Abstract
Background
Young women with coronary artery disease (CAD), a group with high psychosocial burden, were previously shown to have higher levels of interleukin‐6 (IL‐6) compared with men of similar age. We sought to examine IL‐6 response to acute stress in CAD patients across sex and age, and contrast results to healthy controls and other biomarkers known to increase with mental stress (monocyte chemoattractant protein‐1 and matrix metallopeptidase‐9) and known limited stress‐reactivity (high‐sensitivity C‐reactive protein).
Methods and Results
Inflammatory biomarkers were measured at rest and 90 minutes after mental stress (speech task) among 819 patients with CAD and 89 healthy controls. Repeated‐measures models were used to investigate age (continuous) and sex differences across time, before and after adjusting for demographics, CAD risk factors, depressive symptoms, medication use, and CAD severity. Among patients with CAD, the mean age was 60 years (range, 25–79) and 31% were women. Younger women with CAD had significantly higher concentrations of IL‐6 at rest, 90 minutes after mental stress, as well as a higher response to stress, compared with similarly aged men (P<0.05 for sex by age interactions). In contrast, IL‐6 increased with age, and there were no sex differences in IL‐6 levels or response to stress among controls. Inflammatory responses to stress for high‐sensitivity C‐reactive protein, monocyte chemoattractant protein‐1, and matrix metallopeptidase‐9 among CAD patients were similar in women and men.
Conclusions
IL‐6 response to mental stress are higher in young women with CAD than men of similar age.
Keywords: inflammation, interleukin, mental stress, stress test, women
Subject Categories: Inflammation, Biomarkers, Clinical Studies, Cardiovascular Disease, Women
Clinical Perspective
What Is New?
Younger women with coronary artery disease (CAD) had greater inflammation and inflammatory response to mental stress for interleukin‐6 compared with similarly aged men.
These differences persisted after adjustment for CAD risk factors, including depression and CAD severity.
These differences were not observed among older women and men or among community controls without CAD.
What Are the Clinical Implications?
Young women with CAD should be recognized as a vulnerable group in terms of their basal level of circulating levels of interleukin‐6 and of their enhanced immune reactivity to stress.
Findings from this study raise the possibility that mental stress may increase risk of cardiovascular events among young women through an inflammatory pathway involving interleukin‐6.
Introduction
Proinflammatory biomarkers, including circulating cytokine interleukin‐6 (IL‐6), monocyte chemoattractant protein‐1 (MCP‐1), and matrix metallopeptidase‐9 (MMP‐9), together with acute‐phase C‐reactive protein (CRP), have been shown to predict cardiovascular disease.1, 2, 3, 4, 5, 6, 7, 8 A growing number of studies have shown elevations of inflammatory cytokines in response to an acute laboratory stressor, especially IL‐6, suggesting that inflammation may be one potential pathway by which stress contributes to progression of coronary artery disease (CAD) and triggering of acute coronary syndromes.9, 10, 11, 12
Women have been reported to exhibit higher circulating concentrations of inflammatory markers than men13, 14 and heightened cytokine production in response to psychological stressors.15, 16 Sex‐specific differences in inflammatory response to stress may be important in understanding the pathophysiology and prognosis of CAD among women as demonstrated by recent reports examining mental stress–induced myocardial ischemia,17, 18, 19, 20 and especially young women, a group with high psychosocial burden.19, 21, 22, 23 Compared with men of the same age, young women with CAD have poorer prognosis with an increased risk of adverse cardiovascular outcomes.24, 25, 26, 27, 28 Understanding physiological differences in response to stress may help elucidate potential mechanisms that may increase young women's susceptibility to adverse cardiovascular outcomes.
In the current study, based on a large sample of patients with stable CAD with a broad age range, our goal was to extend previous findings29 and contrast results of other biomarkers with known relevance to CAD, which were recently shown to increase with mental stress in this sample (MCP‐1 and MMP‐9),11 and biomarkers with known limited stress‐reactivity (high‐sensitivity C‐reactive protein; hsCRP)9, 10 across sex and age. To determine whether sex differences in inflammatory biomarker levels are specific for CAD patients or reflect differences between women and men in the general population, we also examined inflammatory responses among a sample of healthy community controls. Given our previous findings that young women post‐MI (myocardial infarction) have higher plasma concentrations of IL‐6 before and after stress testing,29 and previous data including 2 meta‐analyses that showed increased circulating and stimulated concentrations of IL‐6 following exposure to mental stress, but not hsCRP,9, 10, 11 we hypothesized that younger women with CAD, but not controls, would have higher concentrations of IL‐6, and possibly also MCP‐1 and MMP‐9, but not hsCRP, compared with men and older patients.
Methods
Study Design and Participants
The data that support the findings of this study are available from the corresponding author on reasonable request. Between June 2011 and March 2016, we enrolled 949 individuals (626 men, 323 women) with stable CAD in 2 parallel studies with similar protocols, the MIPS (Mental Stress Ischemia Prognosis Study; n=636) and the MIMS2 (Myocardial Infarction and Mental Stress Study 2; n=313).11, 20, 30, 31 Both studies included patients with CAD from Emory University–affiliated hospitals and clinics, shared testing and data collection protocols, as well as study staff, investigators, facilities, and equipment, but there were some differences in the inclusion criteria. For the MIPS, patients were eligible for participation if they were aged between 30 and 79 years and had documented CAD, including any of the following: (1) abnormal coronary angiography or intravascular ultrasound demonstrating atherosclerosis with at least luminal irregularities; (2) previous percutaneous or surgical coronary revascularization; (3) documented MI; or (4) positive exercise or pharmacological nuclear stress test or electrocardiographic exercise stress test.31 Although patients in the MIPS could have angiographic (but not necessarily incident) CAD by history, 73% of patients in the MIPS did have a past history of MI, stroke, heart failure, or angina. For the MIMS2, patients were included if they were between the ages of 18 and 60 year, and were hospitalized for an acute MI within the past 8 months. Diagnosis of MI was verified by medical record review based on standard criteria of troponin levels and ECG changes. The MIMS2 also included 50% women by design. Patients were excluded from both studies if they were pregnant; if they were hospitalized in the previous week for unstable angina, decompensated heart failure, or MI; if they had severe psychiatric conditions such as schizophrenia or alcohol or substance abuse; and if they had active malignancy, end‐stage renal disease, or other severe medical problems expected to shorten life expectancy. We excluded 29 patients from MIMS2 who had any chronic inflammatory conditions of chronic infections (eg, HIV, lupus) because these patients were also excluded in the MIPS. Because overall study procedures and results were similar after first analyzing the studies separately (results not shown), we combined the data from these 2 studies, resulting in 920 patients (636 MIPS and 284 MIMS2). Previously published data showed that patients from the MIPS and the MIMS2 had similar inflammatory response to mental stress.11
During the baseline enrollment visits for the study, clinical information, including previous cardiovascular events, risk factors for CAD, and coronary angiography results, were documented as described below. Patients also underwent mental stress testing following standardized procedures. Medications, including beta blockers, calcium‐channel blockers, as well as long‐acting nitrates, xanthine derivatives, and caffeine‐containing products, were withheld for 24 hours before stress testing. All procedures were in accord with institutional guidelines.
Of 920 CAD patients in the pooled MIPS and MIMS2 data set, 101 patients had missing plasma samples, because of technical difficulties in sample drawing or processing, or the patient refused blood draw. Thus, a total of 819 patients with CAD were included in this analysis. As part of the study design for the MIMS2, 112 young and middle‐aged controls were also recruited in the Atlanta area from a community‐based study of individuals without established CAD. Inclusion criteria for controls were aged between 18 and 60 years and no past history of MI, unstable or stable angina pectoris, congestive heart failure, or stroke. Controls were frequency matched for age and sex to MI cases, with the goal of achieving ≈50% women and a similar mean age in both samples. We excluded 2 controls from the analytical sample who had any chronic inflammatory conditions of chronic infections (eg, HIV, lupus) and restricted the analysis to those with available plasma samples (biomarker data were only available for IL‐6), resulting in a total sample of 89 control subjects for comparison. This research was approved by the Emory University Institutional Review Board. Written informed consent was obtained from all patients and controls enrolled in the study. More detailed information on objectives and study design of these research protocols has been described elsewhere.11, 31, 32
Measurements
Mental stress testing procedure
Patients and controls were tested using a standardized public speaking task after a 30‐minute rest period, in a temperature‐controlled, quiet, and dimly lit room. Briefly, participants were asked to imagine a situation in which a close relative had been mistreated in a nursing home. Participants were given 2 minutes to prepare and 3 minutes to deliver a speech in front of an evaluative audience. Blood pressure and heart rate were recorded throughout.
Myocardial perfusion imaging and single‐photon emission computed tomography images
Although both patients and controls underwent mental stress testing, only patients with CAD underwent myocardial perfusion imaging and were given 20 to 30 mCi of Tc99 m radioisotope at 1 minute into the speech.33 Myocardial perfusion imaging with 99 m‐Tc‐sestamibi single‐photon emission computed tomography was performed at rest and 30 to 60 minutes after mental stress following a standardized protocol. Images were interpreted by 2 experienced readers without knowledge of patients’ medical history, demographic characteristics (eg, sex, race/ethnicity), or severity of CAD. Rest and stress images were visually compared for number and severity of perfusion defects using a 17‐segment model. Specifically, each of the segments was scored from 0 (normal uptake) to 4 (no uptake) as previously described.19, 31, 34 Discrepancies in the interpretation of single‐photon emission computed tomography images were further investigated and resolved after consensus.
