190. A major factor contributing to the high-affinity binding of NADP(H) to AKRs is the electrostatic interaction of 2′-phosphate of AMP via salt bridges with R276 and R270 (23). The AKR1C9 R276M mutant displays a large increase in its dissociation constant (Kd) and a decrease in the fluorescence kinetic transient observed upon binding NADP(H) (41) such that the mutant binds NADP(H) with similar affinity to NAD(H). The anchoring of the adenosine 2′-phosphate of NADPH is likely essential for the tight binding of NADPH to AKRs.
Figure 7.
Schema showing binding of the NADP+ cofactor to AKR1C enzymes. The cofactor is in blue. [Reproduced with permission from Jez JM, Bennett MJ, Schlegel BP, et al. Comparative anatomy of the aldo-keto reductase superfamily. BiochemJ. 1997;326(3):625–636.]
A significant increase in affinity for NADPH is observed in some AKRs by the formation of a “safety belt,” where a clamping loop moves to form electrostatic linkages across the pyrophosphate bridge of the cofactor. Crystal structures of human aldose reductase (AKR1B1) display a local conformational change that locks NADPH into the active site, especially in loop 7 (the β-strand 7 to α-helix 7 connection) (42). Residues 210 to 212 of this loop shift 1.0 Å to form van der Waals contacts with the coenzyme, whereas residues 213 to 216 fold over the pyrophosphate bridge and ribose-2′-phosphate portions of NADPH. The main chain residues then rotate to close off the top of the binding cleft forming a tunnel over the adenosine 2′-phosphate of NADPH. In the AKR1C enzymes this clapping loop is absent.
Steroid recognition and binding modes
HSDs were originally described as being positional and stereospecific for the oxidoreduction of hydroxyl groups on the steroid nucleus and side chain. AKRs break these rules in that they demonstrate promiscuity in terms of regiospecificity, positional specificity, and stereospecificity. Thus, whereas rat 3α-HSD (AKR1C9) and 20α-HSD (AKR1C8) show strict positional and stereospecificity, the human enzymes AKR1C1 to AKR1C4 show different ratios of 3-, 17-, and 20-ketosteroid reductase activity with different stereochemical outcomes for the hydroxyl group produced. Attempts have been made to rationalize these findings.
The steroid binding pockets of AKR often consist of 14 residues located on five loops, three of which are on flexible loops labeled A, B, and C (Fig. 8). Successful attempts have been made to change the steroid specificity of AKRs using both chimeras and point mutations.
Figure 8.
Steroid binding residues in AKRs. Top, Table showing sequence alignment of steroid-binding residues in AKR1C9 vs the human AKR1C and AKR1D1 enzymes. Note that the steroid-binding residues are predominately in loops A, B, and the C-terminal loop. Bottom, Superposition of AKR1D1 (yellow), AKR1C9 (red), and AKR1C2 (blue) reveals significant conformational differences in loops A, B, and C. [Reproduced with permission from Di Costano L, Drury J, Penning TM, Christianson DW. Crystal structure of human liver Δ4-3-ketosteroid 5β-reductase (AKR1D1) and implications for substrate binding and catalysis. J Biol Chem. 2008;283(24):16830–16839.]
Several chimeric HSDs were generated where the substrate-binding loops of rat 20α-HSD (AKR1C8) were substituted for those in 3α-HSDs (AKR1C9) (43). Whereas replacement of loop A resulted in a chimeric enzyme with 17β-HSD activity, substitution of loops A, B, and C of 20α-HSD onto 3α-HSD successfully converted 3α-HSD to a stereospecific 20α-HSD chimera. This chimera catalyzed the reduction of progesterone to 20α-hydroxyprogesterone with an increase in catalytic efficiency of 1011-fold (43). However, introduction of point mutations to substitute amino acid residues in the steroid-binding cavity of 3α-HSD with those in 20α-HSD failed to yield the same result, indicating that point mutations alone were insufficient to convert one enzyme into the other (43).
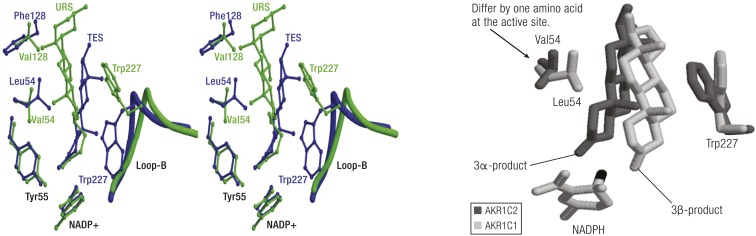
In contrast, human AKR1C1 and AKR1C2 differ from each other in that AKR1C1 exhibits 20α-HSD activity, whereas AKR1C2 exhibits 3α-HSD. Only one of the seven amino acids that is different in these two enzymes is located at the active site at position 54 (44). Replacement of L54 in AKR1C1 with V54 found in AKR1C2 generated an enzyme with identical properties to AKR1C2 (45, 46). The reverse mutation of V54L in AKR1C2 converted the enzyme into AKR1C1 (46). A comprehensive examination of the crystal structures of the ternary complexes of AKR1C1 and AKR1C2 with steroid ligands explains the ability of these two enzymes to alternate their substrate specificity based on this single point mutation.
In the AKR1C1⋅NADP+⋅20α-hydroxyprogesterone ternary complex [Protein Data Bank (PDB) ID: 1MRQ], the d-ring of the steroid substrate binds at the base of the active site, positioning the 20-keto group in the oxyanion hole (47). In this binding pose the C18 and C19 angular methyl groups of the substrate clash with the bulky L54 hydrophobic side chain of AKR1C1. Owing to steric hindrance, the two angular methyl groups flip to face W227 and L308 on the opposite side of the steroid-binding cavity. In this pose, AKR1C1 would reduce progesterone to 20α-hydroxyprogesterone.
Crystal structures of AKR1C2⋅NADP+⋅ursodeoxycholate ternary complexes (PDB ID: 1IHI) provide examples of additional binding modes for steroids (48). The C24 carboxylate of ursodeoxycholate binds in the oxyanion hole. The binding pose of this competitive inhibitor indicates that as well as binding backward (D-ring in the A-ring position), relative to the position of testosterone in the AKR1C9⋅NADP+⋅testosterone ternary complex (PBD 1AFS), the angular methyl groups at C18 and C19 are inverted due to 180° rotation around the C3 to C17 long axis (49). In this pose, the C18 and C19 angular methyl groups of the substrate now point toward V54 in AKR1C2 (Fig. 9, left). In the AKR1C2⋅NADP+⋅progesterone (PDB ID: 4L1W) structure two possible binding modes of progesterone were observed; one resembles the binding mode observed in the AKR1C2⋅NADP+⋅ursodeoxycholate ternary complex whereas another illustrates rotation around the C3 to C17 long axis so that the angular methyl groups show the same facial orientation as testosterone in AKR1C9 (46). In the AKR1C2V54L⋅NADP+⋅progesterone ternary complex the C18 and C19 angular methyl groups point away from L54 as described in the AKR1C1⋅NADP+⋅20α-hydroxyprogesterone complex. These crystal structures show that the backward binding pose and steric hindrance (between the C18 and C19 angular methyl groups with L54) are important determinants for the stereospecific reduction of progesterone in AKR1C1. Because of these different binding poses it is perhaps not surprising that AKR1C1 to AKR1C4 can act as 3-, 17-, and 20-ketosteroid reductases to varying extents.
Figure 9.
Different steroid-binding poses in AKRs. Left, Superimposition of the steroid-binding cavities of AKR1C9⋅NADP+⋅testosterone complex (blue) with the AKR1C2⋅NADP+⋅ursodeoxycholate complex (green). In the first structure, the 3-ketone group of testosterone lies deep in the pocket close to Y55, and the β-face of the steroid and angular methyl groups face W227. This would be a productive binding mode for 3-ketosteroid reduction. In the second structure, ursodeoxycholate has its C24 carboxylate anchored by Y55 and rotation around the steroid long axis from C3 to C17 has occurred. In this structure, the steroid binds backward and upside down relative to testosterone. These alternative binding modes in part explain why human AKR1C enzymes can act as 3-, 17-, and 20-ketosteroid reductases. [Reproduced with permission from Jin Y, Stayrook SE, Albert RH, Penning TM, Lewis M. Crystal structure of human type III 3α-hydroxysteroid dehydrogenase/bile acid binding protein complexed with NAD(P)+ and ursodeoxycholate. Biochemistry. (2001;) 40 (34): 10161–10168. Copyright 2001 American Chemical Society.] Right, Illustration of how AKR1C1 can reduce 3-ketosteroids to 3β-hydroxysteroids and how AKR1C2 can reduce 3-ketosteroids to 3α-hydroxysteroids. In AKR1C1 L54 pushes the α-face of the steroid toward W227 so that hydride transfer occurs to the α-face to produce the 3β-product. In AKR1C2 V54 allows the α-face of the steroid to hug this side of the binding pocket so that hydride transfer will occur to the β-face to produce the 3α-product.
In another example, AKR1C1 and AKR1C2 can reduce 5α-DHT with different stereochemical outcomes; AKR1C1 reduces 5α-DHT to 3β-Adiol whereas AKR1C2 reduces 5α-DHT to 3α-Adiol (25). The substitution of L54 in AKR1C1 with valine in AKR1C2 is sufficient to change the stereochemistry of hydride transfer to the 3-ketone group as shown in AutoDock molecular modeling studies (50). In AKR1C1, the C3-ketone of 5α-DHT binds to the oxyanion hole with its β-face adjacent to W227 due to steric clashing of the C18 and C19 angular methyl groups of L54. The α-face is thus adjacent to the nicotinamide head group of NADPH, which results in a 3β-Adiol product. When L54 is replaced by a less bulky substituent V54 in AKR1C2, 5α-DHT has space to occupy the side of the channel near V54 with the β-face adjacent to the nicotinamide head group of NADPH, which results in a 3α-Adiol product. In this binding pose, the angular C18 and C19 methyl groups still point away from V54, but stereochemical inversion is achieved by a swinging motion of the steroid to present an opposing face to the A-ring of the cofactor (Fig. 9, right).
AKR1C3 contains L54 in the active site, similar to AKR1C1. The enzyme conducts 17-ketosteroid reduction to produce a 17β-hydroxysteroid. The crystal structure of AKR1C3⋅NADP+⋅Δ4-androstene-3.17-dione (PDB ID: 1XF0) reveals substrate binding interactions that closely resemble those of AKR1C1 with its 5α-DHT substrate (51). The 17-keto group of the steroid now occupies the oxyanion hole; in this orientation, the D-ring of the steroid is located in the A-ring position of 5α-DHT in AKR1C1. A distance of 7 Å between the C17-ketone group and the nicotinamide head group indicates a nonproductive binding mode. However, it is proposed that catalysis is able to proceed due to L54 steric forces that push the β-face of the steroid substrate toward W227. This dynamic will arrange the α-face of the steroid to align with the nicotinamide head group of the cofactor, resulting in the 17β-product.
Kinetic and Catalytic Mechanism
Steady-state kinetics
AKR enzymes catalyze a sequential ordered bi bi mechanism (Fig. 10) (52, 53). In this mechanism NADP(H) cofactor binds first, followed by steroid to form a central complex, chemistry occurs, and steroid product leaves, followed by the cofactor to yield free enzyme. For example, in the reduction of 5α-androstane-3,17-dione to yield androsterone, there are four complexes that form: E⋅NADPH, E⋅NADPH⋅5α-androstane-3,17-dione, E⋅NADP+⋅Androsterone, and E⋅NADP+ governed by 10 microscopic rate constants. By conducting ketosteroid reduction reactions with saturating NADPH all the AKR enzymes are present in a single form E⋅NADPH, which permits the estimation of Km (Michaelis-Menten constant) and kcat (turnover number) values [see Table 3 (24, 26, 34, 36, 37, 54–56)]. These constants have been determined in continuous spectrometric assays in which the disappearance of NADPH was monitored and also in discontinuous radiometric assays in which the products were identified. In latter work, products have also been identified by liquid chromatography–mass spectrometry methods.
Figure 10.
Ordered bibi kinetic mechanism of AKRs showing possible inhibitor complexes.
Table 3.
Steady-State Kinetic Parameters for Human AKRs
| Substrate |
Product |
Cofactor |
Reduction |
K
m (μM) |
k
cat (min−1) |
k
cat/Km (min−1 mM−1) |
|---|---|---|---|---|---|---|
| AKR1C1 | ||||||
| ProgesteroneR | 20α-HydroxyP | NADPH | 20-Ketosteroid | 5.7 | 0.93 | 210a |
| Androsterone | 5α-Androstane-3α,17β-diol | NADPH | 17-Ketosteroid | 21 | 0.18 | 9b |
| 5α-DHTR | 5α-Androstane-3α,17β-diol | NADPH | 3-Ketosteroid | 81 | 0.66 | 8b |
| AKR1C2 | ||||||
| 5α-DHTR | 5α-Androstane-3α,17β-diol | NADPH | 3-Ketosteroid | 4.6 | 3.8 | 820a |
| 5α-DHTS | 5α-Androstane-3α,17β-diol | NADPH | 3-Ketosteroid | 2.9 | 1.8 | 620c |
| 5α-DHP | 3α-Hydroxy-5α-pregnane-20-one | NADPH | 3-Ketosteroid | 1.8 | 0.18 | 100d |
| 5β-DHP | 3α-Hydroxy-5β-pregnane-20-one | NADPH | 3-Ketosteroid | 0.6 | 0.5 | 1200e |
| 4-Androstene-3,17-dioneR | Testosterone | NADPH | 17-Ketosteroid | ND | ND | ND |
| AKR1C3 | ||||||
| 5α-DHTR | 5α-Androstane-3α,17β-diol | NADPH | 3-Ketosteroid | 19.8 | 0.26 | 6a |
| 5α-Androstane-3,17-dioneS | Androsterone; | NADPH | 3-Ketosteroid | 5.0 | 7.63 | 56a |
| 5α-DHT | 17-Ketosteroid | |||||
| 4-Androstene-3,17-dioneR | Testosterone | NADPH | 17-Ketosteroid | 13.4 | 0.87 | 65f |
| AndrosteroneS | 5α-Androstane-3α,17β-diol | NADPH | 17-Ketosteroid | 8.9 | 10.2 | 42a |
| EstroneR | 17β-Estradiol | NADPH | 17-Ketosteroid | 9.0 | 0.068 | 7.5g |
| Progesterone | 20α-HydroxyP | NADPH | 20-Ketosteroid | 2.8 | 1.0 | 370f |
| AKR1C4 | ||||||
| 5α-DHTR | 5α-Androstane-3α,17β-diol | NADPH | 3-Ketosteroid | 8.3 | 1.92 | 231a |
| 5β-DHT | 5β-Androstane-3α,17β-diol | NADPH | 3-Ketosteroid | 0.2 | 4.7 | 23,500e |
| 5β-DHP | 3α-Hydroxy-5β-pregnane-20-one; | NADPH | 3-Ketosteroid | 0.3 | 4.9 | 16,300e |
| 5β-Pregnane-3α,20α-diol | 20-Ketosteroid | |||||
| 20α-Hydroxy-5β-pregnane-3-one | 5β-Pregnane-3α,20α-diol | NADPH | 3-Ketosteroid | 0.3 | 4.3 | 14,300e |
| 3α-Hydroxy-5β-pregnane-20-one | 5β-Pregnane-3α,20α-diol | NADPH | 20-Ketosteroid | 0.4 | 1.5 | 3800e |
| AKR1D1 | ||||||
| Testosterone | 5β-Dihydrotestosterone | NADPH | 5β-Reduction | 2.7 | 8.4 | 3120h |
| Cortisol | 5β-Dihydrocortisol | NADPH | 5β-Reduction | 13.1 | 2.7 | 210h |
| Cortisone | 5β-Dihydrocortisone | NADPH | 5β-Reduction | 15.1 | 11.7 | 780h |
| 4-Cholesten-7α-ol-3-one | 7α-Hydroxy-5β-dihydrocholestan-3-one | NADPH | 5β-Reduction | 0.8 | 2.0 | 2530h |
| Cholestenone | 5β−dihydrochlestenone | NADPH | 5β-Reduction | 0.3 | 0.60 | 1980d |
Abbreviation: ND, not determined.
