Abstract
Background
Atrial fibrillation (AF) is common in the elderly, but rare in the young; however, the changes that occur with age that promote AF are not fully understood. Action potential (AP) alternans may be involved in the initiation of AF. Using a translationally relevant model, we investigated whether age‐associated atrial vulnerability to AF was associated with susceptibility to AP alternans.
Methods and Results
AF was induced in conscious young and old sheep using 50 Hz burst pacing. Old sheep were more vulnerable to AF. Monophasic and cellular APs were recorded from the right atrium in vivo and from myocytes isolated from the left and right atrial appendages. AP alternans occurred at lower stimulation frequencies in old sheep than young in vivo (old, 3.0±0.1 Hz; young, 3.3±0.1 Hz; P<0.05) and in isolated myocytes (old, 1.6±0.1 Hz; young, 2.0±0.1 Hz; P<0.05). Simultaneous recordings of [Ca2+]i and membrane potential in myocytes showed that alternans of APs and [Ca2+]i often occurred together. However, at low stimulation rates [Ca2+]i alternans could occur without AP alternans, whereas at high stimulation rates AP alternans could still be observed despite disabling Ca2+ cycling using thapsigargin.
Conclusions
We have shown, for the first time in a large mammalian model, that aging is associated with increased duration of AF and susceptibility to AP alternans. We suggest that instabilities in Ca2+ handling initiate alternans at low stimulation rates, but that AP restitution alone can sustain alternans at higher rates.
Keywords: action potential, aging, alternans, atrial fibrillation, calcium regulation
Subject Categories: Calcium Cycling/Excitation-Contraction Coupling, Electrophysiology, Mechanisms, Myocardial Biology
Clinical Perspective
What Is New?
Atrial fibrillation is commonly observed in older patients, but the mechanisms underlying this are not fully understood.
Action potential alternans, a beat‐to‐beat oscillation in action potential duration and/or amplitude, has been mechanistically associated with arrhythmias, including atrial fibrillation.
We show that older sheep are more susceptible to action potential alternans, which may contribute to their vulnerability to atrial fibrillation.
The action potential alternans observed in old sheep is likely to arise because of changes in atrial calcium cycling.
What Are the Clinical Implications?
The changes in atrial cellular electrophysiology that occur with age described here and by others raise the possibility that older patients might respond differently to antiarrhythmic therapies.
The evidence base for treating atrial fibrillation is derived almost exclusively from trials performed in younger patients, and care should be taken when extrapolating this to older populations.
Introduction
Although atrial fibrillation (AF) is rarely observed in the young, it is endemic in the elderly, affecting greater than 9% of those aged >80 years.1 Although the mechanisms of AF are slowly becoming clearer, despite the epidemic of AF in an aging population we still do not fully understand why the old develop AF.
Structural changes, such as atrial dilatation and fibrosis, and electrophysiological changes, such as shortening of the effective refractory period (ERP) and slowing of conduction, all influence atrial vulnerability to AF.2 Mishandling of intracellular Ca2+ can also promote AF,2 and we have previously shown that this occurs with age in sheep atria.3 Disordered Ca2+ handling can lead to an electrophysiological phenomenon that has been well established as an initiator of arrhythmias: action potential (AP) alternans.4, 5 AP alternans involves the switching of AP duration and/or amplitude between contrasting states on successive beats, that is, a long‐short‐long‐short pattern. This creates a “dispersion of repolarization” in which some regions of tissue can conduct a premature impulse whereas others remain refractory. A properly timed premature impulse can therefore lead to a broken wavefront as some regions conduct while others block, for example, creating the potential for re‐entrant circuits.4
AP alternans is strongly connected with ventricular arrhythmias and has been exploited in clinical practice using microvolt T‐wave alternans to predict arrhythmic risk.5 There is also a link between AP alternans and AF. Rapid pacing of the atria can precipitate AF with a transition preceded by AP alternans.6 Atrial tachycardia remodeling increases the magnitude of atrial endocardial electrogram alternans.7 Furthermore, patients with a history of AF exhibit alternans of atrial monophasic APs of greater magnitude at lower stimulation rates than those without AF.8 We therefore asked whether an increased propensity to AP alternans might be observed in the elderly, and whether this might contribute to their vulnerability to AF. The effects of aging on atrial alternans in small mammals are inconsistent, with increased alternans observed in the pulmonary vein sleeves of old rabbits,9 but decreased alternans in the atria of old mice.10 No studies to date have investigated how alternans susceptibility changes with age in the atria of large mammals, and whether alternans contributes to atrial vulnerability in the old.
The mechanisms by which alternans arises, although long debated, are also incompletely understood.11, 12, 13, 14 AP alternans was originally thought to be driven by membrane currents alone, specifically by how the AP reacts to changes in stimulation rate, known as AP restitution.11 According to this hypothesis, alternans of cytosolic calcium concentration ([Ca2+]i) follows changes in AP shape. This viewpoint was questioned by observations that [Ca2+]i alternans can occur despite a stable AP morphology enforced by an AP clamp,12 suggesting that [Ca2+]i alternans instead drives AP alternans. More‐recent work has suggested that both AP restitution and [Ca2+]i cycling are important in the generation of alternans.13, 15, 16 We therefore asked whether both mechanisms may be important, and whether each mechanism has a dominant effect at different times.
Building on our previous work showing that aging is associated with perturbations of Ca2+ handling in the atria,3 we use a natural sheep model of aging to show that old sheep are both more vulnerable to AF and more susceptible to AP alternans in vivo and in isolated myocytes. We explore the interplay between AP and [Ca2+]i alternans, and show that [Ca2+]i cycling can be important in the generation of alternans, but is not obligatory. This is revealed through experiments that show [Ca2+]i cycling can drive alternans independently of membrane ionic currents at low stimulation rates, but when stimulated rapidly, membrane ionic currents can drive alternans despite [Ca2+]i cycling being disabled.
Methods
The data, analytical methods, and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure upon reasonable request to the corresponding author. All procedures were in accord with The UK Animals (Scientific Procedures) Act (1986) and EU directive 2010/63. Institutional approval was received from The University of Manchester Animal Welfare and Ethical Review Board. Reporting follows the ARRIVE (Animal Research: Reporting of in vivo Experiments) guidelines.17
Animal Model
A total of 60 young adult (≈18 months, first quintile of life) and 38 old (>8 years, fifth quintile of life) female Welsh mountain sheep (32.9±1.3 versus 38.0±2.3 kg; P=not significant) without observable disease were used in this work. Animals were group housed, fed hay, and maintained on a 12‐hour light/12‐hour dark cycle for 1 week before any surgical procedures. Data were included for all animals that completed the predefined experimental protocol in which usable recordings had been obtained. Uninterpretable data or incomplete protocols meant that 2 old and 1 young animal were excluded from analysis.
In Vivo Electrophysiology
Animals were anesthetized using isofluorane (2–4% v/v) in oxygen 4 L·min−1 with mechanical ventilation at 15 to 18 breaths·min−1 (Zoovent; BK Universal, Harvard, UK). Perioperative analgesia was provided by meloxicam 0.5 mg·kg−1, and enrofloxacin 2.5 mg·kg−1 was given as antibiotic prophylaxis.