Measurement of inflammatory responses
Inflammatory biomarkers were measured from venous blood samples collected at rest and 90 minutes post–mental stress testing, including IL‐6, hsCRP, MCP‐1, and MMP‐9. Only inflammatory data for IL‐6 were available for community controls. Plasma collection time points were selected based on previous studies of mental stress testing, and our own pilot testing, indicating that inflammatory response to stress becomes more apparent 1 hour after mental stress onset.10, 15, 29, 35, 36, 37 Venous blood was collected into ice‐cooled citrate tubes and immediately centrifuged at 4°C; obtained plasma was snap‐frozen at −70°C until further processing. We utilized the MesoScale system (Meso Scale Diagnostics, Rockville, MD) using the SECTOR Imager 2400 to quantitate hsCRP, IL‐6, MCP‐1, and MMP‐9 according to the protocols supplied by the manufacturer. The Mesoscale multiplex assay system uses electrochemiluminescence for high sensitivity and broad dynamic range. All biomarkers were in the range of detection. The inter‐assay coefficient of variations for midpoint standards were 3.06% for hsCRP, 5.78% for IL‐6, 4.99% for MCP‐1, and 9.38% for MMP‐9. Intra‐assay coefficient of variations were 2.33% for hsCRP, 3.29% for IL‐6, 3.45% for MCP‐1, and 5.95% for MMP‐9.
Other measurements
Demographic information was obtained using standardized questionnaires. Previous medical history (diabetes mellitus, hypertension, or previous MI) and medication use (eg aspirin, beta blockers) were obtained by study nurses or physicians through medical history, clinical examinations, and by reviewing medical records. Depressive symptoms were assessed with the Beck Depression Inventory‐II,38 a reliable and valid self‐report measure that has been widely used in cardiac as well as noncardiac patients. Lifetime history of major depression, current major depression, and lifetime/current post‐traumatic stress disorder (PTSD) were assessed using the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition39 by a trained research nurse under the supervision of the study psychiatrist (J.D.B.). We also administered the Cohen's Perceived Stress Scale,40 a 10‐item survey of general stress validated in multiethnic populations. Height and weight were objectively measured during the clinical exam and used to calculate body mass index (kg/m2). Angiographic data for CAD patients were obtained from the most recent coronary angiogram in the patient's chart. CAD severity was measured using the Gensini Score.41
Statistical Analyses
Descriptive statistics were compared between men and women using t tests or Mann–Whitney–Wilcoxon tests for continuous variables and chi‐square tests or Fisher's exact test for categorical variables. Given the skewed distributions of inflammatory markers (IL‐6, hsCRP, MCP‐1, and MMP‐9), natural log transformations were used in all analyses. Results for inflammatory markers are presented as geometric means.
We examined concentrations of IL‐6, hsCRP, MCP‐1, and MMP‐9 before and 90 minutes after mental stress testing using linear mixed models for repeated measures. To determine whether baseline levels and inflammatory response to stress differed by age and sex, we included time‐by‐age, time‐by‐sex, age‐by‐sex, and time‐by‐age‐by‐sex interactions in the repeated‐measures analyses. Age was examined as a continuous variable. We estimated linear combinations of the regression coefficients for age across sex and time. We further tested differences in slopes for age between women and men. For descriptive purposes, mean concentrations of IL‐6, hsCRP, MCP‐1, and MMP‐9 were calculated at 40, 50, 60, 70, and 80 years of age from models including age as a continuous variable.
We also expressed the results in terms of inflammatory response to stress for IL‐6, hsCRP, MCP‐1, and MMP‐9, calculated as (natural log) differences between 90 minutes post–mental stress and resting values, in mixed models for repeated measures. The antilog of this difference, or exp(loge(post stress values)−loge(resting values)), is equal to the antilog of the ratio, or exp(loge(post stress value/resting value)), and can be interpreted as the percent increase in inflammatory response with stress from baseline values.
All analyses were conducted before and after adjusting for possible confounding factors considered a priori, including demographics factors (age, race, and education), lifestyle and clinical risk factors known to affect inflammation (current smoking, body mass index, diabetes mellitus, hypertension, previous MI, heart failure, and major depression), as well as medication use (aspirin, beta‐blockers, statins, angiotensin‐converting enzyme inhibitors, and antidepressants). To determine whether inflammatory concentrations were confounded by severity of CAD, a subsequent model further adjusted for summed resting score, a quantitative measure of perfusion defect. Also, to minimize any potential batch effect, we deemed important to include biomarker plate as a random effect in our models to account for any correlation of values related to how the samples were run or prepared in the laboratory. Thus, we further included plate effect as a random intercept in a final set of models. We also adjusted final models for study source. The significance level for main effects was set at P<0.05, whereas the statistical significance of interaction effects was set at P<0.10. In exploratory analysis restricted to only women, we also investigated whether age differences in inflammation and response over time were moderated or explained for by postmenopausal status. We also explored whether adjusting (separately) for other psychosocial variables (current major depression, depressive symptoms, lifetime or current history of PTSD, PTSD symptoms, and perceived stress) would confound or change the results given that young women with CAD have high psychosocial burden.19, 21, 22, 23 All analyses were repeated among community controls. All statistical analyses were conducted using SAS software (version 9.4; SAS Institute Inc, Cary, NC).
Results
Patients With CAD
Descriptive characteristics
The analytic sample included 819 patients, of which 31% were women (n=257). Female sex was significantly associated with younger age, black race, and lower educational attainment (Table 1). Female sex was also significantly associated with higher body mass index, a previous MI, a history of major depression and diabetes mellitus, and taking beta blockers and antidepressants. Male sex, however, was significantly associated with dyslipidemia, a higher Gensini Score (a measure of CAD severity), and taking angiotensin‐converting enzyme inhibitors.
Table 1.
Descriptive Characteristics for Patients and Community Controls, MIPS and MIMS2
| Patients (n=819) | Controls (n=98) | |||||
|---|---|---|---|---|---|---|
| Women | Men | P Valuea | Women | Men | P Valuea | |
| Total | 257 | 562 | 50 | 48 | ||
| Demographics | ||||||
| Age, y, mean (SD) | 57.6 (10.7) | 60.9 (9.7) | <0.0001 | 50.2 (7.9)c | 48.0 (10.7)c | 0.25 |
| Age >50 y, n (%) | 198 (77.0) | 486 (86.5) | 0.001 | 28 (56.0)b | 29 (60.4)c | 0.66 |
| Black, n (%) | 141 (54.9) | 160 (28.4) | <0.0001 | 22 (44.0) | 16 (33.3) | 0.28 |
| Education high school or less | 84 (32.9) | 158 (28.4) | 0.19 | 4 (8.2)b | 5 (10.9)b | 0.73 |
| Psychosocial risk factors | ||||||
| Lifetime history major depression, n (%) | 105 (42.0) | 127 (23.4) | <0.0001 | 11 (22.0)b | 8 (16.7) | 0.50 |
| Current major depression, n (%) | 39 (15.7) | 39 (7.3) | 0.0002 | 1 (2.0)b | 2 (4.2) | 0.61 |
| Beck Depression Inventory, mean (SD) | 12.3 (10.3) | 8.1 (8.5) | <0.0001 | 5.4 (7.2)c | 6.7 (7.3) | 0.38 |
| Lifetime history of PTSD, n (%) | 30 (12.1) | 43 (7.9) | 0.06 | 2 (4.0) | 3 (6.3) | 0.67 |
| Current PTSD, n (%) | 21 (8.4) | 30 (5.5) | 0.12 | 2 (4.0) | 2 (4.2) | 0.99 |
| PTSD symptom checklist, mean (SD) | 31.1 (13.3) | 26.6 (11.9) | <0.0001 | 23.0 (9.7)c | 25.3 (12.3) | 0.31 |
| Perceived Stress Scale, n (%) | 15.9 (8.6) | 12.3 (7.7) | <0.0001 | 10.3 (6.6)c | 10.9 (6.5) | 0.64 |
| Cardiovascular risk factors | ||||||
| BMI, kg/m2, mean (SD) | 31.8 (7.7) | 29.5 (5.0) | <0.0001 | 28.7 (6.9)b | 28.6 (5.0) | 0.93 |
| Current smoker, n (%) | 40 (15.7) | 81 (14.7) | 0.71 | 2 (4.2)b | 2 (4.3)b | 0.99 |
| Diabetes mellitus, n (%) | 98 (38.1) | 170 (30.3) | 0.03 | 4 (8.0)c | 3 (6.3)b | 0.99 |
| Hypertension, n (%) | 206 (80.2) | 422 (75.1) | 0.11 | 15 (30.0)c | 14 (29.2)c | 0.93 |
| Dyslipidemia, n (%) | 199 (77.4) | 471 (83.8) | 0.03 | 17 (34.0)c | 12 (25.0)c | 0.33 |
| Medications | ||||||
| Aspirin, n (%) | 210 (82.0) | 481 (85.9) | 0.16 | 6 (12.5)c | 3 (6.1)c | 0.32 |
| Beta blocker, n (%) | 211 (82.4) | 420 (75.0) | 0.02 | 3 (6.1)c | 2 (4.2)c | 0.99 |
| ACE inhibitors, n (%) | 96 (37.5) | 279 (49.8) | 0.001 | 4 (8.2)c | 8 (16.7)c | 0.23 |
| Antidepressant, n (%) | 78 (30.5) | 106 (18.9) | 0.0003 | 11 (22.5) | 4 (8.3) | 0.09 |
| Statins, n (%) | 210 (82.0) | 487 (87.0) | 0.06 | 6 (12.2)c | 8 (16.7)c | 0.58 |
| Clinical characteristics (patients only) | ||||||
| Previous MI, n (%) | 163 (63.4) | 265 (47.2) | <0.0001 | ··· | ··· | ··· |
| Heart failure, n (%) | 41 (16.0) | 110 (19.6) | 0.22 | ··· | ··· | ··· |
| Revascularization, n (%) | 198 (77.0) | 429 (76.3) | 0.82 | ··· | ··· | ··· |
| Mental stress–induced ischemia, n (%) | 42 (16.8) | 92 (16.5) | 0.92 | ··· | ··· | ··· |
| Gensini Score, median (IQR) | 19.0 (6.0, 47.5) | 30.5 (10.0, 67.0) | 0.0002 | ··· | ··· | ··· |
| Ejection fraction, mean (SD) | 63.2 (16.7) | 60.6 (14.7) | 0.03 | ··· | ··· | ··· |
| SPECT Summed Rest Score, mean (SD) | 3.1 (6.1) | 5.4 (8.8) | <0.0001 | ··· | ··· | ··· |
ACE indicates angiotensin‐converting enzyme; BMI, body mass index; IQR, interquartile range; MI, myocardial infarction; MIMS2, Myocardial Infarction and Mental Stress Study 2; MIPS, Mental Stress Ischemia Prognosis Study; PTSD, post‐traumatic stress disorder; SPECT, single‐photon emission computed tomography.
