Abstract
Background
Late gadolinium enhancement magnetic resonance imaging is an effective tool for assessment of atrial fibrosis. The degree of left atrial fibrosis is a good predictor of atrial fibrillation (AF) ablation success at 1 year, but the association between left atrial fibrosis and long‐term ablation success has not been studied.
Methods and Results
Late gadolinium enhancement magnetic resonance images of sufficient quality to quantify atrial fibrosis were obtained before the first AF ablation in 308 consecutive patients. Left atrial fibrosis was classified in 4 Utah stages (I, 0–10%; II, 10–20%; III, 20–30%; and IV, >30%). Patients were followed up for up to 5 years until the time of first arrhythmia recurrence or second ablation. A total of 308 patients were included; the mean age was 64.5±12.1 years, and 63.4% were men. During follow‐up, 157 patients experienced an arrhythmia recurrence and 106 patients underwent a repeated ablation. A graded effect was observed in which patients with more advanced atrial fibrosis were more likely to experience recurrent AF (hazard ratio for stage IV versus stage I, 2.73; 95% confidence interval, 1.57–4.75) and undergo a repeated ablation (proportional odds ratio for stage IV versus stage I, 5.19; 95% confidence interval, 2.12–12.69).
Conclusions
The degree of left atrial fibrosis predicts the success of AF ablation at up to 5 years follow‐up. In patients with advanced atrial fibrosis, AF ablation is associated with a high procedural failure rate.
Keywords: ablation, atrial fibrillation, fibrosis, magnetic resonance imaging
Subject Categories: Atrial Fibrillation, Fibrosis, Clinical Studies, Magnetic Resonance Imaging (MRI), Quality and Outcomes
Clinical Perspective
What Is New?
This study shows that the degree of left atrial fibrosis predicts the long‐term (up to 5 years follow‐up) success of atrial fibrillation ablation.
In addition, it identifies a group with high procedural failure rate of atrial fibrillation ablation attributable to advanced atrial fibrosis.
The likelihood of repeated ablations increased as the degree of atrial fibrosis increased.
What Are the Clinical Implications?
The degree of atrial fibrosis should be taken into consideration when discussing with patients the success of atrial fibrillation ablation and the likelihood of multiple ablations.
Patients with advanced atrial fibrosis may not benefit from atrial fibrillation ablation, or new ablation strategies may be needed to improve the outcome in this subset of patients.
Introduction
Catheter ablation of atrial fibrillation (AF) represents an important strategy for maintaining sinus rhythm (SR) in patients with symptomatic AF.1 However, successful maintenance of SR is challenging, and recurrence of AF after the procedure is common.2, 3, 4, 5 A major predictor of long‐term treatment success is the baseline severity of the left atrial (LA) fibrotic myopathy that underlies the AF.6, 7 Late gadolinium enhancement magnetic resonance imaging (LGE‐MRI) has been shown to be an effective tool for assessment and quantification of LA fibrosis.8 In a multicenter prospective study (DECAAF [Delayed Enhancement‐MRI Determinant of Successful Catheter Ablation of Atrial Fibrillation]), a high degree of atrial fibrosis, relative to a low degree of atrial fibrosis (Utah stage IV versus stage I), was shown to be independently associated with a >4‐fold increased likelihood of recurrent arrhythmia within 1 year after AF ablation.6
Several studies have reported the 5‐year outcome of catheter ablation of AF2, 3, 4, 5 or even the 10‐year outcomes.9 However, the predictive value of LA fibrosis, a marker of the severity of the atrial fibrotic myopathy, on long‐term outcomes of AF ablation or need for subsequent ablations has not been examined. Therefore, the objective of this study was to characterize the long‐term outcomes after AF ablation stratified by the quantity of LA fibrosis and determine the utility of LA fibrosis as an independent predictive factor of those outcomes.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Design
A retrospective cohort study design was used to compare the association of recurrent atrial arrhythmias and Utah stages of LA fibrosis after catheter ablation. Patients who underwent LGE‐MRI of sufficient quality for LA fibrosis assessment, and subsequently underwent AF ablation, were included in the analysis. The date of the ablation defined the index date, and a 90‐day blanking period was used to allow for optimization of antiarrhythmic medications or reablation, as needed. Patients were followed up observationally until recurrence of atrial arrhythmia, reablation, death, last rhythm assessment, or 5 years from the index, whichever occurred first.
Patient Population
Patient data were obtained from the Atrial Fibrillation Research Registry at the Comprehensive Arrhythmia and Research Management Center, University of Utah (Salt Lake City, UT), between December 2006 and January 2010. This database has been previously described in detail.10 Briefly, this registry is an observational, retrospective database of patients who undergo LGE‐MRI as part of routine arrhythmia care at the University of Utah. The registry contains clinical and demographic data, extracted from electronic medical records, and is a particularly rich source of imaging, AF phenotype, ablation, and rhythm assessment data. Patients within the database were excluded from this study because of unquantifiable fibrosis (poor LGE‐MRI image quality, LGE‐MRI not performed, or LGE‐MRI performed after the ablation procedure), <90 days of follow‐up after ablation (blanking period), and catheter ablation to treat an arrhythmia other than AF. In addition, because LA fibrosis is likely a dynamic measurement that progresses with time, we excluded patients whose LGE‐MRI occurred >90 days preablation. The database study protocol was reviewed and approved by the University of Utah Institutional Review Board and was Health Insurance Portability and Accountability Act compliant. Patient requirement for consent has been waived.
