Abstract
Background
Full-term pregnancy (FTP) is associated with a reduced breast cancer (BC) risk over time, but women are at increased BC risk in the immediate years following an FTP. No large prospective studies, however, have examined whether the number and timing of pregnancies are associated with BC risk for BRCA1 and BRCA2 mutation carriers.
Methods
Using weighted and time-varying Cox proportional hazards models, we investigated whether reproductive events are associated with BC risk for mutation carriers using a retrospective cohort (5707 BRCA1 and 3525 BRCA2 mutation carriers) and a prospective cohort (2276 BRCA1 and 1610 BRCA2 mutation carriers), separately for each cohort and the combined prospective and retrospective cohort.
Results
For BRCA1 mutation carriers, there was no overall association with parity compared with nulliparity (combined hazard ratio [HRc] = 0.99, 95% confidence interval [CI] = 0.83 to 1.18). Relative to being uniparous, an increased number of FTPs was associated with decreased BC risk (HRc = 0.79, 95% CI = 0.69 to 0.91; HRc = 0.70, 95% CI = 0.59 to 0.82; HRc = 0.50, 95% CI = 0.40 to 0.63, for 2, 3, and ≥4 FTPs, respectively, Ptrend < .0001) and increasing duration of breastfeeding was associated with decreased BC risk (combined cohort Ptrend = .0003). Relative to being nulliparous, uniparous BRCA1 mutation carriers were at increased BC risk in the prospective analysis (prospective hazard ration [HRp] = 1.69, 95% CI = 1.09 to 2.62). For BRCA2 mutation carriers, being parous was associated with a 30% increase in BC risk (HRc = 1.33, 95% CI = 1.05 to 1.69), and there was no apparent decrease in risk associated with multiparity except for having at least 4 FTPs vs. 1 FTP (HRc = 0.72, 95% CI = 0.54 to 0.98).
Conclusions
These findings suggest differential associations with parity between BRCA1 and BRCA2 mutation carriers with higher risk for uniparous BRCA1 carriers and parous BRCA2 carriers.
Women carrying mutations in BRCA1 or BRCA2 are at high risk of developing breast cancer (BC) and ovarian cancer with cumulative BC risks to 80 years of 72% (95% CI = 65% to 79%) and 69% (95% CI = 61% to 77%) for BRCA1 and BRCA2 mutation carriers, respectively (1). For women in the general population, it is well established that those who had their first full-term pregnancy (FTP) at a young age (<30 years) have a lower risk of BC than nulliparous women or women who had their first FTP after age 30 years; additional FTPs are associated with even lower risks (2). The consistent association between the number of pregnancies and long-term reduction in BC risk is restricted to FTPs (3–5), as incomplete pregnancies (IP) have not been associated with BC risk [eg, (3)]. While FTPs are associated with a reduced BC risk in the long-term, a short-term increase in BC risk has been consistently observed for women following an FTP (6–8), which may be reduced by breastfeeding (4,9). Thus, in addition to being related to long-term risk reduction, breastfeeding might mitigate a short-term increase in BC risk after FTP (10).
Given the earlier age at which BC risk increases for women carrying a BRCA1 or BRCA2 (BRCA1/2) mutation, it is important to know whether the BC risk for carriers is modified by the number and timing of their pregnancies and/or by breastfeeding. However, the few studies that assessed associations with pregnancies and breastfeeding for BRCA1/2 mutation carriers have reported inconsistent results [for reviews, see (11,12)], ranging from studies supporting a decreased risk from FTP (13,14) to studies supporting no association (15) to studies supporting an increased risk (16). Although more limited in numbers, studies that examined BRCA1 and BRCA2 mutation carriers separately have supported differences in associations by mutation type [eg, higher risk for late age at first FTP or parity in general for BRCA2 mutation carriers (13,16) and lower risk for multiparity for BRCA1 mutation carriers (16) and differences based on breastfeeding (17–19)].
Most studies of BRCA1 and BRCA2 mutation carriers have been retrospective and the few prospective studies have had limited power to examine BRCA1 and BRCA2 mutation carriers separately. To address these issues, we estimated BC risk associations with reproductive history for BRCA1 and BRCA2 mutation carriers separately using an international cohort comprised of 9232 and 3886 women in the retrospective and prospective cohorts, respectively.
Methods
Study Sample
We harmonized information from three prospective cohorts, which included 21 national or center-based prospective follow-up studies conducted in Western countries: the International BRCA1/2 Carrier Cohort Study (IBCCS), the Kathleen Cuningham Foundation Consortium for Research into Familial Breast Cancer (kConFab) Follow-Up Study, and the Breast Cancer Family Registry (BCFR) (20–24). Of the study participants, 84% were enrolled through one of the five major studies: (1) Epidemiological Study of Familial Breast Cancer (EMBRACE) in the United Kingdom and Ireland; (2) Gene Etude Prospective Sein Ovaire (GENEPSO) in France; (3) Hereditary Breast and Ovarian cancer study Netherlands (HEBON) in the Netherlands; (4) kConFab in Australia and New Zealand; and (5) BCFR in North America and Australia.
Study Participants
Women were eligible if they were 18–80 years of age and had a known pathogenic BRCA1 or BRCA2 mutation. Of the cohort participants, 94% were tested in family clinics and 6% were tested in a research setting, and it was unknown whether or when they opted for a clinical test. We defined two subcohorts for the analyses: (1) a prospective cohort comprising women unaffected with BC at baseline, for whom reproductive history data from baseline and, if collected, follow-up questionnaires were combined (2276 BRCA1 and 1610 BRCA2 mutation carriers); and (2) a retrospective cohort comprising both unaffected and affected women at baseline, for whom only data from the baseline questionnaire were used (5707 BRCA1 and 3525 BRCA2 mutation carriers). The kConFab study women were included only in the prospective cohort.
Data Collection
The baseline and follow-up questionnaires collected detailed information on known or suspected risk factors for BC, including reproductive and medical history and surgical interventions. We collected family history of cancer either from the baseline questionnaire or from pedigrees provided by the genetic counselling centers. We collected information on cancer occurrences, which were confirmed by medical records including pathology records, or through linkage to cancer registries for 92% of all cases. The overall response to the follow-up questionnaires was 73% (1). Information on vital status was obtained from municipal, death, or cancer registries or from relatives. Participants provided written informed consent, and each study was approved by a relevant research ethics committee.
Statistical Analysis
We used Cox proportional hazards regression models with age as the timescale to calculate hazard ratios (HRs) to assess the association between pregnancy-related variables (ie, parity, number of FTPs, age at first FTP, number of years since last FTP, breastfeeding history and duration of breastfeeding, incomplete pregnancies (IP) due to either spontaneous or induced abortion, timing of IP relative to the first FTP and BC risk), both prospectively (prospective hazard ratio [HRP]) and retrospectively (retrospective hazard ratio [HRR]). We conducted separate analyses for BRCA1 and BRCA2 mutation carriers. We stratified all analyses for birth cohort (<1950, 1950–1959, 1960–1969, ≥1970) and for study group (EMBRACE, GENEPSO, HEBON, BCFR, kConFab, and others combined) and used robust variance estimation to account for the inclusion of related women. We assessed whether the findings differed by age using attained age analyses for women based on censoring at age 40 years. We counted pregnancies that occurred at least one year before the age at right censoring to exclude pregnancies that may have occurred at the same time as diagnosis. We adjusted for bilateral oophorectomy as a time-varying covariate in all of the primary analyses and performed sensitivity analyses by including the potential confounders use of oral contraceptives (as a time-varying covariate), age at menarche, and family history of BC.
Retrospective Cohort Analysis
For retrospective analyses, we modeled time from birth to the diagnosis of first primary BC (invasive or in situ), censoring individuals at the earliest of the following events: diagnosis of any cancer, risk-reducing mastectomy (RRM), or completion of the baseline questionnaire. All covariates were constructed as time-varying covariates. All analyses of the retrospective cohort were performed using the weighted regression approach described by Antoniou et al. (25) to allow for the oversampling of affected women; cohort members were weighted so that the observed BC incidences in the study sample were consistent with established BC risk estimates for BRCA1 and BRCA2 mutation carriers (26). To evaluate potential survival bias, we also performed sensitivity analyses for the retrospective cohort using only pseudo-incident cases in which we considered only the follow-up from 5 years prior to study recruitment to age at censoring.
Prospective Cohort Analysis
For the prospective analysis, we considered follow-up from the date of the baseline questionnaire to the date of diagnosis of any cancer, RRM, last follow-up questionnaire, last information from external source (eg, linkage), age 80 years, or loss to follow-up or death, whichever came first. We included pregnancies and breastfeeding as time-varying covariates.
Combined Cohort Analyses
We also conducted a combined analysis using both retrospective and prospective data. We modeled time from birth to the date of diagnosis of any cancer, RRM, last follow-up questionnaire, last information from external source, age 80 years, loss to follow-up or death, whichever came first, with time-dependent weights as described by Antoniou et al. (25) for the retrospective period and weights equal to one for the prospective period. Statistical analyses were performed using SAS 9.4.
Results
Tables 1 and 2 summarize the descriptive information for BRCA1 and BRCA2 mutation carriers, respectively.
Table 1.
