Abstract
Parental effects have been shown to buffer the negative effects of within-generation exposure to ocean acidification (OA) conditions on the offspring of shallow water marine organisms. However, it remains unknown if parental effects will be impacted by the presence of diel CO2 cycles that are prevalent in many shallow water marine habitats. Here, we examined the effects that parental exposure to stable elevated (1000 µatm) and diel-cycling elevated (1000 ± 300 µatm) CO2 had on the survival and growth of juvenile coral reef anemonefish, Amphiprion melanopus. Juvenile survival was unaffected by within-generation exposure to either elevated CO2 treatment but was significantly increased (8%) by parental exposure to diel-cycling elevated CO2. Within-generation exposure to stable elevated CO2 caused a significant reduction in juvenile growth (10.7–18.5%); however, there was no effect of elevated CO2 on growth when diel CO2 cycles were present. Parental exposure to stable elevated CO2 also ameliorated the negative effects of elevated CO2 on juvenile growth, and parental exposure to diel CO2 cycles did not alter the effects of diel CO2 cycles on juveniles. Our results demonstrate that within-generation exposure to diel-cycling elevated CO2 and parental exposure to stable elevated CO2 had similar outcomes on juvenile condition. This study illustrates the importance of considering natural CO2 cycles when predicting the long-term impacts of OA on marine ecosystems.
Keywords: parental effects, phenotypic plasticity, acclimation, pH variability, daily fluctuations, coral reefs
1. Background
Parental effects occur when the environmental conditions experienced by the mother and/or father alter the phenotype of their offspring [1–3]. Thus, parental effects represent a form of phenotypic plasticity that spans generations. Parental effects have the potential to increase offspring fitness, although they can also act to reduce offspring performance [4]. Consequently, parental effects have gained interest as a potential mechanism that may assist marine organisms to persist in the face of ongoing rapid climate change and ocean acidification (OA) [5,6]. Many experimental studies have shown that within-generation exposure to OA conditions predicted to occur by the end of the century can negatively impact a range of traits (including survival, development and growth) in early life-stages of various marine taxa [7]. However, in some instances, early life-stage tolerance to OA is increased when parents experience similar conditions to their offspring [8–10].
Many ecologically and economically important marine species live in shallow water coastal habitats. These habitats can experience substantial natural fluctuations in CO2 on a variety of temporal scales [11]. Despite this, most OA experiments to date on shallow water species have used stable elevated CO2 levels consistent with open ocean environments and thus may have limited ecological relevance [12]. Perhaps the most well-known CO2 fluctuations are those that occur over a 24 h period, primarily driven by the net effects of photosynthesis and respiration over a day–night cycle [13]. These diel CO2 cycles occur in many shallow water ecosystems, including coral reefs, and are expected to increase in magnitude with ongoing OA owing to the change in seawater buffering capacity as the ocean absorbs more CO2 [14,15]. Recent studies accounting for diel CO2 cycles have demonstrated that they can either alleviate or intensify the within-generation responses of marine organisms to OA [16–20]. However, it is unknown if diel CO2 cycles will alter the outcome of parental effects on offspring traits, which limits our ability to accurately predict how marine organisms will respond to OA in the long term.
The influence that parental effects have on offspring fitness is related to how predictable variations in environmental conditions are over time [1]. Consequently, the outcome of parental effects in the presence of diel CO2 cycles may differ from those expressed in a stable CO2 environment. To test this, we conditioned adult pairs of coral reef anemonefish, Amphiprion melanopus, to control (500 µatm), stable elevated (1000 µatm) and diel-cycling elevated (1000 ± 300 µatm) CO2 (figure 1 and table 1). Juveniles from control parents were reared in all CO2 treatments, whereas juveniles from the two elevated CO2 treatments were reared in the same conditions as their parents. Comparisons between treatments allowed us to determine the within-generation effects of stable elevated and diel-cycling elevated CO2 on juvenile growth and survival, and how these responses were modified by parental exposure to either stable elevated or diel-cycling elevated CO2.
Figure 1.
