Abstract
Background:
Anterior cruciate ligament (ACL) injuries are catastrophic events that impact athletic careers and lead to long-term, degenerative knee changes. As injuries are believed to occur within the first 50 msec following initial contact during a rapid deceleration task, impact simulators that rapidly deliver impulse loads to cadaveric specimens have been developed. However, no impactor has reproducibly and reliably created ACL injures in a distribution that mimics clinical observation.
Purpose:
The purpose of this investigation was to better understand ACL injury patterns through a cadaveric investigation that applied in vivo measured external loads to the knee during simulated landings.
Study Design:
Controlled laboratory study.
Methods:
A novel mechanical impact simulator reproduced kinetics from in vivo recorded drop landing tasks on 45 cadaveric knees. Specimens were exposed to a randomized order of variable knee abduction moment, anterior tibial shear, and internal tibial rotation loads prior to the introduction of an impulse load at the foot. This process was repeated until hard or soft tissue injury was induced on the joint. Injuries were assessed by an orthopedic surgeon and ligament strain was recorded by implanted strain gauges.
Results:
The mechanical impact simulator induced ACL injuries in 87% of specimens with medial collateral ligament (MCL) injuries in 31%. ACL tear locations were 71% femoral side, 25% midsubstance, and 4% tibial side. Peak strain prior to failure for ACL-injured specimens was 15.3 ± 8.7% for the ACL and 5.1 ± 5.6% for the MCL (P < 0.001).
Conclusion:
The ACL injuries induced by the mechanical impact simulator in the present study have provided clinically relevant in vitro representations of in vivo ACL injury patterns as cited in the literature. Additionally, current ligament strains corroborate with the literature to support disproportionately loading of the ACL relative to the MCL during athletic tasks.
Keywords: Anterior cruciate ligament, jump landing, injury simulation, impact, knee ligament biomechanics
INTRODUCTION
Anterior cruciate ligament (ACL) injuries occur frequently in sports,4, 18, 23 are expensive,5, 19 and involve a lengthy 6–12 month rehabilitation process.16 Only 76% of young athletes return their pre-injury level of competitive sport and only 53% continue to participate 5 years post-injury.37 Therefore, prevention may be the most effective mechanisms to address ACL injures. In order to develop improved injury prevention programs, it is necessary to better understand what movements or combination of movements cause ACL injuries. It is difficult to quantify these mechanical behaviors in vivo, although bi-plane fluoroscopy has been used to quantify differences in tibiofemoral translations between ACL reconstructed and contralateral knees during landing and hoping tasks.14, 19 However, these technical and labor-intensive in vivo investigations lack the ability to examine knee and ligament mechanics in an injurious environment. In addition, a multitude of institutions have utilized robotic manipulators and impact simulators to perform in vitro assessments of intra-articular tibiofemoral mechanics in ACL-intact, ACL-deficient, and ACL-reconstructed knees during simulated clinical exams,5 gait,12, 17 and even athletic tasks.7, 26 Unfortunately, most of these simulations have again failed to create an ACL rupture condition within the in vitro environment.5 The one simulator that has effected consistent ACL rupture (88% of specimens) primarily incurred injuries at the tibial eminence.26 This is not a commonly-observed, clinical ACL failure mechanism, which primarily occur in the midsubstance or at the femoral origin.35, 36 Due to these limitations, our investigative team developed a mechanical impact simulator designed to induce ACL injuries on cadaveric specimens in a manner consistent with clinical observation.9 Validation of the morphology of ACL injures in a simulated in vitro model against clinical presentation would be significant because it would provide researchers with a unique tool to assess efficacy of clinical prevention, surgical intervention, and rehabilitation techniques outside of a patient population.
The purpose of this investigation was to quantify the underlying intra-articular mechanical behaviors that occur during simulated landings tasks that lead up to and induce ACL injuries through a cadaveric investigation that applied in vivo measured external loads to the knee. A laboratory instrument that can consistently manifest ACL injuries in a manner that replicates the clinical display and distribution could offer the potential not only to better quantify underlying intra-articular stresses, strains, and loadings that cannot currently be assessed directly in vivo, but also, to investigate the potential efficacy of injury prevention and surgical techniques in an in vitro athletic environment. The hypothesis tested was that our novel mechanical impactor design would reproducibly incite ACL injury patterns on cadaveric specimens that correlate well with clinically observed failure patterns. In addition, because females are at higher risk for ACL injury, a secondary objective of this investigation was to determine whether male or female specimens share similar peak strains during failure events. It was hypothesized that female specimens that experienced ACL rupture would do so at a higher peak strain than male specimens.
METHODS
Forty-five cadaveric full lower extremity specimens from the femoral head to the toes from 27 different donors were obtained from an anatomical donations program (Anatomy Gifts Registry, Hanover, MD) and subjected to mechanical impact testing. Six specimens were excluded due to failure induced by structural weakness of the specimen during setup (n = 2), substandard stiffness of bone tissue (n = 1), equipment failure / inconsistency in testing execution (n = 3). This left 39 specimens from 25 different donors (19 males, 20 females, age = 41.5 ± 8.4 years, mass = 85.7 ± 25.6 kg, height = 173 ± 11 cm, BMI = 28 ± 8) for analysis.