Characteristics of the BRCA1 mutation carriers in the retrospective and prospective cohort
| Characteristic | Women with breast cancer |
Unaffected women |
||
|---|---|---|---|---|
| Retrospective | Prospective | Retrospective | Prospective | |
| (n = 2544) | (n = 269) | (n = 3163) | (n = 2007) | |
| No. (%) or mean (SD) | No. (%) or mean (SD) | No. (%) or mean (SD) | No. (%) or mean (SD) | |
| Age at start of follow-up, y | — | 40.6 (10.2) | — | 37.5 (11.8) |
| Age at censure, y | 40.1 (8.8) | 44.9 (10.3) | 39.3 (11.5) | 43.1 (12.3) |
| Year of birth | ||||
| <1950 | 805 (31.6) | 35 (13.0) | 526 (16.6) | 205 (10.2) |
| 1950–1959 | 843 (33.1) | 76 (28.3) | 646 (20.4) | 347 (17.3) |
| 1960–1969 | 665 (26.1) | 104 (38.7) | 943 (29.8) | 586 (29.2) |
| ≥1970 | 231 (9.1) | 54 (20.1) | 1048 (33.1) | 869 (43.3) |
| Study group | ||||
| EMBRACE | 746 (29.3) | 41 (15.2) | 814 (25.7) | 432 (21.5) |
| GENEPSO | 325 (12.8) | 46 (17.1) | 691 (21.8) | 442 (22.0) |
| HEBON | 339 (13.3) | 40 (14.9) | 463 (14.6) | 202 (10.1) |
| kConFab | — | 55 (20.4) | — | 270 (13.5) |
| BCFR | 456 (17.9) | 50 (18.6) | 433 (13.7) | 277 (13.8) |
| Others* | 678 (26.7) | 37 (13.8) | 762 (24.1) | 384 (19.1) |
| No. of full-term pregnancies (FTP) | ||||
| Nulliparous (no FTP) | 518 (20.4) | 51 (19.0) | 951 (30.1) | 602 (30.0) |
| 1 | 470 (18.5) | 43 (16.0) | 481 (15.2) | 295 (14.7) |
| 2 | 924 (36.3) | 113 (42.0) | 1040 (32.9) | 652 (32.5) |
| 3 | 430 (16.9) | 49 (18.2) | 467 (14.8) | 292 (14.5) |
| ≥4 | 202 (7.9) | 13 (4.8) | 224 (7.1) | 166 (8.3) |
| Age at 1st full-term pregnancy among parous, y | ||||
| <20 | 286 (14.1) | 26 (11.9) | 244 (11.0) | 148 (10.5) |
| 20–24 | 830 (41.0) | 73 (33.5) | 794 (35.9) | 482 (34.3) |
| 25–29 | 620 (30.6) | 73 (33.5) | 776 (35.1) | 511 (36.4) |
| ≥30 | 290 (14.3) | 46 (21.1) | 398 (18.0) | 264 (18.8) |
| Years since last full-term pregnancy | ||||
| Nulliparous | 518 (20.4) | 51 (19.0) | 951 (30.1) | 602 (30.0) |
| 1–5 | 540 (21.2) | 43 (16.0) | 665 (21.0) | 291 (14.5) |
| 6–20 | 1078 (42.4) | 102 (37.9) | 991 (31.3) | 662 (33.0) |
| ≥21 | 408 (16.0) | 73 (27.1) | 556 (17.6) | 452 (22.5) |
| Breastfeeding duration among women with full-term pregnancy, mo | ||||
| None | 594 (29.3) | 50 (22.9) | 561 (25.4) | 311 (22.1) |
| 1–5 | 602 (29.7) | 59 (27.1) | 620 (28.0) | 388 (27.6) |
| 6–12 | 469 (23.1) | 52 (23.9) | 544 (24.6) | 332 (23.6) |
| 13–24 | 244 (12.0) | 39 (17.9) | 323 (14.6) | 243 (17.3) |
| >24 | 116 (5.7) | 17 (7.8) | 159 (7.2) | 130 (9.3) |
| FTP but stillborn | 1 (0.0) | 1 (0.5) | 5 (0.2) | 1 (0.1) |
| Incomplete pregnancy (IP) | ||||
| No full-term pregnancy or IP | 437 (17.2) | 40 (14.9) | 825 (26.1) | 515 (25.7) |
| Full-term pregnancy, no IP | 1373 (54.0) | 141 (52.4) | 1473 (46.6) | 926 (46.1) |
| Induced abortion only | 281 (11.0) | 32 (11.9) | 334 (10.6) | 216 (10.8) |
| Miscarriage only | 383 (15.1) | 51 (19.0) | 459 (14.5) | 295 (14.7) |
| Induced abortion and miscarriage | 70 (2.8) | 5 (1.9) | 72 (2.3) | 55 (2.7) |
| Incomplete pregnancy relative to first full-term pregnancy | ||||
| No IP | 1833 (72.1) | 184 (68.4) | 2333 (73.8) | 1458 (72.6) |
| Before first FTP or no FTP | 359 (14.1) | 46 (17.1) | 461 (14.6) | 330 (16.4) |
| After first FTP | 352 (13.8) | 39 (14.5) | 369 (11.7) | 219 (10.9) |
| Bilateral oophorectomy | ||||
| No | 2342 (92.1) | 131 (48.7) | 2253 (71.2) | 1215 (60.5) |
| Yes | 202 (7.9) | 138 (51.3) | 909 (28.7) | 792 (39.5) |
| Missing | 0 | 0 | 1 (0.0) | 0 |
| Oral contraceptive use | ||||
| Never | 605 (23.8) | 39 (14.5) | 653 (20.6) | 290 (14.4) |
| Ever | 1820 (71.5) | 226 (84.0) | 2352 (74.4) | 1659 (82.7) |
| Unknown start age | 69 (2.7) | 1 (0.4) | 104 (3.3) | 6 (0.3) |
| Missing | 50 (2.0) | 3 (1.1) | 54 (1.7) | 52 (2.6) |
| Age at menarche, y | ||||
| <12 | 469 (18.4) | 34 (12.6) | 452 (14.3) | 270 (13.5) |
| 12 | 621 (24.4) | 65 (24.2) | 836 (26.4) | 529 (26.4) |
| 13 | 594 (23.3) | 74 (27.5) | 745 (23.6) | 483 (24.1) |
| 14 | 429 (16.9) | 54 (20.1) | 598 (18.9) | 386 (19.2) |
| ≥15 | 380 (14.9) | 39 (14.5) | 474 (15.0) | 313 (15.6) |
| Age missing | 51 (2.0) | 3 (1.1) | 58 (1.8) | 26 (1.3) |
| Never had menstrual period | 0 | 0 | 0 | 0 |
Others included the following studies (at inlusion total number): Medical University of Vienna (MUV) (261), Modifier Study of Quantitative Effects on Disease (MODSQUAD) (228), German Consortium of Hereditary Breast and Ovarian Cancer (GC-HBOC) (178), Lund-BRCA (160), Odense University Hospital (OUH) (105), Hospital Clinico San Carlos (HCSC) (84), INterdisciplinary HEalth Research Internal Team BReast CAncer susceptibility (INHERIT) (66), National Institute of Oncology (NIO) (98), International Hereditary Cancer Center (IHCC) (97), Stockholm-BRCA (71), The Spanish National Cancer Center (CNIO) (40), Milan Italy (33), Hospital Clinico San Carlos (9), German Cancer Research Center (DKFZ) (4), Belgium (3), Dusseldorf Germany (3). EMBRACE = Epidemiological Study of Familial Breast Cancer; GENEPSO = Gene Etude Prospective Sein Ovaire; HEBON = Hereditary Breast and Ovarian cancer study Netherlands; kConFab = Kathleen Cuningham Foundation Consortium for Research into Familial Breast Cancer; BCFR = Breast Cancer Family Registry.
Table 2.
Characteristics of the BRCA2 mutation carriers in the retrospective and prospective cohort
| Characteristic | Women with breast cancer |
Unaffected women |
||
|---|---|---|---|---|
| Retrospective | Prospective | Retrospective | Prospective | |
| (n = 1560) | (n = 157) | (n = 1965) | (n = 1453) | |
| No. (%) or mean (SD) | No. (%) or mean (SD) | No. (%) or mean (SD) | No. (%) or mean (SD) | |
| Age at start, y | 45.1 (10.1) | 40.0 (12.6) | ||
| Age at censure, y | 43.4 (9.1) | 49.0 (10.3) | 41.5 (12.4) | 45.0 (13.0) |
| Year of birth | ||||
| <1950 | 563 (36.1) | 42 (26.8) | 386 (19.6) | 200 (13.8) |
| 1950–1959 | 513 (32.9) | 44 (28.0) | 385 (19.6) | 259 (17.8) |
| 1960–1969 | 387 (24.8) | 55 (35.0) | 570 (29.0) | 433 (29.8) |
| ≥1970 | 97 (6.2) | 16 (10.2) | 624 (31.8) | 561 (38.6) |
| Study group | ||||
| EMBRACE | 615 (39.4) | 42 (26.8) | 740 (37.7) | 441 (30.4) |
| GENEPSO | 161 (10.3) | 18 (11.5) | 437 (22.2) | 307 (21.1) |
| HEBON | 91 (5.8) | 4 (2.5) | 146 (7.4) | 71 (4.9) |
| kConFab | — | 38 (24.2) | — | 250 (17.2) |
| BCFR | 359 (23.0) | 33 (21.0) | 322 (16.4) | 222 (15.3) |
| Others* | 334 (21.4) | 22 (14.0) | 320 (16.3) | 162 (11.1) |
| No. of full-term pregnancy (FTP) | ||||
| Nulliparous (no FTP) | 278 (17.8) | 23 (14.6) | 537 (27.3) | 406 (27.9) |
| 1 | 224 (14.4) | 14 (8.9) | 288 (14.7) | 196 (13.5) |
| 2 | 622 (39.9) | 62 (39.5) | 631 (32.1) | 449 (30.9) |
| 3 | 284 (18.2) | 36 (22.9) | 330 (16.8) | 264 (18.2) |