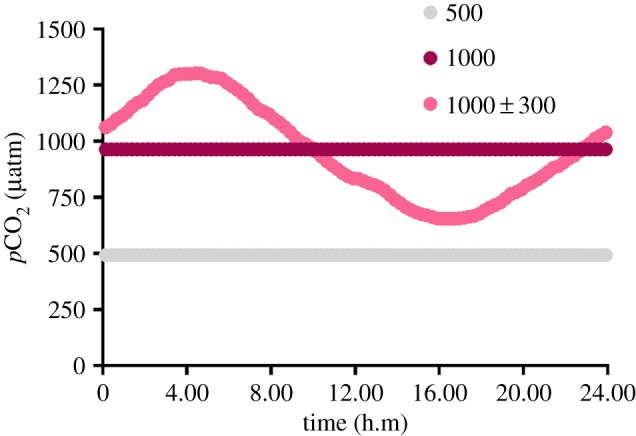
Mean daily pCO2 profiles for the 500, 1000 and 1000 ± 300 µatm CO2 treatments. (Online version in colour.)
Table 1.
Experimental seawater parameters. Values are means ± 1 s.d. for mean, min and max pH (total scale) and pCO2. Means ± 1 s.d. for total alkalinity (TA), temperature and salinity are also shown.
|
pCO2 treatment (µatm) |
|||
|---|---|---|---|
| parameter | 500 | 1000 | 1000 ± 300 |
| mean pHT | 7.99 ± 0.03 | 7.75 ± 0.03 | 7.76 ± 0.09 |
| min. pHT | — | — | 7.63 ± 0.06 |
| max. pHT | — | — | 7.88 ± 0.03 |
| mean pCO2 | 493 ± 24 | 964 ± 50 | 957 ± 233 |
| min. pCO2 | — | — | 681 ± 63 |
| max. pCO2 | — | — | 1270 ± 127 |
| TA (μmol kg−1) | 2495 ± 155 | 2494 ± 150 | 2458 ± 129 |
| temperature (°C) | 28.5 ± 0.07 | 28.5 ± 0.1 | 28.5 ± 0.09 |
| salinity (‰) | 35.4 ± 0.7 | 35.4 ± 0.7 | 35.4 ± 0.8 |
2. Material and methods
(a). Parental conditioning
Adult breeding pairs of the cinnamon anemone fish, Amphiprion melanopus, were collected from the Bramble and Trunk Reef region of the Great Barrier Reef in September 2016. Diel CO2 cycles on shallow coral reefs in the region have been shown to range between ±50 and 200 µatm [21,22], although a threefold amplification is expected to occur by the year 2100 [14]. Breeding pairs were housed in 60 l aquaria and maintained at temperature conditions matching the collection location (22.5°C winter–28.5°C summer) for 1 year prior to the experiment. Pairs were assigned to the control (500 µatm), stable elevated (1000 µatm) and diel-cycling elevated (1000 ± 300 µatm) CO2 treatments at the end of August 2017 (control CO2 = 6 pairs, stable elevated CO2 = 3 pairs and diel-cycling elevated CO2 = 4 pairs). This allowed pairs to be conditioned in their CO2 treatments for three months before the start of the breeding season in December 2017. Two pairs in the diel-cycling elevated CO2 treatment failed to rear enough offspring through to hatching. Therefore, at the beginning of March, three control pairs (pair # 2, 3 and 26), from which offspring had been obtained, were transferred to diel-cycling elevated CO2. These pairs were allowed to produce two clutches before a clutch was taken for the experiment. Breeding pairs were provided with half of a terracotta pot as a shelter and a spawning site. Temperatures were increased from winter temperatures of 22.5°C at a rate of 0.5°C per week until the summer breeding temperature of 28.5°C was reached in the first week of December 2017 and maintained for the rest of the experiment. A total of 22 clutches were sourced from all pairs between January and May 2018.
