Abstract
Background
Delayed enhancement (DE) on magnetic resonance imaging is associated with ventricular arrhythmias, adverse events, and worse left ventricular mechanics. We investigated the impact of DE on cardiac resynchronization therapy (CRT) outcomes and the effect of CRT optimization.
Methods and Results
We studied 130 patients with ejection fraction (EF) ≤40% and QRS ≥120 ms, contrast cardiac magnetic resonance imaging, and both pre‐ and 1‐year post‐CRT echocardiograms. Sixty‐three (48%) patients did not have routine optimization of CRT. The remaining patients were optimized for wavefront fusion by 12‐lead ECG. The primary end point in this study was change in EF following CRT. To investigate the association between electrical dyssynchrony and EF outcomes, the standard deviation of activation times from body‐surface mapping was calculated during native conduction and selected device settings in 52 of the optimized patients. Patients had no DE (n=45), midwall septal stripe (n=30), or scar (n=55). Patients without DE had better ∆EF (13±10 versus 4±10 units; P<0.01). Optimized patients had greater ∆EF in midwall stripe (2±9 versus 12±12 units; P=0.01) and scar (0±7 versus 5±10; P=0.04) groups, but not in the no‐DE group. Patients without DE had greater native standard deviation of activation times (P=0.03) and greater ∆standard deviation of activation times with standard programming (P=0.01). Device optimization reduced standard deviation of activation times only in patients with DE (P<0.01).
Conclusions
DE on magnetic resonance imaging is associated with worse EF outcomes following CRT. Device optimization is associated with improved EF and reduced electrical dyssynchrony in patients with DE.
Keywords: body surface mapping, cardiac magnetic resonance imaging, cardiac resynchronization therapy, heart failure, outcome
Subject Categories: Electrophysiology, Heart Failure, Electrocardiology (ECG), Magnetic Resonance Imaging (MRI), Pacemaker
Clinical Perspective
What Is New?
Delayed enhancement (fibrosis or scar) on cardiac MRI is associated with worse EF improvement with CRT.
Optimization of CRT programming using 12‐lead ECG improves electrical dyssynchrony and often requires LV‐only or LV‐preactivation pacing.
What Are the Clinical Implications?
Optimization of CRT programming using 12‐lead ECG may significantly improve EF response to therapy.
Introduction
Randomized controlled clinical trials have demonstrated that CRT results in improved heart failure symptoms, functional status, exercise capacity, hospitalization rate, and mortality in heart failure patients with a wide QRS.1, 2, 3 However, as many as 40% of CRT recipients fail to respond well to therapy, depending on the method of assessing response and the follow‐up time studied.4, 5 In CRT recipients with baseline electrical dyssynchrony, nonresponse may be attributed to the cardiac structural substrate or suboptimal CRT programming with inadequate reduction of electrical dyssynchrony.
Cardiac magnetic resonance imaging (cMRI) has become an important tool in the evaluation of cardiomyopathy etiology.6, 7 A few studies using gadolinium delayed enhancement (DE) have demonstrated that myocardial scarring from coronary artery disease or fibrosis in the midmyocardial wall of nonischemic cardiomyopathy (NICM) patients is a negative predictor of clinical benefit following CRT.8, 9, 10
We have recently developed a novel body surface activation mapping (BSAM) system to quickly and noninvasively quantify electrical dyssynchrony using a 53‐lead ECG belt. The reduction in standard deviation of activation times (SDAT) from the BSAM system baseline or native rhythm to CRT pacing is associated with increase in acute hemodynamic response11 and reduction in left ventricular (LV) end‐systolic volumes and improvements in ejection fraction (EF) assessed by 6‐month postimplant echocardiography.12 Both native SDAT and the change in SDAT with CRT pacing were better predictors of echo measures of CRT response than native QRS duration and the change in QRS duration. In addition, we recently showed, in 94 patients, that SDAT decreases by 20% with standard traditional CRT pacing, but an additional 26% reduction in electrical synchrony could be achieved with ECG belt–guided optimization.13
Our primary hypothesis was that patients with DE on cMRI would have reduced benefit from CRT compared with those without DE, and that optimization of CRT programming to minimize paced electrical dyssynchrony would result in improved CRT response within groups of patients based on DE status. Secondarily, we hypothesized that improved CRT response in optimized patients would be associated with a greater reduction in electrical dyssynchrony. In our current study, we investigated the effects of both cMRI DE and 12‐lead ECG device optimization on echocardiographic outcomes following CRT.
Methods
The data, analytical methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Population
All consecutive patients implanted with a first‐time CRT device at United Heart & Vascular Clinic (St. Paul, MN) between March 2007 and October 2017 who underwent a cMRI with gadolinium DE pre‐CRT were retrospectively included. One hundred thirty patients met inclusion criteria of pre‐CRT QRS duration ≥120 ms, EF ≤40%, and paired pre‐/post‐CRT echocardiograms. The study was approved by an institutional review board, and informed consent was waived.
Echocardiography
Echocardiographic studies pre‐CRT and ≈1 year following CRT were acquired with standard commercially available ultrasound systems. Studies were analyzed by experienced readers in an echocardiographic core laboratory using commercially available software blinded to baseline characteristic, study time point (pre‐/post‐CRT), and cMRI results. Measurements of LV end‐diastolic and end‐systolic volumes and LV ejection fraction were quantified using the biplane Simpson method.
cMRI Acquisition Protocol and Image Analysis
cMRI studies were performed using a 1.5‐Tesla scanner (Avanto; Siemens, Erlangen, Germany) and a 64‐element coil. The imaging protocol included standard cine and gadolinium DE imaging. Cine images were acquired using steady‐state free precession with nominal parameters: repetition time/echo time, 2.6/1.12 ms, 20 views per segment; field of view, 34×28 cm; matrix, 192×130; slice thickness, 8 mm; and flip angle, 80 degrees. DE was performed using a standard inversion recovery gradient echo pulse sequence and single‐shot steady‐state free precision sequences 10 minutes following intravenous gadolinium contrast (0.2 mmol/kg; Multihance; Bracco, Princeton, NJ). Inversion time mapping sequence (TI scout) was utilized and individually optimized to null the myocardium. DE images were reviewed by 2 experienced level III readers, classified as no DE, midwall fibrosis, or scar (subendocardial or transmural), and the scar level was quantified. Patients with both midwall fibrosis and scar were placed into the scar group.
