Abstract
Background:
Preventive medicine techniques have alleviated billions of dollars in economic burden from the medical care system through the implementation of vaccinations and screenings prior to the onset of disease symptoms. These similar concepts are applicable to non-contact musculoskeletal injuries to reduce the economic burden of sports medicine treatment and enhance the long-term health of athletes. Knowledge of biomechanical tendencies has progressed rapidly over the past 20 years to the point where clinicians can identify, in healthy athletes, the underlying mechanisms that lead to catastrophic injuries such as anterior cruciate ligament rupture.
Purpose:
Illustrate the practical medical benefits that could be gained from preventive biomechanics as well as the need and feasibility for broad implementation of these principles.
Study Design:
Meta-analysis
Methods:
Recent literature pertinent to musculoskeletal injury screening and prevention was reviewed and compiled into a meta-analysis commentary on the current state and applicability of preventive biomechanics.
Results:
Investigators have identified neuromuscular training protocols that screen for and correct the underlying biomechanical deficits that lead to ACL injury. The literature shows that when athletes comply with these prescribed training protocols, the incidence of injury is significantly reduced within that population. Such preventive biomechanics practices employ basic training methods that would be familiar to athletic coaches and have the potential to save billions of dollars in cost in sports medicine.
Conclusion:
Widespread implementation of preventive biomechanics concepts could profoundly impact the field of sports medicine injuries with a minimum of initial investment.
Keywords: anterior cruciate ligament injury, injury risk classification, motion capture, motion analysis, neuromuscular training
INTRODUCTION
We define “Preventive Biomechanics” as the implementation of clinical measures within a standard training setting that demonstrate the capacity to diagnose relative risk and reduce the incidence rate of musculoskeletal injuries prior to onset. In opposition to complex and expensive laboratory assessments, the realization of preventive biomechanics is rooted on the cost effective integration of clinical knowledge and sports performance to protect athletes. The objective of preventive biomechanics is to employ basic training techniques in manners that are clinically proven to reduce injury incidence while enhancing performance. The overarching objective of preventive biomechanics is the widespread reduction of musculoskeletal injuries in athletics.
One particular injury that could benefit from the implementation of preventive biomechanics is anterior cruciate ligament (ACL) rupture. Over the past 17 years, the incidence rate of ACL injury among collegiate athletes in cutting and pivoting sports has declined in the United States.1–3 In NCAA male and female soccer players the incidence rate of ACL injury per 1000 athletic exposures was respectively reported as 0.12 and 0.33 from 1994–1998,1 then as 0.12 and 0.32 from 1989–2004,2 and most recently as 0.07 and 0.25 from 2011–2014.3 Similarly, incidence among NCAA male and female basketball athletes were 0.07 and 0.29 from 1994–1998,1 then 0.08 and 0.28 from 1989–2004,2 and most recently 0.07 and 0.20 from 2011–2014.3 The period of these investigations (1999–2016) represents the advent and rise in popularity of targeted neuromuscular training designed to prevent ACL injuries through the alteration of an athlete’s movement biomechanics.4–8 These preventive measures have gained popularity, especially among female athletes who are more susceptible to ACL injury than their male counterparts, but have yet to realize widespread incorporation across sport and medicine. Despite a lack of widespread adoption, these preventive measures still seem to have made an impact on ACL injury outcomes, as incidence among NCAA female athletes reduced by 22% - 29% between 2004 and 2014. This concept also pertains to lateral epicondylitis, where home exercise relieved pain better than a wait-and-see approach, but not as well as clinic-based exercise program;9 elbow injuries, where poor pitching biomechanics increased varus torque on the ulnar collateral ligament;10 shoulder injuries, where strengthening the scapula stabilizing musculature reduced injury incidence in tennis players;11 as well as ankle sprains, where prophylactic interventions reduced incidence among basketball players.12
The concept of preventive medicine has proliferated since it was introduced by Leavell and Clark in 1965.13 The overarching goal of preventive medicine is to maintain patient health through the institution of and investment in measures that reduce or eliminate a healthy individual’s risk of acquiring a medical complication. The idea is that maintaining health across a whole population represents a reduced financial burden compared to treating individual cases of a medical complication. Indeed, it was estimated that in 2000, approximately 50% of American deaths were from preventable causes.14 This represents a significant, and potentially unnecessary, financial burden on the health care system as well as an emotional and personal burden patients and their families.
The benefits of preventive medicine may be difficult to quantify monetarily because it remains unknown how each patient will respond to preventive interventions and it is impossible to quantify the value of human life. However, from 2000 to 2010, preventable infant deaths in the 42 countries responsible for 90% of the worlds infant deaths were reduced from 9.6 million to 7.6 million.15 Within these countries, medical coverage of standard preventive care measures implemented in developed parts of the world is under 50%.16 It is estimated that an additional 55% of these deaths could be avoided with basic preventive interventions such as improved nutrition, sanitation, and vaccination techniques.16 Within the United States the implementation of the polio vaccine as a preventive measure reduced the burden of this disease from an average of greater than 15,000 paralytic cases per year in the 1950s to complete elimination by 1994.17 Overall, it is estimated that for every $1 spent on polio vaccinations over $5 has been saved in treatment costs. This circumstance is similarly true for vaccines of measles-mumps-rubella (reduced by >98%, saves $16.34 for every $1 spent) and diphtheria-tetanus toxoids-pertussis (reduced by >91%, saves $6.21).18 Similarly, population based medical risk screenings such as mammograms have been widely implemented as early detection of breast cancer can reduce treatment costs from $140,000 to $11,000.19 These, along with numerous other preventive medicine measures, relieve significant financial burden from the medical care system as well as improve the quality of life for patients who avoid these respective complications.
Despite the benefits documented, preventive medicine has, up to this point, experienced limited integration into the subspecialty of sports medicine. Most preventive measures prescribed within sports medicine have been directed around the general incorporation of exercise at a moderate-or-greater level of intensity into daily routine.20,21 Commitments to general exercise exhibit positive influence on overall cardiovascular health and mortality.22 While general exercise and speed training have a slightly positive effect on the overall incidence of lower extremity musculoskeletal injuries, there is little evidence to indicate that such non-targeted training significantly impacts the incidence rate of catastrophic soft injuries such as ACL ruptures that sideline athletes for extended periods of time.23,24 Injuries such as ACL rupture can devastate athletic careers and long-term quality of life.25–27 These injuries occur in non-contact scenarios 70% of the time and are primarily the result of poor mechanical control that leads to the application of high loads within and athlete’s joints, consequently placing them “at risk” for injury.28–34 Recently, significant work has been done to indicate that the incidence rate of these primarily non-contact injuries can be significantly reduced through appropriate training of an athlete’s neuromuscular control.4,5,7,8 With an ever increasing numbers of adolescent and adult sports participants, and the corresponding injury incidence, the widespread application of preventive biomechanics measures stands to have a great impact on athlete health, just as preventive exercise and preventive medicine have had on the general population.
The objective of this commentary is to illustrate the practical medical benefits that could be gained from preventive biomechanics as well as the need and feasibility for broad implementation of these principles. Using data culled from the last 20 years of anterior cruciate ligament (ACL) injury research, this manuscript will outline a prospectus on how advanced laboratory biomechanical measures can be applied in clinical and pre-clinical settings in order the improve the overarching spectrum of sports health.
STAGES OF PREVENTION
According to the literature there are three traditional stages of preventive medicine.13,35 Primary prevention addresses the elimination of disease causes in order to prevent the establishment of the disease condition. Secondary prevention addresses the disease itself in an attempt to disrupt the disease process before symptoms appear. Finally, tertiary prevention focuses on containment in an attempt to limit the physical and social repercussions of a symptomatic disease.