A Blazer electrophysiological catheter (Boston Scientific, Quincy, MA) was advanced transvenously under fluoroscopic guidance to the right atrium. Monophasic action potentials were recorded from the right atrial posterior wall using a PowerLab amplifier and LabChart 7 software (ADInstruments, Colorado Springs, CO). A passive fixation pacing lead (Medtronic, Woburn, MA) was used to stimulate the atrium from the right atrial appendage. Atria were stimulated at increasing rates from 2 to 4 Hz in 10 ms increments with 80 action potentials recorded at each pacing rate. Monophasic APs were beat aligned in the x and y axis using the AP upstroke and maximum diastolic potential, respectively. APs were averaged before calculating AP duration, measured from AP onset to 90% repolarization from the plateau voltage.
Following incremental pacing, the effective refractory period and electrical restitution curve were assessed utilizing an extrastimulus protocol using an 8‐beat S1 400‐ms drivetrain with an S2 decrementing at 10 ms until failure to capture. Restitution curves were analyzed in custom written software. Noise was reduced by using a 4‐pass 11‐point moving average filter approximating to a 44‐point Gaussian kernel. Plots were fitted with single exponential curves to yield the maximum slope before loss of capture and the maximum diastolic interval generating a slope >1. The maximum slope and point at which the fitted curve slope exceeded 1 were calculated by differentiating the fitted curve. Subsequently, an active fixation bipolar pacing lead was secured to the right atrial appendage and connected to an implantable cardioverter‐defibrillator (Medtronic) and buried in a subcutaneous pocket in the neck.
Conscious electrophysiological studies were performed >1 week after surgery under gentle restraint. All animals had a pacing threshold <2 V and a pacing lead impedance of <1200 Ω. The effective refractory period was measured using an extrastimulus protocol at S1S1 400 ms. Atria were stimulated at 50 Hz for increasing durations from 1 to 10 seconds and at increasing voltages from 1 to 8 V. ECGs and endocardial electrograms were recorded using emka (emka Technologies, Paris, France) and analyzed in LabChart 7 (ADInstruments).
Cellular Electrophysiology
Following euthanasia using 20% pentobarbitone (200 mg·kg−1) mixed with heparin (20 000 IU), single myocytes were enzymatically isolated from left and right atrial appendages, as previously described.18, 19, 20 APs were recorded from myocytes using the perforated patch technique under current clamp control. In a subset of myocytes, [Ca2+]i and membrane potential were measured simultaneously using the Ca2+‐sensitive fluorescent indicator, Fluo‐5F AM.
Statistical Analysis
Data are expressed as mean±SEM (N=number of animals, n=number of cells) and presented on a linear scale, unless otherwise specified. Non‐normally distributed data were transformed using log10. Differences between groups in vivo were assessed using a 2‐tailed Student t test for single values or 2‐way repeated‐measures ANOVA. Linear mixed modelling was used in cellular experiments to account for instances where multiple cellular observations were obtained from each animal, as previously described.19, 21 An unstructured covariance matrix was used because this is suitable for all variances and covariances between random effects. Differences in Kaplan–Meier curves were evaluated using the log‐rank test. Categorical variables were compared using the chi‐squared or Fisher's exact test, as appropriate. Pearson moment product correlation coefficient was used to evaluate the association between 2 continuous variables. Differences were considered significant when P<0.05.
Quantification of Alternans
A spectral method was used to quantify alternans using custom‐written software, as described previously.22 Briefly, after discarding the first 10 beats after an abrupt rate change, 32 or 64 APs were beat‐aligned to AP onset. The voltage at a given time point was extracted for each beat in the series. A discrete Fourier transform was applied to this beat series, yielding a magnitude spectrum that enabled identification of oscillations in voltage occurring along the series. Oscillations that occurred with every other beat manifested as a peak in the magnitude spectrum at 0.5 cycles·beat−1. The height of this peak represented the difference in voltage between the average odd and even beat and is referred to as the alternans voltage VAlt. Random nonalternans oscillations or noise was defined as frequency components between 0.33 and 0.49 cycles·beat−1. Likelihood of the presence of alternans was assessed using the k‐score.
where ΣT is the spectral magnitude at 0.5 cycles·beat−1. μnoise is the mean spectral magnitude from 0.33 to 0.49 cycles·beat−1. σnoise is the standard deviation of the spectral magnitude from 0.33 to 0.49 cycles·beat−1.
Alternans was deemed to be present when both a k‐score >3 and a minimum VAlt were found. A minimum VAlt of >0.02 mV was used for monophasic AP recordings and 0.5 mV for transmembrane APs because these approximate to the 10th centile of VAlt values with k>3.
Alternans was classified as affecting amplitude if the mean VAlt of samples within phase 0 to 1 was ≥10% of AP amplitude. This cutoff was consistent with visual classification of the data and approximated to the 75th centile of VAlt to amplitude ratios.
Results
Old Sheep Are More Vulnerable to AF
We first investigated whether old sheep were more vulnerable to AF than younger sheep by applying 50 Hz burst pacing to the right atrium (Figure 1A). The duration of AF resulting from each burst was highly variable between and within animals. Whereas most AF episodes lasted less than 60 seconds, 10% lasted 3 to 15 minutes.
Figure 1.
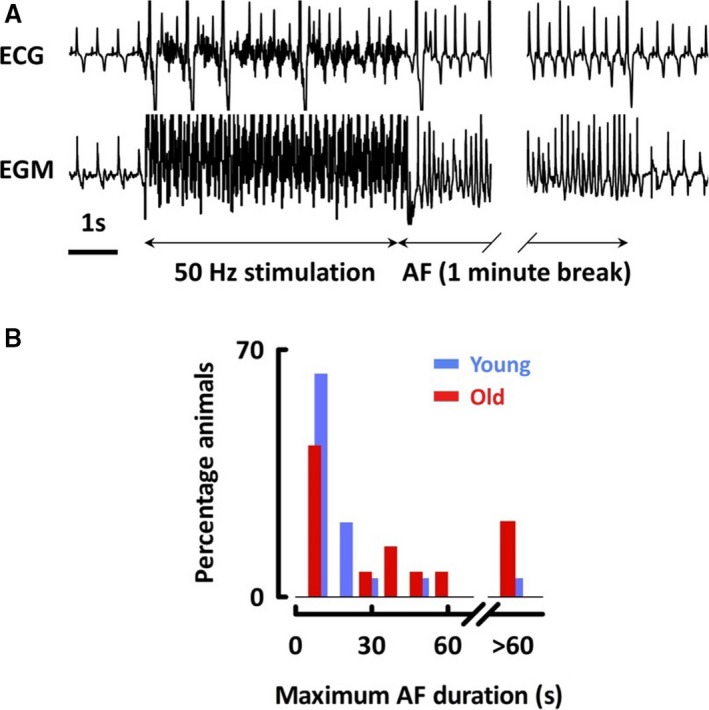
Aged atria are more susceptible to atrial fibrillation. A, Surface ECG and right atrial endocardial electrogram before, during, and after 50 Hz stimulation. B, Histogram of maximum AF duration. AF indicates atrial fibrillation; EGM, endocardial electrogram.
