Abstract
Background
Recent evidence shows an association between the level of physical activity and cardiovascular mortality and morbidity in patients with atrial fibrillation (AF). We sought to assess the impact of AF daily burden on the activity level of the patient who underwent pulmonary vein isolation.
Methods and Results
Patients enrolled in the DISCERN AF (Discerning Symptomatic and Asymptomatic Episodes Pre and Post Radiofrequency Ablation of Atrial Fibrillation) study all had insertable cardiac monitors, which provided the daily burden of atrial tachycardia and atrial fibrillation (AT/AF) and a corresponding activity level. A total of 44 341 daily AT/AF burden points were collected from 50 patients with an average of 887 observations for every patient, with <5 minutes of AT/AF reported on 82.6% of days. The daily burden of AT/AF after ablation ranged between 0 and 1440 minutes. The minimum and maximum daily activity was 0 and 600 minutes per day, respectively. A significant inverse association was detected between activity levels and AF burden (P<0.001; 95% confidence interval, 0.01–0.04). The daily activity starts progressively decreasing after 500 minutes of AF and considerably drops after 1000 minutes. The association between activity level and burden of AT/AF was still statistically significant after adjustment for clinical variables (P =0.02; 95% confidence interval, −003 to 0.04).
Conclusions
Daily activity level correlates with daily AT/AF burden in patients who underwent AF ablation. The daily activity started decreasing after a daily burden of 500 minutes of AF and greatly drops after 1000 minutes. Therefore, the amount of AT/AF burden that may impact the activity level seems to be related to hours and not minutes of arrhythmias.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov. Unique identifier: NCT00745706.
Keywords: ablation, Atrial Fibrillation Burden, atrial fibrillation, exercise, physical exercise
Subject Categories: Atrial Fibrillation, Exercise, Lifestyle
Clinical Perspective
What Is New?
Current guidelines use an episode of atrial fibrillation (AF) lasting >30 seconds as the cutoff for recurrence after AF ablation
The effects of AF burden reduction after ablation are neglected.
Herein, the correlation between AF burden and activity level of the patients are explored.
What Are the Clinical Implications?
In our analysis, daily activity level correlates with daily AF burden in patients who underwent AF ablation.
The daily activity decreases after a daily burden of 400 minutes of AF and greatly drops after 1000 minutes.
AF ablation decreasing the amount of AF burden may improve the activity level of the patients, although it does not abolish recurrence of arrhythmia.
According to the HRS Consensus Statement on Catheter Ablation of Atrial Fibrillation, an episode of atrial fibrillation (AF) lasting >30 seconds is used as the cutoff for recurrence1, 2 This is a very high bar, requiring almost complete elimination of the arrhythmia for procedural success. While this end point serves as a common reference point by which to compare the outcomes of clinical trials, it is known that reductions in AF burden without total AF elimination can improve quality of life.
Data have shown that patients can continue to have up to 2 hours of AF per month and still have significant improvement in their quality of life3 Furthermore, reduction in symptoms and increases in quality‐of‐life measures correlate to improved exercise performance4 This is important because the risk of cardiovascular events affected by AF has been linked with patient exercise activity5
Therefore, it is important to understand the relationship among postablation AF burden, quality of life, and activity level. The DISCERN AF (Discerning Symptomatic and Asymptomatic Episodes Pre and Post Radiofrequency Ablation of Atrial Fibrillation)6 used insertable cardiac monitors (ICMs) and detailed symptom diaries to measure AF burden both before and after ablation and to determine the proportion of symptomatic versus asymptomatic AF episodes. ICMs are also able to provide a measurement of patient activity levels. The aim of this study was to assess the correlation among AF burden, symptoms, and activity level and to possibly identify a critical burden of arrhythmia that might impact a patient's activity level.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Patients and Design
The DISCERN AF study has been described in detail elsewhere6 Briefly, the study was a multicenter, prospective cohort study that enrolled patients (n=50) with symptomatic paroxysmal or persistent AF. Paroxysmal AF was defined as ≥4 episodes of AF in the 6 months before assessment that spontaneously terminated within 7 days; persistent AF was defined as at least 1 episode sustained for >7 days. All patients were undergoing first‐time catheter ablation for AF. The details of the ablation technique adopted were described in the original DISCERN AF study6 The ethics review board at each institution approved the study. All the subjects enrolled in the study gave informed consent.
