Abstract
Background
In older adults undergoing cardiac surgery, prediction of downstream risk is critical. Our objective was to determine the association of 5‐m gait speed with 1‐year mortality and repeat hospitalization following cardiac surgery.
Methods and Results
In this prospective cohort of patients undergoing cardiac surgery at centers participating in the Society of Thoracic Surgeons Database with gait speed recorded, we examined all‐cause mortality using a landmark analysis at 0 to 30, 30 to 365, and >365 days, as well as repeat hospitalization. The cohort consisted of 8287 patients (median age, 74 years; 32% females). At 1 year, survival was 90% in the slow (<0.83 m/s), 95% in the middle (0.83–1.00 m/s), and 97% in the fast (>1.00 m/s) gait speed tertiles, and risk of hospitalization was 45%, 33%, and 27%, respectively (both P<0.0001). After adjustment, gait speed remained predictive of mortality (hazard ratio, 2.16 per 0.1‐m/s decrease in gait speed; 95% confidence interval, 1.59–2.93) and rehospitalization (hazard ratio, 1.71 per 0.1‐m/s decrease in gait speed; 95% confidence interval, 1.45–2.0). In a landmark analysis, the effect of slow gait speed on mortality was most marked from 30 to 365 days after surgery, where each decline in 0.1 m/s of gait speed conferred a 2‐fold increased risk of mortality.
Conclusions
Gait speed is a simple tool to screen for frailty and identify older adults at risk for adverse events in the early and midterm postoperative periods.
Keywords: elderly, function, mortality, surgery
Subject Categories: Aging, Cardiovascular Surgery, Mortality/Survival, Quality and Outcomes, Risk Factors
Clinical Perspective
What Is New?
Building on previous studies that demonstrated an association between gait speed and operative mortality at 30 days, this is the first large‐scale study to demonstrate an association with mortality at 1 year and beyond.
Our landmark survival analysis demonstrated that, in slow walkers, the relative risk of mortality was highest during the time period between 30 days and 1 year following cardiac surgery.
What Are the Clinical Implications?
The utility of measuring gait speed is to screen for frailty in older adults referred for cardiac surgery in order to refine estimates of predicted risk, identify patients in need of further geriatric assessment, and optimize postoperative care during the short and mid term follow‐up period.
Introduction
Frailty, defined by an inability to maintain physiological homeostasis when faced with stressors, is prevalent in 20% to 60% of older adults with cardiovascular disease.1 The utility of frailty has been most marked in the evaluation of patients referred for invasive procedures such as cardiac surgery to predict the risk of mortality, morbidity, disability, and healthcare utilization.2, 3, 4 Gait speed measured over a 5‐m course is recognized as a simple yet powerful measure of frailty for risk stratification of older adults who may warrant further geriatric assessment.5 In patients undergoing coronary artery bypass and heart valve surgery, the association between gait speed and adverse short‐term outcomes was elucidated in the FRAILTY ABCs (Frailty Assessment Before Cardiac Surgery) study6 and, more recently, in the Society of Thoracic Surgeons (STS) Adult Cardiac Surgery Database.7 After adjusting for the STS predicted risk of mortality, each 0.1‐m/s decrement in gait speed conferred an 11% increase in 30‐day mortality.
Athough the evidence for gait speed as a risk factor for 30‐day mortality and morbidity is compelling, the association between gait speed and long‐term postsurgical outcomes has yet to be examined in an adequately powered sample size. Slow walkers who survive the index operation are hypothesized to have a heightened risk of mortality in the ensuing months. The utility of gait speed, accordingly, would not only be to assist operative decision making, but also to guide postoperative interventions such as cardiac rehabilitation.8, 9 Using linked data from the STS and Centers for Medicare and Medicaid Services (CMS) databases, we performed a landmark analysis to determine the prognostic value of 5‐m gait speed in older adults over a cumulative follow‐up period of 1 year or more following cardiac surgery.
Methods
Study Design
A cohort of older adults was assembled within the STS Adult Cardiac Surgery Database as previously described.7 The STS began collecting 5‐m gait speed as of July 2011, and patients undergoing coronary and heart valve surgery between July 2011 and December 2014 were eligible for this analysis. Gait speed, comorbid conditions, operative variables, and vital status during the index hospitalization were extracted from the STS database. Linkage with the CMS database was performed to obtain vital status and hospitalization data from the index discharge to at least 1‐year post‐procedure (December 2015). Multivariable regression was used to determine the effect of gait speed on 1‐year mortality, and a landmark analysis was performed to compare effects at <30, 30 to 365, and >365 days. This article was prepared in accord with the standards set forth by the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines.10 The analytical methods, but not the data, will be made available upon request to other researchers for purposes of reproducing the results or replicating the procedure.