The vast majority of sports injuries are mechanical in nature, which presents an obvious difference in pathology from disease progression. However, the three pillars of preventive medicine can easily be adapted into three similar concepts that form the base principles of preventive biomechanics. The primary prevention measure of preventive biomechanics would be screening for injury risk. As with disease progression, athletic injuries are often preceded by identifiable risk factors that can be assessed in a lab, clinical, or athletic setting.32,33,36–45 The secondary prevention measure for preventive biomechanics would incorporate the training of athletes identified to be at high-risk for injury. Just as not every woman who has a mammogram requires a mastectomy, not every athlete who undergoes biomechanical screening will require corrective neuromuscular training. Certainly, preventive protocols can be applied to the athletic population at-large; however, it has been shown that the athletes who exhibit high-risk movement patterns are the individuals who will register the greatest change in response to corrective biomechanics training.46 Finally, the tertiary prevention measure in preventive biomechanics would focus on evidence-based treatment and rehabilitation of injury occurrences to limit damage to the body and minimize the risk of further re-injury. As with many forms of preventive medicine, while the implementation of primary and secondary preventive biomechanics will reduce injury incidence,4,5,7,8,23 they will not fully eliminate occurrence. At a minimum, these injured athletes need to return to activities of daily living, and often desire a full return to sport. Accordingly, the third pillar of prevention should be to treat injury occurrence in a manner that most efficaciously balances short term recovery and long term health.
INJURY RISK SCREENING
It has been recommended that football injury prevention efforts be focused at the knee and ankle as these joints account for 37.6% and 36.9% of all time-loss injuries at the high school and collegiate levels respectively.47 Within this population, ACLs were the third most common knee injury and second most common knee surgery.48 Incidence and variance of knee injuries in elite college football players] In the NBA, the tibiofemoral joint was the seventh most frequently injured structure of the body (4.4%), but accounted for the greatest time loss due to injury (13.6%).49 Accordingly, for the past three decades, investigators have worked to identify the underlying mechanisms that lead to ACL injury. It is the desire of these investigators to identify treatable, high-risk biomechanical trends within athletes prior to the onset of catastrophic injuries. Indeed, a number of modifiable biomechanical factors have been related to increased risk of ACL injury.32,33,36–45,50 Many of these factors can be organized into four general categories of ligament dominance, quadriceps dominance, leg dominance, and trunk dominance.28–31,51–54 Ligament dominance is characterized by an inhibition to dissipate the impulse reaction forces generated during ground contact in the musculature. Instead, the loads are propagated through the passive restraints in the knee, resulting in larger stresses and strains that may induce rupture.28–31,54 Specifically, during a prospective in vivo investigation, knee abduction moments and angles generated when landing from a jump were found to be significant predictors of subsequent ACL injury status.32 Quadriceps dominance is characterized by an imbalance in muscle recruitment between the knee flexors and extensors.28,30,31,51,52,54,55 The quadriceps muscle works as an antagonist to the ACL as it induces anterior tibial translation and ACL strain when contracted, while the hamstrings serve as a counteracting ACL agonist.56–59 Thus, a quadriceps-to-hamstrings activation ratio below 60% is associated with increased risk of ACL injury as this was prospectively observed in females who went on to rupture and is believed to add strain to the ligament during dynamic activity.60 Leg dominance is characterized by an imbalance of muscle recruitment and dynamic control between contralateral limbs.28,30,31,51,53–55 Athletes with asymmetric quadriceps recruitment have demonstrated similarly asymmetric mechanics in knee flexion excursion, trunk flexion, and knee extension moment.61 These, along with asymmetries in transverse plane hip kinetics, frontal plane knee kinematics, sagittal plane knee moments, and postural stability, are risk factors for subsequent ACL injury.62 Trunk dominance is characterized by poor control of the trunk during dynamic activity that moves the center of mass outside of the base of support of the body.30,31 Approximately 60% of young female athletes demonstrate biomechanical profiles that express deficits in one or more of these four categories, which places them at elevated risk for ACL injury.31
These mechanical risk factors that precede ACL rupture can be used to parse out which athletes are most susceptible to injury prior to onset. In particular, knee abduction moment predicted ACL injury status with 78% sensitivity and 73% specificity (Figure 1).32 Direct laboratory measures of peak knee abduction angle, peak knee extensor moment, knee flexion range of motion, BMI Z-score, and tibia length account for 78% of the variance in knee abduction moment and predict high valgus knee loads (> 2.25 Nm) in an athletes with 85% sensitivity and 93% specificity.63 Similarly, active proprioceptive response has been related to ACL injury risk and was used to predict injury status in a cohort of collegiate athletes with 91% accuracy.64 The inherent problem associated with the identification of these risk factors is cost. Joint kinematics and kinetics are assessed via three dimensional motion analysis techniques that require facilities with access to quarter-million-dollar motion systems and trained personnel to conduct the time-intensive data analyses. Such expenses make these gold standard measures of diagnosing relative ACL injury risk cost-prohibitive to implement on nationwide scale with millions of adolescent athletes at the middle and high school levels.
Figure 1:
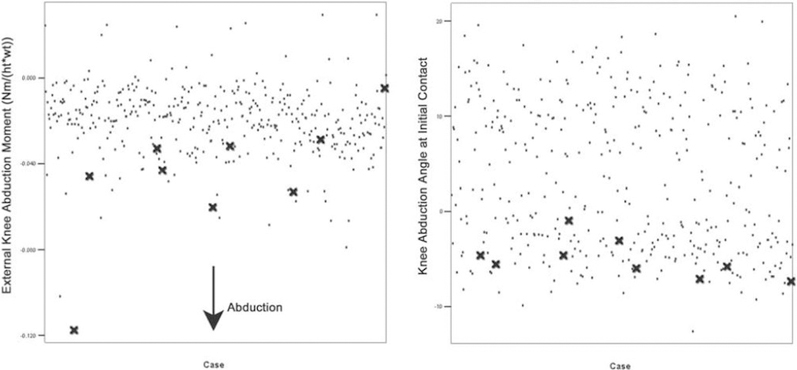
Data stratification of 205 young female athletes who were prospectively followed for ACL injury. The nine subjects who went on to ACL rupture demonstrated greater than average knee abduction angles and moments when landing from drop. This is indicative of the potential for biomechanical behaviors to serve as screening tools to identify those athletes who are most susceptible to particular injuries. Image reproduced with permission from Hewett, et al. 2005. Am J Sports Med, 33(4):492–501.
The cost-inhibition related to widespread implementation of advanced scientific metrics is not lost on sports medicine investigators. Unfortunately, two-dimensional (2D) video has been shown to correlate poorly with 3D laboratory measures and, therefore, cannot be interchanged as a reliable surrogate in a clinical setting.65 Likewise, subjective 2D video analysis of 3D motion and subsequent classification of high and low ACL risk without the use of specific variable measures exhibited “inadequate” levels of sensitivity.66 Using such and undefined protocol, raters failed to detect up to 33% of high-risk individuals. Accordingly, some investigators have worked to develop more clinically-applicable tools that extrapolate similar injury risk predictions without the expense of a full, detailed biomechanical analysis.67–71 The clinic-based algorithm, a prediction tool using clinical surrogates for the laboratory-based 3D variables, predicted high KAM status with 84% sensitivity and 67% specificity using knee valgus motion, knee flexion range of motion, body mass, tibia length, and quadriceps to hamstrings strength ratio (Figure 2).67 A similar prediction model has assessed high KAM status with 73% sensitivity and 70% specificity.68 As illustrated previously, KAM is a leading predictor for risk of ACL injury.32 Each of these simplified screening mechanisms were executed with standard clinical tools including a physician’s scale, measuring tape, camcorder, free Image J software (National Institutes of Health, Bethesda, MD, USA), and an isokinetic dynamometer.72 With these clinical correlates and reduced equipment demands, it is now possible to implement effective biomechanical injury screening within academic and athletic team settings.
