Abstract
Enhanced cognitive ability is beneficial in unpredictable and harsh environments, as it enables animals to respond with flexibility. For animals living in urbanized areas, local environments not only are altered but can rapidly change during their lifetime. Urban residents are therefore challenged with identifying novel dangers and safe refuges in dynamic environments. We demonstrate that the tropical agamid lizard Psammophilus dorsalis experiences dramatically different habitats not only across the rural to urban spatial scale but also over the short temporal scale of a few years in suburban areas. Differences in environmental stability are expected to affect rates of learning and reversal learning in resident lizards. In testing arenas, lizards from these populations were required to choose a designated ‘safe’ refuge instead of an ‘unsafe’ one after simulated predator attacks. The contingency for safety was switched during the reversal learning task. In general, P. dorsalis showed high rates of learning and reversal learning, but lizards from suburban areas were quicker to learn and unlearn the location of the safe refuge than those from rural areas. This demonstrates for the first time to our knowledge that suburban lizards have faster learning and reversal learning skills for a key survival-related behaviour, finding safety in unpredictable environments.
Keywords: antipredator, refuge, reversal learning, urbanization, agama, Psammophilus dorsalis
1. Introduction
Among the numerous anthropogenic alterations to the environment, urbanization is currently one of the most transformative. Rapid urbanization alters key ecological conditions, such as habitats, resources and predator communities, exposing animals to novel selection pressures that are drastically different from those in their natural environments [1,2]. The ability to respond is essential under such challenging or unpredictable conditions, and effective learning enables animals to adjust to altered conditions [3]. Evidence for enhanced learning ability comes from numerous studies of fish [4], birds [5] and mammals [6], wherein populations that live in unpredictable and structurally complex environments demonstrate higher cognitive skills than those in favourable or stable environments. Whether habitat complexity or environmental disturbance affects learning abilities in reptiles remains unknown.
Reptiles show remarkable ability to learn about food, refuges and threats using both associative and non-associative learning skills [7–10]. Since predation is one of the strongest selection pressures, the ability to learn about risk and the location of safe refuges in any environment is expected to be high [11]. However, dynamic changes in habitat structure in anthropogenically disturbed areas pose a challenge for lizards residing in those habitats. For example, in developing suburban environments, typical spatial features or landmarks that serve as cues for navigation are likely to be altered by novel anthropogenic structures. This challenge can be overcome if animals have behavioural flexibility and rapid learning skills, which can arise from selection or experience. Behavioural flexibility allows animals to adjust their existing responses and strategies under novel environmental conditions, such as changes in microhabitat structure or predation risk. For example, reduced neophobia in urban birds compared with rural birds allows them to exploit novel resources [12]. House sparrows from urban areas increase their antipredator responses as they age and gain experience, unlike those from rural areas which maintain similar levels of wariness throughout their lifetime [13]. All lizards must learn the location of suitable refuges in their environment and are expected to flexibly adjust their choice of refuge depending on the current associated contingency of risk, learnt through experience. Given that suburban individuals experience different selection pressures [2,14,15] and a more variable and dynamic environment during their lifetime, we predict that lizards from suburban areas will have faster and more accurate learning abilities compared with lizards from rural areas. Using the Indian rock agama Psammophilus dorsalis, we first demonstrate that populations in the suburban study area have experienced rapid and dramatic changes to their habitat within and across generations which are unlike those experienced by rural lizards that live on undisturbed rocky outcrops. We then use a learning paradigm to test how quickly lizards from both suburban and rural areas can learn to identify safe from unsafe refuges, and whether they can exhibit reversal learning skills by altering their refuge choice when risk changes.
2. Material and methods
We first measured the rate and type of habitat change in rural and suburban areas within and around Bengaluru, India, by quantifying habitat composition from satellite images of the study area from 2013 to 2018 (N = 3 suburban and 3 rural sites which currently have P. dorsalis populations; replicate sites within each habitat were 5–10 km apart, and the suburban and rural study areas were 60 km apart (see electronic supplementary material, S1 for details of study areas and habitat analysis)). For the learning trials, we captured adult males of P. dorsalis (typical lifespan in the wild approximately 1–2 years) from rural (N = 14) and suburban areas (N = 16) and housed them in large enclosures (80 × 45 × 30 cm). Males of P. dorsalis establish larger territories than females [16] and therefore use more perch and refuge sites, making them a good model system to test refuge learning skills. Before the start of the trials, we measured the mass (g) and snout–vent length (SVL, mm) for all lizards using a digital weighing balance and digital callipers, respectively.
