Short abstract
See Article by Kanamori et al
Keywords: Editorials, aortic stenosis, aortic valve replacement, asymptomatic, outcome, severity
Subject Categories: Echocardiography, Valvular Heart Disease, Cardiovascular Surgery
Severe aortic stenosis (AS) is currently defined by an aortic valve area (AVA) <1.0 cm2 and/or a mean transaortic pressure gradient (MPG) >40 mm Hg and/or a peak aortic jet velocity (Vmax) >4 m/s.1, 2
Symptoms represent the central element guiding the management of severe AS, and US and European guidelines1, 2 both recommend aortic valve replacement (AVR) in patients with symptoms clearly related to the valvular obstacle (class I recommendation). The slowly progressive nature of AS combined with the advanced age of the affected population predispose to underreporting and/or underestimation of symptoms. About one third of patients with severe AS considered asymptomatic at diagnosis experience symptoms on exercise testing. Exercise testing should therefore be performed whenever possible to detect patients who are not “truly asymptomatic” and who should undergo AVR when the operative risk is acceptable. The estimated annualized rate of sudden death for asymptomatic patients with severe AS is 1% per year. AS is a lesion that evolves relentlessly, and progression is more rapid when the aortic valve is severely stenotic and calcified. The risk of conservative management must be weighed against the risk of operative mortality associated with AVR (1%–3% in patients <70 years, and 3%–8% in older patients). In addition, patients with prosthetic valves may incur specific life‐threatening complications. It has also been suggested that some patients with severe asymptomatic AS are operated on at an advanced stage of the disease, when myocardial impairment is partially irreversible, resulting in a high risk of mortality and heart failure after successful AVR.
Currently, asymptomatic patients with severe AS associated with left ventricular (LV) dysfunction (left ventricular ejection fraction [LVEF] <50%) or undergoing other cardiac/ascending aortic surgery present a class I indication for AVR.1, 2 However, this recommendation is rarely applicable, because overt LV dysfunction is uncommon in asymptomatic patients with severe AS (<2% of patients).3 AVR should be considered (class IIa recommendation) for asymptomatic patients with severe AS and abnormal blood pressure response on exercise stress testing,1, 2 or for patients with severe valve calcification and rapid (peak velocity rate >0.3 m/s per year) progression (class IIa recommendation in European guidelines, IIb recommendation in US guidelines). AVR is considered reasonable in asymptomatic patients with high Vmax presenting a low surgical risk (class IIa recommendation),1, 2 although the Vmax cut‐off currently used remains controversial (≥5 m/s in US guidelines and >5.5 m/s in European guidelines). Finally, the latest European guidelines add markedly elevated brain natriuretic peptide (BNP) levels and severe pulmonary hypertension at rest (systolic pulmonary artery pressure >60 mm Hg) as incentives for AVR in severe asymptomatic AS with low surgical risk.2
However, the outcome of severe asymptomatic AS under conservative management and the operative risk of these patients are heterogeneous. Given the risk of AVR and the potential complications related to the aortic prosthesis that may negatively impact outcome, there is an unmet need to identify with echocardiography a subset of high‐risk patients with asymptomatic very severe aortic stenosis (VSAS) characterized by a dismal prognosis in the absence of AVR. In such patients who present a significant risk of death with conservative management, the benefit of early surgery should outweigh the potential complications related to the surgical procedure and the prosthetic valve.