Figure 2:
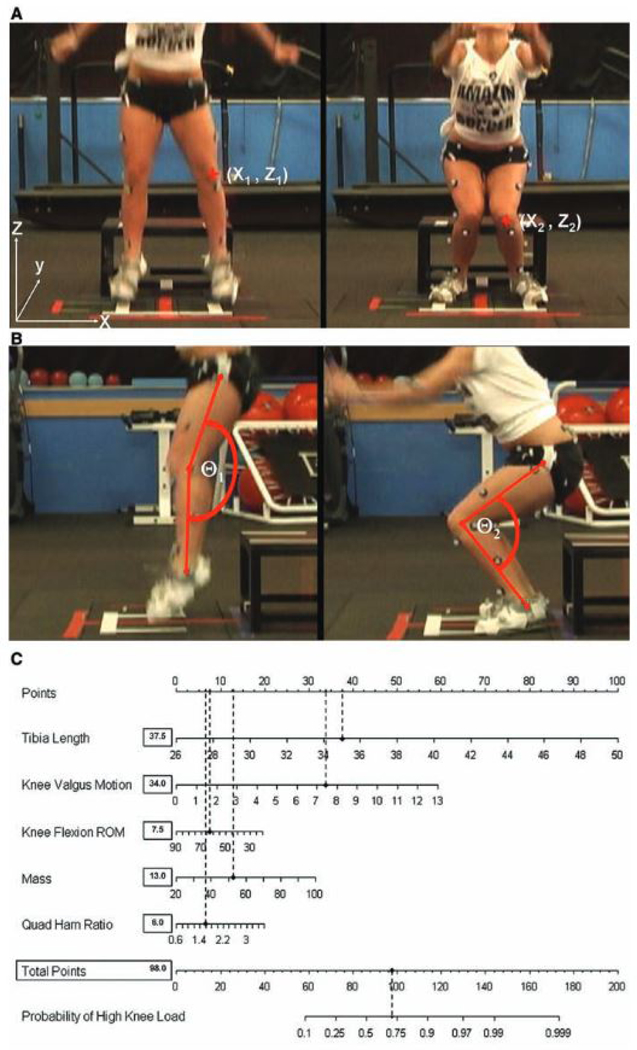
Multiple clinical biomechanical risk factors can be combined into a single algorithm and offer strong predictive screening models that maintain high sensitivity and specificity without the prohibitive expense of obtaining precise laboratory measures. Image reproduced with permission from Myer, et al. 2010. Am J Sports Med, 38(10):2025–2033.
While clinically-based screening tools may accurately predict the presence of underlying mechanisms that lead to ACL injury and contribute to injury risk, there remains room for improvement in the prospective prediction of an actual ACL injury event. The landing error scoring system (LESS), a 2D tool that is reliable in clinical assessment of biomechanical deficiencies during a DVJ task,71,73 was unable to predict non-contact ACL tears across a population of matched high school and collegiate athletes.74 However, poor LESS scores did correspond with increased odds ratio of ACL rupture, which indicated potential for the tool to serve as a screen for relative injury risk. Such a concept was best illustrated within a cohort of 829 elite soccer athletes.75 The LESS system predicted the need for ACL injury-prevention training with 86% sensitivity and 64% specificity within this group, but the 1-season risk of injury was 1.37% in athletes who scored worse than the established LESS threshold and 0.13% in athletes who scored better. A screening tool diagnosis of high injury risk does not guarantee that future ACL incidence will occur.
Muscular strength and activation have provided a second series of ACL injury risk prediction mechanisms for a clinical environment. A model based on EMG data from the vastus medialis, vastus lateralis, rectus femoris, biceps femoris, and semi-tendinosus predicted that the probability of sustaining ACL injury was 50% for athletes that expressed greater than one standard deviation in difference between vastus lateralis and semitendinosus pre-activity (mean 47% ± 14%) during a sidestep cut task.76 Such a concept was later supported by isometric hip strength data that showed athletes who went on to ACL injury had significantly lower hip external rotation and abduction strength than their healthy counterparts.77 The sensitivity and specificity of these hip strength prediction variables were 87–93% and 59–65%, respectively. While most clinical sports medicine environments will have access to isokinetic / isometric dynamometers to evaluate isolated muscle group strength, such equipment may be cost prohibitive at the middle and high school level. Fortunately, it is possible to assess strength deficits with standard weightlifting equipment, though the reliability of these measures have not been correlated to dynamometers.
The wealth of prediction tools presented provides medical practitioners, coaches, trainers, and teachers with a variety of tools to actively screen their patients, athletes, and students in a non-laboratory athletic environment. Widespread application of these tool are plausible as literature has shown a lack of differences in assessment reliability between medical practitioners and other groups in field-based DVJ screening tools.78 The underlying purpose of all these screens is to ascertain easily identifiable biomechanical markers that occur within athletic tasks that can be used to identify athletes who are at high risk of catastrophic injury. In-turn, these high risk athletes can be treated and rehabilitated before an adverse event, or “pre-habilitated”, in order to optimize the probability of injury prevention.
TRAINING
The knee joint performs optimally when it functions as a hinge joint with minimal frontal or transverse plane rotation. Musculoskeletal modeling has demonstrated that no amount of sagittal plane flexion torque can lead to ACL failure;79 however, both frontal and transverse plane moments serve as precursors to ACL failure as they invoke ligament loading.80–83 Athletes who invoke high levels of neuromuscular control during the performance of athletic tasks tend to exhibit smaller magnitudes of excursion and instability outside the sagittal plane and, consequently, reduced loading on the ACL. Conversely, athletes with poor neuromuscular activation lack this control and demonstrate the clinically screenable biomechanical tendencies described above.28,32,84–86 Once an athlete has been identified with high-injury-risk potential, it is possible to correct his or her negative biomechanical tendencies through targeted intervention training. 4,5,7,8 In as little as 6–10 weeks, this pre-habilitation can reduce neuromuscular deficits to the point where it influences injury incidence outcomes.5 Specifically, pre-habilitation training in high-risk athletes has reduced ACL injury incidence compared to untrained controls by upwards of 50% across multitude of investigations, with individual study results as high as 88%.4–8 Within a population of female soccer players, controlled randomized implementation of injury-prevention pre-habilitation programs reduced overall ACL incidence rate from 0.67% in controls to 0.28% in trained athletes.87 However, it should be noted that compliance has a direct impact on the effectiveness of injury prevention programs, as high-compliance athletes demonstrated an 88% reduction in ACL injury rate when compared to low-compliance athletes.87 Implementation of pre-habilitation measures are not just isolated to ACL injuries. Many of the same risk factors for ACL failure are shared by patellofemoral pain syndrome (PFPS) patients, which is the most common disorder of the knee (Figure 3).88,89,90 Correspondingly, pre-habilitation has demonstrated similar effectiveness in PFPS reduction.91
Figure 3:
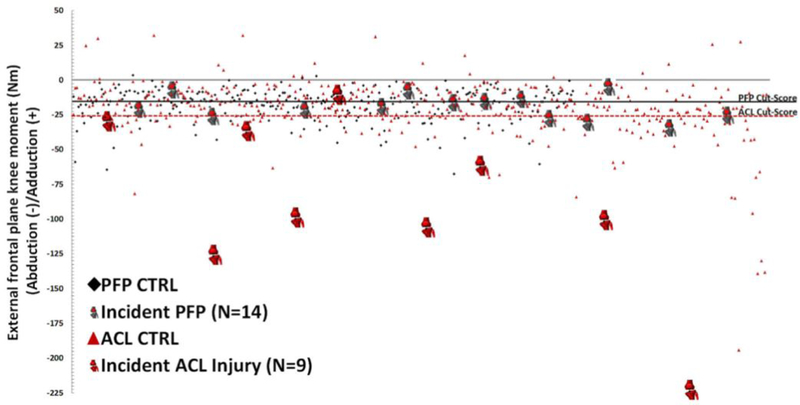
Multiple injury types can share similar mechanism and, therefore, similar biomechanical predictors. This data stratification depicts that knee abduction moment was both a predictor for ACL injury and PFPS. The ACL injury prediction was predicated by a greater abduction moment, so it is also possible that the magnitude of a biomechanical factor can be related to the magnitude of injury that it predicts. Image reproduced with permission from Myer, et al. 2015. Am J Sports Med, 49(2):118–122.