Each animal enclosure had a perch in the middle and two identical refuges constructed from PVC pipes that were cut longitudinally (15 cm long × 5 cm radius) and placed at the two farthest ends. Lizards from both suburban and rural areas used the PVC pipes as refuges with equal propensity (see electronic supplementary material, S1 for details). A red square (7 × 7 cm) was attached to the wall behind one refuge to serve as a local cue for the ‘safe refuge’. Each lizard was subjected to two learning tasks. In task 1 (learning trials), lizards had to learn the location of the safe refuge. We simulated predatory attacks by gently tapping the tail of the perching lizard with a brush which made it seek refuge. Lizards were continually ‘attacked’ until they chose the safe refuge. If the lizard entered the unsafe (incorrect) refuge, we would lift that refuge, which forced the lizard to come out, and would then simulate another attack as soon as the lizard was back on the central perch. The unsafe refuge (incorrect refuge) was returned to the original position before the attack. Each animal experienced learning trials three times in a day with a gap of 3–4 h (trial timings ca 09.00, 13.00 and 16.00). The entire learning task was conducted for 7 days (20 trials). In order to determine which individuals would be subjected to the reversal learning task, we first classified a lizard as having learned if it chose the safe refuge as a first choice in at least five consecutive trials. All lizards that learned (as defined by this criterion) continued to choose the safe refuge as the first choice for all subsequent trials. Only individuals that exhibited learning experienced the reversal learning task, in which the positive and negative contingencies were switched so that individuals had to reverse previously created associations. Thus, the refuge without the local red cue was designated as the new ‘safe’ refuge. Reversal learning trials were also performed for 7 consecutive days (three trials per day) using the same protocol, and a gap of 24 h was given between learning and reversal learning tasks. All trials were conducted during the peak activity hours of the lizard (09.00–12.00 and 15.00–17.00).
During each trial, we recorded the (i) first refuge chosen, (ii) number of incorrect choices made before choosing the safe refuge and (iii) latency to choose the safe refuge. Number of incorrect choices and latency to choose the safe refuge were tightly correlated (Pearson's correlation coefficient = 0.73, t = 26.00, p < 0.001, 95% CI: 0.68, 0.76), so all further analyses to examine the rate of learning were performed using latency to choose the safe refuge as the response variable, with trial number, habitat (suburban or rural) and body condition as predictor variables. Body condition was calculated from SVL and mass as a scaled mass index (per [17]). We included replicate sites and individual lizard identity as random effects in all our models. Additional details regarding animal capture, experimental protocols and statistical analyses are provided in the electronic supplementary material, S1.
3. Results
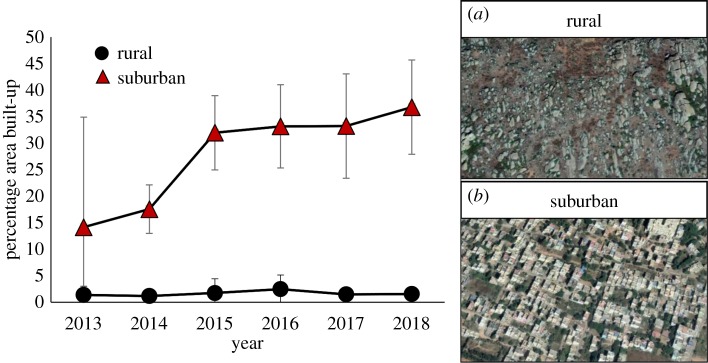
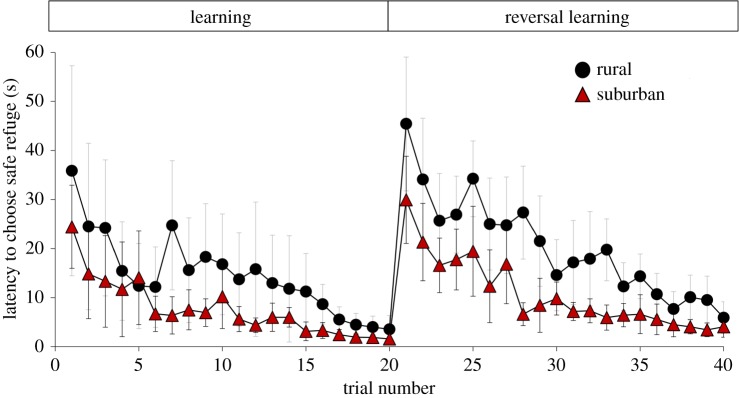