Patients had an ICM (Reveal XT, Medtronic Inc) implanted 3 months before ablation and were followed for a mean of 2.4 years months after ablation. The ICM Reveal XT has proved reliability in AF detection in a prior study where it was tested against an ECG Holter7 Furthermore, in the original DISCERN AF paper, the predictive accuracy for ICM‐detected episodes of atrial tachycardia (AT)/AF episodes was tested versus patient symptoms, intermittent ECG (3 months), and intermittent 48‐hour Holter monitoring (every 3 months).6
During the study, patients kept a detailed symptom diary to record the time and duration of any symptoms they experienced. Patients were followed every 3 months, during which all of the ICM data were downloaded and symptom diaries collected. This was done to avoid ICM memory overflow or loss of symptom diaries.
For each download, the ICM provides both a continuous measure of average burden of AF and AT and a complete tabulation of daily AT/AF burden for each day of follow‐up. For each of these 3‐month blocks of burden, an average daily burden of arrhythmia was calculated.
Activity Level and Primary End Point
The ICM is also capable of collecting the patient's average daily activity level through an embedded accelerometer. It does so by classifying each minute as either “active” or “inactive” based on whether the number of accelerometer counts exceeds a preset threshold. The accuracy of the Medtronic accelerometer to measure daily activity was recently validated in a study comparing it with an external triaxial accelerometer.8
The number of active minutes is then summed over each calendar day and recorded as total number of minutes per day. These data were matched with the related daily burden of AT/AF.
The primary end point of this post hoc analysis was to correlate daily patient activity with daily burden of AT/AF and to investigate how other confounding variables, such as age, sex, hypertension, palpitations, dyspnea, fatigue, and Canadian Cardiovascular Society scale of AF symptoms, influence this correlation.
Statistical Analysis
All data are reported as mean (SD) for continuous variables and frequencies with percentages for categorical variables unless otherwise indicated. To examine the predictors of patient activity according to the burden of AT/AF, a generalized estimating equation was used to account for the correlations between repeated measures on the same patient over time. An autoregressive correlation structure (first‐order autoregression) was set in the generalized estimating equation to assess for within‐subject correlations over time. Identity was specified as a link function, and the distribution was Gaussian. A quadratic transformation was used to uncover any trend of variation in the relationship between activity and AF burden for observation at the edge of values. The model was also tested using different assumptions: random effects and lagged variable modeling.
The quasilikelihood under the independence model criterion statistic was used to check for the best model.9 From the generalized estimating equation model, the marginal effects of AF burden on activity were predicted and plotted against different values of AF burden.
The main outcome, predictors, and confounders were calculated for every measure. A P value of <0.05 was considered significant for all statistical determinations. The analysis was performed using commercially available software (STATA 14, release 2015; Stata Statistical Software, College Station, TX).
Results
A total of 44 341 daily AT/AF burden data points were collected from the 50 patients of the DISCERN AF study. Baseline characteristics of the patients are reported in Table 1. An average of 887 observations were available for every patient. In our population, the daily burden of AT/AF after ablation ranged between 0 and 1440 minutes. The predictive accuracy for ICM‐detected episodes of AT/AF is reported in Table 2.
Table 1.
Baseline Patient Characteristics
| Characteristics | Data |
|---|---|
| Age, mean (SD), y | 57 (11) |
| Male sex | 32 (64) |
| Paroxysmal AF | 40 (80) |
| Canadian Cardiovascular Society Severity of AF score (SD) | 3 (1) |
| No. of failed antiarrhythmics administered before ablation, mean (SD) | 1.2 (0.7) |
| Duration of AF symptoms before ablation, median (range), y | 6.2 (1–32) |
| CHADS index, mean (SD) | 1.4 (0.3) |
| Hypertension | 15 (30) |
| Diabetes mellitus | 5 (10) |
| Sleep apnea | 5 (10) |
| Prior stroke/TIA | 4 (8) |
| Coronary artery disease | 3 (6) |
| Heart failure | 3 (6) |
| Valvular heart disease | 2 (4) |
| Ejection fraction, mean (SD) | 54 (10) |
| Left atrial diameter, mean (SD), mm | 41 (6) |
AF indicates atrial fibrillation; SD, standard deviation; TIA, transient ischemic attack.