Byrns et al., 2008, Biochem Pharmacol (56).
Penning et al., 2000, Biochem J (24).
Jin and Penning, 2006, Biochemistry (54).
Trauger et al., 2002, Biochemistry (55).
Jin et al., 2011, Biochem J (34).
Sharma et al., 2006, Mol Cell Endocrinol (36).
Byrns et al., 2010, J Steroid Biochem Mol Biol (26).
Chen et al., 2011, Steroids (37).
Examination of these kinetic constants shows that the 20-ketone reduction of progesterone catalyzed by AKR1C1 is favored over the 17-ketone reduction of androsterone and the 3-ketone reduction of 5α-DHT by 25-fold, indicating that it is a dominant 20-ketosteroid reductase. The catalytic efficiency for the reduction of the 3-ketone group of 5α-DHT catalyzed by AKR1C2 is higher than that observed for the reduction of progesterone catalyzed by AKR1C1, making it a predominant 3-ketosteroid reductase. Interestingly, the 3-ketosteroid reduction of 5β-dihydroprogesterone (DHP) is favored 12-fold over the 3-ketosteroid reduction of 5α-DHP. The reduction of Δ4-androstene-3,17-dione to testosterone catalyzed by AKR1C3 has a catalytic efficiency 10-fold higher than that observed for the 3-ketone reduction of 5α-DHT. In radiochemical assays, AKR1C3 was the only AKR1C isoform that produced significant amounts of testosterone from Δ4-androstene-3,17-dione, making it a favored 17-ketosteroid reductase in the subfamily. Surprisingly, the catalytic efficiency for the 20-ketone reduction of progesterone catalyzed by AKR1C3 is of the same order of magnitude as that for AKR1C1. AKR1C4 has a high catalytic efficiency for the reduction of the 3-ketone group of 5α- and 5β-dihydrosteroids with a significant preference for the latter. Thus, the reduction of the 3-ketone group of 5β-DHT has a catalytic efficiency that is 100-fold greater than that observed for the reduction of the 3-ketone group of 5α-DHT.
ARK1D1 was found to have high catalytic efficiencies for the 5β-reduction of C19, C21, C24, and C27 Δ4-3-ketosteroids. However, the catalytic efficiencies observed were 0.1 of those measured for the reduction of the 3-ketone group to yield the corresponding 3α,5β-tetrahydrosteroids catalyzed by the liver-specific AKR1C4. For example, the 5β-reduction of testosterone yields a catalytic efficiency of 3120 min−1 mM−1 but the reduction of 5β-DHT by AKR1C4 yields a catalytic efficiency of 23,500 min−1 mM−1. These second-order rate constants demonstrate that steroid 5β-reduction is relatively slow vs the downstream 3-keto reduction.
The formation of the binary and central complexes predicted by the kinetic mechanism are accompanied by conformational changes that occur on the large loops upon the binding and the release of NADP(H) (42, 53, 57). Furthermore, in the binary E⋅NADP+ complex structure the β1 to α1 loop, part of loop B, and the C-terminal tail are disordered in AKR1C9. However, upon binding steroid an ordered binding cavity forms as seen in the abortive ternary complex AKR1C9⋅NADP+⋅testosterone structure and indicates that additional enzyme complexes form along the reaction coordinate to the central complex and is likely applicable to the human AKR1C enzymes.
Transient kinetics
Steady-state kinetic approaches only yield estimates of macroscopic rate constants. In-depth analysis of ligand binding and substrate turnover have been performed using stopped-flow spectrometry on AKR1C9, which is a prototypic AKR1C member, and AKR1C2. Cofactor binding to the AKR enzymes has been measured using stopped-flow spectrometry where the intrinsic W86 fluorescence of the protein is quenched as the cofactor binds (52, 53, 58). Biexponential fitting of the fluorescence kinetic transient fits a three-step binding model for AKR1C9 and AKR1C2 enzymes (52–54). These observations indicate that there are eight enzyme complexes that form in the kinetic mechanism for AKR1C enzymes involving the formation of loose E⋅NADP(H) complexes followed by subsequent isomerization events to form tight binding complexes [E*⋅NADP(H) and E**⋅NADP(H)], and that the kinetic mechanism is governed by 18 microscopic rate constants.
In the three-step binding model of NADP(H) for AKR1C9, an electrostatic linkage between R276 and the 2′-phosphate of AMP facilitates the first conformational change, as the R276M mutant displays a significant reduction in the fluorescent kinetic transient (41). This cofactor anchoring event is thought to initiate the formation of a cofactor tunnel, which involves the organization of loop β1 to α1 and loop B so that the nicotinamide head group can form hydrogen bonds with cofactor binding residues within the AKR enzyme. The cofactor is tightly bound after this second conformational change to from the E**⋅NADPH complex, with an on rate that is 20-fold greater than the off rate, resulting in a 100-fold increase in cofactor affinity.
The rate-determining step in the AKR kinetic mechanism is determined by the rate constants for cofactor binding or release, steroid binding and release, or the chemical step (52, 54, 59, 60). In AKR1C9, the enzyme is capable of catalyzing the oxidation of androsterone using NADP+ and the reduction of 5α-androstane-3,17-dione using NADPH at pH 7.0 (52). Multiple-turnover experiments of the oxidation reaction using stopped-flow spectrometry showed burst-phase kinetics in that the rate of the chemical reaction kchem was found to be 55.8 sec–1, whereas the rate of the release of the NADPH cofactor krNADPH was identical to kcat of 0.77 sec–1. These experiments show that the oxidation reaction catalyzed by AKR1C9 follows an initial period of fast product formation followed by a slow product-release phase, indicating that the slow release of cofactor places an upper limit on kcat. Similar experiments determined a kchem of 0.44 sec–1, rate of the release of the NADPH cofactor krNADPH 0.65 sec–1, and kcat of 0.30 sec–1 for 5α-DHT reduction, and no burst phase kinetics were observed, suggesting that in the reduction of 5α-DHT catalyzed by AKR1C9 chemistry is rate determining. Stopped-flow experiments on the AKR1C2 catalyzed reduction of 5α-DHT revealed a kinetic mechanism in which a series of slow events, including the chemical step (0.12 sec−1), the release of the steroid product (0.081 sec−1), and the release of the cofactor product (0.21 sec−1) all contribute to yield the overall observed low kcat of 0.033 sec−1 (54).
Equilibrium constant and directionality
Direct measurement of the equilibrium constant Keq can be reconciled by the calculation of the Haldane constants, which show that the hydroxysteroid dehydrogenase reaction catalyzed by AKRs overwhelmingly favors the reduction reaction, yielding a Keq of 20 (52, 54). Additionally, the difference in affinity for NADP(H) vs NAD(H) cofactors is vast. The former binds to the AKR enzymes with mid-nanomolar affinity (100 nM) whereas the latter binds to AKR enzymes with mid-micromolar affinity (200 μM). The affinity of the human AKR1C enzymes for the NADP(H) cofactors is so high that it can be difficult to purify the apoenzyme. The high affinity for NADPH is not without consequence. NAD+-dependent oxidation reactions can be completely attenuated by low micromolar concentrations of NADPH that would prevail in the cellular microenvironment, further supporting the role of these enzymes to act as ketosteroid reductases (28). Furthermore, transfection studies into mammalian cell lines (e.g., HEK-293, LNCaP, and MCF-7 cells) shows that expressed enzymes when challenged with the prevailing concentrations of cellular cofactors only act as ketosteroid reductases (9, 26–28). As described, the conserved R276 is critical for preserving the higher affinity of NADP(H) vs NAD(H) to favor ketosteroid reduction. HEK-293 cells stably expressing AKR1C9 mutants R276M and R276G show attenuated equilibrium constants, whereas the negatively charged substitution R276E disfavors NADP(H) binding and reverses the directional preference to oxidation (61).
Catalytic mechanism and site-directed mutagenesis
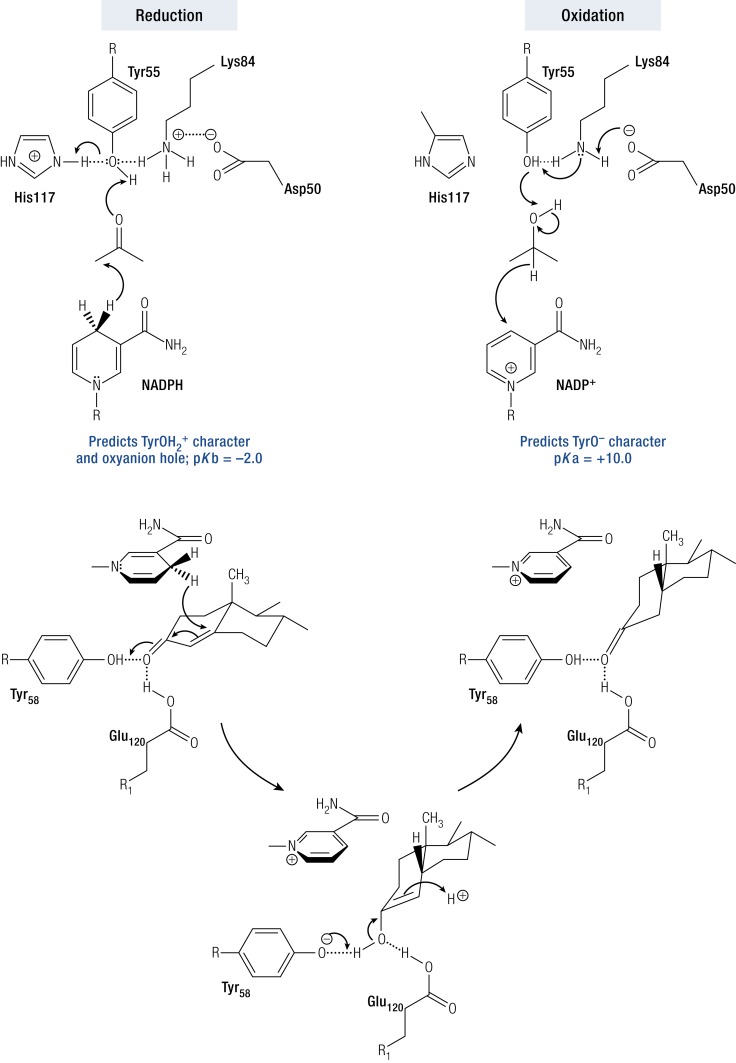
Crystal structures of AKRs indicate conservation of catalytic tetrad residues Y55, D50, K84, and H117 within the superfamily. Site-directed mutagenesis of the tetrad residues in AKR1C9 coupled with pH-kcat profiles for the conversion of [14C]-5α-DHT to [14C]-3α-Adiol provided evidence for a “push–pull” mechanism involving a diprotic enzyme in which Y55 acted as the general acid base, which was facilitated by the protonation state of neighboring tetrad residues. In the reduction direction, the protonated form of Tyr (TyrOH2+) acts as the general acid by participating in a proton relay with the imidazole group of histidine to polarize the steroid carbonyl group to accept a hydride ion from the reduced cofactor. In the oxidation direction, the phenolate form of Tyr (TyrO−) acts as a general base to abstract a proton from the steroid alcohol to facilitate hydride transfer back to the oxidized cofactor. Phenolate ion formation was facilitated by K84, which in turn was deprotonated by D50 acting as a base (Fig. 11a) (62).
Figure 11.
Catalytic mechanisms for AKR enzymes. Top, “Push–pull” mechanism using a diprotic AKR1C enzyme to catalyze ketosteroid reduction and hydroxysteroid oxidation. In the former instance, Y55 has TyrOH2+ character due to its proton relay with H117. In the latter instance, Y55 has phenolate character due to its proton relay with D50 and K84. Bottom, 5β-Reduction of 3-ketosteroids by AKR1D1, where E120 substitutes for H117 and acts as a superacid. E120 also permits the steroid to bind deeper in the pocket to permit hydride transfer to occur at the C5 position. [Reproduced with permission from Di Costano L, Drury J, Penning TM, Christianson DW. Crystal structure of human liver Δ4-3-ketosteroid 5β-reductase (AKR1D1) and implications for substrate binding and catalysis. J Biol Chem. 2008;283(24):16830–16839.]
AKR1D1 catalyzes the irreversible reduction of the steroid C-C double bond in Δ4-3-ketosteroids by acting as a steroid 5β-reductase (30, 63, 64). By reducing the Δ4-ene at the C5 position, a 90° bend is introduced at the A/B-ring junction to produce a 5β-dihydrosteroid (38, 64–66). This reaction is difficult to achieve by synthetic methods. Strong chemical reagents are required to reduce C-C double bonds, whereas reduction of the α,β-unsaturated ketone by lithium aluminum borohydride will lead to formation of the allylic alcohol (67, 68). AKR1D enzymes have a unique catalytic tetrad whereby H117 is replaced by E117. The AKR1C9 H117E mutant displayed 5β-reductase activity instead of 3α-HSD activity, confirming the importance of this substitution (69). Crystal structures of AKR1D1 ternary complex with cortisone and progesterone suggest a dual role for E120 (equivalent of His117) (63, 70). The smaller carboxylic acid side chain of E120 compared with the bulky imidazole side chain allows the steroid to bind deeper into the active site pocket, so that the C5 position of the substrate is in close proximity with the 4-proR hydrogen of NADPH. In addition, the anti-conformation of E120 side chain suggests that this residue will be fully protonated. It is likely that this residue serves as a superacid and promotes acid-catalyzed enolization of the α,β-unsaturated ketone substrate in concert with Y55 (Fig. 11b) (63, 69, 70).
AKR Genomics
Gene structure
The organization of the human AKR steroid-transforming genes is highly conserved they contain 9 to 10 exons with 8 to 9 introns of similar length but have variable 5′- and 3′-untranslated regions. The AKR1B15 gene is located on chromosome 7q33, the AKR1C1 to AKR1C4 genes are located on chromosome 10p15 to 10p14 arranged in a head-to-tail fashion, suggesting that they arose by gene duplication, whereas the AKR1D1 gene is located on 7q32 to 7q33.
Regulation of gene expression
Despite their roles in regulating ligand access to steroid receptors, few studies have been performed on the regulation of AKR1C gene expression by these receptors and whether these same receptors bind to the AKR1C gene promoter or enhancer elements. In contrast, there is extensive literature on changes in transcript and protein expression under pathophysiologic conditions and this is reviewed in later sections. Human AKR1C enzymes are pluripotent and use substrates other than steroids. Among these are the byproducts of lipid peroxidation, for example, 4-hydroxynonenal and 4-oxo-2-nonenal (71). As they are part of the defense system against oxidative stress, it is not surprising that they belong to the antioxidant response gene battery and are regulated by the Keap1-Nrf2 system via antioxidant response elements (AREs) in their gene promoters (40, 72). In fact, SILAC-labeling experiments show that they among the most upregulated genes in this battery (73). Examination of the gene promoters for AKR1B15, AKR1C1, AKR1C2, AKR1C3, AKR1C4, and AKR1D1 identify 4, 10, 15, 4, 2, and 11 AREs, respectively, based on the Nrf2 consensus sequence (40). Whether all of these AREs are functional remains to be determined and chromatin immunoprecipitation sequencing experiments remain to be performed. However, based on the upregulation of the AKR1C genes by electrophiles, reactive oxygen species, and the Nrf2 activator R-sulforaphane, the distinct possibility exists that steroid metabolism will be redox regulated at the gene expression level and influenced by oxidative stress.
