Abstract
Background
Monocular vision loss, attributed to either central retinal artery occlusion (CRAO), branch retinal artery occlusion (BRAO), or ocular ischemic syndrome (OIS), is thought to be associated with an increased prevalence of cerebral infarcts. However, there is a paucity of data substantiating this. We aimed to investigate this relationship in a Canadian center and further understand the importance of associated internal carotid artery stenosis in potential clinical decision making.
Methods and Results
We performed a retrospective cohort study at a comprehensive stroke center of patients presenting initially with CRAO, BRAO, or OIS to a centralized ophthalmology center over a 5‐year period. Patients were followed for 3 years for the occurrence of a hemispheric stroke. We identified 83 affected eyes, with 31 CRAO, 35 BRAO, and 17 OIS patients. Before ocular diagnosis, 32.3%, 11.4%, and 41.2% of CRAO, BRAO, and OIS patients, respectively, experienced a symptomatic stroke. Of the remaining patients, 4.8%, 12.9%, and 40%, respectively, suffered a hemispheric stroke within 3 years of ocular diagnosis. Logistic regressions suggested that for CRAO and BRAO patients together, the degree of ipsilateral internal carotid artery stenosis is unable to predict the occurrence of a stroke (P=0.18), whereas our model correctly predicted a stroke in 82.4% of OIS patients (P=0.005).
Conclusions
CRAO, BRAO, and OIS are associated with significantly increased symptomatic stroke rates. Degree of ipsilateral internal carotid artery stenosis may not be useful in risk stratification for these patients, suggesting that they should be triaged appropriately for stroke risk‐factor management, independent of internal carotid artery stenosis.
Keywords: Branch retinal artery occlusion, Central retinal artery occlusion, cerebral infarct, Ocular ischemic syndrome, retinal ischemia, stenosis, stroke
Subject Categories: Cerebrovascular Disease/Stroke, Ischemic Stroke, Transient Ischemic Attack (TIA), Epidemiology
Clinical Perspective
What Is New?
Central retinal artery occlusion, branch retinal artery occlusion, and ocular ischemic syndrome are associated with high cerebral stroke rates, both before and after diagnosis.
Therefore, the diagnosis of these entities warrants a full stroke workup, likely on a semiurgent basis, and referral to a stroke center.
Internal cerebral artery stenosis ipsilateral to the affected eye does not appear to predict stroke risk in central retinal artery occlusion or branch retinal artery occlusion, but may predict stroke risk in ocular ischemic syndrome.
What Are the Clinical Implications?
Degree of internal cerebral artery stenosis should perhaps not be used to triage patients with a new diagnosis of central retinal artery occlusion or branch retinal artery occlusion.
Introduction
Ischemic stroke presents an exceptionally large medical burden given that it is one of the leading causes of morbidity and mortality and is associated with high healthcare expenditures.1 Through rigorous studies, many independent risk factors for stroke have been identified, including age, smoking, diabetes mellitus, hypertension, and others.1 One of the less‐studied risk factors is monocular visual loss, when occurring secondary to retinal arterial occlusion. The NASCET (North American Symptomatic Carotid Endarterectomy Trial) study provided a substantial amount of data regarding the natural history of symptomatic carotid artery plaques in terms of embolic disease. For example, it was found that the 3‐year stroke risk in patients who presented with amaurosis fugax, a transient monocular blindness, was 10%2; however, amaurosis fugax represents just 1 of several clinical presentations of monocular blindness caused by arterial occlusion. Others, leading to permanent monocular blindness or vision loss, include central retinal artery occlusion (CRAO) and branch retinal artery occlusion (BRAO), caused by arterial embolic events to the retina, or ocular ischemic syndrome (OIS), which is associated with acute or subacute vision loss with severe ipsilateral carotid stenosis.