Over the last 5 years, suburban areas in Bengaluru, India, have changed dramatically from being vegetated or bare (non-built up) to heavily built up with anthropogenic structures, while rural study areas have remained unchanged (glmm results show that the percentage built-up area in suburban and rural sites differed significantly from 2014 to 2018; all z > 2.0 and p < 0.03, figure 1; see detailed results in electronic supplementary material, table S1). When lizards from these areas were tested in learning tasks, we found that a high proportion of suburban and rural individuals were able to learn the location of a safe refuge (suburban = 1.00; rural = 0.85; χ2 = 0.69, p = 0.405) and also show reversal learning for the same task (suburban = 0.87; rural = 0.58; χ2 = 1.75, p = 0.185). For all lizards, the latency to choose the safe refuge during the learning (glmm: z = −22.68, p < 0.001) and reversal learning (z = −31.41, p < 0.001) tasks decreased with the number of trials (figure 2). Notably, suburban lizards were faster to select the safe refuge compared with rural lizards in both the learning (mean ± s.e. = 7.61 ± 0.65 s for suburban; 14.74 ± 1.27 s for rural; z = −2.39, p = 0.017) as well as reversal learning tasks (mean ± s.e. = 10.70 ± 0.68 s for suburban; 20.23 ± 1.09 s for rural; z = −3.43, p < 0.001, figure 2). Body condition was not significantly different between suburban and rural lizards (t-test: t = 0.45, p = 0.651) and did not have an effect on the latency to select the safe refuge during both tasks (glmm: task 1: z = 0.58, p = 0.559; task 2: z = 1.71, p = 0.080). Variation in response due to individual lizard identity and replicate sites (within suburban and rural) was negligible (glmm random effects: s.d. <0.50) and these parameters were not significant predictors in our model. Detailed results are provided in electronic supplementary material, table S2.
Figure 1.
Percentage of area built-up by anthropogenic development in rural and suburban study sites across years. Shown are means ± 95% CI from replicate sites (N = 3 each in rural and suburban). Representative satellite images of rural and suburban areas (2018) are shown in (a,b), respectively. (Online version in colour.)
Figure 2.
Latency to choose the safe refuge across learning and reversal learning trials for rural and suburban lizards. Shown are means ± 95% CI across three replicate sites for suburban (N = 16 lizards) and rural (N = 14 lizards). (Online version in colour.)
4. Discussion
In this study, we find that P. dorsalis is capable of exhibiting rapid and flexible learning when identifying safe from unsafe refuges. The ability to learn about safe refuges has been shown in turtles, snakes and lizards, with different species employing different strategies for learning [8,9,18,19]. When tested in the wild, P. dorsalis is capable of learning about low-risk threats over very short timescales (repeated approaches by humans over 30 min) [20]. From the current laboratory study, P. dorsalis not only shows a high proportion and rate of successful learning but also exhibits rapid reversal learning. Reversal learning paradigms are an effective way to test the adaptive value of cognitive flexibility as they show whether animals can adjust their response optimally under current challenges. By showing successful reversal learning, P. dorsalis exhibits higher cognitive complexity because a reversal task involves a double-order cognitive response. An animal has to first inhibit a previously associated reward response and next form a new stimulus–reward association [21]. Thus, our study adds to the growing body of literature which suggests that lizards are capable of rapid learning and behavioural flexibility that might enable them to explore novel stimuli similarly to other endothermic species.