Ablation Procedure
The LA ablation procedure was previously described in detail.11, 12 Briefly, a 14‐pole catheter (TZMedical [Portland, OR] or Bard EP [Lowell, MA]) was used to record right atrial and coronary sinus electrograms and as the reference catheter for 3‐dimensional electroanatomical mapping with CARTO 3 (Biosense‐Webster, Inc, Diamond Bar, CA). Two transseptal punctures were performed under intracardiac echocardiography guidance using a phased‐array catheter (AccuNav; Siemens Medical Solutions USA, Inc, Mountain View, CA). A circular mapping catheter (Lasso; Biosense Webster Inc) and a radiofrequency ablation catheter (ThermoCool NaviStar; Biosense Webster, Inc) were advanced into the left atrium for mapping and ablation. Radiofrequency energy was delivered with 50 W (30 mL/min open irrigation) at a catheter tip temperature of 50°C for a maximum duration of 15 seconds and was guided by electrogram abolition. Ablation lesions were placed in a circular manner in the pulmonary vein (PV) antral region until electrical isolation of the PVs was achieved. PV isolation (PVI) was successful in all patients acutely. Radiofrequency delivery was interrupted if the impedance increased suddenly or if a burst in microbubble density was seen by intracardiac echocardiography. Additional LA posterior wall ablation was performed as previously described.11 Posterior wall debulking was performed in 90% of the patients, and a cavotricuspid isthmus ablation was performed in 11% of the patients. Bidirectional block across the cavotricuspid line was confirmed in all patients. The decision on the ablation strategy was at the operator's discretion.
MRI Image Acquisition
MRI image acquisition was performed as previously described.11, 12 Briefly, images were acquired with either a 1.5‐T Avanto or a 3‐T Verio clinical MRI scanner (Siemens Healthcare, Erlangen, Germany) using body and spine phased‐array receiver coils. Scans were performed ≈15 minutes after contrast agent injection (0.1 mmol/kg of Multihance; Bracco Diagnostic Inc, Princeton, NJ) using a 3‐dimensional inversion recovery prepared, respiration‐navigated, ECG‐gated, gradient echo pulse sequence. The acquisition parameters were as follows: free breathing using navigator gating, a transverse imaging volume with a voxel size of 1.25×1.25×2.5 mm (reconstructed to 0.625×0.625×1.25 mm), inversion time=270 to 320 ms, and generalized autocalibrating partially parallel acquisition with reduction factor R=2. The other scan parameters for LGE‐MRI at 1.5‐T scanner were as follows: repetition time=5.2 ms, echo time=2.4 ms, and flip angle=20°. Scan parameters for LGE‐MRI at 3‐T scanner were as follows: repetition time/echo time=3.1/1.4 ms and flip angle=14°. Fat saturation was used to suppress fat signal, and an echo time of the LGE scan was chosen such that fat and water signals were approximately out of phase, resulting in a reduced signal intensity of partial volume fat‐tissue voxels and improved delineation of the LA wall boundary. The inversion time was identified using an inversion time scout scan. ECG gating was used to acquire a small subset of phase‐encoding views during the diastolic phase of the LA cardiac cycle. The time interval between the R peak of the ECG and the start of data acquisition was defined using cine images of the LA.
LGE‐MRI Quantification of LA Fibrosis
LA wall volumes were manually segmented from LGE‐MRI images using the Corview image processing software (Marrek Inc, Salt Lake City, UT), as described later. The endocardial border of the LA and the proximal PVs was delineated by manually tracing the LA‐PV blood pool in each slice of the LGE‐MRI volume. Then, the endocardial segmentation was morphologically dilated and manually adjusted to create an assessment of the boundary of the epicardial LA surface. Finally, the endocardial segmentation was subtracted from the epicardial segmentation to define a wall segmentation, which was manually edited to exclude the mitral valve and PVs. Thus, the resulting LA wall segmentation included the 3‐dimensional extent of both the LA wall and the antral regions of the PVs.
Quantification of LA fibrosis was obtained using the methods previously described.8 Briefly, to delineate regions of fibrosis in preablation LGE‐MRI images, we defined enhancement through an intensity threshold. To assist this process, initial visualization used a volume‐rendering tool in Corview that allowed the operator to visualize the distribution of enhancement in 3 dimensions. A custom transfer function allowed the operator to define gradations of enhancements while suppressing blood and normal tissue. The quantity of fibrosis was categorized according to Utah stages of LA fibrosis.6
Outcomes
The primary outcome in this study was a recurrence of an atrial arrhythmia after an AF ablation. Recurrence after ablation was defined as a documented rhythm of atrial tachycardia/AF/atrial flutter or a repeated ablation after a 90‐day blanking period. We also examined time to repeated ablation alone. Ablations of supraventricular tachycardia, premature atrial contractions, or right side only (cavotricuspid isthmus) were not considered to be recurrence or repeated ablation. Documentation of recurrence and repeated ablation was obtained through chart review of results from rhythm assessments (eg, 12‐lead ECG, echocardiographs, or Holter/event monitors) and provider (eg, electrophysiologist, cardiologist, and emergency department physician) notes stating that the patient had experienced a recurrence.
Statistical Analysis
We used means and SDs for continuous variables, and counts and percentages for categorical variables, to describe the distribution of baseline characteristics among patients stratified by Utah stage. Between‐group differences in characteristics were assessed with ANOVA or χ2 tests, as appropriate.
The Kaplan‐Meier method was used to calculate arrhythmia‐free survival by Utah stage of LA fibrosis. Cox proportional hazard regression models were used to calculate univariable‐ and multivariable‐adjusted hazard ratios (HRs) for time to recurrence associated with Utah stage and other clinical and demographic characteristics; Utah stage I served as the reference category in all models. All covariates considered to be potentially related to both the severity of fibrosis and recurrence of arrhythmia on the basis of prior research and clinical judgment were evaluated for inclusion in multivariable models. Four nested models were constructed. Model 1 included adjustment for patient demographics (age, sex, body mass index, smoking status, and AF type). Model 2 included the variables in model 1 and additional adjustment for comorbidities (heart failure, hypertension, diabetes mellitus, and stroke history). Model 3 included the variables in model 2 and additional adjustment for medication use (class I and III antiarrhythmic use, calcium channel blocker use, angiotensin‐converting enzyme inhibitor or angiotensin II receptor blocker use, and β‐blocker use.). Model 4, the fully adjusted model, included the variables in model 3 with additional adjustment for other laboratory and cardiac measurements (left ventricular ejection fraction, LA volume, LA area, left ventricular diameter, estimated glomerular filtration rate, and mitral valve regurgitation). A common rule of thumb in regression modeling is to include ≤10 predictor variables per observed outcome to avoid the risk of model overfitting. However, this number may be overly conservative, and may be relaxed to 5 to 9 outcomes, particularly to demonstrate adequate control of confounding.13 Because model 4 includes <10 outcomes per predictor variable, the results of this model should be interpreted with caution and compared with the results from the more parsimonious models 1 to 3. We tested all models for proportional hazards, both for each model variable and globally, using a formal significance test based on the unscaled and scaled Schoenfeld residuals. No violation of this assumption was observed. Because the hazard during the blanking period is artificially 0 for all groups, day 90 was used as the start of follow‐up in all Cox models. P values for linear trend across Utah stage were calculated by modeling the Utah stage as an ordinal variable in the Cox proportional hazard models. We further explored the relationship between LA fibrosis and risk of arrhythmia recurrence using natural cubic splines. A cubic spline graph with 3 knots was created by calculating a fully adjusted HR for time to recurrence associated with LA fibrosis modeled as a continuous variable, rather than as an ordinal variable. Ordinal logistic regression was used to associate Utah stage with the number of ablations experienced during the 5‐year follow‐up period.