In a study of 116 asymptomatic patients with severe AS, Vmax >5.5 m/s was associated with a 3‐year risk of surgery/death of 89%.4 Our group recently reported that Vmax ≥5 m/s is an independent predictor of mortality in patients with severe AS with preserved LVEF, irrespective of functional status, and is associated with an ≈80% increase in the relative risk of death during follow‐up in asymptomatic patients.5 Moreover, patients with Vmax 5 to 5.5 m/s (≈19% of our study population of severe AS) had an outcome similar to those with Vmax >5.5 m/s. Vmax ≥5 m/s therefore appears as a breakpoint in the progression of asymptomatic severe AS, and values above this cut‐off at the time of AS assessment should represent prompt triggers for AVR referral in patients with low operative risk. MPG is well correlated with Vmax and strongly impacts outcome in severe AS.6 A recent report shows that asymptomatic or minimally symptomatic patients with severe AS and MPG ≥60 mm Hg at diagnosis are at high risk of death during follow‐up with medical and surgical management compared with those with MPG <60 mm Hg.6
In this issue of the Journal of the American Heart Association (JAHA), Kanamori et al report the relationship between AVA and outcomes in a population of 1309 asymptomatic patients with severe AS included in the CURRENT AS (Contemporary Outcomes After Surgery and Medical Treatment in Patients With Severe Aortic Stenosis) registry and managed conservatively.7 Patients were stratified into 3 groups according to AVA: >0.8, 0.6 to 0.8, and <0.6 cm2. The primary end point was a composite of aortic valve–related death or hospitalization for heart failure. Indications for surgery and causes of death during follow‐up were carefully collected. While the cumulative 5‐year incidence of AVR was similar across the 3 groups (≈40%), the 5‐year incidence of the primary end point was high with AVA <0.6 cm2 (48%) and much lower for the other 2 groups (24% for AVA >0.8 cm2 and 29% for AVA 0.6–0.8 cm2). After covariate adjustment, AVA <0.6 cm2 was associated with a substantial increase in the risk of the primary outcome measure (hazard ratio: 2.21 CI [1.56–3.11]) compared with AVA >0.8 cm2, while the excess risk of AVA 0.6 to 0.8 cm2 versus >0.8 cm2 was modest, although statistically significant. Kanamori et al unequivocally demonstrate that asymptomatic patients with severe AS, normal LVEF, and AVA <0.6 cm2 have a dismal outcome when managed conservatively. While the Society of Thoracic Surgeons score of the group with AVA <0.6 cm2 was 4.1%, suggesting moderate operative risk, the annual incidence of the primary outcome measure was unacceptably high (9%). Similarly, the annual incidence of sudden death was ≈3%, slightly higher than that previously reported. The authors conclude that patients with asymptomatic AS and AVA <0.6 cm2 should be promptly referred for surgery. The results of Kanamori et al are in accordance with previous data published by our group based on a smaller series of 199 patients with asymptomatic severe AS.8 In our series, 4‐year event‐free survival (death or need for AVR) was 34% for AVA 0.8 to 1 cm2, 26% for AVA 0.6 to 0.8 cm2, and 11% for AVA ≤0.6 cm2. After covariate adjustment, AVA ≤0.6 cm2 was associated with high mortality risk compared with AVA >0.6 cm2, whereas patients with AVA 0.6 to 0.8 cm2 and AVA 0.8 to 1 cm2 had similar mortality risk. Moreover, we have recently demonstrated that AVA indexation to body size is helpful for risk stratification in asymptomatic AS by identifying a subgroup of patients who are at very high short‐term risk on medical management.9 Asymptomatic patients with severe AS and AVA/body surface area <0.4 cm2/m2 (or AVA/height <0.45 cm2/m) have an unacceptably high risk of events. Furthermore, AVA indexation to height appears to be superior to indexation to body surface area for outcome prediction in asymptomatic AS and certainly deserves further investigation.9 Finally, given the notoriously difficult measurement of LV outflow tract diameter by echocardiography, dimensionless index represents a simple and robust predictor of outcome in asymptomatic/minimally symptomatic AS with risk abruptly increasing below the 0.20 cut‐off.10 In clinical practice, the assessment of the severity of asymptomatic AS must be carefully performed and must be based on a multiparameter approach integrating AVA, MPG, and Vmax. In light of the data presented above, VSAS could be defined by Vmax >5 m/s, MPG ≥60 mm Hg, AVA <0.6 cm2, indexed AVA <0.4 cm2/m2 (<0.45 cm2/m), or dimensionless index <0.20 (Table). Unpublished data from our group show that patients with asymptomatic VSAS and low operative risk (European System for Cardiac Operative Risk Evaluation II [EuroSCORE II] ≤4%) have a significantly greater risk of death during follow‐up with medical and surgical management than patients not meeting the criteria of VSAS (Figure). Prospective randomized controlled trials, such as the ongoing AVATAR (Aortic valve replacement versus conservative treatment in asymptomatic severe aortic stenosis) trial comparing early surgery to a symptom‐driven approach in asymptomatic severe AS, will be useful to confirm the definition of VSAS.11
Table 1.