Table 2.
Predictive Accuracy for ICM‐Detected Episodes of AT/AF
| Predictive Value, % | ||
|---|---|---|
| Positive | Negative | |
| Patient symptoms | 86.9 | 23.6 |
| Intermittent ECG (every 3 mo) | 33.3 | 94.9 |
| Intermittent 48‐h Holter monitoring (every 3 mo) | 0 | 94.4 |
AF indicates atrial fibrillation; AT, atrial tachycardia; ICM, insertable cardiac monitor.
The daily burden of AT/AF did not follow a normal distribution. The daily physical activity showed a normal distribution and was considered a continuous variable. The minimum and maximum daily activity was 0 and 600 minutes per day, respectively. The mean of the daily activity was 244.63±119.75 minutes.
The daily activity significantly correlated with the daily burden of AF (P<0.001; 95% confidence interval [CI], 0.01–0.04). The results were consistent and significant even when different assumptions were made in the model.
The predicted effect of AF burden on the activity level was estimated from the observed data and is displayed in Figure 1. From 0 up to 400 minutes of AF burden, there is a unit increase of activity per unit increase in AF burden. However, starting from 500 minutes, there is a decrease in activity per unit of increase in AF burden.
Figure 1.
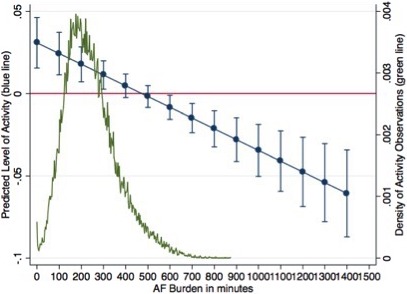
Predicted effects of AF burden on activity level. Blue points represent predicted increase with 95% confidence intervals of activity for a unit increase at different values of AF burden. AF indicates atrial fibrillation.
Figure 2 displays the predicted values of activity with relative CIs, at a different value of AF burden. Accordingly, the activity level shows a slight increase for AF burden between 0 and 400 minutes (6 hours and 40 minutes), moving from 241 minutes of activity per day up to over 256 minutes of activity. After 500 minutes of AF, the daily activity level starts decreasing gradually and markedly decreases after 1000 minutes. It decreases to 222.9 minutes of activity (95% CI, 201–235) for a daily AF burden of 1440 minutes.
Figure 2.
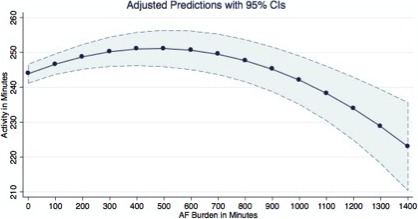
Margins plot reporting the mean activity and relative confidence intervals predicted at different values of AF burden. AF indicates atrial fibrillation.
At a multivariate regression including clinical variables such as age, sex, hypertension, palpitations, dyspnea, fatigue, heart rate variability, and Canadian Cardiovascular Society scale of AF symptoms score showed that clinical symptoms possibly related to AF such as palpitations, dyspnea, and fatigue significantly impact the association between activity and AF/AT burden, but the Canadian Cardiovascular Society scale of AF symptoms score and hypertension did not (Table 3). The association between AF burden and activity level was statistically significant after adjustment for the clinical variable (P=0.02; 95% CI, −0.03 to 0.04).
Table 3.