Statistical tests: categorical variables: chi‐square or Fisher's exact test; continuous variables: Student t test or Wilcoxon–Mann–Whitney U test, when appropriate.
P<0.05 between patients and controls within sex.
P<0.0001 between patients and controls within sex.
Compared with women in the control sample, women with CAD were significantly associated with older age, lower educational attainment, and a more adverse psychosocial profile (more depression, more PTSD symptoms, and more perceived stress). As expected, women with CAD were significantly associated with cardiovascular risk factors and taking aspirin, beta blockers, angiotensin‐converting enzyme inhibitors, and statins. Whereas similar differences in sociodemographic and cardiovascular risk factors were noted comparing men with CAD with male controls, there were no significant differences in distribution of psychosocial factors between these 2 groups.
Descriptive inflammatory profiles of patients at rest and 90 minutes post‐stress by sex are presented in Table 2. At both time points, women had significantly higher levels of IL‐6, hsCRP, and MCP‐1, whereas men had higher levels of MMP‐9.
Table 2.
Descriptive Inflammatory Profiles at Rest and 90 Minutes After Mental Stress by Sex Among Patients With CAD, MIPS and MIMS2 Combined Cohorts
| Women | Men | P Value | |||
|---|---|---|---|---|---|
| N | Geometric Mean (95% CI) | N | Geometric Mean (95% CI) | ||
| IL‐6, pg/mL | |||||
| Rest | 257 | 1.75 (1.62, 1.89) | 561 | 1.40 (1.33, 1.47) | <0.0001 |
| 90 min | 217 | 2.48 (2.27, 2.71) | 515 | 1.82 (1.72, 1.93) | <0.0001 |
| hsCRP, mg/L | |||||
| Rest | 256 | 2.52 (2.14, 2.98) | 559 | 1.60 (1.43, 1.79) | <0.0001 |
| 90 min | 216 | 2.17 (1.82, 2.59) | 512 | 1.51 (1.35, 1.69) | 0.001 |
| MCP‐1, pg/mL | |||||
| Rest | 257 | 136 (132, 141) | 562 | 118 (115, 120) | <0.0001 |
| 90 min | 217 | 142 (136, 147) | 515 | 123 (120, 126) | <0.0001 |
| MMP‐9, ng/mL | |||||
| Rest | 257 | 57.5 (53.2, 62.2) | 562 | 65.3 (61.9, 68.8) | 0.01 |
| 90 min | 217 | 59.6 (54.8, 64.8) | 515 | 72.0 (68.2, 76.0) | 0.0002 |
Values reported are geometric mean concentrations of IL‐6, HsCRP, MCP‐1, and MMP‐9. CAD indicates coronary artery disease; CI, confidence interval; hsCRP, high‐sensitivity C‐reactive protein; IL‐6, interleukin‐6; MCP‐1, monocyte chemoattractant protein‐1; MIMS2, Myocardial Infarction and Mental Stress Study 2; MIPS, Mental Stress Ischemia Prognosis Study; MMP‐9, matrix metallopeptidase 9.
Inflammatory profiles at rest and post‐stress
Sex differences for IL‐6 were more prominent for younger women in unadjusted models at rest and post‐stress (Figures 1 and 2), also showing a steeper age‐related slope in women than men, especially for post‐stress values (Figure 2). After adjusting for additional demographic factors (race and education), lifestyle and clinical risk factors for increased inflammation (current smoking, body mass index, diabetes mellitus, hypertension, previous MI, heart failure, and lifetime history of depression), as well as medication use (aspirin, beta‐blocker, statins, angiotensin‐converting enzyme inhibitors, and antidepressants), concentrations of IL‐6 were significantly higher for women at rest and post‐stress compared with similarly aged men at 40, 50, and 60 years of age, but not at 70 or 80 years of age (Table 3). Substituting other psychosocial variables in place of lifetime history of depression did not substantially change the results. Results were similar in a subsequent model that further adjusted for summed resting score, a quantitative measure of perfusion deficit, plate effect, and study source. Thus, these results did not appear confounded by severity of CAD. Furthermore, in exploratory analysis restricted to only women, we did not find that differences in IL‐6 by age were moderated or explained for by postmenopausal status. Interaction terms of sex‐by‐age, sex‐by‐time, and age‐by‐time had P<0.05, indicating that participants’ inflammatory reactivity to mental stress for IL‐6 across time differed by age and also by sex, that is, the slopes for age were significantly different by sex. Sex‐by‐age‐by‐time interactions were <0.10 in all models, indicating that participants’ inflammatory reactivity for IL‐6 differed by age and sex across time.
Figure 1.
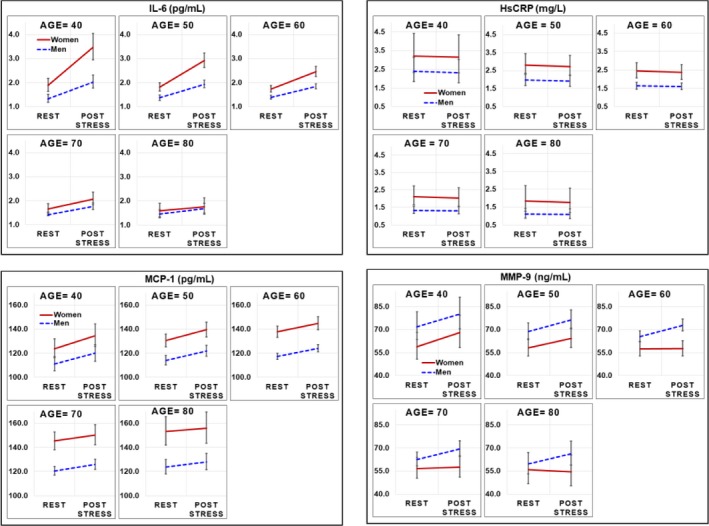
Unadjusted geometric mean plasma concentrations and 95% confidence intervals for IL‐6, hsCRP, MCP‐1, and MMP‐9 at specified values of age (40, 50, 60, 70, and 80 years) across sex and time among women and men with CAD from MIPS and MIMS2 combined cohorts. Repeated‐measures models were used to investigate age and sex differences across time, testing for interaction of age (continuous) and sex. Natural log values modeled and presented as geometric means. CAD indicates coronary artery disease; hsCRP, high‐sensitivity C‐reactive protein; IL‐6, interleukin‐6; MCP‐1, monocyte chemoattractant protein‐1; MIMS2, Myocardial Infarction and Mental Stress Study 2; MIPS, Mental Stress Ischemia Prognosis Study; MMP‐9, matrix metallopeptidase 9.
Figure 2.