CRT Programming
Patients implanted with CRT in 2007–2013 did not have routine 12‐lead ECG optimization of device parameters. CRT programming, including the atrioventricular delay, ventricular‐ventricular offset, and LV pacing vector, was at the discretion of the implanting electrophysiologist, and typically was simultaneous biventricular pacing with an atrioventricular delay of ≈70% of the native PR interval. Patients receiving CRT in 2014–2017 routinely underwent 12‐lead ECG optimization of CRT programming 1 week following implant with a goal of promoting wavefront fusion and cancellation.14, 15 Briefly, wavefront fusion and cancellation was obtained by attempting to find settings with small‐to‐moderate R waves in leads V1 and/or V2, a Q wave in lead 1, and a narrowing of QRS duration. ECGs were obtained at baseline device setting and over a range of ventricular‐ventricular delays (typically simultaneous and LV preactivated by 20, 40, and 60 ms) and with LV‐only pacing over a range of atrioventricular delays (typically 40–90% of native PR interval).12, 13 If a CRT device had the Medtronic AdaptivCRT Algorithm (Medtronic, Minneapolis, MN), the recommended pacing parameters were evaluated with 12‐lead ECG and the algorithm left on, if pacing resulted in adequate wavefront fusion and cancellation, or turned off if manual programming options resulted in significantly better fusion. Simultaneous biventricular (BiV) pacing (ventricular‐ventricular=0 ms) at an AVD chosen by the implanting physician was considered standard CRT programming, whereas nonstandard CRT programming involved either LV‐only pacing or sequential BiV pacing (VV≠0 ms).
BSAM Acquisition
A 53‐electrode ECG belt was used for BSAM in 52 of the patients who had undergone CRT optimization. Details of the system and data acquisition protocol have been previously described.11, 12, 13 The system is comprised of 17 anterior and 36 posterior electrodes covering the torso. Unipolar electrograms were analyzed, and the steepest negative slope determined the activation time at the body surface for each lead. The standard deviation of the 53 activation times (SDAT) quantified electrical dyssynchrony. BSAM data were collected at native underlying rhythm, during standard CRT programming, and at the 12‐lead ECG optimized setting.
Statistical Analysis
Continuous variables were summarized as mean±SD and categorical variables expressed as count (percentage). Comparisons within groups (pre‐/post‐CRT) were performed using paired Student t tests. Comparisons between groups were performed using unpaired Student t tests or 1‐way ANOVA with Bonferroni correction for subsequent pair‐wise comparisons, as appropriate. Categorical proportions between groups were assessed with Fischer's exact test. STATA/MP software (version 14.2; StataCorp LP, College Station, TX) was used for data analysis, and a 2‐sided P<0.05 was considered statistically significant.
Results
Baseline characteristics for the 130 patients are summarized in Table 1. The population was representative of a traditional CRT population: 67±12 years old at implant, 68% male, 95% New York Heart Association class II or III, on optimal medical therapy, 66% left bundle branch block (LBBB) morphology, and QRS duration of 154±20 ms. There were no significant differences in baseline characteristics between 12‐lead ECG optimized patients and those not optimized.
Table 1.
Baseline Demographics and Clinical Characteristics
| Patient Characteristics | All | Not Optimized 2007–2013 | 12‐Lead ECG Optimized 2014–2017 | P Value |
|---|---|---|---|---|
| n=130 | n=63 | n=67 | ||
| Age, y | 67±12 | 66±14 | 69±11 | 0.286 |
| Sex, male | 88 (68%) | 41 (65%) | 47 (70%) | 0.577 |
| NYHA class | 0.477 | |||
| I | 1 (1%) | 1 (2%) | 0 (0%) | |
| II | 35 (27%) | 14 (22%) | 21 (31%) | |
| III | 89 (68%) | 45 (71%) | 44 (66%) | |
| IV | 5 (4%) | 3 (5%) | 2 (3%) | |
| ACE‐I/ARB use | 110 (85%) | 53 (84%) | 57 (85%) | 1.000 |
| Beta‐blocker use | 115 (88%) | 56 (89%) | 59 (88%) | 1.000 |
| QRS duration, ms | 154±20 | 156±21 | 152±19 | 0.272 |
| PR interval, ms | 181±34 | 184±39 | 178±30 | 0.400 |
| Conduction | 0.758 | |||
| LBBB | 86 (66%) | 39 (62%) | 47 (70%) | |
| RBBB | 17 (13%) | 9 (14%) | 8 (12%) | |
| IVCD | 24 (18%) | 13 (21%) | 11 (16%) | |
| RV‐paced | 3 (2%) | 2 (3%) | 1 (1%) | |
| DE characteristic | 0.235 | |||
| No DE | 45 (35%) | 18 (29%) | 27 (40%) | |
| Midwall stripe | 30 (23%) | 18 (29%) | 12 (18%) | |
| Scar | 55 (42%) | 27 (42%) | 28 (42%) | |
| Scar burden, % | 18±13 | 21±16 | 14±9 | 0.050 |
| Lateral/posterolateral LV lead | 123 (95%) | 60 (95%) | 63 (94%) | 1.000 |
| Pre‐CRT LVESV, mL | 120±57 | 120±52 | 119±62 | 0.877 |
| Pre‐CRT LVEDV, mL | 164±63 | 166±60 | 163±66 | 0.760 |
| Pre‐CRT EF, % | 29±7 | 29±7 | 29±8 | 0.639 |
ACE‐I indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; DE, delayed enhancement; EF, ejection fraction; IVCD, intraventricular conduction delay; LBBB, left bundle branch block; LV, left ventricular; LVEDV, left ventricular end‐diastolic volume; LVESV, left ventricular end‐systolic volume; NYHA, New York Heart Association; RV, right ventricular.
