Abstract
Background
Cardiovascular biomarkers constitute promising tools for improved risk stratification and prediction of outcome in patients undergoing transcatheter aortic valve implantation. We examined the association of periprocedural changes of NT‐proBNP (N‐terminal pro–B‐type natriuretic peptide) with survival after transcatheter aortic valve implantation.
Methods and Results
NT‐proBNP levels were measured in 704 patients before transcatheter aortic valve implantation and at discharge. Patients were grouped as responders and nonresponders depending on an NT‐proBNP–based ratio (postprocedural NT‐proBNP at discharge/preprocedural NT‐proBNP). Overall, 376 of 704 patients showed a postprocedural decrease in NT‐proBNP levels (NT‐proBNP ratio <1). Responders and nonresponders differed significantly regarding median preprocedural (2822 versus 1187 pg/mL, P<0.001) and postprocedural (1258 versus 3009 pg/mL, P<0.001) NT‐proBNP levels. Patients in the nonresponder group showed higher prevalence of atrial fibrillation (47.0% versus 39.4%, P=0.042), arterial hypertension (94.2% versus 87.5%, P=0.002), renal impairment (77.4% versus 69.1%, P=0.013), and peripheral artery disease (24.4% versus 14.6%, P=0.001). In contrast, patients in the responder group had higher prevalence of moderately reduced left ventricular ejection fraction (17.3% versus 11.0%, P=0.017), lower calculated aortic valve area (0.7 versus 0.8 cm2, P<0.001), and higher mean pressure gradient (41 versus 35 mm Hg, P<0.001). Median follow‐up was 22.6 months. Kaplan–Meier analysis showed a highly significant survival benefit for the responder group compared with the nonresponder group (log‐rank test, P<0.001).
Conclusions
A ratio based on periprocedural changes of NT‐proBNP is a simple tool for better risk stratification and is associated with survival in patients after transcatheter aortic valve implantation.
Keywords: aortic stenosis, biomarker, NT‐proBNP, risk stratification, transcatheter aortic valve implantation
Subject Categories: Aortic Valve Replacement/Transcather Aortic Valve Implantation, Catheter-Based Coronary and Valvular Interventions
Clinical Perspective
What Is New?
Periprocedural changes of NT‐proBNP (N‐terminal pro–B‐type natriuretic peptide) are significantly associated with survival after transcatheter aortic valve implantation.
What Are the Clinical Implications?
Implementing a ratio based on periprocedural changes of NT‐proBNP might improve risk stratification of patients undergoing transcatheter aortic valve implantation.
Patients without a postprocedural decrease of NT‐proBNP after transcatheter aortic valve implantation should be carefully evaluated for optimized medical treatment and closer follow‐up.
Introduction
Aortic stenosis (AS) is the most frequent type of aortic valve disease in North America and Europe, with an age‐dependent prevalence of ≈5% in the population aged >65 years.1 In recent years, transcatheter aortic valve implantation (TAVI) has emerged as the standard treatment of AS in high‐risk and selected intermediate‐risk patients.2 Especially in light of the expansion of TAVI toward a lower risk and younger patient population, tools for improved risk stratification and evaluation of treatment response are essential. Several biomarkers of cardiovascular disease have been studied in the context of AS in an attempt to reflect and investigate the complex processes involved in its pathophysiology.3 In TAVI, mainly preprocedural measurements of biomarkers such as high‐sensitive troponin T,4 GDF15 (growth differentiation factor 15),5 osteopontin,6 and soluble ST2 (suppression of tumorigenicity 2)7 have been shown to provide robust prognostic information.
Natriuretic peptides (NPs) including BNP (B‐type NP) and its prohormone NT‐proBNP (N‐terminal pro‐BNP) are established biomarkers, especially in the field of heart failure (HF).8 NPs have also been correlated to AS severity as well as symptom onset9 and are of predictive value in patients undergoing aortic valve replacement.10, 11 In TAVI patients, elevated NP levels at baseline and after TAVI have been identified as predictors of adverse outcome.11, 12, 13 However, the impact of periprocedural changes in circulating NT‐proBNP levels after TAVI regarding survival remains unclear. We examined the value of an NT‐proBNP–based ratio (postprocedural NT‐proBNP at discharge/preprocedural NT‐proBNP) in a single‐center study.
Methods
For data protection reasons, individual patient data will not be made available to other researchers. Nevertheless, we are convinced that the calculation of our proposed NT‐proBNP ratio is easily reproducible, and we encourage scientists to validate our findings in other TAVI studies.