| ≥4 | 152 (9.7) | 22 (14.0) | 179 (9.1) | 138 (9.5) |
| Age at 1st full-term pregnancy among parous, y | ||||
| <20 | 154 (12.0) | 11 (8.2) | 173 (12.1) | 113 (10.8) |
| 20–24 | 503 (39.2) | 57 (42.5) | 550 (38.5) | 386 (36.9) |
| 25–29 | 408 (31.8) | 36 (26.9) | 451 (31.6) | 347 (33.1) |
| ≥30 | 217 (16.9) | 30 (22.4) | 254 (17.8) | 201 (19.2) |
| Year since last full-term pregnancy | ||||
| Nulliparous | 278 (17.8) | 23 (14.6) | 537 (27.3) | 406 (27.9) |
| 1–5 | 280 (17.9) | 16 (10.2) | 410 (20.9) | 175 (12.0) |
| 6–20 | 669 (42.9) | 63 (40.1) | 590 (30.0) | 484 (33.3) |
| ≥21 | 333 (21.3) | 55 (35.0) | 428 (21.8) | 388 (26.7) |
| Breastfeeding duration among women with full-term pregnancy | ||||
| None | 357 (27.8) | 26 (19.4) | 408 (28.6) | 263 (25.1) |
| 1–5 mo | 342 (26.7) | 36 (26.9) | 389 (27.2) | 255 (24.4) |
| 6–12 mo | 311 (24.3) | 34 (25.4) | 293 (20.5) | 219 (20.9) |
| 13–24 mo | 186 (14.5) | 18 (13.4) | 220 (15.4) | 186 (17.8) |
| >24 mo | 84 (6.6) | 20 (14.9) | 115 (8.1) | 122 (11.7) |
| FTP but stillborn | 2 (0.2) | 0 | 3 (0.2) | 2 (0.2) |
| Incomplete pregnancy (IP) | ||||
| No full-term pregnancy or IP | 225 (14.4) | 22 (14.0) | 471 (24.0) | 343 (23.6) |
| Full-term pregnancy, no IP | 850 (54.5) | 87 (55.4) | 956 (48.7) | 680 (46.8) |
| Induced abortion only | 154 (9.9) | 10 (6.4) | 199 (10.1) | 157 (10.8) |
| Miscarriage only | 280 (17.9) | 31 (19.7) | 284 (14.5) | 225 (15.5) |
| Induced abortion and miscarriage | 51 (3.3) | 7 (4.5) | 55 (2.8) | 48 (3.3) |
| Incomplete pregnancy relative to first full-term pregnancy | ||||
| No IP | 1087 (69.7) | 110 (70.1) | 1445 (73.5) | 1036 (71.3) |
| Before first FTP or no FTP | 256 (16.4) | 22 (14.0) | 270 (13.7) | 229 (15.8) |
| After first FTP | 217 (13.9) | 25 (15.9) | 250 (12.7) | 188 (12.9) |
| Bilateral oophorectomy | ||||
| No | 1430 (91.7) | 95 (60.5) | 1522 (77.5) | 959 (66.0) |
| Yes | 130 (8.3) | 62 (39.5) | 443 (22.5) | 494 (34.0) |
| Missing | 0 | 0 | 0 | 0 |
| Oral contraceptive use | ||||
| Never | 378 (24.2) | 17 (10.8) | 412 (21.0) | 214 (14.7) |
| Ever | 1106 (70.9) | 136 (86.6) | 1452 (73.9) | 1201 (82.7) |
| Unknown start age | 46 (2.9) | 1 (0.6) | 72 (3.7) | 5 (0.3) |
| Missing | 30 (1.9) | 3 (1.9) | 29 (1.5) | 33 (2.3) |
| Age at menarche, y | ||||
| <12 | 238 (15.3) | 29 (18.5) | 337 (17.2) | 237 (16.3) |
| 12 | 365 (23.4) | 40 (25.5) | 503 (25.6) | 353 (24.3) |
| 13 | 404 (25.9) | 37 (23.6) | 454 (23.1) | 377 (25.9) |
| 14 | 274 (17.6) | 24 (15.3) | 336 (17.1) | 246 (16.9) |
| ≥15 | 247 (15.8) | 27 (17.2) | 303 (15.4) | 214 (14.7) |
| Age missing | 31 (2.0) | 0 | 30 (1.5) | 24 (1.7) |
| Never had menstrual period | 1 (0.1) | 0 | 2 (0.1) | 2 (0.1) |
Others included the following studies (total number): Medical University of Vienna (MUV) (100), Modifier Study of Quantitative Effects on Disease (MODSQUAD) (80), German Consortium of Hereditary Breast and Ovarian Cancer (GC-HBOC) (105), Lund-BRCA (58), Odense University Hospital (OUH) (62), Hospital Clinico San Carlos (HCSC) (65), INterdisciplinary HEalth Research Internal Team BReast CAncer susceptibility (INHERIT) (74), National Institute of Oncology (NIO) (31), International Hereditary Cancer Center (IHCC) (0), Stockholm-BRCA (13), The Spanish National Cancer Center (CNIO) (44), Milan Italy (12), Hospital Clinico San Carlos (10). EMBRACE = Epidemiological Study of Familial Breast Cancer; GENEPSO = Gene Etude Prospective Sein Ovaire; HEBON = Hereditary Breast and Ovarian cancer study Netherlands; kConFab = Kathleen Cuningham Foundation Consortium for Research into Familial Breast Cancer; BCFR = Breast Cancer Family Registry.
BRCA1 Mutation Carriers
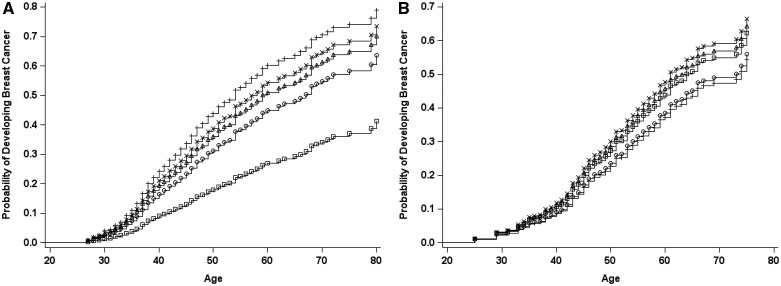
For BRCA1 mutation carriers, there was no overall association of parity compared with nulliparity (combined hazard ratio [HRc] = 0.99, 95% CI = 0.83 to 1.18) (Table 3). Relative to being uniparous, multiparity was associated with decreased BC risk (HRc = 0.79, 95% CI = 0.69 to 0.91; HRc = 0.70, 95% CI = 0.59 to 0.82; HRc = 0.50, 95% CI = 0.40 to 0.63 for 2, 3, and ≥4 FTPs, respectively, Ptrend < .0001). The reduced risk associated with multiparity was still evident after adjusting for age at FTP and other risk factors. Each additional FTP after the first was associated with a 16% (95% CI = 11% to 21%) and 26% (95% CI = 14% to 36%) decreased risk in the retrospective and prospective analyses, respectively. Figure 1 shows the probability of developing BC for the prospective cohort. This decreasing risk with increasing parity was evident across all birth cohorts (Supplementary Figure 1, available online).
Table 3.
Retrospective, prospective, and combined analyses for the BRCA1 mutation carriers
| Characteristic | Retrospective | Ptrend* | Prospective | Ptrend* | Combined | Ptrend* |
|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | ||||
| Parous (at least 1 full-term pregnancy)† | ||||||
| No | Referent | Referent | Referent | |||
| Yes | 0.87 (0.72 to 1.05) | 1.41 (0.94 to 2.10) | 0.99 (0.83 to 1.18) | |||
| No. of full-term pregnancy† (FTP) | ||||||
| Nulliparous (no FTP) | Referent | Referent | Reference | |||
| 1 | 0.98 (0.81 to 1.20) | <.0001 | 1.69 (1.09 to 2.62) | <.0001 | 1.11 (0.92 to 1.34) | <.0001 |
| 2 | 0.77 (0.63 to 0.95) | 1.25 (0.81 to 1.95) | 0.88 (0.73 to 1.07) | |||
| 3 | 0.68 (0.53 to 0.86) | 1.15 (0.70 to 1.90) | 0.77 (0.62 to 0.97) | |||
| ≥4 | 0.54 (0.40 to 0.73) | 0.52 (0.27 to 1.02) | 0.56 (0.42 to 0.74) | |||
| 1 | Referent | Referent | <.0001 | Reference | <.0001 | |
| 2 | 0.78 (0.68 to 0.91) | <.0001 | 0.74 (0.51 to 1.08) | 0.79 (0.69 to 0.91) | ||
| 3 | 0.69 (0.58 to 0.82) | 0.68 (0.44 to 1.05) | 0.70 (0.59 to 0.82) | |||
| ≥4 | 0.55 (0.43 to 0.70) | 0.31 (0.17 to 0.57) | 0.50 (0.40 to 0.63) | |||
| Nulliparous | 1.02 (0.83 to 1.24) | 0.59 (0.38 to 0.92) | 0.90 (0.75 to 1.09) | |||
| No. of full-term pregnancy by attained age† | ||||||
| <40 years | ||||||
| 1 | Referent | Referent | .22 | Reference | <.0001 | |
| 2 | 0.73 (0.61 to 0.87) | <.0001 | 1.08 (0.61 to 1.91) | 0.79 (0.66 to 0.94) | ||
| 3 | 0.68 (0.55 to 0.85) | 0.35 (0.12 to 1.09) | 0.65 (0.52 to 0.82) | |||
| ≥4 | 0.63 (0.45 to 0.87) | 0.67 (0.20 to 2.27) | 0.64 (0.46 to 0.89) | |||
| ≥40 years | ||||||
| 1 | Referent | <.0001 | Referent | <.0001 | Reference | <.0001 |
| 2 | 0.82 (0.66 to 1.03) | 0.61 (0.38 to 0.99) | 0.78 (0.64 to 0.96) | |||
| 3 | 0.68 (0.54 to 0.87) | 0.69 (0.41 to 1.16) | 0.70 (0.56 to 0.88) | |||
| ≥4 | 0.52 (0.39 to 0.70) | 0.24 (0.12 to 0.48) | 0.46 (0.34 to 0.61) | |||
| Age at 1st full-term pregnancy, y‡ | ||||||
| <20 | Referent | .03 | Referent | .95 | Reference | .06 |
| 20–24 | 0.98 (0.81 to 1.19) | 0.84 (0.54 to 1.30) | 0.95 (0.80 to 1.13) | |||
