Abstract
Background
Few previous studies used information on changes in fasting plasma glucose (FPG) assessed at multiple points in time in relationship to cardiovascular disease (CVD) incidence. The present study aimed to identify subgroups of FPG trajectories with assessing CVD incidence.
Methods and Results
The present study was based on the Suita study, a population‐based cohort study in Japan. The primary outcome was incidence of the first CVD events consisting of stroke and coronary heart diseases between 1989 and 2013. The main exposure was FPG assessed every 2 years. We used joint latent class mixed models to derive FPG trajectories over time while evaluating cumulative incidence of CVD, and categorized participants into several subgroups based on those trajectories and cumulative incidence. We observed 356 and 243 CVD events during the median follow‐up of 17.2 and 20.2 years among 3120 men and 3482 women, respectively. The joint latent mixed models found 3 subgroups in men and 2 subgroups in women. Of the 3 subgroups in men, 1 subgroup had FPG levels that increased sharply (96.5–205.0 mg/dL from aged 40 to 80 years) and higher CVD cumulative incidence. Of the 2 subgroups in women, 1 subgroup had FPG levels that increased sharply (97.7–190.5 mg/dL from aged 40 to 80 years) and tended to have slightly higher CVD incidence compared with the other subgroup.
Conclusion
It can be important to manage CVD risk factors especially for people whose FPG trajectories sharply increased to prevent CVD.
Keywords: blood glucose trajectory, cardiovascular diseases, epidemiology
Subject Categories: Cardiovascular Disease, Epidemiology, Risk Factors
Clinical Perspective
What Is New?
Many previous studies did not investigate longitudinal changes (ie, trajectories) of fasting plasma glucose (FPG) in relationship to incidence of cardiovascular diseases (CVD); thus, the present study aimed to identify common subgroups of FPG trajectories by using all of the available FPG values before CVD incidence and to assess the cumulative incidence of CVD within those subgroups.
What Are the Clinical Implications?
The present study showed that a high risk of CVD incidence was observed in people whose FPG trajectories sharply increased, especially in men, and the present study also suggested that management of multiple CVD risk factors over time could be important to prevent CVD incidence, especially in middle‐aged men with high CVD risk factors.
The present study warrants that FPG should be measured at multiple points in time rather than at 1 point to understand the association of FPG with CVD incidence and to predict and prevent CVD incidence.
Introduction
High blood glucose level can influence several etiologies of cardiovascular diseases (CVD) such as atherosclerosis and oxidative stress.1, 2, 3 Previous studies showed that people with high blood glucose had higher CVD incidence than those without,2, 4, 5, 6, 7, 8, 9, 10 and our cohort, the Suita Study, showed similar results.6, 7, 8
Many previous studies did not investigate longitudinal changes (ie, trajectories) of fasting plasma glucose (FPG) in relationship to CVD incidence. In previous studies, FPG was usually used one point in time but not for multiple points in time (during the follow‐up period), even though trajectories of FPG may be useful to identify and prevent CVD incidence early. Types of FPG trajectories were significantly associated with incident myocardial infarction.11 In this study, compared with people with a moderate‐stable trajectory of FPG (4.9–5.1 mmol/L for 4 years), people with an elevated‐stable trajectory of FPG (6.1–6.3 mmol/L for 4 years) developed more myocardial infarction (hazard ratio=1.53).11
However, in this previous study, the types of FPG trajectories were based only on FPG values assessed before the baseline, which encouraged us to use all of the available FPG values before CVD incidence to trajectory types. This can be conducted by joint latent class mixed models.12, 13 The joint latent class mixed models were used to identify long‐term trajectories of blood pressure and to assess stroke incidence within those trajectories.14
The present study aimed to identify common subgroups of FPG trajectories by using all of the available FPG values before CVD incidence and to assess the cumulative incidence of CVD within those subgroups. The present study was based on the Suita study that is an ongoing cohort in a Japanese urban city since 1989 and has assessed CVD onset and those risk factors biennially. We believe that the description of FPG trajectories in relationship to CVD incidence is possibly able to improve guidelines for FPG controls to prevent CVD.
Methods
Data, Materials, and Code Disclosure Statement
Data are available on request from the authors. The data that support the findings of this study are available from the corresponding author and Yoshihiro Miyamoto (s_ogata@ncvc.go.jp, miyamoty@ncvc.go.jp) upon reasonable request.
Study Design and Participants
The present study was based on the Suita Study that is a currently ongoing (in 2018) population‐based cohort study in Suita city as a Japanese urban city.15, 16, 17 The details of the Suita study have been reported elsewhere.15, 16, 17 The present paper used longitudinal data based on the Suita study between 1989 and 2013. As the baseline, 12 200 and 3000 citizens, who lived in Suita city and were between age 30 and 79 years, were randomly selected in 1989 and 1996, respectively, from municipality population registry of Suita city and were stratified into groups by sex and age in 10‐year increments. Of the 12 200 citizens, 6485 participated in the baseline examination between 1989 and 1996. Of the 3000 citizens, 1329 participated in the baseline examination between 1996 and 1998. The Suita study was approved by the Institutional Review Board of the National Cerebral and Cardiovascular Center in Suita, Japan. All participants in the study provided written informed consent.
The exclusion criteria of the present analyses were as follows. We excluded people who had CVD histories before the first assessment (n=355), were unable to be followed up (n=282), moved into other cities before the first assessment (n=21), had a history of transient ischemic attack at the baseline (n=1), had an event before the first assessment (n=1), and had no FPG values (n=378). Of the 6776 eligible participants, 174 participants had missing values of covariates. Thus, 6602 participants were analyzed in the present study.
Ascertainment of CVD
Detailed ascertainment of the outcome has been previously described elsewhere.15, 16, 17 The main outcome was the incidence of the first CVD events consisting of stroke and coronary heart diseases. The health status of each participant was assessed by physicians or nurses on biennial health checkups at the National Cerebral and Cardiovascular Center. Additionally, all participants completed questionnaires annually by mail or telephone. The patients suspected of CVD onset were confirmed by a review of medical records performed by either registered hospital or research physicians. Furthermore, we performed a systematic search of death certificates for fatal CVD. In Japan, all death certificates are forwarded to the Ministry of Health, Welfare, and Labor and coded for the National Vital Statistics.
In the Suita study, the definition of stroke is based on the criteria used by the US National Survey of Stroke.18 Stroke subtypes consisted of ischemic stroke, intracerebral hemorrhage, and subarachnoid hemorrhage, which were determined by the examination of computed tomographic scans, magnetic resonance images, or autopsies. Definite and probable myocardial infarctions were defined according to the criteria by the Monitoring Trends and Determinants in Cardiovascular Disease (MONICA) project.19 In the Suita study, the criteria for the diagnosis of CHD were initial acute myocardial infarction, coronary intervention, and sudden cardiac death.
Measured FPG and Potential Confounders
We performed routine fasting blood collection and immediately measured serum glucose and total cholesterol levels in the same laboratory of the National Cerebral and Cardiovascular Center. In the present analyses, we used FPG levels measured after the participants had fasted for at least 8 hours.