Multivariate Analysis Adjusted for the Confounding Clinical Variables
| Activity | Coefficient | P Value | 95% CI |
|---|---|---|---|
| AF burden | 0.2 | <0.02 | −0.03 to 0. 04 |
| Age | −1.42 | <0.0001 | −1.69 to −1.14 |
| Male sex | 20.2 | <0.0001 | 14 to 26.5 |
| Hypertension | −1.1 | 0.71 | −7.3 to 5 |
| Palpitation | −25 | <0.0001 | −35.9 to −14 |
| Dyspnea | 53.3 | <0.0001 | 47.9 to 58.7 |
| Fatigue | −13.4 | <0.0001 | −20.7 to −6.2 |
| CCSAF score | 2.8 | 0.27 | −2.2 to 7.8 |
| HR variability | 0.91 | <0.0001 | 0.89 to 0.94 |
AF indicates atrial fibrillation; CCSAF, Canadian Cardiovascular Society AF score; CI, confidence interval; HR, heart rate.
The predicted effects of AF burden on activity adjusted for the other clinical variables are displayed in Figure 3. It appears that activity starts decreasing after 300 minutes of AF burden and shows almost a 3‐unit decrease for a single‐unit increase of AF burden at level of 1400 minutes.
Figure 3.
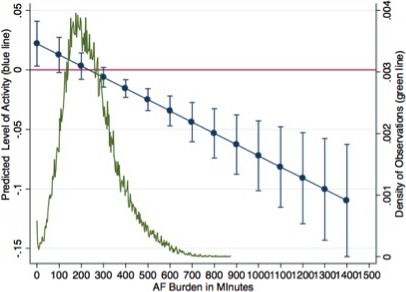
Predicted effects of AF burden on activity level adjusted according to the clinical variables. Blue points represent predicted increase with 95% confidence intervals of activity for a unit increase of AF burden at different AF burden. AF indicates atrial fibrillation.
Adjusting the model for the clinical variables did not significantly change the predictive value of activity on different values of AF burden, although the activity started decreasing earlier than 400 minutes; the lowest value for activity was 183 minutes (95% CI, 159–206) at 1440 minutes of AF/AT burden. Figure 4 shows the predicted values of activity with relative CIs at a different value of AF burden when adjusted for clinical variables.
Figure 4.
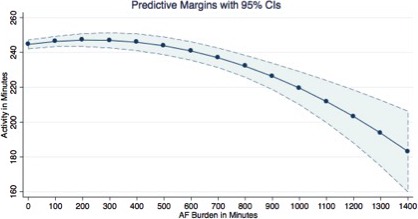
Margins plot reporting the mean activity and relative confidence intervals predicted at different values of AF burden adjusted for clinical variables. AF indicates atrial fibrillation.
Discussion
Our analysis shows a significant inverse correlation between the daily burden of AT/AF and daily activity level. Notably, the daily activity levels starts decreasing with AT/AF burdens >500 minutes and considerably drops after 1000 minutes of arrhythmias; lower activity levels were detected at higher AT/AF burdens. The inverse relationship between daily activity level and AT/AF burden persisted even after adjustment for other covariables. Daily AT/AF burden remained one of the strongest independent predictors of daily activity level in a multivariable analysis.
This suggests that the amount of AT/AF burden that may impact the activity level seems to be related to hours and not minutes of arrhythmias. It could be hypothesized that catheter ablation may improve the activity level of patients with AF by decreasing the burden of arrhythmia as opposed to eliminating it altogether. Of note, there is a trend of slight increases in activity level for small burdens of AT/AF up to 300 minutes, although attenuated in the model adjusted for other clinical covariables. It is difficult to fully explain this finding. It is interesting to hypothesize that activity may serve as a trigger for AF in some patients, so there may be a small correlation between increased activity level and small burdens of AT/AF.
Overall, in the multivariable model, the daily AT/AF burden remained the most important predictor of the decrease in activity level. Clinical variables such as dyspnea, palpitations, and fatigue are important clinical predictors, which would make sense, as all these symptoms may be related to AF; indeed, those patients with more symptoms would be expected to have lower levels of activity. Age was also a significant predictor, presumably because those of older age may have lower levels of activity. It is unclear why sex was a predictor; an increased physical activity in men compared with women can be explained by a higher stress perception of AF in the latter than in the former as shown by Trovato et al10 Heart rate variability was also inversely related to daily activity level. This is perhaps not surprising because patients with lower activity levels would have lower average heart rates. It is unclear if the decreased heart rate variability may be a result of poor activity level or a causative factor.