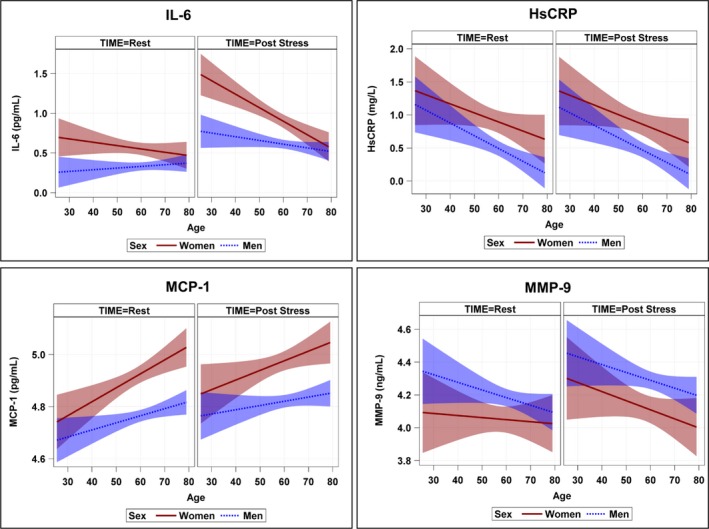
Unadjusted regression slopes for age across sex and time for IL‐6, hsCRP, MCP‐1, and MMP‐9 among women and men with CAD from MIPS and MIMS2 combined cohorts. Natural log values modeled using repeated‐measures analysis for inflammatory outcome measures of IL‐6, hsCRP, MCP‐1, and MMP‐9 with 95% confidence intervals. Repeated‐measures models were used to investigate age and sex differences across time, testing for interaction of age (continuous) and sex. CAD indicates coronary artery disease; hsCRP, high‐sensitivity C‐reactive protein; IL‐6, interleukin‐6; MCP‐1, monocyte chemoattractant protein‐1; MIMS2, Myocardial Infarction and Mental Stress Study 2; MIPS, Mental Stress Ischemia Prognosis Study; MMP‐9, matrix metallopeptidase 9.
Table 3.
Unadjusted and Adjusted Geometric Mean Plasma Concentrations of IL‐6 at Specified Values of Age (40, 50, 60, 70, and 80 years) Across Sex and Time Among Women and Men With CAD, MIPS and MIMS2 Combined Cohortsa
| Rest | 90 Minutes Post‐Stress | |||||
|---|---|---|---|---|---|---|
| Women | Men | P Value | Women | Men | P Value | |
| Geometric Mean (95% CI) | Geometric Mean (95% CI) | Geometric Mean (95% CI) | Geometric Mean (95% CI) | |||
| IL‐6, pg/mL | ||||||
| Model 1 | ||||||
| Age=40 y | 1.89 (1.63, 2.18) | 1.33 (1.18, 1.51) | 0.0004 | 3.46 (2.95, 4.05)c | 2.02 (1.77, 2.31)c | <0.0001 |
| Age=50 y | 1.81 (1.65, 1.98) | 1.36 (1.26, 1.47) | <0.0001 | 2.92 (2.63, 3.23)c | 1.93 (1.78, 2.10)c | <0.0001 |
| Age=60 y | 1.73 (1.60, 1.87) | 1.39 (1.32, 1.47) | <0.0001 | 2.46 (2.26, 2.68)c | 1.84 (1.74, 1.95)c | <0.0001 |
| Age=70 y | 1.66 (1.48, 1.87) | 1.42 (1.32, 1.52) | 0.02 | 2.07 (1.83, 2.35)c | 1.76 (1.63, 1.90)c | 0.03 |
| Age=80 y | 1.59 (1.34, 1.90) | 1.45 (1.30, 1.63) | 0.38 | 1.75 (1.44, 2.12) | 1.68 (1.49, 1.89)b | 0.72 |
| Model 2 | ||||||
| Age=40 y | 1.88 (1.58, 2.25) | 1.43 (1.23, 1.67) | 0.01 | 3.49 (2.89, 4.22)c | 2.19 (1.86, 2.57)c | <0.0001 |
| Age=50 y | 1.92 (1.69, 2.19) | 1.56 (1.39, 1.76) | 0.001 | 3.12 (2.73, 3.58)c | 2.23 (1.97, 2.51)c | <0.0001 |
| Age=60 y | 1.71 (1.54, 1.89) | 1.96 (1.76, 2.20) | 0.01 | 2.80 (2.49, 3.15)c | 2.26 (2.04, 2.51)c | <0.0001 |
| Age=70 y | 2.00 (1.74, 2.31) | 1.86 (1.65, 2.10) | 0.29 | 2.50 (2.15, 2.91)c | 2.30 (2.03, 2.60)c | 0.26 |
| Age=80 y | 2.05 (1.68, 2.49) | 2.03 (1.74, 2.37) | 0.95 | 2.24 (1.81, 2.76) | 2.34 (1.98, 275)b | 0.71 |
| Model 3 | ||||||
| Age=40 y | 1.89 (1.58, 2.25) | 1.44 (1.23, 1.67) | 0.01 | 3.48 (2.89, 4.22)c | 2.20 (1.87, 2.58)c | <0.0001 |
| Age=50 y | 1.93 (1.69, 2.19) | 1.57 (1.40, 1.76) | 0.001 | 3.13 (2.73, 3.58)c | 2.23 (1.98, 2.52)c | <0.0001 |
| Age=60 y | 1.96 (1.75, 2.20) | 1.71 (1.54, 1.90) | 0.01 | 2.80 (2.49, 3.15)c | 2.27 (2.04, 2.52)c | 0.0001 |
| Age=70 y | 2.00 (1.74, 2.30) | 1.87 (1.66, 2.11) | 0.33 | 2.51 (2.16, 2.94)c | 2.31 (2.04, 2.61)c | 0.26 |
| Age=80 y | 2.04 (1.67, 2.48) | 2.04 (1.74, 2.39) | 0.99 | 2.25 (1.82, 2.78) | 2.34 (1.99, 2.77)b | 0.73 |
| Model 4 | ||||||
| Age=40 y | 1.70 (1.40, 2.05) | 1.36 (1.15, 1.61) | 0.01 | 3.14 (2.58, 3.84)c | 2.08 (1.74, 2.48)c | <0.0001 |
| Age=0 y | 1.88 (1.61, 2.19) | 1.58 (1.36, 1.82) | 0.003 | 3.06 (2.62, 3.58)c | 2.25 (1.94, 2.60)c | <0.0001 |
| Age=60 y | 2.08 (1.80, 2.40) | 1.83 (1.59, 2.10) | 0.005 | 2.98 (2.57, 3.45)c | 2.42 (2.10, 2.79)c | <0.0001 |
| Age=70 y | 2.30 (1.95, 2.72) | 2.11 (1.81, 2.47) | 0.17 | 2.90 (2.44, 3.45)c | 2.62 (2.24, 3.06)c | 0.14 |
| Age=80 y | 2.55 (2.06, 3.16) | 2.45 (2.03, 2.95) | 0.68 | 2.83 (2.26, 3.53) | 2.83 (2.33, 3.42)b | 0.99 |
Model 1 adjusted for: age, sex, time, age×sex, age×time, sex×time, and age×sex×time. Model 2 adjusted for: model 1 covariates+race, education, diabetes mellitus, hypertension, body mass index (continuous), lifetime history of depression, smoking status, aspirin, beta blocker, statins, angiotensin‐converting enzyme inhibitors, antidepressants, previous myocardial infarction, and heart failure. Model 3 adjusted for: model 2 covariates+summed rest score. Model 4 adjusted for: model 3 covariates+plate effect and data source. CAD indicates coronary artery disease; CI, confidence interval; IL‐6, interleukin‐6; MIMS2, Myocardial Infarction and Mental Stress Study 2; MIPS, Mental Stress Ischemia Prognosis Study.
A natural log transformation was used for biomarker values as outcome using repeated‐measures analyses.
P<0.05 between baseline and 90 minutes within sex.
P<0.0001 between baseline and 90 minutes within sex.
In contrast to IL‐6, slopes for age regressed on hsCRP, MCP‐1, and MMP‐9 were not significantly different between women and men at either rest or 90 minutes post‐stress (Figure 2). Estimated geometric mean concentrations across time and sex for specified values of age (Table S1) showed that, with the exception of MCP‐1, the other biomarkers of inflammation all tended to decrease with age, in a similar fashion in women and men.
Inflammatory response (difference between post‐stress values and resting values)
When results were expressed in terms of the difference between post‐stress values and resting values within the mixed models for repeated measures, conclusions remained consistent. Inflammatory response for IL‐6 was significantly higher for younger women (at calculated values of age at 40, 50, and 60 years), but not among men (Figure S1), and age slopes were significantly different by sex (Figure S2; Table S2). More specifically, at 40 years of age, inflammatory response for women at 90 minutes post‐stress was 83% higher compared with baseline values, whereas inflammatory response for men was 52% (P=0.01; Table 4). Inflammatory response for women versus men at 50 years of age was 61% versus 42% (P=0.005) and at 60 years of age was 42% versus 32% (P=0.05). Results were similar in adjusted models (Table 4). There were no differences in inflammatory response for other inflammatory biomarkers by age and sex, with the exception of MMP‐9 for older participants (Figures S1 and S2; Tables S2 and S3).