Despite the striking associative findings of increased stroke risk with amaurosis fugax, there is a paucity of published investigations of permanent monocular vision loss (CRAO, BRAO, and OIS) and stroke risk. Moreover, it has been claimed that retinal artery occlusion is associated with a low prevalence of extracranial cerebrovascular disease.3, 4 However, 2 retrospective studies demonstrated a significant co‐occurrence of numerous asymptomatic acute brain infarcts on magnetic resonance imaging (MRI) and first presentation of monocular blindness, secondary to acute retinal artery occlusion, in patients with no history of stroke or transient ischemic attack.5, 6 Furthermore, a 2015 prospective study found that ≈6% of patients with a newly diagnosed acute CRAO had demonstrated new brain infarcts on MRI during routine follow‐up.7 The same study highlighted that 40% of these patients were simultaneously diagnosed with significant ipsilateral carotid stenosis, a known significant risk factor for stroke. These data support the findings from the NASCET subgroup analysis, suggesting that monocular blindness potentially warrants more‐urgent stroke workup and possible treatments. However, the association between monocular vision loss and silent brain infarction does not necessarily correlate with clinical stroke risk. Still fewer studies have examined this potentially significant relationship. We aimed to retrospectively study stroke risk in patients presenting with monocular vision loss secondary to CRAO, BRAO, and OIS to develop a further understanding of this important clinical topic. Furthermore, we investigated the relationship between ipsilateral internal cerebral artery (ICA) stenosis and stroke prevalence. Our hypothesis was that stroke prevalence would be increased in patients with CRAO, BRAO, and OIS compared with the general population, and that severe ipsilateral ICA stenosis would be associated with increased stroke rates. Baseline annual stroke risks of the general population range from ≈0.03% to 1%, with risk increasing with advancing age.7
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. This study was approved by our institutional ethics review board, the Conjoint Health Research Ethics Board. Because of the retrospective nature of this study, no informed consent was required. We conducted a single‐center case series of patients presenting to our centralized retina ophthalmology group with a new diagnosis of CRAO, BRAO, or OIS during a 5‐year period from October 2008 (full implementation of electronic health records system) to September 2013. Diagnoses were established with fundus fluorescein angiography to visualize the arterial occlusive disease, with indocyanine green angiography and/or optical coherence tomography used to verify the diagnosis when able or required. Patients included were at least 18 years of age and had a new diagnosis of either CRAO, BRAO, or OIS at our centralized ophthalmology center. Exclusion criteria included a diagnosis of a confounding vasculitis or lack of 3‐year follow‐up (if cause of death was not stroke). Baseline demographics and stroke risk factors (including hypertension, dyslipidemia, diabetes mellitus, atrial fibrillation, smoking history, and previous stroke) were collected. Patients with no previous stroke diagnosis were retrospectively followed for 3 years, because those that had a previous stroke were already being followed by a neurologist and undergoing risk‐factor modification. Those that died earlier than 3 years from the time of CRAO, BRAO, or OIS diagnosis because of a stroke were included in the analysis. The primary outcome was occurrence of an incident ischemic hemispheric stroke. In the case of a stroke, vascular territory, suspected etiology, and modified Rankin Scale outcome were collected when available. A favorable outcome was defined as a modified Rankin Scale of less than 3 at the time of discharge. Cause of death was recorded for patients who died before the 3‐year follow‐up time point from the date of monocular visual loss diagnosis. ICA stenosis, as measured by any radiographic modality, including computed tomography, Doppler ultrasound, or magnetic resonance imaging, was collected for each patient and categorized into the following 4 groups: insignificant (<50%), moderate (50–69%), severe (70–99%), and occluded as per NASCET criteria.2 These data were collected on all patients included in the study and used to create a logistic regression model to determine the predictive value of degree of ipsilateral ICA stenosis. For this analysis, the analytical sample consisted of each patient's degree of ipsilateral ICA stenosis, with the outcome being the presence of either a prevalent or incident stroke. Any patients with missing carotid stenosis data were excluded from the secondary analysis. The CRAO and BRAO groups were combined for this analysis to allow for a more‐robust analysis with larger sample size and because of the common etiology shared between the 2 disease entities. All statistical testing was carried out using SPSS software (version 25; IBM, Armonk, NY).
Results
Of the 75 666 adult patients in the ophthalmology patient database, 98 (99 eyes) were identified as having a new diagnosis of either CRAO, BRAO, or OIS within the 5‐year inclusion period. One patient had simultaneous diagnoses of BRAO and contralateral OIS. We elected to include this patient in the BRAO group and exclude from the OIS group because the patient presented on account of the acute onset of BRAO, not OIS. Four patients were excluded for confounding vascular diagnoses, including 1 with giant cell arteritis, 2 with retinal vasculitis, and 1 with amaurosis fugax (Figure 1). Three CRAO, 3 BRAO, and 5 OIS patients, totaling 11, were excluded because of death before completion of a 3‐year follow‐up. None of the deaths were related to cerebrovascular events, myocardial infarctions, or other vascular pathology. Thus, 83 patients were included in the study with 31, 35, and 17 patients diagnosed with CRAO, BRAO, and OIS, respectively. Baseline demographics were statistically similar between groups, with an overall male predominance of 57.8% and mean age of 72.5 years (Table 1). Age comparison was performed with a 1‐way ANOVA; binary outcomes were compared using chi‐square or Fisher's exact when contingency table cells contained 5 or fewer occurrences.