The present study considered baseline body mass index (BMI, kg/m2), systolic blood pressure (SBP, mm Hg), non‐high‐density‐lipoprotein cholesterol (non‐HDL‐c, mg/dL), smoking status (ever [current or past]/never as the reference), alcohol drinking status (ever/never as the reference), and medication uses for diabetes mellitus, hypertension, or dyslipidemia (yes/no as the reference) as potential confounders on considering previous studies that investigated risk scores of diabetes mellitus20 and association of glycemic status and CVD incidence.21 Height and weight were measured in light clothing, and BMI was calculated as weight (kg) divided by the square of height (m2). BP was measured 3 times in a sitting position after 5 minutes of rest by well‐trained physicians using a standard mercury sphygmomanometer. The average of the second and the third measurements was used for the analyses. Non‐HDL‐c was calculated by subtracting HDL‐c from the total cholesterol. Participants reported smoking status, alcohol drinking status, and medication uses for hypertension, diabetes mellitus, and dyslipidemia in the questionnaires.
Statistical Analyses
Baseline characteristics of the present study were summarized by means and standard deviations for continuous variables and by n and percent for categorical variables. Joint latent class mixed models were conducted to identify common subgroups of FPG trajectories overage and to assess risks of CVD within those subgroups. Joint latent class mixed models aim to describe associations between longitudinal trajectories of exposure (eg, FPG) assessed at multiple points in time and incidence of an outcome (eg, CVD).12, 13 Joint latent class mixed models also aim to categorize participants into several unmeasured subgroups (ie, latent classes) that are assumed to be mutually exclusive. Joint latent class mixed models assume that the population of participants is heterogeneous while consisting of several homogeneous unmeasured subgroups (ie, latent classes) where participants within a subgroup share the same mean longitudinal trajectories of exposure and incidence of the outcome. Thus, joint latent class mixed models can find common subgroups of FPG trajectories over age and assess risks of CVD within those subgroups. We used Jointlcmm function from the R package lcmm22 in the statistical software R.23
For longitudinal trajectories function, we modeled FPG over age from 30 years old by a class‐specific mixed model with age as time with spline link function, and random intercepts and slopes of age with adjusting for age, BMI, SBP, non‐HDL‐c, smoking status, alcohol drinking status, and medication uses for diabetes mellitus, hypertension, or dyslipidemia at baseline. We determined the optimal number of spline nods based on the lowest Bayesian Information Criterion (BIC) among models with 1 latent class. A smaller BIC indicates a better balance of the model fit to the observed data while considering the simplicity of the model. The optimal numbers of splines were 7 for men and 9 for women. For survival function, we used dates of CVD incidence as the main outcome, dates of CVD‐free deaths as the competing risk, and the last date of the follow‐up or December 31, 2013, as the censored, among which came first. CVD‐free mortality was modeled as the competing risk because this could be 1 of the major competing risks for the CVD incidence. Thus, the survival functions were set by a 2‐parameter Weibull distribution with class‐specific baseline risk functions for CVD as the primary outcome and CVD‐free mortality as the competing risk.13 Linearity assumptions of the covariates in the Weibull models were checked by plotting log survival time versus log[−log(Kaplan–Meier)] that shows linear and parallel lines if the models are adequate (Figure S1). The linearity assumptions were satisfied in all covariates except medication use for dyslipidemia. Thus, for the survival function, all models were adjusted for age, BMI, SBP, non‐HDL‐c, smoking status, alcohol drinking status, and medication uses for diabetes mellitus, or hypertension at baseline. Additionally, all analyses were stratified by sex because FPG levels and its changes over time could largely differ between men and women.
We determined the optimal number of subgroups (ie, latent classes)12 by the smallest BIC for men and women. A smaller BIC indicates a better balance of the model fit to the observed data while considering the simplicity of the model. Additionally, we divided the present data set into 10 data sets for sensitivity analyses to determine the optimal number of the subgroups. From the 10 data sets, we excluded 1 data set, used the remaining 9 data sets to perform the joint latent class mixed models with 1 to 4 subgroups for men and 1 to 3 subgroups for women, and obtained BICs of those models. We repeated this procedure 10 times for both men and women. The BICs were summarized (Tables S1 and S2).
Furthermore, we used a score test method to check an assumption of joint latent class mixed models, the conditional independence between the time to event (ie, CVD onset in the present study) and the repeated risk factors (ie, FPG trajectories in the present study) given the latent classes.24 The null hypothesis of the score test is that independence between the repeated risk factors and the outcome onset given the latent classes; thus, no significant P‐values show that the assumption is satisfied.24 Finally, we checked residuals of the best models in men and women by plotting residuals versus fitted values, and by plotting fitted values versus actual observed values (Figure S2).
We used Monte Carlo method to calculate and plot predicted values of trajectories of FPG and predicted values of cumulative incidences of CVD in each latent class for the following specified profile of covariates: the mean value for BMI, SBP, and non‐HDL‐c at baseline, people without medication uses for diabetes mellitus, hypertension, and dyslipidemia at baseline (because almost all people did not use those medications), women with never smoking and never alcohol drinking at baseline (because almost all women had never smoking and never alcohol drinking), and men with ever (ie, current or past) smoking and ever alcohol drinking at baseline (because almost all men had ever smoking and ever alcohol drinking). The 95% CI of those predicted values was computed by a Monte Carlo approximation of the posterior distribution of the predicted values, and the median, 2.5% and 97.5% percentiles were obtained.22 Models with different random starting values were analyzed to ensure convergence to the global maximum of the model.12
We summarized the baseline characteristics of the present study by means and SD for continuous variables and by n and percent for categorical variables according to the estimated subgroups. We also calculated differences between the first and last assessments of the continuous variables and n and percent for the categorical variables at the last assessment of each participant. The mean differences and 95% CIs in SBP, BMI, non‐HDL‐c, and FPG at baseline among the estimated subgroups were obtained by regression analyses with adjusting for age, BMI, SBP, non‐HDL‐c, smoking status, alcohol drinking status, and medication uses for diabetes mellitus, hypertension, or dyslipidemia at baseline. Additionally, mean differences and 95% CIs among the estimated subgroups in differences of SBP, BMI, non‐HDL‐c, and FPG between the first and last assessments were estimated by regression analyses with adjusting for those covariates. We also obtained odds ratios (OR) and 95% CIs for smoking and alcohol drinking status at baseline and the last assessment among the estimated subgroups by multinomial logistic regression with adjusting for those covariates.
Results
The baseline characteristics of the present participants were summarized in Table 1 for men and Table 2 for women. During the median (interquartile range) follow‐up of 17.2 (12.2) years and 20.2 (8.8) years, we observed 356 (11.4%) and 243 (7.0%) CVD events among 3120 men and 3482 women, respectively.
Table 1.