Table 4.
Unadjusted and Adjusted Inflammatory Response of IL‐6 (pg/mL) by Sex at Specified Values of Age (40, 50, 60, 70, and 80 Years) Among Patients With CAD, MIPS and MIMS2 Combined Cohorts
| Outcome: Inflammatory Responsea | |||
|---|---|---|---|
| Women | Men | P Value | |
| Ratio (95% CI) | Ratio (95% CI) | ||
| Model 1 | |||
| Age=40 y | 1.83 (1.64, 2.04) | 1.52 (1.39, 1.66) | 0.01 |
| Age=50 y | 1.61 (1.50, 1.73) | 1.42 (1.34, 1.50) | 0.01 |
| Age=60 y | 1.42 (1.34, 1.50) | 1.32 (1.27, 1.37) | 0.05 |
| Age=70 y | 1.25 (1.14, 1.36) | 1.24 (1.18, 1.30) | 0.86 |
| Age=80 y | 1.10 (0.96, 1.25) | 1.16 (1.06, 1.25) | 0.51 |
| Model 2 | |||
| Age=40 y | 1.85 (1.66, 2.07) | 1.53 (1.39, 1.68) | 0.01 |
| Age=50 y | 1.62 (1.51, 1.75) | 1.42 (1.34, 1.51) | 0.01 |
| Age=60 y | 1.42 (1.34, 1.51) | 1.33 (1.28, 1.38) | 0.05 |
| Age=70 y | 1.25 (1.14, 1.36) | 1.23 (1.17, 1.30) | 0.83 |
| Age=80 y | 1.09 (0.96, 1.25) | 1.15 (1.06, 1.25) | 0.54 |
| Model 3 | |||
| Age=40 y | 1.85 (1.65, 2.07) | 1.53 (1.39, 1.68) | 0.01 |
| Age=50 y | 1.62 (1.51, 1.74) | 1.42 (1.34, 1.51) | 0.01 |
| Age=60 y | 1.43 (1.35, 1.52) | 1.33 (1.28, 1.38) | 0.04 |
| Age=70 y | 1.26 (1.15, 1.37) | 1.23 (1.17, 1.30) | 0.73 |
| Age=80 y | 1.11 (0.97, 1.27) | 1.15 (1.06, 1.25) | 0.63 |
| Model 4 | |||
| Age=40 y | 1.85 (1.66, 2.07) | 1.53 (1.39, 1.68) | 0.01 |
| Age=50 y | 1.63 (1.52, 1.75) | 1.42 (1.34, 1.51) | 0.01 |
| Age=60 y | 1.43 (1.35, 1.52) | 1.33 (1.28, 1.38) | 0.04 |
| Age=70 y | 1.26 (1.15, 1.38) | 1.24 (1.17, 1.30) | 0.75 |
| Age=80 y | 1.11 (0.97, 1.27) | 1.15 (1.06, 1.26) | 0.61 |
Model 1 adjusted for: age, sex, time, age×sex, age×time, sex×time, and age×sex×time. Model 2 adjusted for: model 1 covariates+race, education, diabetes mellitus, hypertension, body mass index (continuous), lifetime history of depression, smoking status, aspirin, beta blocker, statins, angiotensin‐converting enzyme inhibitors, antidepressants, previous myocardial infarction, and heart failure. Model 3 adjusted for: model 2 covariates+summed rest score. Model 4 adjusted for model 3 covariates+plate effect and data source. CAD indicates coronary artery disease; CI, confidence interval; IL‐6, interleukin‐6; MIMS2, Myocardial Infarction and Mental Stress Study 2; MIPS, Mental Stress Ischemia Prognosis Study.
A natural log transformation was used for biomarker values in analyses. Inflammatory response calculated as: exp(loge(Post stress values)−loge(resting values))=exp(loge(Post stress value/resting value)) and can be interpreted as a ratio.
Community Controls
There were 98 community controls, with a mean age of 49.2 (SD, 9.4; age range, 22–61), of which 51% (n=50) were women and 39% (n=38) were black. There were no significant differences between women and men across demographic or medical and lifestyle factors (Table 1) or for IL‐6 at rest or 90 minutes post‐stress (Table S4). In general, IL‐6 levels were lower in controls than in CAD patients both at baseline and after stress. Concentrations of IL‐6 among control subjects increased with age for both women and men, both at rest and 90 minutes post‐stress, and there were no significant differences in age slopes between women and men at either time point (Table 5; Figure 3A and 3B). IL‐6 response to stress also increased with age in both women and men, with no sex differences across age (Table S5; Figure 3C and 3D).
Table 5.
Unadjusted and Adjusted Geometric Mean Plasma Concentrations of IL‐6 Specified Values of Age (30, 40, 50, and 60 Years) Across Sex and Time Among Community Controls, MIMS2 Cohorta
| Rest | 90 Minutes Post‐Stress | |||||
|---|---|---|---|---|---|---|
| Women | Men | P Value | Women | Men | P Value | |
| Geometric Mean (95% CI) | Geometric Mean (95% CI) | Geometric Mean (95% CI) | Geometric Mean (95% CI) | |||
| IL‐6, pg/mL | ||||||
| Model 1 | ||||||
| Age=30 y | 0.90 (0.55, 1.45) | 0.69 (0.48, 0.98) | 0.38 | 1.42 (0.79, 2.54) | 1.36 (0.91, 2.05)b | 0.91 |
| Age=40 y | 0.98 (0.74, 1.31) | 0.84 (0.67, 1.05) | 0.39 | 1.70 (1.20, 2.39)b | 1.58 (1.22, 2.04)c | 0.74 |
| Age=50 y | 1.08 (0.90, 1.28) | 1.02 (0.85, 1.23) | 0.69 | 2.02 (1.66, 2.47)c | 1.83 (1.49, 2.24)c | 0.48 |
| Age=60 y | 1.18 (0.89, 1.56) | 1.25 (0.96, 1.63) | 0.77 | 2.41 (1.73, 3.36)c | 2.12 (1.56, 2.87)b | 0.57 |
| Model 2 | ||||||
| Age=30 y | 1.00 (0.49, 2.04) | 0.98 (0.54, 1.79) | 0.95 | 1.51 (0.68, 3.34) | 1.91 (1.01, 3.60)b | 0.51 |
| Age=40 y | 1.10 (0.62, 1.93) | 1.10 (0.66, 1.84) | 0.98 | 1.86 (1.02, 3.40)b | 2.05 (1.21, 3.47)c | 0.66 |
| Age=50 y | 1.21 (0.75, 1.95) | 1.24 (0.77, 1.98) | 0.84 | 2.30 (1.41, 3.77)c | 2.20 (1.37, 3.54)c | 0.75 |
| Age=60 y | 1.32 (0.81, 2.17) | 1.39 (0.85, 2.26) | 0.81 | 2.84 (1.67, 4.81)c | 2.36 (1.43, 3.89)b | 0.42 |
| Model 3 | ||||||
| Age=30 y | 1.02 (0.50, 2.07) | 0.99 (0.54, 1.80) | 0.92 | 1.54 (0.70, 3.39) | 1.92 (1.02, 3.61)b | 0.53 |
| Age=40 y | 1.11 (0.63, 1.94) | 1.10 (0.66, 1.84) | 0.98 | 1.88 (1.03, 3.43)c | 2.05 (1.21, 3.46)b | 0.69 |
| Age=50 y | 1.21 (0.75, 1.95) | 1.23 (0.77, 1.97) | 0.88 | 2.30 (1.40, 3.78)c | 2.18 (1.35, 3.52)c | 0.71 |
| Age=60 y | 1.31 (0.80, 2.16) | 1.37 (0.84, 2.24) | 0.83 | 2.81 (1.66, 4.78)c | 2.33 (1.41, 3.85)b | 0.40 |
Model 1 adjusted for: age, sex, time, age×sex, age×time, sex×time, and age×sex×time. Model 2 adjusted for: model 1 covariates+race, education, diabetes mellitus, hypertension, body mass index (continuous), lifetime history of depression, smoking status, aspirin, beta blocker, statins, angiotensin‐converting enzyme inhibitors, and antidepressants. Model 3 adjusted for: model 3 covariates+plate effect. CI indicates confidence interval; IL‐6, interleukin‐6; MIMS2, Myocardial Infarction and Mental Stress Study 2.
A natural log transformation was used for biomarker values as outcome using repeated‐measures analyses.
P<0.05 between baseline and 90 minutes within sex.
P<0.0001 between baseline and 90 minutes within sex.
Figure 3.