Pre-habilitation protocols target modifiable risk factors associated with ACL injury risk.92 Specifically these training programs look to correct neuromuscular deficits through targeted interventions. Neuromuscular training varies between protocols, but programs incorporate a combination of plyometric and agility training, balance and stability training, resistance training, speed training, as well as strengthening of the musculature around the core, hips, pelvis, knees, and ankles.5,92 However, when parsed out, none of these specific components were individually associated with improved ACL injury outcome measures.4 For example strength training alone will improve strength, but has not been shown to alter lower extremity movement patterns.93 This data indicates that the injury reduction observed from pre-habilitation culminates from the correction of a multitude of biomechanical factors that contribute to ACL loading. Apart from biomechanical benefits such as reduced hip internal rotation, knee valgus moment, and knee extensor moments as well as increased joint flexion, hip abduction, and hamstrings muscle activation, these neuromuscular training protocols can also lead to enhanced performances that are directly tangible on the field of play.4 Direct side-effects from pre-habilitation training have included increased strength, jumping performance, speed, and reaction time.5,24,94,95 Though biomechanical improvements have a direct influence on injury prevention, these concepts may appear abstract to an athletic coach or trainer. Accordingly, the enhanced performance attributes ascribed to targeted neuromuscular training should intrigue athletic coaches and trainers in addition to the efficacious health benefits for their athletes.
Muscle activation, specifically hip muscle activation, works to maintain the body’s center of mass in a planar base. Poor neuromuscular control leads to the trunk and center of mass deviating from this planar base; and thus, extends the moment lever arm acting on the knee and other lower extremity joints. This leads to greater frontal plane excursion of the knee57 which, in turn, produces large magnitudes of torque at the joint. Pre-habilitation training incorporates a significant amount of plyometric jump and perturbation tasks that will strengthen an athlete, but also correct the neuromuscular activation pathways that lead to out-of-plane motion at the knee.57 The end objective is enhanced joint control as forces are actively dissipated across the knee via musculature and less likely to be passively absorbed by the ligaments.
A wide variety of exercises can be employed to address each of the modifiable ACL injury risk factors that arise from poor neuromuscular control.57 Muscle activation deficits have been linked to poor control of the lower extremities96,97 and can be modified through single-leg hoping and landing exercises that improve hip strength and necessitate trunk and lower extremity control to minimize frontal plane moments.57,98 These single leg anterior and lateral plyometric jumping tasks can similarly be implemented to gain greater control of postural instability that is associated with ACL injury risk.57,62,99 Excessive trunk motion can increase ACL injury risk;100,101 thus, lateral, prone, and kneeling trunk stability exercises may lead to increased proprioception and decreased frontal plane knee moments.57 Hamstrings serve as an agonist to ACL strain during dynamic activities;56 therefore, hamstring strength training through weightbearing activities such as single leg dead lift can improve quadriceps-to-hamstrings strength rations and contribute to reduced injury risk.57,102 ACL rupture is related to limited and asymmetrical knee flexion;103 thus, lunges, lunge jumps, and tuck jump tasks that encourage symmetrical, deep knee flexion while maintaining frontal plane control of the knee can contribute to injury prevention.57,104 Each the neuromuscular modifying tasks presented are described in detail by Di Stasi, et al. What makes these presented exercises particularly applicable is that each one is already a common athletic training task. As such, coaches and trainers at all levels should be familiar with these activities and easily able to execute them in conjunction in both individual and team settings with minimal equipment or educational investment.
TREATMENT
Knee injuries account for between 10–40% of adolescent sports injuries dependent on definition and criteria.105,106 Additionally, knee injuries are the most common cause of surgery in high school sports, accounting for 60% of sports injury operations.107 ACL injuries specifically account for approximately 20.5–25.4% of these sports related knee injuries and result in the greatest time lost from sport participation in young female athletes.43,106,108 With such high prevalence, it is unlikely that any amount of pre-habilitation will entirely eradicate non-contact ACL injuries. However, prevention of just one ACL rupture saves over $17,000 in medical costs for reconstructive surgery and rehabilitation.109 Further, if the annual estimated incidence of 250,000 ACL ruptures and 125,000 ACL reconstructions in the United States can be reduced by greater than 50% via widespread application of pre-habilitation techniques, the overall saving to the American medical system would be more than $1.1 billion annually in immediate care costs alone.109–112 The cost footprint on the medical system is then further reduced as the long-term pain treatments and total joint replacements necessitated by early onset arthritis, which is expected in 50–90% of patients within 20 years of injury,25–27 may be avoided. Consequently, implementation of pre-habilitation in young, high-injury-risk athletes has the potential to alleviate significant financial burden from the national medical system.
As indicated, even widespread implementation of pre-habilitation training will not eradicate ACL injuries. Those athletes who suffer an initial ACL tear represent the most likely cohort to suffer additional failures as the incidence of secondary ACL failure is as high as 30%.57,113 This secondary failure rate was highest amongst young athletes (<25 years old) who returned to sport following initial reconstruction.114 Correspondingly, injured athletes represent the cohort that stands to benefit the most from preventive biomechanics as they are 5 to 40 times more likely to suffer future injury than healthy counterparts,113–115 and secondary ACL injuries can be devastating to athletic careers. Following primary ACL injury, 63% of athletes successfully return to sport at their previous level of competition,116,117 while only 23% return after a secondary tear.118 Further, following rehabilitation from ACLR, under 50% of young athletes report being able to perform at their pre-injury level.119 In healthy controls, screening measures serve to separate the high-injury-risk athletes from low-injury-risk counterparts to determine need for pre-habilitation. Injury occurrence itself is the gold-standard screening tool as this cohort of athletes exhibits, by far, the highest incidence rate of future injury.113–115
As in healthy athletes, there are both modifiable and non-modifiable risk factors that contribute to secondary ACL injury. The modifiable factors much resemble those indicated in primary failure.57 In particular, it was demonstrated that neuromuscular impairments in both the reconstructed and contralateral limb predict secondary ACL injury with 92% sensitivity and 88% specificity.62 Once again, neuromuscular deficits lead to dynamic knee valgus that precipitates increased loading on the ACL or ACLR graft. The effectiveness of neuromuscular training on the reduction of secondary ACL injuries has not been directly investigated.57 However, as previously described, these practices are well documented in the correction of primary injury biomechanical deficits, 4–8 so it is likely their implementation during ACLR rehabilitation would similarly impact the prevention of secondary tears through a reduction in incidence. A specific protocol for late-phase ACLR rehabilitation was previously proposed, using objective patient-specific performance criteria as qualifiers for enrollment.57 Patients who demonstrated full and pain free ROM relative to the contralateral limb, minimal joint effusion, >70% strength symmetry, and ability to hop in place without pain or apprehension were to being a targeted neuromuscular training with exercises designed to “(1) activate muscles hypothesized to be deficient at the time of sport, (2) utilize surfaces and movements that elicit muscle co-activation capable of modifying mechanics theorized to be related to injury risk, and (3) elicit movement that may replicate conditions experienced during sport.”57 Specifically, the program incorporates the same single leg anterior and lateral hop, lunge, lunge jump, tuck jump, lateral jump, lateral trunk, trunk stability, posterior chain, and single-leg dead lift exercises utilized in pre-habilitaton, to improve ACLR rehabilitation outcomes. These activities are applied bilaterally to target typically-observed post-ACLR deficits and side-to-side asymmetries in the gluteals, trunk, quadriceps, and hamstrings musculature (Figure 4).57 Though the proposed protocol has yet to be validated as efficacious in the prevention of secondary, each incorporated exercise has been shown to alleviate factors related to primary injury.57,98,99,120 As both primary and secondary injuries share biomechanical risk factors it is expected that dynamic exercises designed around the reduction of ligament dominance, quadriceps dominance, trunk dominance, and leg dominance will also have efficacious impact on secondary prevention.