Testing was executed as previously described.9 Briefly, each specimen was resected of soft tissues 20 cm proximal to the tibiofemoral joint line. Skin was then further resected down to 3 cm proximal to the superior aspect of the patella. The tendons of the semitendinosus, semimembranosus, gracilis, biceps femoris, rectus femoris, vastus lateralis, vastus medialis, and vastus intermedius were denuded of muscle tissue and secured into wire clamps. The femur was then resected transversely 20 cm proximal to the superior aspect of the patella, potted in Bondo®, inverted, and secured into a custom mounting fixture such that the long axis of the femur was aligned with the vertical axis of a six degree-of-freedom load cell (Omega160 IP65/IP68, ATI Industrial Automation, Inc., Apex, NC, USA) that was integrated into the novel mechanical impact simulator. Pneumatic pistons (CG5LN40SV-100 and CG5LN50SV-100, SMC Corporation, Tokyo, Japan) mounted on the mechanical impact simulator were then affixed to the tendon clamps with carbon fiber rope (Ø7/64 in.; Amsteel®-Blue, Samson, Ferndale, WA, USA) such that muscle forces could be applied throughout testing. A custom-manufactured compression clamp was then affixed to the tibia in alignment with the long axis of the tibia. This structure allowed additional pneumatic pistons (MQQTL40TN-100DM and CG5LN80SV-100, SMC Corporation, Tokyo, Japan) mounted to the mechanical impact simulator (Figure 1) to apply externally generated anterior tibial shear (ATS), knee abduction rotation (KAM), and internal tibial rotation (ITR) moments to the knee joint. The tibial and femoral components were secured such that the tibia was oriented vertically and 25° flexed from full knee extension, which is representative of the initial contact knee orientation observed in young athletes landing from a jump.3, 24
Figure 1:
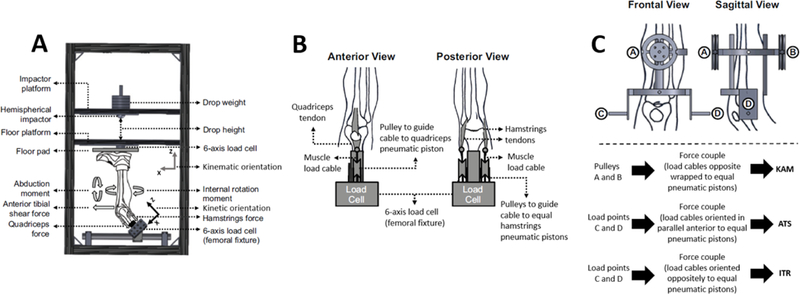
(A) Meta-view of custom designed mechanical impact simulator for creation of ACL ruptures,9 (B) cable pulley system used to deliver pneumatically actuated loads to the quadriceps and hamstrings tendons, (C) external fixation frame attached to the tibia and used to deliver pneumatically actuated KAM, ATS, and ITR loads to each specimen. This figure has been adapted from Kiapour, et al. 2016. Am J Sports Med. 44(8):2087–2096.21 The most apparent design difference between the current mechanical impact simulator and the previous drop-stand testing apparatus is that the external load are now actuated in a 3 sec interval from pneumatic pistons as opposed to static hanging weights individually suspended from a pulley system.9
The mechanical impact simulator then delivered an impulse force to the plantar aspect of each specimen’s foot by releasing a suspended weighted sled from a 31 cm height. Prior to the release of the weight sled, both the tendon and external force pneumatic pistons activated to preload the specimen to the desired landing conditions as determined from previous in vivo evaluations. Each specimen was subjected to a two part testing protocol designated ‘sub-failure’ and ‘failure’ testing. For sub-failure testing, 450 N of force was applied to both the quadriceps and hamstrings tendon clamps in order to create an ideal 1:1 ratio of muscle loading,27 while the weight sled was loaded with a consistent 34.0 kg load. The magnitude of forces applied by the external ATS, KAM, and ITR cylinders were predetermined from in vivo motion analysis data previously collected on a cohort of 67 healthy, young adult athletes (age = 23.2 (3.9) years; mass = 73.3 (13.4) kg) preforming drop vertical jump tasks.9 These external loads were relative to the 0th, 33rd, 67th, and 100th percentile of forces generated in the in vivo cohort and were applied to the specimens in a randomized order such that a potential of 46 sub-failure impacts could be completed on a single specimen.9 The in vivo cohort was captured using a 55 marker modified Helen Hayes marker set, 12 camera retroreflective motion analysis system (Raptor-12 cameras, Motion Analysis Corporation, Santa Rosa, CA, USA), dual in-ground 6-axis force platforms (FP6090–15-2000, Bertec Corporation, Columbus, OH, USA), and a custom biomechanical model (Visual3D, version 5, C-motion Inc., Germantown, MD, USA) to calculate joint kinetics and kinematics.9 Upon completion of sub-failure testing, failure testing would maintain the 1:1 ratio of quadriceps and hamstrings pre-tensioning, adjust the weight sled to 0.5 * bodyweight, and initiate each of the external load pistons at the 100th percentile magnitude. Following each delivered ground impulse, the magnitude of each external load piston would be increased by 20% of the 100th percentile magnitude. This would be repeated until specimen failure was induced or the maximal loading tolerance of the mechanical impact simulator was reached. If an impact generated soft or hard tissue damage at any point during ‘sub-failure’ or ‘failure’ testing, the remaining protocol was aborted and specimen failure was denoted.
Prior to testing, a board certified orthopedic surgeon clinically and arthroscopically examined each specimen to ensure structural integrity. Following testing termination, the same surgeon re-examined each specimen to verify any induced structural damage. A Lachman’s test, anterior drawer test, posterior drawer test, and medial/lateral laxity evaluations were performed by a board certified orthopaedic surgeon (AJK) and scored on a clinical scale from grade 0 (normal) to grade 3 (ruptured, nonfunctional). Specifically, grade 1 was defined as 5 mm laxity, grade 2 was 5 to 10 mm laxity, and grade 3 greater than 10 mm laxity. Due to the mid-femur resection of each specimen, pivot-shift testing could not be completed on the present cohort once they were prepared for simulation. During testing, the six-degree-of-freedom load cell recorded forces and torques at the tibiofemoral joint. A uniaxial load cell (1720ACK-10kN, Interface, Inc., Scottsdale, AZ, USA) mounted to the plantar aspect of the foot recorded the impulse force delivered to the specimen. Additional uniaxial force transducers (MLP-300 and MLP-1K, Transducer Techniques, LLC, Temecula, CA, USA) mounted to the end effectors of the muscle and external force pneumatic pistons verified their magnitude of force application during each trial. On each specimen, portals were opened through the skin and joint capsule into the anterior aspect of the knee to allow for the implantation of a differential variance resistance transducer (DVRT, LORD MicroStrain, Willingston, VT, USA) onto the anteromedial bundle of the ACL to record ACL strain. Similarly, a portal was cut into the medial aspect of the knee to allow for implantation of a DVRT on the midsubstance of the medial collateral ligament (MCL) across the tibiofemoral joint line to record MCL strain. In order to create sufficient intra-articular clearance and prevent bony impingement for the ACL-implanted DVRT, a surgeon-trained biomechanics researcher performed a partial femoral notchplasty on each specimen with an arthroscopic burr (Stryker Corporation, Kalamazoo, MI, USA). During notchplasty, care was taken to avoid damaging any of the articulating cartilage surfaces as a limited amount of bone stock was reamed off of the medial wall of the femoral notch. Tissue removal was not explicitly quantified, but was limited to just enough volume to ensure the DVRT sensor would not impinge on the bone during joint rotation. Neutral strain for the ACL and MCL were determined prior to testing by articulation of the tibia through anterior-posterior and abduction-adduction motions, respectively, with observation of the inflection point of the DVRT. Identification of the neutral strain point for each ligament allows for the reporting of absolute strain as opposed to relative strain. These strain measurement techniques were derived from the literature.6, 9, 11, 15, 22, 26, 31 DVRTs were also used to identify when damage was induced on the ACL or MCL as rupture trials would be depicted by significant change or complete absence of elastic behavior in either ligament.