| 25–29 | 0.87 (0.71 to 1.06) | 0.80 (0.52 to 1.23) | 0.85 (0.71 to 1.02) | |||
| ≥30 | 0.82 (0.65 to 1.04) | 0.95 (0.59 to 1.55) | 0.86 (0.70 to 1.07) | |||
| Year since last full-term pregnancy§ | ||||||
| 0–5 | Referent | .02 | Referent | .002 | Reference | .0002 |
| 6–20 | 1.19 (1.03 to 1.36) | 0.89 (0.59 to 1.35) | 1.14 (0.99 to 1.30) | |||
| ≥21 | 1.48 (1.17 to 1.87) | 1.12 (0.63 to 1.98) | 1.44 (1.15 to 1.81) | |||
| Nulliparous | 1.05 (0.86 to 1.29) | 0.55 (0.33 to 0.89) | 0.92 (0.76 to 1.11) | |||
| Nulliparous | Referent | Referent | Reference | |||
| 0–5 | 0.95 (0.78 to 1.16) | .02 | 1.84 (1.13 to 2.99) | .002 | 1.09 (0.90 to 1.32) | .0002 |
| 6–20 | 1.13 (0.90 to 1.41) | 1.64 (1.02 to 2.64) | 1.24 (1.01 to 1.52) | |||
| ≥21 | 1.41 (1.06 to 1.87) | 2.06 (1.12 to 3.79) | 1.57 (1.20 to 2.06) | |||
| Year since last full-term pregnancy by number of full-term pregnancy† | ||||||
| 1 FTP, 0–5 | Referent | Referent | Reference | |||
| ≥2 FTP, 0–5 | 0.65 (0.53 to 0.80) | 0.75 (0.38 to 1.47) | 0.68 (0.55 to 0.84) | |||
| 1 FTP, 6–20 | 0.94 (0.73 to 1.20) | 0.96 (0.47 to 1.95) | 0.95 (0.75 to 1.21) | |||
| ≥2 FTP, 6–20 | 0.84 (0.67 to 1.04) | 0.65 (0.33 to 1.30) | 0.82 (0.66 to 1.03) | |||
| 1 FTP, ≥21 | 1.55 (1.09 to 2.21) | 1.22 (0.48 to 3.07) | 1.55 (1.11 to 2.17) | |||
| ≥2 FTP, ≥21 | 0.96 (0.72 to 1.30) | 0.81 (0.36 to 1.84) | 0.98 (0.73 to 1.31) | |||
| Breastfeeding duration‖ | ||||||
| None | Referent | .0002 | Referent | .28 | Reference | .0003 |
| 1–5 mo | 0.96 (0.82 to 1.12) | 1.07 (0.73 to 1.56) | 0.97 (0.84 to 1.11) | |||
| 6–12 mo | 0.81 (0.69 to 0.95) | 1.04 (0.70 to 1.54) | 0.84 (0.72 to 0.97) | |||
| 13–24 mo | 0.75 (0.61 to 0.91) | 1.05 (0.68 to 1.63) | 0.80 (0.67 to 0.95) | |||
| > 24 mo | 0.64 (0.48 to 0.86) | 0.75 (0.44 to 1.31) | 0.66 (0.50 to 0.87) | |||
| No. of full-term pregnancy and breastfeeding† | ||||||
| Nulliparous | Referent | Referent | Reference | |||
| 1 FTP, never breastfeeding | 1.15 (0.89 to 1.49) | 2.01 (1.14 to 3.55) | 1.33 (1.04 to 1.70) | |||
| ≥2 FTP, never breastfeeding | 0.85 (0.67 to 1.07) | 1.15 (0.67 to 1.96) | 0.94 (0.76 to 1.18) | |||
| 1 FTP, ever breastfeeding | 0.97 (0.78 to 1.19) | 1.64 (1.00 to 2.70) | 1.09 (0.89 to 1.33) | |||
| ≥2 FTP, ever breastfeeding | 0.73 (0.59 to 0.89) | 1.22 (0.79 to 1.90) | 0.84 (0.69 to 1.02) | |||
| Incomplete pregnancy§ (IP) | ||||||
| No full-term or incomplete pregnancy | Referent | Referent | Reference | |||
| Full-term pregnancy, no IP | 0.96 (0.79 to 1.16) | 1.64 (1.03 to 2.61) | 1.08 (0.90 to 1.29) | |||
| Induced abortion only | 1.02 (0.82 to 1.27) | 1.72 (1.04 to 2.83) | 1.15 (0.93 to 1.41) | |||
| Miscarriage only | 0.97 (0.78 to 1.21) | 1.77 (1.09 to 2.87) | 1.11 (0.91 to 1.36) | |||
| Induced abortion and miscarriage | 1.09 (0.77 to 1.55) | 1.09 (0.40 to 2.94) | 1.11 (0.80 to 1.55) | |||
| Incomplete pregnancy relative to first full-term pregnancy§ | ||||||
| No IP | Referent | Referent | Reference | |||
| Before first FTP or no FTP | 1.05 (0.90 to 1.22) | 1.02 (0.74 to 1.41) | 1.04 (0.91 to 1.19) | |||
| After first FTP | 1.03 (0.89 to 1.20) | 1.32 (0.93 to 1.88) | 1.09 (0.94 to 1.25) | |||
Nulliparous excluded, risk factor as continuous.
Adjusted for bilateral oophorectomy (Yes, No), age at 1st full-term pregnancy (<30, ≥30+nulliparous), strata by birth year and study site.
Adjusted for bilateral oophorectomy, number of full-term pregnancies (0–1, ≥2), strata by birth year and study site.
Adjusted for bilateral oophorectomy, number of full-term pregnancies (0–1, ≥2), age at 1st full-term pregnancy, strata by birth year and study site.
Adjusted for bilateral oophorectomy, number of live births (0–1, ≥2), age at 1st full-term pregnancy, strata by birth year and study site.
Figure 1.
Probability of developing breast cancer in the prospective cohort by parity. A) BRCA1. B) BRCA2. Circles = nulliparous; plus sign, parity = 1; x, parity = 2; triangles, parity = 3; squares, parity = 4 or more.
The increased risk from uniparity was only seen in the prospective analysis (HRp = 1.69, 95% CI = 1.09 to 2.62). There was some suggestion that this association was stronger for women who have never breastfed (HRp = 2.01, 95% CI = 1.14 to 3.55; HRp = 1.64, 95% CI = 1.00 to 2.70 for women who did not and did breastfeed, respectively), but these HRs were not statistically different (Pheterogeneity = .54]. The increased risk, although not statistically significant (HRp = 1.41, 95% CI = 0.94 to 2.10), for overall parity in the prospective cohort was driven mainly by the difference in nulliparity vs uniparity between the two analyses (HRp = 0.59, 95% CI = 0.38 to 0.92, and HRR = 1.02, 95% CI = 0.83 to 1.24, respectively) because the point estimates of each successive pregnancy compared with uniparity were similar in both the retrospective and prospective analyses. Supplementary Figure 2 (available online) illustrates the difference based on penetrance for BRCA1 mutation carriers according to different reproductive life scenarios.
Relative to a recent pregnancy, longer time since last FTP was associated with higher risk in the retrospective analysis. Increasing duration of breastfeeding was associated with decreased BC risk (combined cohort Ptrend = .0003) in the retrospective analysis (Ptrend = .0002), but not in the prospective analysis (Ptrend = .28).
IP was associated with an increased BC risk compared with women without IP or FTP in the prospective analysis (HRp = 1.72, 95% CI = 1.04 to 2.83 and HRp = 1.77, 95% CI = 1.09 to 2.87 for induced abortion only and miscarriage only, respectively), but not in the retrospective analysis (HRR = 1.02, 95% CI = 0.82 to 1.27 and HRR = 0.97, 95% CI = 0.78 to 1.21 for induced abortion only and miscarriage only, respectively). The magnitude of the association with IP was similar to the association for any FTP without IP (HRp = 1.64, 95% CI = 1.03 to 2.61). There was also no difference in association whether the IP was before or after the first FTP in all of the analyses.
BRCA2 Mutation Carriers
For BRCA2 mutation carriers, parity was associated with a 30% increase in BC risk (HRc = 1.33, 95% CI = 1.05 to 1.69) (Table 4). Multiparity was associated with a decreased BC risk (HRc = 0.72, 95% CI = 0.54 to 0.98 for ≥ 4 vs 1 FTP) in the retrospective analysis (HRR = 0.58, 95% CI = 0.42 to 0.79 for ≥4 vs 1 FTP, Ptrend = .0001), but not in the prospective cohort (HRp = 1.68, 95% CI = 0.83 to 3.39 for ≥4 vs 1 FTP, Ptrend = .41 and Pheterogeneity = .006) (Figure 1). Multiparity was associated with a decreased BC risk only prior to age 40 years (HRR = 0.29, 95% CI = 0.16 to 0.52 for ≥4 vs 1 FTP) (Table 4).
Table 4.