Following clinical guidelines, sustained AF was defined as lasting >30 seconds.23 Young sheep were less susceptible to AF, with only 2 of 19 young sheep developing ≥1 episode of sustained AF. However, 7 of 14 old sheep developed sustained AF (P=0.02; Figure 1B). Mean duration of AF from multiple 5 V bursts was >30 seconds in 3 of 14 old and 0 of 19 young sheep (P=0.07). We investigated the minimum voltage required to induce AF, but found that this was not reproducible within individuals. Extrastimulus pacing generated extrasystoles in 7 of 14 old and 4 of 11 young sheep (P=0.68) and nonsustained AF in 2 of 14 old and 0 of 11 young sheep (P=0.49). Overall, old sheep were more vulnerable to AF at clinically relevant arrhythmia durations.
The Atria of Old Sheep Are More Susceptible to Alternans In Vivo
Having shown that old sheep are more vulnerable to sustained AF, we next investigated potential mechanisms. Atrial size, left ventricular systolic function, and atrial ERP have previously been correlated with vulnerability to AF, but we found no difference in left atrial diameter (old, 28±1 mm; young, 28±2 mm; P=0.78), left ventricular fractional area change (old, 60.5±2.9%; young, 65.3±2.1%; P=0.22), nor right atrial ERP (old, 168±10 ms; young, 172±11 ms; P=0.74) between young and old sheep (N=11 old, 14 young).
Instead, because AP alternans has been associated with arrhythmogenesis, we explored whether alternans was more likely to occur in old atria. For in vivo data, repeated‐measures ANOVA was used to combine data recorded at multiple stimulation frequencies in each animal. Stimulating from 2 to 4 Hz, we ascertained the lowest stimulation rate that generated sustained alternans (alternans threshold; Figure 2A) using a spectral method. AP alternans developed at lower rates in the atria of old sheep (old: 3.0±0.1 Hz, N=12; young: 3.3±0.1 Hz, N=13; P<0.05; Figure 2B). We assessed alternans magnitude independently of threshold by including only traces that manifested alternans, and found that magnitude was similar between age groups in vivo (P=0.41; Figure 2C and 2D). Considering all stimulation frequencies together, no differences were found in monophasic AP duration during continuous rate pacing (eg, 2 Hz stimulation; old: 192±9 ms, N=14; young: 219±14 ms, N=15; P=0.09; Figure 2E).
Figure 2.
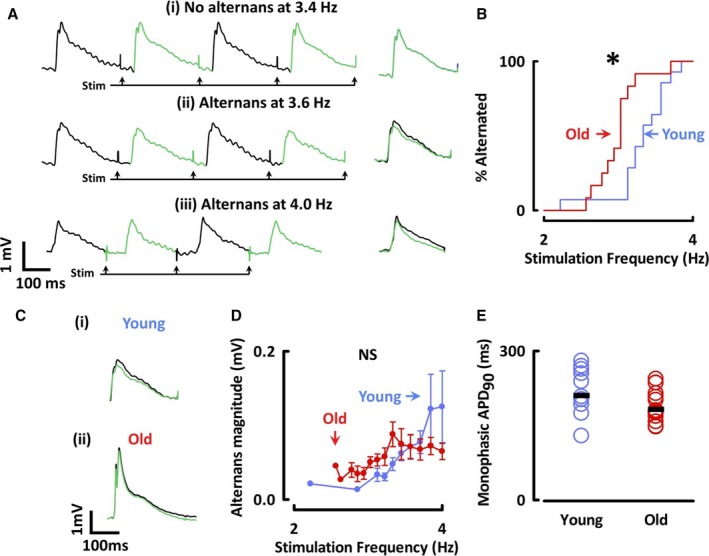
Atria from old animals are more susceptible to action potential alternans. A, Monophasic APs stimulated at 3.4, 3.6, and 4 Hz (left) with averaged odd and even traces (right). B, Kaplan‐Meier curves of atria having shown alternans. C, Representative traces from (i) young and (ii) old sheep. D, Relationship between stimulation frequency and alternans magnitude. E, APD 90 of monophasic action potentials stimulated at 2 Hz. *P<0.05. NS indicates not significant; VA lt, spectral magnitude at 0.5 cycles·beat−1; APD 90, action potential duration at 90% repolarization. Differences assessed using log‐rank test and repeated‐measure ANOVA.
In a subset of 13 animals, we recorded monophasic APs and induced AF with burst pacing. Whereas older animals were more susceptible to AF and had a lower alternans threshold, no significant association was found between AF duration and the threshold for whole‐trace or amplitude alternans within each age group (P=not significant; Figure 3).
Figure 3.
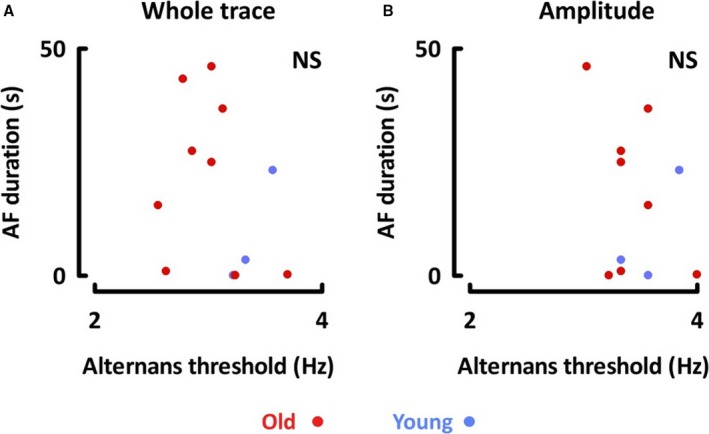
No correlation was noted between duration of AF and threshold for (A) whole trace alternans or (B) amplitude alternans. AF indicates atrial fibrillation; NS, not significant.
In Vivo Alternans Threshold Is Not Associated With Steepness of the Restitution Curve
Having observed that old sheep develop AP alternans at lower stimulation rates, we next explored potential mechanisms. One proposed mechanism is that alternans arises because of the extent to which APs shorten in response to changes in the preceding diastolic interval, known as AP restitution. We therefore asked: Could the propensity to alternans in old sheep be explained by differences in AP restitution?
Decreasing the interval between successive APs leads to shortening of AP duration (Figure 4Ai), which can be presented graphically as the AP restitution curve (Figure 4Aii). Steeper slopes reflect enhanced AP shortening and have been proposed as a mechanism of alternans initiation.11 Accordingly, we plotted restitution curves of AP duration at 90% repolarization against the preceding diastolic interval and fitted the results with a single exponential (Figure 4Aii). The maximum slope of the restitution curve did not differ between age groups (old: 1.2±0.3, N=11; young: 1.9±0.5, N=14; P=0.29; Figure 4Aiii), suggesting that susceptibility to alternans was not attributed to changes in restitution curve.
Figure 4.

In vivo (A) and cellular (B) AP restitution curves do not differ between young and old sheep. (i) Examples of AP shortening in response to decreasing DI. (ii) Representative restitution curve with fitted single exponential. (iii) Maximum slope of AP restitution curves. AP indicates action potential; APD 90, action potential duration at 90% repolarization; DI, diastolic interval; D.U., dimensionless units; NS, not significant.