All data were collected at 10 kHz and filtered through a fourth-order 12 Hz low-pass Butterworth filter. All event timing for the mechanical impact simulator was synchronized via custom LabVIEW code (version 2016, National Instruments, Austin, TX, USA). Data processing was performed via custom MATLAB code (version 2015b, The MathWorks, Inc., Natick, MA, USA). For analysis, data capture began at initial contact and was extended for 1.0 sec following initial contact, which is nearly double the duration of landing phase following a drop landing.2 Initial contact was defined as the point where the vertical ground reaction force recorded by the impactor exceeded 25 N of force. Peak strain for both ligaments was considered to be the maximum strain that was recorded by the respective DVRTs during any impact prior to the failure trial. Differences in pre- and post-clinical exam scores as well as ACL and MCL strain were assessed with a paired t-test (α = 0.05). Sex differences in clinical exam scores, ACL rupture locations, and injury outcomes were assessed with a Fisher’s Exact Test (α = 0.05). A comprehensive analysis of absolute ligament strain and the change in ligament strain from initial contact to peak value required six separate t-tests to examine statistical differences between clinical conditions (whole sample population, ACL injured specimens, MCL injured specimens) and sex (male, female). Therefore, a Bonferroni correction was applied such that α = 0.05/6 = 0.008. Change in ligament strain is important for analysis in addition to absolute strain because between-specimen variability in absolute strains can be relatively large, which can foster large standard deviations and a wide range of rupture values across a specimen population.5, 8, 26 Large standard deviations can mask relevant changes; however, delta strain values relative to a specimen-specific baseline value tend to exhibit greater inter-specimen consistency and can subsequently reveal clinically-significant mechanical patterns that would be overlooked with absolute values.8
RESULTS
Modes of Failure
Anterior cruciate ligament ruptures were observed in 34 specimens (87%; Table 1 & 2). ACL failure location was predominantly proximal or near the femoral insertion, followed by midsubstance rupture, followed by failure at the tibial eminence (Table 3, Figure 2 & 3). In the present cohort, 85% of ACL ruptures were complete tears, while the remaining 15% were partial tears. Additional injuries incurred as a result of impact simulations were MCL rupture (31%), fibular collateral ligament (FCL) rupture (15%), meniscus tear (13%), and long bone fracture (8%). The long bone fractures were one male femur fracture, one female femur fracture, and one male tibial plateau fracture. Concomitant injuries of the MCL, FCL, or menisci were not associated with ACL rupture location in our population cohort (Chi-Squared P-values ≥ 0.43). Score differences between pre-testing and post-testing clinical evaluations were noted for the Lachman’s Exam (Δ = 2.5 ± 1.0; P < 0.001; Table 4), anterior drawer (Δ = 2.3 ± 1.0; P < 0.001), MCL integrity (Δ = 0.6 ± 1.2; P = 0.003), and FCL integrity (Δ = 0.3 ± 0.8; P = 0.027).
Table 1:
Injury pattern for each specimen.
| Specimen | Sex | Side | ACL Disruption |
ACL Location | MCL Disruption |
Damage to Other Structures |
|---|---|---|---|---|---|---|
| DON01 | F | L | Partial | Femoral Side | No Change | |
| DON01 | F | R | Complete | Femoral Side | No Change | |
| DON02 | M | R | Complete | Femoral Side | No Change | |
| DON03 | F | L | Partial | Midsubstance | No Change | |
| DON03 | F | R | Intact | No Change | ||
| DON04 | M | L | Complete | Midsubstance | No Change | |
| DON05 | M | L | Complete | Femoral Side | Complete | |
| DON05 | M | R | Complete | Femoral Side | Complete | |
| DON06 | M | L | Complete | Midsubstance | Complete | FCL, lat. meniscus, PL corner |
| DON06 | M | R | Complete | Midsubstance | Complete | FCL |
| DON07 | F | L | Intact | Complete | ||
| DON07 | F | R | Complete | Femoral Side | Complete | |
| DON08 | F | L | Complete | Femoral Side | No Change | |
| DON08 | F | R | Intact | No Change | Med. meniscus | |
| DON09 | M | L | Complete | Femoral Side | No Change | FCL, lat. meniscus |
| DON09 | M | R | Partial | Femoral Side | No Change | FCL |
| DON10 | M | R | Complete | Femoral Side | No Change | Lat. meniscus, med. meniscus |
| DON11 | M | R | Complete | Femoral Side | No Change | |
| DON12 | F | R | Complete | Femoral Side | Partial | |
| DON13 | F | L | Complete | Femoral Side | No Change | |
| DON13 | F | R | Complete | Femoral Side | Complete | |
| DON14 | M | R | Intact | Complete | ||
| DON15 | M | L | Complete | Femoral Side | No Change | |
| DON15 | M | R | Complete | Femoral Side | No Change | |
| DON16 | F | L | Complete | Femoral Side | No Change | FCL |
| DON17 | M | R | Intact | No Change | ||
| DON18 | F | L | Complete | Midsubstance | No Change | |
| DON19 | F | L | Complete | Femoral Side | No Change | |
| DON19 | F | R | Complete | Femoral Side | No Change | |
| DON20 | F | L | Partial | Midsubstance | No Change | |
| DON20 | F | R | Partial | Midsubstance | No Change | |
| DON21 | M | L | Complete | Femoral Side | Complete | med. meniscus |
| DON21 | M | R | Complete | Femoral Side | No Change | |
| DON22 | F | L | Complete | Tibial Side | No Change | |
| DON23 | M | L | Complete | Tibial Side | No Change | FCL |
| DON24 | M | L | Complete | Femoral Side | Complete | |
| DON24 | M | R | Complete | Femoral Side | No Change | |
| DON25 | F | L | Complete | Femoral Side | No Change | |
| DON25 | F | R | Complete | Tibial Side | Complete | |
Table 2:
Distribution of impactor-induced injuries across structures.