Patients were classified as having no DE (n=44; 34%), septal midwall DE (n=30; 23%), or ischemic pattern scar DE (transmural or subendocardial; n=56; 43%). Figure 1 provides representative images of this classification, and Table 2 compares baseline characteristic between these groups. Patients with scar had shorter QRS durations (150±20 versus 161±17 ms; P=0.010) and less frequently had an LBBB (42% versus 89%; P>0.001) as compared with patients with no fibrosis. Patients with scar also had higher baseline EF than patients with midwall fibrosis (31±6% versus 26±8%; P=0.004).
Figure 1.
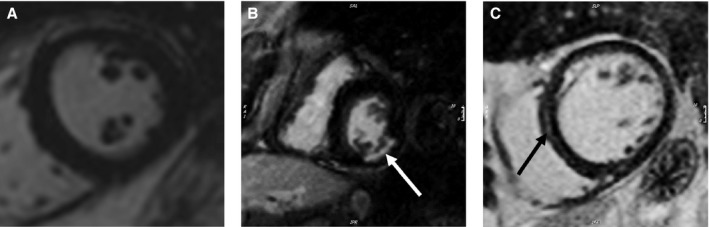
Delayed enhancement on mid ventricular short‐axis images. No delayed enhancement noted (A). Subendocardial delayed enhancement (tip of white arrow) involving the inferolateral wall with 25% to 49% wall thickness involvement (B). Midmyocardial septal stripe/delayed enhancement (tip of black arrow; C).
Table 2.
Demographics and Clinical Characteristics of Groups Based on MRI Results
| Patient Characteristics | All | No Delayed Enhancement | Midwall Stripe | Scar | ANOVA P Value |
|---|---|---|---|---|---|
| n=130 | n=45 | n=30 | n=55 | ||
| Age, y | 67±12 | 66±11 | 64±16 | 70±10 | 0.038 |
| Sex, male | 88 (68%) | 25 (58%) | 19 (63%) | 44 (80%) | 0.029 |
| NYHA class | 0.411 | ||||
| I | 1 (1%) | 1 (2%) | 0 (0%) | 0 (0%) | |
| II | 35 (27%) | 16 (36%) | 5 (17%) | 14 (25%) | |
| III | 89 (68%) | 27 (60%) | 24 (80%) | 38 (69%) | |
| IV | 5 (4%) | 1 (2%) | 1 (3%) | 3 (5%) | |
| ACE‐I/ARB use | 110 (85%) | 41 (91%) | 26 (87%) | 43 (78%) | 0.192 |
| Beta‐blocker use | 115 (88%) | 43 (96%) | 27 (90%) | 45 (82%) | 0.097 |
| QRS duration, ms | 154±20 | 161±17 | 152±22 | 150±20 | 0.011 |
| PR interval, ms | 181±34 | 181±30 | 179±45 | 181±31 | 0.976 |
| Conduction | <0.001 | ||||
| LBBB | 86 (66%) | 40 (89%) | 23 (77%) | 23 (42%) | |
| RBBB | 17 (13%) | 1 (2%) | 1 (3%) | 15 (27%) | |
| IVCD | 24 (18%) | 2 (4%) | 6 (20%) | 16 (29%) | |
| RV‐paced | 3 (2%) | 2 (4%) | 0 (0%) | 1 (2%) | |
| Optimized | 67 (52%) | 27 (60%) | 12 (40%) | 28 (51%) | 0.235 |
| Lateral/posterolateral LV lead | 123 (%) | 42 (93%) | 28 (93%) | 53 (96%) | 0.751 |
| Pre‐CRT LVESV, mL | 120±57 | 121±68 | 132±69 | 112±36 | 0.267 |
| Pre‐CRT LVEDV, mL | 164±63 | 165±76 | 174±76 | 158±40 | 0.553 |
| Pre‐CRT EF, % | 29±7 | 28±8 | 26±8 | 31±6 | 0.004 |
ACE‐I indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; DE, delayed enhancement; EF, ejection fraction; IVCD, intraventricular conduction delay; LBBB, left bundle branch block; LV, left ventricular; LVEDV, left ventricular end‐diastolic volume; LVESV, left ventricular end‐systolic volume; NYHA, New York Heart Association; RV, right ventricular.
Figure 2 shows CRT programming strategies based on 12‐lead ECG optimization status. Standard CRT programming was utilized in 89% of nonoptimized patients and 27% of patients optimized by 12‐lead ECG for wavefront fusion (P<0.001). Nonoptimized patients receiving nonstandard CRT programming had sequential BiV pacing in 6 cases and LV‐only pacing by the AdaptivCRT (Medtronic) in 1 case. The majority (n=34) of the 49 twelve‐lead ECG optimized patients with nonstandard CRT programming received LV‐only pacing appropriately timed to native conduction by either AdaptivCRT (n=27) or by manually turning the right ventricular (RV) lead subthreshold (n=7). The remaining 15 patients had sequential BiV pacing with average LV preactivation of 23±24 ms.
Figure 2.
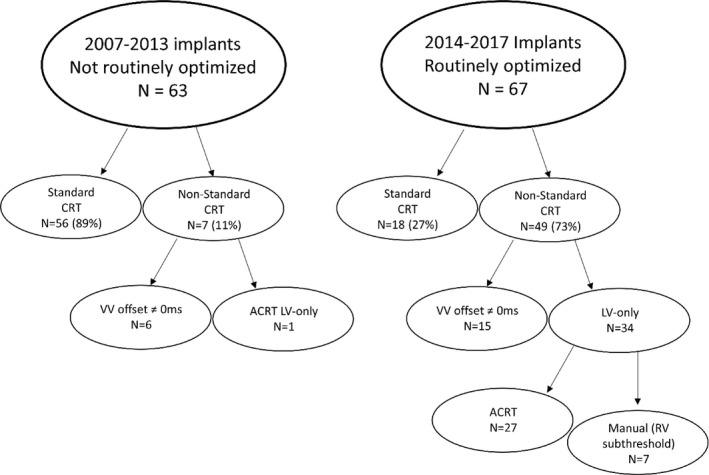
CRT (cardiac resynchronization therapy) programming based on 12‐lead ECG optimization. Patients receiving CRT 2007–2013 did not undergo 12‐lead ECG optimization of CRT settings, and most (89%) patients were programmed to standard CRT settings of simultaneous biventricular pacing. Conversely, patients implanted 2014–2017 were optimized 1 week post‐CRT with 12‐lead ECG to promote wavefront fusion. ECG optimized settings were often (73%) nonstandard, utilizing left ventricular (LV)‐only pacing appropriately timed to native conduction or sequential biventricular pacing. ACRT, adaptive CRT; RV, right ventricular; VV, ventricular‐ventricular.