Population
Inclusion criteria were: (1) age ≥65 years; (2) undergoing coronary artery bypass graft (CABG) surgery, aortic valve surgery, mitral valve surgery, or CABG combined with aortic or mitral valve surgery; and (3) preoperative gait speed recorded in the STS Adult Cardiac Surgery Database. Patients were excluded if they were unable to safely walk or had a critical preoperative status before surgery, defined as emergency or emergency salvage surgery, cardiogenic shock, or requirement for inotropic support or intra‐aortic balloon pump. To set a minimum experience at the center level, centers were excluded if they had recorded gait speed in <10 patients during the study interval. Successful linkage between STS and CMS records was necessary for long‐term follow‐up information. A flow diagram of the study population is shown in Figure 1.
Figure 1.

Flow diagram. The final cohort consisted of 8287 patients aged 65 years and older that underwent nonemergent coronary artery bypass, surgical aortic or mitral valve replacement or repair, or combinations thereof. The study base consisted of 118 centers that were participating in the STS Adult Cardiac Surgery Database, had recorded gait speed data in at least 10 patients, and had linked vital status data from CMS. CMS indicates Centers for Medicare & Medicaid Services; MI, myocardial infarction; STS, Society of Thoracic Surgeons.
5‐m Gait Speed
Before surgery, patients performed the 5‐m gait speed test according to standardized instructions: position the patient with his or her feet behind and just touching the 0‐m start line; instruct to “walk at your comfortable pace” until a few steps past the 5‐m mark (should not start to slow down before); begin each trial on the word “go”; start the timer with the first footfall after the 0‐m line; and stop the timer with the first footfall after the 5‐m line. Three trials were repeated, allowing sufficient time for recuperation between trials, and average gait speed was calculated. Erroneous walking times of <2 or >30 seconds were set to missing (n=38). Gait speed was similarly distributed in CMS‐linked and unlinked populations, with the same cutoffs as previously identified in the 30‐day analysis.7
Outcomes
The primary outcome was all‐cause mortality from the date of the index surgical procedure to the last available follow‐up and, moreover, during the following time intervals: (1) within the first 30 days following the index surgical procedure, (2) from 30 to 365 days, and (3) >365 days. Secondary outcomes were all‐cause rehospitalization, time to cardiovascular hospitalization, and time to noncardiovascular hospitalization. The cause of each hospitalization was ascertained based on a previously validated algorithm using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) codes recorded in the CMS database.11 All‐cause hospitalization was identified as the first occurrence per patient, and cause‐specific (cardiovascular and noncardiovascular) hospitalization was identified as the first occurrence per patient after censoring for other types of hospitalization and death.
Covariates
The covariates included in the ASCERT (American College of Cardiology Foundation–Society of Thoracic Surgeons Collaboration on the Comparative Effectiveness of Revascularization Strategies)12 risk model were extracted from the STS database; these covariates were selected to predict 1‐year mortality after cardiac surgery (as opposed to covariates in the STS‐predicted risk of mortality model that were designed to predict 30‐day outcomes). Covariates from the ASCERT model used for adjustment were: age, sex, race, diabetes mellitus, cerebrovascular disease, peripheral arterial disease, chronic lung disease, current cigarette smoking, dialysis, preoperative creatinine, number of diseased coronary vessels, left ventricular ejection fraction, congestive heart failure, and type of cardiac surgical procedure.
Statistical Analysis
Continuous variables are presented as medians with first quartiles (Q1) and third quartiles (Q3), and categorical variables are presented as numbers (n) and proportions (%). Baseline characteristics and unadjusted outcomes were compared across tertiles of gait speed using the Kruskal–Wallis and chi‐square tests. Missing values were handled by using simple imputation (common category) for categorical covariates, by using the conditional median for continuous covariates. For binary covariates, missing values were treated as not present according to STS analysis standards. Baseline characteristics and observed outcomes were compared for patients with versus without gait speed captured in the STS database and with versus without CMS successful linkage.
Kaplan–Meier curves were plotted for gait speed tertiles and all‐cause mortality, and the 3‐sample log‐rank test was applied to test for a survival difference. Cumulative incidence curves were plotted for gait speed tertiles and hospitalizations. After testing the proportional hazards assumptions using Schoenfeld residuals, Cox multivariable regression models were constructed for the entire follow‐up period and for the specified time intervals (landmark analysis). For each time interval, patients censored before the start of that interval were excluded and events occurring after the end of that interval were not counted. Gait speed is represented as a continuous linear term (because the use of splines did not improve the model's fit) and, secondarily, as an ordinal tertile term. To test its incremental value, model performance was evaluated using c‐statistics and integrated discrimination improvement.13
Results
Baseline Characteristics
The study population consisted of 8287 patients from 118 participating centers with a median age of 74 years (Q1, 69; Q3, 79), including 1809 (22%) octogenarians and 2659 (32%) females. The surgery performed was isolated CABG in 4542 patients (55%), isolated aortic or mitral valve surgery in 2275 (28%), and combined CABG plus aortic or mitral valve surgery in 1470 (18%). Median follow‐up was 605 days (Q1, 349; Q3, 896), with all surviving patients being followed for at least 1 year. Compared with those included, patients excluded on the basis of not having gait speed measured or not having CMS vital status data accessible were similar in terms of age, sex, and comorbidity burden, yet they were more likely to have multivessel coronary disease and undergo isolated CABG surgery (Table S1).