These results are consistent with other studies suggesting that improvement in quality of life can occur even when AF is not totally eliminated. Considering that the main indication for pulmonary vein isolation is symptomatic relief, an increase in the quality‐of‐life score and activity levels without complete elimination of all arrhythmias may be clinically relevant. Mantovan et al11 showed in the STAR AF (Substrate and Trigger Ablation for Reduction of Atrial Fibrillation Trial) population that the degree of impairment in the subscales of the Short Form Health Survey 36‐item questionnaire were related to the burden of arrhythmias, showing a deterioration of physical functioning only for burden of arrhythmias >4.5 hours per month. Similar findings were reported by the MANTRA‐PAF (Medical Antiarrhythmic Treatment or Radiofrequency Ablation in Paroxysmal Atrial Fibrillation) study12 which demonstrated a significant improvement in quality of life in patients treated with ablation versus antiarrhythmic therapy; the main finding reported by the authors is a considerable rise in physical activity scale and a reduction in symptoms even with ongoing arrhythmias. Overall, this evidence is pointing toward an effect of catheter ablation on physical activity along with a decrease in symptoms, which persists even in the presence of a limited amount of arrhythmia recurrence.
It is interesting that activity levels decreased with increasing AT/AF burdens despite the observation in the original DISCERN AF trial that revealed an increase in the proportion of asymptomatic episodes of AT/AF after ablation. Perhaps ongoing AT/AF burden continues to exert limitations on activity tolerance despite obvious perception of symptoms such as palpitations. Indeed, prior studies4, 13, 14, 15, 16 have shown a decrease in exercise performance in patients with AF that can be improved by a rhythm control strategy. Evidence associates AF with higher resting and exercising heart rates, leading to a decrease in the stroke volume17 Studies4, 15 that performed treadmill tests in patients after AF ablation report a lower baseline and exercise heart rate along with an improvement in exercise capacity shown by an increase in maximal oxygen consumption and the metabolic equivalent of maximal oxygen consumption, which are directly related to stroke volume. Therefore, it can be hypothesized that reducing the burden of AT/AF and increasing the time in sinus rhythm restores normal heart physiology that ultimately results in an improvement of exercise performance.
Maintaining activity levels in patients with AF is important. Recently, the EORP‐AF (EURObservational Research Programme on Atrial Fibrillation) study5 (2442 patients with AF across 9 European countries) demonstrated an association between higher physical activity and lower occurrence of cardiovascular death and thromboembolic events. Physical activity also showed a trend toward limiting progression from paroxysmal to persistent AF. Higher activity levels may also contribute to weight loss, which may also produce better outcomes in patients18, 19, 20
Limitations
The main limitation of our study is the small size of the population analyzed. For this reason, the analysis was not meant to correlate AF burden before and after ablation with activity level.
Despite the small sample size, there was the benefit of continuous monitoring of both AF burden and activity with 100% patient compliance. Therefore, even though the sample size is modest, the data are quite robust.
In addition, it must be acknowledged that there is a drop of observations for burden above 400 minutes; however, the total of observations above 400 minutes is 1379, which makes the results trustable.
Finally, this represents a post hoc analysis of a prospective trial originally developed to assess the changes in AF symptoms after ablation.
Conclusion
The results of this post hoc analysis show an inverse association between burden of AT/AF after ablation and the activity level of the patient; the daily activity starts decreasing after a daily burden of 500 minutes and markedly drops after 1000 minutes of AF. Therefore, the amount of AT/AF burden that may impact the activity level seems to be related to hours and not minutes of arrhythmias.
Sources of Funding
The study was supported by a research grant from Medtronic of Canada Inc.
Disclosures
Mr Ziegler is employee and shareholder of Medtronic. Dr Verma has received research grants from Medtronic, Biosense Webster, St. Jude Medical, Bayer, and Bristol‐Myers Squibb and has given presentations for Boeringer Ingelheim and Medtronic. The remaining authors have no disclosures to report.