Baseline Characteristics of the Male Participants, and Mean Differences and ORs in CVD Risk Factors According to Subgroups Estimated by Joint Latent Class Mixed Models in the Suita Study
| Subgroups (Classes) | First | Second | Third | All |
|---|---|---|---|---|
| n (%) | 101 (3.2) | 2766 (88.7) | 253 (8.1) | 3120 (100) |
| Baseline continuous variables, mean (SD) | ||||
| Age at baseline, y | 54.51 (10.87) | 55.84 (13.43) | 53.49 (11.37) | 55.60 (13.21) |
| Body mass index, kg/m2 | 23.27 (3.01) | 22.74 (2.83) | 23.59 (3.00) | 22.83 (2.86) |
| Systolic blood pressure, mm Hg | 129.77 (18.82) | 127.92 (21.02) | 127.76 (19.19) | 127.97 (20.81) |
| Diastolic blood pressure, mm Hg | 81.17 (10.18) | 79.56 (12.05) | 79.74 (12.65) | 79.63 (12.04) |
| Non‐HDL cholesterol, mg/dL | 156.49 (32.57) | 150.72 (35.00) | 152.32 (36.16) | 151.04 (35.03) |
| Fasting plasma glucose, mg/dL | 143.59 (41.57) | 97.75 (12.70) | 118.04 (29.43) | 100.88 (18.97) |
| Baseline categorical variables, n (%) | ||||
| Medication use for diabetes mellitus | 5 (5.0) | 60 (2.2) | 7 (2.8) | 72 (2.3) |
| Medication use for hypertension | 13 (12.9) | 320 (11.6) | 25 (9.9) | 358 (11.5) |
| Medication use for dyslipidemia | 2 (2.0) | 31 (1.1) | 3 (1.2) | 36 (1.2) |
| Smoking status | ||||
| Never | 13 (12.9) | 542 (19.6) | 45 (17.8) | 600 (19.2) |
| Past | 33 (32.7) | 860 (31.1) | 60 (23.7) | 953 (30.5) |
| Current | 55 (54.5) | 1364 (49.3) | 148 (58.5) | 1567 (50.2) |
| Alcohol drinking status | ||||
| Never | 10 (9.9) | 608 (22.0) | 55 (21.7) | 673 (21.6) |
| Past | 3 (3.0) | 93 (3.4) | 10 (4.0) | 106 (3.4) |
| Current | 88 (87.1) | 2065 (74.7) | 188 (74.3) | 2341 (75.0) |
| Mean differences (95% CIs) in baseline CVD risk factors compared with the second subgroup (ie, the major subgroup)a | ||||
| Body mass index, kg/m2 | 0.29 (−0.24, 0.81) | Ref. | 0.75 (0.41, 1.1) | ··· |
| Systolic blood pressure, mm Hg | 1.30 (−2.18, 4.78) | Ref. | 0.75 (−1.51, 3.01) | ··· |
| Non‐HDL cholesterol, mg/dL | 4.96 (−1.71, 11.62) | Ref. | −1.18 (−5.51, 3.15) | ··· |
| Fasting plasma glucose, mg/dL | 43.88 (41.1, 46.66) | Ref. | 19.71 (17.9, 21.51) | ··· |
| ORs (95% CIs) for smoking and alcohol drinking compared with the second subgroup (ie, the major subgroup)b | ||||
| Smoking status | ||||
| Past (vs never) | 1.6 (0.83, 3.1) | Ref. | 0.95 (0.63, 1.43) | ··· |
| Current (vs never) | 1.7 (0.92, 3.16) | Ref. | 1.44 (1.01, 2.05) | ··· |
| Alcohol drinking status | ||||
| Past (vs never) | 1.83 (0.49, 6.88) | Ref. | 1.36 (0.66, 2.79) | ··· |
| Current | 2.4 (1.23, 4.67) | Ref. | 0.91 (0.66, 1.26) | ··· |
CVD, cardiovascular diseases; non‐HDL cholesterol, non‐high‐density‐lipoprotein cholesterol; OR, odds ratio.
Mean differences were adjusted for baseline age, body mass index, systolic blood pressure, non‐HDL cholesterol, smoking status, alcohol drinking, and medication uses for diabetes mellitus, hypertension, and dyslipidemia by multiple regression analyses.
ORs were adjusted for baseline age, body mass index, systolic blood pressure, non‐HDL cholesterol, smoking status, alcohol drinking, and medication uses for diabetes mellitus, hypertension, and dyslipidemia by multinomial logistic regression analyses.
Table 2.
Baseline Characteristics of the Female Participants, and Mean Differences and ORs in CVD Risk Factors According to Subgroups Estimated by Joint Latent Class Mixed Models in the Suita Study
| Subgroups (Classes) | First | Second | All |
|---|---|---|---|
| n(%) | 185 (5.3) | 3297 (94.7) | 3482 (100) |
| Baseline continuous variables, mean (SD) | |||
| Age at baseline, y | 52.97 (11.37) | 54.00 (12.98) | 53.95 (12.90) |
| Body mass index, kg/m2 | 23.49 (3.90) | 22.12 (3.23) | 22.20 (3.28) |
| Systolic blood pressure, mm Hg | 128.03 (20.84) | 124.45 (21.96) | 124.64 (21.91) |
| Diastolic blood pressure, mm Hg | 78.46 (12.11) | 75.45 (11.96) | 75.61 (11.98) |
| Non‐HDL cholesterol, mg/dL | 161.36 (44.04) | 154.00 (38.73) | 154.39 (39.06) |
| Fasting plasma glucose, mg/dL | 126.41 (42.06) | 94.20 (11.24) | 95.91 (16.29) |
| Baseline categorical variables, n (%) | |||
| Medication use for diabetes mellitus | 4 (2.2) | 42 (1.3) | 46 (1.3) |
| Medication use for hypertension | 26 (14.1) | 346 (10.5) | 372 (10.7) |
| Medication use for dyslipidemia | 4 (2.2) | 94 (2.9) | 98 (2.8) |
| Smoking status | |||
| Never | 153 (82.7) | 2784 (84.4) | 2937 (84.3) |
| Past | 10 (5.4) | 122 (3.7) | 132 (3.8) |
| Current | 22 (11.9) | 391 (11.9) | 413 (11.9) |
| Alcohol drinking status | |||
| Never | 129 (69.7) | 2181 (66.2) | 2310 (66.3) |
| Past | 1 (0.5) | 49 (1.5) | 50 (1.4) |
| Current | 55 (29.7) | 1067 (32.4) | 1122 (32.2) |
| Mean differences (95% CI) in baseline CVD risk factors compared with the second subgroup (ie, the major subgroup)a | |||
| Body mass index, kg/m2 | 1.04 (0.59, 1.5) | Ref. | ··· |
| Systolic blood pressure, mm Hg | 1.84 (−0.77, 4.44) | Ref. | ··· |
| Non‐HDL cholesterol, mg/dL | 4.29 (−0.94, 9.52) | Ref. | ··· |
| Fasting plasma glucose, mg/dL | 30.68 (28.79, 32.58) | Ref. | ··· |
| ORs (95% CI) for smoking and alcohol drinking compared with the second subgroup (ie, the major subgroup)b | |||
| Smoking status | |||
| Past (vs never) | 1.63 (0.83, 3.20) | Ref. | ··· |
| Current (vs never) | 1.06 (0.66, 1.69) | Ref. | ··· |
| Alcohol drinking status | |||
| Past (vs never) | 0.35 (0.05, 2.58) | Ref. | ··· |
| Current | 0.80 (0.57, 1.12) | Ref. | ··· |
CVD, cardiovascular diseases; non‐HDL cholesterol, non‐high‐density‐lipoprotein cholesterol; OR, odds ratio.
Mean differences were adjusted for baseline age, body mass index, systolic blood pressure, non‐HDL cholesterol, smoking status, alcohol drinking, and medication uses for diabetes mellitus, hypertension, and dyslipidemia by multiple regression analyses.