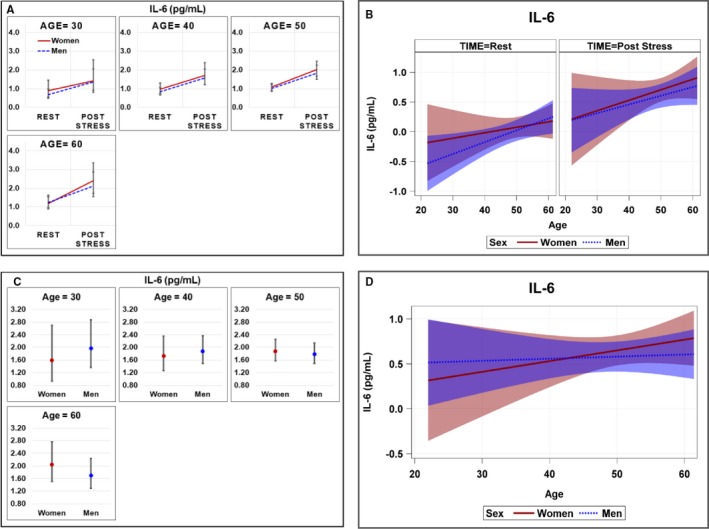
Geometric mean plasma concentrations and regression slopes with 95% confidence intervals of IL‐6 among women and men community controls. A, Geometric mean plasma concentrations at specified values of age (30, 40, 50, and 60 years) across sex and time; (B) regression slopes for age across sex and time; (C) inflammatory response; and (D) regression slopes for inflammatory response across age and sex. Repeated‐measures models were used to investigate age and sex differences across time testing for interaction of age (continuous) and sex. Inflammatory response was calculated as: (exp (loge(Post stress values)−loge(resting values)) within the repeated‐measures model. Natural log values modeled and presented as geometric means in (A) and (C). IL‐6 indicates interleukin‐6.
Discussion
In the present study, we found that younger women with CAD have significantly higher concentrations of IL‐6 before and after mental stress, as well as a higher response to stress, compared with similarly aged men with CAD. These differences persisted after adjustment for CAD risk factors, including depression and CAD severity. Importantly, these differences were not observed among older women and men or among controls without CAD. Although MCP‐1 and MMP‐9 also increased with mental stress among CAD patients, the pattern of increase was similar by sex across age, and there were no significant differences in inflammatory response for age and sex with the exception of MMP‐9 in adjusted models for older participants. HsCRP did not increase with mental stress irrespective of sex and age.
Our results are in agreement with our previous findings in a small sample of post‐MI patients, where we reported, for the first time, that women aged ≤50 years, but not older women, had higher plasma concentrations of IL‐6 before and 90 minutes after mental stress testing compared with age‐matched men.29 In the present analysis, we were able to replicate these findings using a larger sample of patients with CAD with a broader age range, included data from community controls, and examined a larger panel of inflammatory markers. In this comprehensive study, we were able to demonstrate not only that young women with CAD have higher IL‐6 levels than men of similar age, but they also show an enhanced IL‐6 response to mental stress compared with men. Importantly, these results were only found among patients with CAD, and not among the community controls. In contrast to patients with CAD, IL‐6 increased with age in the healthy sample of community controls. Other studies have also found that serum levels of IL‐6 tend to increase with age in healthy populations.42 In addition, we found that patients with CAD have higher baseline levels of IL‐6 and greater inflammatory response to mental stress than controls.
Our results are consistent with the only other study that has investigated inflammatory responses to mental stress among CAD patients and controls.43 That study found IL‐6 increases after mental stress among both patients with CAD and controls. Consistent with our results, they also found a tendency for patients to have a higher inflammatory response than controls. Our findings are also consistent with a recent systematic review and meta‐analysis on the effects of acute psychological stress on circulating and stimulated inflammatory markers among healthy and clinical populations, which found that IL‐6 showed among the most robust and consistent associations with stress.10 Age and sex differences, however, were not investigated within these aforementioned studies. Importantly, in most studies, the largest effect for IL‐6 following stress was at 90 minutes post‐stress,10 which supports our plasma collection time point at 90 minutes.
In our study, we also found that, whereas women and younger patients with CAD had higher concentrations of hsCRP at baseline and 90 minutes after mental stress, there were no significant differences in slopes across age or sex for both baseline values and 90 minutes post‐stress, and virtually no change in hsCRP with stress irrespective of age or sex. Kop et al43 did find an increase in CRP 90 minutes after mental stress challenge among patients with CAD. However, the bulk of the literature points to lack of change in CRP in response to stress. Marsland et al10 examined the results of 6 studies and nine unique samples, and found no evidence for a significant change in CRP from baseline to 0 to 10, 20 to 30, or 20 to 120 minutes post‐stress. CRP is produced primarily in the liver in response to IL‐6.44, 45 Although it is possible that a longer time is needed to capture the peak CRP response to stress, the fact that no effect is noted even at 120 minutes up to 24 hours post‐stress suggests low reactivity rather than a delayed response.37, 46
The underlying explanation for age and sex differences in the proinflammatory cytokine, IL‐6, among CAD patients remains unknown. A possibility may be sex differences in glucocorticoid receptor sensitivity, which may affect proinflammatory cytokine production after stress, and perhaps may be more pronounced in patients with CAD than community controls. A study conducted by Rohleder et al16 showed that men had a significant increase in glucocorticoid sensitivity 1 hour after mental stress, whereas women showed a slight decrease. An increase in glucocorticoid sensitivity would inhibit proinflammatory cytokine production and thereby terminate an inflammatory response timely in men, whereas a decrease in glucocorticoid sensitivity among women would maintain or further stimulate an inflammatory response.16 As a consequence, the male response pattern may protect the body from tissue damage and other adverse effects of systematic elevations of proinflammatory cytokines after acute psychological stress, whereas women may be more susceptible to them.16 Research also suggests that estrogens may enhance cytokine production,47 thereby stimulating secretion of proinflammatory cytokines among women. A decrease in estrogen levels among older women because of their postmenopausal status may thereby result in lower secretion of proinflammatory cytokines, explaining why we did not observe age and sex differences in concentrations of IL‐6 before and after stress testing among older women. However, there is still an unresolved paradox about the role of estrogens in inflammation.42 In exploratory analysis restricted to only women, however, we did not find that differences in IL‐6 by age were moderated or explained by postmenopausal status. Given that postmenopausal status was self‐reported, an investigation of estrogen and other sex hormones as potential mechanisms for age and sex differences in IL‐6 among CAD patients as well as controls deserves further attention. Our results were also independent of lifetime history of depression given that young women with CAD are a group with high psychosocial burden. In exploratory analyses, adjustment for additional psychosocial variables measuring depression, PTSD, and perceived stress were not likely to confound or explain these observed associations. However, the possibility for bidirectional associations between CAD and depression and its relationship with inflammation should not be dismissed.
Our results point to young women with CAD as a vulnerable group in terms of their basal level of circulating levels of IL‐6 and their enhanced immune reactivity to stress. The pronounced sex differences in baseline levels of IL‐6 and IL‐6 response to stress among younger patients, with women showing much higher levels than men, are not paralleled by similar findings among young community controls, where women and men had very similar overall levels as well as responses to stress. These distinctive findings in patients and controls raise the possibility that IL‐6 responses to acute stress are involved in the etiology of early‐onset CAD among women, although this hypothesis requires further research. Furthermore, inflammatory responses to psychological stress involving IL‐6 could increase the development and risk of untoward subsequent events in young women with CAD, potentially affecting their long‐term prognosis. This interleukin is a significant predictor of a plethora of adverse health outcomes, including increased ambulatory blood pressure,48 increased arterial stiffness,49 and increased risk for acute coronary syndromes.50 Previous research has also shown that higher values of IL‐6 increased the risk of clinical outcomes, recurrent coronary events, and all‐cause mortality in stable CAD patients and after acute coronary syndromes,5, 6 and may be a more‐robust risk biomarker than CRP.6 These previous findings suggest that IL‐6 reflects a pathophysiological process for adverse cardiovascular outcomes rather than being a mere risk biomarker.4 Thus, IL‐6 may be an important pathway through which young women with CAD are susceptible to increased risk of CAD and adverse outcomes and deserves further exploration. Importantly, these results did not appear confounded by CAD severity as measured by myocardial perfusion imaging.
A limitation of our study is that we were unable to prospectively explore whether a higher inflammatory response to stress among women was associated with adverse cardiovascular outcomes, although this is a rationale for future research. Another limitation of this study is that we only examined inflammatory biomarkers at 2 time points. However, previous data indicate that the 90 minutes post‐stress time point is optimal for most biomarkers of inflammation.10, 15, 29, 35, 36, 37 Furthermore, we cannot exclude the possibility of unmeasured or residual confounding, which may bias effect estimates. However, there are also several strengths of our study. Our study included a large sample of participants with a large proportion of women and wide age range to examine age and sex differences in inflammatory response to mental stress. The experimental design and inclusion of community controls are also unique strengths of this study.
In a large study of patients with stable CAD undergoing mental stress testing in the laboratory, we showed that younger women exhibited a higher IL‐6 response to acute psychological stress than other groups. Although women with CAD compared with men in our sample had higher levels of other inflammatory biomarkers at baseline, the enhanced stress‐induced inflammatory response among young women was specific to IL‐6. These results were uniquely found among women with CAD and not among a sample of young community controls without CAD. Higher inflammatory cytokines among young women with CAD in response to stress may be an important pathway linking stress and cardiovascular outcomes. Future prospective studies should investigate whether these differences are associated with higher morbidity and mortality among young women with CAD.
