Abstract
Background
Data are scarce on the role of aortic valve area (AVA) to identify those patients with asymptomatic severe aortic stenosis (AS) who are at high risk of adverse events. We sought to explore the prognostic impact of AVA in asymptomatic patients with severe AS in a large observational database.
Methods and Results
Among 3815 consecutive patients with severe AS enrolled in the CURRENT AS (Contemporary Outcomes After Surgery and Medical Treatment in Patients With Severe Aortic Stenosis) registry, the present study included 1309 conservatively managed asymptomatic patients with left ventricular ejection fraction ≥50%. The study patients were subdivided into 3 groups based on AVA (group 1: AVA >0.80 cm2, N=645; group 2: 0.8 cm2 ≥AVA >0.6 cm2, N=465; and group 3: AVA ≤0.6 cm2, N=199). The prevalence of very severe AS patients (peak aortic jet velocity ≥5 m/s or mean aortic pressure gradient ≥60 mm Hg) was 2.0%, 5.8%, and 26.1% in groups 1, 2, and 3, respectively. The cumulative 5‐year incidence of AVR was not different across the 3 groups (39.7%, 43.7%, and 39.9%; P=0.43). The cumulative 5‐year incidence of the primary outcome measure (a composite of aortic valve–related death or heart failure hospitalization) was incrementally higher with decreasing AVA (24.1%, 29.1%, and 48.1%; P<0.001). After adjusting for confounders, the excess risk of group 3 and group 2 relative to group 1 for the primary outcome measure remained significant (hazard ratio, 2.21, 95% CI, 1.56–3.11, P<0.001; and hazard ratio, 1.34, 95% CI, 1.01–1.78, P=0.04, respectively).
Conclusions
AVA ≤0.6 cm2 would be a useful marker to identify those high‐risk patients with asymptomatic severe AS, who might benefit from early AVR.
Clinical Trial Registration
URL: www.umin.ac.jp. Unique identifier: UMIN000012140.
Keywords: aortic valve area, aortic valve replacement, aortic valve stenosis, asymptomatic, echocardiography, prognosis
Subject Categories: Valvular Heart Disease, Aortic Valve Replacement/Transcather Aortic Valve Implantation, Echocardiography, Prognosis
Short abstract
See Editorial by Tribouilloy et al
Clinical Perspective
What Is New?
This is the first large‐scale report exploring the ability of aortic valve area (AVA) to predict the prognosis of asymptomatic patients with severe aortic stenosis in a contemporary multicenter registry of consecutive patients with severe aortic stenosis.
AVA ≤0.60 and 0.8 cm2 ≥AVA >0.6 cm2 as compared with AVA >0.80 cm2 was associated with higher risk for the composite of aortic valve–related death or heart failure hospitalization.
The excess risk of AVA ≤0.60 cm2 relative to AVA >0.80 cm2 remained significant even in patients without very severe AS defined by peak aortic jet velocity ≥5 m/s or mean aortic pressure gradient ≥60 mm Hg, who constituted 74% of those patients with AVA ≤0.6 cm2.
What Are the Clinical Implications?
AVA ≤0.6 cm2 might be an additional objective echocardiographic parameter to identify the high‐risk subsets of patients with asymptomatic severe aortic stenosis.
Initial AVR strategy might be reasonable in patients with AVA ≤0.6 cm2.
Introduction
Current guidelines recommend aortic valve replacement (AVR) for severe aortic stenosis (AS) when the patients have symptoms and/or left ventricular (LV) systolic dysfunction.1, 2 However, we previously reported that the prognosis of asymptomatic patients with severe AS was dismal, and the initial AVR strategy in these patients was associated with better prognosis than the conservative strategy.3 Risk stratification is important to identify the high‐risk subsets of patients with asymptomatic severe AS, who might benefit from early AVR. Current guidelines recommend AVR in asymptomatic patients with very severe AS with peak aortic jet velocity (Vmax) ≥5 m/s or mean aortic pressure gradient (MPG) ≥60 mm Hg, if the surgical risk is low.1, 2 However, there was no recommendation for AVR with respect to aortic valve area (AVA). Several studies reported that AVA was discordant with Vmax in a significant proportion of patients.4, 5, 6 There were a few single‐center studies to investigate the ability of AVA obtained by Doppler echocardiography to identify a subgroup of patients with asymptomatic severe AS who are at high risk of events.7, 8, 9, 10, 11, 12 However, these previous studies were inconclusive about the role of AVA in predicting outcomes of patients with asymptomatic severe AS independent of Vmax and/or MPG.
Therefore, we aimed to explore the ability of AVA to predict the prognosis of asymptomatic patients with severe AS in a large observational database in Japan.
Methods
We will not make the data, methods used in the analysis, and materials used to conduct the research available to any researcher for purposes of reproducing the results or replicating the procedure.
Study Population
The study design, methodologies, and outcomes from the CURRENT AS (Contemporary Outcomes After Surgery and Medical Treatment in Patients With Severe Aortic Stenosis) registry have been described previously.3 Briefly, the CURRENT AS registry is a retrospective, multicenter registry that enrolled 3815 consecutive patients with severe AS among 27 centers in Japan between January 2003 and December 2011. We searched the hospital database of transthoracic echocardiography and enrolled consecutive patients who met the definition of severe AS (Vmax >4.0 m/s, MPG >40 mm Hg, or AVA <1.0 cm2) for the first time during the study period.1, 2 Angina, syncope, and heart failure (HF) symptoms including dyspnea were regarded as AS‐related symptoms.
In the present analysis, we excluded 1197 patients in whom AVR was selected as the initial treatment strategy after the index echocardiography, 1100 patients who had AS‐related symptom, 123 patients with left ventricular ejection fraction (LVEF) <50%, 1 patient whose symptomatic status was not available, 5 patients whose LVEF was unknown, and 80 patients whose AVA was unknown. Therefore, the current study population consisted of 1309 patients with asymptomatic severe AS and LVEF ≥50% who were managed conservatively after the index echocardiography (Figure 1). The number of patients excluded because of reduced LVEF was low because patients with severe AS with reduced LVEF were often symptomatic and/or received initial AVR. The reasons why the AVR was not performed in 123 patients with LVEF <50% at index echocardiography were decision making by the attending physicians in 80 patients (65%), high risk for AVR in 39 patients (32%), and patient's refusal in 4 patients (3%), although the decision regarding the ineligibility for AVR was not uniform in this retrospective study. The current study patients were subdivided into 3 groups based on the AVA: group 1 (AVA >0.80 cm2, N=645), group 2 (0.8 cm2 ≥AVA >0.6 cm2, N=465), and group 3 (AVA ≤0.6 cm2, N=199), based on a previous study of 2427 patients with AVA ≤2.0 cm2 reporting that a Vmax of 4.0 and >5.5 m/s corresponded to an AVA of 0.82 and 0.59 cm2, respectively4 (Figure 1). We compared the baseline characteristics and 5‐year clinical outcomes among the 3 groups. Follow‐up was commenced on the day of index echocardiography unless specified otherwise. All the institutional review boards approved the protocol. Written informed consent was waived because of the retrospective nature of this study, and no patients refused to participate in the study when contacted for follow‐up.
Figure 1.
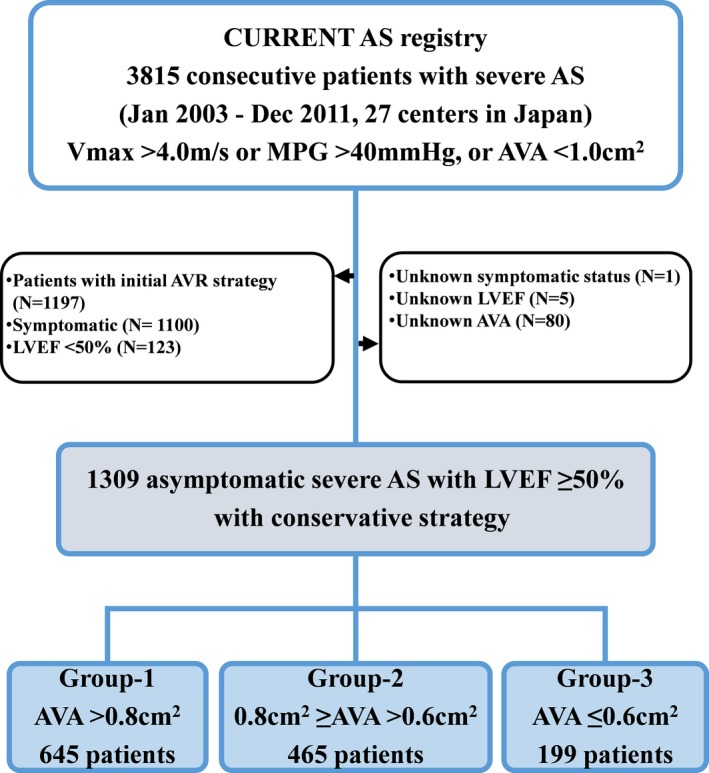
Study patient flow. AS indicates aortic stenosis; AVA, aortic valve area; AVR, aortic valve replacement; LVEF, left ventricular ejection fraction; MPG, mean aortic pressure gradient; Vmax, peak aortic jet velocity.