Compared with patients in the fast gait speed tertile of >1.00 m/s, those in the slow gait speed tertile of <0.83 m/s were older (76 versus 72 years) and more likely to be female (46% versus 20%), have lower hematocrit (37.1% versus 40.4%), lower serum albumin (3.8 versus 4.1 g/dL), lower creatinine clearance (66.2 versus 75.4 mL/min per 1.73 m2), and lower left ventricular ejection fraction (56.0% versus 60.0%), and have a higher prevalence of diabetes mellitus (47% versus 33%), hypertension (93% versus 85%), cerebrovascular disease (22% versus 13%), peripheral arterial disease (18% versus 12%), chronic lung disease (35% versus 23%), and recent heart failure (35% versus 24%; Table 1).
Table 1.
Baseline Characteristics by Tertiles of Gait Speed
| Slow Tertile <0.83 m/s (N=2686) | Middle Tertile 0.83 to 1.00 m/s (N=3179) | Fast Tertile >1.00 m/s (N=2422) | P Value | |
|---|---|---|---|---|
| Demographic | ||||
| Age, y (Q1, Q3) | 76 (70, 81) | 73 (69, 78) | 72 (68, 77) | <0.001 |
| Female sex | 1203 (46) | 934 (29) | 495 (20) | <0.001 |
| Race | <0.001 | |||
| White | 2396 (89) | 2895 (91) | 2143 (88) | |
| Black | 131 (5) | 119 (4) | 48 (2) | |
| Asian | 43 (2) | 58 (2) | 126 (5) | |
| Hispanic | 54 (2) | 54 (2) | 44 (2) | |
| Native American | 6 (<1) | 3 (<1) | 5 (<1) | |
| Other | 47 (2) | 40 (1) | 45 (2) | |
| Missing | 9 (<1) | 10 (<1) | 11 (<1) | |
| Clinical | ||||
| BMI, median (Q1, Q3) | 28.8 (25.2, 33.2) | 28.5 (25.5, 32.3) | 27.9 (25.1, 31.04) | <0.001 |
| Diabetes mellitus | 1263 (47) | 1239 (39) | 792 (33) | <0.001 |
| Hypertension | 2486 (93) | 2827 (89) | 2066 (85) | <0.001 |
| Cigarette smoker | 461 (17) | 565 (18) | 448 (19) | 0.46 |
| Severe CAD | 1418 (53) | 1641 (52) | 1237 (51) | 0.16 |
| Congestive heart failure | 947 (35) | 846 (27) | 580 (24) | <0.001 |
| LVEF, % (Q1, Q3) | 56 (50, 60) | 58 (50, 63) | 60 (50, 63) | <0.001 |
| Cancer within 5 y | 206 (8) | 246 (8) | 173 (7) | 0.67 |
| Previous stroke | 295 (11) | 237 (7) | 106 (4) | <0.001 |
| Cerebrovascular disease | 578 (22) | 564 (18) | 320 (13) | <0.001 |
| Peripheral arterial disease | 472 (18) | 463 (15) | 296 (12) | <0.001 |
| Chronic lung disease | 941 (35) | 901 (28) | 555 (23) | <0.001 |
| Chronic kidney disease | 655 (24) | 575 (18) | 292 (12) | <0.001 |
| Creatinine, mg/dL (Q1, Q3) | 1.0 (0.8, 1.3) | 1.0 (0.9, 1.2) | 1.0 (0.9, 1.2) | 0.07 |
| Creatinine clearance, mL/min/1.73 m2 (Q1, Q3) | 66.2 (50.5, 87.0) | 72.0 (55.4, 92.1) | 75.4 (60.7, 93.5) | <0.001 |
| Serum albumin, g/dL (Q1, Q3) | 3.8 (3.4, 4.1) | 4.0 (3.6, 4.3) | 4.1 (3.8, 4.3) | <0.001 |
| Hematocrit, % (Q1, Q3) | 37.1 (34.0, 40.7) | 39.2 (36.0, 42.0) | 40.4 (37.6, 43.0) | <0.001 |
| Procedural | ||||
| STS‐PROM, % (Q1, Q3) | 0.03 (0.02, 0.05) | 0.02 (0.01, 0.03) | 0.02 (0.01, 0.03) | <0.001 |
| Previous cardiac surgery | 942 (35) | 1046 (32.9) | 806 (33.3) | 0.19 |
| Urgent surgery | 1122 (42) | 768 (24) | 520 (21) | <0.001 |
| Surgery performed | 0.09 | |||
| Isolated CABG | 1501 (56) | 1718 (54) | 1323 (55) | |
| Isolated valve | 694 (26) | 883 (28) | 698 (29) | |
| CABG plus valve | 491 (18) | 578 (18) | 401 (17) | |
Proportions listed as N (%). BMI indicates body mass index; CABG, coronary artery bypass graft surgery; CAD, coronary artery disease (severe CAD defined as 3‐vessel disease); LVEF, left ventricular ejection fraction; Q1, quartile 1; Q3, quartile 3; STS‐PROM, Society of Thoracic Surgeons Predicted Risk of Mortality.