All P values are 2 sided, and all analyses were performed using Stata, version 15.0 (StataCorp, College Station, TX).
Results
Patient Characteristics
A total of 308 patients met the inclusion criteria (Figure 1). Overall, the median duration of follow‐up was 1.1 years (interquartile range, 0.5–3.0 years). The average patient age was 64.5±12.1 years, and 63.4% were men. Of the patients, 67% had hypertension, 13% had heart failure, 15% had diabetes mellitus, and 22% had a prior transient ischemic attack or stroke; 25% were taking class IA or III antiarrhythmic drugs before ablation. The clinical characteristics of the study population, stratified by Utah stage and by the occurrence of censoring from the study before 5 years, are presented in Tables 1 and 2, respectively.
Figure 1.
Patient selection flowchart. AF indicates atrial fibrillation; CARMA, Comprehensive Arrhythmia and Research Management; LAF, left atrial fibrosis; LGE‐MRI, late gadolinium enhancement magnetic resonance imaging.
Table 1.
Patient Demographics by Utah Stages of LA Fibrosis
| Baseline Characteristic | Utah Stages of LA Fibrosis | P Value | |||
|---|---|---|---|---|---|
| I (N=105) | II (N=108) | III (N=61) | IV (N=34) | ||
| Age, y | 62.9±12.3 | 64.5±10.9 | 65.3±13.6 | 66.9±12.2 | 0.080 |
| Male sex | 69 (65.7) | 76 (70.4) | 34 (55.7) | 17 (50.0) | 0.038 |
| Body mass index, kg/m2 | 28.8±6.3 | 29.8±5.4 | 29.6±6.5 | 28.6±5.7 | 0.887 |
| Smoker | 32 (30.5) | 25 (23.1) | 14 (23.0) | 11 (32.4) | 0.850 |
| Paroxysmal AF | 46 (43.8) | 47 (43.5) | 25 (41.0) | 5 (14.7) | 0.004 |
| AF duration, mo | 24.7 (6.4–60.6) | 33.4 (8.7–79.9) | 25.5 (8.1–124.2) | 61.6 (15.9–101.2) | 0.143 |
| Congestive heart failure | 12 (11.4) | 12 (11.1) | 12 (19.7) | 5 (14.7) | 0.385 |
| Hypertension | 76 (72.4) | 74 (68.5) | 32 (52.5) | 26 (76.5) | 0.981 |
| Diabetes mellitus | 12 (11.4) | 14 (13.0) | 14 (23.0) | 6 (17.6) | 0.189 |
| Prior stroke | 9 (8.6) | 10 (9.3) | 7 (11.5) | 8 (23.5) | 0.024 |
| CHA2DS2VASc score | |||||
| 0 | 8 (7.6) | 10 (9.3) | 8 (13.1) | 3 (8.8) | 0.69 |
| 1 | 40 (38.1) | 40 (37) | 10 (16.4) | 3 (8.8) | <0.001 |
| ≥2 | 57 (54.3) | 58 (53.7) | 43 (70.5) | 28 (82.4) | 0.001 |
| AAD class | |||||
| I | 14 (13.3) | 11 (10.2) | 9 (14.8) | 5 (14.7) | 0.663 |
| III | 14 (13.3) | 14 (13.0) | 3 (4.9) | 6 (17.6) | 0.971 |
| Calcium channel blockers | 29 (27.6) | 33 (30.6) | 17 (27.9) | 12 (35.3) | 0.472 |
| β Blockers | 55 (52.4) | 47 (43.5) | 29 (47.5) | 21 (61.8) | 0.294 |
| ACEI or ARB | 45 (42.9) | 36 (33.3) | 24 (39.3) | 12 (35.3) | 0.585 |
| LV ejection fraction, % | 58.2±9.2 | 56.5±11 | 57.9±9.7 | 55.6±10.3 | 0.302 |
| LA volume, mL | 97±37.1 | 104.8±40.3 | 104±43.9 | 128±50.2 | <0.001 |
| LA area, cm2 | 27.4±7.2 | 26.9±6.6 | 27.3±5.7 | 31.9±9.7 | 0.002 |
| LV diastolic diameter, cm | 5.1±0.6 | 5±0.5 | 5.1±0.6 | 5.1±0.7 | 0.611 |
| GFR, mL/min per 1.73 m2 | 74.2±16.3 | 73.1±18.1 | 72.1±24 | 71.4±20.5 | 0.417 |
| MVR | 7 (6.7) | 3 (2.8) | 5 (8.2) | 4 (11.8) | 0.155 |
Data are given as mean±SD, median (interquartile range), or count (percentage). AAD indicates antiarrhythmic drug; ACEI, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin II receptor blocker; GFR, glomerular filtration rate; LA, left atrial; LV, left ventricular; MVR, mitral valve regurgitation (mild or greater).
Table 2.