| ACL | MCL | FCL | PCL | Meniscus | Fracture | Survival | |
|---|---|---|---|---|---|---|---|
| Specimen (n = 39) | |||||||
| N | 34 | 12 | 6 | 0 | 5 | 3 | 1 |
| Percent | 87% | 31% | 15% | 0% | 13% | 8% | 3% |
| Males (n = 19) | |||||||
| N | 17 | 7 | 5 | 0 | 4 | 2 | 0 |
| Percent | 89% | 37% | 26% | 0% | 21% | 11% | 0% |
| Females (n = 20) | |||||||
| N | 17 | 5 | 1 | 0 | 1 | 1 | 1 |
| Percent | 85% | 25% | 5% | 0% | 5% | 5% | 5% |
Table 3:
Distribution of location and type for ACL injuries.
| Femoral Side |
Mid- substance |
Tibial Side |
Complete | Partial | |
|---|---|---|---|---|---|
| ACL Ruptures (n = 34) | |||||
| N | 24 | 7 | 3 | 29 | 5 |
| Percent | 71% | 21% | 9% | 85% | 15% |
| Males (n = 17) | |||||
| N | 13 | 3 | 1 | 16 | 1 |
| Percent | 76% | 18% | 6% | 94% | 6% |
| Females (n = 17) | |||||
| N | 11 | 4 | 2 | 13 | 4 |
| Percent | 65% | 24% | 12% | 76% | 24% |
Figure 2:
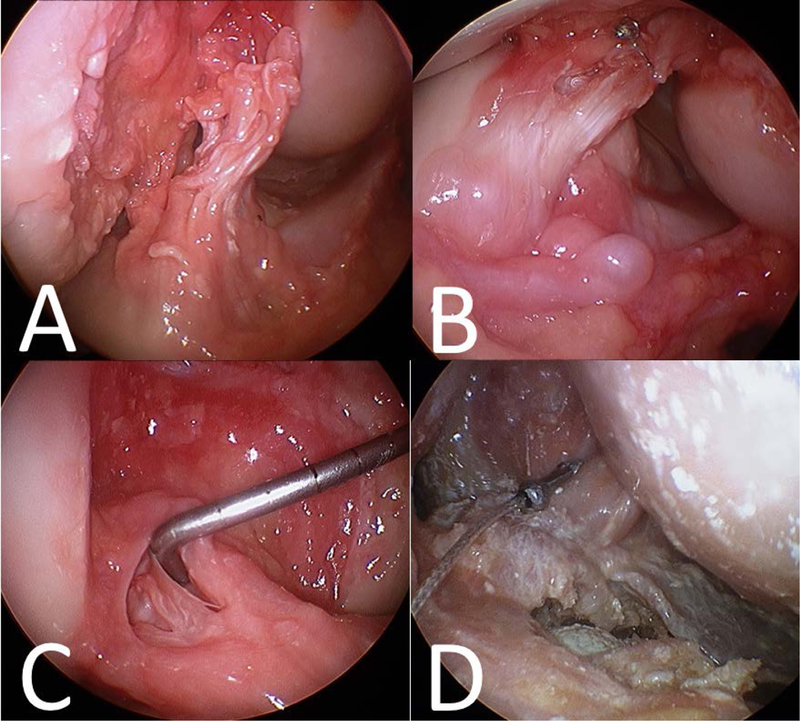
Examples of ACL injury patterns induced by the mechanical impact simulator (A) complete femoral side rupture, (B) complete midsubstance rupture, (C) partial midsubstance rupture, (D) complete tibial side rupture.
Figure 3:
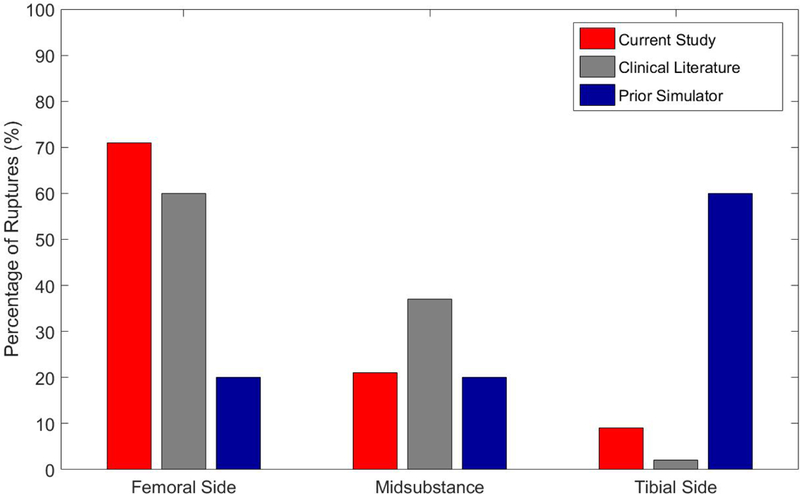
Distribution of ACL tear location as a percentage for the current mechanical impact simulator data (red), average from clinical literature (gray),35, 36 and a previous impact simulator (blue).26 The mechanical impact simulator is significantly closer to matching the clinical distribution of injuries than the previous impactor.