| Severe AS | VSAS | |
|---|---|---|
| Peak aortic jet velocity | >4 m/s | >5 m/s |
| MPG | >40 mm Hg | ≥60 mm Hg |
| Aortic valve area | <1 cm2 | <0.6 cm2 |
| Indexed aortic valve area | <0.6 cm2/m2 | <0.4 cm2/m2 (<0.45 cm2/m) |
| Dimensionless index | <0.25 | <0.20 |
AS indicates aortic stenosis; MPG, mean transaortic pressure gradient; VSAS, very severe aortic stenosis.
Figure 1.
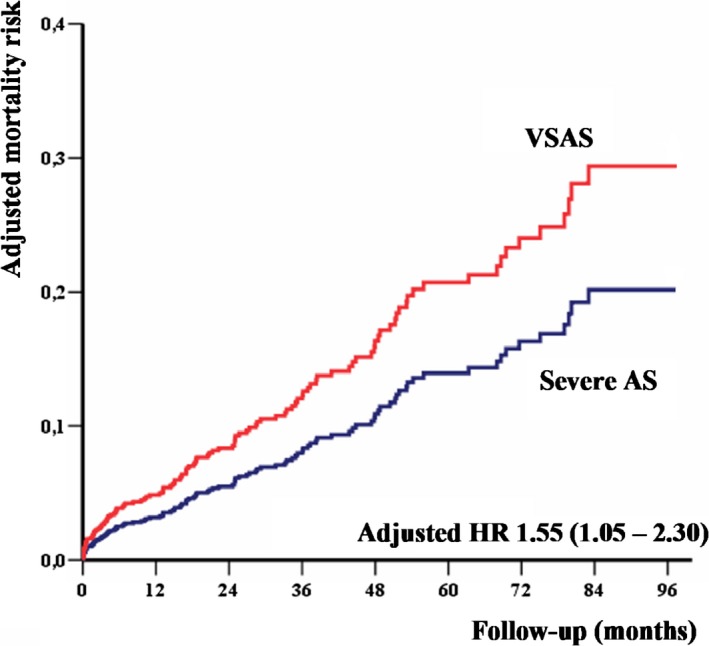
Mortality analysis of 785 asymptomatic patients (mean age 73 years, 53% males) with severe AS (AVA <1 cm2), preserved ejection fraction (LVEF ≥ 50%), and low surgical risk (EuroSCORE II ≤4%) managed medically and surgically in 3 European centers (Amiens, France; Lille, France; and Brussels, Belgium). The figure depicts the adjusted mortality of patients with VSAS (Vmax >5 m/s, or MPG ≥60 mm Hg, or AVA <0.6 cm2, or indexed AVA <0.4 cm2/m2, n=365, 46.5%) compared with that of patients with severe AS not fulfilling any of the VSAS diagnostic criteria (n=420, 53.5%). Curves are adjusted for age, sex, EuroSCORE II, coronary artery disease, atrial fibrillation, LVEF, and AVR using a time‐dependent methodology (Tribouilloy et al, unpublished data, 2019). AS indicates aortic stenosis; AVA, aortic valve area; AVR, aortic valve replacement; HR, hazard ratio; LVEF, left ventricular ejection fraction; MPG, mean transaortic pressure gradient; VSAS, very severe aortic stenosis.