Patient Demographics by Censoring During Follow‐Up
| Baseline Characteristic | Censored Before 5 y of Follow‐Up | P Value | |
|---|---|---|---|
| Yes (N=204) | No (N=104) | ||
| Age, y | 62.7±13 | 65.2±11.5 | 0.084 |
| Male sex | 67 (64.4) | 129 (63.2) | 0.838 |
| Body mass index, kg/m2 | 28.9±5.7 | 29.4±6.1 | 0.473 |
| Smoker | 21 (20.2) | 61 (29.9) | 0.068 |
| Paroxysmal AF | 55 (52.9) | 68 (33.3) | <0.001 |
| AF duration, mo | 29.3 (8.3–96.2) | 25.3 (9.7–73.1) | 0.198 |
| Congestive heart failure | 7 (6.7) | 34 (16.7) | 0.015 |
| Hypertension | 59 (56.7) | 149 (73.0) | 0.004 |
| Diabetes mellitus | 13 (12.5) | 33 (16.2) | 0.392 |
| Prior stroke | 9 (8.7) | 25 (12.3) | 0.34 |
| CHA2DS2VASc score | |||
| 0 | 14 (13.5) | 15 (7.4) | 0.083 |
| 1 | 40 (38.5) | 53 (26.0) | 0.024 |
| ≥2 | 50 (48.1) | 136 (66.7) | 0.002 |
| AAD class | |||
| I | 14 (13.5) | 25 (12.3) | 0.763 |
| III | 9 (8.7) | 28 (13.7) | 0.195 |
| Calcium channel blockers | 32 (30.8) | 59 (28.9) | 0.737 |
| β Blockers | 50 (48.1) | 102 (50.0) | 0.75 |
| ACEI or ARB | 28 (26.9) | 89 (43.6) | 0.004 |
| LV ejection fraction, % | 57.1±9.7 | 57.3±10.3 | 0.843 |
| LA volume, mL | 97.8±37.4 | 108±43.8 | 0.044 |
| LA area, cm2 | 28.1±6.4 | 27.5±7.5 | 0.546 |
| LV diastolic diameter, cm | 5.1±0.6 | 5.0±0.6 | 0.753 |
| GFR, mL/min per 1.73 m2 | 74.5±18.3 | 72.4±19.5 | 0.347 |
| MVR | 5 (4.8) | 14 (6.9) | 0.478 |
Data are given as mean±SD, median (interquartile range), or count (percentage). AAD indicates antiarrhythmic drug; ACEI, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin II receptor blocker; GFR, glomerular filtration rate; LA, left atrial; LV, left ventricular; MVR, mitral valve regurgitation (mild or greater).
Imaging
Preprocedure LGE‐MRI was performed at 3 (interquartile range, 1–15) days preablation. The distribution of fibrosis values in the population was skewed, with more patients presenting with smaller quantities of fibrosis. Overall, there were 105 patients (34.1%) with stage I, 108 patients (35.1%) with stage II, 61 patients (19.8%) with stage III, and 34 patients (11.0%) with stage IV fibrosis. The percentage of patients with paroxysmal AF decreased as the stage of fibrosis increased: stage I, 43.8%; stage II, 43.5%; stage III, 41.0%; and stage IV, 14.7%.
Arrhythmia‐Free Survival After a Single Procedure
Overall, 157 patients experienced an arrhythmia recurrence during the 5‐year follow‐up period. The HRs for individual demographic and clinical factors relating to the time to arrhythmia recurrence are reported in Table 3. In univariable analysis, LA fibrosis, tobacco use, paroxysmal AF (versus persistent AF), history of stroke, LA volume, LA area, and mitral valve regurgitation had statistically significant associations with arrhythmia recurrence. In multivariable analysis, the incidence of atrial recurrence was progressively higher as fibrosis increased (Figure 2 and Table 4). After full multivariable adjustment, patients with stage IV versus stage I had a statistically significantly higher risk of arrhythmia recurrence (HR, 2.73; 95% confidence interval [CI], 1.57–4.75). When considering LA fibrosis as a continuous variable, there was an observed 45% increased risk of recurrence for every 10% increase in atrial fibrosis (HR, 1.45; 95% CI, 1.20–1.76; P<0.001).
Table 3.
Univariable Association of Baseline Characteristics With Arrhythmia Recurrence and Repeated Ablation
| Baseline Characteristics | Recurrence of Atrial Arrhythmia or Repeated Ablation | |
|---|---|---|
| HR (95% CI) | P Value | |
| Left atrial fibrosis (per 10%) | 1.45 (1.24–1.68) | <0.001 |
| Age (per 10 y) | 1.06 (0.91–1.24) | 0.467 |
| Male sex | 0.98 (0.70–1.35) | 0.879 |
| Body mass index (per kg/m2) | 1.02 (1.00–1.05) | 0.086 |
| Smoker | 1.41 (1.00–1.98) | 0.047 |
| Paroxysmal AF | 0.53 (0.38–0.75) | <0.001 |
| Congestive heart failure | 1.06 (0.70–1.62) | 0.781 |
| Hypertension | 1.42 (0.99–2.03) | 0.057 |
| Diabetes mellitus | 1.25 (0.84–1.88) | 0.274 |
| Prior stroke | 2.04 (1.32–3.17) | 0.001 |
| CHA2DS2‐VASc score | ||
| 0 | 1.00 | … |
| 1 | 1.24 (0.62–2.49) | 0.547 |
| ≥2 | 1.84 (0.96–3.51) | 0.066 |
| AAD class | ||
| I | 1.01 (0.63–1.62) | 0.962 |
| III | 1.41 (0.91–2.17) | 0.125 |
| Calcium channel blockers | 1.08 (0.77–1.52) | 0.651 |
| β Blockers | 1.04 (0.76–1.42) | 0.816 |
| ACEI or ARB | 1.34 (0.97–1.83) | 0.073 |
| LV ejection fraction, % | 1.00 (0.99–1.02) | 0.987 |
| LA volume, mL | 1.01 (1.00–1.01) | <0.001 |
| LA area, cm2 | 1.04 (1.02–1.06) | <0.001 |
| LV diastolic diameter, cm | 1.26 (0.95–1.68) | 0.111 |
| GFR, mL/min per 1.73 m2 | 1.00 (0.99–1.01) | 0.939 |
| MVR | 1.95 (1.11–3.45) | 0.021 |
AAD indicates antiarrhythmic drug; ACEI, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin II receptor blocker; CI, confidence interval; GFR, glomerular filtration rate; HR, hazard ratio; LA, left atrial; LV, left ventricular; MVR, mitral valve regurgitation (mild or greater).
Figure 2.