Retrospective, prospective, and combined analyses for the BRCA2 mutation carriers
| Characteristic | Retrospective | Ptrend* | Prospective | Ptrend* | Combined | Ptrend* |
|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | ||||
| Parous (at least 1 full-term pregnancy)† | ||||||
| No | Referent | Referent | Referent | |||
| Yes | 1.26 (0.99 to 1.62) | 1.44 (0.83 to 2.49) | 1.33 (1.05 to 1.69) | |||
| No. of full-term pregnancy† (FTP) | ||||||
| Nulliparous | Referent | Referent | Referent | |||
| 1 | 1.28 (0.98 to 1.67) | .0001 | 1.08 (0.55 to 2.14) | .41 | 1.29 (1.01 to 1.66) | .005 |
| 2 | 1.32 (1.00 to 1.73) | 1.63 (0.91 to 2.92) | 1.42 (1.09 to 1.85) | |||
| 3 | 1.04 (0.76 to 1.44) | 1.72 (0.89 to 3.34) | 1.22 (0.89 to 1.66) | |||
| ≥4 | 0.73 (0.51 to 1.07) | 1.82 (0.91 to 3.64) | 0.93 (0.66 to 1.33) | |||
| 1 | Referent | .0001 | Referent | .41 | Referent | .005 |
| 2 | 1.03 (0.83 to 1.28) | 1.51 (0.85 to 2.66) | 1.10 (0.90 to 1.35) | |||
| 3 | 0.82 (0.63 to 1.06) | 1.59 (0.83 to 3.04) | 0.94 (0.74 to 1.20) | |||
| ≥4 | 0.58 (0.42 to 0.79) | 1.68 (0.83 to 3.39) | 0.72 (0.54 to 0.98) | |||
| Nulliparous | 0.78 (0.60 to 1.02) | 0.92 (0.47 to 1.82) | 0.78 (0.60 to 0.99) | |||
| No. of full-term pregnancy by attained age† | ||||||
| <40 years | ||||||
| 1 | Referent | <.0001 | Referent | .98 | Referent | .0008 |
| 2 | 0.81 (0.62 to 1.06) | 2.36 (0.47 to 11.83) | 0.88 (0.67 to 1.16) | |||
| 3 | 0.79 (0.56 to 1.13) | 1.25 (0.14 to 11.55) | 0.81 (0.56 to 1.19) | |||
| ≥4 | 0.29 (0.16 to 0.52) | 1.31 (0.09 to 19.54) | 0.33 (0.17 to 0.63) | |||
| ≥40 years | ||||||
| 1 | Referent | .005 | Referent | .39 | Referent | .04 |
| 2 | 1.28 (0.95 to 1.73) | 1.33 (0.71 to 2.48) | 1.26 (0.97 to 1.65) | |||
| 3 | 0.96 (0.69 to 1.34) | 1.51 (0.77 to 2.96) | 1.07 (0.79 to 1.45) | |||
| ≥4 | 0.77 (0.53 to 1.12) | 1.57 (0.76 to 3.25) | 0.90 (0.64 to 1.26) | |||
| Age at 1st full-term pregnancy, y‡ | ||||||
| <20 | Referent | .0003 | Referent | .12 | Referent | <.0001 |
| 20–24 | 1.13 (0.87 to 1.47) | 1.60 (0.85 to 2.98) | 1.25 (0.97 to 1.60) | |||
| 25–29 | 1.39 (1.05 to 1.84) | 1.26 (0.63 to 2.51) | 1.39 (1.06 to 1.83) | |||
| ≥30 | 1.64 (1.20 to 2.24) | 1.95 (0.95 to 3.98) | 1.77 (1.30 to 2.40) | |||
| Year since last full-term pregnancy§ | ||||||
| 0–5 | Referent | .57 | Referent | .06 | Referent | .40 |
| 6–20 | 0.97 (0.79 to 1.18) | 0.82 (0.42 to 1.59) | 0.96 (0.79 to 1.17) | |||
| ≥21 | 0.92 (0.67 to 1.25) | 0.71 (0.31 to 1.64) | 0.88 (0.65 to 1.19) | |||
| Nulliparous | 0.74 (0.56 to 0.97) | 0.79 (0.35 to 1.77) | 0.73 (0.56 to 0.94) | |||
| Nulliparous | Referent | Referent | Referent | |||
| 0–5 | 1.36 (1.03 to 1.78) | .57 | 1.27 (0.57 to 2.86) | .06 | 1.37 (1.06 to 1.78) | .40 |
| 6–20 | 1.31 (0.98 to 1.76) | 1.04 (0.51 to 2.14) | 1.32 (1.01 to 1.74) | |||
| ≥21 | 1.24 (0.86 to 1.79) | 0.90 (0.38 to 2.15) | 1.21 (0.86 to 1.70) | |||
| Breastfeeding duration§ | ||||||
| None | Referent | .002 | Referent | .59 | Referent | .01 |
| 1–5 mo | 1.00 (0.82 to 1.24) | 1.14 (0.69 to 1.88) | 1.05 (0.87 to 1.28) | |||
| 6–12 mo | 1.16 (0.93 to 1.43) | 1.28 (0.77 to 2.13) | 1.17 (0.96 to 1.43) | |||
| 13–24 mo | 0.85 (0.66 to 1.09) | 0.74 (0.40 to 1.35) | 0.82 (0.64 to 1.04) | |||
| > 24 mo | 0.61 (0.43 to 0.86) | 1.03 (0.58 to 1.81) | 0.74 (0.55 to 1.00) | |||
| No. of full-term pregnancy and breastfeeding† | ||||||
| Nulliparous | Referent | Referent | Referent | |||
| 1 FTP, never breastfeeding | 1.33 (0.91 to 1.93) | 1.90 (0.77 to 4.72) | 1.45 (1.02 to 2.06) | |||
| ≥2 FTP, never breastfeeding | 1.25 (0.91 to 1.72) | 1.32 (0.66 to 2.65) | 1.31 (0.98 to 1.77) | |||
| 1 FTP, ever breastfeeding | 1.33 (1.00 to 1.78) | 0.79 (0.35 to 1.80) | 1.27 (0.98 to 1.66) | |||
| ≥2 FTP, ever breastfeeding | 1.19 (0.90 to 1.57) | 1.71 (0.97 to 3.03) | 1.35 (1.03 to 1.76) | |||
| Incomplete pregnancy (IP)§ | ||||||
| No full-term or Incomplete pregnancy | Referent | Referent | Referent | |||
| Full-term pregnancy, no IP | 1.35 (1.05 to 1.75) | 0.82 (0.40 to 1.68) | 1.28 (1.00 to 1.64) | |||
| Induced abortion only | 1.38 (1.01 to 1.89) | 0.47 (0.18 to 1.17) | 1.15 (0.85 to 1.56) | |||
| Miscarriage only | 1.52 (1.13 to 2.04) | 0.88 (0.43 to 1.79) | 1.40 (1.06 to 1.84) | |||
| Induced abortion and miscarriage | 1.87 (1.19 to 2.92) | 0.87 (0.35 to 2.15) | 1.61 (1.07 to 2.42) | |||
| Incomplete pregnancy relative to first full-term pregnancy§ | ||||||
| No IP | Referent | Referent | Referent | |||
| Before first FTP or no FTP | 1.34 (1.10 to 1.63) | 0.78 (0.50 to 1.21) | 1.17 (0.97 to 1.40) | |||
| After first FTP | 1.01 (0.82 to 1.24) | 0.99 (0.65 to 1.53) | 0.99 (0.82 to 1.20) | |||
Nulliparous excluded, risk factor as continuous.
Adjusted for bilateral oophorectomy (Yes, No), age at 1st full-term pregnancy (<30, ≥30 nulliparous), strata by birth year and study site.
Adjusted for bilateral oophorectomy, number of full-term pregnancies (0–1, ≥2), strata by birth year and study site.
Adjusted for bilateral oophorectomy, number of full-term pregnancies (0–1, ≥2), age at 1st full-term pregnancy, strata by birth year and study site.
Adjusted for bilateral oophorectomy, number of live births (0–1, ≥2), age at 1st full-term pregnancy, strata by birth year and study site.
We observed an increase in risk with increasing age at first FTP in the retrospective analysis (Ptrend = .0003). There was some suggestion of a similar trend in the prospective cohort (Ptrend = .12; HRp = 1.95, 95% CI = 0.95 to 3.98 for a first FTP at age ≥30 years vs <20 years). Recent pregnancy was associated with BC risk (≤5 years relative to nulliparous; HRR = 1.36, 95% CI = 1.03 to 1.78; HRp = 1.27, 95% CI = 0.57 to 2.86; HRc = 1.37, 95% CI = 1.06 to 1.78). Increasing duration of breastfeeding was associated with decreased BC risk in the retrospective analysis (Ptrend= .002), but not in the prospective cohort (Ptrend = .59). Any pregnancy, including IP, was associated with BC risk but only in the retrospective cohort (Table 4).
We performed sensitivity analyses that further adjusted for age at menarche, oral contraceptive use, and family history of BC or excluding in situ BC. The estimates were very similar to those in the main analysis (Supplementary Tables 1–3, available online). Analysis based on the pseudo-incidence retrospective cohort also gave very similar estimates to those based on the entire retrospective cohort (Supplementary Table 4, available online).
Discussion
Using data from the largest international cohort study of BRCA1 and BRCA2 mutation carriers to date, we found that overall parity was not associated with BC risk for BRCA1 mutation carriers but was associated with BC risk for BRCA2 mutation carriers. Nulliparous and multiparous BRCA1 mutation carriers had lower BC risk compared with uniparous women. Longer duration of breastfeeding also was associated with a reduced risk for BRCA1 mutation carriers. There was some suggestion that uniparous women who subsequently breastfed may have a decrease in BC risk compared with those that did not. for BRCA2 mutation carriers, multiparity reduced risk, particularly prior to age 40 years, and late age at first FTP was associated with increased risk.
Previous epidemiological studies investigating modifiable factors for BRCA1 and BRCA2 mutation carriers have had limited power to examine gene-specific associations and have primarily been retrospective (8,11,19). Our cohort provides the first large-scale prospective evaluation of parity separately for BRCA1 and BRCA2 mutation carriers. Overall, we found that increasing parity beyond the first child was associated with a decrease in BC risk for BRCA1 mutation carriers in both the retrospective and prospective analyses. This association with multiparity in BRCA1 mutation carriers was consistent with a meta-analysis that reported a 17% decrease for each additional birth (11). Curiously, however, nulliparity was associated with a reduced risk of BC in comparison with uniparity; this association was particularly marked in the prospective analysis.
Increasing age at FTP was associated with reduced BC risk for BRCA1 mutation carriers but only in the retrospective analysis. Moreover, the effect size was smaller than that reported in the meta-analysis by Friebel et al. (11) (for pregnancy after age 30 years vs before 25 years, relative risk [RR] = 0.65, 95% CI = 0.42 to 0.99). The pattern of association is clearly different from that seen in the general population, where increased age at first FTP is associated with increased BC risk (27).
For BRCA2 mutation carriers, we observed a positive association with overall parity in both the retrospective and prospective analyses not driven by uniparity as observed for BRCA1 mutation carriers. We also observed an increased risk of BC with later age at first FTP, which is more consistent with the association seen in the general population, but in contrast to the results of the Friebel et al. (11) meta-analysis, which found no association. We also found an association between multiparity and a reduced risk of BC particularly for women who had four or more pregnancies in the retrospective analysis. We also observed a modest increase in risk associated with recent pregnancies (≤5 years, relative to nulliparous) in BRCA2 mutation carriers in both retrospective and prospective analyses (36% and 27%, respectively). for BRCA1 mutation carriers, the risk was also higher in the first five years, relative to nulliparous women, but this was observed only in the prospective cohort. However, there was no difference by attained age even in the prospective analysis where women are slightly older and no evidence that BC risk declined by time since pregnancy, and in opposite, the risk increased with time since last pregnancy in both the retrospective and prospective cohorts.