Data Collection and Definitions of Outcome Measures
The collection of the baseline clinical information was conducted through review of hospital charts and database. All patients at each participating center underwent comprehensive 2‐dimensional and Doppler echocardiographic evaluations. Vmax and the MPG were calculated using the simplified Bernoulli equation. AVA was calculated using the standard continuity equation.13
The primary outcome measure in the present analysis was a composite of aortic valve–related death or HF hospitalization. Other outcome measures included all‐cause death, cardiovascular death, aortic valve–related death, aortic valve procedure death, sudden death, emerging symptoms related to AS, and HF hospitalization. The causes of death were classified according to the Valve Academic Research Consortium definitions, and were adjudicated by a clinical event committee.14, 15 Aortic valve–related deaths included aortic valve procedure death, sudden death, and death caused by HF possibly related to AS. HF hospitalization was defined as hospitalization because of worsening HF requiring intravenous drug therapy. Sudden death was defined as unexplained death in previously stable patients. Other definitions of clinical events have been described previously.3
Statistical Analysis
Continuous variables were expressed as the mean±standard deviation or median with the interquartile range. On the basis of their distributions, we compared continuous variables using 1‐way ANOVA or the Kruskal–Wallis test. Categorical variables were presented as numbers and percentages and were compared using the chi‐squared test. The cumulative incidences of events were estimated by the Kaplan–Meier method, and the differences were assessed with the log‐rank test. We also had evaluated the Gray's test for secondary outcomes (AVR, emerging new symptoms–related AS, and HF hospitalization), with death as competing risk because the secondary outcomes may be biased by death. The outcomes of group 2 and group 3 were compared with those of group 1 (reference) in the multivariable Cox proportional hazard models. Consistent with our previous report, we used the 20 clinically relevant risk‐adjusting variables (age, sex, body mass index, hypertension, current smoking, diabetes mellitus on insulin, coronary artery disease, prior myocardial infarction, prior symptomatic stroke, atrial fibrillation or flutter, aorta/peripheral artery disease, serum creatinine, hemodialysis, anemia, liver cirrhosis, malignancy currently under treatment, chronic lung disease, any valvular disease, LVEF ≥68%, and tricuspid regurgitation pressure gradient ≥40 mm Hg) to adjust for the differences in baseline characteristics (Table 1). The additive analysis for the prognostic impact of AVA over a multivariate model including very severe AS (Vmax ≥5.0 m/s or MPG ≥60 mm Hg) were performed to show how much AVA add to the prognosis value of AS severity as compared with Vmax or MPG. With the exception of age, continuous risk‐adjusting variables were dichotomized using clinically meaningful reference values or median values. We treated age as a continuous variable in the Cox proportional hazards models. The center was incorporated as the stratification variable. The risk of group 2 and group 3 relative to group 1 for the clinical outcomes were expressed as hazard ratios (HRs) and their 95% CI. We also assessed the effects of AVA on long‐term outcomes censored on the day of AVR. Subgroup analyses were also performed in patients with or without other valvular disease (moderate to severe aortic regurgitation, mitral regurgitation, and mitral stenosis), and high‐gradient AS (Vmax >4.0 m/s or MPG >40 mm Hg). Sensitivity analysis was performed in patient without very severe AS (Vmax ≥5.0 m/s or MPG ≥60 mm Hg). All statistical analyses were performed with the statistical software R 3.1.1 (The R Foundation for Statistical Computing, Vienna, Austria), SAS 9.4 (SAS Institute Inc, Cary, NC), or SPSS Statistics 19.0.0 (IBM Corp., Armonk, NY). All reported P values were 2‐tailed, and P<0.05 was considered significant.
Table 1.
Baseline Characteristics and Echocardiographic Parameters
| Group 1: AVA >0.8 cm2 (N=645) | Group 2: 0.8 cm2 ≥AVA >0.6 cm2 (N=465) | Group 3: AVA ≤0.6 cm2 (N=199) | P Value | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age, y* | 76±9 | 78±9 | 81±9 | <0.001 |
| Age ≥80 y | 250 (38.8) | 233 (50.1) | 115 (57.8) | <0.001 |
| Male* | 296 (45.9) | 151 (32.6) | 56 (28.1) | <0.001 |
| BMI, kg/m² | 22.5±3.7 | 21.8±4.0 | 21.2±3.3 | <0.001 |
| BMI <22 kg/m²* | 338 (52.4) | 283 (60.9) | 142 (71.4) | <0.001 |
| BSA, m² | 1.50±0.18 | 1.44±0.17 | 1.40±0.17 | <0.001 |
| Hypertension* | 463 (71.8) | 333 (71.6) | 125 (62.8) | 0.04 |
| Current smoking* | 40 (6.2) | 14 (3.0) | 7 (3.5) | 0.03 |
| History of smoking | 159 (24.7) | 87 (18.7) | 29 (14.6) | 0.003 |
| Dyslipidemia | 250 (38.8) | 148 (31.8) | 62 (31.2) | 0.03 |
| On statin therapy | 200 (31.0) | 101 (21.7) | 41 (20.6) | <0.001 |
| Diabetes mellitus | 162 (25.1) | 113 (24.3) | 38 (19.1) | 0.21 |
| On insulin therapy* | 33 (5.1) | 19 (4.1) | 11 (5.5) | 0.64 |
| Prior myocardial infarction* | 41 (6.4) | 36 (7.7) | 14 (7.0) | 0.67 |
| Prior PCI | 112 (17.4) | 75 (16.1) | 23 (11.6) | 0.15 |
| Prior CABG | 31 (4.8) | 25 (5.4) | 8 (4.0) | 0.75 |
| Prior open heart surgery | 52 (8.1) | 50 (10.8) | 17 (8.5) | 0.29 |
| Prior symptomatic stroke* | 102 (15.8) | 65 (14.0) | 26 (13.1) | 0.54 |
| Atrial fibrillation or flutter* | 115 (17.8) | 91 (19.6) | 45 (22.6) | 0.31 |
| Aortic/peripheral vascular disease* | 94 (14.6) | 86 (18.5) | 29 (14.6) | 0.18 |
| Serum creatinine, mg/dL* | 0.9 (0.7–1.1) | 0.8 (0.7–1.2) | 0.8 (0.7–1.1) | 0.69 |
| Creatinine level >2 mg/dL | 73 (11.3) | 62 (13.3) | 26 (13.1) | 0.56 |
| Hemodialysis* | 56 (8.7) | 52 (11.2) | 23 (11.6) | 0.29 |
| Anemia*, † | 278 (43.1) | 219 (47.1) | 105 (52.8) | 0.05 |
| Liver cirrhosis (Child‐Pugh B or C)* | 4 (0.6) | 4 (0.9) | 1 (0.5) | 0.84 |
| Malignancy | 95 (14.7) | 75 (16.1) | 26 (13.1) | 0.58 |