Splice variants
The availability of RNA sequencing (RNAseq) has led to the identification of a number of splice variants for human steroid-transforming AKR genes. The AKR1B15 gene encodes for two splice variants (33). The AKR1B15.1 variant (AKR1B15-201) has 91% sequence similarity to small intestine aldose reductase AKR1B10 and encodes for AKR1B15 (33). The AKR1B15.2 variant (AKR1B15-001; CCDS 47715) differs in that it has an additional 28 amino acids at the N terminus of AKR1B15.1 (74). This difference in the two isoforms is responsible for differences in their expression levels, subcellular localization, and enzymatic activity. AKR1B15.1 is a mitochondrial 17β-HSD and is expressed at higher amounts than AKR1B15.2. The 17β-HSD activity is not observed in the cytosolic AKR1B15.2 even though it contains the four conserved catalytic tetrad residues (D72, Y77, K106, and H139). AKR1B15.1 also reduces 3-keto-acyl-CoAs and because of its mitochondrial localization it is thought to function primarily as a 3-keto-acyl-CoA reductase (33).
The AKR1C2 gene also gives rise to three splice variants. Two of these variants (AKR1C2-001; CCDS7062 and AKR1C2-201; CCDS7062) differ in the length of their 5′-untranslated region but give rise to the same full-length AKR1C2 protein of 323 amino acids. The third variant (AKR1C2-203; CCDS44350) has lost five exons and would form a 139–amino acid protein that would be inactive.
The AKR1C3 gene gives rise to two potential splice variants: P42330-1, which lacks amino acids 1 to 119, and P42330-2, which contains only the first 204 amino acids. Evidence that these transcripts are translated into proteins is lacking and neither is predicted to be catalytically active.
The AKR1D1 gene also gives rise to three splice variants. One variant (AKR1D1-002; CCDS5846) gives rise to the full-length AKR1D1 protein of 326 amino acids. The remaining two variants give rise to truncated proteins of 285 amino acids (AKR1D1-001; CCDS5170) and 290 amino acids (AKR1D1-006; CCDS55169), and both are predicted to be inactive due to the absence of the C terminus.
Although multiple transcripts exist for the steroid-transforming AKRs, in general only one transcript per AKR gene gives rise to active protein. However, the challenge is that depending on primer design, real-time quantitative polymerase chain reaction may give a false estimate of the expression of mature transcripts that would be translated to full-length proteins, and thus RNAseq measurements will be more reliable for expression studies.
Polymorphic variants
The National Center for Biotechnology Information (NCBI) database lists single nucleotide polymorphisms (SNPs) that exist in the AKR1 genes and nonsynonymous SNPs (nsSNPs) in their coding regions. Some of these SNPs have been identified in candidate gene studies and genome-wide association studies of disease incidence. However, the number of functional studies to support the identified disease associations is small in number.
SNPs in the promoter region of AKR1C3 have been associated with disease state. The AKR1C3 SNPs c-71A>G and c-210A>C have been associated with 21-hydroxylase deficiency genotypes and may contribute to the external genital virilization observed in females due to increased fetal androgen biosynthesis mediated by AKR1C3 (75). The intron variant rs1937845, which has a global minor allelic frequency (MAF) of 42%, was associated with a significant increase in polycystic ovarian syndrome (PCOS) in Chinese women, where again this could be due to an increase in androgen production (76). Nevertheless, other studies using cohorts from the United States failed to identify an association between PCOS and common AKR1C3 polymorphisms in these populations.
A complete list of the nsSNPs with an MAF of >0.1% are shown in Table 4. The availability of AKR1 crystal structures that show conservation in cofactor binding site residues, as well as conservation in amino acid positions used in steroid binding, coupled with knowledge of the active site tetrad, permits amino acid changes predicted by these nsSNPs to be mapped to these structures to predict change in function. Unfortunately, most nsSNPs that fall into these categories are beneath the 0.1% cut-off for MAF and indicate that the effect of these allelic variants will be rare. Another method of nsSNP analysis is to determine whether the amino acids are evolutionary conserved in the AKR superfamily. Bioinformatic tools, for example, SIFT and PolyPhen, predict that when amino acids in evolutionary conserved amino acids are mutated within a protein superfamily this would be deleterious to function (77–80).
Table 4.
nsSNPs in Human Steroid-Transforming AKR Genes (MAF >0.1%)
| Cofactor Binding Residue | Steroid Substrate Binding Residue | AKR1B15 | MAF | AKR1C1 | MAF | AKR1C2 | MAF | AKR1C3 | MAF | AKR1C4 | MAF | AKR1D1 | MAF |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T24 | 52 | G54→S | 0.004 | K39→R | 0.0046 | F46→Ya | 0.0649 | H5→Q*a | 0.42 | G135→Ea | 0.0270 | N/A | N/A |
| D50 | 54 | A95→T | 0.001 | T147→I | 0.0022 | A70→V | 0.0034 | E36→ns | 0.24 | S145→Ca | 0.1028 | ||
| S166 | 55 | I103→V | 0.003 | R170→H | 0.0028 | D71→H | 0.0026 | I42→V | 0.002 | G181→V | 0.0010 | ||
| N167 | 86 | F111→I | 0.010 | N280→Kb | 0.0076 | V122→I | 0.0026 | R47→H | 0.002 | A243→T | 0.0084 | ||
| Q190 | 117 | K271→T | 0.001 | T323→ns | Q279→Hb | 0.0010 | I49→T | 0.002 | L311→Va | 0.1024 | |||
| Y216 | 118 | T273→A | 0.001 | R258→Cc | 0.064 | E59→K | 0.004 | ||||||
| L219 | 120 | R66→Q | 0.023 | ||||||||||
| S221 | 128 | R76→G | 0.004 | ||||||||||
| R270 | 129 | E77→Ga | 0.037 | ||||||||||
| S271 | 227 | R91→ns | 0.004 | ||||||||||
| R276 | 306 | P91→A | 0.002 | ||||||||||
| Q279 | 308 | K104→Da | 0.152 | ||||||||||
| N280 | 310 | L122→V | 0.001 | ||||||||||
| C145→Y | 0.005 | ||||||||||||
| I163→T | 0.004 | ||||||||||||
| P180→Sc | 0.086 | ||||||||||||
| K183→Rc | 0.026 | ||||||||||||
| Q190→ns | 0.024 | ||||||||||||
| R199→Wc | 0.004 | ||||||||||||
| R199→Qc | 0.004 | ||||||||||||
| S208→L | 0.001 | ||||||||||||
| R250→Q | 0.002 | ||||||||||||
| R258→Cc | 0.033 | ||||||||||||
| P315→T | 0.002 |
Other AKR1C2 alleles studied in DHT metabolism include: V38A (ND), V38I (0.0002; 0%), H47R (ND; 0%), S87C (ND), V111A (ND), H170R (not in NCBI), L172Q (ND), K179E (ND), K185E (ND), and R258C (ND).
Abbreviations: N/A, not available; ND, not determined; ns, not significant.
Amino acids examined for exemestane turnover.
Amino acids involved in cofactor binding.
Evolutionary conserved amino acids.
The NCBI database lists four nsSNPs in AKR1C2 (T23I, P119T, K185E, and R258C) that are evolutionary conserved, where R258C has an MAF of 0.064. In AKR1C3 there are seven nsSNPs that in fit into this category (L85F, P180S, K183R, R199W, R199Q, R258C, and M293I) where P180S, K183R, R199W, R199Q, and R258C have MAFs of 0.086, 0.026, 0.004, 0.004, and 0.033, respectively. In AKR1C4, there are nsSNPs in four evolutionary conserved amino acids (R76T, E192A, Q262R, and R263H); however, none of these have an MAF >0.001.
The effect of allelic variation in AKR1C2 on the in vitro metabolism of 5α-DHT has been examined (81). Unfortunately, the authors examined the effect of these variants following their expression in Sf9 insect cell lysates and used a catalytic inactive mutant Y55F as a control. Under these conditions a significant background turnover of 5α-DHT was noted in the presence of the Y55F mutant, making it difficult to interpret these data. The authors concluded that F46Y (0.0649 MAF) and L172Q (not in NCBI) reduced the apparent maximum velocity (Vmax) and that L172Q, K185E, and R258C all reduced the apparent Km. However, their effect on the utilization ratio Vmax/Km for reduction of 5α-DHT by these variants was modest and varied by only two to three fold.
The effect of allelic variation in AKR1C3 was also examined for differences in the reduction of the 17-ketone group of the aromatase inhibitor exemestane using purified recombinant enzymes (82). AKR1C3 H5Q (0.42 MAF), AKR1C3 E77G (0.037 MAF), AKR1C3 P180S (0.086 MAF), and AKR1C3 R258C (0.033 MAF) all decreased the 17-keto reduction of exemestane by 20- to 40-fold with little variance in Km except for R258C where the Km increased by sevenfold. Thus, each of these major allelic variants had a profound effect on the catalytic efficiency for this reaction. However, the kcat for the reduction of the 17-ketone group of exemestane by AKR1C3 was 0.003 min−1 ompared with a kcat of 0.87 min−1 for the reduction of Δ4-androstene-3,17-dione. Whether these differences in catalytic efficiency observed in the variants will hold for the reduction of physiologic substrates for AKR1C3 remains to be determined.
Genetic deficiencies
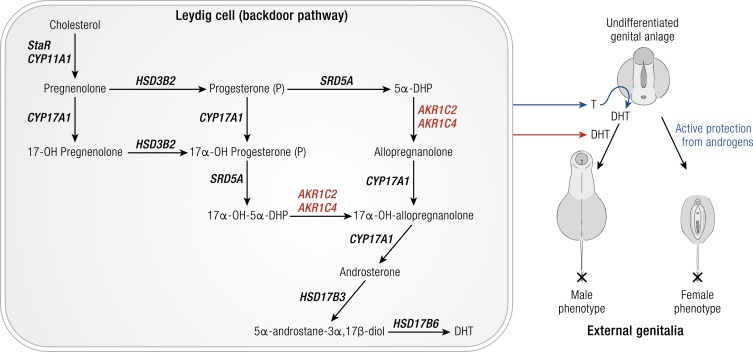
5α-DHT is required for the virilization of the male genitalia in the neonate. Disease-causing mutations exist in AKR1C2 and AKR1C4 that are associated with undervirilized male genitalia (83). These mutations result in decreased production of 5α-DHT via the “backdoor pathway” (84–86). In the fetal testis Leydig cells, this pathway begins with progesterone being reduced to 5α-DHP catalyzed by SRD5A or a parallel pathway starting with the 5α-reduction of 17α-hydroxyprogesterone. 5α-DHP or 17-OH-5α-DHP are then reduced to allopregnanolone (3α−hydroxy-5α-pregnane-20-one) or 17-OH-allopregnanolone by AKR1C2, and CYP17A1 converts these pregnanes to androsterone, which is reduced to 3α-Adiol by HSD17B3 (Fig. 12). Two 46,XY individuals from the same family with failure to develop male genitalia were raised as females. These individuals were compound AKR1C2 heterozygotes and contained either a I79V plus H90G mutation or a I79V plus N300T mutation (83). Each of the AKR1C2 mutations resulted in a significant reduction in the Vmax/Km ratio for the reduction of 5α-DHP to allopregnanolone. Nevertheless, the reduction in utilization ratio was too small to account for the loss of virilization. In these instances, an AKR1C4 mutant was also present in which exon 2 that contains the catalytic tetrad was deleted (83). These disease-causing mutations support the importance of the backdoor pathway in androgen biosynthesis in the developing neonate to promote normal formation of the male genitalia. These studies also indicate an important and unexpected role for the AKR1C4 gene in this pathway because its expression was thought to be liver specific. Both AKR1C2 and AKR1C4 transcripts were found to be expressed in the developing fetal testis. These studies showed that the severity of the defect depended on the mutations of two genes within the same pathway.
Figure 12.
Role of AKR1C2 and AKR1C4 in male virilization. Left panel, Fetal testis Leydig cell steroidogenesis. Only the backdoor pathway to DHT is shown. CYP11A1 represents cholesterol side-chain cleavage enzyme. CYP17A1, 17α-hydroxylase/17,20-lyase; HSDB2, 3β-hydroxysteroid dehydrogenase type 2; HSD17B3, androgenic 17β-hydroxysteroid dehydrogenase type 3; HSD17B6, 17β-hydroxysteroid dehydrogenase type 6; SRD5A, steroid 5α-reductase; StAR, steroid acute regulatory protein. Italics refer to gene names. Right panel, DHT synthesized in the fetal Leydig cells is required for the formation of the male genitalia and virilization. Loss-of-function mutations in AKR1C2 and AKR1C4 prevent the formation of DHT by the backdoor pathway and actively protect the undifferentiated genital anlage from androgens so that the female phenotype predominates. “X” marks the end of the anogenital distance, which is longer in boys.
In a different family, a 46,XY individual was diagnosed 7 weeks after birth to have disordered sexual development. One allele of this individual had a hybrid AKR1C1/1C2 gene and a paternal copy of AKR1C1, and the other allele had a hybrid AKR1C1/1C2 gene and a maternal copy of AKR1C2 with an H22Q mutation. As a result, neither allele encoded an authentic AKR1C2 gene. These studies identified a distinct monogenic disorder in AKR1C2 that resulted in loss of male virilization.
AKR1D1 is essential for the synthesis of 5β-reduced cholestanes in the bile acid biosynthesis pathway. Bile acid deficiency can result from cholestatic liver disease or liver damage or from a primary genetic defect in AKR1D1 (87–89). AKR1D1 deficiency (MIM604741) can be best diagnosed when an abnormal urinary bile acid profile is present in which 3-oxo-Δ4-bile acids and their conjugates represent >70% of the total profile, and this is followed by genetic testing. Bile acid deficiency due to inherited mutations in AKR1D1 is often misdiagnosed as liver disease without examining its genetic underpinning, and thus the frequency of AKR1D1 mutations is likely underestimated. Bile acid deficiency is often neonatal fatal because bile acids are essential for the absorption of lipids and fat-soluble vitamins. Although this syndrome can be treated with dietary replacement of primary bile acids (90), it often requires liver transplantation. A defect in AKR1D1 activity causes the inadvertent buildup of hepatotoxic allo-bile acids (5α-reduced cholanic acids), which is exacerbated by the absence of bile acids that would bind to the farnesoid X receptor (FXR) to repress CYP7A1 (7α-hydroxylase) expression, which catalyzes the rate-determining step in bile acid biosynthesis (87–89, 91, 92). Thus, a feed-forward mechanism is created that will further stimulate allo-bile acid production (Fig. 13).
Figure 13.
Role of AKR1D1 in bile acid deficiency. Pivotal roles of AKR1D1 and AKR1C4 in human liver bile acid biosynthesis. In AKR1D1 deficiency, lack of feedback inhibition by primary bile acids on the expression of CYP7A1 mediated by FXR leads to diversion of 7α-hydroxy-cholest-4-ene-3-one to hepatoxic allo-bile acids. CYP7A1, steroid 7α-hydroxylase; SRD5A, steroid 5α-reductase type 1. Arrows to chenodeoxycholate and cholic acid represent the multiple steps required to convert C27 cholestanes to C24 cholanes. Italics refer to gene names.
Inherited point mutations in AKR1D1 (L106F, P133R, P198L, G223E, and R261C) associated with bile-acid deficiency have been identified. Mapping of these mutations to the X-ray crystal structure failed to determine why they might cause loss of function (93). Alignment of all 50 members of the AKR1 family showed that these residues were evolutionary conserved and likely perform critical functions to maintain the protein fold (94). Of these mutations, P133R was the only one that could be purified in sufficient quantities to determine its effect on AKR1D1 activity (95). The P133R mutation caused a >40-fold increase in Kd values for the NADP(H) cofactors and increased the rate of release of NADP+ from the enzyme by 2 orders of magnitude when compared with the wild-type enzyme. In contrast, the effect of the mutation on Kd values for steroids were 10-fold or less. The reduced affinity for the cofactor suggests that the mutant exists largely in the less stable cofactor-free form in the cell. Using stopped-flow spectroscopy, a significant reduction in the rate of the chemical step was observed in multiple turnover reactions catalyzed by the P133R mutant, possibly due to the altered position of NADPH. Thus, impaired NADPH binding and hydride transfer are the molecular bases for bile acid deficiency in patients with the P133R mutation. All the mutant proteins were expressed in HEK-293 cells and shown to be expressed at lower amounts than wild-type AKR1D1, had lower activity for the 5β-reduction of testosterone, and showed a shorter half-life under cycloheximide pulse-chase conditions (93, 94).