Although multiparity relative to nulliparity reduced risk in both BRCA1 and BRCA2 mutation carriers, late age at first FTP was only associated with increased risk for BRCA2 mutation carriers. The differences we observed between BRCA1 and BRCA2 mutation carriers might reflect their difference in the estrogen receptor (ER) status distribution that has been reported by mutation type (28). We did not have hormonal receptor status for our pooled cohort, but we expect the differences we observed reflect both hormonal status as well as age-related differences between BRCA1 and BRCA2 mutation carriers. For example, as we recently reported, BRCA1 and BRCA2 mutation carriers have different BC risk distributions. For BRCA1 mutation carriers, there is a rapid increase in BC incidence until ages 30 to 40 years, whereas the risk for BRCA2 mutation carriers continues to increase until approximately age 50 years, similar to the distribution in the general population (1). Therefore, one can expect that risk factors may be different or act differently between BRCA1 and BRCA2 mutation carriers because of their timing. In particular, given the later peak in incidence for BRCA2 mutation carriers, later age at FTP may increase risk in the short-term similar to the transient increase from pregnancy seen in the general population.
Retrospective analyses generally have substantially more power but may be potentially biased for selected risk factors given that risk factors are ascertained after diagnosis, or might motivate study participation. Prospective cohorts have the advantage of collecting information prior to knowing the outcome, but often have more limited statistical power compared to retrospective studies. FTPs, however, are unlikely to have substantial information bias when collected retrospectively, and for prospective analyses, the mean age at start of follow-up has mostly passed the reproductive life period. Similar findings between the two designs also support that selection bias may be less of a concern as selection bias often operates differently in retrospective and prospective studies. We were limited, however, to addressing confounding by only established risk factors that have been collected across all of the studies. We formally tested for homogeneity across the two cohorts using meta-analytic techniques, and both random and fixed effects models suggested that the inferences in both retrospective and prospective analyses were not different from each other. Thus we were able to provide more precise estimates by combining both cohorts. We also investigated heterogeneity across birth cohorts (Supplementary Figure 1, available online) and geographic study sites and observed similar inferences.
The increased risk for uniparous BRCA1 mutation carriers (and perhaps for BRCA2 carriers) is inconsistent with the pattern for the general population. However, the lack of a protective effect of parity in BRCA1 mutation carriers who develop primarily ER-negative tumor is consistent with the weaker association with parity and age at first FTP observed for ER-negative BC in the general population (28). It suggests that many of the key driver events may have already occurred in adolescence, such that the first FTP increases the risk of BC due to stimulation of partially transformed mammary cells. This risk may be stronger for first pregnancy for those most susceptible based on prior exposures and then decline after FTP given increased cell differentiation in the late phase of pregnancy and lactation and postpartum gland involution (29–33), thus the lower risk for the uniparous women who breastfeed than women who do not may be explained by the differential rates of mammary gland involution.
Nulliparous and multiparous BRCA1 mutation carriers have lower BC risk compared with uniparous women. Long duration of breastfeeding decreased risk for BRCA1 mutation carriers. For BRCA2 mutation carriers, multiparity seems to reduce risk, although this was limited to the retrospective cohort analyses, and late age at first FTP increased risk. These findings might help refine the BC risk estimates and make it possible to adapt the surveillance of mutation carriers according to their reproductive life history.
Funding
This work was supported by Cancer Research UK grants C1287/A17523, C1287/23382, C1287/A16563, C12292/A20861, and C12292/A11174.
The Breast Cancer Family Registry (BCFR) was supported by the US National Institute of Health (grant number RO1CA159868). The Australian BCFR was supported in Australia by the National Health and Medical Research Council, the New South Wales Cancer Council, the Victorian Health Promotion Foundation, the Victorian Breast Cancer Research Consortium, Cancer Australia, and the National Breast Cancer Foundation. The six sites of the Breast Cancer Family Registry (BCFR) were supported by grant UM1 CA164920 from the US National Cancer Institute. The content of this manuscript does not necessarily reflect the views or policies of the National Cancer Institute or any of the collaborating centers in the BCFR, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government or the BCFR.
The Spanish National Cancer Centre (CNIO) was partially supported by the Spanish Ministry of Economy and Competitiveness (MINECO) SAF2014-57680-R, the Spanish Research Network on Rare diseases (CIBERER). CNIO was also partially supported by FISPI16/00440.
INHERIT was supported by the Canadian Institutes of Health Research (CIHR) for the CIHR Team in Familial Risks of Breast Cancer program (grant number CRN-87521) and the Ministry of Economic Development, Innovation and Export Trade (grant number PSR-SIIRI-701). The PERSPECTIVE project was supported by the Government of Canada through Genome Canada and the Canadian Institutes of Health Research (GPH-129344), the Ministère de l’Économie, de la Science et de l’ Innovation du Québec through Genome Québec, and the Quebec Breast Cancer Foundation.
Jacques Simard is Chairholder of the Canada Research Chair in Oncogenetics.
The DKFZ study was supported by the Dresden and German Cancer Research Center (DKFZ).
The Epidemiological Study of Familial Breast Cancer (EMBRACE) is supported by Cancer Research UK grants C1287/A23382 and C1287/A16563.
D. Gareth Evans is supported by a National Institute for Health Research (NIHR) grant to the Biomedical Research Centre, Manchester. The investigators at the Institute of Cancer Research and the Royal Marsden NHS Foundation Trust are supported by an NIHR grant to the Biomedical Research Centre at the Institute of Cancer Research and the Royal Marsden NHS Foundation Trust. Ros Eeles and Elizabeth Bancroft are supported by Cancer Research UK grant C5047/A8385. Ros Eeles is also supported by NIHR support to the Biomedical Research Centre at the Institute of Cancer Research and the Royal Marsden NHS Foundation Trust. Antonis C. Antoniou is funded by Cancer Research UK grants C12292/A20861 and C12292/A11174. MT is funded by the European Union Seventh Framework Program (2007– 013)/European Research Council (310018).
The German Consortium of Hereditary Breast and Ovarian Cancer (GC-HBOC) is supported by the German Cancer Aid (grant number 110837, Rita K. Schmutzler).
The national French cohort, GENEPSO, had been supported by a grant from the Fondation de France and the Ligue Nationale Contre le Cancer and is being supported by a grant from INCa as part of the European program ERA-NET on Translational Cancer Research (TRANSCAN-JTC2012, n°2014-008).
Hospital Clinico San Carlos (HCSC) was supported by CIBERONC 161200301 from ISCIII (Spain), partially supported by European Regional Development FEDER funds.
The HEBON study is supported by the Dutch Cancer Society grants NKI1998-1854, NKI2004-3088, NKI2007-3756; the Netherlands Organisation of Scientific Research grant NWO 91109024; the Pink Ribbon grants 110005 and 2014-187.WO76; the BBMRI grant NWO 184.021.007/CP46; and the Transcan grant JTC 2012 Cancer 12-054.
The International Hereditary Cancer Center (IHCC) was supported by grant PBZ_KBN_122/P05/2004 and ERA-NET TRANSAN JTC 2012 Cancer 12-054 (ERA-NET-TRANSCAN/07/2014).
This work was supported by grants to kConFab and the kConFab Follow-Up Study from Cancer Australia (809195); the Australian National Breast Cancer Foundation (IF 17); the National Health and Medical Research Council (454508, 288704, 145684); the US National Institute of Health (1RO1CA159868); the Queensland Cancer Fund; the Cancer Councils of New South Wales, Victoria, Tasmania, and South Australia; and the Cancer Foundation of Western Australia.
MODSQUAD Czech Republic, Brno was supported by MH CZ—DRO (MMCI, 00209805) and by MEYS—NPS I - LO1413 to LF, MN.
The Hungarian Breast and Ovarian Cancer Study was supported by Hungarian Research grants KTIA-OTKA CK-80745 and NKFI OTKA K-112228 and the Norwegian EEA Financial Mechanism HU0115/NA/2008-3/ÖP-9.
Lund-BRCA collaborators are supported by the Swedish Cancer Society, Lund Hospital Funds, and European Research Council Advanced grant ERC-2011-294576. Stockholm-BRCA collaborators are supported by the Swedish Cancer Society.