In all 130 patients, ∆EF over the ≈1 year follow‐up period was 7±11 units (P<0.001). Patients without fibrosis (no DE) improved their EF more than those with fibrosis (13±10 versus 4±10 units; P<0.001). Patients undergoing ECG optimization had better EF response than those not optimized (10±11 versus 5±10 units; P=0.013). Figure 3 shows change in EF in each of the 3 groups without and with 12‐lead ECG optimization. Patients with no DE had a large and similar (P=0.452) improvement in EF regardless of whether they did not (15±9 units) or did (12±11 units) undergo 12‐lead ECG optimization. In contrast, patients with NICM and midwall stripe (2±9 versus 12±12 units; P=0.014) and patients with scar (0±7 versus 6±11 units; P=0.021) had poor EF response without optimization and a significantly improved EF response if they underwent 12‐lead ECG optimization.
Figure 3.
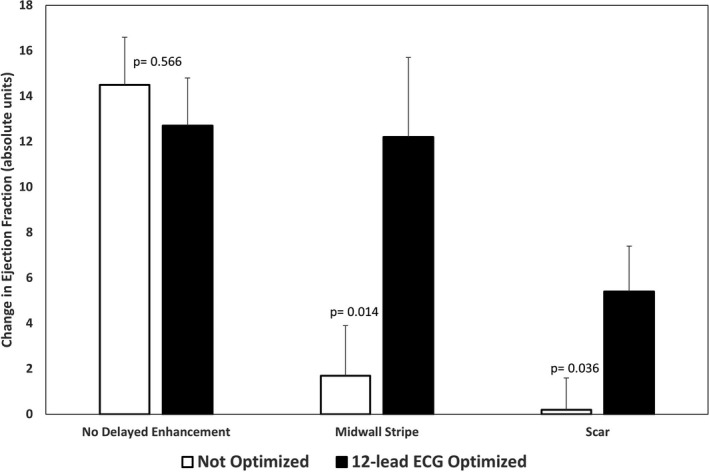
Changes in echocardiographic response based on delayed enhancement (DE) categorization. The change in ejection fraction (EF) 1‐year post‐CRT (cardiac resynchronization therapy) was not altered by CRT optimization in patients without DE. However, patients with DE had improved echocardiographic outcomes following 12‐lead ECG optimization of settings for wavefront fusion. Patients with midwall stripe improved EF from 2±9 to 12±12 units (P=0.014), and those with scar improved from 0±7 vs 5±10 units (P=0.036).
Patients with no DE, as compared with those with DE, had greater native electrical dyssynchrony by 12‐lead ECG QRSd (161±17 versus 151±21 ms; P=0.005). Patients with and without DE had similar reductions in QRSd from native to standard CRT programming (−9.1±15% versus 1.2±17%; P=0.140), but patients without DE had a greater reduction in QRSd with 12‐lead ECG optimization (−12.1±14% versus 2.4±23%; P=0.005).
In those patients who underwent BSAM, SDAT was significantly greater in those without fibrosis (42±10 versus 35±11 ms; P=0.030). Figure 4 shows changes in SDAT with and without 12‐lead ECG optimization. Patients with no DE had a greater reduction in SDAT from native rhythm to standard CRT programming (−14±10 versus −4±15 ms; P=0.009), but lesser reduction in SDAT with 12‐lead ECG optimization (−1±6 versus −6±10 ms; P=0.038). Figure 5 shows BSAM, SDAT and 12‐lead ECGs in a patient with LBBB and previous myocardial infarction. 12‐lead ECG optimization by preactivating the LV lead by 40 ms resulted in improved BSAMs, SDAT, and EF.
Figure 4.
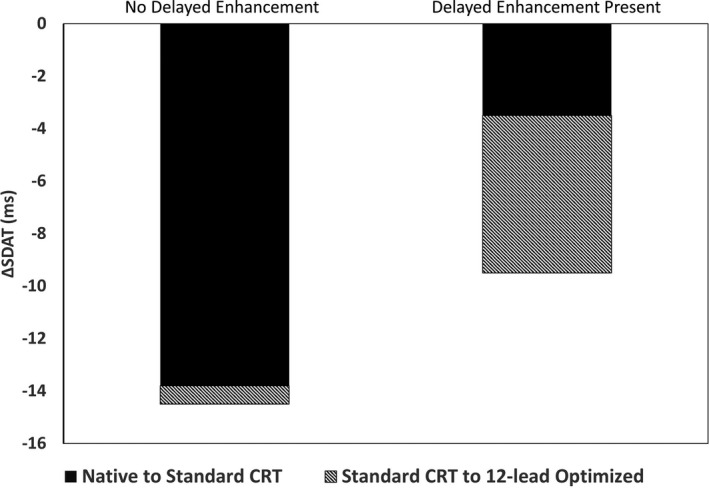
Changes in electrical dyssynchrony (SDAT) with standard and optimized CRT. Patients without delayed enhancement (DE) had a greater reduction in SDAT compared with those with DE (midwall fibrosis or scar) when standard simultaneous biventricular cardiac resynchronization therapy (CRT) was utilized (−14±10 vs −4±15 ms; P<0.01). Patients with DE had greater reductions in SDAT through 12‐lead ECG optimization of settings compared with patients without DE (−6±10 vs −1±6 ms; P=0.04). SDAT indicates standard deviation of activation times.