Distribution in Steroid Target Tissues
The expression of steroid-transforming AKR enzymes in male and human target tissues is summarized in Fig. 3. AKR1 expression has been examined by measurement of RNA (e.g., Northern analysis, PCR, semiquantitative PCR, real-time quantitative PCR, and RNAseq) and by measurement of protein levels (e.g., immunoblot analysis and immunohistochemistry) in normal tissues, pathological specimens, and in normal and transformed cell lines. Early semiquantitative RT-PCR using pooled poly(A)+ RNA from eight human tissues coupled with Southern blot analysis followed by normalization to β-actin showed high expression of AKR1C1 to AKR1C4 in human liver (24). AKR1C4 was found to be liver specific, consistent with its role in hepatic steroid metabolism and bile acid synthesis (24, 96, 97). AKR1C1, AKR1C2, and AKR1C3 were all expressed in lung and in prostate and mammary gland. In the latter two tissues, AKR1C3 was more highly expressed than AKR1C1 and AKR1C2, consistent with its 17-ketosteroid reduction of androgens and estrogens (24).
Subsequent studies showed that AKR1C1 was expressed in liver, prostate, testis, adrenal gland, brain, uterus, mammary gland, and keratinocytes. The highest basal levels were found in liver, mammary gland, and brain (98). AKR1C2 is also expressed in the fetal testes and in fetal and adult adrenal glands (83), as well as in regions of the brain, including the medulla, spinal cord, frontotemporal lobes, thalamus, subthalamic nuclei, and amygdala. Weaker expression was found in the hippocampus, substantia nigra, and caudate (99). Subsequent studies confirmed that AKR1C3 is expressed in many tissues, including adrenal gland, ovary, brain, kidney, liver, lung, mammary gland, placenta, small intestine, colon, spleen, prostate, and testis. It is one of the dominant HSDs in prostate and mammary gland. In the prostate, higher levels were found in the epithelial cells than in stromal cells (96, 100–103).
Roles in Steroid Hormone Action and Disease
Many studies have been performed on AKR1C expression levels in human tissues and cells in concert with changes in other steroidogenic enzymes and interpreted in terms of changes in steroid flux in steroidogenic pathways. However, functional readouts of changes in steroid metabolism that may result due to changes in AKR1C expression have not always been performed and have relied on radiochemical assays. Radiochemical assays often use radiochromatographic methods in which product identity is based on retention time but do not provide structural identity. These assays are also limited by the specific radioactivity of the isotopically labeled steroid available and may prevent assays being performed using the most relevant concentrations of steroid found in human serum. These problems can be circumvented with the advent of liquid chromatography–mass spectrometry assays, which have the increased sensitivity and specificity desired. With the application of this approach more progress will be made to relate changes in gene expression to changes in steroid flux. Roles of individual AKR1C isoforms have been further elaborated by transfection studies as models of overexpression, by RNA interference (e.g., small interfering-RNA or short hairpin RNA), and by pharmacological approaches using isoform-specific inhibitors.
Breast cancer
Hormone-dependent breast cancer responds well to ER antagonists (e.g., tamoxifen) and aromatase inhibitors, consistent with estrogen-dependent disease (104, 105). Additionally, the presence of both ER and PR predicts a greater response to hormone ablative therapy than either receptor alone (106). The ratio of ligands for ER and PR is likely controlled by AKR1C expression. Selective loss of AKR1C1 and AKR1C2 was found in 24 paired breast cancer samples as compared with paired normal tissues from the same individuals. The loss of AKR1C1 and AKR1C2 in breast cancer was predicted to result in decreased conversion of progesterone to 20α-hydroxyprogesterone, which, in combination with increased PR expression, may exacerbate progesterone signaling by its nuclear receptors (107). Studies by Lewis et al. (108) in breast cancer cell lines confirmed the loss of AKR1C1 and AKR1C2 and were found to be correlated with elevated SRD5A1 and SRD5A2 expression. It was concluded that these changes in gene expression would elevate 5α-DHP levels, which promotes cell proliferation and detachment (108). Whether this is a cause or consequence of the breast cancer proliferative phenotype is unknown.
AKR1C3 is overexpressed in some but not all breast cancer patients and its overexpression correlates to poor prognosis (109, 110). AKR1C3 was detected in ductal carcinoma in situ by immunohistochemistry in sections of paraffin-embedded mammary gland using a monoclonal antibody where it was found that the cancerous cells were strongly immunoreactive (109, 110). The consequences of AKR1C3 overexpression was modeled in MCF-7 cells. Although MCF-7 cells are AKR1C3-null, MCF7-AKR1C3 stably transfected with AKR1C3 to mimic its overexpression in breast cancer showed rapid conversion of progesterone to 20α-hydroxyprogesterone, reduction of Δ4-androstene-3,17-dione to testosterone and rapid reduction of estrone to 17β-estradiol. These studies suggest that AKR1C3 acts to provide a peripheral source of 17β-hydroxy-C19 steroid substrates for aromatase and a mechanism to generate 17β-estradiol, which is likely important in postmenopausal women who no longer have functional ovaries (26) (Fig. 14).
Figure 14.
Role of AKRs in steroidogenesis in breast cancer. CYP19A1, aromatase; DHEA, dehydroepiandrosterone; HSD3B1, 3β-hydroxysteroid dehydrogenase type 1; HSD17B1, 17β-hydroxysteroid dehydrogenase type 1 (estrogenic 17β-HSD); HSD17B2, 17β-hydroxysteroid dehydrogenase type 2; STS, steroid sulfatase. Italics refer to gene names.
Prostate cancer
AKR1C enzymes are poised to play a pivotal role in androgen biosynthesis in the prostate. AKR1C3 was originally cloned from a human prostate cDNA library (111). It can catalyze the conversion of Δ4-androstene-3,17-dione to testosterone via the canonical pathway to 5α-DHT (27, 112); it also catalyzes the conversion of 5α-androstane-3,17-dione to 5α-DHT by the alternate pathway that bypasses testosterone (111, 113); AKR1C3 also catalyzes the conversion of androsterone to 3α-Adiol, the penultimate step in the backdoor pathway to 5α-DHT (24, 82); and it catalyzes the conversion of dehydroepiandrosterone to Δ5-androstene-3β,17β-diol, an immediate precursor of testosterone. Thus, AKR1C3 is a pivotal enzyme in all pathways to 5α-DHT in the prostate (Fig. 15).
Figure 15.
Role of AKR1C3 in androgen biosynthesis in CRPC. 3α-Adiol, 5α-androstane-3α,17β-diol; 3β-Adiol, 5α-androstane-3β,17β-diol; 5α-adione, 5α-andostane-3,17-dione; Δ5-Adiol, 5-androstene-3β,17β-diol; CYP17A1, 17α-hydroxylase/17,20-lyase; HSD3B1, 3β-hydroxysteroid dehydrogenase type 1; HSD17B6, 17β-hydroxysteroid dehydrogenase type 6 (also known as RODH); SRD5A, steroid 5α-reductase, type 1 and type 2. Reactions in the box occur in the prostate tumor. Italics refer to gene names.
The pathway to 5α-DHT in the prostate that predominates may vary by prostate cancer cell line and by tissue biopsy due to the heterogeneity of the tumor. Irrespective of the pathway used, AKR1C3 is poised to play a central role in all of them. Additionally, AKR1C2 inactivates 5α-DHT to yield 3α-Adiol, which has no affinity for AR, and AKR1C1 converts 5α-DHT to 3β-Adiol, a proapoptotic ligand for ERβ (114). Based on these considerations, there are a large number of reports describing the expression of AKR1C1, AKR1C2, and AKR1C3 in prostate cancer.
AKR1C expression has been measured in normal and diseased prostate. Normal prostate epithelial cells (n = 14) had higher levels of AKR1C1 (10-fold, P < 0.001), AKR1C2 (115-fold, P < 0.001), and AKR1C3 (6-fold, P < 0.001) transcripts than normal prostate stromal cells (n = 15), suggesting that reductive androgen metabolism may predominate in epithelial cells. In contrast, normal prostate stromal cells had higher levels of AR (8-fold, P < 0.001) and HSD17B6 (21-fold, P < 0.001) than normal prostate epithelial cells, suggesting that 3α-Adiol is converted to 5α-DHT by the backdoor pathway to activate AR in these cells (100).
In prostate cancer, selective loss of AKR1C1 and AKR1C2 expression was observed in tumors vs paired benign tissues and was correlated with decreased metabolism of 5α-DHT (115). After 4 h of incubation with benign tissue samples, [3H]-5α-DHT was predominantly catabolized to 3α-Adiol; however, reduced capacity to metabolize 5α-DHT was observed in tumor samples from four of five freshly isolated pairs of tissue samples (116), which paralleled loss of AKR1C1 and AKR1C2 expression. It was concluded that levels of 5α-DHT in prostate cancer may be elevated due to AKR1C2 loss.
AKR1C3 expression has been measured by immunohistochemistry in sections of paraffin-embedded prostate (117). In normal prostate, immunoreactivity was limited to stromal cells with only faint staining in epithelial cells. In adenocarcinoma of the prostate, elevated staining was observed in the endothelial cells and carcinoma cells (117). Subsequently, AKR1C3 was determined to be the most highly overexpressed steroidogenic gene in castration-resistant prostate cancer (CRPC) in both the tumor and soft tissue metastasis. Expression was confirmed by microarray, real-time quantitative PCR, and immunohistochemistry (118). CRPC is the fatal form of prostate cancer and remains androgen-dependent despite castrate levels of circulating androgens. One mechanism for castration resistance is the intracrine formation of potent androgens mediated by AKR1C3 (119, 120).
AKR1C3 overexpression is an adaptive response to low serum androgens so that the tumor can make its own androgens, and this is seen in prostate cancer cells cultured in androgen-deprived media, in prostate cancer cell xenografts grown in castrate mice, and in CRPC patients (121–123). AKR1C3 is repressed by AR and AR agonists, but this repression can be surmounted by the expression of the fusion protein TMPRSS-ERG, which appears in late stage disease as determined by high Gleason grade. In the proposed model, TMPRSS-ERG displaces AR from the AKR1C3 promoter to induce AKR1C3 expression. Androgens made by AKR1C3 can further induce TMPRSS-ERG expression providing a feed-forward model for AKR1C3 expression and enhanced intratumoral androgen biosynthesis (124).
AKR1C3 has been examined as a promising biomarker for prostate cancer progression where its high expression was measured by immunohistochemistry in 60 human prostate needle biopsies and 10 LNCaP xenografts grown in castrate male mice. Positive correlations were found between Gleason grade and AKR1C3 expression and in the xenografts of castrated mice (125). In another study, high AKR1C3 levels were observed in a subset of CRPC patients and were found useful as a biomarker for active intratumoral steroidogenesis in biopsy or transurethral resection of prostate specimens (121). Gene expression profiling in 20 normal prostate tissue samples, 127 primary prostate carcinomas, and 19 metastatic prostate cancer specimens followed by real-time quantitative PCR showed high expression of AKR1C3 in the metastatic prostate cancer specimens and in circulating tumor cells, further validating its biomarker potential (123). Collectively, these observations support a critical role for AKR1C3 in intratumoral androgen biosynthesis in CRPC that could be exploited therapeutically.
Endometrial cancer and endometriosis
Endometrial cancer is a disease caused by exposure to unopposed estrogens. AKR1C3 may play a role in this disease due to its peripheral formation of testosterone (an aromatase substrate); its ability to convert estrone to 17β-estradiol; and by its ability to inactive progesterone by converting it to 20α-hydroxyprogesterone or further metabolize 5α-DHP. The expression of AKR1C1 and AKR1C3 was measured in 16 paired specimens of endometrial cancer and adjacent normal endometrium (9). Quantification by isoform-specific real-time PCR revealed higher expression of AKR1C1 in nine specimens and higher expression of AKR1C3 in four specimens of endometrial cancer, respectively. Importantly, upregulation of both enzymes in the same specimen was observed. Because AKR1C1 inactivates progesterone, its elevated expression in diseased endometrium may contribute to diminished protection by progesterone, whereas elevated expression of AKR1C3, which forms 17β-estradiol in vivo, may contribute to the enhanced estrogen action. It is suggested that the expression of AKR1C1 and AKR1C3 in endometrial cancer will govern the progesterone/17β-estradiol ratio (9). These studies were extended into endometrial cancer cells. In Ishikawa and HEC-1A cells, expression of AKR1C2 was 110-fold and 6800-fold greater, respectively, than the expression of AKR1C1, which suggests that 20-ketosteroid reduction of 5α-pregnanes and 4-pregnenes is catalyzed mainly by the residual 20-ketosteroid reductase activity of AKR1C2. AKR1C1/AKR1C2 gene silencing showed decreased progesterone metabolism in both endometrial cell lines, further supporting the significant role of AKR1C2. Silencing of SRD5A1 had the most pronounced effects, leading to a decreased rate of progesterone metabolism, and consequently higher concentrations of unmetabolized progesterone. These data show that in model cell lines of endometrial cancer, AKR1C2 and SRD5A1 play crucial roles in progesterone metabolism, and their inhibition may represent a novel therapeutic approach for endometrial cancer treatment (126).
Endometriosis is characterized by endometrial tissue growth outside the uterine cavity. The estimated prevalence in the general population is 6% to 10% but can reach 30% to 50% in women who are infertile and is characterized by increased 17β-estradiol synthesis and dysregulated progesterone signaling. Levels of PRs A and B and progesterone metabolizing enzymes were examined in 31 specimens of ovarian endometriosis and 28 specimens of normal endometrium (127). Real-time PCR analysis revealed significantly decreased mRNA levels of PRs A and B, HSD17B2, and SRD5A2, significantly increased mRNA levels of AKR1C1, AKR1C2, AKR1C3 and SRD5A1, and negligible mRNA levels of AKR1D1. Immunohistochemistry staining of endometriotic tissue compared with control endometrium showed significantly lower PR B levels in epithelial cells and no significant differences in stromal cells, there were no significant differences in the expression of AKR1C3, and significantly higher AKR1C2 levels were seen only in stromal cells. These expression data suggest that in endometriosis there is enhanced metabolism of progesterone by SRD5A1 and by the 20α-HSD and 3α/β-HSD activities of AKR1C1, AKR1C2, and AKR1C3 (127).
PCOS
PCOS is a metabolic syndrome that affects 6% to 19% of women of reproductive age depending on diagnostic criteria. PCOS is associated with androgen excess (AE), oligo-ovulatory/anovultaory inferttility and a high incidence of insulin resistance and dyslipidemia. PCOS was originally thought to be a disorder of ovarian origin but it is now recognized that there are contributions from the adrenal and peripheral tissues leading to AE. Because the efficient androgenic 17β-HSD type 3 is expressed only in the Leydig cells of the testis, AKR1C3 is likely required for the production of all active endogenous androgens in women, including those with PCOS and other AE conditions. Early studies examined the expression of AKR1C3 in theca cells of patients with PCOS vs normal theca cells. In these studies increased androgen production was ascribed to the upregulation of CYP17A1, and 3β-HSD but not AKR1C3 (128). One explanation is that AKR1C3 is necessary for ovarian androgen synthesis but is not rate limiting. Similarly, the adrenal gland produces only small amounts of testosterone but large amounts of dehydroepiandrosterone sulfate, as well as other C19 androgen precursors, particularly in a subset of women with PCOS. AKR1C3 is expressed in the adrenal zona reticularis (129), which accounts for the biosynthesis of testosterone, Δ5-androstene-3β,17β-diol and its sulfate, and other active androgens directly from the adrenal (130).