ORs were adjusted for baseline age, body mass index, systolic blood pressure, non‐HDL cholesterol, smoking status, alcohol drinking, and medication uses for diabetes mellitus, hypertension, and dyslipidemia by multinomial logistic regression analyses.
To decide the optimal number of subgroups (ie, latent classes) for men, we compared BICs obtained by joint latent class mixed models. The BICs were 118 521.5, 118 375.1, 118 321.1, and 118 360.2 for models with 1 to 4 subgroups. Based on the lowest BIC, the optimal number of subgroups was 3 for men. Sensitivity analyses also showed that the optimal number of subgroups was 3 for men based on BIC in Table S1. The model with 3 subgroups showed means of posterior class membership probabilities (ie, the probability that a person most likely belongs to his class): 74.4%, 91.0%, and 73.0% for the first, second, and third subgroups, respectively.
Additionally, the score test showed conditional independence between repeated measurements of FPG and the CVD onset (P=0.120). Thus, we selected the model with 3 subgroups as the best model. Characteristics of men according to the 3 subgroups were summarized in Tables 1 and 3.
Table 3.
Descriptive Statistics of Differences in Continuous CVD Risk Factors Between the First and Last Assessments With Mean Differences, and Descriptive Statistics of Categorical CVD Risk Factors at the Last Assessment With ORs According to Subgroups Estimated by Joint Latent Class Mixed Models in Men of the Suita Study
| Subgroups (Classes) | First | Second | Third | All |
|---|---|---|---|---|
| n (%)a | 89 (3.5) | 2248 (88.5) | 203 (8.0) | 2540 (100) |
| Differences between the first and last assessments of each participant, mean (SD) | ||||
| Body mass index, kg/m2 | −0.85 (1.98) | 0.03 (1.90) | 0.05 (2.15) | 0.00 (1.93) |
| Systolic blood pressure, mm Hg | 1.86 (19.65) | 2.78 (19.90) | 5.51 (21.67) | 2.97 (20.05) |
| Non‐HDL cholesterol, mg/dL | −18.51 (31.81) | −9.99 (32.42) | −6.40 (42.09) | −10.00 (33.31) |
| Fasting plasma glucose, mg/dL | −0.34 (61.77) | 4.02 (14.18) | 35.73 (41.62) | 6.40 (22.88) |
| Categorical variables at the last assessment, n (%) | ||||
| Treatment for diabetes mellitus | 46 (51.7) | 105 (4.7) | 69 (34.0) | 220 (8.7) |
| Treatment for hypertension | 32 (36.0) | 697 (31.0) | 67 (33.0) | 796 (31.3) |
| Treatment for dyslipidemia | 15 (16.9) | 233 (10.4) | 30 (14.8) | 278 (10.9) |
| Smoking status | ||||
| Never | 14 (15.7) | 547 (24.4) | 36 (17.7) | 597 (23.5) |
| Past | 47 (52.8) | 1027 (45.7) | 100 (49.3) | 1174 (46.3) |
| Current | 28 (31.5) | 672 (29.9) | 67 (33.0) | 767 (30.2) |
| Alcohol drinking habit | ||||
| Never | 18 (20.2) | 706 (31.4) | 62 (30.5) | 786 (31.0) |
| Past | 8 (9.0) | 153 (6.8) | 10 (4.9) | 171 (6.7) |
| Current | 63 (70.8) | 1387 (61.8) | 131 (64.5) | 1581 (62.3) |
| Mean differences (95% CIs) in the differences in the CVD risk factors between the first and last assessments compared with the second subgroup (ie, the major subgroup)b | ||||
| Body mass index, kg/m2 | −0.81 (−1.20, −0.42) | Ref. | −0.03 (−0.30, 0.23) | ··· |
| Systolic blood pressure, mm Hg | 0.40 (−3.24, 4.05) | Ref. | 2.57 (0.08, 5.07) | ··· |
| Non‐HDL cholesterol, mg/dL | −6.46 (−12.44, −0.48) | Ref. | 5.15 (1.06, 9.24) | ··· |
| Fasting plasma glucose, mg/dL | −4.71 (−9.14, −0.28) | Ref. | 29.77 (26.74, 32.80) | ··· |
| ORs (95% CIs) for smoking and alcohol drinking at the last assessment compared with the second subgroup (ie, the major subgroup)c | ||||
| Smoking status | ||||
| Past (vs never) | 1.33 (0.59, 3.00) | Ref. | 1.44 (0.79, 2.62) | ··· |
| Current (vs never) | 1.07 (0.40, 2.81) | Ref. | 1.08 (0.54, 2.15) | ··· |
| Alcohol drinking habit | ||||
| Past (vs never) | 1.53 (0.60, 3.88) | Ref. | 0.90 (0.42, 1.93) | ··· |
| Current | 1.23 (0.62, 2.44) | Ref. | 1.13 (0.71, 1.82) | ··· |
CVD indicates cardiovascular diseases; non‐HDL cholesterol, non‐high‐density‐lipoprotein cholesterol; OR, odds ratio.
Of the 3120 men, 580 did not have values of those variables at >1 point in time (ie, they had those values at baseline only and dates of CVD onset).
Mean differences were adjusted for baseline age, body mass index, systolic blood pressure, non‐HDL cholesterol, smoking status, alcohol drinking, and treatment for diabetes mellitus, hypertension, and dyslipidemia by multiple regression analyses.
ORs were adjusted for baseline age, body mass index, systolic blood pressure, non‐HDL cholesterol, smoking status, alcohol drinking, and treatment for diabetes mellitus, hypertension, and dyslipidemia by multinomial logistic regression analyses.
Among the best model for men, longitudinal trajectories of FPG and CVD cumulative incidence in the 3 subgroups were shown in Figure 1. The estimated median values of FPG levels in the major subgroup (ie, the second class) started from 91.76 (90.98, 92.46) mg/dL at age 40 years and gradually increased to 106.54 (104.54, 108.79) mg/dL at age 80 years, which was associated with CVD cumulative incidence that increased gradually (the cumulative incidence rate [95% CI]: 0.46 [0.15, 1.18] % at age 40 years and 13.10 [6.70, 22.90] % at 80 years). Compared with the major group, the third subgroups had sharply increasing FPG levels associated with higher cumulative incidences of CVD. In the first class, higher FPG levels at middle age and stable over time were observed, which was associated with low CVD cumulative incidence (Table 4).
Figure 1.