Figure 1.
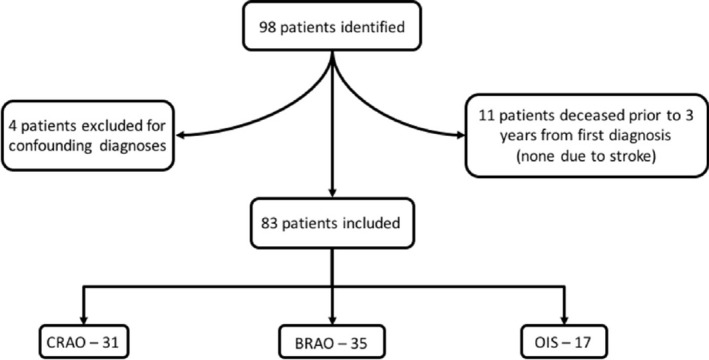
Patient enrollment flowchart. Ninety‐eight patients were identified in our clinical database. Four patients were excluded because of confounding diagnoses (2 for retinal vasculitis, 1 for giant cell arteritis, and 1 for amaurosis fugax). Eleven patients died before the 3‐year follow‐up time point from the diagnosis of CRAO, BRAO, or OIS. None of these deaths were attributed to stroke. Thus, 83 patients were included in the study, with 31 diagnosed with CRAO, 35 with BRAO, and 17 with OIS. BRAO indicates branch retinal artery occlusion; CRAO, central retinal artery occlusion; OIS, ocular ischemic syndrome.
Table 1.
Baseline Demographics for Included Patients
| Factor | CRAO | BRAO | OIS | P Value |
|---|---|---|---|---|
| No. of patients | 31 | 35 | 17 | |
| Age, y | 74.7 (48–90) | 71.4 (46–94) | 70.9 (50–87) | 0.296 |
| Male | 20 (64.5%) | 17 (48.6%) | 11 (64.7%) | 0.35 |
| OD affected | 11 (35.5%) | 20 (57.1%) | 7 (41.2%) | 0.13 |
| Hypertension | 23 (74.2%) | 26 (74.3%) | 16 (94.1%) | 0.212 |
| Dyslipidemia | 15 (48.4%) | 16 (45.7%) | 12 (70.6%) | 0.22 |
| Diabetes mellitus | 7 (22.6%) | 9 (25.7%) | 3 (17.6%) | 0.795 |
| Atrial fibrillation | 3 (9.7%) | 6 (17.1%) | 1 (5.9%) | 0.611 |
| Smoking | 6 (19.4%) | 5 (14.3%) | 4 (23.5%) | 0.76 |
| Previous stroke | 10 (32.3%) | 4 (11.4%) | 7 (41.2%) | 0.036 |
BRAO indicates branch retinal artery occlusion; CRAO, central retinal artery occlusion; OD, oculus dexter; OIS, ocular ischemic syndrome.
For patients diagnosed with CRAO, 10 (32.3%) had evidence of a previous stroke, leaving 21 with no stroke history. Of these, 1 patient (4.8%) presented with a stroke of uncertain embolic etiology within 3 years of CRAO diagnosis (8 months) and had a favorable outcome. This stroke was ipsilateral to the side of the ocular diagnosis. Four (11.4%) of the patients initially presenting with BRAO had a previous stroke. Of the remaining 31 patients, 4 (12.9%) had subsequent strokes with a mean latency of ≈15 months. Two strokes were ipsilateral to the affected eye. An ICA embolic event was responsible for 1, cardiac embolic for another, and the remaining 2 were uncertain embolic sources. Half had a favorable outcome, whereas 1 died as a direct result of the stroke. Seven (41.2%) of the OIS patients had previous strokes, leaving 10 for follow‐up. Of these, 4 (40%) suffered a subsequent stroke with a mean latency of ≈14 months. All strokes were ipsilateral to the affected eye and were found to be ICA embolic etiologies. Only 1 had a favorable outcome, whereas 2 died from the stroke (Figure 2). Chi‐square analysis suggested that there was a statistically significant difference between stroke prevalence of these 3 groups at the time of ocular diagnosis (χ2 (2)=6.625; P=0.036). A hazard function for stroke incidence after ocular diagnosis was calculated for each group, while simultaneously right‐censoring the 11 patients who died from causes other than stroke before the 3‐year follow‐up (Figure 3). Log‐rank testing revealed a statistically significant difference between the 3 groups (χ2 (2)=6.986; P=0.03).