Study Design
We conducted a retrospective analysis of 704 patients who underwent TAVI at our institution for symptomatic severe AS between January 2011 and March 2017. A ratio was calculated based on periprocedural changes of NT‐proBNP (postprocedural NT‐proBNP at discharge/preprocedural NT‐proBNP).
Before TAVI, patients were discussed by our interdisciplinary heart team and precluded from surgical aortic valve replacement because of significantly elevated surgical risk. We used a few first‐generation CoreValve valves (Medtronic) and mostly second‐generation Sapien XT (Edwards Lifesciences, Irvine, California) and third‐generation Sapien 3 and CoreValve Evolut R systems. The choice of the respective valve type was made at the discretion of the implanting physician. The default TAVI access route was transfemoral; otherwise, a transaortic or transapical approach was used. Procedures were typically performed under conscious sedation, with the exception of transaortic and transapical TAVI.
Data Collection
The data collection was conducted in accordance with the Declaration of Helsinki and approved by the local ethics committee at the University of Kiel. All patients provided informed consent to the procedure and the data acquisition.
Blood samples and patient data were usually collected 1 day before TAVI. Depending on the date of discharge, postprocedural blood samples were taken 3 to 7 days after TAVI. NT‐proBNP was measured using a system obtained from Roche Diagnostics (proBNP II). In addition to biomarker levels, the following data were recorded: age, sex, body mass index, history of atrial fibrillation, presence of coronary artery disease, chronic obstructive pulmonary disease, diabetes mellitus, dyslipidemia, arterial hypertension, peripheral artery disease, pulmonary arterial hypertension, cerebrovascular disease, history of smoking, chronic kidney disease based on glomerular filtration rate, hemodynamic variables, logistic EuroSCORE, and TAVI procedural details. Patient outcomes were analyzed following the Valve Academic Research Consortium 2 (VARC‐2) system.14 The primary outcome of our study was survival. Follow‐up after discharge usually included an in‐person visit in our cardiology outpatient clinic 3 months after TAVI. Thereafter, a phone‐call follow‐up was obtained on an annual basis either by calling the patients or their general practitioner or cardiologist. This was done systematically for all patients who had given written consent to participate in our TAVI registry.
Statistical Analyses
Based on periprocedural changes in NT‐proBNP levels, patients were stratified into 2 groups: those who had a decrease in NT‐proBNP (NT‐proBNP ratio <1) after TAVI were defined as responders, whereas patients who had postprocedurally increased or unchanged NT‐proBNP levels (NT‐proBNP ratio ≥1) were defined as nonresponders. Results are summarized using standard statistical evaluations. Continuous data are presented as median and interquartile range, and categorical data are expressed as counts (percentages). Variables were statistically tested for using the χ2 test and the Mann–Whitney U test. Survival data were visualized by Kaplan–Meier plots and assessed using the log‐rank test as well as Cox regression analysis. For the Cox regression model, all preprocedural factors significantly linked to mortality in the log‐rank test were included. Backward selection was based on the likelihood ratio criteria. Cox regression results are presented as adjusted hazard ratios with 95% CIs. For each covariate, the proportional hazards assumption was approved by testing for interactions between Schoenefeld residuals and the log‐transformed time (function “cox.zph()”). All statistical analyses were performed using the statistical software RStudio v1.1.453 (package “survival”) and GraphPad PRISM v7.
Results
Baseline Patient Characteristics
A total of 704 patients were available for analysis. Based on the NT‐proBNP ratio, 376 patients were assigned to the responder group (NT‐proBNP ratio <1) and 328 were assigned to the nonresponder group (NT‐proBNP ≥1). Baseline patient characteristics are summarized in Table 1. The distribution of the NT‐proBNP ratio is visualized in Figure S1.
Table 1.