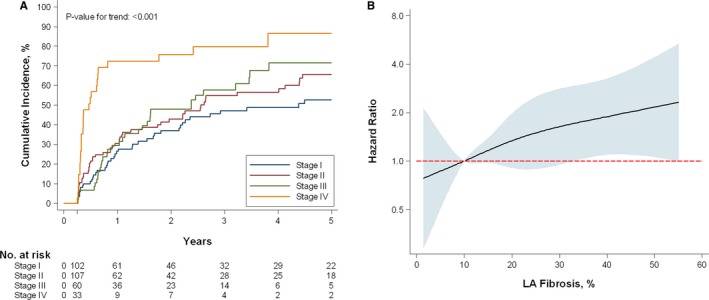
Incidence of atrial fibrillation after a single‐catheter ablation attempt, by left atrial (LA) fibrosis severity. A, Kaplan‐Meier cumulative incidence curve of recurrent atrial arrhythmia or repeated ablation procedure. B, The multivariable‐adjusted hazard ratio (HR) associating left atrial fibrosis (as a continuous variable) with recurrent atrial arrhythmia or repeated ablation after a single‐catheter ablation procedure. The solid line represents the HR, and the shaded area represents the 95% confidence interval. The model includes full covariate adjustment (model 4).
Table 4.
Arrhythmia Recurrence and Repeated Ablation by Utah Stage of LA Fibrosis
| Recurrence of Atrial Arrhythmia or Repeated Ablation | Utah Stages of LA Fibrosis | P Value for Trend | |||
|---|---|---|---|---|---|
| I | II | III | IV | ||
| No. of events | 42 | 54 | 34 | 27 | |
| Incidence rate, per 10 000 person‐years | 5.7 | 8.0 | 9.2 | 25.9 | |
| Hazard ratio (95% CI) | |||||
| Model 1a | 1.00 | 1.34 (0.89–2.03) | 1.39 (0.87–2.22) | 3.17 (1.88–5.33) | <0.001 |
| Model 2b | 1.00 | 1.40 (0.92–2.12) | 1.45 (0.90–2.33) | 2.89 (1.69–4.94) | <0.001 |
| Model 3c | 1.00 | 1.45 (0.96–2.20) | 1.47 (0.90–2.38) | 3.04 (1.76–5.26) | <0.001 |
| Model 4d | 1.00 | 1.55 (1.00–2.40) | 1.36 (0.83–2.25) | 2.73 (1.57–4.75) | 0.001 |
CI indicates confidence interval; LA, left atrial.
Adjusted for age, sex, body mass index, smoking status, and atrial fibrillation type.
Includes variables in model 1 and additional adjustment for heart failure, hypertension, diabetes mellitus, and stroke history.
Includes variables in model 2 and additional adjustment for class I and III antiarrhythmic use, calcium channel blocker use, angiotensin‐converting enzyme inhibitor or angiotensin II receptor blocker use, and β‐blocker use.
Includes variables in model 3 and additional adjustment for left ventricular ejection fraction, LA volume, LA area, left ventricular diameter, estimated glomerular filtration rate, and mitral valve regurgitation.
Repeated Ablation
Overall, at least one repeated ablation was performed in 106 patients (34.4%); multiple repeated ablations were performed in 16 patients (5.2%) (Table 5). The likelihood of repeated ablations increased as fibrosis increased. Of 5 patients with stage IV fibrosis, 3 underwent at least one repeated procedure. Patients with stage IV fibrosis were 5 times more likely to have a repeated ablation performed than patients with stage I fibrosis. This relationship remained significant in the fully adjusted model.
Table 5.
Number of Ablations by Utah Stage of LA Fibrosis
| No. of AF Ablations in 5‐y Period | Utah Stages of LA Fibrosis | P Value for Trend | |||
|---|---|---|---|---|---|
| I | II | III | IV | ||
| Mean±SD | 1.28±0.56 | 1.32±0.51 | 1.34±0.48 | 1.79±0.77 | |
| Proportional odds ratio (95% CI)a | 1.00 | 1.52 (0.78–2.95) | 1.77 (0.82–3.82) | 5.19 (2.12–12.69) | <0.001 |
| Adjusted proportion, %b | |||||
| 1 Ablation | 76 | 69 | 66 | 43 | |
| 2 Ablations | 22 | 29 | 31 | 50 | |
| 3 or 4 Ablations | 2 | 2 | 3 | 8 | |
Values represent the total number of AF ablations that were observed while observing patients retrospectively and observationally for up to 5 years. AF indicates atrial fibrillation; CI, confidence interval; LA, left atrial.
Proportional odds ratio calculated from ordinal logistic regression model using full covariate adjustment (model 4).
Calculated using predictive margins after the multivariable‐adjusted ordinal logistic regression.
Discussion
In this retrospective analysis of patients with quantified atrial fibrosis by LGE‐MRI who underwent AF ablation, we demonstrated a strong association between the degree of atrial fibrosis and the long‐term outcome of AF ablation up to 5 years postprocedure. To the best of our knowledge, the present study is the first to extend the finding that the degree of atrial fibrosis is associated with AF recurrence to beyond the first year postprocedure.