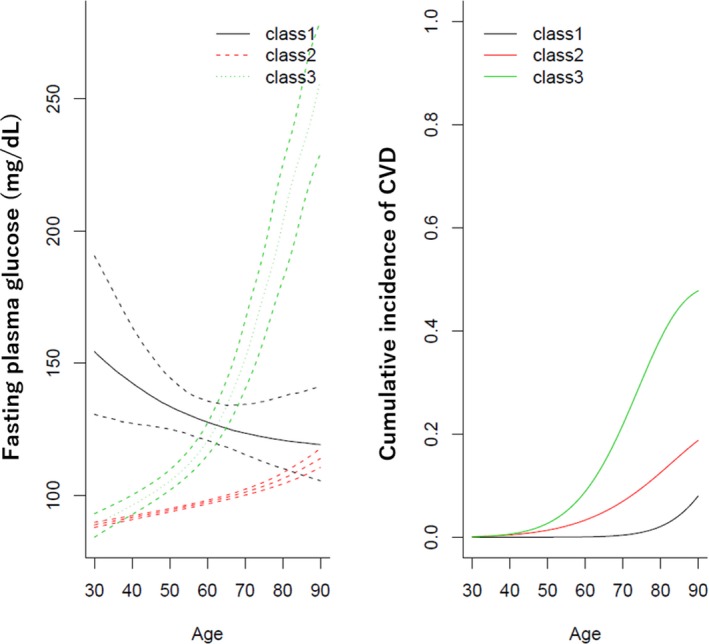
Trajectories of fasting plasma glucose (mg/dL) and cumulative incidence of cardiovascular diseases in men. The best‐fitted model of the joint latent mixed model shows 3 subgroups based on trajectories of fasting plasma glucose and cumulative incidence of cardiovascular diseases. The solid line represents the average fasting plasma glucose in each of the 3 classes for the mean of baseline age, body mass index, systolic blood pressure, non‐high‐density‐lipoprotein cholesterol in people with smoking (current or past) and alcohol drinking (current or past), and people without medication uses for diabetes mellitus, hypertension, and dyslipidemia at baseline. Dotted lines represent 95% CIs obtained by the Monte Carlo method. The right figure shows the average cumulative incidence of cardiovascular diseases in each of the 3 classes for the mean of baseline age, body mass index, systolic blood pressure, non‐high‐density‐lipoprotein cholesterol in people with smoking (current or past) and alcohol drinking (current or past), and people without medication uses for diabetes mellitus and hypertension at baseline.
Table 4.
Estimation of FPG Levels and CVD Cumulative Incidence (95% CI) According to Subgroups and Age Estimated by Joint Latent Class Mixed Models in Men
| Subgroups (Classes) | First | Second | Third |
|---|---|---|---|
| n (%) | 101 (3.2) | 2766 (88.7) | 253 (8.1) |
| FPG (mg/dL) at 40 y | 142.26 (127.23, 163.11) | 91.76 (90.98, 92.46) | 96.54 (93.07, 100.41) |
| FPG (mg/dL) at 60 y | 127.60 (120.68, 135.61) | 97.65 (96.89, 98.30) | 121.25 (115.76, 128.10) |
| FPG (mg/dL) at 80 y | 120.86 (110.11, 137.57) | 106.54 (104.54, 108.79) | 204.99 (182.64, 226.64) |
| CVD cumulative incidence (%) at 40 y | 0.00 (0.00, 0.78) | 0.46 (0.15, 1.18) | 0.62 (0.10, 2.88) |
| CVD cumulative incidence (%) at 60 y | 0.06 (0.00, 4.91) | 3.39 (1.51, 7.16) | 9.39 (3.16, 20.46) |
| CVD cumulative incidence (%) at 80 y | 2.68 (0.21, 17.68) | 13.10 (6.70, 22.90) | 39.81 (22.24, 56.29) |
Note that this table corresponds to Figure 1. CVD indicates cardiovascular diseases; FPG, fasting plasma glucose.
To select the optimal number of subgroups for women, BICs of models were compared as follows. The BICs were 127 647.7, 127 514.4, and 127 555.0 for models with 1 to 3 subgroups in women, showing that the model with 2 subgroups was the best model by the lowest BIC among the 3 models. Sensitivity analyses also showed that the optimal number of subgroups was 2 for women based on BIC in Table S2. The model with 2 subgroups showed means of posterior class membership probabilities (ie, the probability that a person most likely belongs to her class): 80.4% and 96.7% for the first and second subgroups, respectively. Additionally, the score test showed conditional independence between repeated measurements of FPG and the CVD onset (P=0.503). Thus, we selected the model with 2 subgroups as the best model. Tables 2 and 5 show characteristics for women stratified by the 2 subgroups.
Table 5.
Descriptive Statistics of Differences in Continuous CVD Risk Factors Between the First and Last Assessments With Mean Differences, and Descriptive Statistics of Categorical CVD Risk Factors at the Last Assessment With ORs According to Subgroups Estimated by Joint Latent Class Mixed Models in Women of the Suita Study
| Subgroups (Classes) | First | Second | All |
|---|---|---|---|
| n (%)a | 141 (5) | 2746 (95) | 2887 (100) |
| Differences between the first and last assessments of each participant, mean (SD) | |||
| Body mass index, kg/m2 | −0.23 (2.01) | −0.04 (1.96) | −0.05 (1.96) |
| Systolic blood pressure, mm Hg | 3.07 (18.13) | 3.77 (20.30) | 3.74 (20.19) |
| Non‐HDL cholesterol, mg/dL | −5.01 (42.28) | −4.56 (37.95) | −4.59 (38.17) |
| Fasting plasma glucose, mg/dL | 24.41 (35.70) | 3.95 (12.21) | 4.95 (14.94) |
| Categorical variables at the last assessment, n (%) | |||
| Treatment for diabetes mellitus | 67 (47.5) | 78 (2.8) | 145 (5.0) |
| Treatment for hypertension | 49 (34.8) | 805 (29.3) | 854 (29.6) |
| Treatment for dyslipidemia | 24 (17.0) | 544 (19.8) | 568 (19.7) |
| Smoking status | |||
| Never | 123 (87.2) | 2353 (85.9) | 2476 (86.0) |
| Past | 7 (5.0) | 181 (6.6) | 188 (6.5) |
| Current | 11 (7.8) | 204 (7.5) | 215 (7.5) |
| Alcohol drinking habit | |||
| Never | 108 (76.6) | 1996 (72.8) | 2104 (73.0) |
| Past | 3 (2.1) | 59 (2.2) | 62 (2.2) |
| Current | 30 (21.3) | 687 (25.1) | 717 (24.9) |
| Mean differences (95% CI) in the differences in the CVD risk factors between the first and last assessments compared with the second subgroup (ie, the major subgroup)b | |||
| Body mass index, kg/m2 | −0.13 (−0.45, 0.20) | Ref. | ··· |
| Systolic blood pressure, mm Hg | −0.53 (−3.46, 2.39) | Ref. | ··· |
| Non‐HDL cholesterol, mg/dL | 1.97 (−2.99, 6.92) | Ref. | ··· |
| Fasting plasma glucose, mg/dL | 19.77 (17.35, 22.19) | Ref. | ··· |
| ORs (95% CI) for smoking and alcohol drinking at the last assessment compared with the second subgroup (ie, the major subgroup)c | |||
| Smoking status | |||
| Past (vs never) | 0.48 (0.17, 1.37) | Ref. | ··· |
| Current (vs never) | 0.57 (0.19, 1.71) | Ref. | ··· |
| Alcohol drinking habit | |||
| Past (vs never) | 0.83 (0.25, 2.82) | Ref. | ··· |
| Current | 0.67 (0.39, 1.14) | Ref. | ··· |
CVD, cardiovascular diseases; non‐HDL cholesterol, non‐high‐density‐lipoprotein cholesterol; OR, odds ratio.
Of the 3482 women, 595 did not have values of those variables at >1 point in time (ie, they had those values at baseline only and dates of CVD onset).