(b). Larval and juvenile rearing
Breeding pairs were checked daily for the presence of eggs. On the night of hatching, pots were removed from the 60 l breeding tanks and transferred to an aerated 100 l larval rearing aquarium. During the 12-day pelagic larval stage that occurs immediately after hatching, larvae from pairs reared under diel-cycling elevated CO2 were reared under stable CO2 conditions (1000 µatm) to represent the more stable pelagic ocean environments they occupy. Larvae from control and stable elevated CO2 pairs were reared under the same CO2 conditions as their parents. Larvae were reared following standard protocols (electronic supplementary material).
At 12 days post hatch (dph), settlement stage fish (identified by the transition to dark coloration) were randomly transferred into 40 l juvenile rearing tanks (5–12 fish per tank). Juveniles from control CO2 pairs were split into control (control–control), stable elevated (control–stable) and diel-cycling elevated (control–cycling) CO2 conditions. Juveniles from stable elevated and diel-cycling elevated CO2 pairs were only reared in the same conditions as their parents (stable–stable and cycling–cycling). CO2 treatments were duplicated and juveniles from each clutch were split between duplicate tanks (i.e. one tank per replicate CO2 treatment). In some cases, there were insufficient larvae to stock duplicate tanks and so another clutch from that pair was used, with juveniles being placed into a tank on the other replicate CO2 treatment. Juveniles were reared for 28 days. They were fed a combination of freshly hatched Artemia nauplii (100 ml from a stock of 1.5 l in which one teaspoon of cysts were hatched) and weaning fish feed (0.05 g) daily for the first two dph. From 3–7 dph, they were fed once daily (0.05 g) on the weaning feed. From 8–28 dph, juveniles were fed twice daily (0.05 g each time) on the weaning feed. At the end of the rearing period, juveniles were euthanized with clove oil anaesthetic. The number of fish remaining in each tank was recorded. Individuals were then blotted dry, weighed (nearest mg) on an analytical balance (AX224, Sartorius, Bradford, USA) and photographed in a lateral position next to a ruler. Standard length (SL) to the nearest 0.1 mm was estimated for each fish from the digital photographs using ImageJ software (http://rsb.info.nih.gov/ij/).
For full details on the experimental system and seawater manipulation, please refer to the electronic supplementary material.
(c). Statistical analyses
The effect of CO2 treatment on juvenile survival was tested using a general linearized model fitted with a binomial distribution, which was weighted to the number of fish initially stocked into each tank. The effects of CO2 treatment on wet weight and SL were tested using linear mixed effects models. Pair, clutch and tank were included as random effects, with tank nested within clutch nested within a pair. The number of fish remaining in a tank at the end of the experiment was included as a covariate to account for density-dependent effects. Additive models were used (i.e. number + CO2 treatment) based on Akaike information criterion. Pairwise comparisons were made between the control–control and other CO2 treatments based on the linear model summary outputs. All analyses were conducted using R v. 3.4.0. [23] using the ‘lme4’ and ‘nlme’ packages.
3. Results
Juvenile survival ranged from 82.2% to 96.7% in the control–stable and cycling–cycling treatments, respectively, and was significantly affected by CO2 treatment (figure 2a, χ2 = 17.68, d.f. = 4, p = 0.001). Survival in the control–control treatment (88.7 ± 3.6%, mean ± s.e.) was significantly lower than in the cycling–cycling treatment (96.7 ± 2.2%) (z = 2.09, p = 0.037). Juvenile wet weight and SL were also significantly affected by CO2 treatment (figure 2b,c, min. F4,31 = 3.48, p = 0.019). Juvenile exposure to stable elevated CO2 (control–stable) caused an 18.5% and 10.7% reduction in weight wet and SL, respectively, compared to the control–control treatment (max. t = −2.08, p = 0.045). However, there were no significant differences between the control–control and other treatment groups (min. t = −2.19, p = 0.051), indicating that both diel CO2 cycles and parental exposure to elevated CO2 restored growth to the same level as control. The number of fish remaining in a tank at the end of the experiment had a significant effect on wet weight and SL (min. F4,31 = 5.97, p = 0.020). Positive relationships between the number of fish in each tank and wet weight/SL were observed (min. F1,454 = 17.59, R2 = 0.037, p < 0.0001). The full model summary outputs are available in the electronic supplementary material.