Figure 5.
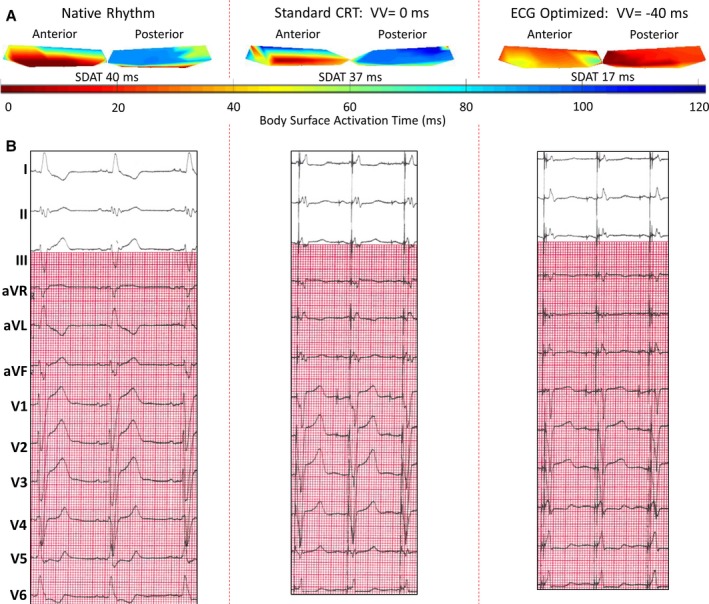
Case example: patient with inferolateral scar and optimized cardiac resynchronization therapy (CRT). Patient with previous myocardial infarction resulting in subendocardial delayed enhancement (DE) ˃75% of the basal and midinferior and inferolateral walls. The patient had an underlying left bundle branch block (LBBB) and was optimized with 12‐lead ECG to have the left ventricular (LV) lead pace 40 ms preceding the right ventricular (RV) lead. A, Body‐surface activation maps during native rhythm, at standard cardiac resynchronization therapy (CRT) programming with LV and RV leads pacing simultaneously, and at the 12‐lead ECG optimized setting. Native map shows delayed posterior electrode activation as compared with anterior electrode activation, corresponding to a dyssynchronous LBBB with a high standard deviation of activation times (SDAT) of 40 ms. Standard CRT programming resulted in only a mild benefit, with nearly similar on pattern and SDAT of 37 ms. Twelve‐lead optimization resulted in near synchronous activation and SDAT of only 17 ms. B, Changes in QRS width and morphology associated with pacing. The patient's ejection fraction (EF) increased from 22% pre‐CRT to 33% after 6 months of CRT. aVF indicates augmented vector foot; aVL, augmented vector left; aVR, augmented vector right; VV, ventricular‐ventricular.
Discussion
The primary result of this study was that a group of recently implanted CRT patients who underwent routine 12‐lead ECG‐based optimization exhibited improved echocardiographic response as compared with a similar group of patients implanted before the implementation of routine optimization. Differences in echocardiographic response were driven by improved response in patients with DE on cMRI, and especially in those with findings of midwall fibrosis. We also demonstrate in a subgroup of optimized patients that heart failure patients with fibrosis (midmyocardial stripe or scar on DE cMRI) have less native electrical dyssynchrony by ECG belt activation mapping and a markedly reduced echocardiographic response to CRT when programmed at standard device settings. Patients with DE who underwent 12‐lead ECG optimization (often by pacing LV‐only appropriately timed to native conduction or with LV preactivation) by using 12‐lead ECG to improve wavefront fusion and cancellation have a significantly improved response to CRT. The improved echocardiographic response in these patients is associated with a significant decrease in electrical dyssynchrony with optimization as measured by SDAT. In contrast, patients without DE have a large improvement in echocardiographic response and reduction in electrical dyssynchrony at standard device settings. These data may explain why patients with DE have previously been noted to have a worse prognosis following CRT, and offers a potential strategy of individualized CRT optimization to improve electrical dyssynchrony and outcomes.
Effects of Scar on Response to CRT
Patients with ischemic cardiomyopathy have been shown in multiple studies to have a reduced response to CRT as compared with those patients with an NICM.16, 17, 18 One potential explanation for this difference is that patients with NICM have greater electrical dyssynchrony or a higher percentage of LBBB pre‐CRT and thus a more‐favorable substrate for CRT to ameliorate. In the MADIT‐CRT (Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy) study, patients with NICM had greater electrical dyssynchrony as measured by QRSd (160 versus 152 ms) and a greater percentage of patients with LBBB (88% versus 58%) as compared with patients with ischemic cardiomyopathy.16 Our study similarly showed greater electrical dyssynchrony in NICM patients, with a wider QRSd and higher SDAT in this patient group. This may, in part, be attributed to a lower percentage of LBBB patients in the DE group, similar to what was found in the MADIT‐CRT. An alternative explanation for the better response to CRT in NICM, as compared with ischemic cardiomyopathy patients, is that scar extent or burden impairs the ability to resynchronize the heart. A number of studies have demonstrated that the amount of scar in patients with ischemic cardiomyopathy correlates with lack of response to CRT.9, 19, 20 In addition, the location of scar and its relationship to the LV lead impacts CRT response. Patients with posterolateral scar have a reduced response to CRT.8 Patients with an LV lead in scar do worse than those who have a lead adjacent to scar, and both groups do worse than those whose LV lead is distant from scar.20, 21 Our study is consistent with these studies in that our patients with DE who did not undergo 12‐lead ECG optimization had a much worse echocardiographic response to CRT as compared with those patients without DE. Of note, in studies assessing CRT response in patients with and without scar, CRT programming was not optimized and studies were typically performed with patients at standard CRT settings and without LV‐only pacing. Our study is unique in that we studied the effect of scar on EF response to CRT in patients at standard settings and in different patients optimized to mostly nonstandard settings (often with LV preactivation or LV‐only pacing). Our data showing a much improved EF response in optimized patients suggest that it is not the scar itself that is the main or perhaps even the sole cause of poor CRT response, but rather the absence of adequate electrical resynchronization that can be achieved with individualized CRT programming in this group of patients.