Figure 2.
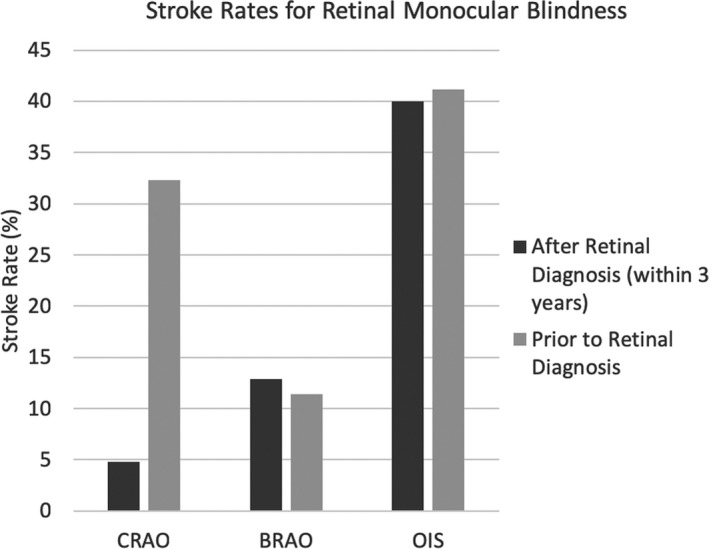
Stroke rates before and after diagnosis of CRAO, BRAO, and OIS. Postdiagnosis stroke rate represents patients with no previous history of stroke or transient ischemic attack. The majority of CRAO patients who had a stroke at some point had it before their diagnosis of monocular vision loss. In BRAO and OIS patients, strokes occurred approximately equally before and after monocular vision loss diagnosis. BRAO inidicates branch retinal artery occlusion; CRAO, central retinal artery occlusion; OIS, ocular ischemic syndrome.
Figure 3.
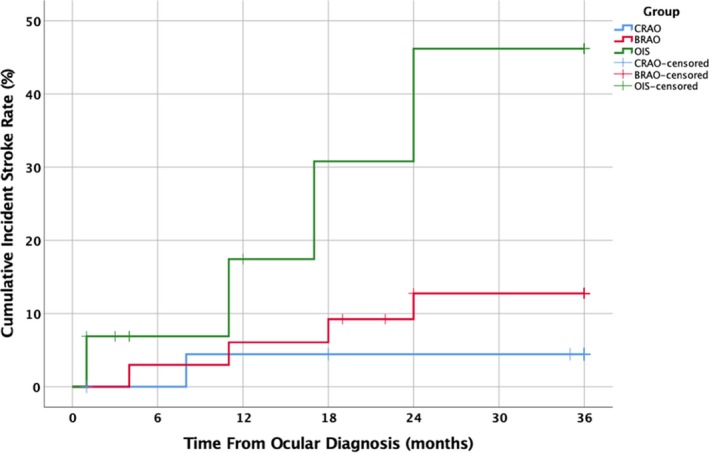
Hazard function of cumulative incident stroke rate after initial diagnosis of CRAO, BRAO or OIS with no previous history of stroke. Log‐rank testing revealed a statistically significant difference between stroke rates (χ2(2)=6.986; P=0.03). BRAO indicates branch retinal artery occlusion; CRAO, central retinal artery occlusion; OIS, ocular ischemic syndrome.
With regard to ipsilateral ICA imaging in cases of CRAO, 4 patients had no carotid imaging available, 1 of which had a history of stroke. Of the remaining 27 patients, 1 (3.7%) had ipsilateral ICA occlusion, 2 (7.4%) had 70% to 99% ipsilateral ICA stenosis, 3 (11.1%) had 50% to 69% stenosis, and 21 (77.8%) had insignificant stenosis (defined as less than 50%). No CRAO patients underwent a carotid endarterectomy or carotid stenting. Seven BRAO patients had no available ipsilateral ICA imaging, 1 of which developed a stroke within 3 years of initial diagnosis. Of the remaining 28 patients, none had ICA occlusion, 1 (3.6%) had 70% to 99% ICA occlusion, 1 had 50% to 69% occlusion, and 26 had insignificant stenosis. The BRAO patient with severe ICA stenosis underwent a carotid endarterectomy 22 months after ocular diagnosis, and neither had a previous stroke nor developed a subsequent stroke. Finally, all patients with OIS had ipsilateral ICA imaging. Five (29.4%) had an occluded ipsilateral ICA, 7 (41.2%) had ipsilateral 70% to 99% stenosis, none had ipsilateral 50% to 69% stenosis, whereas 5 (29.4%) had insignificant stenosis (Figure 4). No OIS patients underwent carotid endarterectomy or carotid stenting.