Figure 2.
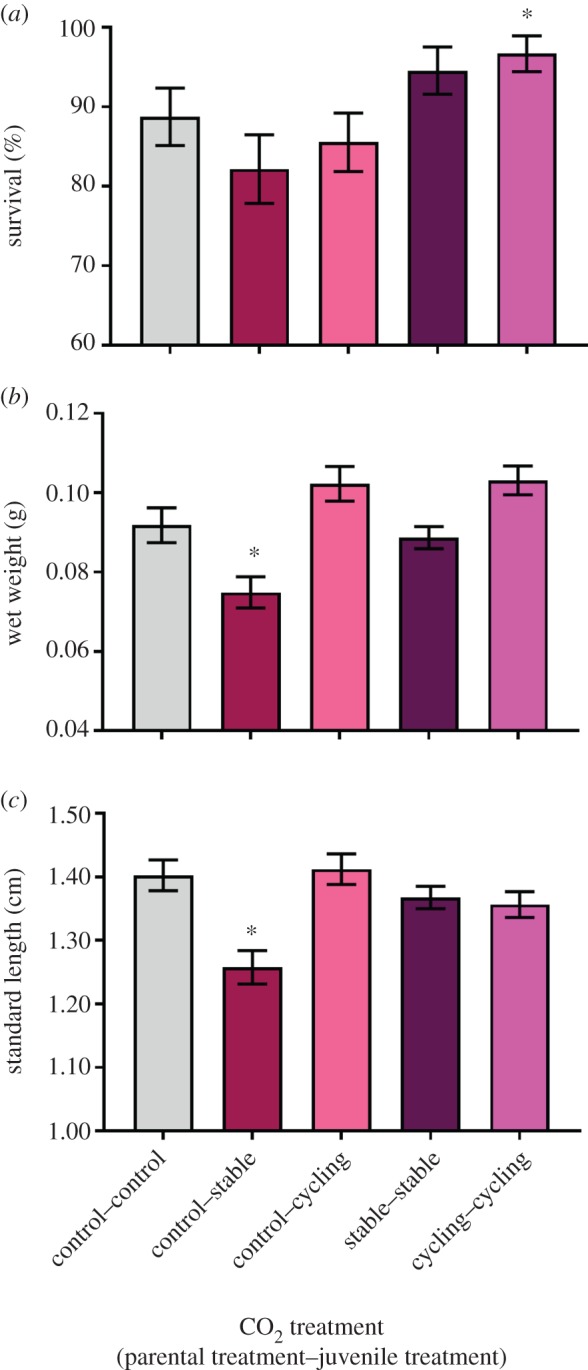
Effects of within-generation and parental exposure to stable elevated and diel-cycling elevated CO2 on (a) survival, (b) wet weight and (c) SL of juvenile cinnamon anemone fish, Amphiprion melanopus. Bars represent means ± s.e. Asterisks (*) denote a significant difference compared to the control–control treatment. (Online version in colour.)
4. Discussion
Our findings show that both parental effects and diel CO2 cycles can significantly modify the growth of a coral reef fish under OA conditions. Parental exposure to stable elevated CO2 (1000 µatm) alleviated the negative effect of high CO2 on the growth of juvenile A. melanopus. This is consistent with past work on the same species [8] and the Atlantic silverside, Menidia menidia [9]. In contrast to juvenile fish reared at stable elevated CO2, no negative effect on growth was observed in juveniles that experienced within-generation exposure to diel-cycling elevated CO2 (1000 ± 300 µatm). Diel CO2 cycles were also shown to alleviate the negative effect of elevated CO2 on the growth of pink salmon, Oncorhynchus gorbuscha, larvae [17]. Importantly, the beneficial effect of diel CO2 cycles under elevated CO2 on the growth of juvenile fish in our study was unchanged by parental exposure to the same treatment, suggesting that a diel-cycling regime is predictable enough to prevent negative parental effects from occurring.