Mean differences were adjusted for baseline age, body mass index, systolic blood pressure, non‐HDL cholesterol, smoking status, alcohol drinking, and treatment for diabetes mellitus, hypertension, and dyslipidemia by multiple regression analyses.
ORs were adjusted for baseline age, body mass index, systolic blood pressure, non‐HDL cholesterol, smoking status, alcohol drinking, and treatment for diabetes mellitus, hypertension, and dyslipidemia by multinomial logistic regression analyses.
Longitudinal trajectories of FPG and CVD cumulative incidence in the 2 subgroups of the best model for women were shown in Figure 2. The estimated median values of FPG levels in the major subgroup (ie, the second class) started from 87.96 (87.48, 88.43) mg/dL at age 40 years, and gradually increased to 101.35 (100.44, 102.24) mg/dL at age 80 years, which was associated with CVD cumulative incidence that increased gradually (the cumulative incidence rate [95% CI]: 0.1 [0.0, 0.2] % at age 40 years and 4.6 [2.4, 8.4] % at age 80 years). Compared with the major group, the first subgroup had sharply increasing FPG levels (97.65 [94.80, 100.87] mg/dL and 190.45 [170.16, 218.15] mg/dL for ages 40 and 80 years, respectively). The higher cumulative incidence of CVD was also observed (0.2 [0.0, 1.5] % and 8.9 [3.9, 17.6] % at ages 40 and 80 years, respectively) in the first subgroup compared with the major subgroup. For the details, please see Table 6.
Figure 2.

Trajectories of fasting plasma glucose (mg/dL) and cumulative incidence of cardiovascular diseases in women. The best‐fitted model of the joint latent mixed model shows 2 subgroups based on trajectories of fasting plasma glucose and cumulative incidence of cardiovascular diseases. The solid line represents the average fasting plasma glucose in each of the 2 classes for the mean of baseline age, body mass index, systolic blood pressure, non‐high‐density‐lipoprotein cholesterol in people without smoking and alcohol drinking, and people without medication uses for diabetes mellitus, hypertension, and dyslipidemia at baseline. Dotted lines represent 95% Cis obtained by the Monte Carlo method. The right figure shows the average cumulative incidence of cardiovascular diseases in each of the 2 classes for the mean of baseline age, body mass index, systolic blood pressure, non‐high‐density‐lipoprotein cholesterol in people without smoking and alcohol drinking, and people without medication uses for diabetes mellitus and hypertension at baseline.
Table 6.
Estimation of FPG Levels and CVD Cumulative Incidence (95% CI) According to Subgroups and Age Estimated by Joint Latent Class Mixed Models in Women
| Subgroups (Classes) | First | Second |
|---|---|---|
| n (%) | 185 (5.3) | 3297 (94.7) |
| FPG (mg/dL) at 40 y | 97.65 (94.80, 100.87) | 87.96 (87.48, 88.43) |
| FPG (mg/dL) at 60 y | 120.04 (115.02, 125.82) | 93.68 (93.25, 94.06) |
| FPG (mg/dL) at 80 y | 190.45 (170.16, 218.15) | 101.35 (100.44, 102.24) |
| CVD cumulative incidence (%) at 40 y | 0.2 (0.0, 1.5) | 0.1 (0.0, 0.2) |
| CVD cumulative incidence (%) at 60 y | 1.8 (0.5, 6.0) | 0.9 (0.4, 1.7) |
| CVD cumulative incidence (%) at 80 y | 8.9 (3.9, 17.6) | 4.6 (2.4, 8.4) |
Note that this table corresponds to Figure 2. CVD indicates cardiovascular diseases; FPG, fasting plasma glucose.
Discussion
The present study showed that men and women were categorized into 3 and 2 subgroups, respectively, based on the trajectories of FPG and CVD incidence by joint latent mixed models. Of the 3 subgroups in men, 1 subgroup had FPG levels that increased sharply and higher CVD cumulative incidence than the other groups (8.1% of men). Of the 3 subgroups in men, the other subgroups had low CVD cumulative incidence with high FPG levels at middle age and stable over time (3.2% of men) and with low FPG levels at middle age and stable over time (88.7% of men). In women, 1 subgroup had low FPG levels that were stable over time (94.7% of women), and the other subgroup had FPG levels that increased sharply (5.3% of women). Although the 2 subgroups had similar CVD cumulative incidence, the subgroup with FPG levels that increased sharply tended to have a little higher CVD incidence. To our best knowledge, the present study was the first study that identified common subgroups of FPG trajectories with calculating cumulative incidence of CVD within those subgroups and showed that high risk of CVD incidence was observed in people whose FPG trajectories sharply increased.
FPG trajectory types can be important to predict and/or prevent CVD incidence considering previous and present studies. In a previous study investigating associations of FPG trajectories with MI incidence, compared with people in a moderate‐stable trajectory of FPG (4.9–5.1 mmol/L) during 4 years before the baseline, MI incidence during 4 years after the baseline was much observed (hazard ratio=1.53) in an elevated‐stable trajectory of FPG (6.1–6.3 mmol/L), and less observed (hazard ratio=0.61) in an elevated‐decreasing trajectory (6.0–5.4 mmol/L).11 This previous study derived FPG trajectories based only on FPG values assessed before the baseline. Thus, the present study has newly shown associations between types of FPG trajectories and the CVD cumulative incidence by utilizing all of the available FPG values before CVD incidence in the joint latent class mixed models. Both the previous and present studies showed importance to assess FPG levels at multiple points in time to predict and prevent CVD incidence. Note that FPG measured at one point in time is still a significant risk factor.25, 26
Interestingly, the present study suggests that management of multiple CVD risk factors over time could lead to the prevention of CVD incidence. The present study showed the cumulative CVD incidence in the first subgroup of men, although FPG levels were higher and stable over time, was as low as that of the second subgroup with relatively lower risk. In the first group, levels of CVD risk factors decreased more (−0.34 mg/dL for FPG, −18.51 mg/dL for non‐HDL‐c, and −0.85 kg/m2 for BMI) or increased less (1.86 mm Hg for SBP) between the first and the last assessments compared with the other subgroups (Table 3). The proportions of medication uses for hypertension, diabetes mellitus, and dyslipidemia were also highest at the last assessment. The first subgroup of men may have been well intervened with medication uses or lifestyle modification and succeeded in improvement. In fact, previous studies showed that CVD was prevented in patients with type 2 diabetes mellitus by a multifactorial intervention that aims to decrease multiple CVD risk factors at the same time such as levels of blood glucose, blood pressure, and cholesterol.27, 28 Thus, continuous management of multiple CVD risk factors could be important in preventing CVD in middle‐aged men at higher risk from increased FPG, though further research is necessary for a conclusive statement.
In the present study, the number of subgroups based on trajectories of FPG and CVD incidence by joint latent mixed models was 3 in men, but 2 in women. This sex difference could be explained by sex differences of CVD risk profiles. Women, especially middle‐aged women, have been reported to have better CVD risk profiles compared with men such as lower prevalence of obesity, diabetes mellitus, hypertension, and dyslipidemia, and lower levels of SBP, FPG, and triglyceride.29, 30 In the present study, compared with men, women had lower levels of the baseline SBP (124.6 mm Hg for women and 127.97 mm Hg for men) and FPG (95.9 mg/dL for women and 100.9 mg/dL for men). In the present study, women also had lower prevalence of the baseline current smoking (11.9% for women and 50.2% for men) and current alcohol drinking (32.2% for women and 75.0% for men) compared with men. However, as the present study was observational, it was unable to reveal the sex differences.