Figure 4.
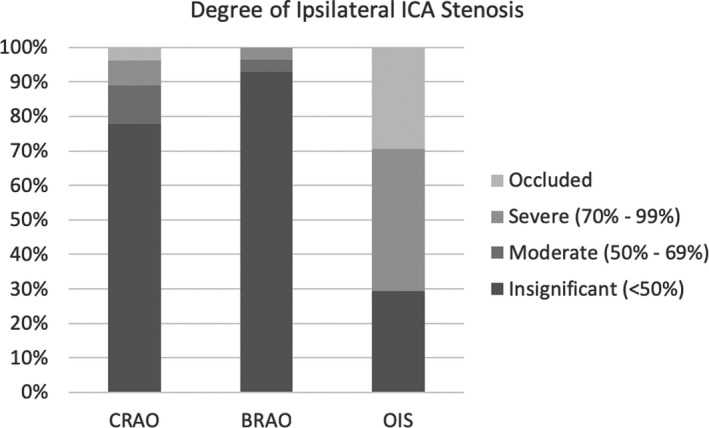
Degree of ICA stenosis ipsilateral to affected eye for CRAO, BRAO, and OIS patients. Of the 31 CRAO patients, 27 had carotid imaging available; 28 of 35 BRAO patients had imaging; all 17 OIS patients had imaging available. The majority of CRAO and BRAO patients had insignificant ipsilateral ICA stenosis. No BRAO patients had ICA occlusion. No OIS patients exhibited moderate stenosis. BRAO indicates branch retinal artery occlusion; CRAO, central retinal artery occlusion; ICA, internal carotid artery; OIS, ocular ischemic syndrome.
A logistic regression analysis was performed on all patients with either CRAO or BRAO to determine whether degree of ipsilateral ICA stenosis could predict stroke (Table 2). A test of the model against a constant‐only model was statistically insignificant, suggesting that degree of stenosis was unable to determine the occurrence of a stroke (χ2 (3)=4.893; P=0.18). Performing the same analysis of OIS patients resulted in a model that, when tested against a constant‐only model, was statistically significant in predicting the occurrence of a stroke (χ2 (2)=7.843; P=0.005; Table 3). Nagelkerke's R 2 was 0.508, indicating a moderate relationship between degree of ipsilateral ICA stenosis and stroke, with the model correctly predicting stroke occurrence in 82.4% of cases. The model found that for every increase in ipsilateral ICA degree of stenosis category, there was an increase in odds of stroke by 4.2 in OIS patients (95% confidence interval, 1.23–14.36).
Table 2.
Case Frequency Table for Stroke Occurrence Stratified by Degree of Ipsilateral ICA Stenosis Used in the CRAO/BRAO and OIS Logistic Regression Models
| Degree of Ipsilateral ICA Stenosis | Stroke | Total | |
|---|---|---|---|
| Yes (%) | No (%) | ||
| CRAO/BRAO | |||
| Insignificant | 17 (36.2) | 30 (63.8) | 47 |
| Moderate | 1 (25) | 3 (75) | 4 |
| Severe | 0 (0) | 3 (100) | 3 |
| Occluded | 1 (100) | 0 (0) | 1 |
| Total | 19 (34.5) | 36 (65.5) | 55 |
| OIS | |||
| Insignificant | 1 (20) | 4 (80) | 5 |
| Moderate | 0 | 0 | 0 |
| Severe | 5 (71.4) | 2 (28.6) | 7 |
| Occluded | 5 (100) | 0 (0) | 5 |
| Total | 11 (64.7) | 6 (35.3) | 17 |
BRAO indicates branch retinal artery occlusion; CRAO, central retinal artery occlusion; ICA, internal carotid artery; insignificant, <50% stenosis; moderate, 50% to 69% stenosis; occluded, 100% stenosis; OIS, ocular ischemic syndrome; severe, 70% to 99% stenosis.
Table 3.