Our work demonstrates that parental exposure to stable elevated CO2 and within-generation exposure to diel-cycling elevated CO2 both alleviate the negative effect of elevated CO2 on juvenile growth of a marine fish. One possible explanation for this is that energetic costs for juvenile fish living under elevated CO2 are reduced when their parents have experienced the same CO2 conditions and when diel CO2 cycles are present. Consistent with this hypothesis, a previous study on A. melanopus showed that parental exposure restored juvenile resting metabolic rates and size under elevated CO2, demonstrating that the cost of living was lower if parents had experienced the same conditions [8]. Possible mechanisms for the restoration of metabolic rates across generations are epigenetic modification of gene expression [24] or the inheritance of acclimated mitochondria from mothers [25], as has been observed in marine fish exposed to elevated temperature. Finally, it is important to mention that improved juvenile growth, caused by altered metabolic rates/energy re-allocation, could have negative consequences later in life as a result of trade-offs between traits [26,27].
The physiological mechanisms responsible for the beneficial effect of diel CO2 cycles on the growth of juvenile fishes under elevated CO2 are uncertain. However, there is evidence to suggest that acclimation of metabolic rates may be responsible. For example, in addition to alleviating the negative effects of elevated CO2 on growth, a diel-cycling CO2 regime also restored maximal metabolic rates of O. gorbuscha larvae to levels seen in control fish [17]. Alterations in metabolic rates under elevated CO2 are thought to be linked to the costs associated with defending acid–base status [28]. Under elevated CO2 fishes actively increase intracellular and extracellular HCO3− concentrations to prevent plasma and tissue acidosis, a process that takes place over a time-scale of hours to days depending on the level of acidification experienced [29]. Consequently, the presence of diel CO2 cycles could mean that elevated CO2 levels are not experienced for long enough to allow complete acid–base regulation to occur. This could potentially result in more energy being available for other processes and warrants further investigation.
This study shows that parental effects are not required to improve juvenile fish growth under elevated CO2 if diel CO2 cycles are present. Thus, our research adds to a growing body of literature that highlights the importance of incorporating natural CO2 variability in OA experiments to accurately predict the responses of shallow water coastal marine species to rising CO2 levels [16–20]. Further work is needed to determine how parental exposure modifies the responses of other marine organisms to diel-cycling elevated CO2, especially those that are more sensitive to OA than most fishes, as a more challenging parental environment might lead to negative outcomes for offspring fitness in some species [4].
Supplementary Material
Acknowledgements
We thank Ben Lawes, Simon Wever and Andrew Thompson for their technical support with the aquarium systems at JCU. We also thank Blake Spady, Donald Warren, Bridie Allan, Daniel Roberts and Leela Chakravarti for assistance with fish collection.
Ethics
This research was carried out following approval from the James Cook University animal ethics committee (permit no. A2210).
Data accessibility
Tropical Research Data Hub: http://.doi.org/10.25903/5c0f3a323a749 [30].
Authors' contributions
M.D.J. and P.L.M. designed the experiment. M.D.J. carried out the experiment, data collection and analysis. M.D.J. wrote the manuscript with input from P.L.M. Both authors agree to be held accountable for the content herein and approve the final version of the manuscript.
Funding
This project was funded by the ARC Centre of Excellence for Coral Reefs Studies and an ARC Future Fellowship awarded to P.L.M.
Competing interests
We declare we have no competing interests.