| Malignancy currently under treatment* | 35 (5.4) | 30 (6.5) | 5 (2.5) | 0.12 |
| Chest wall irradiation | 5 (0.8) | 1 (0.2) | 1 (0.5) | 0.45 |
| Immunosuppressive therapy | 27 (4.2) | 15 (3.2) | 4 (2.0) | 0.32 |
| Chronic lung disease (moderate or severe)* | 15 (2.3) | 13 (2.8) | 7 (3.5) | 0.65 |
| Coronary artery disease* | 175 (27.1) | 116 (24.9) | 46 (23.1) | 0.47 |
| Logistic EuroSCORE, % | 7.7 (4.8–11.5) | 9.2 (5.8–15.5) | 10.7 (6.6–15.2) | <0.001 |
| EuroSCORE II, % | 2.1 (1.3–3.3) | 2.7 (1.7–3.8) | 2.9 (2.0–4.1) | <0.001 |
| STS score (PROM), % | 3.0 (2.0–4.6) | 3.7 (2.3–5.6) | 4.1 (2.8–5.9) | <0.001 |
| Etiology of aortic stenosis | 0.18 | |||
| Degenerative | 573 (88.8) | 414 (89.0) | 181 (91.0) | |
| Congenital | 43 (6.7) | 26 (5.6) | 9 (4.5) | |
| Rheumatic | 24 (3.7) | 23 (4.9) | 5 (2.5) | |
| Infective endocarditis | 0 (0.0) | 0 (0.0) | 1 (0.5) | |
| Other | 5 (0.8) | 2 (0.4) | 3 (1.5) | |
| Echocardiographic variables | ||||
| Vmax, m/s | 3.6±0.6 | 3.8±0.7 | 4.4±0.7 | <0.001 |
| Vmax ≥5 m/s | 12 (1.9) | 23 (4.9) | 47 (23.6) | <0.001 |
| 5.0 m/s >Vmax ≥4.5 m/s | 35 (5.5) | 48 (10.3) | 47 (23.6) | |
| 4.5 m/s >Vmax ≥4 m/s | 128 (19.9) | 121 (26.0) | 53 (26.6) | |
| Vmax <4 m/s | 467 (72.7) | 273 (58.7) | 52 (26.1) | |
| Peak aortic PG, mm Hg | 53±18 | 61±22 | 80±26 | <0.001 |
| MPG, mm Hg | 29±11 | 35±13 | 47±17 | <0.001 |
| MPG ≥60 mm Hg | 7 (1.3) | 18 (4.5) | 40 (23.4) | <0.001 |
| 60 mm Hg >MPG ≥40 mm Hg | 82 (14.7) | 107 (26.8) | 74 (43.3) | |
| MPG <40 mm Hg | 470 (84.1) | 274 (68.7) | 57 (33.3) | |
| Very severe AS (Vmax ≥5 m/s or MPG ≥60 mm Hg) | 13 (2.0) | 27 (5.8) | 52 (26.1) | <0.001 |
| AVA (equation of continuity), cm² | 0.92±0.08 | 0.73±0.06 | 0.52±0.07 | <0.001 |
| AVA index, cm²/m² | 0.62±0.08 | 0.52±0.07 | 0.38±0.06 | <0.001 |
| LV end‐diastolic diameter, mm | 45±6 | 44±5 | 43±5 | <0.001 |
| LV end‐systolic diameter, mm | 28±5 | 27±5 | 27±5 | 0.005 |
| LVEF, %* | 68±8 | 68±8 | 67±7 | 0.06 |
| IVST in diastole, mm | 10.8±2.1 | 11.0±2.0 | 11.5±2.3 | <0.001 |
| PWT in diastole, mm | 10.4±1.8 | 10.6±1.8 | 11.2±2.1 | <0.001 |
| Any combined valvular disease (moderate or severe)* | 218 (33.8) | 128 (27.5) | 62 (31.2) | 0.08 |
| Moderate or severe AR | 127 (19.7) | 56 (12.0) | 23 (11.6) | 0.001 |
| Moderate or severe MS | 21 (3.3) | 11 (2.4) | 5 (2.5) | 0.65 |
| Moderate or severe MR | 76 (11.8) | 43 (9.2) | 26 (13.1) | 0.26 |
| Moderate or severe TR | 82 (12.7) | 58 (12.5) | 32 (16.1) | 0.41 |
| TR pressure gradient ≥40 mm Hg* | 56 (8.7) | 52 (11.2) | 24 (12.1) | 0.24 |
Values are expressed as mean±SD, and number (%), or median (interquartile range). AR indicates aortic regurgitation; AS, aortic stenosis; AVA, aortic valve area; BMI, body mass index; BSA, body surface area; CABG, coronary artery bypass grafting; IVST, interventricular septum thickness; LV, left ventricular; LVEF, left ventricular ejection fraction; MPG, mean aortic pressure gradient; MR, mitral regurgitation; MS, mitral stenosis; PCI, percutaneous coronary intervention; PG, pressure gradient; PROM, predicted risk of mortality; PWT, posterior wall thickness; STS, Society of Thoracic Surgeons; TR, tricuspid regurgitation; Vmax, peak aortic jet velocity.
Risk‐adjusting variables selected for the multivariable Cox proportional hazards models.
Anemia was defined by the World Health Organization criteria (hemoglobin <12.0 g/dL in women and <13.0 g/dL in men).
Results
Baseline Characteristics
Baseline clinical characteristics and echocardiographic parameters were substantially different across the 3 groups (Table 1). With decreasing AVA from group 1 to group 3, the patients became older and more often were female and had a smaller body mass index or body surface area and higher surgical risk scores, while they less often had dyslipidemia or were smokers (Table 1). Regarding the echocardiographic variables, patients with lower AVA more often had higher Vmax or MPG, smaller LV dimension, and LV hypertrophy. LVEF was comparable across the 3 groups (Table 1). The full distribution of AVA was provided in Figure 2, and the relationship between AVA and Vmax or MPG was shown in Figure 3. Three quarters of patients with AVA <0.6 cm2 were not included in the very severe AS defined by Vmax or MPG criteria (Figure 3 and Table 1). Consequently, the prevalence of very severe AS defined by Vmax or MPG was 2.0%, 5.8%, and 26.1%, in groups 1, 2, and 3, respectively (Table 1).
Figure 2.

Distribution of AVA. AVA indicates aortic valve area.
Figure 3.
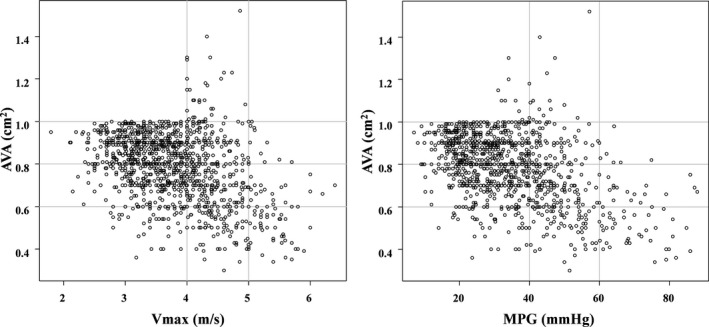
Scatterplot for Vmax vs AVA, and for MPG vs AVA. AVA indicates aortic valve area; MPG, mean aortic pressure gradient; Vmax, peak aortic jet velocity.
Clinical Outcomes
The median follow‐up duration of the surviving patients was 1203 (interquartile range, 773–1575) days with 93% follow‐up completed at 2 years. A total of 414 (32%) out of 1309 patients died, with HF (68 patients) and sudden death (62 patients) being the dominant cardiac causes (Table 2). During follow‐up, 339 patients (25.9%) underwent surgical AVR and 14 patients (1.1%) underwent transcatheter aortic valve implantation. The cumulative 5‐year incidence of AVR or transcatheter aortic valve implantation was not different across the 3 groups (Figure 4). Among 353 patients referred for AVR during follow‐up, 311 (88%) patients had formal indications for AVR; emerging symptoms related to AS in 241 patients (61%), rapid hemodynamic progression in 77 patients (22%), very severe AS (Vmax >5.0 m/s or mean aortic pressure gradient >60 mm Hg) in 11 patients (3%), candidates for other cardiac surgery in 8 patients (2%), and LV dysfunction (defined as LVEF <50%) in 1 patient (0.3%). A total of 380 (39%) of 965 patients without AVR died, with HF (67 patients) and sudden death (60 patients) being the dominant cardiac causes. On the other hand, 34 (9.6%) of 353 patients with AVR died, with aortic valve procedure death (15 patients) and infection (6 patients) being the dominant causes (Table 2).