Learning and memory allow animals to adjust their behaviour to adapt to changing environments and thus cope with unpredictability. In habitats that are stable, with little change in structure or resources, individuals might show low flexibility in learning skills with strong long-term memory [22]. In dynamically altered environments, short-term memories about relevant resources that can be replaced with new information are more useful [23]. For P. dorsalis, the undisturbed boulder fields of rural areas are diametrically different from suburban areas, where habitat structure and anthropogenic disturbance change frequently during the lifetime of an individual lizard (1–2 years). The flexibility of this species is reflected in the fact that the lizards use perch and refuge sites that are available to them; in suburban areas, this includes novel anthropogenic structures, whereas in rural areas they use rocks and boulders [20]. Because the suburban habitat changes during their lifetime, lizards must learn and unlearn the location of safe refuges. In support of our prediction, we found that populations of P. dorsalis from suburban areas were faster in both learning and reversal learning tasks compared with rural lizards. Though the proportion of individuals that exhibited learning and reversal learning by the end of the experiment was not different between suburban and rural habitats, the time taken to learn about safe refuges was significantly faster in males from suburban areas during both tasks. Our results are in contrast to [24], where no differences in learning abilities of urban and rural populations of Lampropholis delicata were found. Differences in learning abilities between suburban and rural animals could be attributed to the differences in type and intensity of selection pressures in the urban environment, which could include predation pressure and availability of refuges [25,26]. The rapid and dynamic changes to habitat structure that P. dorsalis from suburban areas experience within their lifetime likely play a strong role in shaping their rates of learning about refuges. Notably, the suburban and rural habitats of P. dorsalis do not seem to differ in predation pressure (M. Amdekar & M. Thaker 2018, unpublished data), yet escape strategies of lizards in the wild differ, as suburban lizards seek shelter in closer refuges when attacked compared with rural lizards [20]. Similar to studies in birds in which better learning capabilities were found in individuals living in novel environments [5,26], lizards living in the novel urbanized habitat are also faster learners. This increased cognitive ability could be a result of microevolutionary differences between populations, or individual lifetime experience or a combination of both, and suggests that the dynamic and unstable environment of urbanized areas supports faster learning abilities.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Madhura Amdekar and Abhijit Kumar for help in the field.
Ethics
This species is not covered under the Schedules of the Indian Wildlife (Protection) Act; therefore, collection permits were not required. All capture, handling and experimental protocols were approved by the Institutional Animal Ethics Committee at the Indian Institute of Science (CAF/Ethics/394/2014).
Data accessibility
All data associated with the manuscript have been uploaded as part of electronic supplementary material, S2.
Authors' contributions
A.B. and M.T. conceived the study. A.B. collected and analysed the data. A.B. and M.T. wrote the manuscript. A.B. and M.T. approved the final version of the manuscript and agree to be held accountable for the content of therein.
Competing interests
The authors have no competing interests.
Funding
This work was supported by DBT-IISc Partnership Program (funding for MT) and University Grants Commission (fellowship for A.B.).
References
- 1.Sih A. 2013. Understanding variation in behavioural responses to human-induced rapid environmental change: a conceptual overview. Anim. Behav. 85, 1077–1088. ( 10.1016/j.anbehav.2013.02.017) [DOI] [Google Scholar]
- 2.Ouyang JQ, Isaksson C, Schmidt C, Hutton P, Bonier F, Dominoni D. 2018. A new framework for urban ecology: an integration of proximate and ultimate responses to anthropogenic change. Integr. Comp. Biol. 58, 915–928. ( 10.1093/icb/icy110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dukas R. 1998. Cognitive ecology: the evolutionary ecology of information processing and decision making. Chicago, IL: University of Chicago Press. [DOI] [PubMed] [Google Scholar]
- 4.Brydges NM, Heathcote RJP, Braithwaite VA. 2008. Habitat stability and predation pressure influence learning and memory in populations of three-spined sticklebacks. Anim. Behav. 75, 935–942. ( 10.1016/j.anbehav.2007.08.005) [DOI] [PubMed] [Google Scholar]