Effects of Midwall Fibrosis on Response to CRT
Presence of midwall fibrosis in patients with NICM is a very poor prognostic sign,22, 23, 24, 25, 26 Midwall fibrosis in patients treated with CRT predicts hospitalization for heart failure and cardiovascular mortality, hospitalization for major adverse cardiac and total mortality, as well as cardiovascular mortality independent of multiple other variables such as New York Heart Association class, QRSd, LV size and function, and radial mechanical dyssynchrony.22 It also has been shown to adversely affect LV function by impairing LV global circumferential strain, strain rate, torsion, and diastolic strain rate.26 This has been proposed as a reason for the reduced LV remodeling and EF response,22 as well as poorer clinical outcomes following CRT.23 Our data from patients who did not undergo 12‐lead ECG optimization showed a poor echocardiographic response to CRT in patients with midwall fibrosis with a mean absolute EF improvement of 2%. However, in patients undergoing 12‐lead ECG optimization, the EF improvement rose to 12%, a value similar to that found in the NICM patients without midwall fibrosis. Thus, our data suggest that 12‐lead ECG optimization may be an effective strategy for improving systolic function in this group of patients known to typically have a poor response to CRT.
Electrical Dyssynchrony and 12‐Lead ECG Optimization
The ability of QRSd reduction to predict response to CRT is controversial, with some studies showing a poor predictive value12, 27 and others showing a strong predictive value.28 More‐recent studies have shown that the 12‐lead ECG provides a great deal of information in addition to QRSd that can be used to understand the effect of CRT on electrical synchrony. Sweeney et al demonstrated that waveform fusion and cancellation as a result of interaction among native, RV‐paced, and LV‐paced electrical wavefronts can be detected on 12‐lead ECG and used to predict LV remodeling response to CRT.15 Findings of increased wavefront fusion and cancellation, such as a narrower QRS, a Q wave in lead I, and an R wave in V1, were predictive of a better response to CRT. Cooper et al also showed that the 12‐lead ECG could be used to optimize CRT, with 70% of their patients needing LV preactivation with a mean LV preactivation time of 30 ms.14
In our study, only 11% of patients in the group implanted between 2007 and 2013 had LV preactivation or LV‐only pacing fused to native conduction. In contrast, 73% of patients in the group implanted between 2014 and early 2017 were programmed to sequential biventricular pacing with LV preactivation or LV‐only settings. We believe that the large differences in EF response to CRT in the more recently implanted group are predominantly related to the routine use of 12‐lead ECG optimization that began as part of our standard clinical practice in 2014. There are a number of reasons that support this assertion. Baseline characteristics, including QRSd and EF, in the patients in both time frames were similar, suggesting that patient selection did not change greatly with time. Lead position was also similar (95% placed lateral or posterolateral) in the 2 groups of patients, arguing against a large change in implanter behavior or skill. Quadripolar LV leads were implanted in none of the patients before 2014 and in 82% of those implanted in 2014–2017. However, the 2 most basal electrodes were only used for pacing in 52% of patients whereas the traditionally available bipolar electrodes were used in the remaining 48% of patients with quadripolar leads. The LV pacing vector was rarely changed from what was programmed during implant, and, if changed, was attributed to high thresholds or phrenic nerve stimulation and not because of 12‐lead ECG optimization given that we did not evaluate different LV vectors with 12‐lead ECG. Thus, pacing from a better location is unlikely to be the cause of the better response in the latter group.
The main differences between the patients with fibrosis and those without fibrosis related to baseline electrical dyssynchrony and change in dyssynchrony with CRT both at standard and 12‐lead ECG optimized settings. We measured electrical dyssynchrony using a novel body‐surface mapping technology. This technology generates body‐surface activation maps to qualitatively assess spatial electrical heterogeneity (by map patterns) and to objectively quantify it (with SDAT). We have previously shown that change in SDAT with pacing from different LV sites at CRT implantation has better accuracy than QRSd or RV‐LV sensing delays in identifying sites with improvements in acute hemodynamic response.11 Additionally, we have shown that native SDAT and change in SDAT with CRT are better predictors of 6‐month LV remodeling response than are standard variables such as native QRSd or morphology or change in QRSd with pacing.12 Changes in SDAT parallel changes in EF in the optimized groups. We hypothesize that the improvements in EF and SDAT with optimization (often with LV preactivation or appropriately timed LV‐only pacing) were attributed to adjusting for LV latency or slow conduction as a result of fibrosis that required more‐precise timing of CRT parameters. CRT in LBBB patients involves fusing native right bundle branch conduction and/or the RV‐paced wavefront with the LV‐paced wavefront. Native right bundle branch conduction is relatively fast, and the RV lead is placed in the septal myocardium. The LV lead, on the other hand, is placed in an epicardial vein, and this may result in a delay between the timing of LV pacing stimulus and activation of the nearby myocardium. Alternatively, the LV lead may be closer to areas of scar or delayed conduction or further from fibers that can increase conduction velocity. We hypothesize that the typical tendency of the right‐sided native and RV‐paced wavefronts to be ahead of the LV‐paced wavefront is accentuated by the presence of fibrosis or scar in the LV. Thus, optimal fusion often requires LV‐preactivation or fusion of the LV‐paced wavefront with native right bundle conduction.