AKR1C3 is also the only 17β-HSD found in adipose tissue that can contribute to AE in subcutaneous fat. Women with PCOS were found to have increased intra-adipose concentrations of testosterone (P = 0.0006) and 5α-DHT (P = 0.01), which correlated with increased expression of AKR1C3 (P = 0.04) in subcutaneous adipose tissue. After acute dehydroepinandrosterone exposure in vivo, microdialysis revealed suppression of lipolysis and glycerol levels in subcutaneous adipose tissue. Using primary subcutaneous adipocytes, AKR1C3 upregulation was observed with insulin treatment and led to an increase in testosterone production and de novo lipogenesis. Mirroring this trend, nontargeted serum metabolomics revealed prolipogenic effects of androgens in women with PCOS (131, 132). The in vitro effects of AKR1C3 observed in primary adipocytes could be reversed by a selective AKR1C3 inhibitor, 3(4-trifluoromethyl)phenylamino benzoic acid (132, 133). The insulin resistance observed in PCOS leads to increased insulin production, provides a feed-forward mechanism to upregulate AKR1C3, and causes AE and promotes a lipotoxic lipidome, which can be reversed by pharmacologic AKR1C3 inhibition.
In addition to the traditional androgen testosterone, the adrenal also contains CYP11B1 (11β-hydroxylase), for which both Δ4-androstene-3,17-dione and testosterone are excellent substrates. In adrenal vein serum samples, the third most abundant steroid behind dehydroepiandrosterone sulfate and cortisol is 11β-hydroxy-Δ4-androstene-3,17-dione (134), demonstrating that these 11-oxyandrogens are significant adrenal products. The adrenal also produces some 11β-hydroxytestosterone and 11-ketotestosterone via AKR1C3, but most of these active 11-oxyandrogens are also produced in peripheral tissues, by AKR1C3. Data are emerging that 11-oxo-androgens of adrenal origin (primarily 11β-hydroxy-Δ4-androstene-3,17-dione and 11-ketotestosterone) are among the most prominent androgens formed in women with PCOS (135) and even more so in patients with classic 21-hydroxylase deficiency, a more severe form of adrenal-derived AE (136, 137). Both 11-ketotestosterone and 11-keto-DHT are potent agonists of the AR in reporter gene assays, and similar to testosterone and 5α-DHT, their formation may be dependent on AKR1C3 (138) (Fig. 16).
Figure 16.
Role of AKR1C3 in PCOS. (a) Schematic representation of the proposed mechanistic link between AE, insulin resistance, and lipotoxicity in PCOS, and (b) graphical representation of the major human androgen biosynthesis pathways. AKR1C3 plays a central gatekeeping role in androgen activation in the classic androgen synthesis pathway and the alternative (backdoor) pathway to 5α-DHT and the 11-oxygenated androgen synthesis pathway. Active androgens capable of activating the androgen receptor are highlighted in blue boxes and white font. [Reproduced with permission from O'Reilly MW, Kempegowda P, Jenkinson C, et al. 11-Oxygenated C19 steroids are the predominant androgens in polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102:840–848.]
Central nervous system, anxiety, and depression
Allopregnanolone is a product of the 3-ketosteroid reduction of 5α-DHP catalyzed by AKR1C2. The steroid acts as a positive modulator of the GABAa receptor (139) and as such has anxiolytic, anticonvulsant, and anesthetic properties. The decline in allopregnanolone levels at the end of menstrual cycle and at postpartum has been associated with premenstrual syndrome and postpartum depression, respectively (140, 141). AKR1C2 has the correct regional distribution in the central nervous system to be the enzyme responsible for the formation of allopregnanolone in the central nervous system (99). The selective serotonin uptake inhibitor fluoxetine has proved beneficial in the treatment of premenstrual syndrome, and it was proposed that selective serotonin uptake inhibitors allosterically modulate AKR1C2 activity to decrease the Km for 5α-DHP and increase allopregnanolone production (99). Follow-up studies with recombinant AKR1C2 failed to confirm the direct allosteric modulation of the enzyme by fluoxetine (55, 142). Despite these data, allopregnanolone analogs are being developed by Sage Pharmaceuticals as neurosteroids (143).
AKR Inhibitors as Chemical Probes and Therapeutics
Inhibitors of human steroid-transforming AKR enzymes are sought both as chemical probes to distinguish their roles in pathophysiology from other steroidogenic enzymes as well as potential therapeutics. One potential challenge is AKR isoform specificity because AKR1C1 to AKR1C4 exhibit 86% sequence identity, and because AKR1C1 and AKR1C2 differ by only one amino acid at the active site. Additionally, different patterns of enzyme inhibition are possible based on the kinetic mechanism (see Fig. 10). First, in the direction of ketosteroid reduction, both E⋅NADPH⋅I and E⋅NADP+⋅I complexes can form, giving rise to either competitive or uncompetitive inhibition patterns, respectively, when steroid substrate is varied. Second, formation of the E⋅NADP+⋅I complex can be favored when the substrates have high turnover number (56). Third, using the same inhibitor in the reduction and oxidation directions, the formation of the E⋅NADPH⋅I and E⋅NADP+⋅I complexes, respectively, will give rise to competitive inhibition patterns. Because these two complexes are different, the 50% inhibitory concentration values and Ki values will not be equivalent when inhibition screens are conducted in the reduction and oxidation directions. Often the E⋅NADP+⋅I complex gives a tighter binding complex and lower Ki enzyme-inhibitor dissociation constant than in the E⋅NADPH⋅I complex. It is important to consider these differences in inhibitor screens.
Despite these challenges a number of isoform-specific inhibitors as well as pan-AKR1C inhibitors exist. Pan-AKR1C inhibitors include 6-medroxyprogesterone acetate as well as N-phenylanthranilates represented by the nonsteroidal anti-inflammatory drugs (NSAIDs) meclofenamic and flufenamic acid (56). This in turn led to a screen of the major classes of NSAIDs for AKR1C isoform specificity. This screen identified salicylates as potent inhibitors of AKR1C1 and AKR1C2 but not AKR1C3, aryl propionic acids (e.g., S-naproxen) as potent inhibitors of AKR1C2 and AKR1C3 but not AKR1C1, and indole acetic acids represented by indomethacin as potent and selective inhibitors of AKR1C3 (56).
AKR1C1 inhibitors
Given the dominant 20-ketosteroid reductase activity of this enzyme, inhibitors of this enzyme could be beneficial in maintaining progesterone levels. These inhibitors could be used alone or in combination with synthetic progestins for the treatment of endometriosis and dysmenorrhea, to prevent threatened abortion or miscarriage, or premenstrual syndrome. In the latter instance, elevation of progesterone could lead to increased 5α-DHP levels and eventually higher levels of allopregnanolone that could bind to the GABA receptor to have anxiolytic properties (139, 144, 145). Based on the potent inhibition of AKR1C1 by salicylates, salicylate analogs [e.g., 3-chloro-5-phenylsalicylic acid (Ki = 0.86 nM) (146), 3-bromo-5-phenylsalicylate, and 3,5-dichlorosalicylate] have been developed that are selective for this enzyme and crystal structures of the enzyme–inhibitor complexes reported (147).
“A number of isoform-specific inhibitors as well as pan-AKR1C inhibitors exist.”
AKR1C2 inhibitors
Given the central role of AKR1C2 in eliminating 5α-DHT, inhibitors of this enzyme could be used to treat androgen insufficiency syndromes. AKR1C2 was originally discovered as bile acid–binding protein and has nanomolar affinity for secondary bile acids, for example, ursodeoxycholate (48, 148).
AKR1C3 inhibitors
Given the central role of AKR1C3 in the production of testosterone and 5α-DHT in peripheral tissues, inhibitors of this enzyme could have multiple uses. For example, they could be of benefit in men with CRPC, but in women they could be used for the treatment of breast and endometrial cancer to prevent the formation of 17β-hydroxy-C19 substrates for aromatase, and they could be used for the treatment of PCOS. AKR1C3 inhibitors may also be widely used in other syndromes of AE. NSAIDs have been repurposed with these goals in mind based on N-phenylaminobenzoates (133), N-naphthylaminobenzoates (149), indole acetic acids (150), as well as aryl-propionic acids (151). The repurposed compounds are specific for the inhibition of AKR1C3, they have nanomolar affinity for the target, they do not inhibit COX-1 or COX-2, and they block the production of testosterone in LNCaP-AKR1C3 cells. Crystal structures of AKR1C3 ternary complexes containing NSAIDs and these repurposed compounds have been reported (149, 150). It is apparent that their specificity for AKR1C3 over AKR1C1 and AKR1C2 exists because of the occupancy of subpockets SP1, SP2, and SP3 that are either unavailable in AKR1C1 or AKR1C2 or the residues in these pockets cause steric hindrance with the drug (152).
Additionally, the two new drugs for the treatment of CRPC abiraterone acetate (153–155) and enzalutamide (156, 157) are associated with the emergence of drug resistance and a component of that drug resistance is overexpression of AKR1C3. When modeled in murine xenografts of human prostate cancer cells, this drug resistance can be surmounted by indomethacin (119, 120). Because of these findings and the broad appeal of AKR1C3 as a therapeutic target, there has been further intense interest in AKR1C3 inhibitors as potential therapeutics from the pharmaceutical industry. Some noteworthy compounds are ASP9521 (158, 159), GTx-560 (160), and BMT4-158 (149).
ASP9521 is a potent competitive inhibitor of AKR1C3 developed by Astellas that was taken through preclinical development and into a Phase 1/11b clinical trial for CRPC patients. The drug proved to be well tolerated but without efficacy, with the clinical endpoint being decreased serum prostate-specific antigen and progression-free survival. Unfortunately, 6 of 13 patients withdrew from the trial and patients were never screened at biopsy for overexpression of the target before trial entry (159).
GTx-560 was developed as a competitive inhibitor of AKR1C3 and was effective in xenografts of prostate cancer. This compound also revealed that AKR1C3 unexpectedly functioned as a coactivator protein of the AR and this compound blocked this function. The coactivator domain of AKR1C3 was located to a distinct region of the protein that was separate from the cofactor and steroid binding site and active site residues. The importance of this coactivator function in CRPC remains to be determined (160).
BMT4-158 is an N-naphthylaminobenzoate that was developed as a competitive inhibitor of AKR1C3 but in addition was found to act as an AR antagonist and AR degrader (149). Many of the AKR1C3 inhibitors are covered by patents, and a recent patent review further elaborates their status (161).
AKR1C4 inhibitors
Because of the central role of AKR1C4 in the hepatic metabolism of steroids and its role in bile acid biosynthesis, inhibition of this enzyme should be avoided, despite its possible involvement in the backdoor pathway to 5α-DHT. It is noteworthy that phenolphthalein is a potent AKR1C4 selective inhibitor that can be used as a chemical probe (162).
AKR1D1 inhibitors
AKR1D1 is essential for bile acid biosynthesis, and elimination of this activity leads to bile acid deficiency and loss or ligands for FXR. Therefore, its inhibition under normal physiologic conditions should be avoided. Additionally, 5β-dihydrosteroids produced by AKR1D1 have other benefits. 5β-Androstanes enhance growth and survival of colony forming unit–erythroid and induce heme biosynthesis and can be considered as an alternative in stimulating erythropoiesis (163, 164), and 5β-pregnanes are potent tocolytic agents and may be responsible for uterine quiescence maintained by progesterone in pregnancy (165, 166).
Conclusions and Future Directions
The structure–function relationships in human steroid-transforming AKR1 enzymes have been thoroughly studied in terms of X-ray crystal structures, site-directed mutagenesis, and steady-state and transient kinetics. These studies reveal that different binding poses exist for steroid substrates and inhibitors, making it difficult to predict modes of ligand binding for rational drug design. The triose phosphate isomerase barrel structure for these enzymes also contains three large loops at the back of the barrel, and comparison of apoenzyme, binary, and ternary complexes shows that loop movement occurs and therefore more will be learned from solution structures using nuclear magnetic resonance approaches.
At the genomic level, functional analysis of many of the more common nsSNPs still needs to be explored because differences in enzyme activity cannot be predicted by mapping amino acid changes to crystal structures. Less is known about how AKR1 genes are regulated in terms of transcription factors, signaling pathways, and epigenetic regulation. This becomes an important topic as both the role of the AKR enzymes in pathophysiology evolves further and our understanding of the biological properties of steroid metabolites continues to reveal surprises. The role of AKR enzymes in physiology/pathophysiology can also be elaborated with targeted gene disruption/gene editing (e.g., CRISPR/Cas9). The case can be made for the development of AKR1C1, AKR1C2, and AKR1C3 inhibitors to treat hormone-dependent malignancies and endocrine disorders. However, many require preclinical development and likely optimization.
Acknowledgments
Financial Support: This work was supported by National Institute of Environmental Health Sciences Grant P30-ES013508 to T.M.P. and by National Institute of General Medical Sciences Grant R01GM086596 to R.J.A.
Disclosure Summary: T.M.P. is founder and Director of Penzymes, LLC, has been a recipient of a sponsored research agreement from Forendo, has conducted fee-for-service work for Constellation, and performs consultant work for Sage and Tokai Pharmaceuticals and the Research Institute for Fragrance Materials. T.M.P. is an inventor on US patents 9271961-B2 and 9346803-B2. The remaining authors have nothing to declare.