The present results could be supported by the following possible biological mechanisms. Insulin resistance in pre‐diabetes mellitus and diabetes mellitus can promote atherogenesis by influencing endothelium, vascular wall, smooth muscle cells, and so on, which likely contributes to the elevated risk of CVD.1 Additionally, a possible mechanism for the association can be oxidative stress, 1 of the risk factors for CVD.2 Insulin resistance, hyperglycemia, and glycemic variability can increase oxidative stress by overproduction of reactive oxygen species, and activate pathways leading to diabetes mellitus complications such as microvascular and macrovascular complications, including CVD.1, 2, 3 Furthermore, coexisting risk factors may cause CVD incidence in people with elevated FPG. People with high prediabetes mellitus usually have hypertension, dyslipidemia, obesity, and metabolic syndrome as classic CVD risk factors.31 However, the present study used an observational design and was unable to reveal mechanisms of the association between FPG trajectories and CVD incidence.
The present study has the following strengths. First, we measured FPG at multiple points in time during long‐term follow‐up. This allowed us to find subgroups based on FPG trajectories and CVD incidence by latent class joint mixed models. Second, our participants were randomly selected from the municipality population registry of Suita City, an urban city in Japan. Thus, the present results can be generalized to Japanese people living in urban cities corresponding to two thirds of the Japanese population.
The present study was limited by the following points. First, the outcome of the present study was all CVD combining stroke and CHD because the number of incident CVD was too small to evaluate CVD types. Second, the median number of FPG measurements were 4 for men and 5 for women, which could imply that more FPG measurement points in time allow us to obtain models more precisely and to find different trajectories. However, we considered that the present study could have a sufficient number of measurement points in time to find subgroups with high risks for CVD based on FPG trajectories. Additionally, the present study can warrant further studies to assess trajectories of CVD risk factors measured at more points in time to predict and prevent CVD events and to understand associations of CVD risk factors’ trajectories with CVD incidence. Third, the present study was unable to reveal biological mechanisms associated with FPG trajectories and CVD incidence, and it was also unable to reveal sex differences that may have been indicated in relation. This was because the present study was an observational study. Thus, we warrant future studies to investigate pathogenic importance of FPG changes on CVD incidence, especially because control of FPG elevation can be a new strategy to prevent CVD events if biological mechanisms support trajectories and/or change rates of FPG are causal risk factors for CVD events (ie, not just a reflection of lifestyle changes). Note that the aims of the present study were to identify common subgroups of FPG trajectories and to assess the cumulative incidence of CVD within those subgroups, but were neither to develop nor optimize prediction models for CVD. Thus, knowledge suggested by the present study may not be useful to improve the prediction models for CVD.
In conclusion, the present study showed that a high risk of CVD incidence was observed in people whose FPG trajectories sharply increased, especially in men. We also suggested that management of multiple CVD risk factors over time could be important to prevent CVD incidence, especially in middle‐aged men with high CVD risk factors. The present study warrants that FPG should be measured at multiple points in time rather than at one point to understand the association of FPG with CVD incidence and to predict and prevent CVD incidence.
Author Contributions
Miyamoto and Ogata conceptualized the present study. Watanabe, Kokubo, Higashiyama, Nakao, Takegami, Nishimura, and Miyamoto collected data. Ogata, Watanabe, Kokubo, Higashiyama, and Nakai managed data. Ogata and Watanabe analyzed data. Ogata, Watanabe, Kiyoshige, Kokubo, Higashiyama, Nakao, Takegami, Nishimura, Nakai, Hosoda, Okamura, and Miyamoto interpreted analyses results. Ogata, Watanabe, and Miyamoto primally wrote the manuscript. Kokubo, Kiyoshige, Higashiyama, Nakao, Takegami, Nishimura, Nakai, Hosoda, and Okamura reviewed the manuscript. All authors agreed to submit the manuscript.
Sources of Funding
The present study was supported by the Intramural Research Fund (27‐4‐3) for Cardiovascular Diseases of National Cerebral and Cardiovascular Center.
Disclosures
None.
Supporting information
Table S1. BICs Obtained by Joint Latent Class Mixed Models for Men Based on Data Randomly Derived From the Original Data Set
Table S2. BICs Obtained by Joint Latent Class Mixed Models for Women Based on Data Randomly Derived From the Original Data Set
Figures S1. (1) Assumption checks for the Weibull models in men. (2) Assumption checks for the Weibull models for women.
Figure S2. Plotting residuals, fitted values, and actual observation values for the trajectories of FPG.
Acknowledgments
We sincerely appreciate members of the Suita Medical Foundation and Suita City Health Center. We also thank Satuki‐Junyukai, the society members of the Suita study. We acknowledge Kei Matsumaru for proofreading the present manuscript by improving the sentences written in the English language.
(J Am Heart Assoc. 2019;8:e010628 DOI: 10.1161/JAHA.118.010628.)