Although 12‐lead ECG optimization resulted in doubling of the mean EF response from 5% to 10% over a 1‐year follow‐up period, this was likely not the maximal potential improvement that could be achieved with CRT optimization. Twelve‐lead ECG optimization decreased our measure of electrical dyssynchrony, SDAT, from 38 ms native to 27 ms in the entire patient cohort. However, in a previous study, we showed that the lowest SDAT achieved, on average, by testing multiple device settings in 94 CRT patients was 20 ms.13 The device settings (eg, pacing configuration like biventricular or LV‐only pacing, and timing parameters like atrioventricular or VV delays) that help achieve this optimal electrical activation with desirable fusion of intrinsic and paced wavefronts are variable depending on patient‐specific substrate and conduction patterns. Noninvasive BSAM maps and related metrics of electrical heterogeneity could provide useful qualitative as well as quantitative guidance in optimal tailoring of device programming in individual patients. Twelve‐lead ECG optimization in the present study thus did not likely achieve optimal electrical synchrony. Use of the ECG belt for optimization, rather than 12‐lead ECG, in future studies offers the potential for additional improvements in electrical dyssynchrony and therefore even better EF improvements as compared with standard CRT programming.
Study Limitations
This was a nonrandomized retrospective study of 130 patients. Although we showed an association between 12‐lead ECG optimization and improved EF response in DE patients, we cannot prove causality. It is recognized that the recommendations for CRT implantation evolved over the duration of this study, and that refinements in patient selection might explain some of the results of this study. However, Table 1 shows that the optimized and nonoptimized groups were well matched, and we expect that any changes in patient selection over the study period were small and unlikely to have accounted for the findings reported in this study. Similarly, given that most of the optimized patients and none of the nonoptimized patients were implanted with quadripolar leads, it is difficult to determine what role quadripolar leads may have played in the results. We found no significant differences in EF response when comparing the small groups of optimized patients with or without quadripolar leads (data not shown), so we expect that our results were primarily explained by the optimization protocol. A future randomized trial is warranted, however, in order to account for these differences in patient selection and lead design. We did not assess lead location with respect to scar location in this study, and thus we cannot determine whether this variable impacted the effect of optimization on EF response. CRT optimization was only based on 12‐lead ECG and not the ECG belt. Further investigations programming patients to settings with lowest possible SDAT are warranted to evaluate true potential for CRT response in patients with and without DE.
Conclusions
DE on cMRI is associated with worse EF outcomes 1 year following CRT. However, ECG optimization, often resulting in nonstandard CRT programming utilizing LV‐only or sequential BiV pacing, improves EF in patients with midwall stripe or scar DE. These findings may partially explain the observed 20% to 30% nonresponse rate to CRT in patients receiving standard, traditional CRT programming. The use of 12‐lead ECG or ECG belt optimized programming offers the possibility to improve CRT outcomes despite the presence of myocardial fibrosis/scar.
Disclosures
Mr Gage, Ms Curtin, and Dr Bank have consulting services agreements with Medtronic. Dr Ghosh and Mr Gillberg are employees of Medtronic. The remaining authors have no disclosures to report.
(J Am Heart Assoc. 2018;7:e009559 DOI: 10.1161/JAHA.118.009559)
References
- 1. Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM; Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators . Cardiac‐resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. [DOI] [PubMed] [Google Scholar]
- 2. Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L; Cardiac Resynchronization‐Heart Failure (CARE‐HF) Study Investigators . The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. [DOI] [PubMed] [Google Scholar]
- 3. Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, Estes NA III, Foster E, Greenberg H, Higgins SL, Pfeffer MA, Solomon SD, Wilber D, Zareba W; MADIT‐CRT Trial Investigators . Cardiac‐resynchronization therapy for the prevention of heart‐failure events. N Engl J Med. 2009;361:1329–1338. [DOI] [PubMed] [Google Scholar]
- 4. Kass DA. Ventricular resynchronization: pathophysiology and identification of responders. Rev Cardiovasc Med. 2003;4(suppl 2):S3–S13. [PubMed] [Google Scholar]
- 5. Zhang Q, Zhou Y, Yu CM. Incidence, definition, diagnosis, and management of the cardiac resynchronization therapy nonresponder. Curr Opin Cardiol. 2015;30:40–49. [DOI] [PubMed] [Google Scholar]
- 6. Delfino JG, Fornwalt BK, Oshinski JN, Lerakis S. Role of MRI in patient selection for CRT. Echocardiography. 2008;25:1176–1185. [DOI] [PubMed] [Google Scholar]
- 7. Lardo AC, Abraham TP, Kass DA. Magnetic resonance imaging assessment of ventricular dyssynchrony: current and emerging concepts. J Am Coll Cardiol. 2005;46:2223–2228. [DOI] [PubMed] [Google Scholar]
- 8. Bleeker GB, Kaandorp TA, Lamb HJ, Boersma E, Steendijk P, de Roos A, van der Wall EE, Schalij MJ, Bax JJ. Effect of posterolateral scar tissue on clinical and echocardiographic improvement after cardiac resynchronization therapy. Circulation. 2006;113:969–976. [DOI] [PubMed] [Google Scholar]
- 9. Chalil S, Foley PW, Muyhaldeen SA, Patel KC, Yousef ZR, Smith RE, Frenneaux MP, Leyva F. Late gadolinium enhancement‐cardiovascular magnetic resonance as a predictor of response to cardiac resynchronization therapy in patients with ischaemic cardiomyopathy. Europace. 2007;9:1031–1037. [DOI] [PubMed] [Google Scholar]