Glossary
Abbreviations
- AE
androgen excess
- AKR
aldo-keto reductase
- AR
androgen receptor
- ARE
antioxidant response element
- CRPC
castration-resistant prostate cancer
- DHP
dihydroprogesterone
- ER
estrogen receptor
- FXR
farnesoid X receptor
- HSD
hydroxysteroid dehydrogenase
- Kd
dissociation rate constant
- kcat
turnover number
- Keq
equilibrium constant
- Ki
enzyme-inhibitor dissociation constant
- Km
Michaelis-Menton constant
- MAF
minor allelic frequency
- NADP
NAD phosphate
- NADPH
reduced form of NAD phosphate
- NCBI
National Center for Biotechnology Information
- NSAID
nonsteroidal anti-inflammatory drug
- nsSNP
nonsynonymous single nucleotide polymorphism
- PCOS
polycystic ovarian syndrome
- PDB
Protein Data Bank
- PR
progesterone receptor
- RNAseq
RNA sequencing
- SDR
short-chain dehydrogenase/reductase
- SNP
single nucleotide polymorphism
- Vmax
maximum velocity
References
- 1. Funder JW, Pearce PT, Smith R, Smith AI. Mineralocorticoid action: target tissue specificity is enzyme, not receptor, mediated. Science. 1988;242(4878):583–585. [DOI] [PubMed] [Google Scholar]
- 2. Penning TM. Hydroxysteroid dehydrogenases and pre-receptor regulation of steroid hormone action. Hum Reprod Update. 2003;9(3):193–205. [DOI] [PubMed] [Google Scholar]
- 3. Penning TM, Jin Y, Rizner TL, Bauman DR. Pre-receptor regulation of the androgen receptor. Mol Cell Endocrinol. 2008;281(1–2):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Labrie F. Intracrinology. Mol Cell Endocrinol. 1991;78:C113–C118. [DOI] [PubMed] [Google Scholar]
- 5. Penning TM, Bauman DR, Jin Y, Rizner TL. Identification of the molecular switch that regulates access of 5α-DHT to the androgen receptor. Mol Cell Endocrinol. 2007;265-266:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Draper N, Stewart P.. 11β-Hydroxysteroid dehydrogenase and the pre-receptor regulation of corticosteroid hormone action. J Endcorinol. 2005;186(2):251–271. [DOI] [PubMed] [Google Scholar]
- 7. Whorwood CB, Franklyn JA, Sheppard MC, Stewart PM. Tissue localization of 11β-hydroxysteroid dehydrogenase and its relationship to the glucocorticoid receptor. J Steroid Biochem Mol Biol. 1992;41(1):21–28. [DOI] [PubMed] [Google Scholar]
- 8. Labrie F, Luu-The V, Lin SX, Simard J, Labrie C, El-Alfy M, Pelletier G, Bélanger A. Intracrinology: role of the family of 17 beta-hydroxysteroid dehydrogenases in human physiology and disease. J Mol Endocrinol. 2000;25(1):1–16. [DOI] [PubMed] [Google Scholar]
- 9. Rižner TL, Smuc T, Rupreht R, Sinkovec J, Penning TM. AKR1C1 and AKR1C3 may determine progesterone and estrogen ratios in endometrial cancer. Mol Cell Endocrinol. 2006;248(1–2):126–135. [DOI] [PubMed] [Google Scholar]
- 10. Jörnvall H, Persson B, Krook M, Atrian S, Gonzàlez-Duarte R, Jeffery J, Ghosh D. Short-chain dehydrogenases/reductases (SDR). Biochemistry. 1995;34(18):6003–6013. [DOI] [PubMed] [Google Scholar]
- 11. Kallberg Y, Oppermann U, Jörnvall H, Persson B. Short-chain dehydrogenases/reductases (SDRs). Eur J Biochem. 2002;269(18):4409–4417. [DOI] [PubMed] [Google Scholar]
- 12. Jin Y, Penning TM. Aldo-keto reductases and bioactivation/detoxication. Annu Rev Pharmacol Toxicol. 2007;47(1):263–292. [DOI] [PubMed] [Google Scholar]
- 13. Penning TM, Drury JE. Human aldo-keto reductases: function, gene regulation, and single nucleotide polymorphisms. Arch Biochem Biophys. 2007;464(2):241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lukacik P, Kavanagh KL, Oppermann U. Structure and function of human 17β-hydroxysteroid dehydrogenases. Mol Cell Endocrinol. 2006;248(1–2):61–71. [DOI] [PubMed] [Google Scholar]
- 15. Moeller G, Adamski J. Integrated view on 17β-hydroxysteroid dehydrogenases. Mol Cell Endocrinol. 2009;301(1–2):7–19. [DOI] [PubMed] [Google Scholar]
- 16. Bauman DR, Steckelbroeck S, Williams MV, Peehl DM, Penning TM. Identification of the major oxidative 3α-hydroxysteroid dehydrogenase in human prostate that converts 5α-andostane-3α,17β-diol to 5α-dihydrotestosterone: a potential therapeutic target for androgen dependent disease. Mol Endocrinol. 2006;20(2):444–458. [DOI] [PubMed] [Google Scholar]
- 17. Biswas MG, Russell DW. Expression cloning and characterization of oxidative 17β- and 3α-hydroxysteroid dehydrogenases from rat and human prostate. J Biol Chem. 1997;272(25):15959–15966. [DOI] [PubMed] [Google Scholar]
- 18. Kavanagh KL, Jörnvall H, Persson B, Oppermann U. Medium- and short-chain dehydrogenase/reductase gene and protein families. The SDR superfamily: functional and structural diversity within a family of metabolic and regulatory enzymes. Cell Mol Life Sci. 2008;65(24):3895–3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bhatia C, Oerum S, Bray J, Kavanagh KL, Shafqat N, Yue W, Oppermann U. Towards a systematic analysis of human short-chain dehydrogenases/reductases (SDR): ligand identification and structure-activity relationships. Chem Biol Interact. 2015;234:114–125. [DOI] [PubMed] [Google Scholar]
- 20. Persson B, Kallberg Y, Bray JE, Bruford E, Dellaporta SL, Favia AD, Duarte RG, Jörnvall H, Kavanagh KL, Kedishvili N, Kisiela M, Maser E, Mindnich R, Orchard S, Penning TM, Thornton JM, Adamski J, Oppermann U. The SDR (short-chain dehydrogenase/reductase and related enzymes) nomenclature initiative. Chem Biol Interact. 2009;178(1–3):94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jez JM, Bennett MJ, Schlegel BP, Lewis M, Penning TM. Comparative anatomy of the aldo-keto reductase superfamily. Biochem J. 1997;326(Pt 3):625–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hyndman D, Bauman DR, Heredia VV, Penning TM. The aldo-keto reductase superfamily homepage. Chem Biol Interact. 2003;143–144:621–631. [DOI] [PubMed] [Google Scholar]
- 23. Bennett MJ, Schlegel BP, Jez JM, Penning TM, Lewis M. Structure of 3α-hydroxysteroid/dihydrodiol dehydrogenase complexed with NADP+. Biochemistry. 1996;35(33):10702–10711. [DOI] [PubMed] [Google Scholar]
- 24. Penning TM, Burczynski ME, Jez JM, Hung C-F, Lin H-K, Ma H, Moore M, Palackal N, Ratnam K. Human 3α-hydroxysteroid dehydrogenase isoforms (AKR1C1–AKR1C4) of the aldo-keto reductase superfamily: functional plasticity and tissue distribution reveals roles in the inactivation and formation of male and female sex hormones. Biochem J. 2000;351(Pt 1):67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Steckelbroeck S, Jin Y, Gopishetty S, Oyesanmi B, Penning TM. Human cytosolic 3α-hydroxysteroid dehydrogenases of the aldo-keto reductase superfamily display significant 3β-hydroxysteroid dehydrogenase activity: implications for steroid hormone metabolism and action. J Biol Chem. 2004;279(11):10784–10795. [DOI] [PubMed] [Google Scholar]
- 26. Byrns MC, Duan L, Lee S-H, Blair IA, Penning TM. Aldo-keto reductase 1C3 expression in MCF-7 cells reveals roles in steroid hormone and prostaglandin metabolism that may explain its over-expression in breast cancer. J Steroid Biochem Mol Biol. 2010;118(3):177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Byrns MC, Mindnich R, Duan L, Penning TM. Overexpression of aldo-keto reductase 1C3 (AKR1C3) in LNCaP cells diverts androgen metabolism towards testosterone resulting in resistance to the 5α-reductase inhibitor finasteride. J Steroid Biochem Mol Biol. 2012;130(1–2):7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rižner TL, Lin HK, Peehl DM, Steckelbroeck S, Bauman DR, Penning TM. Human type 3 3α-hydroxysteroid dehydrogenase (aldo-keto reductase 1C2) and androgen metabolism in prostate cells. Endocrinology. 2003;144(7):2922–2932. [DOI] [PubMed] [Google Scholar]
- 29. Rižner TL, Penning TM. Role of aldo–keto reductase family 1 (AKR1) enzymes in human steroid metabolism. Steroids. 2014;79:49–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kondo KH, Kai MH, Setoguchi Y, Eggertsen G, Sjöblom P, Setoguchi T, Okuda KI, Björkhem I. Cloning and expression of cDNA of human Δ4-3-oxosteroid 5β-reductase and substrate specificity of the expressed enzyme. Eur J Biochem. 1994;219(1–2):357–363. [DOI] [PubMed] [Google Scholar]
- 31. Jez JM, Flynn TG, Penning TM. A new nomenclature for the aldo-keto reductase superfamily. Biochem Pharmacol. 1997;54(6):639–647. [DOI] [PubMed] [Google Scholar]
- 32. Veliça P, Davies NJ, Rocha PP, Schrewe H, Ride JP, Bunce CM. Lack of functional and expression homology between human and mouse aldo-keto reductase 1C enzymes: implications for modelling human cancers. Mol Cancer. 2009;8(1):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Weber S, Salabei JK, Möller G, Kremmer E, Bhatnagar A, Adamski J, Barski OA. Aldo-keto reductase 1B15 (AKR1B15): a mitochondrial human aldo-keto reductase with activity toward steroids and 3-keto-acyl-CoA conjugates. J Biol Chem. 2015;290(10):6531–6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jin Y, Mesaros AC, Blair IA, Penning TM. Stereospecific reduction of 5β-reduced steroids by human ketosteroid reductases of the AKR (aldo-keto reductase) superfamily: role of AKR1C1–AKR1C4 in the metabolism of testosterone and progesterone via the 5β-reductase pathway. Biochem J. 2011;437(1):53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 2003;72(1):137–174. [DOI] [PubMed] [Google Scholar]
- 36. Sharma KK, Lindqvist A, Zhou XJ, Auchus RJ, Penning TM, Andersson S. Deoxycorticosterone inactivation by AKR1C3 in human mineralocorticoid target tissues. Mol Cell Endocrinol. 2006;248(1–2):79–86. [DOI] [PubMed] [Google Scholar]
- 37. Chen M, Drury JE, Penning TM. Substrate specificity and inhibitor analyses of human steroid 5β-reductase (AKR1D1). Steroids. 2011;76(5):484–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tomkins GM. A mammalian 3α-hydroxysteroid dehydrogenase. J Biol Chem. 1956;218(1):437–447. [PubMed] [Google Scholar]
- 39. Barski OA, Tipparaju SM, Bhatnagar A. The aldo-keto reductase superfamily and its role in drug metabolism and detoxification. Drug Metab Rev. 2008;40(4):553–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tebay LE, Robertson H, Durant ST, Vitale SR, Penning TM, Dinkova-Kostova AT, Hayes JD. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic Biol Med. 2015;88(Pt B):108–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ratnam K, Ma H, Penning TM. The arginine 276 anchor for NADP(H) dictates fluorescence kinetic transients in 3α-hydroxysteroid dehydrogenase, a representative aldo-keto reductase. Biochemistry. 1999;38(24):7856–7864. [DOI] [PubMed] [Google Scholar]
- 42. Borhani DW, Harter TM, Petrash JM. The crystal structure of the aldose reductase⋅NADPH binary complex. J Biol Chem. 1992;267(34):24841–24847. [DOI] [PubMed] [Google Scholar]
- 43. Ma H, Penning TM. Conversion of mammalian 3α-hydroxysteroid dehydrogenase to 20α-hydroxysteroid dehydrogenase using loop chimeras: changing specificity from androgens to progestins. Proc Natl Acad Sci USA. 1999;96(20):11161–11166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hara A, Matsuura K, Tamada Y, Sato K, Miyabe Y, Deyashiki Y, Ishida N. Relationship of human liver dihydrodiol dehydrogenases to hepatic bile-acid-binding protein and an oxidoreductase of human colon cells. Biochem J. 1996;313(Pt 2):373–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Matsuura K, Deyashiki Y, Sato K, Ishida N, Miwa G, Hara A. Identification of amino acid residues responsible for differences in substrate specificity and inhibitor sensitivity between two human liver dihydrodiol dehydrogenase isoenzymes by site-directed mutagenesis. Biochem J. 1997;323(Pt 1):61–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang B, Zhu DW, Hu XJ, Zhou M, Shang P, Lin SX. Human 3-alpha hydroxysteroid dehydrogenase type 3 (3α-HSD3): the V54L mutation restricting the steroid alternative binding and enhancing the 20α-HSD activity. J Steroid Biochem Mol Biol. 2014;141:135–143. [DOI] [PubMed] [Google Scholar]
- 47. Couture J-F, Legrand P, Cantin L, Luu-The V, Labrie F, Breton R. Human 20α-hydroxysteroid dehydrogenase: crystallographic and site-directed mutagenesis studies lead to the identification of an alternative binding site for C21-steroids. J Mol Biol. 2003;331(3):593–604. [DOI] [PubMed] [Google Scholar]
- 48. Jin Y, Stayrook SE, Albert RH, Palackal NT, Penning TM, Lewis M. Crystal structure of human type III 3α-hydroxysteroid dehydrogenase/bile acid binding protein complexed with NADP+ and ursodeoxycholate. Biochemistry. 2001;40(34):10161–10168. [DOI] [PubMed] [Google Scholar]
- 49. Bennett MJ, Albert RH, Jez JM, Ma H, Penning TM, Lewis M. Steroid recognition and regulation of hormone action: crystal structure of testosterone and NADP+ bound to 3α-hydroxysteroid/dihydrodiol dehydrogenase. Structure. 1997;5(6):799–812. [DOI] [PubMed] [Google Scholar]
- 50. Jin Y, Penning TM. Molecular docking simulations of steroid substrates into human cytosolic hydroxysteroid dehydrogenases (AKR1C1 and AKR1C2): insights into positional and stereochemical preferences. Steroids. 2006;71(5):380–391. [DOI] [PubMed] [Google Scholar]
- 51. Qiu W, Zhou M, Labrie F, Lin SX. Crystal structures of the multispecific 17β-hydroxysteroid dehydrogenase type 5: critical androgen regulation in human peripheral tissues. Mol Endocrinol. 2004;18(7):1798–1807. [DOI] [PubMed] [Google Scholar]
- 52. Cooper WC, Jin Y, Penning TM. Elucidation of a complete kinetic mechanism for a mammalian hydroxysteroid dehydrogenase (HSD) and identification of all enzyme forms on the reaction coordinate: the example of rat liver 3α-HSD (AKR1C9). J Biol Chem. 2007;282(46):33484–33493. [DOI] [PubMed] [Google Scholar]
- 53. Grimshaw CE, Bohren KM, Lai CJ, Gabbay KH. Human aldose reductase: rate constants for a mechanism including interconversion of ternary complexes by recombinant wild-type enzyme. Biochemistry. 1995;34(44):14356–14365. [DOI] [PubMed] [Google Scholar]
- 54. Jin Y, Penning TM. Multiple steps determine the overall rate of the reduction of 5α-dihydrotestosterone catalyzed by human type 3 3α-hydroxysteroid dehydrogenase: implications for the elimination of androgens. Biochemistry. 2006;45(43):13054–13063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Trauger JW, Jiang A, Stearns BA, LoGrasso PV. Kinetics of allopregnanolone formation catalyzed by human 3α-hydroxysteroid dehydrogenase type III (AKR1C2). Biochemistry. 2002;41(45):13451–13459. [DOI] [PubMed] [Google Scholar]