Association Between 5‐m Gait Speed and Outcomes
One‐year survival was 90%, 95%, and 97% in the slow, middle, and fast gait speed tertiles, respectively (P<0.0001; Figure 2). Compared with the fast tertile, there was a graded increase in adjusted all‐cause mortality among patients in the middle tertile (hazard ratio [HR], 1.27; 95% confidence interval [CI], 1.03–1.56) and the slow tertile (HR, 1.73; 95% CI, 1.34–2.24). After adjusting for baseline covariates, continuous gait speed was also predictive of 1‐year mortality (HR, 2.16 per 0.1‐m/s decrease in gait speed; 95% CI, 1.59–2.93). There was no interaction for the association between gait speed and mortality based on patient sex, type of surgical procedure, or other prespecified covariates (interaction P>0.1).
Figure 2.

Adjusted Kaplan–Meier survival curves by tertiles of gait speed. Slow gait speed was associated with reduced survival.
Risk of hospitalization was 45%, 33%, and 27% at 1 year; and it was 19%, 14%, and 10% at 30 days in these tertiles (P<0.0001; Figure 3). There was an increased risk of both cardiovascular and noncardiovascular hospitalizations in slow walkers, although noncardiovascular hospitalizations were more frequently observed. Compared with the fast tertile, there was a graded increase in adjusted all‐cause hospitalization among patients in the middle tertile (HR, 1.17; 95% CI, 1.06–1.29) and the slow tertile (HR, 1.43; 95% CI, 1.29–1.59). After adjusting for baseline covariates, continuous gait speed was also predictive of all‐cause hospitalization (adjusted HR, 1.71 per 0.1‐m/s decrease in gait speed; 95% CI, 1.45–2.02).
Figure 3.
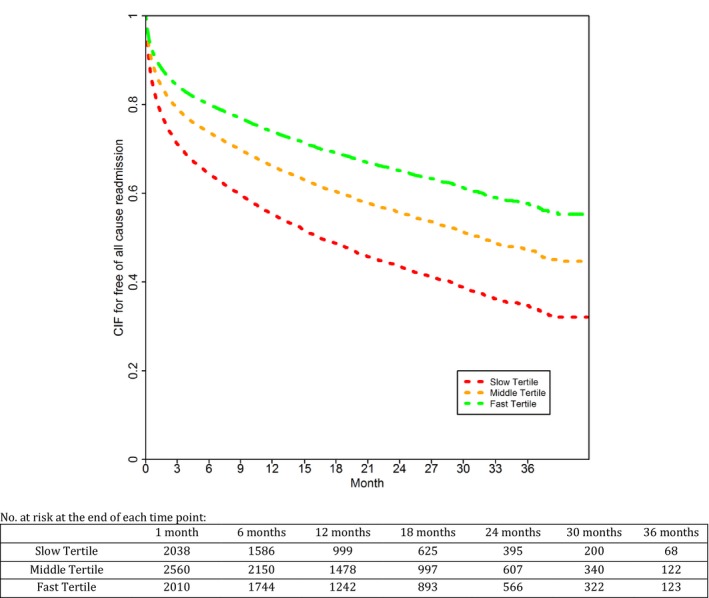
Cumulative incidence curves for hospitalization by tertiles of gait speed. Slow gait speed was associated with increased hospitalizations. CIF indicates cumulative incidence function.
Landmark Analysis
In the landmark survival analysis, slow walkers had the greatest relative risk of all‐cause mortality during the time interval of 30 to 365 days after surgery (adjusted HR, 2.28; 95% CI, 1.57–3.32). An association was also observed, albeit with a smaller effect size, during the time intervals of <30 days after surgery (adjusted HR, 1.57; 95% CI, 0.97–2.53) and >365 days after surgery (adjusted HR, 1.41; 95% CI, 1.00–1.99). Similarly, continuous gait speed was associated with a greater relative risk of all‐cause mortality during the time interval of 30 to 365 days after surgery. Time‐interval–stratified HRs and survival functions are shown in Table 2 and Figure 4.
Table 2.