Other parameters validated as strong predictors of poor outcome in asymptomatic severe AS might represent incentives for AVR referral: indexed stroke volume by Doppler echocardiography <30 mL/m2,12 LVEF between 50% and 55%,13 and left atrial enlargement14 (left atrial volume >95 mL or indexed left atrial volume >50 mL/m2 in patients in sinus rhythm). The role of exercise echocardiography is still debated. Although the clinical manifestations of AS are mainly related to the consequences of the valvular obstacle on the left ventricle, the relationship between the severity of stenosis and LV structure and function has rarely been studied in asymptomatic AS. Patients with concentric LV hypertrophy or remodeling, extensive LV fibrosis, or a marked decrease of LV strain may be at high risk of irreversible myocardial damage and clinical events. Baseline blood levels of cardiac biomarkers, such as brain natriuretic peptide, ST2, high‐sensitivity troponin T, and their changes over time may also be of interest. Further studies with careful follow‐up, extensive multimodality imaging, and laboratory assessment are needed to identify additional markers of risk in asymptomatic VSAS.
Based on the evidence presented above, patients with asymptomatic VSAS (Table), preserved LVEF, and low surgical risk should not be managed conservatively because this approach will result in an unacceptably high risk of death. Additional factors supporting prompt surgery in VSAS are the fact that the more severe the valvular lesion, the faster the progression and the continuously increasing operative risk in aging patients managed conservatively. Kicking the can down the road because patients have trouble expressing symptoms may be more comfortable to physicians because physicians are “risk‐averse” and do not want to impose an immediate risk to their patients. However, this comfortable attitude is the source of undertreatment of AS and of excess mortality while the patients remains under “observation” and secondarily when (and if) AVR is ultimately performed. This is why we believe it is time to consider a more proactive approach to AVR in patients with severe AS, and VSAS is a starting point for this call to action. In our opinion, patients with VSAS and low operative risk should be promptly referred for surgical AVR. Transcatheter aortic valve replacement is not currently recommended for asymptomatic severe AS with low surgical risk. The results of ongoing randomized trials in low‐risk patients might extend the use of transcatheter aortic valve replacement as a therapeutic solution for asymptomatic VSAS in elderly patients.
Disclosures
None.
(J Am Heart Assoc. 2019;8:e011724 DOI: 10.1161/JAHA.118.011724.)
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
References
- 1. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM III, Thomas JD. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:e57–e185. [DOI] [PubMed] [Google Scholar]
- 2. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Muñoz D, Rosenhek R, Sjögren J, Tornos Mas P, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–2791. [DOI] [PubMed] [Google Scholar]
- 3. Henkel DM, Malouf JF, Connolly HM, Michelena HI, Sarano ME, Schaff HV, Scott CG, Pellikka PA. Asymptomatic left ventricular systolic dysfunction in patients with severe aortic stenosis: characteristics and outcomes. J Am Coll Cardiol. 2012;60:2325–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rosenhek R, Zilberszac R, Schemper M, Czerny M, Mundigler G, Graf S, Bergler‐Klein J, Grimm M, Gabriel H, Maurer G. Natural history of very severe aortic stenosis. Circulation. 2010;121:151–156. [DOI] [PubMed] [Google Scholar]