Having found no difference in restitution between age groups, we then questioned the importance of AP restitution for initiation of atrial AP alternans. According to the restitution hypothesis, alternans arises when the slope of the restitution curve exceeds 1.11 However, in our experiments, alternans occurred at a diastolic interval corresponding with a mean slope of 0.3±0.1. If AP restitution was responsible for initiation of alternans, then alternans should not occur at such shallow slopes. Although alternans occurred in every animal, the restitution slope remained <1 at all diastolic intervals in 5 of 11 old and 4 of 11 young animals, and no difference was noted in the diastolic interval at which this occurred (old, 32±11 ms; young, 30±10 ms; P=0.84). Furthermore, alternans occurred in every animal at a diastolic interval at which the slope of the restitution curve was greater than 1 (N=22). Finally, no correlation could be found between alternans threshold and maximum restitution slope or diastolic interval where the slope exceeded 1.
These findings contradict the hypothesis that alternans is initiated by the slope of the restitution curve exceeding 1, and instead suggest that another factor, such as Ca2+ cycling, might be responsible for alternans at low stimulation rates.
Atrial Myocytes From Old Sheep Are Also More Susceptible to AP Alternans
We next sought to investigate whether similar phenomena could be found at a cellular level by recording APs from single atrial myocytes isolated from old and young sheep. In order to minimize perturbations to the intracellular milieu, we used the perforated patch technique. For cellular data, linear mixed modeling was used to combine data recorded from multiple cells from multiple animals.
In agreement with the in vivo observations, myocytes from old animals alternated at lower stimulation rates than those from young animals (old, 1.6±0.1 Hz; young, 2.0±0.1 Hz; P<0.05, n=14–17, N=5–8 each age and atrial side; Figure 5A). Furthermore, the magnitude of alternans was greater in myocytes from old animals (2 Hz stimulation; old, 6.9±0.1 mV; young, 4.2±0.7 mV; P<0.05; Figure 5B and 5C). Similar to in vivo observations, the difference in cellular AP alternans threshold was not associated with any difference in the maximum slope of the cellular AP restitution curve between age groups (median old, 0.4; interquartile range, 0.2–0.5; young, 0.4; interquartile range, 0–0.6; P=0.29, n=9–11, N=5–10; Figure 4B), again suggesting that differences in alternans susceptibility were not attributed to differences in AP restitution.
Figure 5.
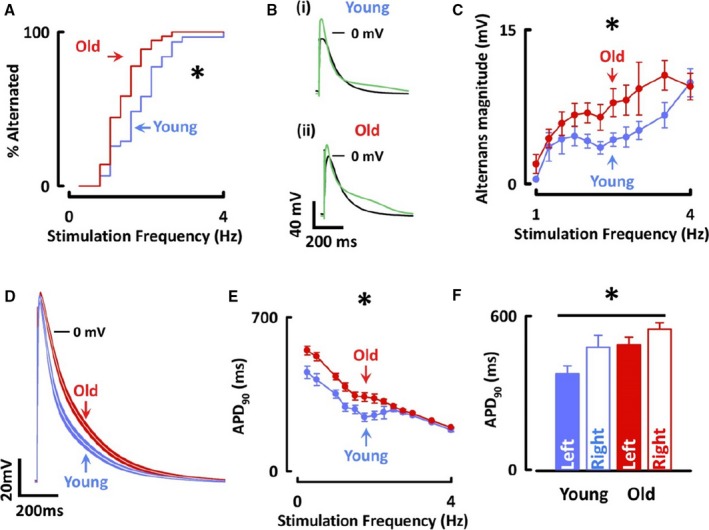
Alternans threshold is lower and action potential duration is prolonged in atrial myocytes from old sheep. A, Kaplan–Meier curve of cells having shown alternans. B, Representative traces showing mean of odd and even sweeps from (i) young and (ii) old atrial myocytes. C, Alternans magnitude from 1 to 4 Hz. D, Mean action potentials±SEMs from left atrial myocytes from young and old sheep. E, APD 90 stimulated from 0.25 to 4 Hz. F, APD 90 from myocytes stimulated at 0.5 Hz stratified by age and laterality. *P<0.05 for difference between age groups in both atria; P<0.05 for difference between left and right atria in both age groups. APD 90 indicates action potential duration at 90% repolarization. Differences assessed using log‐rank test and linear mixed modeling.
In contrast to monophasic APs, cellular AP duration was 23% longer in atrial myocytes of old compared with young sheep over a range of stimulation frequencies (old, 430±14 ms; young, 350±19 ms at 1 Hz; P<0.05; n=20–32, N=6–13 each age and atrial side; Figure 5D through 5F). AP duration was longer in right atrial than left atrial myocytes when stimulated at rates of 1 Hz or less, lower than the threshold for alternans in any cell (right, 513±24 ms; left, 431±23 ms at 0.5 Hz; P<0.05; Figure 5F). This difference disappeared at stimulation rates faster than 1 Hz, and similar effects were observed in both age groups. No difference was found in the alternans threshold between left and right atrial myocytes (right, 1.7±0.1 Hz; left, 1.8±0.1 Hz; P=0.91). Furthermore, a correlation was found between AP duration and alternans magnitude (R=+0.61; P<0.001), but not between AP duration and alternans threshold (Figure 6).
Figure 6.
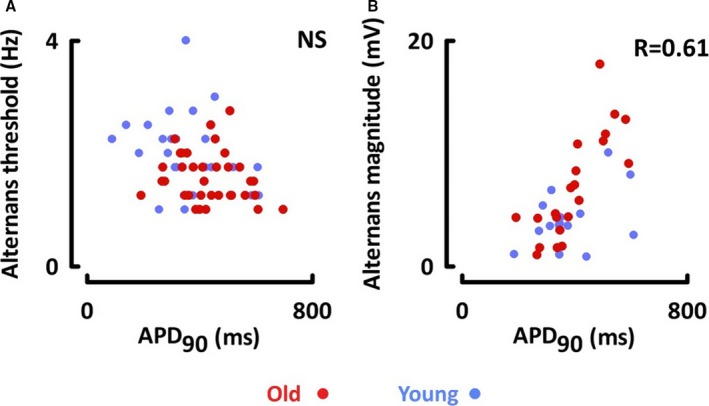
A, No correlation was noted between cellular alternans threshold and action potential duration. B, Alternans magnitude was positively correlated with action potential duration. APD 90 indicates action potential duration at 90% repolarization; NS, not significant.
These findings in isolated myocytes support the in vivo observations that older sheep are more susceptible to atrial AP alternans. We next turned our attention to the mechanisms underlying alternans by exploring which phases of the AP were most affected.
Depolarization and Repolarization Alternans Occur Separately and Not Necessarily Sequentially
AP alternans, at times, only affects repolarization (leading to alternating long and short APs), but it can also affect AP amplitude (leading to alternating high and low amplitude APs). In the ventricle, alternans that affects AP amplitude occurs at faster stimulation rates than alternans that affects repolarization alone.24 Furthermore, the occurrence of amplitude alternans is a better predictor of ventricular fibrillation than the occurrence of repolarization alternans.24 We explored whether this pattern was also observed in the atria.
Alternans was classified as affecting amplitude if the mean VAlt of samples within phase 0 to 1 was ≥5% of AP amplitude for in vivo (Figure 7A and 7B) and ≥10% for cellular recordings (Figure 7D and 7E). Cellular recordings showing amplitude alternans could be further subdivided into cases where all stimuli generated APs, marked by a depolarization occurring after the stimulation artefact (Figure 7Ei), and cases where every other stimulation failed to produce an AP, generating only a subthreshold response (Figure 7Eii), from now on referred to as 2:1 refractoriness.