- 56. Byrns MC, Steckelbroeck S, Penning TM. An indomethacin analogue, N-(4-chlorobenzoyl)-melatonin, is a selective inhibitor of aldo-keto reductase 1C3 (type 2 3α-HSD, type 5 17β-HSD, and prostaglandin F synthase), a potential target for the treatment of hormone dependent and hormone independent malignancies. Biochem Pharmacol. 2008;75(2):484–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wilson DK, Bohren KM, Gabbay KH, Quiocho FA. An unlikely sugar substrate site in the 1.65 A structure of the human aldose reductase holoenzyme implicated in diabetic complications. Science. 1992;257(5066):81–84. [DOI] [PubMed] [Google Scholar]
- 58. Kubiseski TJ, Hyndman DJ, Morjana NA, Flynn TG. Studies on pig muscle aldose reductase. Kinetic mechanism and evidence for a slow conformational change upon coenzyme binding. J Biol Chem. 1992;267(10):6510–6517. [PubMed] [Google Scholar]
- 59. Grimshaw CE, Bohren KM, Lai CJ, Gabbay KH. Human aldose reductase: pK of tyrosine 48 reveals the preferred ionization state for catalysis and inhibition. Biochemistry. 1995;34(44):14374–14384. [DOI] [PubMed] [Google Scholar]
- 60. Heredia VV, Cooper WC, Kruger RG, Jin Y, Penning TM. Alanine scanning mutagenesis of the testosterone binding site of rat 3α-hydroxysteroid dehydrogenase demonstrates contact residues influence the rate-determining step. Biochemistry. 2004;43(19):5832–5841. [DOI] [PubMed] [Google Scholar]
- 61. Papari-Zareei M, Brandmaier A, Auchus RJ. Arginine 276 controls the directional preference of AKR1C9 (rat liver 3α-hydroxysteroid dehydrogenase) in human embryonic kidney 293 cells. Endocrinology. 2006;147(4):1591–1597. [DOI] [PubMed] [Google Scholar]
- 62. Schlegel BP, Jez JM, Penning TM. Mutagenesis of 3α-hydroxysteroid dehydrogenase reveals a “push-pull” mechanism for proton transfer in aldo-keto reductases. Biochemistry. 1998;37(10):3538–3548. [DOI] [PubMed] [Google Scholar]
- 63. Di Costanzo L, Drury JE, Penning TM, Christianson DW. Crystal structure of human liver Δ4-3-ketosteroid 5β-reductase (AKR1D1) and implications for substrate binding and catalysis. J Biol Chem. 2008;283(24):16830–16839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Okuda A, Okuda K. Purification and characterization of delta 4-3-ketosteroid 5 β-reductase. J Biol Chem. 1984;259(12):7519–7524. [PubMed] [Google Scholar]
- 65. Berséus O. Conversion of cholesterol to bile acids in rat: purification and properties of a delta-4-3-ketosteroid 5-β-reductase and a 3-α-hydroxysteroid dehydrogenase. Eur J Biochem. 1967;2(4):493–502. [DOI] [PubMed] [Google Scholar]
- 66. Onishi Y, Noshiro M, Shimosato T, Okuda K. Molecular cloning and sequence analysis of cDNA encoding delta 4-3-ketosteroid 5β-reductase of rat liver. FEBS Lett. 1991;283(2):215–218. [DOI] [PubMed] [Google Scholar]
- 67. Norymberski JK, Woods GF. Partial reduction of steroid hormones and related substances. J Chem Soc. 1955;76:3426–3430. [Google Scholar]
- 68. March J. Advanced Organic Chemistry: Reactions, Mechanisms and Structure. New York, NY: John Wiley & Sons; 2001: 5th Edition [Google Scholar]
- 69. Jez JM, Penning TM. Engineering steroid 5β-reductase activity into rat liver 3α-hydroxysteroid dehydrogenase. Biochemistry. 1998;37(27):9695–9703. [DOI] [PubMed] [Google Scholar]
- 70. Di Costanzo L, Drury JE, Christianson DW, Penning TM. Structure and catalytic mechanism of human steroid 5β-reductase (AKR1D1). Mol Cell Endocrinol. 2009;301(1–2):191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Burczynski ME, Sridhar GR, Palackal NT, Penning TM. The reactive oxygen species—and Michael acceptor-inducible human aldo-keto reductase AKR1C1 reduces the α,β-unsaturated aldehyde 4-hydroxy-2-nonenal to 1,4-dihydroxy-2-nonene. J Biol Chem. 2001;276(4):2890–2897. [DOI] [PubMed] [Google Scholar]
- 72. Burczynski ME, Lin HK, Penning TM. Isoform-specific induction of a human aldo-keto reductase by polycyclic aromatic hydrocarbons (PAHs), electrophiles, and oxidative stress: implications for the alternative pathway of PAH activation catalyzed by human dihydrodiol dehydrogenase. Cancer Res. 1999;59(3):607–614. [PubMed] [Google Scholar]
- 73. Agyeman AS, Chaerkady R, Shaw PG, Davidson NE, Visvanathan K, Pandey A, Kensler TW. Transcriptomic and proteomic profiling of KEAP1 disrupted and sulforaphane-treated human breast epithelial cells reveals common expression profiles. Breast Cancer Res Treat. 2012;132(1):175–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Salabei JK, Li XP, Petrash JM, Bhatnagar A, Barski OA. Functional expression of novel human and murine AKR1B genes. Chem Biol Interact. 2011;191(1–3):177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kaupert LC, Gomes LG, Brito VN, Lemos-Marini SH, de Mello MP, Longui CA, Kochi C, de Castro M, Guerra G Jr, Mendonca BB, Bachega TA. A single nucleotide variant in the promoter region of 17β-HSD type 5 gene influences external genitalia virilization in females with 21-hydroxylase deficiency. Horm Res Paediatr. 2016;85(5):333–338. [DOI] [PubMed] [Google Scholar]
- 76. Ju R, Wu W, Fei J, Qin Y, Tang Q, Wu D, Xia Y, Wu J, Wang X. Association analysis between the polymorphisms of HSD17B5 and HSD17B6 and risk of polycystic ovary syndrome in Chinese population. Eur J Endocrinol. 2015;172(3):227–233. [DOI] [PubMed] [Google Scholar]
- 77. Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc. 2009;4(7):1073–1081. [DOI] [PubMed] [Google Scholar]
- 78. Kumar S, Suleski MP, Markov GJ, Lawrence S, Marco A, Filipski AJ. Positional conservation and amino acids shape the correct diagnosis and population frequencies of benign and damaging personal amino acid mutations. Genome Res. 2009;19(9):1562–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Tyler KM, Wagner GK, Wu Q, Huber KT. Functional significance may underlie the taxonomic utility of single amino acid substitutions in conserved proteins. J Mol Evol. 2010;70(4):395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Yandell M, Moore B, Salas F, Mungall C, MacBride A, White C, Reese MG. Genome-wide analysis of human disease alleles reveals that their locations are correlated in paralogous proteins. PLOS Comput Biol. 2008;4(11):e1000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Takahashi RH, Grigliatti TA, Reid RE, Riggs KW. The effect of allelic variation in aldo-keto reductase 1C2 on the in vitro metabolism of dihydrotestosterone. J Pharmacol Exp Ther. 2009;329(3):1032–1039. [DOI] [PubMed] [Google Scholar]
- 82. Platt A, Xia Z, Liu Y, Chen G, Lazarus P. Impact of nonsynonymous single nucleotide polymorphisms on in-vitro metabolism of exemestane by hepatic cytosolic reductases. Pharmacogenet Genomics. 2016;26(8):370–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Flück CE, Meyer-Böni M, Pandey AV, Kempná P, Miller WL, Schoenle EJ, Biason-Lauber A. Why boys will be boys: two pathways of fetal testicular androgen biosynthesis are needed for male sexual differentiation. Am J Hum Genet. 2011;89(2):201–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Auchus RJ. The backdoor pathway to dihydrotestosterone. Trends Endocrinol Metab. 2004;15(9):432–438. [DOI] [PubMed] [Google Scholar]
- 85. Shaw G, Renfree MB, Leihy MW, Shackleton CH, Roitman E, Wilson JD. Prostate formation in a marsupial is mediated by the testicular androgen 5α-androstane-3α,17β-diol. Proc Natl Acad Sci USA. 2000;97(22):12256–12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wilson JD, Auchus RJ, Leihy MW, Guryev OL, Estabrook RW, Osborn SM, Shaw G, Renfree MB. 5α-Androstane-3α,17β-diol is formed in tammar wallaby pouch young testes by a pathway involving 5α-pregnane-3α,17α-diol-20-one as a key intermediate. Endocrinology. 2003;144(2):575–580. [DOI] [PubMed] [Google Scholar]
- 87. Gonzales E, Cresteil D, Baussan C, Dabadie A, Gerhardt M-F, Jacquemin E. SRD5B1 (AKR1D1) gene analysis in Δ4-3-oxosteroid 5β-reductase deficiency: evidence for primary genetic defect. J Hepatol. 2004;40(4):716–718. [DOI] [PubMed] [Google Scholar]
- 88. Lemonde HA, Custard EJ, Bouquet J, Duran M, Overmars H, Scambler PJ, Clayton PT. Mutations in SRD5B1 (AKR1D1), the gene encoding Δ4-3-oxosteroid 5β-reductase, in hepatitis and liver failure in infancy. Gut. 2003;52(10):1494–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Setchell KD, Suchy FJ, Welsh MB, Zimmer-Nechemias L, Heubi J, Balistreri WF. Delta 4-3-oxosteroid 5 beta-reductase deficiency described in identical twins with neonatal hepatitis. A new inborn error in bile acid synthesis. J Clin Invest. 1988;82(6):2148–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Clayton PT, Mills KA, Johnson AW, Barabino A, Marazzi MG. Delta 4-3-oxosteroid 5 beta-reductase deficiency: failure of ursodeoxycholic acid treatment and response to chenodeoxycholic acid plus cholic acid. Gut. 1996;38(4):623–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sumazaki R, Nakamura N, Shoda J, Kurosawa T, Tohma M. Gene analysis in δ4-3-oxosteroid 5β-reductase deficiency. Lancet. 1997;349(9048):329. [DOI] [PubMed] [Google Scholar]
- 92. Ueki I, Kimura A, Chen HL, Yorifuji T, Mori J, Itoh S, Maruyama K, Ishige T, Takei H, Nittono H, Kurosawa T, Kage M, Matsuishi T. SRD5B1 gene analysis needed for the accurate diagnosis of primary 3-oxo-Δ4-steroid 5β-reductase deficiency. J Gastroenterol Hepatol. 2009;24(5):776–785. [DOI] [PubMed] [Google Scholar]
- 93. Drury JE, Mindnich R, Penning TM. Characterization of disease-related 5β-reductase (AKR1D1) mutations reveals their potential to cause bile acid deficiency. J Biol Chem. 2010;285(32):24529–24537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Mindnich R, Drury JE, Penning TM. The effect of disease associated point mutations on 5β-reductase (AKR1D1) enzyme function. Chem Biol Interact. 2011;191(1–3):250–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Chen M, Jin Y, Penning TM. In-depth dissection of the P133R mutation in steroid 5β-reductase (AKR1D1): a molecular basis of bile acid deficiency. Biochemistry. 2015;54(41):6343–6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Khanna M, Qin K-N, Wang RW, Cheng K-C. Substrate specificity, gene structure, and tissue-specific distribution of multiple human 3α-hydroxysteroid dehydrogenases. J Biol Chem. 1995;270(34):20162–20168. [DOI] [PubMed] [Google Scholar]
- 97. Dufort I, Labrie F, Luu-The V. Human types 1 and 3 3α-hydroxysteroid dehydrogenases: differential lability and tissue distribution. J Clin Endocrinol Metab. 2001;86(2):841–846. [DOI] [PubMed] [Google Scholar]
- 98. Zhang Y, Dufort I, Rheault P, Luu-The V. Characterization of a human 20α-hydroxysteroid dehydrogenase. J Mol Endocrinol. 2000;25(2):221–228. [DOI] [PubMed] [Google Scholar]
- 99. Griffin LD, Mellon SH. Selective serotonin reuptake inhibitors directly alter activity of neurosteroidogenic enzymes. Proc Natl Acad Sci USA. 1999;96(23):13512–13517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bauman DR, Steckelbroeck S, Peehl DM, Penning TM. Transcript profiling of the androgen signal in normal prostate, benign prostatic hyperplasia, and prostate cancer. Endocrinology. 2006;147(12):5806–5816. [DOI] [PubMed] [Google Scholar]
- 101. Suzuki-Yamamoto T, Nishizawa M, Fukui M, Okuda-Ashitaka E, Nakajima T, Ito S, Watanabe K. cDNA cloning, expression and characterization of human prostaglandin F synthase. FEBS Lett. 1999;462(3):335–340. [DOI] [PubMed] [Google Scholar]
- 102. Penning TM, Burczynski ME, Jez JM, Lin H-K, Ma H, Moore M, Ratnam K, Palackal N. Structure-function aspects and inhibitor design of type 5 17β-hydroxysteroid dehydrogenase (AKR1C3). Mol Cell Endocrinol. 2001;171(1–2):137–149. [DOI] [PubMed] [Google Scholar]
- 103. Dufort I, Rheault P, Huang X-F, Soucy P, Luu-The V. Characteristics of a highly labile human type 5 17β-hydroxysteroid dehydrogenase. Endocrinology. 1999;140(2):568–574. [DOI] [PubMed] [Google Scholar]
- 104. Brodie A. Aromatase inhibitors in breast cancer. Trends Endocrinol Metab. 2002;13(2):61–65. [DOI] [PubMed] [Google Scholar]
- 105. Jordan VC. Long-term adjuvant tamoxifen therapy for breast cancer. Breast Cancer Res Treat. 1990;15(3):125–136. [DOI] [PubMed] [Google Scholar]
- 106. Allegra JC, Lippman ME, Thompson EB, Simon R, Barlock A, Green L, Huff KK, Do HM, Aitken SC. Distribution, frequency, and quantitative analysis of estrogen, progesterone, androgen, and glucocorticoid receptors in human breast cancer. Cancer Res. 1979;39(5):1447–1454. [PubMed] [Google Scholar]
- 107. Ji Q, Aoyama C, Nien YD, Liu PI, Chen PK, Chang L, Stanczyk FZ, Stolz A. Selective loss of AKR1C1 and AKR1C2 in breast cancer and their potential effect on progesterone signaling. Cancer Res. 2004;64(20):7610–7617. [DOI] [PubMed] [Google Scholar]
- 108. Lewis MJ, Wiebe JP, Heathcote JG. Expression of progesterone metabolizing enzyme genes (AKR1C1, AKR1C2, AKR1C3, SRD5A1, SRD5A2) is altered in human breast carcinoma. BMC Cancer. 2004;4(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Jansson AK, Gunnarsson C, Cohen M, Sivik T, Stål O. 17β-Hydroxysteroid dehydrogenase 14 affects estradiol levels in breast cancer cells and is a prognostic marker in estrogen receptor-positive breast cancer. Cancer Res. 2006;66(23):11471–11477. [DOI] [PubMed] [Google Scholar]
- 110. Suzuki T, Miki Y, Moriya T, Akahira J, Hirakawa H, Ohuchi N, Sasano H. In situ production of sex steroids in human breast carcinoma. Med Mol Morphol. 2007;40(3):121–127. [DOI] [PubMed] [Google Scholar]
- 111. Lin H-K, Jez JM, Schlegel BP, Peehl DM, Pachter JA, Penning TM. Expression and characterization of recombinant type 2 3α-hydroxysteroid dehydrogenase (HSD) from human prostate: demonstration of bifunctional 3α/17β-HSD activity and cellular distribution. Mol Endocrinol. 1997;11(13):1971–1984. [DOI] [PubMed] [Google Scholar]
- 112. Fankhauser M, Tan Y, Macintyre G, Haviv I, Hong MKH, Nguyen A, Pedersen JS, Costello AJ, Hovens CM, Corcoran NM. Canonical androstenedione reduction is the predominant source of signaling androgens in hormone-refractory prostate cancer. Clin Cancer Res. 2014;20(21):5547–5557. [DOI] [PubMed] [Google Scholar]
- 113. Chang KH, Li R, Papari-Zareei M, Watumull L, Zhao YD, Auchus RJ, Sharifi N. Dihydrotestosterone synthesis bypasses testosterone to drive castration-resistant prostate cancer. Proc Natl Acad Sci USA. 2011;108(33):13728–13733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Guerini V, Sau D, Scaccianoce E, Rusmini P, Ciana P, Maggi A, Martini PG, Katzenellenbogen BS, Martini L, Motta M, Poletti A. The androgen derivative 5α-androstane-3β,17β-diol inhibits prostate cancer cell migration through activation of the estrogen receptor beta subtype. Cancer Res. 2005;65(12):5445–5453. [DOI] [PubMed] [Google Scholar]