References
- 1.Burgess SC, Marshall DJ. 2014. Adaptive parental effects: the importance of estimating environmental predictability and offspring fitness appropriately. Oikos 123, 769–776. ( 10.1111/oik.01235) [DOI] [Google Scholar]
- 2.Lane A, Campanati C, Dupont S, Thiyagarajan V. 2015. Trans-generational responses to low pH depend on parental gender in a calcifying tubeworm. Sci. Rep. 5, 10847 ( 10.1038/srep10847) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salinas S, Munch SB. 2012. Thermal legacies: transgenerational effects of temperature on growth in a vertebrate. Ecol. Lett. 15, 159–163. ( 10.1111/j.1461-0248.2011.01721.x) [DOI] [PubMed] [Google Scholar]
- 4.Marshall DJ, Uller T. 2007. When is a maternal effect adaptive? Oikos 116, 1957–1963. ( 10.1111/j.2007.0030-1299.16203.x) [DOI] [Google Scholar]
- 5.Munday PL. 2014. Transgenerational acclimation of fishes to climate change and ocean acidification. F1000 Prime Rep. 6, 99 ( 10.12703/P6-99) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ross PM, Parker L, Byrne M. 2016. Transgenerational responses of molluscs and echinoderms to changing ocean conditions. ICES J. Mar. Sci. 73, 537–539. ( 10.1093/icesjms/fsv254) [DOI] [Google Scholar]
- 7.Kroeker KJ, Kordas RL, Crim R, Hendriks IE, Ramajo L, Singh GS, Duarte CM, Gattuso JP. 2013. Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Glob. Chang. Biol. 19, 1884–1896. ( 10.1111/gcb.12179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller GM, Watson S-A, Donelson JM, McCormick MI, Munday PL. 2012. Parental environment mediates impacts of increased carbon dioxide on a coral reef fish. Nat. Clim. Chang. 2, 858–861. ( 10.1038/nclimate1599) [DOI] [Google Scholar]
- 9.Murray C, Malvezzi A, Gobler C, Baumann H. 2014. Offspring sensitivity to ocean acidification changes seasonally in a coastal marine fish. Mar. Ecol. Prog. Ser. 504, 1–11. ( 10.3354/meps10791) [DOI] [Google Scholar]
- 10.Parker LM, O'Connor WA, Raftos DA, Pörtner H, Ross PM. 2015. Persistence of positive carryover effects in the oyster, Saccostrea glomerata, following transgenerational exposure to ocean acidification. PLoS ONE 10, e0132276 ( 10.1371/journal.pone.0132276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duarte CM, Hendriks IE, Moore TS, Olsen YS, Steckbauer A, Ramajo L, Carstensen J, Trotter JA, McCulloch M. 2013. Is ocean acidification an open-ocean syndrome? Understanding anthropogenic impacts on seawater pH. Estuar. Coasts 36, 221–236. ( 10.1007/s12237-013-9594-3) [DOI] [Google Scholar]
- 12.Wahl M, Saderne V, Sawall Y. 2016. How good are we at assessing the impact of ocean acidification in coastal systems? Limitations, omissions and strengths of commonly used experimental approaches with special emphasis on the neglected role of fluctuations. Mar. Freshw. Res. 67, 25–36. ( 10.1071/MF14154) [DOI] [Google Scholar]
- 13.Falter JL, Lowe RJ, Zhang Z, Mcculloch M. 2013. Physical and biological controls on the carbonate chemistry of coral reef waters: effects of metabolism, wave forcing, sea level, and geomorphology. PLoS ONE 8, e53303 ( 10.1371/journal.pone.0053303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaw EC, McNeil BI, Tilbrook B, Matear R, Bates ML. 2013. Anthropogenic changes to seawater buffer capacity combined with natural reef metabolism induce extreme future coral reef CO2 conditions. Glob. Chang. Biol. 19, 1632–1641. ( 10.1111/gcb.12154) [DOI] [PubMed] [Google Scholar]