- 10. White JA, Yee R, Yuan X, Krahn A, Skanes A, Parker M, Klein G, Drangova M. Delayed enhancement magnetic resonance imaging predicts response to cardiac resynchronization therapy in patients with intraventricular dyssynchrony. J Am Coll Cardiol. 2006;48:1953–1960. [DOI] [PubMed] [Google Scholar]
- 11. Johnson WB, Vatterott PJ, Peterson MA, Bagwe S, Underwood RD, Bank AJ, Gage RM, Ramza B, Foreman BW, Splett V, Haddad T, Gillberg JM, Ghosh S. Body surface mapping using an ECG belt to characterize electrical heterogeneity for different left ventricular pacing sites during cardiac resynchronization: relationship with acute hemodynamic improvement. Heart Rhythm. 2017;14:385–391. [DOI] [PubMed] [Google Scholar]
- 12. Gage RM, Curtin AE, Burns KV, Ghosh S, Gillberg JM, Bank AJ. Changes in electrical dyssynchrony by body surface mapping predict left ventricular remodeling in patients with cardiac resynchronization therapy. Heart Rhythm. 2017;14:392–399. [DOI] [PubMed] [Google Scholar]
- 13. Bank AJ, Gage RM, Curtin AE, Burns KV, Gillberg JM, Ghosh S. Body surface activation mapping of electrical dyssynchrony in cardiac resynchronization therapy patients: potential for optimization. J Electrocardiol. 2018;51:534–541. [DOI] [PubMed] [Google Scholar]
- 14. Cooper JM, Patel RK, Emmi A, Wang Y, Kirkpatrick JN. RV‐only pacing can produce a Q wave in lead 1 and an R wave in V1: implications for biventricular pacing. Pacing Clin Electrophysiol. 2014;37:585–590. [DOI] [PubMed] [Google Scholar]
- 15. Sweeney MO, Hellkamp AS, van Bommel RJ, Schalij MJ, Borleffs CJ, Bax JJ. QRS fusion complex analysis using wave interference to predict reverse remodeling during cardiac resynchronization therapy. Heart Rhythm. 2014;11:806–813. [DOI] [PubMed] [Google Scholar]
- 16. Barsheshet A, Goldenberg I, Moss AJ, Eldar M, Huang DT, McNitt S, Klein HU, Hall WJ, Brown MW, Goldberger JJ, Goldstein RE, Schuger C, Zareba W, Daubert JP. Response to preventive cardiac resynchronization therapy in patients with ischaemic and nonischaemic cardiomyopathy in MADIT‐CRT. Eur Heart J. 2011;32:1622–1630. [DOI] [PubMed] [Google Scholar]
- 17. Gasparini M, Mantica M, Galimberti P, Genovese L, Pini D, Faletra F, Marchesina UL, Mangiavacchi M, Klersy C, Gronda E. Is the outcome of cardiac resynchronization therapy related to the underlying etiology? Pacing Clin Electrophysiol. 2003;26:175–180. [DOI] [PubMed] [Google Scholar]
- 18. Wikstrom G, Blomstrom‐Lundqvist C, Andren B, Lonnerholm S, Blomstrom P, Freemantle N, Remp T, Cleland JG; CARE‐HF study investigators . The effects of aetiology on outcome in patients treated with cardiac resynchronization therapy in the CARE‐HF trial. Eur Heart J. 2009;30:782–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Adelstein EC, Saba S. Scar burden by myocardial perfusion imaging predicts echocardiographic response to cardiac resynchronization therapy in ischemic cardiomyopathy. Am Heart J. 2007;153:105–112. [DOI] [PubMed] [Google Scholar]
- 20. Leyva F, Foley PW, Chalil S, Ratib K, Smith RE, Prinzen F, Auricchio A. Cardiac resynchronization therapy guided by late gadolinium‐enhancement cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2011;13:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Khan FZ, Virdee MS, Palmer CR, Pugh PJ, O'Halloran D, Elsik M, Read PA, Begley D, Fynn SP, Dutka DP. Targeted left ventricular lead placement to guide cardiac resynchronization therapy: the target study: a randomized, controlled trial. J Am Coll Cardiol. 2012;59:1509–1518. [DOI] [PubMed] [Google Scholar]
- 22. Leyva F, Taylor RJ, Foley PW, Umar F, Mulligan LJ, Patel K, Stegemann B, Haddad T, Smith RE, Prasad SK. Left ventricular midwall fibrosis as a predictor of mortality and morbidity after cardiac resynchronization therapy in patients with nonischemic cardiomyopathy. J Am Coll Cardiol. 2012;60:1659–1667. [DOI] [PubMed] [Google Scholar]
- 23. Leyva F, Zegard A, Acquaye E, Gubran C, Taylor R, Foley PWX, Umar F, Patel K, Panting J, Marshall H, Qiu T. Outcomes of cardiac resynchronization therapy with or without defibrillation in patients with nonischemic cardiomyopathy. J Am Coll Cardiol. 2017;70:1216–1227. [DOI] [PubMed] [Google Scholar]
- 24. Leong DP, Chakrabarty A, Shipp N, Molaee P, Madsen PL, Joerg L, Sullivan T, Worthley SG, De Pasquale CG, Sanders P, Selvanayagam JB. Effects of myocardial fibrosis and ventricular dyssynchrony on response to therapy in new‐presentation idiopathic dilated cardiomyopathy: insights from cardiovascular magnetic resonance and echocardiography. Eur Heart J. 2012;33:640–648. [DOI] [PubMed] [Google Scholar]
- 25. Assomull RG, Prasad SK, Lyne J, Smith G, Burman ED, Khan M, Sheppard MN, Poole‐Wilson PA, Pennell DJ. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol. 2006;48:1977–1985. [DOI] [PubMed] [Google Scholar]
- 26. Taylor RJ, Umar F, Lin EL, Ahmed A, Moody WE, Mazur W, Stegemann B, Townend JN, Steeds RP, Leyva F. Mechanical effects of left ventricular midwall fibrosis in non‐ischemic cardiomyopathy. J Cardiovasc Magn Reson. 2016;18:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gold MR, Thebault C, Linde C, Abraham WT, Gerritse B, Ghio S, St John Sutton M, Daubert JC. Effect of qrs duration and morphology on cardiac resynchronization therapy outcomes in mild heart failure: results from the resynchronization reverses remodeling in systolic left ventricular dysfunction (REVERSE) study. Circulation. 2012;126:822–829. [DOI] [PubMed] [Google Scholar]
- 28. Rickard J, Popovic Z, Verhaert D, Sraow D, Baranowski B, Martin DO, Lindsay BD, Varma N, Tchou P, Grimm RA, Wilkoff BL, Chung MK. The QRS narrowing index predicts reverse left ventricular remodeling following cardiac resynchronization therapy. Pacing Clin Electrophysiol. 2011;34:604–611. [DOI] [PubMed] [Google Scholar]