Baseline Characteristics of Patients Undergoing TAVI
| Total (n=704) | Responders (n=376) | Nonresponders (n=328) | P Value | |
|---|---|---|---|---|
| Age, y | 81.6 (77.6–86.0) | 81.3 (77.5–85.7) | 81.8 (78.0–86.3) | 0.204 |
| Female | 386 (54.8) | 200 (53.2) | 186 (56.7) | 0.350 |
| BMI, kg/m2 | 26.2 (23.6–29.4) | 26.2 (23.7–29.0) | 26.5 (23.5–30.0) | 0.236 |
| Atrial fibrillation | 302 (42.9) | 148 (39.4) | 154 (47.0) | 0.042 |
| CAD | 510 (72.4) | 271 (72.1) | 239 (72.9) | 0.815 |
| COPD | 115 (16.3) | 56 (14.9) | 59 (18.0) | 0.268 |
| Diabetes mellitus | 230 (32.7) | 120 (31.9) | 110 (33.5) | 0.647 |
| Dyslipidemia | 358 (50.9) | 188 (50.0) | 170 (51.8) | 0.628 |
| Hypertension | 638 (90.6) | 329 (87.5) | 309 (94.2) | 0.002 |
| PAD | 135 (19.2) | 55 (14.6) | 80 (24.4) | 0.001 |
| CVD | 133 (18.9) | 62 (16.5) | 71 (21.6) | 0.081 |
| PAH | 132 (18.8) | 85 (22.6) | 47 (14.3) | 0.005 |
| LVEF | ||||
| <35% | 67 (9.5) | 41 (10.9) | 26 (7.9) | 0.179 |
| 35–45% | 101 (14.3) | 65 (17.3) | 36 (11.0) | 0.017 |
| 45–55% | 131 (18.6) | 72 (19.1) | 59 (18.0) | 0.693 |
| >55% | 405 (57.5) | 198 (52.7) | 207 (63.1) | 0.005 |
| GFR | ||||
| <30 mL/min | 76 (10.8) | 36 (9.6) | 40 (12.2) | 0.264 |
| 30–45 mL/min | 157 (22.3) | 79 (21.0) | 78 (23.8) | 0.378 |
| 45–60 mL/min | 281 (39.9) | 145 (38.6) | 136 (41.5) | 0.433 |
| >60 mL/min | 190 (27.0) | 116 (30.9) | 74 (22.6) | 0.013 |
| History of smoking | 157 (22.3) | 78 (20.7) | 79 (24.1) | 0.288 |
| Log. EuroSCORE (%) | 18.8 (11.9–28.4) | 18.6 (11.6–28.4) | 19.0 (12.7–28.5) | 0.721 |
| NT‐proBNP, pg/mL | 1991 (746–4235) | 2822 (1113–5575) | 1187 (507–2782) | <0.001 |
| AVA, cm2 | 0.7 (0.6–0.9) | 0.7 (0.5–0.8) | 0.8 (0.6–0.9) | <0.001 |
| MPG, mm Hg | 37 (28–49) | 41 (30–53) | 35 (25–43) | <0.001 |
| Diastolic dysfunction ≥ II | 442 (62.8) | 234 (62.2) | 208 (63.4) | 0.746 |
| RV dysfunction | 110 (15.6) | 61 (16.2) | 49 (14.9) | 0.640 |
| MR III–IV | 36 (5.1) | 20 (5.3) | 16 (4.9) | 0.791 |
| TR III–IV | 47 (6.7) | 23 (6.1) | 24 (7.3) | 0.525 |
Values are presented as count (percentage) or median (interquartile range). AVA indicates aortic valve area; BMI, body mass index; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CVD, cerebrovascular disease; GFR, glomerular filtration rate; LVEF, left ventricular ejection fraction; MPG, mean pressure gradient; MR, mitral regurgitation; NT‐proBNP, N‐terminal pro–B‐type natriuretic peptide; PAD, peripheral artery disease; PAH, pulmonary arterial hypertension; RV, right ventricle; TAVI, transcatheter aortic valve implantation; TR, tricuspid regurgitation.
No significant differences between groups were observed in terms of age (median: 81.6 years), female sex (54.8%), body mass index (median: 26.2), coronary artery disease (72.4%), chronic obstructive pulmonary disease (16.3%), diabetes mellitus (32.7%), dyslipidemia (50.9%), history of smoking (22.3%), and cerebrovascular disease (18.9%). However, patients in the responder group showed significantly lower prevalence of atrial fibrillation (39.4% versus 47.0%, P=0.042), arterial hypertension (87.5% versus 94.2%, P=0.002), peripheral artery disease (14.6% versus 24.4%, P=0.001), pulmonary arterial hypertension (22.6% versus 14.3%, P=0.005), and renal impairment (69.1% versus 77.4%, P=0.013). With respect to left ventricular ejection fraction (LVEF), responders had higher prevalence of moderately reduced LVEF (17.3% versus 11.0%, P=0.017) and significantly higher NT‐proBNP levels at baseline (2822 versus 1187 pg/mL, P<0.001). Moreover, responders showed lower aortic valve areas (0.7 versus 0.8 cm2, P<0.001) and higher mean pressure gradients (41 versus 35 mm Hg, P<0.001) as echocardiographic features of more advanced AS.