Notes
Affiliations of authors: Department of Epidemiology, Columbia University, New York, NY (MBT, YL, JAM); Herbert Irving Comprehensive Cancer Center, Columbia University Medical Center, New York, NY (MBT, YL, JAM); Department of Gynecology and Obstetrics, Medical Faculty and University Hospital Carl Gustav Carus, Technische Universität Dresden, Dresden, Germany (KK); National Center for Tumor Diseases (NCT), Partner Site Dresden, Dresden, Germany (KK); German Cancer Consortium (DKTK), Dresden and German Cancer Research Center (DKFZ), Heidelberg, Germany (KK); Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (ACA, DB, DF, DFE); Strangeways Research Laboratory, Worts Causeway, University of Cambridge, Cambridge, UK (ACA, DB, DF, DFE); Department of Epidemiology, Netherlands Cancer Institute, Amsterdam, The Netherlands (TMM, MJRB, FL, MR); Institute for Medical Informatics, Statistics and Epidemiology, University of Leipzig, Leipzig, Germany (CE); Institut Paoli Calmette, Department d’Anticipation et de Suivi du Cancer, Pôle Clinique Consultations d’Oncologie Génétique, Marseille, France (CN, JMS); Institut Curie, Service de Génétique Médicale, Paris, France (BB); CLCC Antoine Lacassagne, Département d’Hématologie - Oncologie médicale, Nice, France (VM); CLCC Institut Claudius Regaud, IUCT Oncopole, Toulouse, France (LG); CHR Metz-Thionville, Hôpital de Mercy, Metz, France (EL); Institute of Genetic Medicine, Centre for Life, Newcastle Upon Tyne Hospitals NHS Trust, Newcastle upon Tyne, UK (AH); Department of Clinical Genetics, Royal Devon and Exeter Hospital, Exeter, UK (CB); Genomic Medicine, Manchester Academic Health Sciences Centre, Institute of Human Development, Manchester University, Central Manchester University Hospitals NHS Foundation Trust, Manchester, UK (DGE); University of Southampton Faculty of Medicine, Southampton University Hospitals NHS Trust, Southampton, UK (DE); Sheffield Clinical Genetics Service, Sheffield Children’s Hospital, Sheffield, UK (JC); West Midlands Regional Genetics Service, Birmingham Women’s Hospital Healthcare NHS Trust, Edgbaston, Birmingham, UK (KRO); Clinical Genetics, Guy’s and St. Thomas’ NHS Foundation Trust, London, UK (LI); North East Thames Regional Genetics Service, Great Ormond Street Hospital for Children NHS Trust, London, UK (MA); Northern Ireland Regional Genetics Centre, Belfast City Hospital, Belfast, UK (PJM); Amsterdam UMC, Vrije Universiteit Amsterdam, Department of Clinical Genetics. The Netherlands (CJD); Department of Genetics, University Medical Center, University of Groningen, Groningen, The Netherlands (JCO); Division Laboratories, Pharmacy and Biomedical Genetics, Department of Genetics, University Medical Center Utrecht. The Netherlands (MGEMA); Department of Medical Oncology, Erasmus MC Cancer Institute, Rotterdam, The Netherlands (MK); Department of Medicine, University of Utah Health Sciences Center, Huntsman Cancer Institute, Salt Lake City, UT (SSB); Department of Molecular Genetics, University of Toronto, Toronto, ON, Canada (ILA); Lunenfeld-Tanenbaum Research Institute, Sinai Health System, Toronto, ON, Canada (ILA); Department of Medicine and Stanford Cancer Institute, Stanford University School of Medicine, Stanford, CA (EMJ); Department of Clinical Genetics, Fox Chase Cancer Center, Philadelphia, PA (MD); Department of Medical Oncology, Prince of Wales Hospital, Randwick, NSW, Australia (MF); Division of Cancer Medicine, Peter MacCallum Cancer Centre, Melbourne, VIC, Australia (MF); Department of Medicine, St Vincent’s Hospital, The University of Melbourne, Parkville, VIC, Australia (SAM); Department of Medical Oncology, St Vincent’s Hospital, Fitzroy, VIC, Australia (SAM); Human Genetics Group, Spanish National Cancer Centre (CNIO) and Biomedical Network on Rare Diseases (CIBERER), Madrid, Spain (AO); Molecular Oncology Laboratory, Hospital Clinico San Carlos, IdISSC, CIBERONC (ISCIII) (TC); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ); Genomics Center, Centre Hospitalier Universitaire de Québec – Université Laval Research Center, Quebec City, QC, Canada (JS); Department of OB/GYN and Comprehensive Cancer Center, Medical University of Vienna, Vienna, Austria (CFS, YT); Department of Molecular Genetics, National Institute of Oncology, Budapest, Hungary (EO); Department of Cancer Epidemiology and Genetics, Masaryk Memorial Cancer Institute, Brno, Czech Republic (MN, LF); Department of Clinical Genetics, Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark (AMG); Department of Oncology and Pathology, Karolinska Institute, Stockholm, Sweden (BA); Department of Oncology, Lund University Hospital, Lund, Sweden (BA, HO); Center for Familial Breast and Ovarian Cancer, Center for Integrated Oncology (CIO), Medical Faculty, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne (CMMC), University of Cologne, Cologne, Germany (RKS); Centre for Epidemiology and Biostatistics, School of Population and Global Health, The University of Melbourne, Melbourne, VIC, Australia (JLH, RLM); Department of Dermatology, University of Utah School of Medicine, Salt Lake City, UT (DG); Cancer Epidemiology and Intelligence Division, Cancer Council Victoria, Melbourne, VIC, Australia (RLM); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (DFE); Strangeways Research Laboratory, Worts Causeway, University of Cambridge, Cambridge, UK (DFE); INSERM, U900, Paris, France (NA); Institut Curie, Paris, France (NA); Mines Paris Tech, Fontainebleau, France (NA); PSL Research University, Paris, France (NA); Sir Peter MacCallum Department of Oncology, The University of Melbourne, Melbourne, VIC, Australia (on behalf of EMBRACE, GENEPSO, BCFR, HEBON, kConFab and IBCCS); the Research Department, Peter MacCallum Cancer Centre, Melbourne, VIC, Australia (on behalf of EMBRACE, GENEPSO, BCFR, HEBON, kConFab and IBCCS).
MBT and NA drafted the initial manuscript. The complete writing group consisted of MBT, NA, KK, MAR, and DFE. MBT, YL, and NA performed and are responsible for the statistical analyses, and the complete analysis group additionally consists of JLH, HO, ACA, CE, RM, KK, MBT, YL, MAR, DFE, and NA. TMM and MRB are the international database managers. MAR coordinated the collaborative study, and DEG, DFE, NA, CN, MBT, JLH, and MAR initiated and coordinated the original studies. CN, BB, VM, FE, LG, EL, AH, CB, DGE, DE, JC, KRO, LI, MA, PJM, CJD, JCO, MGEMA, MK, SB, IA, EMJ, MD, MF, SAM, AO, TC, AJ, JS, CFS, YT, EO, MN, LF, AMG, BA, KO, and RKS invited patients and collected data. All authors read and approved the final manuscript.
The funders had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
We acknowledge the EMBRACE Centres: the Coordinating Centre: University of Cambridge and the Collaborating Centres: Guy’s and St. Thomas’ NHS Foundation Trust, London: Louise Izatt; Central Manchester University Hospitals NHS Foundation Trust, Manchester: Gareth Evans; Chapel Allerton Hospital, Leeds: Julian Adlard; The Institute of Cancer Research and Royal Marsden NHS Foundation Trust, Sutton: Ros Eeles; Birmingham Women’s Hospital Healthcare NHS Trust, Birmingham: Kai-Ren Ong; South Glasgow University Hospitals, Glasgow: Rosemarie Davidson; Addenbrooke’s Hospital, Cambridge: Marc Tischkowitz; St. Georges, London: Katie Snape; Royal Devon and Exeter Hospital, Exeter: Carole Brewer; Southampton University Hospitals NHS Trust, Southampton: Diana Eccles; Sheffield Children’s Hospital, Sheffield: Jackie Cook; Newcastle Upon Tyne Hospitals NHS Trust, Newcastle: Alex Henderson; Great Ormond Street Hospital for Children NHS Trust, London: Munaza Ahmed; Churchill Hospital, Oxford: Lisa Walker; Western General Hospital, Edinburgh: Mary Porteous; St Michael’s Hospital, Bristol: Alan Donaldson; Belfast City Hospital, Belfast: Patrick Morrison; Nottingham University Hospitals NHS Trust, Nottingham: Jacqueline Eason; University Hospital of Wales, Cardiff: Mark Rogers; Alder Hey Hospital, Liverpool: Claire Miller; Kennedy Galton Centre, Harrow: Angela Brady; Trinity College Dublin and St James’s Hospital, Dublin: M John Kennedy; University Hospitals of Leicester NHS Trust, Leicester: Julian Barwell; NHS Grampian and University of Aberdeen, Aberdeen: Helen Gregory; Glan Clwyd Hospital, Rhyl: Caroline Pottinger; Singleton Hospital, Swansea: Alex Murray.
The BCFR wish to thank members and participants in the Breast Cancer Family Registry from the New York, northern California, Ontario, Philadelphia, and Utah sites for their contributions to the study. BCFR-Australia wish to acknowledge Maggie Angelakos, Gillian Dite, and Helen Tsimiklis. The CNIO thanks Alicia Barroso, Rosario Alonso, and Guillermo Pita for their assistance.
We acknowledge the GENEPSO Centers: the Coordinating Center: Institut Paoli Calmettes, Marseille: Catherine Noguès, Emmanuel Breysse, Pauline Pontois, Lilian Laborde, and the Collaborating Centers: Institut Curie, Paris: Dominique Stoppa-Lyonnet, Marion Gauthier-Villars; Bruno Buecher, Institut Gustave Roussy, Villejuif: Olivier Caron; Hôpital René Huguenin/Institut Curie, Saint Cloud: Emmanuelle Fourme-Mouret; Centre Paul Strauss, Strasbourg: Jean-Pierre Fricker; Centre Léon Bérard, Lyon: Christine Lasset, Valérie Bonadona; Hôtel Dieu - Centre Hospitalier, Chambery: Sandra Fert-Ferrer; Centre François Baclesse, Caen: Pascaline Berthet; CHRU Dupuytren, Limoges: Laurence Venat-Bouvet; CHU La Milétrie, Poitiers: Brigitte Gilbert-Dussardier; Hôpital d’Enfants CHU Dijon – Centre Georges François Leclerc, Dijon: Laurence Faivre; Centre Alexis Vautrin, Vandoeuvre-les-Nancy: Elisabeth Luporsi; Institut Claudius Regaud, Toulouse: Laurence Gladieff; Réseau Oncogénétique Poitou Charente, Niort: Paul Gesta; Institut Paoli-Calmettes, Marseille: Catherine Noguès, Hagay Sobol, François Eisinger; Institut Bergonié, Bordeaux: Michel Longy, Centre Eugène Marquis, Rennes: Catherine Dugast†; GH Pitié Salpétrière, Paris: Chrystelle Colas, Florent Soubrier; CHU Arnaud de Villeneuve, Montpellier: Isabelle Coupier, Pascal Pujol, Carole Corsini; Centres Paul Papin, and Catherine de Sienne, Angers, Nantes: Alain Lortholary; Centre Oscar Lambret, Lille: Philippe Vennin†, Claude Adenis; Institut Jean Godinot, Reims: Tan Dat Nguyen; Institut Jean-Godinot and ICC Courlancy, Reims: Clotilde Penet; Centre René Gauducheau, Nantes: Capucine Delnatte; Centre Henri Becquerel, Rouen: Julie Tinat, Isabelle Tennevet; Hôpital Civil, Strasbourg: Jean-Marc Limacher; Christine Maugard; Polyclinique Courlancy, Reims: Liliane Demange†; Clinique Sainte Catherine, Avignon: Hélène Dreyfus; Hôpital Saint-Louis, Paris: Odile Cohen-Haguenauer; Couple-Enfant-CHU de Grenoble: Dominique Leroux; Hôpital de la Timone, Marseille: Hélène Zattara-Cannoni;, CHU Fort de France, Fort de France: Odile Bera.