Figure 4:

Progressive, bilateral training involving plyometric, perturbation, and stability that is used to incrementally adapt an athlete to positive neuromuscular patterns that are likely to reduce risk of biomechanically induced injury. The progressive model starts the athlete at a basic task level and incrementally increases difficulty as the subject’s mechanics adapt. Image reproduced with permission from Di Stasi, et al. 2013. J Ortho Sports Phys Ther, 43(11):777-A11.
CONCLUSION
ACL injury and rehabilitation are the number one cause for time loss due to injury in NCAA athletics. These injuries exact an exorbitant cost on both patients and the medical community at large. This cost is not limited to just monetary expense as ACL ruptures frequently lead to decreased quality of life for the patient. The efficacious impact of pre-habilitation techniques on the reduction of ACL injury incidence is well documented in the literature. This reduction of incidence is possible through the combination of screening, training, and treatment measures into an overarching preventive biomechanics program. Nationwide implementation of preventive biomechanics techniques at the level of young, adolescent, and collegiate could be executed at a minimum of cost with the potential for a high-reward return on investment. Coaches, athletic trainers, and physical therapists already possess the knowledge and much of the equipment necessary to appropriately execute effective preventive biomechanics. As coaches and trainers begin to observe that preventive biomechanics methods not only preserve their athletes’ health, but also improve their performance measures, a natural progression of general acceptance within the sporting community should follow. Further, preventive biomechanics are not limited to just the ACL rupture condition as these same principles have been applied to reduce PFPS. Accordingly, it is expected that preventive biomechanics could be uniquely adapted to sport-specific needs in order to more effectively reduce incidence of additional traumatic and overuse injuries such as ankle sprains, epicondylitis, ulnar collateral ligament tears, or lower back pain. Widespread implementation of preventive biomechanics in today’s athletic community is feasible and could significantly improve health outcomes as well as reduce medical expenditures.
REFERENCES
- 1.Arendt EA, Agel J, Dick R. Anterior Cruciate Ligament Injury Patterns Among Collegiate Men and Women. J. Athl. Train. 1999;34:86–92. [PMC free article] [PubMed] [Google Scholar]
- 2.Mihata LC, Beutler AI, Boden BP. Comparing the incidence of anterior cruciate ligament injury in collegiate lacrosse, soccer, and basketball players: implications for anterior cruciate ligament mechanism and prevention. Am. J. Sports Med. June 2006;34(6):899–904. [DOI] [PubMed] [Google Scholar]
- 3.Stanley LE, Kerr ZY, Dompier TP, Padua DA. Sex Differences in the Incidence of Anterior Cruciate Ligament, Medial Collateral Ligament, and Meniscal Injuries in Collegiate and High School Sports: 2009–2010 Through 2013–2014. Am. J. Sports Med. March 3 2016. [DOI] [PubMed] [Google Scholar]
- 4.Donnell-Fink LA, Klara K, Collins JE, et al. Effectiveness of Knee Injury and Anterior Cruciate Ligament Tear Prevention Programs: A Meta-Analysis. PLoS ONE. 2015;10(12):e0144063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Myer GD, Sugimoto D, Thomas S, Hewett TE. The influence of age on the effectiveness of neuromuscular training to reduce anterior cruciate ligament injury in female athletes: a meta-analysis. Am. J. Sports Med. January 2013;41(1):203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herman K, Barton C, Malliaras P, Morrissey D. The effectiveness of neuromuscular warm-up strategies, that require no additional equipment, for preventing lower limb injuries during sports participation: a systematic review. BMC Med. 2012;10:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sugimoto D, Myer GD, Foss KD, Hewett TE. Dosage effects of neuromuscular training intervention to reduce anterior cruciate ligament injuries in female athletes: meta- and sub-group analyses. Sports Med. April 2014;44(4):551–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sugimoto D, Myer GD, McKeon JM, Hewett TE. Evaluation of the effectiveness of neuromuscular training to reduce anterior cruciate ligament injury in female athletes: a critical review of relative risk reduction and numbers-needed-to-treat analyses. Br. J. Sports Med. November 2012;46(14):979–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menta R, Randhawa K, Cote P, et al. The effectiveness of exercise for the management of musculoskeletal disorders and injuries of the elbow, forearm, wrist, and hand: a systematic review by the Ontario Protocol for Traffic Injury Management (OPTIMa) collaboration. J. Manipulative Physiol. Ther. September 2015;38(7):507–520. [DOI] [PubMed] [Google Scholar]
- 10.Fleisig GS, Andrews JR. Prevention of elbow injuries in youth baseball pitchers. Sports Health. September 2012;4(5):419–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kibler WB, McQueen C, Uhl TL. Fitness evaluations and fitness findings in competitive junior tennis players. Clin. Sports Med. 1988;7(2):403–416. [PubMed] [Google Scholar]
- 12.Taylor JB, Ford KR, Nguyen AD, Terry LN, Hegedus EJ. Prevention of Lower Extremity Injuries in Basketball: A Systematic Review and Meta-Analysis. Sports Health. Sep-Oct 2015;7(5):392–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katz DL, Ali A. Preventive medicine, integrative medicine & the health of the public. Summit on Integrative Medicine and the Health of the Public; February, 2009, 2009. [Google Scholar]
- 14.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Correction: Actual causes of death in the United States 2000. Journal of the American Medical Association. 2005;293(3):293. [DOI] [PubMed] [Google Scholar]
- 15.Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. The Lancet. 2012;379(9832):2151–2161. [DOI] [PubMed] [Google Scholar]
- 16.Jones G, Steketee RW, Black RE, Bhutta ZA, Morris SS. How many child deaths can we prevent this year? The Lancet. 2003;362(9377):65–71. [DOI] [PubMed] [Google Scholar]
- 17.Thompson KM, Duintjer Tebbens RJ. Retrospective cost-effectiveness analyses for polio vaccination in the United States. Risk Anal. 2006;26(6):1423–1440. [DOI] [PubMed] [Google Scholar]
- 18.Prevention CfDCa. An Ounce of Prevention.... What are the Returns. In: Services USDoHaH, ed. Second ed Atlanta, Georgia: 1999. [Google Scholar]
- 19.Shaw LJ, Raggi P, Berman DS, Callister TQ. Cost effectiveness of screening for cardiovascular disease with measures of coronary calcium. Prog. Cardiovasc. Dis. 2003;46(2):171–184. [DOI] [PubMed] [Google Scholar]
- 20.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med. Sci. Sports Exerc August 2007;39(8):1423–1434. [DOI] [PubMed] [Google Scholar]
- 21.Pate RR, Pratt M, Blair SN, et al. Physical activity and publich health: A recommendation from the Centers for Disease Control and prevention and the American College of Sports Medicine. Journal of the American Medical Association. 1995;273(5):402–407. [DOI] [PubMed] [Google Scholar]
- 22.Kesaniemi YA, Danforth E Jr., Jensen MD, Kopelman PG, Lefebvre P, Reeder BA. Dose-response issues concerning physical activity and health: An evidence-based symposium. Med. Sci. Sports Exerc. 2001:S352–S358. [DOI] [PubMed] [Google Scholar]
- 23.Heidt RS Jr., Sweeterman LM, Carlonas RL, Traub JA, Tekulve FX. Avoidance of soccer injuries with preseason conditioning. Am. J. Sports Med. Sep-Oct 2000;28(5):659–662. [DOI] [PubMed] [Google Scholar]