Table 4:
Pre- and post-impact clinical exam scores (0 = intact, 3 = ruptured, non-functional).
| Lachman | Ant. Drawer |
Post. Drawer |
MCL | FCL | |
|---|---|---|---|---|---|
| Specimens (n = 39) | |||||
| Pre-Test | 0.0 ± 0.2 | 0.0 ± 0.2 | 0.0 ± 0.0 | 0.2 ± 0.7 | 0.0 ± 0.0 |
| Post-Test | 2.5 ± 1.0 | 2.4 ± 1.1 | 0.0 ± 0.0 | 0.8 ± 1.2 | 0.3 ± 0.8 |
| Males (n = 17) | |||||
| Pre-Test | 0.1 ± 0.2 | 0.1 ± 0.2 | 0.0 ± 0.0 | 0.3 ± 0.7 | 0.1 ± 0.2 |
| Post-Test | 2.6 ± 1.0 | 2.6 ± 1.0 | 0.0 ± 0.0 | 1.0 ± 1.2 | 0.6 ± 1.0 |
| Females (n = 17) | |||||
| Pre-Test | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.2 ± 0.7 | 0.0 ± 0.0 |
| Post-Test | 2.4 ± 1.1 | 2.2 ± 1.1 | 0.0 ± 0.0 | 0.7 ± 1.1 | 0.1 ± 0.4 |
The peak strain before failure in the 34 ACL-injured specimens was 15.3 ± 8.7% for the ACL and 5.1 ± 5.6% for the MCL (P < 0.001; Table 5). For the ACL-injured specimens, the change in strain was greater in the ACL than the MCL both from initial contact (IC; P = 0.003) and from baseline (P = 0.002). The peak strain before failure in the 12 MCL-injured specimens was 17.6 ± 8.5% for the ACL and 6.3 ± 5.1% for the MCL (P < 0.001). For the MCL-injured specimens, there were no significant change in strain differences between the ACL and MCL (P > 0.350).
Table 5:
ACL and MCL peak strain and change in strain prior to failure.
| Peak ACL strain |
ACL Δ IC | Peak MCL strain |
MCL Δ IC | |
|---|---|---|---|---|
| SPECIMENS | ||||
| ACL failure | 15.3 ± 8.7% * | 5.6 ± 4.1% * | 5.1 ± 5.6% | 2.4 ± 4.2% |
| MCL failure | 17.6 ± 8.5% * | 5.8 ± 3.9% | 6.3 ± 5.1% | 5.0 ± 6.6% |
| MALES | ||||
| ACL failure | 13.9 ± 8.5% * | 5.6 ± 3.8% | 5.3 ± 6.3% | 3.0 ± 5.7% |
| MCL failure | 16.6 ± 9.2% * | 5.8 ± 3.2% | 8.7 ± 8.7% | 6.0 ± 8.2% |
| FEMALES | ||||
| ACL failure | 17.0 ± 9.0% * | 5.7 ± 4.6% * | 4.5 ± 4.8% | 2.0 ± 2.4% |
| MCL failure | 16.3 ± 5.3% * | 5.8 ± 4.0% | 8.2 ± 6.2% | 3.7 ± 4.0% |
Δ IC = Change in strain from the point of initial contact through peak strain
indicates significant difference from corresponding MCL measurements
indicates significant difference between corresponding male and female measurements
Sex Differences
There were sex differences present between specimen mass (male = 95.4 ± 19.3 kg, female = 76.5 ± 27.8 kg; P = 0.019) and height (male = 182 ± 5 cm, female = 165 ± 8 cm; P < 0.001). There were no between-sex differences in BMI (P = 0.609) or age (P = 0.247) within the specimen population. No sex differences were noticed in failure occurrence for the ACL (P = 1.000; Table 2), MCL (P = 0.501), PCL (P = 1.000), FCL (P = 0.092), meniscus (P = 0.182), or bony fracture (P = 0.605). No sex differences were observed in the locations of ACL ruptures (P = 0.876; Table 3). No sex differences were observed for the pre-testing clinical examinations (all P-values ≥ 0.224; Table 4) or post-testing clinical examinations (all P-values ≥ 0.107).
The peak ligament strain before failure and change in ligament strain before failure separated by sex and injury group are displayed in Table 5. No sex differences were reported in ACL strain, MCL strain, or delta strain values for the ACL-injured specimen population (P ≥ 0.128), or MCL-injured specimen population (P ≥ 0.450). For ACL-injured specimens, peak ACL strain was greater than peak MCL strain in both sexes (P < 0.003). The ACL expressed greater change in strain from initial contact than the MCL in the female specimens (P = 0.005), but not the male specimens (P = 0.127). For the MCL-injured specimens, peak ACL strain was greater than peak MCL strain in both sexes (P < 0.003). However, there were no significant differences between ligaments relative to change in strain from IC (P > 0.536).
DISCUSSION
The data obtained supports the hypothesis that our mechanical impactor reproducibly incited ACL injury patterns on a cohort of 39 cadaveric specimens and aligned well with clinically observed failure patterns. The strong associations between injury presentation in our mechanical impact simulator and clinical ACL injures offers clinicians and researchers alike a unique and novel opportunity to directly investigate intra-articular and ligament mechanics during a representative injury event. Such a model could henceforth be employed to estimate clinical efficacy of treatment or prevention interventions in vitro prior to in vivo clinical trial. The mechanical impact simulator produced ACL ruptures in 87% of cadaveric specimens tested, as supported by a similar device that produced ACL rupture in 88% of specimens.26 However, 60% of ruptures in the previous simulator were documented on the tibial side of the joint, which is not indicative of the clinically observed condition. Literature-reported, clinical presentation of ACL rupture locations have been reported as 43% femoral side, 52% mid substance, and 4% tibial side.36 In patients who underwent ACL reconstruction these ratios were 77% femoral side, 22% midsubstance, and 0% tibial side.35 Averaging these datasets, clinical presentation of ACL ruptures occur an estimated 60% on the femoral side, 37% midsubstance, and 2% on the tibial side. This echoes the distribution of ruptures presently recorded from our mechanical impact simulator (Figure 3). Thus, we have presented the first mechanical impactor validated to produce a clinically-representative distribution of ACL tears in cadaveric specimens.