Table 2.
Causes of Death
| Number of Patients (Proportion) | |||
|---|---|---|---|
| All (N=1309) | Patients With AVR (N=353) | Patients Without AVR (N=956) | |
| All‐cause death | 414 | 34 | 380 |
| Cardiovascular death | 242 (58.5%) | 22 (64.7%) | 220 (57.9%) |
| Heart failure | 68 (16.4%) | 1 (2.9%) | 67 (17.6%) |
| Sudden death | 62 (15.0%) | 2 (5.9%) | 60 (15.8%) |
| Stroke | 24 (5.8%) | 1 (2.9%) | 23 (6.1%) |
| Aortic valve procedure death | 15 (3.6%) | 15 (44.1%) | 0 (0%) |
| Aortic/peripheral vascular disease | 15 (3.6%) | 0 (0%) | 15 (3.9%) |
| Renal failure | 10 (2.4%) | 0 (0%) | 10 (2.6%) |
| Myocardial infarction | 6 (1.4%) | 0 (0%) | 6 (1.6%) |
| Other cardiac cause | 6 (1.4%) | 0 (0%) | 6 (1.6%) |
| Unknown death | 36 (8.7%) | 3 (8.8%) | 33 (8.7%) |
| Noncardiovascular death | 172 (41.5%) | 12 (35.3%) | 160 (42.1%) |
| Infection | 64 (15.5%) | 6 (17.6%) | 58 (15.3%) |
| Malignancy | 57 (13.8%) | 5 (14.7%) | 52 (13.7%) |
| Respiratory failure | 10 (2.4%) | 0 (0%) | 10 (2.6%) |
| Bleeding | 4 (1.0%) | 0 (0%) | 4 (1.1%) |
| Liver failure | 4 (1.0%) | 0 (0%) | 4 (1.1%) |
| Trauma | 4 (1.0%) | 0 (0%) | 4 (1.1%) |
| Others | 29 (7.0%) | 1 (2.9%) | 28 (7.4%) |
AVR indicates aortic valve replacement.
Figure 4.

Cumulative incidence of AVR. AVA indicates aortic valve area; AVR, aortic valve replacement.
The cumulative 5‐year incidence of the primary outcome measure (aortic valve–related death or HF hospitalization) was incrementally higher, with lower AVA from group 1 to group 3 (24.1%, 29.1%, and 48.1%; P<0.001) (Figure 5 and Table 3). After adjusting for confounders, the excess risk of group 3 and group 2 relative to group 1 for the primary outcome measure remained significant (HR, 2.21, 95% CI, 1.56–3.11, P<0.001; and HR, 1.34, 95% CI, 1.01–1.78, P=0.04, respectively) (Table 3). The excess risk of AVA as a continuous variable for the primary outcome measure remained significant (HR, 0.82/0.1 cm2 AVA increment, 95% CI, 0.76–0.88; P<0.001). In addition to AVA, age, LVEF, any combined valvular disease, atrial fibrillation or flutter, chronic lung disease, chronic renal failure, and coronary artery disease were associated with higher risk for the primary outcome measure (Table S1). The result for the primary outcome measure was consistent even after censoring at AVR (Figure 6). The cumulative incidences of all‐cause death and the other secondary outcome measures, including cardiovascular death, aortic valve–related death, aortic valve procedure death, emerging symptoms related to AS, and HF hospitalization, followed the same trend as that for the primary outcome measure (Figures S1–S6 and Table 3). On the other hand, the difference in the incidence of sudden death was not significant (Figure S7 and Table 3). After adjusting for confounders, the excess risks of group 3 relative to group 1 remained significant for all‐cause death and the other individual secondary outcome measures, whereas the risks of group 2 relative to group 1 were significant for all‐cause death and the other individual secondary outcome measures except for HF hospitalization (Table 3). The cumulative 5‐year incidence of all‐cause death starting the follow‐up at the time of AVR in patients with AVR tended to be higher in group 3 than in groups 1 and 2 (12.3%, 12.9%, and 24.8%; P=0.06). Furthermore, after incorporating very severe AS (Vmax ≥5.0 m/s or MPG ≥60 mm Hg) for the confounder, the excess risks of group 3 relative to group 1 remained significant for all individual outcome measures, whereas the risks of group 2 relative to group 1 were not significant for the individual outcome measures except for all‐cause death, cardiovascular death, and aortic valve–related death (Table S2). The additive analysis for secondary outcomes (AVR, emerging new symptoms–related AS and HF hospitalization) with death as competing risk were consistent with the initial analysis (Figures S8–S10 and Table S3). The AVR‐free survival was incrementally lower, with lower AVA from group 1 to group 3 (71.2%, 53.8%, and 32.6%; P<0.001) (Figure S11). The symptom‐free survival was incrementally lower, with lower AVA from group 1 to group 3 (77.2%, 71.8%, and 53.3%; P=0.002) (Figure S12).
Figure 5.
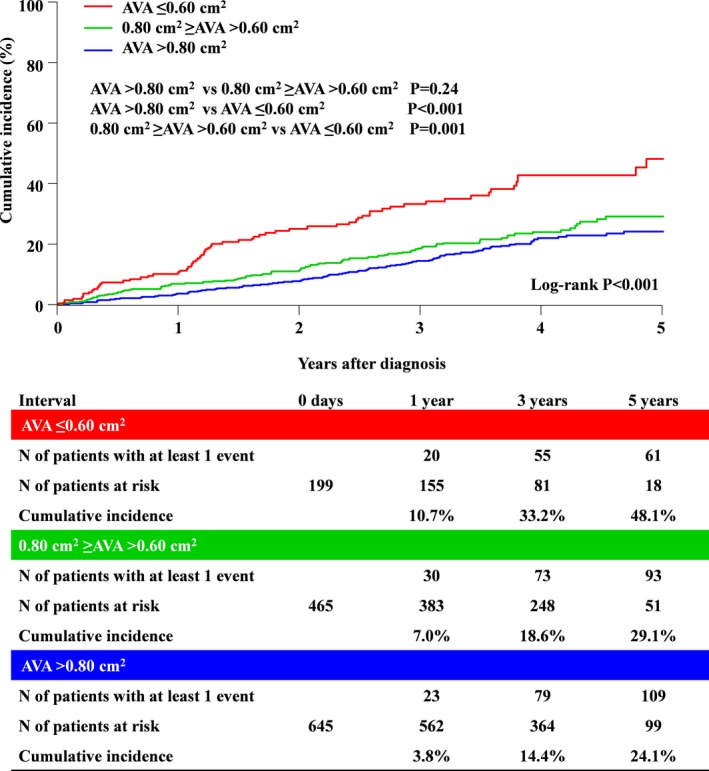
Cumulative incidence of the primary outcome measure (aortic valve–related death or HF hospitalization). AVA indicates aortic valve area; HF, heart failure.
Table 3.