The predictive value of atrial fibrosis on the outcome of AF ablation has been previously reported in short‐term follow‐up studies. A retrospective study performed in 426 patients with a follow‐up of 1 year has shown a strong correlation between recurrent arrhythmias and the degree of atrial fibrosis (stage I, 21%; stage II, 29.3%; stage III, 33.8%; and stage IV, 71.4%).14 In a multivariable analysis, the best predictors of ablation outcome were atrial fibrosis stage (HR, 4.89; P<0.0001) and diabetes mellitus (HR, 1.64; P=0.036).14 Increased LA volume and persistent AF were not significant predictors when adjusting for fibrosis.14 The DECAAF study, a multicenter prospective study, has reported the association between the amount of atrial fibrosis and arrhythmia recurrence after AF ablation at 475 days in 272 patients.6 Arrhythmia recurrences were significantly associated with the degree of atrial fibrosis on presentation (stage I, 15%; stage II, 36%; stage III, 46%; and stage IV, 69%).6 In a multivariable analysis, atrial fibrosis was the only variable that was significantly associated with arrhythmia recurrence post‐AF ablation.6 Another study performed in 165 patients with a follow‐up of 10.2±5.7 months has shown that baseline LGE extent was independently associated with AF recurrence after adjusting for confounders (HR, 1.5 per 10% increased LGE; P<0.001) and that the HR for AF recurrence progressively increased as a function of LGE extent.15
Other studies have reported long‐term outcomes without examining the predictive value of atrial fibrosis. A retrospective study performed in 100 patients with a 5‐year follow‐up showed that an arrhythmia‐free survival rate after a single catheter ablation procedure was 29%, whereas arrhythmia‐free survival after the last catheter ablation procedure was 63% at 5 years.2 The median number of procedures per patient was 2.2 The recurrence rate was higher in patients with long‐standing persistent AF than those with paroxysmal or persistent AF (HR, 1.9; 95% CI, 1.0–3.5; P=0.0462).2 Valvular heart disease (HR, 6.0; 95% CI, 2.0–17.6; P=0.0012) and nonischemic dilated cardiomyopathy (HR, 34.0; 95% CI, 6.3–182.1; P=0.0001) were independent predictors of recurrences.2
In a retrospective study, examining time to the outcome of long‐standing persistent AF ablation in 202 patients with 56 months of follow‐up (range, 49–67 months), the success rate was 20% and 45% with single and multiple ablation procedures, respectively.4 PVI was the initial ablation strategy, whereas additional ablation was performed if direct current cardioversion failed after PVI. Persistent AF duration was an independent predictor of arrhythmia recurrence (HR, 1.09; 95% CI, 1.04–1.13; P=0.001). In addition, acute PVI responders had a reduced risk of recurrence (HR, 0.57; 95% CI, 0.41–0.78; P=0.001) after the first ablation.
Scherr et al5 reported arrhythmia‐free survival rates after a single procedure of 35.3%, 28.0%, and 16.8% at 1, 2, and 5 years, respectively, in a retrospective study performed in 150 patients with persistent AF. The goal of AF termination with a stepwise ablation approach (PVI, electrogram guided, and linear ablation) was achieved in 120 patients (80%). A repeated ablation was performed for recurrent AF or atrial tachycardia. The reported arrhythmia‐free survival rates after the last procedure (mean, 2.1±1.0 procedures) were 89.7%, 79.8%, and 62.9%, at 1, 2, and 5 years, respectively.
These studies report different ablation strategies that may modify the success of one or multiple procedures. When ostial PVI and cavotricuspid isthmus ablation were used, the arrhythmia‐free survival rate after a single catheter ablation procedure was only 29%.2 LA linear ablation of the roof and mitral isthmus, generally performed in patients with persistent, long‐standing AF and recurrent cases, resulted in an arrhythmia‐free survival after the last catheter ablation procedure of 63% at 5 years.2 PVI combined with electrogram‐guided ablation of LA and RA with the goal to achieve SR was reported to have a success rate of 27% and 79% with a single procedure and multiple procedures, respectively, at 5 years in patients with persistent AF.3 Linear ablation was selectively performed only in patients who organized to macro‐reentrant arrhythmia.3 PVI followed by cardioversion to SR or Complex Fractionated Atrial Electrogram (CFAE) ablation if not able to convert to SR with cardioversion was reported to have a success rate of 20% in patients with long‐standing, persistent AF.4 Repeated ablation strategy included reisolation of PVs, atrial tachycardia ablation, and CFAE in patients with failed direct current cardioversion after reisolation of PVs or patients with no PV reconnection, which resulted in a success rate of 45% at 5 years.4 A stepwise ablation strategy with the end point of AF ablation, including PVI, cavotricuspid isthmus line, and electrogram‐based ablation of LA, followed by coronary sinus, roofline, mitral isthmus line, and electrogram‐based ablation of RA, had a success rate of 16.8% at 5 years with a procedure despite 80% conversion to SR during ablation.5 Arrhythmia‐free survival after the last catheter ablation procedure improved to 62.9% at 5 years.5 These studies suggest the success rate of AF ablation is low with one procedure regardless of the ablation strategy at 5 years, but it can be improved with multiple ablations. Several risk factors that may contribute to arrhythmia recurrence have been identified: type of AF (paroxysmal, persistent, or long‐standing persistent), LA size, valvular disease, cardiomyopathy, and left ventricular fibrosis, but not the underlying atrial fibrosis substrate.2, 3, 4, 5, 16, 17 In our current study, we identify LA fibrosis as an independent risk factor for recurrence at 5 years of follow‐up. Virtually all patients with Utah stage IV have a recurrence after AF ablation at 5 years of follow‐up. Targeting fibrosis during AF ablation has not been examined in this study. However, a secondary analysis of the DECAAF study suggests that residual fibrosis (HR, 1.09; 95% CI, 1.05–1.13; P<0.001) was associated with atrial arrhythmia recurrence.18 Similarly, in a study performed in 172 patients, baseline and high residual fibrosis were significant predictors of recurrence (HR, 2.2 [P<0.01] and HR, 3.2 [P<0.01], respectively).19 Low‐voltage areas, frequently but not always defined as <0.5 mV, have been used as a surrogate for atrial fibrosis. Low‐voltage areas were shown to be a predictor of AF recurrence after PVI, and low‐voltage area–based substrate modification along with PVI was shown to improve outcomes in patients with persistent AF.20, 21, 22 New ablation strategies, perhaps involving substrate modification, may be needed to improve the long‐term outcomes of AF ablation.