Differences between the mechanical impact simulator presented here and a previous injury-inducing impactor have been explicitly described in the literature.9 Briefly, the mechanical impact simulator initiates external loads from pneumatic cylinders as opposed to static hanging weights manually suspended from a pulley system.21, 26, 31 As such, loading in the mechanical impact simulator is achieved in less than 1.0 sec9 as opposed to minutes of weight setup per trial that allow the viscoelastic tissues of the specimen to creep under the strain of the applied external loads. Additionally, these pneumatics were actively responsive as they would attempt to maintain a consistent level of loading throughout each trial. This provided a dampening effect relative to the impulse force that is more representative of an active muscle than the inertial response of static hanging weights.9 Further, the drop weight in the mechanical impact simulator was comprised of lead pellets instead of solid metal weights, which reduced reverberation upon impact.9 These alterations enhanced the physiologic accuracy of each simulated landing and likely contributed to more clinically representative outcomes than were observed in the previous injury impactor. As compared to a modified Withrow-Oh testing apparatus that limited external loading to ITR,10 the current impactor allowed for manipulation of KAM, ITR, and ATS inputs. These inputs were used to elicit the multi-planer loading effects associated with increased ACL strain5, 8, 34 and are likely responsible for the increased rate of ACL rupture observed in the current impactor (88%) as compared to the previous apparatus (31%).
The similarities in failure modality between sexes observed in the current data indicate that injury type and location are likely not the root of the disparity in relative injury risk reported between male and female athletes. When exposed to ATS, KAM, and ITR external loading and an impulse force, both the male and female ACLs give way with comparable mechanisms of failure.
The secondary hypothesis that female specimens would fail at lower ligament strains than male specimens was unsupported as there were no statistically-significant sex-differences between peak ACL strain and peak MCL strain for the ACL-injured specimens (Table 5). These findings are representative of previous robotically controlled simulations of dynamic athletic tasks where sex differences were not present in either ACL or MCL strains.7 However, those previous simulations were not conducted in rupture scenarios. Under failure conditions, sex-differences have been reported in intra-articular knee loading.32, 33 Specifically, the change in strain within the female ACL has been reported to be higher than the male ACL.
In the present study, peak ACL strain was approximately three times greater than peak MCL strain. Such a behavior is reflective of previous literature where the ACL was demonstrated to have a significantly greater rate of strain than the MCL during simulations of both injury-inducing impacts and controlled athletic tasks.7, 31 Additionally, the ACL has been found to support a larger magnitude of total knee forces than the MCL during both gait and athletic tasks.7, 28 This evidence of predisposed loading in the ACL despite both ligaments sharing a similar uniaxial failure strain (ACL failure at 15.0%; MCL failure at 17.1%)13, 30 and loading mechanism through knee abduction8 justifies why concomitant MCL injuries only occur in 20–40% of ACL ruptures.25, 29 This rate of concomitant ACL-MCL injury was maintained in our mechanical impact simulator (32%, Table 1) which serves to further validate the clinical relevance of our outcomes.
Change in strain was significantly greater for the ACL than for the MCL in female specimens that experienced ACL-injury, but not in male specimens that experienced ACL-injury (Table 5). This may indicate that the ratio of relative ACL:MCL contribution to knee restraint is relatively higher in the female knee than the male knee. Such a theory would lead to the female ACL achieving failure strain under less strenuous circumstances than the male knee, which could account for some of the sex disparity in ACL injury incidence. Further, investigation would be necessary to validate this theory.
As with all cadaveric simulations, the present investigation faced limitations. One limitation was related to the variability of DVRTs implanted in a soft-tissue structure.7, 11 As such, care was taken to maintain consistency in the procedure and location of DVRT implants in order to minimize their variability as much as possible. Second, as each cadaveric specimens were exposed to a maximum of 57 impact trials, there was potential for tissue fatigue. In order to reduce this potential each specimen was viscoelastically preconditioned prior to testing through a series of knee flexion, anterior-posterior tibial translation, and knee frontal plane rotation tasks. Additionally, a brief delay was afforded between each impact to allow the viscoelastic tissues to rebound. Despite these preparations, two specimens still failed at the attachment sites for the tibial fixture and were consequently excluded from analysis. Ultimately, fatigue is a factor that can influence biomechanics during in vivo athletics, but its role in relation to ACL injuries has yet to be comprehensively defined;1 therefore, controlling for estimated cadaveric fatigue outside of randomization would be unsupported. Similarly, it was assumed that sub-failure simulations did not influence the ultimate failure mechanics of each specimen; however, this assumption remains untested in the literature. Further, femoral notchplasty was performed as necessary to prevent DVRT impingement during simulations. While care was taken to minimally influence contact geometry, and consequently contact mechanics, the influence of notchplasty relative to the ACL and its capacity to naturally impinge on the inner wall of the medial femoral condyle remains unknown. The literature lacks evidence that notchplasty is useful in avoiding impingement post ACL reconstruction;20 however, in the present study notchplasty was necessitated as investigators physically noted impingement between implanted DVRT sensors and the medial femoral condyle when the tibial was rotated internally. Such impingement would have skewed the primary outcomes of this study (ACL failure and strain) and femoral notchplasty relieved this impingement. Finally, this study was limited in that clinical exam scoring is a subjective measure of structural integrity that, while used extensively in a clinical setting, is not calibrated to a specimen-specific response and was not performed with a precision robotically-controlled manipulator. In order to control reliability within this limitation, all of our clinical exams were performed by the same board-certified orthopaedic surgeon who has extensive experience of having performed hundreds of examinations on live patients. Also, the functional integrity of each ligament was secondarily verified by visual and physical arthroscopic examination following each clinical exam both before and after impact simulation.