Procedural Outcomes, HF Medication, and Their Association With Responder Status
Procedural parameters and patient outcomes are presented in Table 2. Patients in the responder group were treated more frequently using a transfemoral access (76.6% versus 50.3%, P<0.001). Median procedural duration was also significantly lower in the responder group (62 versus 72 minutes, P=0.008). There were no significant differences regarding VARC‐2–related outcomes between groups. Data regarding HF medication at discharge are presented in Table 3 and demonstrate no statistically significant difference between groups.
Table 2.
Procedural Variables and Outcomes
| Total (n=704) | Responders (n=376) | Nonresponders (n=328) | P Value | |
|---|---|---|---|---|
| Valve size, mm | ||||
| 23 | 161 (22.9) | 83 (22.1) | 78 (23.8) | 0.591 |
| 26 | 342 (48.6) | 181 (48.1) | 161 (49.1) | 0.802 |
| 29 | 195 (27.7) | 108 (28.7) | 87 (26.5) | 0.515 |
| 34 | 6 (0.9) | 4 (1.1) | 2 (0.6) | 0.513 |
| TF access | 453 (64.3) | 288 (76.6) | 165 (50.3) | <0.001 |
| Procedural duration, min | 66 (50–95) | 62 (50–92) | 72 (52–101) | 0.008 |
| Contrast agent, mL | 80 (60–102) | 80 (65.0–102) | 80 (60–102) | 0.904 |
| VARC‐2 | ||||
| Myocardial infarction | 6 (0.9) | 1 (0.3) | 5 (1.5) | 0.070 |
| Disabling stroke | 10 (1.4) | 4 (1.1) | 6 (1.8) | 0.392 |
| Life‐threatening bleeding | 27 (3.8) | 13 (3.5) | 14 (4.3) | 0.576 |
| Major access complications | 39 (5.5) | 22 (5.9) | 17 (5.2) | 0.699 |
| New pacemaker | 57 (8.1) | 25 (6.6) | 32 (9.8) | 0.132 |
| Conversion to open surgery | 5 (0.7) | 1 (0.3) | 4 (1.2) | 0.133 |
| AKIN stage 3 | 23 (3.3) | 15 (4.0) | 8 (2.4) | 0.248 |
| Residual AR ≥ moderate | 18 (2.6) | 9 (2.4) | 9 (2.7) | 0.769 |
| NT‐proBNP at discharge, pg/mL | 1886 (788–4367) | 1258 (561–2592) | 3009 (1362–6561) | <0.001 |
Values are presented as count (percentage) or median (interquartile). AKIN indicates Acute Kidney Injury Network; AR, aortic regurgitation; NT‐proBNP, N‐terminal pro–B‐type natriuretic peptide; TF, transfemoral; VARC‐2, Valve Academic Research Consortium 2.
Table 3.
HF Medication at Discharge
| Total (n=695) | Responders (n=371) | Nonresponders (n=324) | P Value | |
|---|---|---|---|---|
| ACE‐I or ARB | 550 (79.1) | 303 (81.7) | 247 (76.2) | 0.078 |
| β‐Blocker | 529 (76.1) | 288 (77.6) | 241 (74.4) | 0.317 |
| MR antagonist | 94 (13.5) | 54 (14.6) | 40 (12.3) | 0.395 |
| Loop diuretics | 525 (75.5) | 271 (73.0) | 254 (78.4) | 0.102 |
| Other diuretic agents | 115 (16.5) | 61 (16.4) | 54 (16.7) | 0.937 |
| ARNI | 2 (0.3) | 2 (0.5) | 0 | ··· |
| Digitoxin/digoxin | 49 (7.1) | 23 (6.2) | 26 (8.0) | 0.348 |
| Ivabradin | 5 (0.7) | 3 (0.8) | 2 (0.6) | 0.766 |
| Dihydropyridine CCBs | 289 (41.6) | 155 (41.8) | 134 (41.4) | 0.911 |
Values are presented as count (percentage). Data are available for 695 patients. ACE‐I indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; CCB, calcium channel blocker; HR, heart failure; MR, mineralocorticoid receptor.