Landmark Survival Analysis
| Unadjusted HR (95% CI) | Adjusted HR (95% CI) | |
|---|---|---|
| Time interval: <30 d | ||
| Per 0.1‐m/s decrease in continuous gait speed | 1.13 (1.01, 1.21) | 1.07 (1.00, 1.15) |
| Slow tertile | 2.45 (1.56, 3.83) | 1.57 (0.97, 2.53) |
| Middle tertile | 1.27 (0.84, 1.92) | 0.95 (0.62, 1.46) |
| Fast tertile | Referent | Referent |
| Time interval: 30 to 365 d | ||
| Per 0.1‐m/s decrease in continuous gait speed | 1.21 (1.16, 1.26) | 1.11 (1.06, 1.17) |
| Slow tertile | 3.90 (2.82, 5.40) | 2.28 (1.57, 3.32) |
| Middle tertile | 2.05 (1.47, 2.85) | 1.67 (1.17, 2.37) |
| Fast tertile | Referent | Referent |
| Time interval: >365 d | ||
| Per 0.1‐m/s decrease in continuous gait speed | 1.10 (1.06, 1.15) | 1.05 (1.00, 1.10) |
| Slow tertile | 1.98 (1.43, 2.74) | 1.41 (1.00, 1.99) |
| Middle tertile | 1.33 (0.96, 1.86) | 1.17 (0.83, 1.65) |
| Fast tertile | Referent | Referent |
CI indicates confidence interval; HR, hazard ratio.
Figure 4.

Landmark analysis with survival function by tertiles of gait speed. The association between slow gait speed and mortality was most marked during the time interval from 30 to 365 days after surgery.
Incremental Value of Gait Speed
The c‐statistic of the mortality risk model with baseline covariates was 0.7242; after adding gait speed to the model, the c‐statistic improved to 0.7306 (P=0.0001) and the integrated discrimination improvement was 0.012 (P=0.0001).
Discussion
By linking the CMS and STS Adult Cardiac Surgery databases, this study examined the prognostic value of gait speed for midterm outcomes following cardiac surgery in a large, population‐based cohort. The findings can be summarized as follows: (1) The 5‐m gait speed test was predictive of 1‐year survival in a multivariable model adjusting for cardiac status, comorbidities, and type of surgical procedure; (2) slow walkers were at greatest relative risk for death during the intermediate time period (30 days to 1 year) following surgery; and (3) gait speed was predictive of rehospitalization. These results build naturally on our previous work that demonstrated an association between gait speed and short‐term outcomes,7 and now extends and emphasizes the incremental value of gait speed in cardiac surgery.
The paradigm of frailty is predicated on age‐related perturbations in inflammatory, metabolic, and hematologic axes, as well as subclinical impairments in numerous organ systems.14, 15 Our data are consistent with this multifaceted construct, in which patients classified as frail according to the 5‐m gait speed test had a greater burden of comorbidities such as diabetes mellitus, peripheral and cerebrovascular disease, and lung disease. Furthermore, frail patients had a greater burden of clinical and subclinical biochemical derangements, such as lower hematocrit, lower serum albumin, and higher serum creatinine, as previously reported.16, 17 These results highlight the association between gait speed and other indicators of frailty, and reflects the distinct information provided by gait speed above traditional risk factors such as those encompassed in the ASCERT model.18
Given its association with repeat hospitalizations, the finding of slow gait speed may serve as a simple stratifier to identify at‐risk patients and implement strategies to mitigate this risk. Because of the multifaceted nature of frailty and coexistence of multiple chronic conditions, it was not surprising to observe a large proportion of hospitalizations stemming from noncardiovascular causes in frail patients. The rising incidence of noncardiovascular events in patients with established cardiovascular disease is increasingly recognized,19 notably in heart failure.19, 20 This medical complexity has direct implications for the design of interventions aimed at treating frail patients following cardiac surgery.20, 21 Existing models of postoperative cardiovascular care tend to focus on cardiocentric goals such as relief of angina and recovery of left ventricular ejection fraction, with less attention to recovery of energy and mobility. In order to promote functional recovery after cardiac surgery, coordinated multidisciplinary care is needed with the involvement of primary care practitioners, geriatricians, and rehabilitation specialists.
The results of our landmark analysis suggest that patients with slow gait speed are especially at risk for adverse events 30 days to 1 year following surgery. Early deaths are uncommon, ranging from 1.1% to 3.4% for fast and slow walkers,7 respectively, and appear to be driven mainly by critical preoperative factors and unexpected operative or perioperative complications. Once the (rare) early hazard has been averted, frail patients are discharged from the hospital and tasked with the challenge of rebuilding lost muscle mass, strength, and endurance, facing continued (nonrare) competing risks from a multitude of comorbidities. Frail patients are more likely to decompensate, sometimes fatally, when their comorbid conditions become destabilized. Furthermore, frail patients with sarcopenia and slow gait speed preceding surgery enter a vicious cycle that contributes to further losses of muscle mass and strength following surgery.22 This lack of muscle reserves is exacerbated by the catabolic stress of surgery, leading to deconditioning and disability that can be protracted and lead to repeat hospitalizations, institutionalization, and death.23 The deconditioning of frail patients is best counteracted by cardiac rehabilitation after, and in selected patients before, surgery.24, 25
Gait speed was a significant risk factor for mortality after adjusting for ASCERT risk factors; however, used as a solitary measure of frailty, its incremental discriminatory value was modest (as measured by the c‐statistic change and integrated discrimination improvement). Similar results were noted in our 30‐day STS study7 and in 2 large transcatheter aortic valve replacement studies,26, 27 wherein the addition of gait speed to a well‐performing clinical risk score yielded a c‐statistic change of <0.02 to predict mortality despite a statistically significant multivariable‐adjusted effect. Conversely, multifaceted frailty scales, such as the Essential Frailty Toolset, yielded more impactful c‐statistic changes up to 0.07 in the FRAILTY‐AVR (Frailty Aortic Valve Replacement) study.28 The Essential Frailty Toolset scale is scored 0 to 5 and consists of 5 timed chair rises, a brief cognitive assessment, serum albumin, and hemoglobin. Therefore, combining mobility with other indicators of frailty increases discrimination of multidimensional risk.