(J Am Heart Assoc. 2018;7:e010256 DOI: 10.1161/JAHA.118.010256.)
References
- 1. Calkins H, Kuck KH, Cappato R, Brugada J, John Camm A, Chen SA, Crijns HJG, Damiano RJ, Davies DW, DiMarco J, Edgerton J, Ellenbogen K, Ezekowitz MD, Haines DE, Haissaguerre M, Hindricks G, Iesaka Y, Jackman W, Jalife J, Jais P, Kalman J, Keane D, Kim YH, Kirchhof P, Klein G, Kottkamp H, Kumagai K, Lindsay BD, Mansour M, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Nakagawa H, Natale A, Nattel S, Packer DL, Pappone C, Prystowsky E, Raviele A, Reddy V, Ruskin JN, Shemin RJ, Tsao HM, Wilber D. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow‐up, definitions, endpoints, and research trial design. J Interv Card Electrophysiol. 2012;33:171–257. [DOI] [PubMed] [Google Scholar]
- 2. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW; ACC/AHA Task Force Members . 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–2104. [DOI] [PubMed] [Google Scholar]
- 3. Kochhauser S, Joza J, Essebag V, Proietti R, Koehler J, Tsang B, Wulffhart Z, Pantano A, Khaykin Y, Ziegler PD, Verma A. The impact of duration of atrial fibrillation recurrences on measures of health‐related quality of life and symptoms. Pacing Clin Electrophysiol. 2016;39:166–172. [DOI] [PubMed] [Google Scholar]
- 4. Fiala M, Wichterle D, Bulková V, Sknouril L, Nevralová R, Toman O, Dorda M, Januska J, Spinar J. A prospective evaluation of haemodynamics, functional status, and quality of life after radiofrequency catheter ablation of long‐standing persistent atrial fibrillation. Europace. 2014;16:15–25. [DOI] [PubMed] [Google Scholar]
- 5. Proietti M, Boriani G, Laroche C, Diemberger I, Popescu MI, Rasmussen LH, Sinagra G, Dan G‐A, Maggioni AP, Tavazzi L, Lane DA. Self‐reported physical activity and major adverse events in patients with atrial fibrillation: a report from the EURObservational Research Programme Pilot Survey on Atrial Fibrillation (EORP‐AF) General Registry. Europace. 2017;19:535–543. [DOI] [PubMed] [Google Scholar]
- 6. Verma A, Champagne J, Sapp J, Essebag V, Novak P, Skanes A, Morillo CA, Khaykin Y, Birnie D. Discerning the incidence of symptomatic and asymptomatic episodes of atrial fibrillation before and after catheter ablation (DISCERN AF): a prospective, multicenter study. JAMA Intern Med. 2013;173:149–156. [DOI] [PubMed] [Google Scholar]
- 7. Hindricks G, Pokushalov E, Urban L, Taborsky M, Kuck K‐H, Lebedev D, Rieger G, Pürerfellner H. Performance of a new leadless implantable cardiac monitor in detecting and quantifying atrial fibrillation‐results of the XPECT trial. Circ Arrhythmia Electrophysiol. 2010;3:141–147. [DOI] [PubMed] [Google Scholar]
- 8. Shoemaker MJ, Cartwright K, Hanson K, Serba D, Dickinson MG, Kowalk A. Concurrent validity of daily activity data from medtronic ICD/CRT devices and the actigraph GT3X Triaxial accelerometer: a pilot study. Cardiopulm Phys Ther J. 2017;28:3–11. [Google Scholar]
- 9. Cui J. QIC program and model selection in GEE analyses. Stata J. 2007;7:209–220. [Google Scholar]
- 10. Trovato GM, Pace P, Cangemi E, Martines GF, Trovato FM, Catalano D. Gender, lifestyles, illness perception and stress in stable atrial fibrillation. Clin Ter. 2012;163:281–286. [PubMed] [Google Scholar]