- 24.Myer GD, Ford KR, Hewett TE. Methodological approaches and rationale for training to prevent anterior cruciate ligament injuries in female athletes. Scand. J. Med. Sci. Sports. October 2004;14(5):275–285. [DOI] [PubMed] [Google Scholar]
- 25.Lohmander LS, Ostenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. October 2004;50(10):3145–3152. [DOI] [PubMed] [Google Scholar]
- 26.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am. J. Sports Med. October 2007;35(10):1756–1769. [DOI] [PubMed] [Google Scholar]
- 27.von Porat A, Roos EM, Roos H. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Ann. Rheum. Dis. March 2004;63(3):269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ford KR, Myer GD, Hewett TE. Valgus knee motion during landing in high school female and male basketball players. Med. Sci. Sports Exerc. October 2003;35(10):1745–1750. [DOI] [PubMed] [Google Scholar]
- 29.Andrews JR, Axe MJ. The classification of knee ligament instability. Orthop. Clin. North Am. 1985;16(1):69–82. [PubMed] [Google Scholar]
- 30.Hewett TE, Ford KR, Hoogenboom BJ, Myer GD. Understanding and preventing ACL injuries: Current biomechanical and epidemiologic considerations - Update 2010. North American Journal of Sports Physical Therapy. 2010;5(4):234–251. [PMC free article] [PubMed] [Google Scholar]
- 31.Pappas E, Shiyko MP, Ford KR, Myer GD, Hewett TE. Biomechanical Deficit Profiles Associated with ACL Injury Risk in Female Athletes. Med. Sci. Sports Exerc. January 2016;48(1):107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hewett TE, Myer GD, Ford KR, et al. Biomechanical Measures of Neuromuscular Control and Valgus Loading of the Knee Predict Anterior Cruciate Ligament Injury Risk in Female Athletes: A Prospective Study. Am. J. Sports Med. February 8 2005;33(4):492–501. [DOI] [PubMed] [Google Scholar]
- 33.Boden BP, Dean GS, Feagin JA, Garrett WE. Mechanisms of anterior cruciate ligament injury. Orthopedics. 2000;23(6):573–578. [DOI] [PubMed] [Google Scholar]
- 34.McNair PJ, Marshall RN, Matheson JA. Important features associated with acute anterior cruciate ligament injury. N. Z. Med. J. November 14 1990;103(901):537–539. [PubMed] [Google Scholar]
- 35.Leavell HR, Clark EG. Preventive medicine for the doctor in his community. 3rd ed New York: McGraw-Hill Book Company; 1965. [Google Scholar]
- 36.Shaw KA, Dunoski B, Mardis N, Pacicca D. Knee morphometric risk factors for acute anterior cruciate ligament injury in skeletally immature patients. Journal of children’s orthopaedics. April 2015;9(2):161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peterson JR, Krabak BJ. Anterior cruciate ligament injury: mechanisms of injury and strategies for injury prevention. Phys. Med. Rehabil. Clin. N. Am. November 2014;25(4):813–828. [DOI] [PubMed] [Google Scholar]
- 38.LaBella CR, Hennrikus W, Hewett TE, Council on Sports M, Fitness, Section on O. Anterior cruciate ligament injuries: diagnosis, treatment, and prevention. Pediatrics. May 2014;133(5):e1437–1450. [DOI] [PubMed] [Google Scholar]
- 39.Smith HC, Vacek P, Johnson RJ, et al. Risk factors for anterior cruciate ligament injury: a review of the literature - part 1: neuromuscular and anatomic risk. Sports Health. January 2012;4(1):69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Serpell BG, Scarvell JM, Ball NB, Smith PN. Mechanisms and risk factors for noncontact ACL injury in age mature athletes who engage in field or court sports: A summary of the literature since 1980. Journal of Strength and Conditioning Research. 2012;26(11):3160–3176. [DOI] [PubMed] [Google Scholar]
- 41.Boden BP, Sheehan FT, Torg JS, Hewett TE. Non-contact ACL injuries: mechanisms and risk factors. J. Am. Acad. Orthop. Surg. 2010;18(9):520–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quatman CE, Kiapour A, Ford KR, et al. Simulated ACL Injury Mechanisms: The Effects of Knee Abduction and Anterior Tibial Shear Loads On Passive Knee Restraints. Paper presented at: ACL study Group Annual Conference2010; Phuket, Thailand. [Google Scholar]
- 43.Alentorn-Geli E, Myer GD, Silvers HJ, et al. Prevention of non-contact anterior cruciate ligament injuries in soccer players. Part 1: Mechanisms of injury and underlying risk factors. Knee Surg. Sports Traumatol. Arthrosc. July 2009;17(7):705–729. [DOI] [PubMed] [Google Scholar]
- 44.Yu B, Garrett WE. Mechanisms of non-contact ACL injuries. Br. J. Sports Med. August 2007;41 Suppl 1:i47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hewett TE, Myer GD, Ford KR. Anterior Cruciate Ligament Injuries in Female Athletes: Part 1, Mechanisms and Risk Factors. Am. J. Sports Med. February 2006;34(2):299–311. [DOI] [PubMed] [Google Scholar]
- 46.Myer GD, Ford KR, Brent JL, Hewett TE. Differential neuromuscular training effects on ACL injury risk factors in “high-risk” versus “low-risk” athletes. BMC Musculoskelet Disord. 2007;8(39):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shankar PR, Fields SK, Collins CL, Dick RW, Comstock RD. Epidemiology of high school and collegiate football injuries in the United States, 2005–2006. Am. J. Sports Med. August 2007;35(8):1295–1303. [DOI] [PubMed] [Google Scholar]
- 48.Nader P, Bradley R, Houts R, McRitchie S, O’Brien M. Moderate to vigorous physical activity from ages 9 to 15 years. . Journal of the American Medical Association. 2008;300:295–305. [DOI] [PubMed] [Google Scholar]
- 49.Starkey C. Injuries and illnesses in the national basketball association: A 10-year perspective. J. Athl. Train. 2000;32(2):161–167. [PMC free article] [PubMed] [Google Scholar]
- 50.<Griffin_2000_JAAOS.pdf>.
- 51.Hewett TE, Stroupe AL, Nance TA, Noyes FR. Plyometric training in female athletes. Decreased impact forces and increased hamstring torques. Am. J. Sports Med. 1996;24(6):765–773. [DOI] [PubMed] [Google Scholar]
- 52.Huston LJ, Wojtys EM. Neuromuscular performance characteristics in elite female athletes. Am. J. Sports Med. 1996;24(4):427–436. [DOI] [PubMed] [Google Scholar]
- 53.Knapik JJ, Bauman CL, Jones BH, Harris JM, Vaughan L. Preseason strength and flexibility imbalances associated with athletic injuries in female collegiate athletes. Am. J. Sports Med. 1991;19(1):76–81. [DOI] [PubMed] [Google Scholar]
- 54.Hewett TE. Biomechanical issues related to the gender bias in ACL injuries. 2001; Lexington, Ky. [Google Scholar]
- 55.Hewett TE, Myer GD, Ford KR. Prevention of anterior cruciate ligament injuries. Curr. Womens Health Rep 2001 2001;1(3):218–224. [PubMed] [Google Scholar]
- 56.Renstrom P, Arms SW, Stanwyck TS, Johnson RJ, Pope MH. Strain within the anterior cruciate ligament during hamstring and quadriceps activity. Am. J. Sports Med. Jan-Feb 1986;14(1):83–87. [DOI] [PubMed] [Google Scholar]
- 57.Di Stasi S, Myer GD, Hewett TE. Neuromuscular training to target deficits associated with second anterior cruciate ligament injury. J. Orthop. Sports Phys. Ther November 2013;43(11):777–792, A771–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Withrow TJ, Huston LJ, Wojtys EM, Ashton-Miller JA. The relationship between quadriceps muscle force, knee flexion, and anterior cruciate ligament strain in an in vitro simulated jump landing. Am. J. Sports Med. February 2006;34(2):269–274. [DOI] [PubMed] [Google Scholar]
- 59.Withrow TJ, Huston LJ, Wojtys EM, Ashton-Miller JA. Effect of varying hamstring tension on anterior cruciate ligament strain during in vitro impulsive knee flexion and compression loading. J. Bone Joint Surg. Am. April 2008;90(4):815–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Myer GD, Ford KR, Barber Foss KD, Liu C, Nick TG, Hewett TE. The relationship of hamstrings and quadriceps strength to anterior cruciate ligament injury in female athletes. Clinical journal of sport medicine : official journal of the Canadian Academy of Sport Medicine. January 2009;19(1):3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ithurburn MP, Paterno MV, Ford KR, Hewett TE, Schmitt LC. Young Athletes With Quadriceps Femoris Strength Asymmetry at Return to Sport After Anterior Cruciate Ligament Reconstruction Demonstrate Asymmetric Single-Leg Drop-Landing Mechanics. Am. J. Sports Med. November 2015;43(11):2727–2737. [DOI] [PubMed] [Google Scholar]