Figure 7.
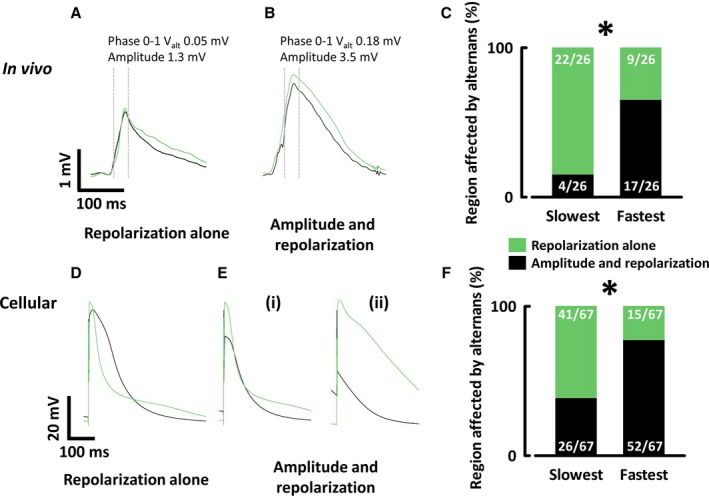
Alternans can affect the repolarization and/or amplitude of action potentials. Monophasic action potentials with: A, Repolarization alternans. B, Amplitude and repolarization alternans. C, Proportion of in vivo traces showing amplitude alternans at the slowest and fastest stimulation rates generating alternans. Transmembrane action potentials with (D) repolarization alternans, (E) (i) amplitude and repolarization alternans (ii) 2:1 refractoriness. F, Proportion of cellular traces showing amplitude alternans at the slowest and fastest stimulation rates generating alternans. *P<0.05.
Within each animal or cell, the lowest stimulation frequency that generated alternans was assessed as to whether alternans affected amplitude or repolarization alone. At the alternans threshold, the repolarization phase of the AP was affected in all traces, but alternans of the AP amplitude was only observed in 4 of 26 animals. When stimulation rate accelerated, the proportion of animals manifesting amplitude alternans increased, although in some animals alternans disappeared at very high stimulation rates. At the highest stimulation rate that still generated alternans, 17 of 26 animals experienced amplitude alternans (P<0.05). These findings were similar between age groups. Similar results were observed in experiments on isolated myocytes with amplitude alternans found in 26 of 67 cells at alternans threshold and 52 of 67 cells at the highest stimulation rate still generating alternans (Figure 7C and 7F). AP alternans therefore predominantly affects repolarization at low stimulation rates and affects amplitude to progressively greater extents as stimulation rates increase.
So far, we have shown that, in the majority of cases, alternans at low stimulation rates involves the repolarization phase only. Furthermore, at these rates, alternans is not associated with steep AP restitution, suggesting that a factor other than AP restitution is responsible for repolarization alternans at low stimulation rates. We therefore asked whether alternans driven by disordered [Ca2+]i cycling could be responsible for the repolarization alternans observed at low stimulation rates.
[Ca2+]i Alternans Drives AP Repolarization Alternans, but AP Amplitude Alternans Can Exist Despite Disabling [Ca2+]i Cycling
The relationship between AP alternans and [Ca2+]i alternans was investigated by simultaneously recording APs and [Ca2+]i using the low‐affinity fluorescent indicator, Fluo‐5F, to minimize disturbances in intracellular Ca2+ buffering. Similar effects were observed in both age groups, and therefore combined data have been presented. Mean alternans threshold was similar between indicator‐loaded and ‐unloaded myocytes (loaded, 1.73 Hz; unloaded, 1.75 Hz; P=0.90), but AP duration at 90% repolarization was slightly prolonged (loaded, 448 ms; unloaded, 413 ms; P<0.05), and repolarization alternans was observed less frequently in indicator‐loaded myocytes (loaded, 8 of 26; unloaded, 41 of 67; P<0.05).
In 4 of 26 myocytes, [Ca2+]i alternans occurred at a low stimulation rate without detectable AP alternans (Figure 8A), demonstrating that alternans can be Ca2+‐driven. Increasing the stimulation rate produced simultaneous [Ca2+]i and AP alternans (Figure 8B). In the remaining 22 of 26 cells, [Ca2+]i and AP alternans appeared at the same time.
Figure 8.
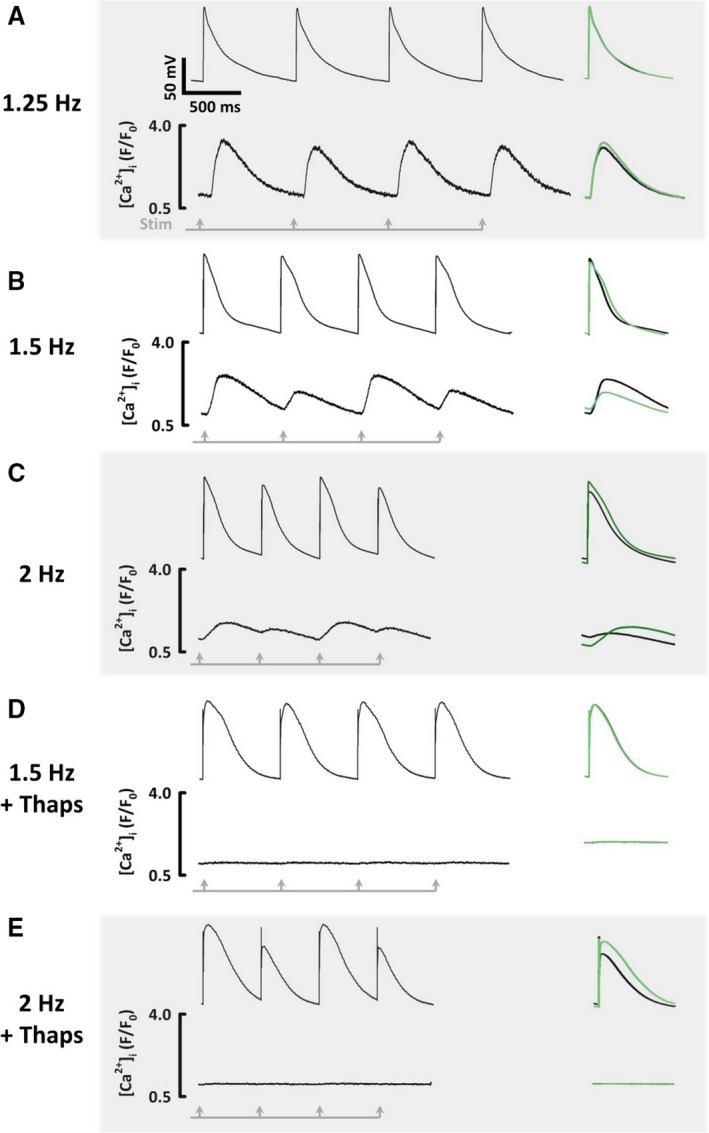
The relationship between action potential and [Ca2+]i alternans. All recordings made sequentially from the same atrial myocyte. A, [Ca2+]i alternans without AP alternans. B, [Ca2+]i alternans with AP repolarization alternans alone. C, [Ca2+]i alternans with AP amplitude alternans. D, After application of thapsigargin, no alternans of AP or [Ca2+]i at low stimulation rates. E, After thapsigargin, at high stimulation rates, AP alternans still occurs without [Ca2+]i alternans. AP indicates action potential.