Logistic Regression Analysis Results for Ocular Ischemic Syndrome Model
| Stenosis | Odds Ratio | SE | 95% Confidence Interval | |
|---|---|---|---|---|
| Degree of stenosis | 4.2 | 2.63 | 1.23 | 14.36 |
Logistic regression model for central retinal artery occlusion and branch retinal artery occlusion was not statistically different from a constant‐only model (P=0.18).
Discussion
CRAO, BRAO, and OIS are conditions of retinal ischemia secondary to the sequelae of pathological changes of the extracranial cardiovascular system. Therefore, these conditions are often observed in vasculopathic patients, and it is thus not surprising to see that the baseline medical demographics of the patients enrolled in this study include a relatively high prevalence of stroke risk factors. Based on known stroke epidemiology, these patient cohorts may be at an increased risk of cerebral infarction when compared with healthy age‐matched controls.1 Although these conditions are not the only etiologies of monocular vision loss, their pathogeneses are akin to that of thromboembolic stroke, as opposed to other etiologies such as venous occlusions. Thus, in the context of stroke risk, CRAO, BRAO, and OIS are the most relevant and warrant further investigation.
The primary outcome for this study was 3‐year stroke risk after initial diagnosis of monocular vision loss. The focus was on patients with no previous stroke history, because there is a paucity of data for this specific subgroup. Extensive research has established guidelines for the management of patients with a newly diagnosed stroke.1 So, those with a history of stroke presenting with a new diagnosis of monocular vision loss will have already been managed by a stroke specialist with regard to risk‐modification strategies. The question addressed by this study is whether patients with CRAO, BRAO, or OIS in the absence of previous stroke warrant urgent stroke assessment. Interestingly, we found that only 4.8% of CRAO patients without previous stroke or transient ischemic attack developed a stroke during the follow‐up period, whereas 12.9% of stroke‐free BRAO patients subsequently had a stroke. Yet, almost one third of CRAO patients had a history of stroke compared with a much smaller 11.4% for BRAO patients. Stroke prevalence at any time (up to 3 years after retinal ischemia diagnosis) was 35.5% and 22.9% for CRAO and BRAO, respectively. This suggests that, overall, CRAO has a higher association with stroke than does BRAO, with stroke generally presenting earlier in CRAO patients than in BRAO. This may be indicative of both the vascular anatomy and thromboembolic burden for these patients. Branch retinal arteries are, by definition, smaller in caliber than the more‐proximal central retinal artery, implying that a larger embolism would be required to occlude the central retinal artery than for a branch retinal artery. A similar principle applies to the brain, with smaller arteries supplying smaller territories than larger arteries. Probabilistically speaking, it is more likely for a large embolism to lead to a symptomatic stroke than a smaller embolism. Assuming no difference in the number of thromboembolic events between these groups, it would potentially be more likely for these events in BRAO patients to be asymptomatic. This hypothesis is supported by 2 studies investigating the prevalence of infarctions on MRI in nonarteritic retinal artery occlusions. Both studies reported ≈25% of CRAO and BRAO patients having acute infarcts on diffusion‐weighted imaging, with most patients having multiple infarcts (as many as 84).5, 6 Therefore, there is good evidence to suggest that CRAO and BRAO patients are subject to many thromboembolic events, with many of them being clinically silent.
The 3‐year stroke rates for CRAO and BRAO patients without a stroke history are similar to the few reports in the literature.8, 9 A UK study found a 7.4% stroke rate in the first year after retinal diagnosis, with a 2.5% annual risk thereafter.10 A Taiwanese study reported a 3‐year stroke rate of 19.61% compared with 10.05% for randomly selected controls.11 A Korean study found a 12‐year stroke rate of 15% for retinal artery occlusion patients compared with 8% in age‐matched controls.12 Finally, a subgroup analysis of the EAGLE (European Assessment Group for Lysis in the Eye) study found that 6% of 83 patients developed a stroke within 4 weeks of CRAO diagnosis.13
OIS patients were found to have similar pre‐ and postdiagnosis stroke rates, which were much higher than in CRAO and BRAO. The pathogenesis of OIS involves the gradual occlusion of blood flow through the carotid system, thereby increasing retinal reliance on collateral circulation. By definition, the presence of retinal symptoms indicates insufficient collaterals. Thus, it is not surprising that incidence of stroke is quite high, given that the cerebral circulation must also increasingly rely on collateral circulation.