Sources of Funding
This work was supported by the National Institutes of Health (P01HL101398, P20HL113451‐01, P01HL086773‐06A1, R56HL126558‐01, R01HL109413, R01HL109413‐02S1, R01HL125246, UL1TR000454, KL2TR000455, K24HL077506, K24MH076955, K23HL127251, T32HL130025, and K12HD085850). The authors of this article are solely responsible for the content of this article. The funding agency had no role in the design and conduct of this study, in the collection, analysis, and interpretation of the data, or in the preparation, review, or approval of this manuscript.
Disclosures
None.
Supporting information
Table S1. Unadjusted and Adjusted Geometric Mean Plasma Concentrations of hsCRP, MCP‐1, and MMP‐9 at Specified Values of Age (40, 50, 60, 70, and 80 Years) Across Sex and Time Among Women and Men With CAD, MIPS and MIMS2 Combined Cohorts*
Table S2. Linear Regression Results Reporting Beta Coefficients Per 10‐Year Increase in Age by Sex With Inflammatory Response Modeled as the Outcome for IL‐6, hsCRP, MCP‐1, and MPP‐9 Among Patients With CAD, MIPS and MIMS2 Combined Cohorts
Table S3. Unadjusted and Adjusted Inflammatory Response of hsCRP, MCP‐1, and MPP‐9 by Sex at Specified Values of Age (40, 50, 60, 70, and 80 Years) Among Patients With CAD, MIPS and MIMS2 Combined Cohorts
Table S4. Descriptive Inflammatory Profiles at Rest and 90 Minutes After Mental Stress by Sex Among Controls (n=98), MIMS2. Values reported are geometric mean concentrations of IL‐6
Table S5. Unadjusted and Adjusted Inflammatory Response of IL‐6 (pg/mL) by Sex at Specified Values of Age (30, 40, 50, and 60 Years) Among Community Controls, MIMS2
Figure S1. Inflammatory response for IL‐6, HsCRP, MCP‐1, and MMP‐9 with 95% confidence intervals among women and men with CAD from MIPS and MIMS2 combined cohorts.
Figure S2. Regression slopes for age and sex showing inflammatory response for IL‐6, HsCRP, MCP‐1, and MMP‐9 with 95% confidence intervals among women and men with CAD from MIPS and MIMS2 combined cohorts.
(J Am Heart Assoc. 2018;7:e010329 DOI: 10.1161/JAHA.118.010329.)
References
- 1. Yabluchanskiy A, Ma Y, Iyer RP, Hall ME, Lindsey ML. Matrix metalloproteinase‐9: many shades of function in cardiovascular disease. Physiology (Bethesda). 2013;28:391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blankenberg S, Rupprecht HJ, Poirier O, Bickel C, Smieja M, Hafner G, Meyer J, Cambien F, Tiret L; AtheroGene Investigators . Plasma concentrations and genetic variation of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease. Circulation. 2003;107:1579–1585. [DOI] [PubMed] [Google Scholar]
- 3. de Lemos JA, Morrow DA, Blazing MA, Jarolim P, Wiviott SD, Sabatine MS, Califf RM, Braunwald E. Serial measurement of monocyte chemoattractant protein‐1 after acute coronary syndromes: results from the A to Z trial. J Am Coll Cardiol. 2007;50:2117–2124. [DOI] [PubMed] [Google Scholar]
- 4. Daniels LB. Pretenders and contenders: inflammation, C‐reactive protein, and interleukin‐6. J Am Heart Assoc. 2017;6:e007490 DOI: 10.1161/JAHA.117.007490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fanola CL, Morrow DA, Cannon CP, Jarolim P, Lukas MA, Bode C, Hochman JS, Goodrich EL, Braunwald E, O'Donoghue ML. Interleukin‐6 and the risk of adverse outcomes in patients after an acute coronary syndrome: observations from the SOLID‐TIMI 52 (stabilization of plaque using darapladib‐thrombolysis in myocardial infarction 52) trial. J Am Heart Assoc. 2017;6:e005637 DOI: 10.1161/JAHA.117.005637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Held C, White HD, Stewart RAH, Budaj A, Cannon CP, Hochman JS, Koenig W, Siegbahn A, Steg PG, Soffer J, Weaver WD, Ostlund O, Wallentin L; STABILITY Investigators . Inflammatory biomarkers interleukin‐6 and C‐reactive protein and outcomes in stable coronary heart disease: experiences from the STABILITY (stabilization of atherosclerotic plaque by initiation of darapladib therapy) trial. J Am Heart Assoc. 2017;6:e005077 DOI: 10.1161/JAHA.116.005077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ridker PM, Hennekens CH, Buring JE, Rifai N. C‐reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. [DOI] [PubMed] [Google Scholar]
- 8. Ridker PM. Inflammation, C‐reactive protein, and cardiovascular disease: moving past the marker versus mediator debate. Circ Res. 2014;114:594–595. [DOI] [PubMed] [Google Scholar]
- 9. Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta‐analysis. Brain Behav Immun. 2007;21:901–912. [DOI] [PubMed] [Google Scholar]
- 10. Marsland AL, Walsh C, Lockwood K, John‐Henderson NA. The effects of acute psychological stress on circulating and stimulated inflammatory markers: a systematic review and meta‐analysis. Brain Behav Immun. 2017;64:208–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hammadah M, Sullivan S, Pearce B, Al Mheid I, Wilmot K, Ramadan R, Tahhan AS, O'Neal WT, Obideen M, Alkhoder A, Abdelhadi N, Mohamed Kelli H, Ghafeer MM, Pimple P, Sandesara P, Shah AJ, Hosny KM, Ward L, Ko YA, Sun YV, Weng L, Kutner M, Bremner JD, Sheps DS, Esteves F, Raggi P, Vaccarino V, Quyyumi AA. Inflammatory response to mental stress and mental stress induced myocardial ischemia. Brain Behav Immun. 2018;68:90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Libby P, Tabas I, Fredman G, Fisher EA. Inflammation and its resolution as determinants of acute coronary syndromes. Circ Res. 2014;114:1867–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khera A, McGuire DK, Murphy SA, Stanek HG, Das SR, Vongpatanasin W, Wians FH Jr, Grundy SM, de Lemos JA. Race and gender differences in C‐reactive protein levels. J Am Coll Cardiol. 2005;46:464–469. [DOI] [PubMed] [Google Scholar]
- 14. Woloshin S, Schwartz LM. Distribution of C‐reactive protein values in the United States. N Engl J Med. 2005;352:1611–1613. [DOI] [PubMed] [Google Scholar]
- 15. Edwards KM, Burns VE, Ring C, Carroll D. Sex differences in the interleukin‐6 response to acute psychological stress. Biol Psychol. 2006;71:236–239. [DOI] [PubMed] [Google Scholar]
- 16. Rohleder N, Schommer NC, Hellhammer DH, Engel R, Kirschbaum C. Sex differences in glucocorticoid sensitivity of proinflammatory cytokine production after psychosocial stress. Psychosom Med. 2001;63:966–972. [DOI] [PubMed] [Google Scholar]
- 17. Vaccarino V, Shah AJ, Rooks C, Ibeanu I, Nye JA, Pimple P, Salerno A, D'Marco L, Karohl C, Bremner JD, Raggi P. Sex differences in mental stress‐induced myocardial ischemia in young survivors of an acute myocardial infarction. Psychosom Med. 2014;76:171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Samad Z, Boyle S, Ersboll M, Vora AN, Zhang Y, Becker RC, Williams R, Kuhn C, Ortel TL, Rogers JG, O'Connor CM, Velazquez EJ, Jiang W; REMIT Investigators . Sex differences in platelet reactivity and cardiovascular and psychological response to mental stress in patients with stable ischemic heart disease: insights from the REMIT study. J Am Coll Cardiol. 2014;64:1669–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vaccarino V, Bremner JD. Behavioral, emotional and neurobiological determinants of coronary heart disease risk in women. Neurosci Biobehav Rev. 2017;74:297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vaccarino V, Wilmot K, Al Mheid I, Ramadan R, Pimple P, Shah AJ, Garcia EV, Nye J, Ward L, Hammadah M, Kutner M, Long Q, Bremner JD, Esteves F, Raggi P, Quyyumi AA. Sex differences in mental stress‐induced myocardial ischemia in patients with coronary heart disease. J Am Heart Assoc. 2016;5:e003630 DOI: 10.1161/JAHA.116.003630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Beckie TM, Fletcher G, Groer MW, Kip KE, Ji M. Biopsychosocial health disparities among young women enrolled in cardiac rehabilitation. J Cardiopulm Rehabil Prev. 2015;35:103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Low CA, Thurston RC, Matthews KA. Psychosocial factors in the development of heart disease in women: current research and future directions. Psychosom Med. 2010;72:842–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shah AJ, Ghasemzadeh N, Zaragoza‐Macias E, Patel R, Eapen DJ, Neeland IJ, Pimple PM, Zafari AM, Quyyumi AA, Vaccarino V. Sex and age differences in the association of depression with obstructive coronary artery disease and adverse cardiovascular events. J Am Heart Assoc. 2014;3:e000741 DOI: 10.1161/JAHA.113.000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Andrikopoulos GK, Tzeis SE, Pipilis AG, Richter DJ, Kappos KG, Stefanadis CI, Toutouzas PK, Chimonas ET; Investigators of the Hellenic Study of AMI . Younger age potentiates post myocardial infarction survival disadvantage of women. Int J Cardiol. 2006;108:320–325. [DOI] [PubMed] [Google Scholar]