Examining the pattern of alternans more closely, in 8 of 26 myocytes AP alternans affecting repolarization alone was observed, with concomitant alternans of peak and diastolic [Ca2+]i (Figure 8B). Large Ca2+ transients coincided with a low preceding diastolic [Ca2+]i and long AP duration at 90% repolarization, but a short AP duration at 50% repolarization. Alternans of AP amplitude was observed in all cells at higher stimulation rates, during which large amplitude APs coincided with large Ca2+ transients (Figure 8C).
Having observed that [Ca2+]i alternans can exist without AP alternans at low stimulation rates, we explored whether the converse was true: can AP alternans exist without [Ca2+]i alternans at higher stimulation rates? In a subset of 9 myocytes, after incrementally stimulating the cell as before, the sarco/endoplasmic reticulum Ca ATPase inhibitor, thapsigargin (5 μmol·L−1), was applied to disable Ca2+ cycling, suppressing Ca2+ transient amplitude by >90%. At low stimulation rates, which had previously produced repolarization alternans, thapsigargin abolished alternans (Figure 8D). In addition, thapsigargin prolonged the AP plateau, increasing AP duration at 50% repolarization by 38% (P<0.05), but shortening the terminal phase of repolarization, decreasing AP duration at 90% repolarization by 19% (P<0.05). The effect of abolishing the Ca2+ transient with thapsigargin was therefore reminiscent of the shape of the AP associated with small Ca2+ transients in alternating myocytes, consistent with the idea that alternation of [Ca2+]i might drive repolarization alternans.
Interestingly, even in the presence of thapsigargin, faster stimulation could still elicit amplitude alternans (Figure 8E). Alternans was still observed in 7 of 9 myocytes following the application of thapsigargin, but this never affected repolarization alone. Instead, AP amplitude alternans was observed in all myocytes with 2:1 refractoriness in 1 of 7 myocytes initially. As the pacing rate increased, 2:1 refractoriness was observed more often, occurring in 5 of 7 myocytes at the maximum stimulation rate. Thapsigargin increased the alternans threshold from 1.6 to 2.1 Hz (P<0.05).
These experiments suggest that whereas AP and [Ca2+]i alternans interact, [Ca2+]i alternans is responsible for driving repolarization alternans at low stimulation rates, whereas AP restitution can sustain alternans at higher rates despite disabling [Ca2+]i cycling. Whereas these fundamental mechanisms of alternans apply to both young and old animals, the lower alternans threshold observed in old animals represents a difference in alternans behavior at low stimulation rates and is therefore most likely to be attributed to differences in [Ca2+]i handling.
Discussion
We demonstrate that (1) old sheep are more vulnerable to AF induced by burst pacing; (2) old sheep are more susceptible to AP alternans both in vivo and in isolated myocytes; (3) alternans is first observed at low stimulation rates corresponding with a shallow AP restitution slope; (4) at low stimulation rates, [Ca2+]i alternans can occur without AP alternans and, as this becomes more pronounced, leads to AP repolarization alternans; and (5) at high stimulation rates, AP amplitude alternans can still occur at despite disabling [Ca2+]i cycling.
Old Sheep Are More Vulnerable to AF and Both In Vivo and Cellular AP Alternans
Old sheep experience more AF in response to burst pacing. Whereas many old sheep have a similar propensity to AF as young animals, 20% to 30% of old animals experience sustained AF. This is similar to humans, in whom 1 of 5 of the oldest cohort experience AF.1 We also, for the first time, show that aging is associated with a reduction in the threshold for AP alternans in a large mammal, both in vivo and in isolated myocytes. Magnitude of AP alternans increased with age in isolated myocytes, although a difference between age groups was not observed in vivo.
The increased vulnerability to AF observed in age is likely to be multifactorial, but we suggest that AP alternans may be one of these factors. This view is supported by simulation studies suggesting a causative role for alternans in promoting AF,25 and in vivo work showing that AF occurs more readily when alternans is observed at lower stimulation rates.26 Whereas the magnitude of alternans seen in vivo is small, it is comparable to the magnitude of alternans detectable on the surface ECG using microvolt T‐wave alternans techniques, which have been used to predict ventricular arrhythmias.5 The difference in alternans thresholds between age groups is similar to the difference observed between patients with paroxysmal and persistent atrial fibrillation,27 suggesting that the differences noted here are physiologically relevant.
The Electrical Restitution Curve, ERP, and Monophasic AP Duration Do Not Change With Age
The slope of the atrial electrical restitution curve, which, if steep enough, can theoretically generate AP alternans,11 was similar between old and young sheep. Consistent with some,28 but not all,29 studies in humans, we found no difference in atrial ERP or monophasic AP duration in sheep with age in vivo. However, AP duration was longer in atrial myocytes isolated from old sheep, potentially because isolated myocytes are not subject to important modulators of AP duration found in vivo, such as autonomic tone30 and the electrotonic influence from myofibroblasts.31 It is unknown whether the effects of these modulators become more pronounced with age.
Cellular AP duration was associated with alternans magnitude. If APs are short and repolarization has completed well before the next stimulus arrives, small changes in the terminal phase of repolarization caused by [Ca2+]i oscillation will have little effect on the next AP. However, if baseline AP duration is longer, similar oscillations will perturb the recovery from inactivation of I Na leading to amplitude alternans. This may explain why the longer AP duration observed in old myocytes is associated with an increase in cellular alternans magnitude, whereas the similar AP durations between age groups in vivo are associated with similar alternans magnitudes.
Repolarization Alternans Is Observed at Low Stimulation Rates in Association With [Ca2+]i Alternans but Amplitude Alternans Is Observed at High Stimulation Rates Despite Disabling [Ca2+]i Cycling
What is driving alternans in the atria? Others have shown that repolarization alternans generally occurs at lower stimulation rates whereas amplitude alternans and intermittent refractoriness are typically observed at higher rates.32 We found this to be the case in sheep atria, although alternans affected AP amplitude in nearly 15% of in vivo and 40% of cellular recordings at threshold. A decrease in alternans threshold is therefore generally attributed to a decrease in threshold for repolarization alternans.
Two lines of evidence suggest that repolarization alternans is driven by Ca2+ cycling. First, low‐amplitude [Ca2+]i alternans could be found in some myocytes without any AP alternation at low stimulation rates. This confirms that AP restitution is not responsible for [Ca2+]i alternans at these low rates, corroborating previous work showing that [Ca2+]i alternans persists despite an AP clamp.33 Second, disabling Ca2+ cycling with thapsigargin abolished repolarization alternans in all cells studied. During repolarization alternans, large Ca2+ transients always corresponded with APs showing a shorter plateau, but longer terminal phase of repolarization. This is consistent with previous work in the ventricle showing that larger transients increase Ca2+‐dependent inactivation of I Ca(L), decreasing Ca2+ influx and shortening the AP plateau on these beats, whereas large transients enhance I NCX, increasing Ca2+ efflux at the more‐negative membrane potentials observed at the end of the AP, thereby prolonging terminal repolarization.14 However, complementary mechanisms, such as a Ca2+‐dependent Cl− current, may contribute in the atria.33
However, at high rates, when alternans affects AP amplitude, intracellular [Ca2+]i cycling is not required. We suggest that amplitude alternans is membrane‐driven given that amplitude alternans could still be elicited at high stimulation rates even after [Ca2+]i cycling had been disrupted by thapsigargin. The increasing role of membrane‐driven alternans at higher rates could arise because, at these rates, the AP restitution slope is steeper, but alternatively could reflect an inability of the AP to shorten adequately at these rates. In this scenario, a large‐amplitude AP will not fully repolarize by the next stimulation, causing impaired recovery from inactivation of I Na. Supporting this hypothesis, in every recording in which AP amplitude alternans was present, alternans of the maximum diastolic potential was also noted. The subsequent AP, with less I Na available, will have lower amplitude and will repolarize faster, leading to a more‐complete recovery from inactivation of I Na. During amplitude alternans, high‐amplitude APs corresponded with large Ca2+ transients in every case. This is likely to occur because high‐amplitude APs lead to greater activation of I Ca(L), triggering more Ca2+‐induced Ca2+ release, thus generating larger Ca2+ transients.