We then assessed the correlation between degree of ipsilateral ICA stenosis and stroke in the cohorts. Our logistic regression model predicted stroke with reasonable accuracy in OIS patients. This is not surprising given that, again, the entire cranial component of the central nervous system is reliant on collateral circulation. However, we found that logistic regression modeling was unable to predict stroke in CRAO and BRAO patients based on degree of ipsilateral ICA stenosis. Perhaps this suggests as well that cardioembolic or cryptogenic thromboemboli are responsible for the observed symptomatic infarcts. Combined with a relatively high short‐term stroke risk, these data suggest that these patients should be assessed urgently by a stroke service regardless of degree of ipsilateral ICA stenosis. Interestingly, a US survey of various relevant specialists revealed that whereas 73% and 86% of neurologists and neuro‐ophthalmologists have sent urgently referred CRAO patients to an emergency department for immediate evaluation, only 35% of ophthalmologists have done the same.14 A more‐recent survey of neurologists and retina specialists in the United States reported that for CRAO patients presenting within 12 hours of symptom onset, 75% of neurologists, but only 18% of retina specialists, recommend urgent hospital‐based workup. Between 24 and 48 hours after symptom onset, the recommendation dropped to 46% and 8%, respectively.15 Regional practice pattern variations certainly exist when it comes to these disease entities, as is exemplified in a 2018 survey by Youn et al of CRAO treatment, who found that only 20% of US vascular neurology centers outlined a formal treatment flowchart. Furthermore, less than 50% offered intravenous fibrinolysis in the acute setting, whereas 9% of centers offered no treatment. As it pertains to the current study, they also found that 11% of centers did not perform any carotid artery imaging.16 Through these surveys, there is clearly some degree of disconnect between retina specialists and stroke specialists on the acute management of retinal artery occlusions, as well as many inconsistencies with regard to treatment and sense of urgency. An interesting finding in our study is that very few patients with severe ipsilateral ICA stenosis underwent a carotid endarterectomy or carotid artery stenting. Although the details behind this were not evident in our chart review, it is conceivable that because this patient population largely presented initially to an ophthalmological physician, the same referral patterns observed in the above studies occurred in our patient population, resulting in many of them not seeing a neurologist or neurosurgeon. This further emphasizes the lack of standardization of treatment for CRAO, BRAO, and OIS. A consensus on the management of amaurosis fugax has been established, with a 3‐year stroke risk of ≈10% in patients without stroke or transient ischemic attack history.2 Yet, none seemingly exists for these arguably higher risk diseases.
This study has a few limitations worth mentioning. First, the data were collected from a single center in Canada. However, our local system contains centralized retinal ophthalmology and stroke services for the catchment area, maximizing our patient inclusion rate. Furthermore, this minimizes our loss to follow‐up. Despite this, there may have been patients included that developed a stroke while travelling abroad or moved to a different region before 3 years postretinal diagnosis. Therefore, our presented stroke rates may be falsely low. Second, the retrospective study design inherently results in an incomplete data set. Fortunately, complete data were available on 87% of enrolled patients. For example, 13.3% had no documentation available of carotid imaging. This could have been attributed to carotid imaging being performed at a private radiology facility to which we did not have access, because of a lack of imaging being performed, or because of patients moving out of our catchment area. Next, we did not include an assessment of brain MRIs for previous infarcts in our included patients. This almost certainly would have revealed a far larger number of patients with previous or subsequent hemispheric infarcts, but would not have necessarily been symptomatic.4, 5, 6 Although asymptomatic, radiographic infarcts are potentially very important for prognostication purposes in the setting of monocular vision loss, it arguably would not have added to the purpose of this study, which is to determine whether patients diagnosed with CRAO, BRAO, or OIS warrant urgent stroke workup. In this case, the presence of symptomatic infarcts is more clinically relevant. Finally, because the pathologies studied are relatively rare, sample size was smaller than other nation‐wide studies. A Canadian nation‐wide study would be of great benefit to increase the power of the study and its conclusions.
Conclusion
We report on the first Canadian study on stroke risk in patients with CRAO, BRAO, and OIS. To the best of our knowledge, this is also the first study investigating the predictive ability of the degree of ipsilateral ICA stenosis in these patient cohorts on stroke incidence. Our findings suggest that CRAO, BRAO, and OIS result in increased stroke rate, suggesting that these patients warrant urgent stroke assessment and risk‐factor modification, regardless of degree of ipsilateral ICA stenosis. In an age where there is a systems‐level focus on increasing stroke management efficiency, CRAO, BRAO, and OIS should be recognized as urgent cases as well and be managed acutely.