- 62.Paterno MV, Schmitt LC, Ford KR, et al. Biomechanical measures during landing and postural stability predict second anterior cruciate ligament injury after anterior cruciate ligament reconstruction and return to sport. The American journal of sports medicine. October 2010;38(10):1968–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Myer GD, Ford KR, Khoury J, Succop P, Hewett TE. Biomechanics laboratory-based prediction algorithm to identify female athletes with high knee loads that increase risk of ACL injury. Br. J. Sports Med. June 17 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zazulak BT, Hewett TE, Reeves NP, Goldberg B, Cholewicki J. The Effects of Core Proprioception on Knee Ligament Injury: A Prospective Biomechanical-Epidemiological Study. Accepted AOSSM Specialty Day San Diego, California. 2007. [DOI] [PubMed] [Google Scholar]
- 65.Stensrud S, Myklebust G, Kristianslund E, Bahr R, Krosshaug T. Correlation between two-dimensional video analysis and subjective assessment in evaluating knee control among elite female team handball players. Br. J. Sports Med. June 2011;45(7):589–595. [DOI] [PubMed] [Google Scholar]
- 66.Ekegren CL, Miller WC, Celebrini RG, Eng JJ, Macintyre DL. Reliability and validity of observational risk screening in evaluating dynamic knee valgus. J. Orthop. Sports Phys. Ther September 2009;39(9):665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Myer GD, Ford KR, Khoury J, Succop P, Hewett TE. Development and validation of a clinic-based prediction tool to identify female athletes at high risk for anterior cruciate ligament injury. The American journal of sports medicine. October 2010;38(10):2025–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Myer GD, Ford KR, Khoury J, Succop P, Hewett TE. Clinical correlates to laboratory measures for use in non-contact anterior cruciate ligament injury risk prediction algorithm. Clin Biomech. August 2010;25(7):693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Myer GD, Ford KR, Hewett TE. New method to identify athletes at high risk of ACL injury using clinic-based measurements and freeware computer analysis. Br. J. Sports Med. November 16 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Myer GD, Ford KR, Khoury J, Hewett TE. Three-dimensional motion analysis validation of a clinic-based nomogram designed to identify high ACL injury risk in female athletes. Phys Sportsmed. February 2011;39(1):19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Padua DA, Marshall SW, Boling MC, Thigpen CA, Garrett WE Jr., Beutler AI. The Landing Error Scoring System (LESS) Is a valid and reliable clinical assessment tool of jump-landing biomechanics: The JUMP-ACL study. Am. J. Sports Med. October 2009;37(10):1996–2002. [DOI] [PubMed] [Google Scholar]
- 72.Hewett TE, Myer GD, Ford KR, Paterno MV, Quatman CE. The 2012 ABJS Nicolas Andry Award: The sequence of prevention: a systematic approach to prevent anterior cruciate ligament injury. Clin Orthop Relat Res. October 2012;470(10):2930–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Padua DA, Boling MC, DiStefano LJ, Onate JA, Beutler AI, Marshall SW. Reliability of the landing error scoring system-real time, a clinical assessment tool of jump-landing biomechanics. J Sport Rehabil. 2011;20:145–156. [DOI] [PubMed] [Google Scholar]
- 74.Smith HC, Johnson RJ, Shultz SJ, et al. A prospective evaluation of the Landing Error Scoring System (LESS) as a screening tool for anterior cruciate ligament injury risk. Am. J. Sports Med. March 2012;40(3):521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Padua DA, DiStefano LJ, Beutler AI, de la Motte SJ, DiStefano MJ, Marshall SW. The Landing Error Scoring System as a Screening Tool for an Anterior Cruciate Ligament Injury-Prevention Program in Elite-Youth Soccer Athletes. J. Athl. Train. June 2015;50(6):589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zebis MK, Andersen LL, Bencke J, Kjaer M, Aagaard P. Identification of athletes at future risk of anterior cruciate ligament ruptures by neuromuscular screening. Am. J. Sports Med. October 2009;37(10):1967–1973. [DOI] [PubMed] [Google Scholar]
- 77.Khayambashi K, Ghoddosi N, Straub RK, Powers CM. Hip Muscle Strength Predicts Noncontact Anterior Cruciate Ligament Injury in Male and Female Athletes: A Prospective Study. Am. J. Sports Med. February 2016;44(2):355–361. [DOI] [PubMed] [Google Scholar]
- 78.Redler LH, Watling JP, Dennis ES, Ahmad CS. Relibility of a field-based drop vertical jump screening test for ACL injury risk assessment. The Physician and Sportsmedicine. 2015;44(1):46–52. [DOI] [PubMed] [Google Scholar]
- 79.McLean SG, Huang X, Su A, van den Bogert AJ. Sagittal plane biomechanics cannot injure the ACL during sidestep cutting. Clin Biomech. 2004;19:828–838. [DOI] [PubMed] [Google Scholar]
- 80.Markolf KL, Burchfield DM, Shapiro MM, Shepard MF, Finerman GA, Slauterbeck JL. Combined knee loading states that generate high anterior cruciate ligament forces. J. Orthop. Res. November 1995;13(6):930–935. [DOI] [PubMed] [Google Scholar]
- 81.Shin CS, Chaudhari AM, Andriacchi TP. Valgus plus internal rotation moments increase anterior cruciate ligament strain more than either alone. Med. Sci. Sports Exerc. August 2011;43(8):1484–1491. [DOI] [PubMed] [Google Scholar]
- 82.Withrow TJ, Huston LJ, Wojtys EM, Ashton-Miller JA. The effect of an impulsive knee valgus moment on in vitro relative ACL strain during a simulated jump landing. Clin Biomech. November 2006;21(9):977–983. [DOI] [PubMed] [Google Scholar]
- 83.Oh YK, Kreinbrink JL, Wojtys EM, Ashton-Miller JA. Effect of axial tibial torque direction on ACL relative strain and strain rate in an in vitro simulated pivot landing. J. Orthop. Res. April 2012;30(4):528–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ford KR, Myer GD, Smith RL, Vianello RM, Seiwert SL, Hewett TE. A comparison of dynamic coronal plane excursion between matched male and female athletes when performing single leg landings. Clin Biomech. 2006;21(1):33–40. [DOI] [PubMed] [Google Scholar]
- 85.Ford KR, Myer GD, Schmitt LC, Uhl TL, Hewett TE. Preferential quadriceps activation in female athletes with incremental increases in landing intensity. J Appl Biomech. August 2011;27(3):215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hewett TE, Myer GD, Ford KR. Decrease in neuromuscular control about the knee with maturation in female athletes. J. Bone Joint Surg. Am. 2004;86-A(8):1601–1608. [DOI] [PubMed] [Google Scholar]
- 87.Walden M, Atroshi I, Magnusson H, Wagner P, Hagglund M. Prevention of acute knee injuries in adolescent female football players: cluster randomised controlled trial. BMJ. 2012;344:e3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Myer GD, Ford KR, Di Stasi SL, Foss KD, Micheli LJ, Hewett TE. High knee abduction moments are common risk factors for patellofemoral pain (PFP) and anterior cruciate ligament (ACL) injury in girls: is PFP itself a predictor for subsequent ACL injury? Br. J. Sports Med. January 2015;49(2):118–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Weiss K, Whatman C. Biomechancis associated with patellofemoral pain and ACL injuries in sports. Sports Med. 2015;45(9):1325–1337. [DOI] [PubMed] [Google Scholar]