The ACL injuries induced by the mechanical impact simulator in the present study have provided clinically relevant in vitro representations of in vivo ACL injury patterns as cited in the literature. Validations of injury location, concomitant MCL incidence, and ligament loading ratio exemplify that the mechanical impact simulator is an accurate model for the intra-articular assessment of ACL injury mechanics. Additionally, current ligament strains corroborate with the literature to support disproportionately loading of the ACL relative to the MCL during athletic tasks. These present findings indicate that the mechanical impact simulator is an appropriate model for examination of independent mechanical variables, treatment techniques, and preventive interventions during athletic tasks leading up to and including ACL injury. Accordingly, this system can be utilized to further determine the factors that contribute to ACL injury as well as to assess the potential shortcomings or advantages of existing and novel ACL reconstruction techniques in a dynamic, simulated environment that is truly representative of in vivo injury scenarios.
Clinical Relevance:
These findings indicate that the mechanical impact simulator is an appropriate model for examining independent mechanical variables, treatment techniques, and preventative interventions during athletic tasks leading up to and including ACL injury. Accordingly, this system can be utilized to further parse out contributing factors to ACL injury as well as assess the shortcomings of ACL reconstruction technique in a dynamic, simulated environment that is better representative of in vivo injury scenarios.
What is known about the subject:
-
−
ACL injuries are the result of multiplaner loading from several potential risk factors occurring at the knee during rapid deceleration or change in direction tasks.
-
−
Several mechanical impactors have been developed in order to deliver rapid impulse forces to cadaveric specimens and study the resulting knee mechanics.
-
−
None of these impactors have produced a distribution of ACL ruptures that mimics what is observed clinically prior to ACL reconstruction.
What this study adds to existing knowledge:
-
−
This is the first mechanical impact simulator to induce a distribution of ACL injuries on cadaveric specimens that is comparable to the clinical distribution of ACL injuries.
-
−
Additionally, the presented impact simulator generated a clinically-representative of concomitant MCL injuries.
-
−
The peak strain to failure supports previously reported ACL failure strains, but in a dynamic task environment.
-
−
In both ACL failure and MCL failure specimens, the ACL peak strain to failure was significantly greater than the peak MCL strain, supporting that the ACL is predisposed to greater loading than the MCL.
-
−
Clinically, this validation provides researchers with a valuable tool to more accurately assess ACL injury mechanisms and ACL reconstruction techniques.
ACKNOWLEDGEMENTS
All authors declare no conflicts of interest. We acknowledge NIH funding from the National Institute of Arthritis and Musculoskeletal and Skin Diseases R01AR056259, R01AR055563, L30AR070273, and T32AR056950, as well as the National Institute of Child Health and Human Development K12HD065987.
Footnotes
CONFLICTS OF INTEREST: None
All authors have read and approved the final submitted manuscript.
REFERENCES
- 1.Alentorn-Geli E, Myer GD, Silvers HJ, et al. Prevention of non-contact anterior cruciate ligament injuries in soccer players. Part 1: Mechanisms of injury and underlying risk factors. Knee Surg Sports Traumatol Arthrosc. 2009;17(7):705–729. [DOI] [PubMed] [Google Scholar]
- 2.Bates NA, Ford KR, Myer GD, Hewett TE. Impact differences in ground reaction force and center of mass between the first and second landing phases of a drop vertical jump and their implications for injury risk assessment. J Biomech. 2013;46(7):1237–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bates NA, Ford KR, Myer GD, Hewett TE. Kinetic and kinematic differences between first and second landings of a drop vertical jump task: Implications for injury risk assessments. Clin Biomech (Bristol, Avon). 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bates NA, McPherson AL, Rao MB, Myer GD, Hewett TE. Characteristics of inpatient anterior cruciate ligament reconstructions and concomitant injuries. Knee Surg Sports Traumatol Arthrosc. 2016;24(9):2778–2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bates NA, Myer GD, Shearn JT, Hewett TE. Anterior cruciate ligament biomechanics during robotic and mechanical simulations of physiologic and clinical motion tasks: a systematic review and meta-analysis. Clin Biomech (Bristol, Avon). 2015;30(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bates NA, Nesbitt RJ, Shearn JT, Myer GD, E. HT. A Novel Methodology for the Simulation of Athletic Tasks on Cadaveric Knee Joints with Respect to In Vivo Kinematics. Ann Biomed Eng. 2015;43(10):2456–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bates NA, Nesbitt RJ, Shearn JT, Myer GD, Hewett TE. Relative strain in the anterior cruciate ligament and medial collateral ligament during simulated jump landing and sidestep cutting tasks: implications for injury risk. Am J Sports Med. 2015;43(9):2259–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bates NA, Nesbitt RJ, Shearn JT, Myer GD, Hewett TE. Knee Abduction Affects Greater Magnitude of Change in ACL and MCL Strains Than Matched Internal Tibial Rotation In Vitro. Clin Orthop Relat Res. 2017;475:2385–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bates NA, Schilaty ND, Nagelli CV, Krych AJ, Hewett TE. Novel mechanical impact simulator designed to generate clinically relevant anterior cruciate ligament ruptures. Clin Biomech (Bristol, Avon). 2017;44:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beaulieu ML, Wojtys EM, Ashton-Miller JA. Risk of anterior cruciate ligament fatigue failure is increased by limited internal femoral rotation during in vitro repeated pivot landings. Am J Sports Med. 2015;43(9):2233–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beynnon B, Howe JG, Pope MH, Johnson RJ, Fleming BC. The measurement of anterior cruciate ligament strain in vivo. Int Orthop. 1992;16(1):1–12. [DOI] [PubMed] [Google Scholar]