We therefore suggest that repolarization alternans is Ca2+‐driven whereas amplitude alternans is driven by the AP itself. These experimental results are the first to show the dual role of [Ca2+]i cycling and the AP itself in the generation of alternans in atria, and are consistent with observations made in isolated ventricular myocytes and in silico ventricular myocyte models.13, 15 Although previous work has shown the role of [Ca2+]i cycling to be of greatest importance at low stimulation rates, but that this role declines as stimulation rate increases,13 this is the first study to directly associate [Ca2+]i alternans with AP repolarization alternans.
What Is Responsible for the Lower Alternans Threshold Observed in Older Sheep?
If either aberrant Ca2+ handling or membrane ion channels alone have the potential to drive alternans, what is responsible for the decreased alternans threshold observed in old animals? It is unlikely that differences in the AP are responsible given that no differences in restitution were observed between age groups, nor were differences in monophasic AP duration detected. Instead, it is more likely that age‐associated differences in Ca2+ handling are responsible for the lower alternans threshold observed in older animals. If [Ca2+]i alternans drives the repolarization alternans most commonly observed at low stimulation rates, then differences in [Ca2+]i handling are more likely to be responsible for changes in threshold.
We have previously shown, in this sheep model of aging, that age is associated with decreased peak I Ca(L), increased sarcoplasmic reticulum Ca2+ content, decreased Ca2+ transient amplitude, and slower reuptake of Ca2+ into the sarcoplasmic reticulum driven by increased Ca2+ buffering.3 Increased Ca2+ buffering is a plausible mechanism for the propensity to alternans observed in old sheep, given that increasing buffering by increasing myofilament sensitivity to calcium has previously been shown to promote AP alternans and arrhythmias.34 However, other aspects of the disordered calcium regulation we have demonstrated in old atria could also contribute—decreased I Ca(L) can promote alternans,35 as can disordered Ca2+ release and disordered Ca2+ uptake.4 The question of whether these differences in [Ca2+]i regulation are responsible for the greater propensity to alternans observed in old sheep will be answered in future work.
Although, in other disease models, a low alternans threshold has been associated with increased vulnerability to AF,26 in this work the animals that showed most alternans were not the animals who developed the most AF. Why did we not find a relationship between these factors? One explanation could lie within the method used to generate AF. Alternans has primarily been proposed as a mechanism for initiating AF by promoting a dispersion of refractoriness, but may play a lesser role in sustaining AF. Fibrillation is induced in all atria in which 50 Hz stimulation is applied, but in some, terminates as soon as stimulation ceases. How long AF persists after stopping stimulation may therefore be a marker of how well AF is sustained rather than how easily AF can be initiated. The alternans threshold might correlate better with a measure of AF initiation, such as extrastimulus‐induced AF. In this work, extrastimuli only rarely generated AF, making it highly unlikely that an association would be found.
Limitations
Although old sheep were more susceptible to both AF and AP alternans, AF is dependent on many factors. The degree to which AP alternans contributes to vulnerability to AF cannot be established by this work.
All in vivo work was performed in the right atrium because of the technical difficulties obtaining access to the left atrium. It is possible that a different pattern of alternans may be observed in the left atrium; however, alternans threshold and magnitude do not differ between the left and right atria in humans.27
We used an extrastimulus protocol to generate restitution curves. Curves generated through continuous rapid pacing yield steeper slopes, which may correspond better with alternans onset.
The longer APs recorded from isolated myocytes than those observed in intact tissue mean that amplitude alternans occurred at lower stimulation rates than might have occurred with physiologically shorter APs. However, this is unlikely to affect the increased propensity to alternans observed in older sheep.
Conclusions
In this study, we have shown that old sheep are more vulnerable to induced AF and are also more prone to action potential alternans in vivo despite similar restitution curves, ERPs, and monophasic AP duration. Similar differences in alternans thresholds are observed in isolated myocytes. We have also shown that repolarization alternans generally precedes amplitude alternans and appears to be driven by [Ca2+]i cycling, whereas AP amplitude alternans occurs at higher stimulation rates and can be driven by membrane voltage alone. The decrease in alternans threshold is therefore likely to be driven by the differences in [Ca2+]i regulation that we have previously shown to occur with age. These insights may help to explain why otherwise healthy older patients are more susceptible to AF and may, in the future, enable better targeted antiarrhythmic therapy for this group.
Sources of Funding
This work was supported by the British Heart Foundation (Intermediate Research Fellowship FS0900226487, Clinical Research Training Fellowship FS123429565, Project Grant PG128929970 and Senior Research Fellowship FS125729717).
Disclosures
None.
Acknowledgments
We wish to thank Medtronic Inc (UK), Boston Scientific and Professor W.J. Lederer (University of Maryland) for the donation of equipment used for in vivo electrophysiological studies.
(J Am Heart Assoc. 2018;7:e009972 DOI: 10.1161/JAHA.118.009972.)