References
- 1. Laakso M. Cardiovascular disease in type 2 diabetes from population to man to mechanisms: the Kelly West Award Lecture 2008. Diabetes Care. 2010;33:442–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kayama Y, Raaz U, Jagger A, Adam M, Schellinger I, Sakamoto M, Suzuki H, Toyama K, Spin J, Tsao P. Diabetic cardiovascular disease induced by oxidative stress. Int J Mol Sci. 2015;16:25234–25263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saisho Y. Glycemic variability and oxidative stress: a link between diabetes and cardiovascular disease? Int J Mol Sci. 2014;15:18381–18406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a high‐risk state for diabetes development. Lancet. 2012;379:2279–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Perreault L, Færch K. Approaching pre‐diabetes. J Diabetes Complications. 2014;28:226–233. [DOI] [PubMed] [Google Scholar]
- 6. Turin TC, Okamura T, Rumana N, Afzal AR, Watanabe M, Higashiyama A, Nakao YM, Nakai M, Takegami M, Nishimura K, Kokubo Y, Okayama A, Miyamoto Y. Diabetes and lifetime risk of stroke and subtypes in an urban middle‐aged population. J Diabetes Complications. 2017;31:831–835. [DOI] [PubMed] [Google Scholar]
- 7. Turin TC, Okamura T, Rumana N, Afzal AR, Watanabe M, Higashiyama A, Nakao YM, Nakai M, Takegami M, Nishimura K, Kokubo Y, Okayama A, Miyamoto Y. Diabetes and lifetime risk of coronary heart disease. Prim Care Diabetes. 2017;11:461–466. [DOI] [PubMed] [Google Scholar]
- 8. Kokubo Y, Okamura T, Watanabe M, Higashiyama A, Ono Y, Miyamoto Y, Furukawa Y, Kamide K, Kawanishi K, Okayama A, Yoshimasa Y. The combined impact of blood pressure category and glucose abnormality on the incidence of cardiovascular diseases in a Japanese urban cohort: the Suita study. Hypertens Res. 2010;33:1238–1243. [DOI] [PubMed] [Google Scholar]
- 9. Shah AD, Langenberg C, Rapsomaniki E, Denaxas S, Pujades‐Rodriguez M, Gale CP, Deanfield J, Smeeth L, Timmis A, Hemingway H. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1.9 million people. Lancet Diabetes Endocrinol. 2015;3:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ford ES, Zhao G, Li C. Pre‐diabetes and the risk for cardiovascular disease. J Am Coll Cardiol. 2010;55:1310–1317. [DOI] [PubMed] [Google Scholar]
- 11. Jin C, Chen S, Vaidya A, Wu Y, Wu Z, Hu FB, Kris‐Etherton P, Wu S, Gao X. Longitudinal change in fasting blood glucose and myocardial infarction risk in a population without diabetes. Diabetes Care. 2017;40:1565–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Proust‐Lima C, Séne M, Taylor JM, Jacqmin‐Gadda H. Joint latent class models for longitudinal and time‐to‐event data: a review. Stat Methods Med Res. 2014;23:74–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Proust‐Lima C, Dartigues J‐F, Jacqmin‐Gadda H. Joint modeling of repeated multivariate cognitive measures and competing risks of dementia and death: a latent process and latent class approach. Stat Med. 2016;35:382–398. [DOI] [PubMed] [Google Scholar]
- 14. Portegies MLP, Mirza SS, Verlinden VJA, Hofman A, Koudstaal PJ, Swanson SA, Ikram MA. Mid‐ to late‐life trajectories of blood pressure and the risk of stroke: the Rotterdam Study. Hypertension. 2016;67:1126–1132. [DOI] [PubMed] [Google Scholar]
- 15. Kokubo Y, Watanabe M, Higashiyama A, Nakao YM, Kusano K, Miyamoto Y. Development of a basic risk score for incident atrial fibrillation in a Japanese General Population—the Suita study. Circ J. 2017;81:1580–1588. [DOI] [PubMed] [Google Scholar]
- 16. Okamura T, Kokubo Y, Watanabe M, Higashiyama A, Ono Y, Miyamoto Y, Yoshimasa Y, Okayama A. Triglycerides and non‐high‐density lipoprotein cholesterol and the incidence of cardiovascular disease in an urban Japanese cohort: the Suita study. Atherosclerosis. 2010;209:290–294. [DOI] [PubMed] [Google Scholar]
- 17. Kokubo Y, Kamide K, Okamura T, Watanabe M, Higashiyama A, Kawanishi K, Okayama A, Kawano Y. Impact of high‐normal blood pressure on the risk of cardiovascular disease in a Japanese urban cohort: the Suita study. Hypertension. 2008;52:652–659. [DOI] [PubMed] [Google Scholar]
- 18. Walker AE, Robins M, Weinfeld FD. The National Survey of Stroke. Clinical findings. Stroke. 1981;12:I13–I44. [PubMed] [Google Scholar]
- 19. Tunstall‐Pedoe H, Kuulasmaa K, Amouyel P, Arveiler D, Rajakangas AM, Pajak A. Myocardial infarction and coronary deaths in the World Health Organization MONICA Project. Registration procedures, event rates, and case‐fatality rates in 38 populations from 21 countries in four continents. Circulation. 1994;90:583–612. [DOI] [PubMed] [Google Scholar]
- 20. Nanri A, Nakagawa T, Kuwahara K, Yamamoto S, Honda T, Okazaki H, Uehara A, Yamamoto M, Miyamoto T, Kochi T, Eguchi M, Murakami T, Shimizu C, Shimizu M, Tomita K, Nagahama S, Imai T, Nishihara A, Sasaki N, Hori A, Sakamoto N, Nishiura C, Totsuzaki T, Kato N, Fukasawa K, Huanhuan H, Akter S, Kurotani K, Kabe I, Mizoue T, Sone T, Dohi S; Japan Epidemiology Collaboration on Occupational Health Study Group for the JEC on OHS . Development of risk score for predicting 3‐year incidence of type 2 diabetes: Japan Epidemiology Collaboration on Occupational Health Study. PLoS One. 2015;10:e0142779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Watanabe M, Kokubo Y, Higashiyama A, Ono Y, Miyamoto Y, Okamura T. Serum 1,5‐anhydro‐D‐glucitol levels predict first‐ever cardiovascular disease: an 11‐year population‐based cohort study in Japan, the Suita study. Atherosclerosis. 2011;216:477–483. [DOI] [PubMed] [Google Scholar]
- 22. Proust‐Lima C, Philipps V, Liquet B. Estimation of extended mixed models using latent classes and latent processes: the R package lcmm. J Stat Softw. 2017;78:1–56. [Google Scholar]
- 23. R Core Team . R: A Language and Environment for Statistical Computing. 2017. Available at: https://www.r-project.org/. Accessed April 25, 2017. [Google Scholar]
- 24. Jacqmin‐Gadda H, Proust‐Lima C, Taylor JMG, Commenges D. Score test for conditional independence between longitudinal outcome and time to event given the classes in the joint latent class model. Biometrics. 2010;66:11–19. [DOI] [PubMed] [Google Scholar]
- 25. Levitan EB, Song Y, Ford ES, Liu S. Is nondiabetic hyperglycemia a risk factor for cardiovascular disease? Arch Intern Med. 2004;164:2147. [DOI] [PubMed] [Google Scholar]
- 26. Huang Y, Cai X, Mai W, Li M, Hu Y. Association between prediabetes and risk of cardiovascular disease and all cause mortality: systematic review and meta‐analysis. BMJ. 2016;355:i5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ueki K, Sasako T, Okazaki Y, Kato M, Okahata S, Katsuyama H, Haraguchi M, Morita A, Ohashi K, Hara K, Morise A, Izumi K, Ishizuka N, Ohashi Y, Noda M, Kadowaki T; J‐DOIT3 Study Group . Effect of an intensified multifactorial intervention on cardiovascular outcomes and mortality in type 2 diabetes (J‐DOIT3): an open‐label, randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5:951–964. [DOI] [PubMed] [Google Scholar]
- 28. Gæde P, Lund‐Andersen H, Parving H‐H, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–591. [DOI] [PubMed] [Google Scholar]
- 29. Bu S, Ruan D, Yang Z, Xing X, Zhao W, Wang N, Xie L, Yang W. Sex‐specific prevalence of diabetes and cardiovascular risk factors in the middle‐aged population of China: a subgroup analysis of the 2007–2008 China National Diabetes and Metabolic Disorders Study. PLoS One. 2015;10:e0139039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim SH, Reaven G. Sex differences in insulin resistance and cardiovascular disease risk. J Clin Endocrinol Metab. 2013;98:E1716–E1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grundy SM. Pre‐diabetes, metabolic syndrome, and cardiovascular risk. J Am Coll Cardiol. 2012;59:635–643. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. BICs Obtained by Joint Latent Class Mixed Models for Men Based on Data Randomly Derived From the Original Data Set
Table S2. BICs Obtained by Joint Latent Class Mixed Models for Women Based on Data Randomly Derived From the Original Data Set
Figures S1. (1) Assumption checks for the Weibull models in men. (2) Assumption checks for the Weibull models for women.
Figure S2. Plotting residuals, fitted values, and actual observation values for the trajectories of FPG.