- 25. Koek HL, de Bruin A, Gast F, Gevers E, Kardaun JW, Reitsma JB, Grobbee DE, Bots ML. Short‐ and long‐term prognosis after acute myocardial infarction in men versus women. Am J Cardiol. 2006;98:993–999. [DOI] [PubMed] [Google Scholar]
- 26. Vaccarino V, Parsons L, Every NR, Barron HV, Krumholz HM. Sex‐based differences in early mortality after myocardial infarction. National Registry of Myocardial Infarction 2 Participants. N Engl J Med. 1999;341:217–225. [DOI] [PubMed] [Google Scholar]
- 27. Vaccarino V, Parsons L, Peterson ED, Rogers WJ, Kiefe CI, Canto J. Sex differences in mortality after acute myocardial infarction: changes from 1994 to 2006. Arch Intern Med. 2009;169:1767–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wokhlu A, Pepine CJ. Mental stress and myocardial ischemia: young women at risk. J Am Heart Assoc. 2016;5:e004196 DOI: 10.1161/JAHA.116.004196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rooks CR, Ibeanu I, Shah A, Pimple P, Murrah N, Shallenberger L, Pace T, Douglas Bremner J, Raggi P, Vaccarino V. Young women post‐MI have higher plasma concentrations of interleukin‐6 before and after stress testing. Brain Behav Immun. 2016;51:92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hammadah M, Al Mheid I, Wilmot K, Ramadan R, Abdelhadi N, Alkhoder A, Obideen M, Pimple PM, Levantsevych O, Kelli HM, Shah A, Sun YV, Pearce B, Kutner M, Long Q, Ward L, Ko YA, Hosny Mohammed K, Lin J, Zhao J, Bremner JD, Kim J, Waller EK, Raggi P, Sheps D, Quyyumi AA, Vaccarino V. Telomere shortening, regenerative capacity, and cardiovascular outcomes. Circ Res. 2017;120:1130–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hammadah M, Al Mheid I, Wilmot K, Ramadan R, Shah AJ, Sun Y, Pearce B, Garcia EV, Kutner M, Bremner JD, Esteves F, Raggi P, Sheps DS, Vaccarino V, Quyyumi AA. The Mental Stress Ischemia Prognosis Study: objectives, study design, and prevalence of inducible ischemia. Psychosom Med. 2017;79:311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vaccarino V, Sullivan S, Hammadah M, Wilmot K, Al Mheid I, Ramadan R, Elon L, Pimple PM, Garcia EV, Nye J, Shah AJ, Alkhoder A, Levantsevych O, Gay H, Obideen M, Huang M, Lewis TT, Bremner JD, Quyyumi AA, Raggi P. Mental stress‐induced‐myocardial ischemia in young patients with recent myocardial infarction: sex differences and mechanisms. Circulation. 2018;137:794–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goldberg AD, Becker LC, Bonsall R, Cohen JD, Ketterer MW, Kaufman PG, Krantz DS, Light KC, McMahon RP, Noreuil T, Pepine CJ, Raczynski J, Stone PH, Strother D, Taylor H, Sheps DS. Ischemic, hemodynamic, and neurohormonal responses to mental and exercise stress. Experience from the Psychophysiological Investigations of Myocardial Ischemia Study (PIMI). Circulation. 1996;94:2402–2409. [DOI] [PubMed] [Google Scholar]
- 34. Holly TA, Abbott BG, Al‐Mallah M, Calnon DA, Cohen MC, DiFilippo FP, Ficaro EP, Freeman MR, Hendel RC, Jain D, Leonard SM, Nichols KJ, Polk DM, Soman P; American Society of Nuclear Cardiology . Single photon‐emission computed tomography. J Nucl Cardiol. 2010;17:941–973. [DOI] [PubMed] [Google Scholar]
- 35. Mendham AE, Donges CE, Liberts EA, Duffield R. Effects of mode and intensity on the acute exercise‐induced IL‐6 and CRP responses in a sedentary, overweight population. Eur J Appl Physiol. 2011;111:1035–1045. [DOI] [PubMed] [Google Scholar]
- 36. Moldoveanu AI, Shephard RJ, Shek PN. Exercise elevates plasma levels but not gene expression of IL‐1beta, IL‐6, and TNF‐alpha in blood mononuclear cells. J Appl Physiol (1985). 2000;89:1499–1504. [DOI] [PubMed] [Google Scholar]
- 37. Steptoe A, Willemsen G, Owen N, Flower L, Mohamed‐Ali V. Acute mental stress elicits delayed increases in circulating inflammatory cytokine levels. Clin Sci (Lond). 2001;101:185–192. [PubMed] [Google Scholar]
- 38. Beck AT, Steer RA, Brown GK. Beck Depression Inventory: BDI‐II: Manual. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- 39. First M, Spitzer R, Williams J, Gibbon M. Structured Clinical Interview for DSM IV‐Patient Edition (SCID‐P). Washington, DC: American Psychiatric Press; 1995. [Google Scholar]
- 40. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 41. Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983;51:606. [DOI] [PubMed] [Google Scholar]
- 42. Wyczalkowska‐Tomasik A, Czarkowska‐Paczek B, Zielenkiewicz M, Paczek L. Inflammatory markers change with age, but do not fall beyond reported normal ranges. Arch Immunol Ther Exp (Warsz). 2016;64:249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kop WJ, Weissman NJ, Zhu J, Bonsall RW, Doyle M, Stretch MR, Glaes SB, Krantz DS, Gottdiener JS, Tracy RP. Effects of acute mental stress and exercise on inflammatory markers in patients with coronary artery disease and healthy controls. Am J Cardiol. 2008;101:767–773. [DOI] [PubMed] [Google Scholar]
- 44. Heinrich PC, Castell JV, Andus T. Interleukin‐6 and the acute phase response. Biochem J. 1990;265:621–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kerr R, Stirling D, Ludlam CA. Interleukin 6 and haemostasis. Br J Haematol. 2001;115:3–12. [DOI] [PubMed] [Google Scholar]
- 46. Shah R, Burg MM, Vashist A, Collins D, Liu J, Jadbabaie F, Graeber B, Earley C, Lampert R, Soufer R. C‐reactive protein and vulnerability to mental stress‐induced myocardial ischemia. Mol Med. 2006;12:269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Da Silva JA. Sex hormones and glucocorticoids: interactions with the immune system. Ann N Y Acad Sci. 1999;876:102–117; discussion, 117–118. [DOI] [PubMed] [Google Scholar]
- 48. Brydon L, Steptoe A. Stress‐induced increases in interleukin‐6 and fibrinogen predict ambulatory blood pressure at 3‐year follow‐up. J Hypertens. 2005;23:1001–1007. [DOI] [PubMed] [Google Scholar]
- 49. Mahmud A, Feely J. Arterial stiffness is related to systemic inflammation in essential hypertension. Hypertension. 2005;46:1118–1122. [DOI] [PubMed] [Google Scholar]
- 50. Blake GJ, Ridker PM. C‐reactive protein and other inflammatory risk markers in acute coronary syndromes. J Am Coll Cardiol. 2003;41(4 Suppl S):37S–42S. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Unadjusted and Adjusted Geometric Mean Plasma Concentrations of hsCRP, MCP‐1, and MMP‐9 at Specified Values of Age (40, 50, 60, 70, and 80 Years) Across Sex and Time Among Women and Men With CAD, MIPS and MIMS2 Combined Cohorts*
Table S2. Linear Regression Results Reporting Beta Coefficients Per 10‐Year Increase in Age by Sex With Inflammatory Response Modeled as the Outcome for IL‐6, hsCRP, MCP‐1, and MPP‐9 Among Patients With CAD, MIPS and MIMS2 Combined Cohorts
Table S3. Unadjusted and Adjusted Inflammatory Response of hsCRP, MCP‐1, and MPP‐9 by Sex at Specified Values of Age (40, 50, 60, 70, and 80 Years) Among Patients With CAD, MIPS and MIMS2 Combined Cohorts
Table S4. Descriptive Inflammatory Profiles at Rest and 90 Minutes After Mental Stress by Sex Among Controls (n=98), MIMS2. Values reported are geometric mean concentrations of IL‐6
Table S5. Unadjusted and Adjusted Inflammatory Response of IL‐6 (pg/mL) by Sex at Specified Values of Age (30, 40, 50, and 60 Years) Among Community Controls, MIMS2
Figure S1. Inflammatory response for IL‐6, HsCRP, MCP‐1, and MMP‐9 with 95% confidence intervals among women and men with CAD from MIPS and MIMS2 combined cohorts.
Figure S2. Regression slopes for age and sex showing inflammatory response for IL‐6, HsCRP, MCP‐1, and MMP‐9 with 95% confidence intervals among women and men with CAD from MIPS and MIMS2 combined cohorts.