Kaplan–Meier survival curves are shown in Figure 1 with a median follow‐up period of 22.6 months. Survival curves of responders and nonresponders substantially diverged, revealing superior outcomes of NT‐proBNP responders in terms of survival (log‐rank test, P<0.001).
Figure 1.
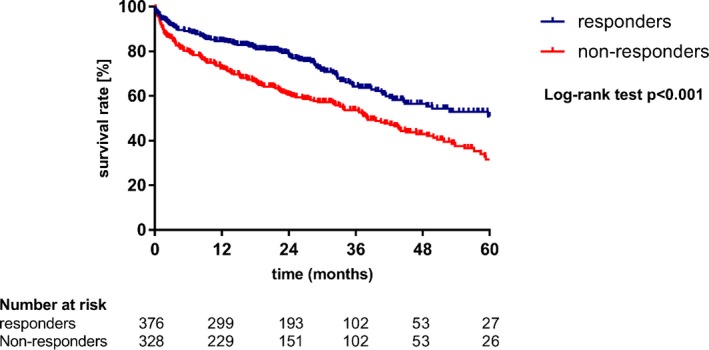
Periprocedural changes in NT‐proBNP (N‐terminal pro–B‐type natriuretic peptide) are associated with survival. Kaplan–Meier survival curves for overall‐survival comparing responders (NT‐proBNP ratio <1) and nonresponders (NT‐proBNP ratio ≥1).
In a further analysis—illustrated in Figure 2—patients with high baseline NT‐proBNP levels (upper quartile) and an NT‐proBNP ratio ≥1 showed the worst survival.
Figure 2.
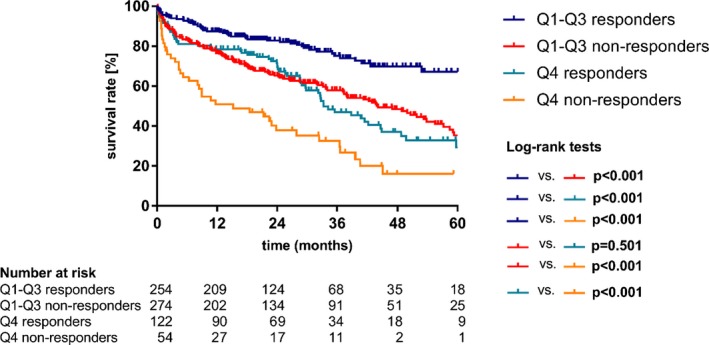
Relevance of a combination of periprocedural NT‐proBNP (N‐terminal pro–B‐type natriuretic peptide) changes and baseline NT‐proBNP levels. Kaplan–Meier survival curves for overall survival of subgroups based on responder status and baseline NT‐proBNP levels. (Q4=upper quartile). Q indicates quartile.
Variables Significantly Associated With Outcome After TAVI
Table 4 summarizes variables that were significantly associated with mortality after a median follow‐up of 22.6 months, using log‐rank tests. These included an NT‐proBNP ratio ≥1 and elevated baseline and postprocedural NT‐proBNP levels (upper quartile, respectively). In addition, age older than the median (81.6 years), atrial fibrillation, chronic obstructive pulmonary disease, diabetes mellitus, dyslipidemia, pulmonary arterial hypertension, and renal impairment were linked to mortality. Regarding VARC‐2–defined outcomes, disabling stroke, life‐threatening bleeding, periprocedural myocardial infarction, and major access complications were also significant parameters. Following backward selection based on the likelihood criteria, multivariable Cox regression analysis was used to identify variables that show statistically significant associations with adverse TAVI outcome. These data are presented in Table 5. The proportional hazards assumption was met (Schoenfeld individual test for each covariate P≥0.05, global Schoenfeld test P=0.250). Of the aforementioned parameters, both diabetes mellitus and dyslipidemia did not reach statistical significance.
Table 4.
Significant Mortality‐Associated Factors (Log‐Rank Test)
| P Value | |
|---|---|
| Nonresponder status (NT‐proBNP ratio ≥1) | <0.001 |
| Preprocedural NT‐proBNP >4235 pg/mL (Q4) | <0.001 |
| Postprocedural NT‐proBNP >4367 pg/mL (Q4) | <0.001 |
| Age older than median (81.6 y) | 0.009 |
| Atrial fibrillation | <0.001 |
| COPD | <0.001 |
| Diabetes mellitus | 0.048 |
| Dyslipidemia | 0.007 |
| PAH | 0.015 |
| Renal impairment (GFR <60 mL/min) | <0.001 |
| Disabling stroke | <0.001 |
| Life‐threatening bleeding | <0.001 |
| Myocardial infarction | 0.003 |
| Major access complication | 0.013 |
COPD indicates chronic obstructive pulmonary disease; GFR, glomerular filtration rate; NT‐proBNP, N‐terminal pro–B‐type natriuretic peptide; PAH, pulmonary arterial hypertension; Q, quartile; Q4, upper quartile.