- 5. Bohbot Y, Rusinaru D, Delpierre Q, Marechaux S, Tribouilloy C. Risk stratification of severe aortic stenosis with preserved left ventricular ejection fraction using peak aortic jet velocity: an outcome study. Circ Cardiovasc Imaging. 2017;10:e006760. [DOI] [PubMed] [Google Scholar]
- 6. Bohbot Y, Kowalski C, Rusinaru D, Ringle A, Marechaux S, Tribouilloy C. Impact of mean transaortic pressure gradient on long‐term outcome in patients with severe aortic stenosis and preserved left ventricular ejection fraction. J Am Heart Assoc. 2017;6:e005850 DOI: 10.1161/JAHA.117.005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kanamori N, Tomohiko T, Morimoto T, Watanabe H, Shiomi H, Ando K, Murata K, Kitai T, Kawase Y, Izumi C, Miyake M, Mitsuoka H, Kato M, Hirano Y, Matsuda S, Nagao K, Inada T, Mabuchi H, Takeuchi Y, Yamane K, Toyofuku M, Ishii M, Minamino‐Muta E, Kato T, Inoko M, Ikeda T, Komasa A, Ishii K, Hotta K, Higashitani N, Kato Y, Inuzuka Y, Maeda C, Jinnai T, Morikami Y, Saito N, Minatoya K, Aoyama T, Kimura T. Prognostic impact of aortic valve area in conservatively managed patients with asymptomatic severe aortic stenosis with preserved ejection fraction. J Am Heart Assoc. 2019;8:e010198 DOI: 10.1161/JAHA.118.010198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maréchaux S, Ringle A, Rusinaru D, Debry N, Bohbot Y, Tribouilloy C. Prognostic value of aortic valve area by Doppler echocardiography in patients with severe asymptomatic aortic stenosis. J Am Heart Assoc. 2016;5:e003146 DOI: 10.1161/JAHA.115.003146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tribouilloy C, Bohbot Y, Maréchaux S, Debry N, Delpierre Q, Peltier M, Diouf M, Slama M, Messika‐Zeitoun D, Rusinaru D. Outcome implication of aortic valve area normalized to body size in asymptomatic aortic stenosis. Circ Cardiovasc Imaging. 2016;9:e005121. [DOI] [PubMed] [Google Scholar]
- 10. Rusinaru D, Malaquin D, Maréchaux S, Debry N, Tribouilloy C. Relation of dimensionless index to long‐term outcome in aortic stenosis with preserved LVEF. JACC Cardiovasc Imaging. 2015;8:766–775. [DOI] [PubMed] [Google Scholar]
- 11. Banovic M, Iung B, Bartunek J, Asanin M, Beleslin B, Biocina B, Casselman F, da Costa M, Deja M, Gasparovic H, Kala P, Labrousse L, Loncar Z, Marinkovic J, Nedeljkovic I, Nedeljkovic M, Nemec P, Nikolic SD, Pencina M, Penicka M, Ristic A, Sharif F, Van Camp G, Vanderheyden M, Wojakowski W, Putnik S. Rationale and design of the Aortic Valve replAcemenT versus conservative treatment in Asymptomatic seveRe aortic stenosis (AVATAR trial): a randomized multicenter controlled event‐driven trial. Am Heart J. 2016;174:147–153. [DOI] [PubMed] [Google Scholar]
- 12. Rusinaru D, Bohbot Y, Ringle A, Maréchaux S, Diouf M, Tribouilloy C. Impact of low stroke volume on mortality in patients with severe aortic stenosis and preserved left ventricular ejection fraction. Eur Heart J. 2018;39:1992–1999. [DOI] [PubMed] [Google Scholar]
- 13. Bohbot Y, de Meester de Ravenstein C, Chadha G, Rusinaru D, Belkhir K, Trouillet C, Pasquet A, Marechaux S, Vanoverschelde JL, Tribouilloy C. Relationship between left ventricular ejection fraction and mortality in asymptomatic and minimally symptomatic patients with severe aortic stenosis. JACC Cardiovasc Imaging. 2019;12:38–48. [DOI] [PubMed] [Google Scholar]
- 14. Rusinaru D, Bohbot Y, Kowalski C, Ringle A, Maréchaux S, Tribouilloy C. Left atrial volume and mortality in patients with aortic stenosis. J Am Heart Assoc. 2017;6:e006615 DOI: 10.1161/JAHA.117.006615. [DOI] [PMC free article] [PubMed] [Google Scholar]