Clinical Outcomes
| No. of Patients With Events (Cumulative 5‐Year Incidence) | Log‐Rank P Value | Unadjusted HR (95% CI) | P Value | Adjusted HR (95% CI) | P Value | |
|---|---|---|---|---|---|---|
| Primary outcome measure | ||||||
| Composite of aortic valve–related death or hospitalization due to HF | <0.001 | |||||
| Group 1 (AVA >0.8 cm2) | 124 (24.1%) | Reference | Reference | |||
| Group 2 (0.8 cm2 ≥AVA >0.6 cm2) | 106 (29.1%) | 1.26 (0.98–1.64) | 0.08 | 1.34 (1.01–1.78) | 0.04 | |
| Group 3 (AVA ≤0.6 cm2) | 67 (48.1%) | 2.22 (1.65–2.98) | <0.001 | 2.21 (1.56–3.11) | <0.001 | |
| Secondary outcome measures | ||||||
| All‐cause death | <0.001 | |||||
| Group 1 (AVA >0.8 cm2) | 160 (27.4%) | Reference | Reference | |||
| Group 2 (0.8 cm2 ≥AVA >0.6 cm2) | 160 (40.0%) | 1.47 (1.18–1.83) | <0.001 | 1.49 (1.17–1.89) | 0.001 | |
| Group 3 (AVA ≤0.6 cm2) | 94 (59.5%) | 2.28 (1.77–2.94) | <0.001 | 2.61 (1.96–3.47) | <0.001 | |
| Cardiovascular death | <0.001 | |||||
| Group 1 (AVA >0.8 cm2) | 91 (16.9%) | Reference | Reference | |||
| Group 2 (0.8 cm2 ≥AVA >0.6 cm2) | 85 (24.2%) | 1.38 (1.02–1.85) | 0.03 | 1.48 (1.07–2.05) | 0.02 | |
| Group 3 (AVA ≤0.6 cm2) | 66 (47.9%) | 2.83 (2.06–3.88) | <0.001 | 3.36 (2.34–4.83) | <0.001 | |
| Aortic valve–related death | <0.001 | |||||
| Group 1 (AVA >0.8 cm2) | 46 (10.0%) | Reference | Reference | |||
| Group 2 (0.8 cm2 ≥AVA >0.6 cm2) | 56 (16.3%) | 1.80 (1.22–2.66) | 0.003 | 2.01 (1.31–3.08) | 0.001 | |
| Group 3 (AVA ≤0.6 cm2) | 42 (34.1%) | 3.60 (2.37–5.47) | <0.001 | 4.53 (2.79–7.34) | <0.001 | |
| Aortic valve procedure death | 0.01 | |||||
| Group 1 (AVA >0.8 cm2) | 6 (1.3%) | Reference | ||||
| Group 2 (0.8 cm2 ≥AVA >0.6 cm2) | 3 (1.1%) | 0.73 (0.18–2.91) | 0.65 | N/A | ||
| Group 3 (AVA ≤0.6 cm2) | 6 (4.7%) | 3.72 (1.20–11.5) | 0.02 | N/A | ||
| Sudden death | 0.08 | |||||
| Group 1 (AVA >0.8 cm2) | 26 (5.8%) | Reference | ||||
| Group 2 (0.8 cm2 ≥AVA >0.6 cm2) | 22 (5.2%) | 1.23 (0.70–2.17) | 0.47 | N/A | ||
| Group 3 (AVA ≤0.6 cm2) | 14 (14.8%) | 2.12 (1.11–4.07) | 0.02 | N/A | ||
| Emerging symptoms related to AS | <0.001 | |||||
| Group 1 (AVA >0.8 cm2) | 186 (18.5%) | Reference | Reference | |||
| Group 2 (0.8 cm2 ≥AVA >0.6 cm2) | 151 (44.1%) | 1.20 (0.97–1.49) | 0.09 | 1.27 (1.01–1.56) | 0.045 | |
| Group 3 (AVA ≤0.6 cm2) | 77 (63.0%) | 1.77 (1.36–2.31) | <0.001 | 1.82 (1.35–2.45) | <0.001 | |
| HF hospitalization | <0.001 | |||||
| Group 1 (AVA >0.8 cm2) | 97 (19.3%) | Reference | Reference | |||
| Group 2 (0.8 cm2 ≥AVA >0.6 cm2) | 83 (23.9%) | 1.27 (0.95–1.70) | 0.11 | 1.33 (0.96–1.83) | 0.08 | |
| Group 3 (AVA ≤0.6 cm2) | 50 (37.7%) | 2.14 (1.52–3.01) | <0.001 | 1.95 (1.31–2.92) | 0.001 | |
| AVR | 0.43 | |||||
| Group 1 (AVA >0.8 cm2) | 178 (39.7%) | Reference | ||||
| Group 2 (0.8 cm2 ≥AVA >0.6 cm2) | 125 (43.7%) | 1.08 (0.86–1.35) | 0.53 | N/A | ||
| Group 3 (AVA ≤0.6 cm2) | 50 (39.9%) | 1.23 (0.90–1.68) | 0.20 | N/A | ||
The number of patients with at least 1 event was counted through the entire follow‐up period, while cumulative incidence was estimated at 5 years. Aortic valve–related death included aortic procedure–related death, sudden death, and death due to HF. HF hospitalization was defined as hospitalization due to worsening HF requiring intravenous drug therapy. Risk‐adjusting variables: age, sex, body mass index, hypertension, current smoking, diabetes mellitus on insulin, coronary artery disease, prior myocardial infarction, prior symptomatic stroke, atrial fibrillation or flutter, aorta/peripheral artery disease, serum creatinine, hemodialysis, anemia, liver cirrhosis, malignancy currently under treatment, chronic lung disease, any valvular disease, LVEF ≥68% and TR pressure gradient ≥40 mm Hg. AS indicates aortic stenosis; AVA, aortic valve area; AVR, aortic valve replacement; HF, heart failure; HR, hazard ratio; LVEF, left ventricular ejection fraction; N/A, not assessed; TR, tricuspid regurgitation.
Figure 6.
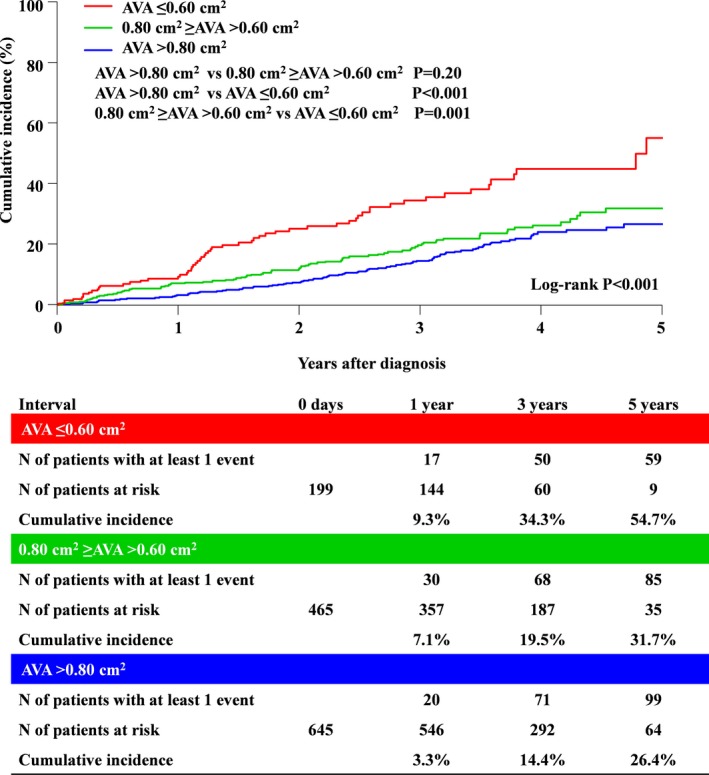
Cumulative incidence of the primary outcome measure (aortic valve–related death or HF hospitalization) with censoring at AVR. AVA indicates aortic valve area; AVR, aortic valve replacement; HF, heart failure.
In the subgroup analysis, the excess risk of group 3 relative to group 1 for the primary outcome measure was consistently seen without any interaction in the subgroups with or without other valvular disease, and high‐ or low‐gradient AS. In patients with high‐gradient AS, the excess risk of group 3 relative to group 1 was significant (HR, 1.75, 95% CI, 1.00–3.05; P=0.048). In the sensitivity analysis, even in patients without very severe AS defined by Vmax or MPG criteria, the excess risk of group 3 relative to group 1 was highly significant (HR, 1.81, 95% CI, 1.21–2.71; P<0.001), whereas the excess risk of group 2 relative to group 1 was no longer significant (HR, 1.27, 95% CI, 0.95–1.70; P=0.11).
Discussion
The main findings of the present study evaluating the prognostic impact of AVA in asymptomatic patients with severe AS were as follows: (1) AVA ≤0.60 and 0.8 cm2 ≥AVA >0.6 cm2 as compared with AVA >0.80 cm2 was associated with higher risk for the composite of aortic valve–related death or HF hospitalization; (2) the excess risk of AVA ≤0.60 cm2 relative to AVA >0.80 cm2 remained significant even in patients without very severe AS defined by Vmax or MPG, who constituted 74% of those patients with AVA ≤0.6 cm2.