Whereas the 5‐m gait speed test remains a highly validated measure of frailty, its role in the setting of cardiac surgery appears to be one of a first‐line screening test to efficiently identify patients who require further geriatric evaluation; this 2‐tiered approach (gait speed and, if slow, multifaceted geriatric assessment) has been adopted in clinical care pathways.29 Ease of use, low cost, and ability to rapidly deploy and interpret the gait speed test make it ideal to screen for frailty. Fast gait speeds provide reassurance for short‐ and mid term outcomes, whereas slow gait speeds signal a potential red flag to probe deeper in individual patients. Thus, gait speed is a pivotal first step in the evaluation of older patients referred for cardiac surgery.
There are a number of limitations in this study. The 5‐m gait speed test was not performed in all STS centers. Most patients in the STS registry were not included in the analysis because gait speed had not been measured preoperatively; a comparison of included and excluded patients suggested that the primary reason for not measuring gait speed was logistical barriers rather than systematic bias. A secondary reason was the inability of the frailest group to complete the walking test. Although this iteration of STS data collection forms did not capture the reason for nonmeasurement of gait speed, previous studies have shown that 5% to 10% of patients are too physically weak or ill to complete the test and that these patients face the highest risk of mortality. Inclusion of these nonambulatory patients in this study would have expectedly strengthened the predictive ability of the gait speed test.30 Other measures of frailty, such as cognitive function, grip strength, and chair rise time, were not available in the STS registry, nor was postoperative gait speed, which would have been of interest to explore the longitudinal trajectory and implications for recovery.31 Nonavailable covariates may represent a source of residual confounding given the poorer global health status of patients with slow gait speed. Cause of death was not adjudicated and hence not reliably available, although cause of hospitalization was available and demonstrated that a substantial proportion of adverse events were noncardiovascular in nature. Finally, the study population was of older patients with CMS linkage; although necessary to obtain long‐term follow‐up and relevant to the population at risk for frailty, observations may not be generalizable to a younger population undergoing cardiac surgery.
Conclusions
Gait speed is a predictor of midterm mortality and hospitalization following cardiac surgery. The intermediate time period 30 to 365 days after hospital discharge has the highest relative risk of fatal adverse events among frail patients. The 5‐m gait speed test is an efficient screening test to identify frail patients; the use of such a strategy should help target those who may benefit from geriatric assessment, medical optimization, and cardiac rehabilitation. Clinical trials are needed to develop effective interventions to improve survival and promote functional recovery in frail older adults undergoing cardiac surgery.
Author Contributions
The Duke Clinical Research Institute had full access to all of the data in the study. Drs Afilalo, Sharma, Alexander, Zhang, and O'Brien take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Afilalo, Sharma, Alexander. Acquisition of data: Shahian. Analysis and interpretation of data: Afilalo, Sharma, Alexander, Zhang, O'Brien. Drafting of the manuscript: Afilalo, Sharma, Alexander. Critical revision of the manuscript for important intellectual content: Afilalo, Sharma, Zhang, O'Brien, Brennan, McClurken, Cleveland, Mack, Edwards, Smith, Shahian, Peterson, Alexander. Statistical analysis: Zhang, O'Brien. Obtained funding: Afilalo, Alexander. Administrative, technical, or material support: Alexander. Study supervision: Afilalo, Alexander.
Sources of Funding
Dr Afilalo holds a Clinical Research Scholars Junior II Career Award from the Fonds de la Recherche en Santé du Québec (FRSQ) and a New Investigator Award from the Canadian Institutes of Health Research. Dr Sharma is supported by the Alberta Innovates Health Solution Clinician Scientist Research Grant. The sponsors had no role in the design and conduct of the study; in the collection, management, analysis, and interpretation of the data; in the preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Disclosures
None.