- 5.Roth TC, Pravosudov VV. 2009. Hippocampal volumes and neuron numbers increase along a gradient of environmental harshness: a large-scale comparison. Proc. R. Soc. B 276, 401–405. ( 10.1098/rspb.2008.1184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sol D, Bacher S, Reader SM, Lefebvre L. 2008. Brain size predicts the success of mammal species introduced into novel environments. Am. Nat. 172, 63–71. ( 10.1086/588304) [DOI] [PubMed] [Google Scholar]
- 7.Saidapur S, Ammanna V, Shanbhag B. 2010. Associative learning in hatchlings of the lizard Calotes versicolor: taste and colour discrimination. Amphibia Reptilia 31, 475–481. ( 10.1163/017353710X518432) [DOI] [Google Scholar]
- 8.Davis KM, Burghardt GM. 2011. Turtles (Pseudemys nelsoni) learn about visual cues indicating food from experienced turtles. J. Comp. Psychol. 125, 404–410. ( 10.1037/a0024784) [DOI] [PubMed] [Google Scholar]
- 9.Noble DWA, Carazo P, Whiting MJ. 2012. Learning outdoors: male lizards show flexible spatial learning under semi-natural conditions. Biol. Lett. 8, 946–948. ( 10.1098/rsbl.2012.0813) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark BF, Amiel JJ, Shine R, Noble DWA, Whiting MJ. 2013. Colour discrimination and associative learning in hatchling lizards incubated at ‘hot’ and ‘cold’ temperatures. Behav. Ecol. Sociobiol. 68, 239–247. ( 10.1007/s00265-013-1639-x) [DOI] [Google Scholar]
- 11.Thaker M, Vanak AT, Lima SL, Hews DK. 2010. Stress and aversive learning in a wild vertebrate: the role of corticosterone in mediating escape from a novel stressor. Am. Nat. 175, 50–60. ( 10.1086/648558) [DOI] [PubMed] [Google Scholar]
- 12.Tryjanowski P, et al. 2016. Urbanization affects neophilia and risk-taking at bird-feeders. Sci. Rep. 6, 28575 ( 10.1038/srep28575) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seress G, Bókony V, Heszberger J, Liker A. 2011. Response to predation risk in urban and rural house sparrows. Ethology 117, 896–907. ( 10.1111/j.1439-0310.2011.01944.x) [DOI] [Google Scholar]
- 14.Halfwerk W, et al. In press Adaptive changes in sexual signalling in response to urbanization. Nat. Ecol. Evol. ( 10.1038/s41559-018-0751-8) [DOI] [PubMed] [Google Scholar]
- 15.Lazerte SE, Slabbekoorn H, Otter KA. 2016. Learning to cope: vocal adjustment to urban noise is correlated with prior experience in black-capped chickadees. Proc. R. Soc. B 283, 20161058 ( 10.1098/rspb.2016.1058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deodhar S. 2017. Sexual selection and personality in Psammophilus dorsalis. Doctoral dissertation, Indian Institute of Science, Bangalore, India. [Google Scholar]
- 17.Peig J, Green AJ. 2009. New perspectives for estimating body condition from mass/length data: the scaled mass index as an alternative method. Oikos 118, 1883–1891. ( 10.1111/j.1600-0706.2009.17643.x) [DOI] [Google Scholar]
- 18.Holtzman D, Harris T, Aranguren G, Bostock E. 1999. Spatial learning of an escape task by young corn snakes, Elaphe guttata guttata . Anim. Behav. 57, 51–60. ( 10.1006/anbe.1998.0971) [DOI] [PubMed] [Google Scholar]
- 19.LaDage LD, Roth TC, Cerjanic AM, Sinervo B, Pravosudov VV. 2012. Spatial memory: are lizards really deficient? Biol. Lett. 8, 939–941. ( 10.1098/rsbl.2012.0527) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Batabyal A, Balakrishna S, Thaker M. 2017. A multivariate approach to understanding shifts in escape strategies of urban lizards. Behav. Ecol. Sociobiol. 71, 83 ( 10.1007/s00265-017-2307-3) [DOI] [Google Scholar]
- 21.Lai ZC, Moss MB, Killiany RJ, Rosene DL, Herndon JG. 1995. Executive system dysfunction in the aged monkey: spatial and object reversal learning. Neurobiol. Aging 16, 947–954. ( 10.1016/0197-4580(95)02014-4) [DOI] [PubMed] [Google Scholar]
- 22.Hughes RN, Mackney PA. 1995. Foraging behaviour and memory window in sticklebacks. Behaviour 132, 1241–1253. ( 10.1163/156853995X00559) [DOI] [Google Scholar]
- 23.Girvan JR, Braithwaite VA. 1998. Population differences in spatial learning in three-spined sticklebacks. Proc. R. Soc. Lond. B 265, 913–918. ( 10.1098/rspb.1998.0378) [DOI] [Google Scholar]
- 24.Kang F, Goulet CT, Chapple DG. 2018. The impact of urbanization on learning ability in an invasive lizard. Biol. J. Linn. Soc. 123, 55–62. ( 10.1093/biolinnean/blx131) [DOI] [Google Scholar]
- 25.Gering JC, Blair RB. 1999. Predation on artificial bird nests along an urban gradient: predatory risk or relaxation in urban environments? Ecography 22, 532–541. ( 10.1111/j.1600-0587.1999.tb01283.x) [DOI] [Google Scholar]
- 26.Møller AP. 2012. Urban areas as refuges from predators and flight distance of prey. Behav. Ecol. 23, 1030–1035. ( 10.1093/beheco/ars067) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data associated with the manuscript have been uploaded as part of electronic supplementary material, S2.