Current guidelines recommend AVR for the asymptomatic severe AS patients, if they have LVEF <50%, very severe AS with low surgical risk, and decreased exercise tolerance or fall in systemic blood pressure during exercise.1, 2 Very severe AS was defined using Vmax and MPG, while there is no description with regard to AVA.1, 2 These guidelines were based on the previous single‐center studies suggesting that a peak aortic velocity, but not AVA, was associated with a high risk of events in asymptomatic severe AS patients.7, 8 Nevertheless, AVA decreases with increasing severity of AS, and therefore AVA might theoretically be used to define very severe AS. Our present study clearly demonstrated that patients with AVA ≤0.6 cm2 had a poorer prognosis compared with AVA >0.8 cm2. The cumulative 5‐year incidence of a composite outcome measure of aortic valve–related death or HF hospitalization, sudden death, and AVR were high in patients with AVA ≤0.6 cm2 (48.1%, 14.8%, and 39.9%, respectively).
Previous studies were inconclusive about the role of AVA in predicting outcomes of patients with asymptomatic severe AS independent of Vmax and/or MPG. Rosenhek et al7 prospectively followed 116 consecutive asymptomatic patients with very severe isolated AS defined by Vmax >5.0 m/s and reported that the outcome of patients with AVA <0.6 cm2 was not significantly different from the outcome of those with AVA ≥0.6 cm2. Pellikka et al8 reported that in 622 isolated asymptomatic AS with Vmax >4 m/s who did not undergo surgery at the initial evaluation, the relative risk of developing symptom or sustaining a cardiac event per 0.2 cm2 decrease in AVA was 1.26 and 1.20, respectively. Lancellotti et al9 examined the prognosis of 69 patients with severe asymptomatic AS (AVA <1 cm2) and reported that AVA <0.75 cm2 was one of the main predictors of outcome. More recently, Maréchaux et al12 reported that among 199 asymptomatic severe AS patients, the event rate including AVR and mortality was >80% in 39 patients with AVA ≤0.60 cm2 during 2 years of follow‐up. On the other hand, there was no difference in terms of mortality between patients with an AVA between 0.6 and 0.8 cm2 and those with an AVA between 0.8 and 1.0 cm2.12 The present study evaluating a much larger number of patients with asymptomatic severe AS unequivocally demonstrated the poor prognosis of those patients with AVA ≤0.6 cm2. In addition, our results showed that the mortality of the patients with an AVA between 0.6 and 0.8 cm2 was higher than those with an AVA >0.8 cm2 significantly. Therefore, patients with an AVA between 0.6 and 0.8 cm2 also might be a high‐risk group of events. The cumulative 5‐year incidence of the primary outcome measure in patients with AVA ≤0.60 cm2 in the present study (48.1%) was comparable to that in patients with Vmax ≥5.0 m/s (47.7%) in our previous report.16 Recently, Bohbot et al17 reported that 559 asymptomatic or minimal symptomatic patients with severe AS and preserved LVEF with MPG ≥60 mm Hg (median AVA of 0.65 cm2, interquartile range, 0.55–0.75 cm2) at the time of diagnosis represented a high‐risk group with >70% increase in all‐cause mortality during 4 years of follow‐up. The surgical risk score in our patients with AVA ≤0.6 cm2 was moderate (Society of Thoracic Surgeons score, 4.1%), while the annual incidences of the primary outcome measure and sudden death were unacceptably high (9% and 3%, respectively). AVR during follow‐up would be the critical determinant on the prognosis of patients with severe AS who were initially managed with the conservative strategy. Previous reports suggested AVR during follow‐up increased with decreasing AVA.12 In the present study, however, the proportion of patients who underwent AVR during follow‐up was not influenced by baseline AVA. With decreasing AVA from group 1 to group 3, the patients became older and had higher surgical risk scores, suggesting that the proportion of patients at high risk for surgical AVR also increased with decreasing AVA. The increase in patients at high risk for surgical AVR might have offset the potentially higher likelihood of AVR during follow‐up with decreasing AVA. Thus, the initial AVR strategy might be reasonable in patients with AVA ≤0.6 cm2. In the present study, only 26% of patients among the 199 patients (15%) with AVA ≤0.6 cm2 were classified as very severe AS defined by Vmax or MPG criteria. Inconsistencies between Vmax and AVA have been reported in previous studies.4, 5, 6 Vmax and MPG are strongly influenced by volume flow rate. Therefore, even with the same valve area, these parameters increase with anemia, decreasing peripheral vascular resistance and hyperthyroidism, and decrease with mitral regurgitation, LV dysfunction, and low stroke volume.13 Furthermore, Vmax and MPG values might be underestimated due to a measurement error, although accurate data recording mandates multiple acoustic windows in order to obtain the highest velocity.13 Actually, the average Vmax and MPG values were 4.4±0.7 m/s and 47±17 mm Hg in our patients with AVA ≤0.6 cm2, suggesting underestimation of the severity of AS in a substantial proportion of patients. However, the impact of AVA ≤0.6 cm² in terms of composite criteria was found only in patients with high‐gradient severe AS, although no interaction was found. Hence, whether AVA truly adds prognostic information over Vmax or MPG is not clear in patients with low‐gradient severe AS, although the relationship between AVA and outcome was found in patients without very severe AS. On the other hand, data of stroke volume, which are a component of the calculation of AVA with the continuity equation, are not available in this registry. Previous reports suggested that patients with an AVA ≤0.6 cm2 are often in a low‐flow state, which may explain their low‐gradient AS.12 Therefore, data of stroke volume might have refined the predictive value of AVA.11, 18, 19 Consequently, severity of AS would be better determined using a multiparametric approach incorporating data derived from less flow‐dependent parameter such as AVA in addition to transaortic velocities.
Although the severe AS was dominant pathology in the majority of patients, 408 of 1309 (31%) patients had other valvular disease. However, the prevalence of combined valvular disease was not different across the 3 groups except for the significantly higher prevalence of AR in group 1 (Table 1). In the real clinical practice, we have to make a decision for AVR based on the echocardiographic parameters such as Vmax or AVA in the presence of other valvular disease. Therefore, we prefer to stick to analysis in the whole study population as the main analysis, conducting a subgroup analysis based on the presence or absence of concomitant valvular disease.
In real clinical practice, some patients with severe AS may not complain recognizable symptoms, if daily living activity is decreased. Furthermore, many patients are unable to perform an exercise test for noncardiac reasons (eg, orthopedic, vascular, respiratory, and obese). Thus, physicians may underestimate the presence of symptoms in patients with severe AS.20 Therefore, the availability of objective echocardiographic parameters may be useful for identifying subjects at a higher risk of adverse events among asymptomatic patients with severe AS. We should further pursue implementation of early AVR in selected asymptomatic patients with severe AS.
Limitations
Our study has several limitations. First, echocardiographic data were site reported, and we had no echocardiographic core laboratory. The quality of echocardiographic examination might be variable across centers. Therefore, we could not deny the possibility for measurement error in some patients. However, the echocardiographic measurement was performed according to the guidelines by the experienced cardiologists and/or ultrasonographers in each participating center. Second, the calculation of the AVA by the continuity equation is prone to errors because of the difficulty in measuring LV outflow tract cross‐sectional area owing to noncircular geometry of LV outflow tract.21 Third, data of stroke volume, which are a component of the calculation of AVA with the continuity equation, are not available in this registry. Fourth, we did not assess the changes of AVA during follow‐up. Fifth, we could not exclude the possibility of ascertainment bias for symptoms related to AS at baseline, although we thoroughly reviewed all patient charts and referred to the hospital database to evaluate symptomatic status. An exercise test was rarely performed to ensure that patients were truly asymptomatic. Finally, most of this study period was in the era before transcatheter aortic valve implantation introduction in Japan. Therefore, transcatheter aortic valve implantation could not be performed in a majority of high‐risk patients.
Conclusions
AVA ≤0.60 cm2 would be a useful marker to identify those high‐risk patients with asymptomatic severe AS, who might be benefit from early AVR.
Sources of Funding
This work was partially supported by an educational grant from the Research Institute for Production Development (Kyoto, Japan).
Disclosures
None.