Supporting information
Table S1. Baseline Characteristics for Included and Excluded Patients
(J Am Heart Assoc. 2018;7:e010139 DOI: 10.1161/JAHA.118.010139)
References
- 1. Afilalo J, Alexander KP, Mack MJ, Maurer MS, Green P, Allen LA, Popma JJ, Ferrucci L, Forman DE. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol. 2014;63:747–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sepehri A, Beggs T, Hassan A, Rigatto C, Shaw‐Daigle C, Tangri N, Arora RC. The impact of frailty on outcomes after cardiac surgery: a systematic review. J Thorac Cardiovasc Surg. 2014;148:3110–3117. [DOI] [PubMed] [Google Scholar]
- 3. Kim DH, Kim CA, Placide S, Lipsitz LA, Marcantonio ER. Preoperative frailty assessment and outcomes at 6 months or later in older adults undergoing cardiac surgical procedures: a systematic review. Ann Intern Med. 2016;165:650–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goldfarb M, Bendayan M, Rudski LG, Morin JF, Langlois Y, Ma F, Lachapelle K, Cecere R, DeVarennes B, Tchervenkov CI, Brophy JM, Afilalo J. Cost of cardiac surgery in frail compared with nonfrail older adults. Can J Cardiol. 2017;33:1020–1026. [DOI] [PubMed] [Google Scholar]
- 5. Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, Nevitt M, Visser M, Kritchevsky S, Badinelli S, Harris T, Newman AB, Cauley J, Ferrucci L, Guralnik J. Gait speed and survival in older adults. JAMA. 2011;305:50–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Afilalo J, Eisenberg MJ, Morin JF, Bergman H, Monette J, Noiseux N, Perrault LP, Alexander KP, Langlois Y, Dendukuri N, Chamoun P, Kasparian G, Robichaud S, Gharacholou SM, Boivin JF. Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J Am Coll Cardiol. 2010;56:1668–1676. [DOI] [PubMed] [Google Scholar]
- 7. Afilalo J, Kim S, O'Brien S, Brennan JM, Edwards FH, Mack MJ, McClurken JB, Cleveland JC Jr, Smith PK, Shahian DM, Alexander KP. Gait speed and operative mortality in older adults following cardiac surgery. JAMA Cardiol. 2016;1:314–321. [DOI] [PubMed] [Google Scholar]
- 8. Schopfer DW, Forman DE. Cardiac rehabilitation in older adults. Can J Cardiol. 2016;32:1088–1096. [DOI] [PubMed] [Google Scholar]
- 9. Grazzi G, Mazzoni G, Myers J, Codecà L, Pasanisi G, Napoli N, Guerzoni F, Volpato S, Conconi F, Chiaranda G. Improved walking speed is associated with lower hospitalisation rates in patients in an exercise‐based secondary prevention programme. Heart. 2016;102:1902–1908. [DOI] [PubMed] [Google Scholar]
- 10. Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, Poole C, Schlesselman JJ, Egger M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Ann Intern Med. 2007;147:W163–W194. [DOI] [PubMed] [Google Scholar]
- 11. Hammill BG, Hernandez AF, Peterson ED, Fonarow GC, Schulman KA, Curtis LH. Linking inpatient clinical registry data to Medicare claims data using indirect identifiers. Am Heart J. 2009;157:995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shahian DM, O'Brien SM, Sheng S, Grover FL, Mayer JE, Jacobs JP, Weiss JM, Delong ER, Peterson ED, Weintraub WS, Grau‐Sepulveda MV, Klein LW, Shaw RE, Garratt KN, Moussa ID, Shewan CM, Dangas GD, Edwards FH. Predictors of long‐term survival after coronary artery bypass grafting surgery: results from the Society of Thoracic Surgeons Adult Cardiac Surgery Database (the ASCERT study). Circulation. 2012;125:1491–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pencina MJ, D'agostino RB, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172; discussion, 207–212. [DOI] [PubMed] [Google Scholar]
- 14. Walston J. Frailty—the search for underlying causes. Sci Aging Knowledge Environ. 2004;2004:pe4. [DOI] [PubMed] [Google Scholar]
- 15. Newman AB, Gottdiener JS, Mcburnie MA, Hirsch CH, Kop WJ, Tracy R, Walston JD, Fried LP; Cardiovascular Health Study Research Group . Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. 2001;56:M158–M166. [DOI] [PubMed] [Google Scholar]
- 16. Chaves PH, Semba RD, Leng SX, Woodman RC, Ferrucci L, Guralnik JM, Fried LP. Impact of anemia and cardiovascular disease on frailty status of community‐dwelling older women: the Women's Health and Aging Studies I and II. J Gerontol A Biol Sci Med Sci. 2005;60:729–735. [DOI] [PubMed] [Google Scholar]