References
- 1. Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. [DOI] [PubMed] [Google Scholar]
- 2. Schotten U, Verheule S, Kirchhof P, Goette A. Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol Rev. 2011;91:265–325. [DOI] [PubMed] [Google Scholar]
- 3. Clarke JD, Caldwell JL, Pearman CM, Eisner DA, Trafford AW, Dibb KM. Increased Ca buffering underpins remodelling of Ca(2+) handling in old sheep atrial myocytes. J Physiol. 2017;595:6263–6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weiss JN, Nivala M, Garfinkel A, Qu Z. Alternans and arrhythmias: from cell to heart. Circ Res. 2011;108:98–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Verrier RL, Klingenheben T, Malik M, El‐Sherif N, Exner DV, Hohnloser SH, Ikeda T, Martinez JP, Narayan SM, Nieminen T, Rosenbaum DS. Microvolt T‐wave alternans physiological basis, methods of measurement, and clinical utility—consensus guideline by International Society for Holter and Noninvasive Electrocardiology. J Am Coll Cardiol. 2011;58:1309–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hiromoto K, Shimizu H, Furukawa Y, Kanemori T, Mine T, Masuyama T, Ohyanagi M. Discordant repolarization alternans‐induced atrial fibrillation is suppressed by verapamil. Circ J. 2005;69:1368–1373. [DOI] [PubMed] [Google Scholar]
- 7. Monigatti‐Tenkorang J, Jousset F, Pascale P, Vesin JM, Ruchat P, Fromer M, Narayan SM, Pruvot E. Intermittent atrial tachycardia promotes repolarization alternans and conduction slowing during rapid rates, and increases susceptibility to atrial fibrillation in a free‐behaving sheep model. J Cardiovasc Electrophysiol. 2014;25:418–427. [DOI] [PubMed] [Google Scholar]
- 8. Lalani GG, Schricker AA, Clopton P, Krummen DE, Narayan SM. Frequency analysis of atrial action potential alternans: a sensitive clinical index of individual propensity to atrial fibrillation. Circ Arrhythm Electrophysiol. 2013;6:859–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wongcharoen W, Chen YC, Chen YJ, Chen SY, Yeh HI, Lin CI, Chen SA. Aging increases pulmonary veins arrhythmogenesis and susceptibility to calcium regulation agents. Heart Rhythm. 2007;4:1338–1349. [DOI] [PubMed] [Google Scholar]
- 10. Valli H, Ahmad S, Fraser JA, Jeevaratnam K, Huang CL. Pro‐arrhythmic atrial phenotypes in incrementally paced murine Pgc1beta(‐/‐) hearts: effects of age. Exp Physiol. 2017;102:1619–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nolasco JB, Dahlen RW. A graphic method for the study of alternation in cardiac action potentials. J Appl Physiol. 1968;25:191–196. [DOI] [PubMed] [Google Scholar]
- 12. Chudin E, Goldhaber J, Garfinkel A, Weiss J, Kogan B. Intracellular Ca2+ dynamics and the stability of ventricular tachycardia. Biophys J. 1999;77:2930–2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Groenendaal W, Ortega FA, Krogh‐Madsen T, Christini DJ. Voltage and calcium dynamics both underlie cellular alternans in cardiac myocytes. Biophys J. 2014;106:2222–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wan X, Cutler M, Song Z, Karma A, Matsuda T, Baba A, Rosenbaum DS. New experimental evidence for mechanism of arrhythmogenic membrane potential alternans based on balance of electrogenic I(NCX)/I(Ca) currents. Heart Rhythm. 2012;9:1698–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Prudat Y, Madhvani RV, Angelini M, Borgstom NP, Garfinkel A, Karagueuzian HS, Weiss JN, de Lange E, Olcese R, Kucera JP. Stochastic pacing reveals the propensity to cardiac action potential alternans and uncovers its underlying dynamics. J Physiol. 2016;594:2537–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kanaporis G, Blatter LA. Membrane potential determines calcium alternans through modulation of SR Ca(2+) load and L‐type Ca(2+) current. J Mol Cell Cardiol. 2017;105:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Voigt N, Pearman CM, Dobrev D, Dibb KM. Methods for isolating atrial cells from large mammals and humans. J Mol Cell Cardiol. 2015;86:187–198. [DOI] [PubMed] [Google Scholar]
- 19. Clarke JD, Caldwell JL, Horn MA, Bode EF, Richards MA, Hall MC, Graham HK, Briston SJ, Greensmith DJ, Eisner DA, Dibb KM, Trafford AW. Perturbed atrial calcium handling in an ovine model of heart failure: potential roles for reductions in the L‐type calcium current. J Mol Cell Cardiol. 2014;79C:169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dibb KM, Clarke JD, Horn MA, Richards MA, Graham HK, Eisner DA, Trafford AW. Characterization of an extensive transverse tubular network in sheep atrial myocytes and its depletion in heart failure. Circ Heart Fail. 2009;2:482–489. [DOI] [PubMed] [Google Scholar]
- 21. Caldwell JL, Smith CE, Taylor RF, Kitmitto A, Eisner DA, Dibb KM, Trafford AW. Dependence of cardiac transverse tubules on the BAR domain protein amphiphysin II (BIN‐1). Circ Res. 2014;115:986–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pearman CM. An Excel‐based implementation of the spectral method of action potential alternans analysis. Physiol Rep. 2014;2:e12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al‐Attar N, Hindricks G, Prendergast B, Heidbuchel H, Alfieri O, Angelini A, Atar D, Colonna P, De Caterina R, De Sutter J, Goette A, Gorenek B, Heldal M, Hohloser SH, Kolh P, Le Heuzey JY, Ponikowski P, Rutten FH. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Europace. 2010;12:1360–1420. [DOI] [PubMed] [Google Scholar]
- 24. Myles RC, Burton FL, Cobbe SM, Smith GL. Alternans of action potential duration and amplitude in rabbits with left ventricular dysfunction following myocardial infarction. J Mol Cell Cardiol. 2011;50:510–521. [DOI] [PubMed] [Google Scholar]
- 25. Chang KC, Trayanova NA. Mechanisms of arrhythmogenesis related to calcium‐driven alternans in a model of human atrial fibrillation. Sci Rep. 2016;6:36395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Narayan SM, Bode F, Karasik PL, Franz MR. Alternans of atrial action potentials during atrial flutter as a precursor to atrial fibrillation. Circulation. 2002;106:1968–1973. [DOI] [PubMed] [Google Scholar]
- 27. Narayan SM, Franz MR, Clopton P, Pruvot EJ, Krummen DE. Repolarization alternans reveals vulnerability to human atrial fibrillation. Circulation. 2011;123:2922–2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Taneja T, Mahnert BW, Passman R, Goldberger J, Kadish A. Effects of sex and age on electrocardiographic and cardiac electrophysiological properties in adults. Pacing Clin Electrophysiol. 2001;24:16–21. [DOI] [PubMed] [Google Scholar]
- 29. Kistler PM, Sanders P, Fynn SP, Stevenson IH, Spence SJ, Vohra JK, Sparks PB, Kalman JM. Electrophysiologic and electroanatomic changes in the human atrium associated with age. J Am Coll Cardiol. 2004;44:109–116. [DOI] [PubMed] [Google Scholar]
- 30. Sosunov EA, Anyukhovsky EP, Rosen MR. Adrenergic‐cholinergic interaction that modulates repolarization in the atrium is altered with aging. J Cardiovasc Electrophysiol. 2002;13:374–379. [DOI] [PubMed] [Google Scholar]
- 31. MacCannell KA, Bazzazi H, Chilton L, Shibukawa Y, Clark RB, Giles WR. A mathematical model of electrotonic interactions between ventricular myocytes and fibroblasts. Biophys J. 2007;92:4121–4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pastore JM, Girouard SD, Laurita KR, Akar FG, Rosenbaum DS. Mechanism linking T‐wave alternans to the genesis of cardiac fibrillation. Circulation. 1999;99:1385–1394. [DOI] [PubMed] [Google Scholar]
- 33. Kanaporis G, Blatter LA. Calcium‐activated chloride current determines action potential morphology during calcium alternans in atrial myocytes. J Physiol. 2016;594:699–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Baudenbacher F, Schober T, Pinto JR, Sidorov VY, Hilliard F, Solaro RJ, Potter JD, Knollmann BC. Myofilament Ca2+ sensitization causes susceptibility to cardiac arrhythmia in mice. J Clin Invest. 2008;118:3893–3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li Y, Diaz ME, Eisner DA, O'Neill S. The effects of membrane potential, SR Ca2+ content and RyR responsiveness on systolic Ca2+ alternans in rat ventricular myocytes. J Physiol. 2009;587:1283–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]