Disclosures
None.
Acknowledgments
The authors acknowledge Geoff Williams, Michael Fielden, Anna Ells, Feisal Adatia, and Patrick Mitchell.
(J Am Heart Assoc. 2019;8:e010509 DOI: 10.1161/JAHA.118.010509)
References
- 1. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics—2015 update. A report from the American Heart Association. Circulation. 2015;131:e29–e322. [DOI] [PubMed] [Google Scholar]
- 2. Benavente O, Eliasziw M, Streifler JY, Fox AJ, Barnett HJM, Meldrum H. Prognosis after transient monocular blindness associated with carotid‐artery stenosis. N Engl J Med. 2001;345:1084–1090. [DOI] [PubMed] [Google Scholar]
- 3. Allan BD, Gregory SK, Vikram SK. The fate of patients with retinal artery occlusion and Hollenhorst plaque. J Vasc Surg. 2007;46:1125–1129. [DOI] [PubMed] [Google Scholar]
- 4. Hayreh SS, Podhajsky PA, Zimmerman MB. Retinal artery occlusion; associated systemic and ophthalmic abnormalities. Ophthalmology. 2009;116:1928–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Helenius J, Arsava EM, Goldstein JN, Cestari DM, Buonanno FS, Rosen BR, Ay H. Concurrent acute brain infarcts in patients with monocular visual loss. Ann Neurol. 2012;72:286–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee J, Kim SW, Lee SC, Kwon OW, Kim YD, Byeon SH. Co‐occurrence of acute retinal artery occlusion and acute ischemic stroke: diffusion‐weighted magnetic resonance imaging study. Am J Ophthalmol. 2014;157:1231–1238. [DOI] [PubMed] [Google Scholar]
- 7. Ovbiagele B, Nguyen‐Huynh MN. Stroke epidemiology: advancing our understanding of disease mechanism and therapy. Neurotherapeutics. 2011;8:319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Klein R, Klein BEK, Moss SE, Meuer SM. Retinal emboli and cardiovascular disease. Arch Ophthalmol. 2003;121:1446–1451. [DOI] [PubMed] [Google Scholar]
- 9. Park SJ, Choi NK, Yang BR, Park KH, Lee J, Jung SY, Woo SJ. Risk and risk periods for stroke and acute myocardial infarction in patients with central retinal artery occlusion. Ophthalmology. 2015;122:2336–2343. [DOI] [PubMed] [Google Scholar]
- 10. Hankey GJ, Slattery JM, Warlow CP. Prognosis and prognostic factors of retinal infarction: a prospective cohort study. BMJ. 1991;302:499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chang YS, Jan RL, Weng SF, Wang JJ, Chio CC, Wei FT, Chu CC. Retinal artery occlusion and the 3‐year risk of stroke in Taiwan: a nationwide population‐based study. Am J Ophthalmol. 2012;154:645–652. [DOI] [PubMed] [Google Scholar]
- 12. Rim TH, Han J, Choi YS, Hwang SS, Lee CS, Lee SC, Kim SS. Retinal artery occlusion and the risk of stroke development: twelve‐year nationwide cohort study. Stroke. 2016;47:376–382. [DOI] [PubMed] [Google Scholar]
- 13. Callizo J, Feltgen N, Pantenburg S, Wolf A, Neubauer AS, Jurklies B, Wachter R, Schmoor C, Schumacher M, Junker B, Pielen A; European Assessment Group for Lysis in the Eye . Cardiovascular risk factors in central retinal artery occlusion: results of a prospective and standardized medical examination. Ophthalmology. 2015; 122:1881–1888. [DOI] [PubMed] [Google Scholar]
- 14. Atkins EJ, Bruce BB, Newman NJ, Biousse V. Translation of clinical studies to clinical practice: survey on the treatment of central retinal artery occlusion. Am J Ophthalmol. 2009;148:172–173. [DOI] [PubMed] [Google Scholar]
- 15. Abel AS, Suresh S, Hussein HM, Carpenter AF, Montezuma SR, Lee MS. Practice patterns after acute embolic retinal artery occlusion. Asia Pac J Ophthalmol. 2017;6:37–39. [DOI] [PubMed] [Google Scholar]
- 16. Youn TS, Lavin P, Patrylo M, Schnidler J, Kirshner H, Greer DM, Schrag M. Current treatment of central retinal artery occlusion: a national survey. J Neurol. 2018;265:330–335. [DOI] [PubMed] [Google Scholar]