- 17. Shimura T, Yamamoto M, Kano S, Kagase A, Kodama A, Koyama Y, Otsuka T, Kohsaka S, Tada N, Yamanaka F, Naganuma T, Araki M, Shirai S, Mizutani K, Tabata M, Ueno H, Takagi K, Higashimori A, Watanabe Y, Hayashida K. Impact of frailty markers on outcomes after transcatheter aortic valve replacement: insights from a Japanese multicenter registry. Ann Cardiothorac Surg. 2017;6:532–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Afilalo J, Mottillo S, Eisenberg MJ, Alexander KP, Noiseux N, Perrault LP, Morin JF, Langlois Y, Ohayon SM, Monette J, Boivin JF, Shahian DM, Bergman H. Addition of frailty and disability to cardiac surgery risk scores identifies elderly patients at high risk of mortality or major morbidity. Circ Cardiovasc Qual Outcomes. 2012;5:222–228. [DOI] [PubMed] [Google Scholar]
- 19. Sharma A, de Souza Brito F, Sun JL, Thomas L, Haffner S, Holman RR, Lopes RD. Noncardiovascular deaths are more common than cardiovascular deaths in patients with cardiovascular disease or cardiovascular risk factors and impaired glucose tolerance: insights from the Nateglinide and Valsartan in Impaired Glucose Tolerance Outcomes Research (NAVIGATOR) trial. Am Heart J. 2017;186:73–82. [DOI] [PubMed] [Google Scholar]
- 20. Bibas L, Levi M, Bendayan M, Mullie L, Forman DE, Afilalo J. Therapeutic interventions for frail elderly patients: part I. Published randomized trials. Prog Cardiovasc Dis. 2014;57:134–143. [DOI] [PubMed] [Google Scholar]
- 21. Bendayan M, Bibas L, Levi M, Mullie L, Forman DE, Afilalo J. Therapeutic interventions for frail elderly patients: part II. Ongoing and unpublished randomized trials. Prog Cardiovasc Dis. 2014;57:144–151. [DOI] [PubMed] [Google Scholar]
- 22. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ferrucci L, Maggio M, Ceda GP, Beghi C, Valenti G, De Cicco G. Acute postoperative frailty. J Am Coll Surg. 2006;203:134–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vigorito C, Abreu A, Ambrosetti M, Belardinelli R, Corrà U, Cupples M, Davos CH, Hoefer S, Iliou MC, Schmid JP, Voeller H, Doherty P. Frailty and cardiac rehabilitation: a call to action from the EAPC Cardiac Rehabilitation Section. Eur J Prev Cardiol. 2017;24:577–590. [DOI] [PubMed] [Google Scholar]
- 25. Stammers AN, Kehler DS, Afilalo J, Avery LJ, Bagshaw SM, Grocott HP, Légaré JF, Logsetty S, Metge C, Nguyen T, Rockwood K, Sareen J, Sawatzky JA, Tangri N, Giacomantonio N, Hassan A, Duhamel TA, Arora RC. Protocol for the PREHAB study‐pre‐operative rehabilitation for reduction of hospitalization after coronary bypass and valvular surgery: a randomised controlled trial. BMJ Open. 2015;5:e007250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alfredsson J, Stebbins A, Brennan JM, Matsouaka R, Afilalo J, Peterson ED, Vemulapalli S, Rumsfeld JS, Shahian D, Mack MJ, Alexander KP. Gait speed predicts 30‐day mortality after transcatheter aortic valve replacement: results from the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Circulation. 2016;133:1351–1359. [DOI] [PubMed] [Google Scholar]
- 27. Kano S, Yamamoto M, Shimura T, Kagase A, Tsuzuki M, Kodama A, Koyama Y, Kobayashi T, Shibata K, Tada N, Naganuma T, Araki M, Yamanaka F, Shirai S, Mizutani K, Tabata M, Ueno H, Takagi K, Higashimori A, Otsuka T, Watanabe Y, Hayashida K. Gait speed can predict advanced clinical outcomes in patients who undergo transcatheter aortic valve replacement: insights from a Japanese multicenter registry. Circ Cardiovasc Interv. 2017;10:e005088. [DOI] [PubMed] [Google Scholar]
- 28. Afilalo J, Lauck S, Kim DH, Lefèvre T, Piazza N, Lachapelle K, Martucci G, Lamy A, Labinaz M, Peterson MD, Arora RC, Noiseux N, Rassi A, Palacios IF, Généreux P, Lindman BR, Asgar AW, Kim CA, Trnkus A, Morais JA, Langlois Y, Rudski LG, Morin JF, Popma JJ, Webb JG, Perrault LP. Frailty in older adults undergoing aortic valve replacement: the FRAILTY‐AVR study. J Am Coll Cardiol. 2017;70:689–700. [DOI] [PubMed] [Google Scholar]
- 29. Lilamand M, Dumonteil N, Nourhashemi F, Hanon O, Marcheix B, Toulza O, Elmalem S, van Kan GA, Raynaud‐Simon A, Vellas B, Afilalo J, Cesari M. Gait speed and comprehensive geriatric assessment: two keys to improve the management of older persons with aortic stenosis. Int J Cardiol. 2014;173:580–582. [DOI] [PubMed] [Google Scholar]
- 30. Afilalo J, Forman DE. Gait speed assessment in transcatheter aortic valve replacement: a step in the right direction. Circ Cardiovasc Interv. 2017;10:e005746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alexander KP. Walking as a window to risk and resiliency. Circulation. 2017;136:644–645. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline Characteristics for Included and Excluded Patients