Limitations
This is a retrospective study from one center. Although this study demonstrated an association between atrial fibrosis and arrhythmia recurrence after AF ablation, it is not currently known whether a modification of the ablation strategy to include targeting of areas of fibrosis may result in improvement of clinical outcomes. This hypothesis will be tested in the upcoming DECAAF II clinical study. As with other studies of LA fibrosis that is quantified via LGE‐MRI, the results herein can only be applied to patients who are able to obtain an LGE‐MRI with sufficient quality to quantify fibrosis. LGE‐MRI of LA for fibrosis/scar visualization continues to be a nontrivial MRI examination. This imaging technique requires well‐trained MRI personnel, modern MRI equipment, and specialized pulse sequences and scan protocols. In comparison with conventional LGE‐MRI of the left ventricle, LGE‐MRI of LA requires significantly better spatial resolution, patient‐specific optimization of scan parameters, strict requirements on contrast dose, and delay between contrast injection and image acquisition.23, 24 The DECAAF multicenter (15 centers) study has demonstrated that LGE‐MRI of LA can be successfully used in clinical centers with various degree of expertise in cardiac MRI.6 Bois et al have reported a low incidence of LA enhancement in patients with AF, but their MRI techniques involved significant differences: significantly lower spatial resolution, image acquisition during a significantly longer proportion of the cardiac cycle, and acquisition with a higher gadolinium dose and a shorter imaging delay.25 In developing our cohort, 63 patients were excluded because they did not undergo LGE‐MRI and 32 were excluded because the image quality was too poor to quantify fibrosis. Although LGE‐MRI–identified LA enhancement has been correlated with atrial fibrosis by histological characteristics in human biopsy specimens, inflammation cannot be completely excluded as a source of LGE.
Conclusions
The degree of LA fibrosis is a strong predictor of the long‐term maintenance of SR after AF ablation. Future research is needed to develop an optimal technique for improving outcomes in patients with high degrees of atrial fibrosis.
Disclosures
Chelu reports research support from Biotronik, Medtronic, Boston Scientific, and Wavelet Health. Kholmovski is a shareholder for Marrek Inc. Han reports research funding from Boston Scientific and St Jude Medical and honoraria from Biotronik. Marrouche reports ownership interest in Marrek Inc and Cardiac Designs; contracted research with Biosense Webster, Medtronic, St Jude Medical, and Boston Scientific; and consulting fees from Biotronik and Preventice. The remaining authors have no disclosures to report.
(J Am Heart Assoc. 2018;7:e006313 DOI: 10.1161/JAHA.117.006313.)
References
- 1. Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, Damiano RJ Jr, Davies DW, DiMarco J, Edgerton J, Ellenbogen K, Ezekowitz MD, Haines DE, Haissaguerre M, Hindricks G, Iesaka Y, Jackman W, Jalife J, Jais P, Kalman J, Keane D, Kim YH, Kirchhof P, Klein G, Kottkamp H, Kumagai K, Lindsay BD, Mansour M, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Nakagawa H, Natale A, Nattel S, Packer DL, Pappone C, Prystowsky E, Raviele A, Reddy V, Ruskin JN, Shemin RJ, Tsao HM, Wilber D; Heart Rhythm Society Task Force on Catheter and Surgical Ablation of Atrial Fibrillation . 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow‐up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation: developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS): endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm. 2012;9:632–696.e21. [DOI] [PubMed] [Google Scholar]
- 2. Weerasooriya R, Khairy P, Litalien J, Macle L, Hocini M, Sacher F, Lellouche N, Knecht S, Wright M, Nault I, Miyazaki S, Scavee C, Clementy J, Haissaguerre M, Jais P. Catheter ablation for atrial fibrillation: are results maintained at 5 years of follow‐up? J Am Coll Cardiol. 2011;57:160–166. [DOI] [PubMed] [Google Scholar]
- 3. Rostock T, Salukhe TV, Steven D, Drewitz I, Hoffmann BA, Bock K, Servatius H, Mullerleile K, Sultan A, Gosau N, Meinertz T, Wegscheider K, Willems S. Long‐term single‐ and multiple‐procedure outcome and predictors of success after catheter ablation for persistent atrial fibrillation. Heart Rhythm. 2011;8:1391–1397. [DOI] [PubMed] [Google Scholar]
- 4. Tilz RR, Rillig A, Thum AM, Arya A, Wohlmuth P, Metzner A, Mathew S, Yoshiga Y, Wissner E, Kuck KH, Ouyang F. Catheter ablation of long‐standing persistent atrial fibrillation: 5‐year outcomes of the Hamburg Sequential Ablation Strategy. J Am Coll Cardiol. 2012;60:1921–1929. [DOI] [PubMed] [Google Scholar]
- 5. Scherr D, Khairy P, Miyazaki S, Aurillac‐Lavignolle V, Pascale P, Wilton SB, Ramoul K, Komatsu Y, Roten L, Jadidi A, Linton N, Pedersen M, Daly M, O'Neill M, Knecht S, Weerasooriya R, Rostock T, Manninger M, Cochet H, Shah AJ, Yeim S, Denis A, Derval N, Hocini M, Sacher F, Haissaguerre M, Jais P. Five‐year outcome of catheter ablation of persistent atrial fibrillation using termination of atrial fibrillation as a procedural endpoint. Circ Arrhythm Electrophysiol. 2015;8:18–24. [DOI] [PubMed] [Google Scholar]
- 6. Marrouche NF, Wilber D, Hindricks G, Jais P, Akoum N, Marchlinski F, Kholmovski E, Burgon N, Hu N, Mont L, Deneke T, Duytschaever M, Neumann T, Mansour M, Mahnkopf C, Herweg B, Daoud E, Wissner E, Bansmann P, Brachmann J. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA. 2014;311:498–506. [DOI] [PubMed] [Google Scholar]
- 7. Gal P, Marrouche NF. Magnetic resonance imaging of atrial fibrosis: redefining atrial fibrillation to a syndrome. Eur Heart J. 2017;38:14–19. [DOI] [PubMed] [Google Scholar]