- 115. Ji Q, Chang L, VanDenBerg D, Stanczyk FZ, Stolz A. Selective reduction of AKR1C2 in prostate cancer and its role in DHT metabolism. Prostate. 2003;54(4):275–289. [DOI] [PubMed] [Google Scholar]
- 116. Ji Q, Chang L, Stanczyk FZ, Ookhtens M, Sherrod A, Stolz A. Impaired dihydrotestosterone catabolism in human prostate cancer: critical role of AKR1C2 as a pre-receptor regulator of androgen receptor signaling. Cancer Res. 2007;67(3):1361–1369. [DOI] [PubMed] [Google Scholar]
- 117. Fung KM, Samara EN, Wong C, Metwalli A, Krlin R, Bane B, Liu CZ, Yang JT, Pitha JV, Culkin DJ, Kropp BP, Penning TM, Lin HK. Increased expression of type 2 3α-hydroxysteroid dehydrogenase/type 5 17β-hydroxysteroid dehydrogenase (AKR1C3) and its relationship with androgen receptor in prostate carcinoma. Endocr Relat Cancer. 2006;13(1):169–180. [DOI] [PubMed] [Google Scholar]
- 118. Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM, Febbo PG, Balk SP. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66(5):2815–2825. [DOI] [PubMed] [Google Scholar]
- 119. Liu C, Lou W, Zhu Y, Yang JC, Nadiminty N, Gaikwad NW, Evans CP, Gao AC. Intracrine androgens and AKR1C3 activation confer resistance to enzalutamide in prostate cancer. Cancer Res. 2015;75(7):1413–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Liu C, Armstrong CM, Lou W, Lombard A, Evans CP, Gao AC. Inhibition of AKR1C3 activation overcomes resistance to abiraterone in advanced prostate cancer. Mol Cancer Ther. 2017;16(1):35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Hamid AR, Pfeiffer MJ, Verhaegh GW, Schaafsma E, Brandt A, Sweep FC, Sedelaar JP, Schalken JA. Aldo-keto reductase family 1 member C3 (AKR1C3) is a biomarker and therapeutic target for castration-resistant prostate cancer. Mol Med. 2013;18:1449–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Hofland J, van Weerden WM, Dits NFJ, Steenbergen J, van Leenders GJ, Jenster G, Schröder FH, de Jong FH. Evidence of limited contributions for intratumoral steroidogenesis in prostate cancer. Cancer Res. 2010;70(3):1256–1264. [DOI] [PubMed] [Google Scholar]
- 123. Mitsiades N, Sung CC, Schultz N, Danila DC, He B, Eedunuri VK, Fleisher M, Sander C, Sawyers CL, Scher HI. Distinct patterns of dysregulated expression of enzymes involved in androgen synthesis and metabolism in metastatic prostate cancer tumors. Cancer Res. 2012;72(23):6142–6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Powell K, Semaan L, Conley-LaComb MK, Asangani I, Wu YM, Ginsburg KB, Williams J, Squire JA, Maddipati KR, Cher ML, Chinni SR. ERG/AKR1C3/AR constitutes a feed-forward loop for AR signaling in prostate cancer cells. Clin Cancer Res. 2015;21(11):2569–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Tian Y, Zhao L, Zhang H, Liu X, Zhao L, Zhao X, Li Y, Li J. AKR1C3 overexpression may serve as a promising biomarker for prostate cancer progression. Diagn Pathol. 2014;9(1):42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Sinreih M, Anko M, Zukunft S, Adamski J, Rižner TL. Important roles of the AKR1C2 and SRD5A1 enzymes in progesterone metabolism in endometrial cancer model cell lines. Chem Biol Interact. 2015;234:297–308. [DOI] [PubMed] [Google Scholar]
- 127. Hevir N, Vouk K, Sinkovec J, Ribič-Pucelj M, Rižner TL. Aldo-keto reductases AKR1C1, AKR1C2 and AKR1C3 may enhance progesterone metabolism in ovarian endometriosis. Chem Biol Interact. 2011;191(1–3):217–226. [DOI] [PubMed] [Google Scholar]
- 128. Nelson VLQK, Qin KN, Rosenfield RL, Wood JR, Penning TM, Legro RS, Strauss JF III, McAllister JM. The biochemical basis for increased testosterone production in theca cells propagated from patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 2001;86(12):5925–5933. [DOI] [PubMed] [Google Scholar]
- 129. Nakamura Y, Hornsby PJ, Casson P, Morimoto R, Satoh F, Xing Y, Kennedy MR, Sasano H, Rainey WE. Type 5 17β-hydroxysteroid dehydrogenase (AKR1C3) contributes to testosterone production in the adrenal reticularis. J Clin Endocrinol Metab. 2009;94(6):2192–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Rege J, Karashima S, Lerario AM, Smith JM, Auchus RJ, Kasa-Vubu JZ, Sasano H, Nakamura Y, White PC, Rainey WE. Age-dependent increases in adrenal cytochrome b5 and serum 5-androstenediol-3-sulfate. J Clin Endocrinol Metab. 2016;101(12):4585–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. O’Reilly M, Gathercole L, Capper F, Arlt W, Tomlinson J. Effect of insulin on AKR1C3 expression in female adipose tissue: in-vivo and in-vitro study of adipose androgen generation in polycystic ovary syndrome. Lancet. 2015;385(Suppl 1):S16. [DOI] [PubMed] [Google Scholar]
- 132. O’Reilly MW, Kempegowda P, Walsh M, Taylor AE, Manolopoulos KN, Allwood JW, Semple RK, Hebenstreit D, Dunn WB, Tomlinson JW, Arlt W. AKR1C3-mediated adipose androgen generation drives lipotoxicity in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102(9):3327–3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Adeniji AO, Twenter BM, Byrns MC, Jin Y, Chen M, Winkler JD, Penning TM. Development of potent and selective inhibitors of aldo-keto reductase 1C3 (type 5 17β-hydroxysteroid dehydrogenase) based on N-phenyl-aminobenzoates and their structure-activity relationships. J Med Chem. 2012;55(5):2311–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Rege J, Nakamura Y, Satoh F, Morimoto R, Kennedy MR, Layman LC, Honma S, Sasano H, Rainey WE. Liquid chromatography-tandem mass spectrometry analysis of human adrenal vein 19-carbon steroids before and after ACTH stimulation. J Clin Endocrinol Metab. 2013;98(3):1182–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. O’Reilly MW, Kempegowda P, Jenkinson C, Taylor AE, Quanson JL, Storbeck KH, Arlt W. 11-Oxygenated C19 steroids are the predominant androgens in polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102(3):840–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Turcu AF, Nanba AT, Chomic R, Upadhyay SK, Giordano TJ, Shields JJ, Merke DP, Rainey WE, Auchus RJ. Adrenal-derived 11-oxygenated 19-carbon steroids are the dominant androgens in classic 21-hydroxylase deficiency. Eur J Endocrinol. 2016;174(5):601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Turcu AF, Mallappa A, Elman MS, Avila NA, Marko J, Rao H, Tsodikov A, Auchus RJ, Merke DP. 11-Oxygenated androgens are biomarkers of adrenal volume and testicular adrenal rest tumors in 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2017;102(8):2701–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Pretorius E, Arlt W, Storbeck KH. A new dawn for androgens: novel lessons from 11-oxygenated C19 steroids. Mol Cell Endocrinol. 2017;441:76–85. [DOI] [PubMed] [Google Scholar]
- 139. Lambert JJ, Peters JA, Sturgess NC, Hales TG. Steroid modulation of the GABAA receptor complex: electrophysiological studies. Ciba Found Symp. 1990;153:56–71. [DOI] [PubMed] [Google Scholar]
- 140. Wang M, Seippel L, Purdy RH, Bãckström T. Relationship between symptom severity and steroid variation in women with premenstrual syndrome: study on serum pregnenolone, pregnenolone sulfate, 5 alpha-pregnane-3,20-dione and 3 alpha-hydroxy-5 alpha-pregnan-20-one. J Clin Endocrinol Metab. 1996;81(3):1076–1082. [DOI] [PubMed] [Google Scholar]
- 141. Gilbert Evans SE, Ross LE, Sellers EM, Purdy RH, Romach MK. 3α-Reduced neuroactive steroids and their precursors during pregnancy and the postpartum period. Gynecol Endocrinol. 2005;21(5):268–279. [DOI] [PubMed] [Google Scholar]
- 142. Wood SH, Mortola JF, Chan YF, Moossazadeh F, Yen SS. Treatment of premenstrual syndrome with fluoxetine: a double-blind, placebo-controlled, crossover study. Obstet Gynecol. 1992;80(3 Pt 1):339–344. [PubMed] [Google Scholar]
- 143. Martinez Botella G, Salituro FG, Harrison BL, Beresis RT, Bai Z, Blanco MJ, Belfort GM, Dai J, Loya CM, Ackley MA, Althaus AL, Grossman SJ, Hoffmann E, Doherty JJ, Robichaud AJ. Neuroactive steroids. 2. 3α-Hydroxy-3β-methyl-21-(4-cyano-1H-pyrazol-1′-yl)-19-nor-5β-pregnan-20-one (SAGE-217): a clinical next generation neuroactive steroid positive allosteric modulator of the (γ-aminobutyric acid)A receptor. J Med Chem. 2017;60(18):7810–7819. [DOI] [PubMed] [Google Scholar]
- 144. Belelli D, Bolger MB, Gee KW. Anticonvulsant profile of the progesterone metabolite 5α-pregnan-3α-ol-20-one. Eur J Pharmacol. 1989;166(2):325–329. [DOI] [PubMed] [Google Scholar]
- 145. Belelli D, Gee KW. 5α-Pregnan-3α,20α-diol behaves like a partial agonist in the modulation of GABA-stimulated chloride ion uptake by synaptoneurosomes. Eur J Pharmacol. 1989;167(1):173–176. [DOI] [PubMed] [Google Scholar]
- 146. El-Kabbani O, Dhagat U, Soda M, Endo S, Matsunaga T, Hara A. Probing the inhibitor selectivity pocket of human 20α-hydroxysteroid dehydrogenase (AKR1C1) with X-ray crystallography and site-directed mutagenesis. Bioorg Med Chem Lett. 2011;21(8):2564–2567. [DOI] [PubMed] [Google Scholar]
- 147. El-Kabbani O, Scammells PJ, Day T, Dhagat U, Endo S, Matsunaga T, Soda M, Hara A. Structure-based optimization and biological evaluation of human 20α-hydroxysteroid dehydrogenase (AKR1C1) salicylic acid-based inhibitors. Eur J Med Chem. 2010;45(11):5309–5317. [DOI] [PubMed] [Google Scholar]
- 148. Stolz A, Hammond L, Lou H, Takikawa H, Ronk M, Shively JE. cDNA cloning and expression of the human hepatic bile acid-binding protein. A member of the monomeric reductase gene family. J Biol Chem. 1993;268(14):10448–10457. [PubMed] [Google Scholar]
- 149. Chen M, Adeniji AO, Twenter BM, Winkler JD, Christianson DW, Penning TM. Crystal structures of AKR1C3 containing an N-(aryl)amino-benzoate inhibitor and a bifunctional AKR1C3 inhibitor and androgen receptor antagonist. Therapeutic leads for castrate resistant prostate cancer. Bioorg Med Chem Lett. 2012;22(10):3492–3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Liedtke AJ, Adeniji AO, Chen M, Byrns MC, Jin Y, Christianson DW, Marnett LJ, Penning TM. Development of potent and selective indomethacin analogues for the inhibition of AKR1C3 (type 5 17β-hydroxysteroid dehydrogenase/prostaglandin F synthase) in castrate-resistant prostate cancer. J Med Chem. 2013;56(6):2429–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Adeniji A, Uddin MJ, Zang T, Tamae D, Wangtrakuldee P, Marnett LJ, Penning TM. Discovery of (R)-2-(6-methoxynaphthalen-2-yl)butanoic acid as a potent and selective aldo-keto reductase 1C3 inhibitor. J Med Chem. 2016;59(16):7431–7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Byrns MC, Jin Y, Penning TM. Inhibitors of type 5 17β-hydroxysteroid dehydrogenase (AKR1C3): overview and structural insights. J Steroid Biochem Mol Biol. 2011;125(1–2):95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Attard G, Reid AHM, Yap TA, Raynaud F, Dowsett M, Settatree S, Barrett M, Parker C, Martins V, Folkerd E, Clark J, Cooper CS, Kaye SB, Dearnaley D, Lee G, de Bono JS. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26(28):4563–4571. [DOI] [PubMed] [Google Scholar]
- 154. Danila DC, Morris MJ, de Bono JS, Ryan CJ, Denmeade SR, Smith MR, Taplin ME, Bubley GJ, Kheoh T, Haqq C, Molina A, Anand A, Koscuiszka M, Larson SM, Schwartz LH, Fleisher M, Scher HI. Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. J Clin Oncol. 2010;28(9):1496–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB Jr, Saad F, Staffurth JN, Mainwaring P, Harland S, Flaig TW, Hutson TE, Cheng T, Patterson H, Hainsworth JD, Ryan CJ, Sternberg CN, Ellard SL, Fléchon A, Saleh M, Scholz M, Efstathiou E, Zivi A, Bianchini D, Loriot Y, Chieffo N, Kheoh T, Haqq CM, Scher HI; COU-AA-301 Investigators. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, de Wit R, Mulders P, Chi KN, Shore ND, Armstrong AJ, Flaig TW, Fléchon A, Mainwaring P, Fleming M, Hainsworth JD, Hirmand M, Selby B, Seely L, de Bono JS; AFFIRM Investigators. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–1197. [DOI] [PubMed] [Google Scholar]
- 157. Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, Wongvipat J, Smith-Jones PM, Yoo D, Kwon A, Wasielewska T, Welsbie D, Chen CD, Higano CS, Beer TM, Hung DT, Scher HI, Jung ME, Sawyers CL. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324(5928):787–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Kikuchi A, Furutani T, Azami H, Watanabe K, Niimi T, Kamiyama Y, Kuromitsu S, Baskin-Bey E, Heeringa M, Ouatas T, Enjo K. In vitro and in vivo characterisation of ASP9521: a novel, selective, orally bioavailable inhibitor of 17β-hydroxysteroid dehydrogenase type 5 (17βHSD5; AKR1C3). Invest New Drugs. 2014;32(5):860–870. [DOI] [PubMed] [Google Scholar]
- 159. Loriot Y, Fizazi K, Jones RJ, Van den Brande J, Molife RL, Omlin A, James ND, Baskin-Bey E, Heeringa M, Baron B, Holtkamp GM, Ouatas T, De Bono JS. Safety, tolerability and anti-tumour activity of the androgen biosynthesis inhibitor ASP9521 in patients with metastatic castration-resistant prostate cancer: multi-centre phase I/II study. Invest New Drugs. 2014;32(5):995–1004. [DOI] [PubMed] [Google Scholar]
- 160. Yepuru M, Wu Z, Kulkarni A, Yin F, Barrett CM, Kim J, Steiner MS, Miller DD, Dalton JT, Narayanan R. Steroidogenic enzyme AKR1C3 is a novel androgen receptor-selective coactivator that promotes prostate cancer growth. Clin Cancer Res. 2013;19(20):5613–5625. [DOI] [PubMed] [Google Scholar]
- 161. Penning TM. Aldo-keto reductase (AKR) 1C3 inhibitors: a patent review. Expert Opin Ther Pat. 2017;27(12):1329–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Ohta T, Ishikura S, Shintani S, Usami N, Hara A. Kinetic alteration of a human dihydrodiol/3α-hydroxysteroid dehydrogenase isoenzyme, AKR1C4, by replacement of histidine-216 with tyrosine or phenylalanine. Biochem J. 2000;352(Pt 3):685–691. [PMC free article] [PubMed] [Google Scholar]
- 163. Levere RD, Kappas A, Granick S. Stimulation of hemoglobin synthesis in chick blastoderms by certain 5β androstane and 5β pregnane steroids. Proc Natl Acad Sci USA. 1967;58(3):985–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Urabe A, Sassa S, Kappas A. The influence of steroid hormone metabolites on the in vitro development of erythroid colonies derived from human bone marrow. J Exp Med. 1979;149(6):1314–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Sheehan PM, Rice GE, Moses EK, Brennecke SP. 5β-Dihydroprogesterone and steroid 5β-reductase decrease in association with human parturition at term. Mol Hum Reprod. 2005;11(7):495–501. [DOI] [PubMed] [Google Scholar]
- 166. Thornton S, Terzidou V, Clark A, Blanks A. Progesterone metabolite and spontaneous myometrial contractions in vitro. Lancet. 1999;353(9161):1327–1329. [DOI] [PubMed] [Google Scholar]