Authors’ Affiliations
From the Division of Cardiology, Shimada Municipal Hospital, Shimada, Japan (N.K., T.A.); Departments of Cardiovascular Medicine (T.T., H.W., H.S., S.M., E.M.‐M., T. Kato, N.S., T. Kimura) and Cardiovascular Surgery (K. Minatoya), Kyoto University Graduate School of Medicine, Kyoto, Japan; Department of Clinical Epidemiology, Hyogo College of Medicine, Nishinomiya, Japan (T.M.); Department of Cardiology, Kokura Memorial Hospital, Kokura, Japan (K.A.); Department of Cardiology, Shizuoka City Shizuoka Hospital, Shizuoka, Japan (K. Murata); Department of Cardiovascular Medicine, Kobe City Medical Center General Hospital, Kobe, Japan (T. Kitai); Department of Cardiovascular Medicine, Kurashiki Central Hospital, Kurashiki, Japan (Y. Kawase); Department of Cardiology, Tenri Hospital, Tenri, Japan (C.I., M.M.); Division of Cardiology, Nara Hospital, Kinki University Faculty of Medicine, Ikoma, Japan (H. Mitsuoka); Department of Cardiology, Mitsubishi Kyoto Hospital, Kyoto, Japan (M.K.); Department of Cardiology, Kinki University Hospital, Osakasayama, Japan (Y.H.); Department of Cardiovascular Center, Osaka Red Cross Hospital, Osaka, Japan (K.N., T. Inada); Department of Cardiology, Koto Memorial Hospital, Higashiomi, Japan (H. Mabuchi); Department of Cardiology, Shizuoka General Hospital, Shizuoka, Japan (Y.T.); Department of Cardiology, Nishikobe Medical Center, Kobe, Japan (K.Y.); Department of Cardiology, Japanese Red Cross Wakayama Medical Center, Wakayama, Japan (M.T.); Department of Cardiology, National Hospital Organization Kyoto Medical Center, Kyoto, Japan (M. Ishii); Cardiovascular Center, The Tazuke Kofukai Medical Research Institute, Kitano Hospital, Osaka, Japan (M. Inoko); Department of Cardiology, Hikone Municipal Hospital, Hikone, Japan (T. Ikeda); Department of Cardiology, Kansai Electric Power Hospital, Osaka, Japan (A.K., K.I.); Department of Cardiology, Hyogo Prefectural Amagasaki General Medical Center, Amagasaki, Japan (K.H.); Department of Cardiology, Japanese Red Cross Otsu Hospital, Otsu, Japan (N.H., T.J.); Department of Cardiology, Saiseikai Noe Hospital, Osaka, Japan (Y. Kato); Department of Cardiology, Shiga Medical Center for Adults, Moriyama, Japan (Y.I.); Department of Cardiology, Hamamatsu Rosai Hospital, Hamamatsu, Japan (C.M.); Department of Cardiology, Hirakata Kohsai Hospital, Hirakata, Japan (Y.M.).
Supporting information
Appendix S1. The members of the CURRENT AS Registry Investigators.
Table S1. Clinical Factors Associated With Composite of Aortic Valve–Related Death or Hospitalization Due to HF in the Multivariable Analyses
Table S2. Clinical Outcomes Incorporating Very Severe AS (Peak Aortic Jet Velocity ≥5 m/s or Mean Aortic Pressure Gradient ≥60 mm Hg) for Confounder
Table S3. Secondary Outcomes With Death as Competing Risk
Figure S1. Cumulative incidence of all‐cause death. AVA indicates aortic valve area.
Figure S2. Cumulative incidence of cardiovascular death. AVA indicates aortic valve area.
Figure S3. Cumulative incidence of aortic valve‐related death. AVA indicates aortic valve area.
Figure S4. Cumulative incidence of aortic valve procedure death. AVA indicates aortic valve area.
Figure S5. Cumulative incidence of emerging symptoms related to AS. AS indicates aortic stenosis; AVA, aortic valve area.
Figure S6. Cumulative incidence of HF hospitalization. AVA indicates aortic valve area; HF, heart failure.
Figure S7. Cumulative incidence of sudden death. AVA indicates aortic valve area.
Figure S8. Cumulative incidence of AVR with competing risk. AVA indicates aortic valve area; AVR, aortic valve replacement.
Figure S9. Cumulative incidence of emerging symptoms related AS with competing risk. AS indicates aortic stenosis; AVA, aortic valve area.
Figure S10. Cumulative incidence of HF hospitalization with competing risk. AVA indicates aortic valve area; HF, heart failure.
Figure S11. AVR‐free survival. AVA indicates aortic valve area; AVR, aortic valve replacement.
Figure S12. Symptom‐free survival. AVA indicates aortic valve area.
(J Am Heart Assoc. 2019;8:e010198 DOI: 10.1161/JAHA.118.010198.)
The authors' affiliations are listed at the end of the article.
Contributor Information
Takeshi Kimura, Email: taketaka@kuhp.kyoto-u.ac.jp.
the CURRENT AS Registry Investigators:
Ryuzo Sakata, Masao Imai, Junichi Tazaki, Toshiaki Toyota, Hirooki Higami, Tetsuma Kawaji, Shinichi Shirai, Kengo Korai, Takeshi Arita, Shiro Miura, Kyohei Yamaji, Kitae Kim, Keiichiro Iwasaki, Hiroshi Miyawaki, Ayumi Misao, Akimune Kuwayama, Masanobu Ohya, Takenobu Shimada, Hidewo Amano, Masashi Amano, Yusuke Takahashi, Yusuke Yoshikawa, Shunsuke Nishimura, Maiko Kuroda, Tetsu Mizoguchi, Takafumi Yokomatsu, Akihiro Kushiyama, Hidenori Yaku, Toshimitsu Watanabe, Sachiko Sugioka, Naoki Takahashi, Kohei Fukuchi, Teruki Takeda, Tomoko Sakaguchi, Keiko Maeda, Masayuki Yamaji, Motoyoshi Maenaka, Yutaka Tadano, Makoto Motooka, Ryusuke Nishikawa, Mitsunori Kawato, Minako Kinoshita, Kenji Aida, Kousuke Takahashi, Euihong Ko, Nobutoyo Masunaga, Hisashi Ogawa, Moritake Iguchi, Takashi Unoki, Kensuke Takabayashi, Yasuhiro Hamatani, Yugo Yamashita, Shuhei Tsuji, Soji Nishio, Jyunya Seki, Miho Yamada, Akira Kawamoto, Kouji Sogabe, Michiya Tachiiri, Yukiko Matsumura, Chihiro Ota, Kenji Minakata, Michiya Hanyu, Fumio Yamazaki, Tadaaki Koyama, Tatsuhiko Komiya, Kazuo Yamanaka, Noboru Nishiwaki, Hiroyuki Nakajima, Motoaki Ohnaka, Hiroaki Osada, Katsuaki Meshii, Toshihiko Saga, Masahiko Onoe, Hitoshi Kitayama, Shogo Nakayama, Genichi Sakaguchi, Atsushi Iwakura, Kotaro Shiraga, Koji Ueyama, Keiichi Fujiwara, Atsushi Fukumoto, Senri Miwa, Junichiro Nishizawa, Mitsuru Kitano, Kenji Nakatsuma, and Tomoki Sasa
References
- 1. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM III, Thomas JD; ACC/AHA Task Force Members . 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:e521–e643. [DOI] [PubMed] [Google Scholar]
- 2. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Muñoz D, Rosenhek R, Sjögren J, Tornos Mas P, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL; ESC Scientific Document Group . 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–2791. [DOI] [PubMed] [Google Scholar]
- 3. Taniguchi T, Morimoto T, Shiomi H, Ando K, Kanamori N, Murata K, Kitai T, Kawase Y, Izumi C, Miyake M, Mitsuoka H, Kato M, Hirano Y, Matsuda S, Nagao K, Inada T, Murakami T, Takeuchi Y, Yamane K, Toyofuku M, Ishii M, Minamino‐Muta E, Kato T, Inoko M, Ikeda T, Komasa A, Ishii K, Hotta K, Higashitani N, Kato Y, Inuzuka Y, Maeda C, Jinnai T, Morikami Y, Sakata R, Kimura T; CURRENT AS Registry Investigators . Initial surgical versus conservative strategies in patients with asymptomatic severe aortic stenosis. J Am Coll Cardiol. 2015;66:2827–2838. [DOI] [PubMed] [Google Scholar]
- 4. Minners J, Allgeier M, Gohlke‐Baerwolf C, Kienzle RP, Neumann FJ, Jander N. Inconsistencies of echocardiographic criteria for the grading of aortic valve stenosis. Eur Heart J. 2008;29:1043–1048. [DOI] [PubMed] [Google Scholar]