- 90.Boling MC, Padua DA, Marshall SW, Guskiewicz K, Pyne S, Beutler A. A Prospective Investigation of Biomechanical Risk Factors for Patellofemoral Pain Syndrome: The Joint Undertaking to Monitor and Prevent ACL Injury (JUMP-ACL) Cohort. The American Journal of Sports Medicine. 2009;37(11):2108–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ford KR, Nguyen AD, Dischiavi SL, Hegedus EJ, Zuk EF, Taylor JB. An evidence-based review of hip-focused neuromuscular exercise interventions to address dynamic lower extremity valgus. Open access journal of sports medicine. 2015;6:291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Alentorn-Geli E, Myer GD, Silvers HJ, et al. Prevention of non-contact anterior cruciate ligament injuries in soccer players. Part 2: a review of prevention programs aimed to modify risk factors and to reduce injury rates. Knee Surg. Sports Traumatol. Arthrosc. August 2009;17(8):859–879. [DOI] [PubMed] [Google Scholar]
- 93.Herman DC, Weinhold PS, Guskiewicz KM, Garrett WE, Yu B, Padua DA. The Effects of Strength Training on the Lower Extremity Biomechanics of Female Recreational Athletes During a Stop-Jump Task. Am. J. Sports Med. January 22 2008. [DOI] [PubMed] [Google Scholar]
- 94.Abt JP, Sell TC, Laudner KG, et al. Neuromuscular and biomechanical characteristics do not vary across the menstrual cycle. Knee Surg. Sports Traumatol. Arthrosc. July 2007;15(7):901–907. [DOI] [PubMed] [Google Scholar]
- 95.Chappell JD, Limpisvasti O. Effect of a neuromuscular training program on the kinetics and kinematics of jumping tasks. Am. J. Sports Med. June 2008;36(6):1081–1086. [DOI] [PubMed] [Google Scholar]
- 96.Willson JD, Kernozek TW, Arndt RL, Reznichek DA, Scott Straker J. Gluteal muscle activation during running in females with and without patellofemoral pain syndrome. Clin Biomech. August 2011;26(7):735–740. [DOI] [PubMed] [Google Scholar]
- 97.Willson JD, Petrowitz I, Butler RJ, Kernozek TW. Male and female gluteal muscle activity and lower extremity kinematics during running. Clin Biomech. December 2012;27(10):1052–1057. [DOI] [PubMed] [Google Scholar]
- 98.Myer GD, Chu DA, Brent JL, Hewett TE. Trunk and hip control neuromuscular training for the prevention of knee joint injury. Clin. Sports Med. July 2008;27(3):425–448, ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Paterno MV, Myer GD, Ford KR, Hewett TE. Neuromuscular training improves single-limb stability in young female athletes. J. Orthop. Sports Phys. Ther. 2004;34(6):305–317. [DOI] [PubMed] [Google Scholar]
- 100.Zazulak BT, Hewett TE, Reeves NP, Goldberg B, Cholewicki J. Deficits in neuromuscular control of the trunk predict knee injury risk: a prospective biomechanical-epidemiologic study. Am. J. Sports Med. July 2007;35(7):1123–1130. [DOI] [PubMed] [Google Scholar]
- 101.Zazulak BT, Hewett TE, Reeves NP, Goldberg B, Cholewicki J. The effects of core proprioception on knee injury: a prospective biomechanical-epidemiological study. Am. J. Sports Med. March 2007;35(3):368–373. [DOI] [PubMed] [Google Scholar]
- 102.Begalle RL, Distefano LJ, Blackburn T, Padua DA. Quadriceps and hamstrings coactivation during common therapeutic exercises. J. Athl. Train. Jul-Aug 2012;47(4):396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fagenbaum R, Darling WG. Jump landing strategies in male and female college athletes and the implications of such strategies for anterior cruciate ligament injury. Am. J. Sports Med. Mar-Apr 2003;31(2):233–240. [DOI] [PubMed] [Google Scholar]
- 104.Stroube BW, Myer GD, Brent JL, Ford KR, Heidt RS Jr, Hewett TE. Effects of task-specific augmented feedback on deficit modification during performance of the tuc-jump exercise. J Sport Rehabil. 2013;22(1):7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Louw QA, Manilall J, Grimmer KA. Epidemiology of knee injuries among adolescents: a systematic review. Br. J. Sports Med. January 2008;42(1):2–10. [DOI] [PubMed] [Google Scholar]
- 106.Joseph AM, Collins CL, Henke NM, Yard EE, Fields SK, Comstock RD. A multisport epidemiologic comparison of anterior cruciate ligament injuries in high school athletics. J. Athl. Train. Nov-Dec 2013;48(6):810–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ingram JG, Fields SK, Yard EE, Comstock RD. Epidemiology of knee injuries among boys and girls in US high school athletics. Am. J. Sports Med. June 2008;36(6):1116–1122. [DOI] [PubMed] [Google Scholar]
- 108.Swenson DM, Collins CL, Best TM, Flanigan DC, Fields SK, Comstock RD. Epidemiology of knee injuries among U.S. high school athletes, 2005/2006–2010/2011. Med. Sci. Sports Exerc. March 2013;45(3):462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hewett TE, Lindenfeld TN, Riccobene JV, Noyes FR. The effect of neuromuscular training on the incidence of knee injury in female athletes. A prospective study. Am. J. Sports Med. Nov-Dec 1999;27(6):699–706. [DOI] [PubMed] [Google Scholar]
- 110.Bates NA, McPherson AL, Rao MB, Myer GD, Hewett TE. Characteristics of inpatient anterior cruciate ligament reconstructions and concomitant injuries. Knee Surg. Sports Traumatol. Arthrosc. December 16 2015;in-press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kim S, Bosque J, Meehan JP, Jamali A, Marder R. Increase in outpatient knee arthroscopy in the United States: a comparison of National Surveys of Ambulatory Surgery, 1996 and 2006. J. Bone Joint Surg. Am. June 1 2011;93(11):994–1000. [DOI] [PubMed] [Google Scholar]
- 112.Griffin LY, Albohm MJ, Arendt EA, et al. Understanding and preventing noncontact anterior cruciate ligament injuries: a review of the Hunt Valley II meeting, January 2005. Am. J. Sports Med. September 2006;34(9):1512–1532. [DOI] [PubMed] [Google Scholar]
- 113.Paterno MV, Rauh MJ, Schmitt LC, Ford KR, Hewett TE. Incidence of Second ACL Injuries 2 Years After Primary ACL Reconstruction and Return to Sport. Am. J. Sports Med. April 21 2014;42(7):1567–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wiggins AJ, Grandhi RK, Schneider DK, Stanfield D, Webster KE, Myer GD. Risk of Secondary Injury in Younger Athletes After Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-analysis. Am. J. Sports Med. January 15 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Paterno MV, Rauh MJ, Schmitt LC, Ford KR, Hewett TE. Incidence of contralateral and ipsilateral anterior cruciate ligament (ACL) injury after primary ACL reconstruction and return to sport. Clin. J. Sport Med. March 2012;22(2):116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ardern CL, Webster KE, Taylor NF, Feller JA. Return to sport following anterior cruciate ligament reconstruction surgery: a systematic review and meta-analysis of the state of play. Br. J. Sports Med. March 11 2011. [DOI] [PubMed] [Google Scholar]
- 117.Shah VM, Andrews JR, Fleisig GS, McMichael CS, Lemak LJ. Return to play after anterior cruciate ligament reconstruction in National Football League athletes. Am. J. Sports Med. November 2010;38(11):2233–2239. [DOI] [PubMed] [Google Scholar]
- 118.Faltstrom A, Hagglund M, Kvist J. Patient-reported knee function, quality of life, and activity level after bilateral anterior cruciate ligament injuries. Am. J. Sports Med. December 2013;41(12):2805–2813. [DOI] [PubMed] [Google Scholar]
- 119.McCullough KA, Phelps KD, Spindler KP, et al. Return to high school- and college-level football after anterior cruciate ligament reconstruction: a Multicenter Orthopaedic Outcomes Network (MOON) cohort study. Am. J. Sports Med. November 2012;40(11):2523–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Paterno MV, Myer GD, Ford KR, Hewett TE. Neuromuscular training improves postural stability in young female athletes. Med Sci Sport Exerc. 2004;36(5). [DOI] [PubMed] [Google Scholar]