- 8. Oakes RS, Badger TJ, Kholmovski EG, Akoum N, Burgon NS, Fish EN, Blauer JJ, Rao SN, DiBella EV, Segerson NM, Daccarett M, Windfelder J, McGann CJ, Parker D, MacLeod RS, Marrouche NF. Detection and quantification of left atrial structural remodeling with delayed‐enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. 2009;119:1758–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Steinberg JS, Palekar R, Sichrovsky T, Arshad A, Preminger M, Musat D, Shaw RE, Mittal S. Very long‐term outcome after initially successful catheter ablation of atrial fibrillation. Heart Rhythm. 2014;11:771–776. [DOI] [PubMed] [Google Scholar]
- 10. Daccarett M, Badger TJ, Akoum N, Burgon NS, Mahnkopf C, Vergara G, Kholmovski E, McGann CJ, Parker D, Brachmann J, Macleod RS, Marrouche NF. Association of left atrial fibrosis detected by delayed‐enhancement magnetic resonance imaging and the risk of stroke in patients with atrial fibrillation. J Am Coll Cardiol. 2011;57:831–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Segerson NM, Daccarett M, Badger TJ, Shabaan A, Akoum N, Fish EN, Rao S, Burgon NS, Adjei‐Poku Y, Kholmovski E, Vijayakumar S, DiBella EV, MacLeod RS, Marrouche NF. Magnetic resonance imaging‐confirmed ablative debulking of the left atrial posterior wall and septum for treatment of persistent atrial fibrillation: rationale and initial experience. J Cardiovasc Electrophysiol. 2010;21:126–132. [DOI] [PubMed] [Google Scholar]
- 12. Badger TJ, Daccarett M, Akoum NW, Adjei‐Poku YA, Burgon NS, Haslam TS, Kalvaitis S, Kuppahally S, Vergara G, McMullen L, Anderson PA, Kholmovski E, MacLeod RS, Marrouche NF. Evaluation of left atrial lesions after initial and repeat atrial fibrillation ablation: lessons learned from delayed‐enhancement MRI in repeat ablation procedures. Circ Arrhythm Electrophysiol. 2010;3:249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression [published online ahead of print December 20, 2006]. Am J Epidemiol. 2007;165:710–718. [DOI] [PubMed] [Google Scholar]
- 14. McGann C, Akoum N, Patel A, Kholmovski E, Revelo P, Damal K, Wilson B, Cates J, Harrison A, Ranjan R, Burgon NS, Greene T, Kim D, Dibella EV, Parker D, Macleod RS, Marrouche NF. Atrial fibrillation ablation outcome is predicted by left atrial remodeling on MRI. Circ Arrhythm Electrophysiol. 2014;7:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Khurram IM, Habibi M, Gucuk Ipek E, Chrispin J, Yang E, Fukumoto K, Dewire J, Spragg DD, Marine JE, Berger RD, Ashikaga H, Rickard J, Zhang Y, Zipunnikov V, Zimmerman SL, Calkins H, Nazarian S. Left atrial LGE and arrhythmia recurrence following pulmonary vein isolation for paroxysmal and persistent AF. JACC Cardiovasc Imaging. 2016;9:142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Suksaranjit P, Akoum N, Kholmovski EG, Stoddard GJ, Chang L, Damal K, Velagapudi K, Rassa A, Bieging E, Challa S, Haider I, Marrouche NF, McGann CJ, Wilson BD. Incidental LV LGE on CMR imaging in atrial fibrillation predicts recurrence after ablation therapy. JACC Cardiovasc Imaging. 2015;8:793–800. [DOI] [PubMed] [Google Scholar]
- 17. Neilan TG, Mongeon FP, Shah RV, Coelho‐Filho O, Abbasi SA, Dodson JA, McMullan CJ, Heydari B, Michaud GF, John RM, Blankstein R, Jerosch‐Herold M, Kwong RY. Myocardial extracellular volume expansion and the risk of recurrent atrial fibrillation after pulmonary vein isolation. JACC Cardiovasc Imaging. 2014;7:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Akoum N, Wilber D, Hindricks G, Jais P, Cates J, Marchlinski F, Kholmovski E, Burgon N, Hu N, Mont L, Deneke T, Duytschaever M, Neumann T, Mansour M, Mahnkopf C, Hutchinson M, Herweg B, Daoud E, Wissner E, Brachmann J, Marrouche NF. MRI assessment of ablation‐induced scarring in atrial fibrillation: analysis from the DECAAF study. J Cardiovasc Electrophysiol. 2015;26:473–480. [DOI] [PubMed] [Google Scholar]
- 19. Akoum N, Morris A, Perry D, Cates J, Burgon N, Kholmovski E, MacLeod R, Marrouche N. Substrate modification is a better predictor of catheter ablation success in atrial fibrillation than pulmonary vein isolation: an LGE‐MRI study. Clin Med Insights Cardiol. 2015;9:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yamaguchi T, Tsuchiya T, Nagamoto Y, Miyamoto K, Murotani K, Okishige K, Takahashi N. Long‐term results of pulmonary vein antrum isolation in patients with atrial fibrillation: an analysis in regards to substrates and pulmonary vein reconnections. Europace. 2014;16:511–520. [DOI] [PubMed] [Google Scholar]
- 21. Yamaguchi T, Tsuchiya T, Nakahara S, Fukui A, Nagamoto Y, Murotani K, Eshima K, Takahashi N. Efficacy of left atrial voltage‐based catheter ablation of persistent atrial fibrillation. J Cardiovasc Electrophysiol. 2016;27:1055–1063. [DOI] [PubMed] [Google Scholar]
- 22. Blandino A, Bianchi F, Grossi S, Biondi‐Zoccai G, Conte MR, Gaido L, Gaita F, Scaglione M, Rametta F. Left atrial substrate modification targeting low‐voltage areas for catheter ablation of atrial fibrillation: a systematic review and meta‐analysis. Pacing Clin Electrophysiol. 2017;40:199–212. [DOI] [PubMed] [Google Scholar]
- 23. Siebermair J, Kholmovski EG, Marrouche N. Assessment of left atrial fibrosis by late gadolinium enhancement magnetic resonance imaging: methodology and clinical implications. JACC Clin Electrophysiol. 2017;3:791–802. [DOI] [PubMed] [Google Scholar]
- 24. Chubb H, Aziz S, Karim R, Sohns C, Razeghi O, Williams SE, Whitaker J, Harrison J, Chiribiri A, Schaeffter T, Wright M, O'Neill M, Razavi R. Optimization of late gadolinium enhancement cardiovascular magnetic resonance imaging of post‐ablation atrial scar: a cross‐over study. J Cardiovasc Magn Reson. 2018;20:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bois JP, Glockner J, Young PM, Foley TA, Sheldon S, Newman DB, Lin G, Packer DL, Brady PA. Low incidence of left atrial delayed enhancement with MRI in patients with AF: a single‐centre experience. Open Heart. 2017;4:e000546. [DOI] [PMC free article] [PubMed] [Google Scholar]