- 11. Mantovan R, Macle L, De Martino G, Chen J, Morillo CA, Novak P, Calzolari V, Khaykin Y, Guerra PG, Nair G, Torrecilla EG, Verma A. Relationship of quality of life with procedural success of atrial fibrillation (AF) ablation and postablation AF burden: substudy of the STAR AF randomized trial. Can J Cardiol. 2013;29:1211–1217. [DOI] [PubMed] [Google Scholar]
- 12. Walfridsson H, Walfridsson U, Cosedis Nielsen J, Johannessen A, Raatikainen P, Janzon M, Levin LA, Aronsson M, Hindricks G, Kongstad O, Pehrson S, Englund A, Hartikainen J, Mortensen LS, Hansen PS. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation: results on health‐related quality of life and symptom burden. The MANTRA‐PAF trial. Europace. 2014;17:215–221. [DOI] [PubMed] [Google Scholar]
- 13. Gosselink ATM, Bulsma EB, Landsman MLJ, Cruns H, Lie KI. Long‐term effect of cardioversion on peak oxygen consumption in chronic atrial fibrillation: a 2‐year follow‐up. Eur Heart J. 1994;15:1368–1372. [DOI] [PubMed] [Google Scholar]
- 14. Agostoni P, Emdin M, Corrà U, Veglia F, Magrì D, Tedesco CC, Berton E, Passino C, Bertella E, Re F, Mezzani A, Belardinelli R, Colombo C, La Gioia R, Vicenzi M, Giannoni A, Scrutinio D, Giannuzzi P, Tondo C, Di Lenarda A, Sinagra G, Piepoli MF, Guazzi M. Permanent atrial fibrillation affects exercise capacity in chronic heart failure patients. Eur Heart J. 2008;29:2367–2372. [DOI] [PubMed] [Google Scholar]
- 15. Mohanty S, Santangeli P, Mohanty P, Di Biase L, Holcomb S, Trivedi C, Bai R, Burkhardt D, Hongo R, Hao S, Beheiry S, Santoro F, Forleo G, Gallinghouse JG, Horton R, Sanchez JE, Bailey S, Hranitzky PM, Zagrodzky J, Natale A. Catheter ablation of asymptomatic longstanding persistent atrial fibrillation: impact on quality of life, exercise performance, arrhythmia perception, and arrhythmia‐free survival. J Cardiovasc Electrophysiol. 2014;25:1057–1064. [DOI] [PubMed] [Google Scholar]
- 16. Jones DG, Haldar SK, Hussain W, Sharma R, Francis DP, Rahman‐Haley SL, McDonagh TA, Underwood SR, Markides V, Wong T. A randomized trial to assess catheter ablation versus rate control in the management of persistent atrial fibrillation in heart failure. J Am Coll Cardiol. 2013;61:1894–1903. [DOI] [PubMed] [Google Scholar]
- 17. Hegbom F, Stavem K, Sire S, Heldal M, Orning OM, Gjesdal K. Effects of short‐term exercise training on symptoms and quality of life in patients with chronic atrial fibrillation. Int J Cardiol. 2007;116:86–92. [DOI] [PubMed] [Google Scholar]
- 18. Pathak RK, Elliott A, Middeldorp ME, Meredith M, Mehta AB, Mahajan R, Hendriks JML, Twomey D, Kalman JM, Abhayaratna WP, Lau DH, Sanders P. Impact of CARDIOrespiratory FITness on arrhythmia recurrence in obese individuals with atrial fibrillation the CARDIO‐FIT Study. J Am Coll Cardiol. 2015;66:985–996. [DOI] [PubMed] [Google Scholar]
- 19. Pathak RK, Middeldorp ME, Lau DH, Mehta AB, Mahajan R, Twomey D, Alasady M, Hanley L, Antic NA, McEvoy RD, Kalman JM, Abhayaratna WP, Sanders P. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST‐AF cohort study. J Am Coll Cardiol. 2014;64:2222–2231. [DOI] [PubMed] [Google Scholar]
- 20. Elliott AD, Mahajan R, Pathak RK, Lau DH, Sanders P. Exercise training and atrial fibrillation: further evidence for the importance of lifestyle change. Circulation. 2016;133:457–459. [DOI] [PubMed] [Google Scholar]