- 15.Pacella SR, Brown CA, Waldbusser GG, Labiosa RG, Hales B. 2018. Seagrass habitat metabolism increases short-term extremes and long-term offset of CO2 under future ocean acidification. Proc. Natl Acad. Sci. USA 115, 3870–3875. ( 10.1073/pnas.1703445115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jarrold MD, Humphrey C, McCormick MI, Munday PL. 2017. Diel CO2 cycles reduce severity of behavioural abnormalities in coral reef fish under ocean acidification. Sci. Rep. 7, 10153 ( 10.1038/s41598-017-10378-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ou M, et al. 2015. Responses of pink salmon to CO2-induced aquatic acidification. Nat. Clim. Chang. 5, 950–955. ( 10.1038/NCLIMATE2694) [DOI] [Google Scholar]
- 18.Cornwall CE, Hepburn CD, McGraw CM, Currie KI, Pilditch CA, Hunter KA, Boyd PW, Hurd CL. 2013. Diurnal fluctuations in seawater pH influence the response of a calcifying macroalga to ocean acidification. Proc. R. Soc. B 280, 20132201 ( 10.1098/rspb.2013.2201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wahl M, Schneider Covachã S, Saderne V, Hiebenthal C, Müller JD, Pansch C, Sawall Y. 2018. Macroalgae may mitigate ocean acidification effects on mussel calcification by increasing pH and its fluctuations. Limnol. Oceanogr. 63, 3–21. ( 10.1002/lno.10608) [DOI] [Google Scholar]
- 20.Mangan S, Urbina MA, Findlay HS, Wilson RW, Lewis C. 2017. Fluctuating seawater pH/pCO2 regimes are more energetically expensive than static pH/pCO2 levels in the mussel Mytilus edulis. Proc. R. Soc. B 284, 20171642 ( 10.1098/rspb.2017.1642) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albright R, Langdon C, Anthony KRN. 2013. Dynamics of seawater carbonate chemistry, production, and calcification of a coral reef flat, Central Great Barrier Reef. Biogeosciences 10, 6747–6758. ( 10.5194/bg-10-6747-2013) [DOI] [Google Scholar]
- 22.Kline DI, et al. 2015. Six month in situ high-resolution carbonate chemistry and temperature study on a coral reef flat reveals asynchronous pH and temperature anomalies. PLoS ONE 10, e0127648 ( 10.1371/journal.pone.0127648) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.R Core Team. R. 2017. A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.R-project.org/. [Google Scholar]
- 24.Ryu T, Veilleux HD, Donelson JM, Munday PL, Ravasi T. 2018. The epigenetic landscape of transgenerational acclimation to ocean warming. Nat. Clim. Chang. 8, 504–509. ( 10.1038/s41558-018-0159-0) [DOI] [Google Scholar]
- 25.Shama LNS, Strobel A, Mark FC, Wegner KM. 2014. Transgenerational plasticity in marine sticklebacks: maternal effects mediate impacts of a warming ocean. Funct. Ecol. 28, 1482–1493. ( 10.1111/1365-2435.12280) [DOI] [Google Scholar]
- 26.Marshall DJ, Morgan SG. 2011. Ecological and evolutionary consequences of linked life-history stages in the sea. Curr. Biol. 21, R718–R725. ( 10.1016/j.cub.2011.08.022) [DOI] [PubMed] [Google Scholar]
- 27.Pechenik JA. 2006. Larval experience and latent effects—metamorphosis is not a new beginning. Integr. Comp. Biol. 46, 323–333. ( 10.1093/icb/icj028) [DOI] [PubMed] [Google Scholar]
- 28.Pörtner HO, Knust R. 2007. Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science 315, 95–98. ( 10.1126/science.1135471) [DOI] [PubMed] [Google Scholar]
- 29.Heuer RM, Grosell M. 2014. Physiological impacts of elevated carbon dioxide and ocean acidification on fish. AJP Regul. Integr. Comp. Physiol. 307, R1061–R1084. ( 10.1152/ajpregu.00064.2014) [DOI] [PubMed] [Google Scholar]
- 30.Jarrold M. 2018. Data from: Diel CO2 cycles and parental effects have similar benefits to growth of a coral reef fish under ocean acidification Tropical Research Data Hub. ( 10.25903/5c0f3a323a749) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Jarrold M. 2018. Data from: Diel CO2 cycles and parental effects have similar benefits to growth of a coral reef fish under ocean acidification Tropical Research Data Hub. ( 10.25903/5c0f3a323a749) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Tropical Research Data Hub: http://.doi.org/10.25903/5c0f3a323a749 [30].