- 12.Boguszewski DV. Characterizing the porcine knee as a biomechanical surrogate model of the human knee to study the anterior cruciate ligament [PhD dissertation]. Cincinnati, OH, USA: Biomedical Engineering, University of Cincinnati; 2012. [Google Scholar]
- 13.Butler DL, Kay MD, Stouffer DC. Comparison of material properties in fascicle-bone units from human patellar tendon and knee ligaments. J Biomech. 1986;19(6):425–432. [DOI] [PubMed] [Google Scholar]
- 14.Deneweth JM, Bey MJ, McLean SG, Lock TR, Kolowich PA, Tashman S. Tibiofemoral joint kinematics of the anterior cruciate ligament-reconstructed knee during a single-legged hop landing. Am J Sports Med. 2010;38(9):1820–1828. [DOI] [PubMed] [Google Scholar]
- 15.Fleming BC, Beynnon BD, Nichols CE, Johnson RJ, Pope MH. An in vivo comparison of anterior tibial translation and strain in the anteromedial band of the anterior cruciate ligament. J Biomech. 1993;26(1):51–58. [DOI] [PubMed] [Google Scholar]
- 16.Harris JD, Abrams GD, Bach BR, et al. Return to sport after ACL reconstruction. Orthopedics. 2014;37(2):e103–108. [DOI] [PubMed] [Google Scholar]
- 17.Herfat ST, Boguszewski DV, Shearn JT. Applying simulated in vivo motions to measure human knee and ACL kinetics. Ann Biomed Eng. 2012;40(7):1545–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hewett TE, Lindenfeld TN, Riccobene JV, Noyes FR. The effect of neuromuscular training on the incidence of knee injury in female athletes. A prospective study. Am J Sports Med. 1999;27(6):699–706. [DOI] [PubMed] [Google Scholar]
- 19.Hofbauer M, Thorhauer ED, Abebe E, Bey M, Tashman S. Altered tibiofemoral kinematics in the affected knee and compensatory changes in the contralateral knee after anterior cruciate ligament reconstruction. Am J Sports Med. 2014;42(11):2715–2721. [DOI] [PubMed] [Google Scholar]
- 20.Keklikci K, Yapici C, Kim D, Linde-Rosen M, Smolinski P, Fu FH. The effect of notchplasty in anterior cruciate ligament reconstruction: a biomechanical study in the porcine knee. Knee Surg Sports Traumatol Arthrosc. 2013;21(8):1915–1921. [DOI] [PubMed] [Google Scholar]
- 21.Kiapour AM, Demetropoulos CK, Kiapour A, et al. Strain Response of the Anterior Cruciate Ligament to Uniplanar and Multiplanar Loads During Simulated Landings: Implications for Injury Mechanism. Am J Sports Med. 2016;44(8):2087–2096. [DOI] [PubMed] [Google Scholar]
- 22.Kiapour AM, Quatman CE, Goel VK, et al. A novel technique to simulate landing biomechanics: a cadaveric model of ACL injury. Orthopaedic Research Society; San Francisco, CA, USA; 2012. [Google Scholar]
- 23.Kim S, Bosque J, Meehan JP, Jamali A, Marder R. Increase in outpatient knee arthroscopy in the United States: a comparison of National Surveys of Ambulatory Surgery, 1996 and 2006. J Bone Joint Surg Am. 2011;93(11):994–1000. [DOI] [PubMed] [Google Scholar]
- 24.Koga H, Nakamae A, Shima Y, et al. Mechanisms for noncontact anterior cruciate ligament injuries: knee joint kinematics in 10 injury situations from female team handball and basketball. Am J Sports Med. 2010;38(11):2218–2225. [DOI] [PubMed] [Google Scholar]
- 25.LaPrade RF, Wentorf FA, Fritts H, Gundry C, Hightower CD. A prospective magnetic resonance imaging study of the incidence of posterolateral and multiple ligament injuries in acute knee injuries presenting with a hemarthrosis. Arthroscopy. 2007;23(12):1341–1347. [DOI] [PubMed] [Google Scholar]
- 26.Levine JW, Kiapour AM, Quatman CE, et al. Clinically relevant injury patterns after an anterior cruciate ligament injury provide insight into injury mechanisms. Am J Sports Med. 2013;41(2):385–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Myer GD, Ford KR, Barber Foss KD, Liu C, Nick TG, Hewett TE. The relationship of hamstrings and quadriceps strength to anterior cruciate ligament injury in female athletes. Clin J Sport Med. 2009;19(1):3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nesbitt RJ, Herfat ST, Boguszewski DV, Engel AJ, Galloway MT, Shearn JT. Primary and secondary restraints of human and ovine knees for simulated in vivo gait kinematics. J Biomech. 2014;47(9):2022–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pressman A, Johnson DH. A review of ski injuries resulting in combined injury to the anterior cruciate ligament and medial collateral ligaments. Arthroscopy. 2003;19(2):194–202. [DOI] [PubMed] [Google Scholar]
- 30.Quapp KM, Weiss JA. Material characterization of human medial collateral ligament. J Biomech Eng. 1998;120(6):757–763. [DOI] [PubMed] [Google Scholar]
- 31.Quatman CE, Kiapour AM, Demetropoulos CK, et al. Preferential loading of the ACL compared with the MCL during landing: a novel in sim approach yields the multiplanar mechanism of dynamic valgus during ACL injuries. Am J Sports Med. 2014;42(1):177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schilaty ND, Bates NA, Nagelli C, Krych AJ, Hewett TE. Sex differences of knee forces and moments that occur with anterior cruciate rupture on cadaveric impact simulations. Am J Sport Med. 2017;submitted. [Google Scholar]
- 33.Schilaty ND, Bates NA, Nagelli C, Krych AJ, Hewett TE. Sex differences of medial collateral and anterior cruciate ligament strains with cadaveric impact simulations. Am J Sport Med. 2017;submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shin CS, Chaudhari AM, Andriacchi TP. Valgus plus internal rotation moments increase anterior cruciate ligament strain more than either alone. Med Sci Sports Exerc. 2011;43(8):1484–1491. [DOI] [PubMed] [Google Scholar]
- 35.van der List JP, DiFelice GS. Preoperative magnetic resonance imaging predicts eligibility for arthroscopic primary anterior cruciate ligament repair. Knee Surg Sports Traumatol Arthrosc. 2017. [DOI] [PubMed] [Google Scholar]
- 36.van der List JP, Mintz DN, DiFelice GS. The Location of Anterior Cruciate Ligament Tears: A Prevalence Study Using Magnetic Resonance Imaging. Orthop J Sports Med. 2017;5(6):2325967117709966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Webster KE, Feller JA, Whitehead TS, Myer GD, Merory PB. Return to Sport in the Younger Patient With Anterior Cruciate Ligament Reconstruction. Orthop J Sports Med. 2017;5(4):2325967117703399. [DOI] [PMC free article] [PubMed] [Google Scholar]