- 5. Minners J, Allgeier M, Gohlke‐Baerwolf C, Kienzle RP, Neumann FJ, Jander N. Inconsistent grading of aortic valve stenosis by current guidelines: haemodynamic studies in patients with apparently normal left ventricular function. Heart. 2010;96:1463–1468. [DOI] [PubMed] [Google Scholar]
- 6. Castel AL, Maréchaux S, Laaouaj J, Rusinaru D, Levy F, Tribouilloy C. Relationship between cutoff values of peak aortic valve velocity and those of other Doppler echocardiographic parameters of severity in patients with aortic stenosis and normal flow. Echocardiography. 2012;29:1150–1156. [DOI] [PubMed] [Google Scholar]
- 7. Rosenhek R, Zilberszac R, Schemper M, Czerny M, Mundigler G, Graf S, Bergler‐Klein J, Grimm M, Gabriel H, Maurer G. Natural history of very severe aortic stenosis. Circulation. 2010;121:151–156. [DOI] [PubMed] [Google Scholar]
- 8. Pellikka PA, Sarano ME, Nishimura RA, Malouf JF, Bailey KR, Scott CG, Barnes ME, Tajik AJ. Outcome of 622 adults with asymptomatic, hemodynamically significant aortic stenosis during prolonged follow‐up. Circulation. 2005;111:3290–3295. [DOI] [PubMed] [Google Scholar]
- 9. Lancellotti P, Lebois F, Simon M, Tombeux C, Chauvel C, Pierard LA. Prognostic importance of quantitative exercise Doppler echocardiography in asymptomatic valvular aortic stenosis. Circulation. 2005;112:1377–1382. [DOI] [PubMed] [Google Scholar]
- 10. Malouf J, Le Tourneau T, Pellikka P, Sundt TM, Scott C, Schaff HV, Enriquez‐Sarano M. Aortic valve stenosis in community medical practice: determinants of outcome and implications for aortic valve replacement. J Thorac Cardiovasc Surg. 2012;144:1421–1427. [DOI] [PubMed] [Google Scholar]
- 11. Capoulade R, Le Ven F, Clavel MA, Dumesnil JG, Dahou A, Thébault C, Arsenault M, O'Connor K, Bédard É, Beaudoin J, Sénéchal M, Bernier M, Pibarot P. Echocardiographic predictors of outcomes in adults with aortic stenosis. Heart. 2016;102:934–942. [DOI] [PubMed] [Google Scholar]
- 12. Maréchaux S, Ringle A, Rusinaru D, Debry N, Bohbot Y, Tribouilloy C. Prognostic value of aortic valve area by Doppler echocardiography in patients with severe asymptomatic aortic stenosis. J Am Heart Assoc. 2016;5:e003146 DOI: 10.1161/JAHA.115.003146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, Iung B, Otto CM, Pellikka PA, Quinones M; EAE/ASE . Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. Eur J Echocardiogr. 2009;10:1–25. [DOI] [PubMed] [Google Scholar]
- 14. Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem NM, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE, van Es GA, Hahn RT, Kirtane AJ, Krucoff MW, Kodali S, Mack MJ, Mehran R, Rodés‐Cabau J, Vranckx P, Webb JG, Windecker S, Serruys PW, Leon MB. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium‐2 consensus document. J Am Coll Cardiol. 2012;60:1438–1454. [DOI] [PubMed] [Google Scholar]
- 15. Leon MB, Piazza N, Nikolsky E, Blackstone EH, Cutlip DE, Kappetein AP, Krucoff MW, Mack M, Mehran R, Miller C, Morel MA, Petersen J, Popma JJ, Takkenberg JJ, Vahanian A, van Es GA, Vranckx P, Webb JG, Windecker S, Serruys PW. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: a consensus report from the Valve Academic Research Consortium. J Am Coll Cardiol. 2011;57:253–269. [DOI] [PubMed] [Google Scholar]
- 16. Nakatsuma K, Taniguchi T, Morimoto T, Shiomi H, Ando K, Kanamori N, Murata K, Kitai T, Kawase Y, Izumi C, Miyake M, Mitsuoka H, Kato M, Hirano Y, Matsuda S, Inada T, Nagao K, Murakami T, Takeuchi Y, Yamane K, Toyofuku M, Ishii M, Minamino‐Muta E, Kato T, Inoko M, Ikeda T, Komasa A, Ishii K, Hotta K, Higashitani N, Kato Y, Inuzuka Y, Maeda C, Jinnai T, Morikami Y, Saito N, Minatoya K, Kimura T; CURRENT AS Registry Investigators . Prognostic impact of peak aortic jet velocity in conservatively managed patients with severe aortic stenosis: an observation from the CURRENT AS Registry. J Am Heart Assoc. 2017;6:e005524 DOI: 10.1161/JAHA.117.005524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bohbot Y, Kowalski C, Rusinaru D, Ringle A, Marechaux S, Tribouilloy C. Impact of mean transaortic pressure gradient on long‐term outcome in patients with severe aortic stenosis and preserved left ventricular ejection fraction. J Am Heart Assoc. 2017;6:e005850 DOI: 10.1161/JAHA.117.005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hachicha Z, Dumesnil JG, Bogaty P, Pibarot P. Paradoxical low‐flow, low‐gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation. 2007;115:2856–2864. [DOI] [PubMed] [Google Scholar]
- 19. Eleid MF, Sorajja P, Michelena HI, Malouf JF, Scott CG, Pellikka PA. Flow‐gradient patterns in severe aortic stenosis with preserved ejection fraction: clinical characteristics and predictors of survival. Circulation. 2013;128:1781–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Redfors B, Pibarot P, Gillam LD, Burkhoff D, Bax JJ, Lindman BR, Bonow RO, O'Gara PT, Leon MB, Généreux P. Stress testing in asymptomatic aortic stenosis. Circulation. 2017;135:1956–1976. [DOI] [PubMed] [Google Scholar]
- 21. Jabbour A, Ismail TF, Moat N, Gulati A, Roussin I, Alpendurada F, Park B, Okoroafor F, Asgar A, Barker S, Davies S, Prasad SK, Rubens M, Mohiaddin RH. Multimodality imaging in transcatheter aortic valve implantation and postprocedural aortic regurgitation: comparison among cardiovascular magnetic resonance, cardiac computed tomography, and echocardiography. J Am Coll Cardiol. 2011;58:2165–2173. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. The members of the CURRENT AS Registry Investigators.
Table S1. Clinical Factors Associated With Composite of Aortic Valve–Related Death or Hospitalization Due to HF in the Multivariable Analyses
Table S2. Clinical Outcomes Incorporating Very Severe AS (Peak Aortic Jet Velocity ≥5 m/s or Mean Aortic Pressure Gradient ≥60 mm Hg) for Confounder
Table S3. Secondary Outcomes With Death as Competing Risk
Figure S1. Cumulative incidence of all‐cause death. AVA indicates aortic valve area.
Figure S2. Cumulative incidence of cardiovascular death. AVA indicates aortic valve area.
Figure S3. Cumulative incidence of aortic valve‐related death. AVA indicates aortic valve area.
Figure S4. Cumulative incidence of aortic valve procedure death. AVA indicates aortic valve area.
Figure S5. Cumulative incidence of emerging symptoms related to AS. AS indicates aortic stenosis; AVA, aortic valve area.
Figure S6. Cumulative incidence of HF hospitalization. AVA indicates aortic valve area; HF, heart failure.
Figure S7. Cumulative incidence of sudden death. AVA indicates aortic valve area.
Figure S8. Cumulative incidence of AVR with competing risk. AVA indicates aortic valve area; AVR, aortic valve replacement.
Figure S9. Cumulative incidence of emerging symptoms related AS with competing risk. AS indicates aortic stenosis; AVA, aortic valve area.
Figure S10. Cumulative incidence of HF hospitalization with competing risk. AVA indicates aortic valve area; HF, heart failure.
Figure S11. AVR‐free survival. AVA indicates aortic valve area; AVR, aortic valve replacement.
Figure S12. Symptom‐free survival. AVA indicates aortic valve area.