The HCSC acknowledges Paula Diaque for her technical assistance.
The representative authors of the Hereditary Breast and Ovarian Cancer Research Group Netherlands (HEBON) thank the other collaborators: Netherlands Cancer Institute (coordinating center), Amsterdam, NL: F.B.L. Hogervorst, M.A. Adank, M.K. Schmidt, N.S. Russell, D.J. Jenner; Erasmus Medical Center, Rotterdam, NL: J.M. Collée, A.M.W. van den Ouweland, M.J. Hooning, C.M. Seynaeve, C.H.M. van Deurzen, I.M. Obdeijn; Leiden University Medical Center, NL: C.J. van Asperen, P. Devilee; Radboud University Nijmegen Medical Center, NL: C.M. Kets, A.R. Mensenkamp; University Medical Center Utrecht, NL: M.J. Koudijs; Amsterdam Medical Center, NL: C.M. Aalfs; VU University Medical Center, Amsterdam, NL: K. van Engelen, J.J.P. Gille; Maastricht University Medical Center, NL: E.B. Gómez-Garcia, M.J. Blok; University of Groningen, NL: A.H. van der Hout, M.J. Mourits, G.H. de Bock; The Netherlands Comprehensive Cancer Organisation (IKNL): S. Siesling, J. Verloop; The nationwide network and registry of histo- and cytopathology in The Netherlands (PALGA): A.W. van den Belt-Dusebout. HEBON thanks the study participants and the registration teams of IKNL and PALGA for part of the data collection.
INHERIT would like to thank Dr Martine Dumont for sample management and skillful assistance.
We thank Eveline Niedermayr, all the kConFab research nurses and staff, the heads and staff of the Family Cancer Clinics, and the many families who contribute to kConFab for their contributions to this resource.
Czech Republic, MMCI, Brno: many thanks to Dita Hanouskova, research nurse, and Sarka Rathouska, research technician, for data collection and management.
We wish to thank the Hungarian Breast and Ovarian Cancer Study Group members (Maria Balogh, Janos Papp, Matrai Zoltan, Judit Franko Department of Molecular Genetics, National Institute of Oncology, Budapest, Hungary) and the clinicians and patients for their contributions to this study.
†Deceased.
Supplementary Material
References
- 1. Kuchenbaecker KB, Hopper JL, Barnes DR, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317(23):2402–2416. [DOI] [PubMed] [Google Scholar]
- 2. Kelsey JL, Gammon MD, John EM.. Reproductive factors and breast cancer. Epidemiol Rev. 1993;15(1):36–47. [DOI] [PubMed] [Google Scholar]
- 3. Beral V, Bull D, Doll R, et al. Breast cancer and abortion: collaborative reanalysis of data from 53 epidemiological studies, including 83 000 women with breast cancer from 16 countries. Lancet. 2004;363(9414):1007–1016. [DOI] [PubMed] [Google Scholar]
- 4. Palmer JR, Wise LA, Adams-Campbell LL, et al. A prospective study of induced abortion and breast cancer in African-American women. Cancer Causes Control. 2004;15(2):105–111. [DOI] [PubMed] [Google Scholar]
- 5. Mahue-Giangreco M, Ursin G, Sullivan-Halley J, et al. Induced abortion, miscarriage, and breast cancer risk of young women. Cancer Epidemiol Biomarkers Prev. 2003;12(3):209–214. [PubMed] [Google Scholar]
- 6. Lambe M, Hsieh C-C, Trichopoulos D, et al. Transient increase in the risk of breast cancer after giving birth. N Engl J Med. 1994;331(1):5–9. [DOI] [PubMed] [Google Scholar]
- 7. Albrektsen G, Heuch I, Hansen S, et al. Breast cancer risk by age at birth, time since birth and time intervals between births: exploring interaction effects. Br J Cancer. 2005;92(1):167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sun X, Nichols HB, Tse C-K, et al. Association of parity and time since last birth with breast cancer prognosis by intrinsic subtype. Cancer Epidemiology Biomarkers Prev. 2016;25(1):60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Work ME, John EM, Andrulis IL, et al. Reproductive risk factors and oestrogen/progesterone receptor-negative breast cancer in the Breast Cancer Family Registry. Br J Cancer. 2014;110(5):1367–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beral V, Bull D, Doll R, et al. Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50 302 women with breast cancer and 96 973 women without the disease. Lancet. 2002;360(9328):187–195. [DOI] [PubMed] [Google Scholar]
- 11. Friebel TM, Domchek SM, Rebbeck TR.. Modifiers of cancer risk in BRCA1 and BRCA2 mutation carriers: systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju091.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pan H, He Z, Ling L, et al. Reproductive factors and breast cancer risk among BRCA1 or BRCA2 mutation carriers: results from ten studies. Cancer Epidemiol. 2014;38(1):1–8. [DOI] [PubMed] [Google Scholar]
- 13. Antoniou AC, Shenton A, Maher ER, et al. Parity and breast cancer risk among BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. 2006;8(6):R72.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee E, Ma H, McKean-Cowdin R, et al. Effect of reproductive factors and oral contraceptives on breast cancer risk in BRCA1/2 mutation carriers and noncarriers: results from a population-based study. Cancer Epidemiol Biomarkers Prev. 2008;17(11):3170–3178. [DOI] [PubMed] [Google Scholar]
- 15. Milne RL, Osorio A, Ramon y Cajal T, et al. Parity and the risk of breast and ovarian cancer in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res Treat. 2010;119(1):221–232. [DOI] [PubMed] [Google Scholar]
- 16. Cullinane CA, Lubinski J, Neuhausen SL, et al. Effect of pregnancy as a risk factor for breast cancer in BRCA1/BRCA2 mutation carriers. Int J Cancer. 2005;117(6):988–991. [DOI] [PubMed] [Google Scholar]
- 17. Jernstrom H, Lubinski J, Lynch HT, et al. Breast-feeding and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2004;96(14):1094–1098. [DOI] [PubMed] [Google Scholar]
- 18. Kotsopoulos J, Lubinski J, Salmena L, et al. Breastfeeding and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. 2012;14(2):R42.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Andrieu N, Goldgar DE, Easton DF, et al. Pregnancies, breast-feeding, and breast cancer risk in the International BRCA1/2 Carrier Cohort Study (IBCCS). J Natl Cancer Inst. 2006;98(8):535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goldgar D, Bonnardel C, Renard H, et al. The International BRCA1/2 Carrier Cohort Study: purpose, rationale, and study design. Breast Cancer Res. 2000;2(6):E10. [Google Scholar]
- 21. John EM, Hopper JL, Beck JC, et al. The Breast Cancer Family Registry: an infrastructure for cooperative multinational, interdisciplinary and translational studies of the genetic epidemiology of breast cancer. Breast Cancer Res. 2004;6(4):R375–R389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Phillips KA, Butow PN, Stewart AE, et al. Predictors of participation in clinical and psychosocial follow-up of the kConFab breast cancer family cohort. Fam Cancer. 2005;4(2):105–113. [DOI] [PubMed] [Google Scholar]
- 23. Thorne H, Mitchell G, Fox S, et al. kConFab: a familial breast cancer consortium facilitating research and translational oncology. J Natl Cancer Inst Monogr. 2011;2011(43):79–81. [DOI] [PubMed] [Google Scholar]
- 24. Terry MB, Phillips KA, Daly MB, et al. Cohort profile: the Breast Cancer Prospective Family Study Cohort (ProF-SC). Int J Epidemiol. 2015. 10.1093/ije/dyv118. 45(3):683-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Antoniou AC, Goldgar DE, Andrieu N, et al. A weighted cohort approach for analysing factors modifying disease risks in carriers of high-risk susceptibility genes. Genet Epidemiol. 2005;29(1):1–11. [DOI] [PubMed] [Google Scholar]
- 26. Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72(5):1117–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reeves GK, Pirie K, Green J, et al. Comparison of the effects of genetic and environmental risk factors on in situ and invasive ductal breast cancer. Int J Cancer. 2012;131(4):930–937. [DOI] [PubMed] [Google Scholar]
- 28. Anderson KN, Schwab RB, Martinez ME.. Reproductive risk factors and breast cancer subtypes: a review of the literature. Breast Cancer Res Treat. 2014;144(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Russo J, Russo IH.. DNA labeling index and structure of the rat mammary gland as determinants of its susceptibility to carcinogenesis. J Natl Cancer Inst. 1978;61(6):1451–1459. [PubMed] [Google Scholar]
- 30. Russo J, Tay LK, Russo IH.. Differentiation of the mammary gland and susceptibility to carcinogenesis. Breast Cancer Res Treat. 1982;2(1):5–73. [DOI] [PubMed] [Google Scholar]
- 31. Britt K, Ashworth A, Smalley M.. Pregnancy and the risk of breast cancer. Endocr Relat Cancer. 2007;14(4):907–933. [DOI] [PubMed] [Google Scholar]
- 32. Dall G, Risbridger G, Britt K.. Mammary stem cells and parity-induced breast cancer protection - new insights. J Steroid Biochem Mol Biol. 2017;170:54–60. [DOI] [PubMed] [Google Scholar]
- 33. Faupel-Badger JM, Arcaro KF, Balkam JJ, et al. Postpartum remodeling, lactation, and breast cancer risk: summary of a National Cancer Institute-sponsored workshop. J Natl Cancer Inst. 2013;105(3):166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.