Table 5.
Cox Regression Analysis
| Variable | Crude HR (95% CI) | P Value | Adjusted HR (95% CI) | P Value |
|---|---|---|---|---|
| Nonresponder status (NT‐proBNP ratio ≥1) | 1.67 (1.32–2.11) | <0.001 | 1.68 (1.27–2.22) | <0.001 |
| Preprocedural NT‐proBNP >4235 pg/mL (Q4) | 1.83 (1.43–2.33) | <0.001 | 1.46 (1.06–1.99) | 0.019 |
| Postprocedural NT‐proBNP >4367 pg/mL (Q4) | 2.48 (1.96–3.14) | <0.001 | 1.52 (1.12–2.05) | 0.007 |
| Age older than median (81.6 y) | 1.36 (1.08–1.72) | 0.010 | 1.38 (1.08–1.76) | 0.010 |
| Atrial fibrillation | 1.77 (1.41–2.24) | <0.001 | 1.48 (1.17–1.88) | 0.001 |
| COPD | 2.14 (1.64–2.79) | <0.001 | 2.19 (1.66–2.88) | <0.001 |
| PAH | 1.40 (1.07–1.85) | 0.016 | 1.47 (1.10–1.96) | 0.009 |
| Renal impairment (GFR <60 mL/min) | 2.23 (1.62–3.07) | <0.001 | 1.63 (1.17–2.28) | 0.004 |
| Disabling stroke | 4.57 (2.43–8.6) | <0.001 | 3.57 (1.83–6.97) | <0.001 |
| Life‐threatening bleeding | 2.34 (1.45–3.78) | <0.001 | 2.63 (1.58–4.38) | <0.001 |
| Myocardial infarction | 3.57 (1.47–8.69) | 0.005 | 4.81 (1.92–12.02) | <0.001 |
| Major access complication | 1.69 (1.11–2.57) | 0.014 | 1.69 (1.07–2.65) | 0.024 |
| Diabetes mellitus | 1.27 (1.0–1.62) | 0.048 | 1.22 (0.95–1.57) | 0.113 |
| Dyslipidemia | 0.73 (0.58–0.92) | 0.007 | 0.80 (0.63–1.02) | 0.076 |
COPD indicates chronic obstructive pulmonary disease; GFR, glomerular filtration rate; HR, hazard ratio; NT‐proBNP, N‐terminal pro–B‐type natriuretic peptide; PAH, pulmonary arterial hypertension; Q, quartile; Q4, upper quartile.
In conclusion, an NT‐proBNP ratio ≥1 was significantly associated with mortality after TAVI (hazard ratio: 1.68; 95% CI, 1.27–2.22; P<0.001).
Discussion
In this analysis of 704 patients, we examined the association between periprocedural changes of NT‐proBNP levels and survival after TAVI. Based on an NT‐proBNP ratio (postprocedural NT‐proBNP at discharge/preprocedural NT‐proBNP) patients were divided into responder (NT‐proBNP ratio <1) and nonresponder (NT‐proBNP ratio ≥1) groups. Our study shows that the NT‐proBNP ratio is significantly associated with survival after TAVI.
NPs and Biomarker‐Guided Therapy in Patients With HF
NPs are essential biomarkers in the context of HF because they reflect disease severity and predict adverse outcomes.15, 16, 17 Although normal plasma concentrations of NPs (cutoff for NT‐proBNP <125 pg/mL in a nonacute setting and <300 pg/mL in an acute setting) have an excellent predictive value for excluding HF,8 elevated NPs may be associated with various cardiovascular and noncardiovascular diseases including valvular heart disease.18, 19 In addition to the prognostic implications of NPs, the concept of a biomarker‐guided treatment strategy has also been evaluated in HF patients. In the UPSTEP (Use of Peptides in Tailoring Heart Failure Project) study,20 279 patients with worsening HF, LVEF <40%, and elevated levels of BNP were randomized to either conventional HF treatment or BNP‐guided therapy. Although no significant differences were noted between groups regarding morbidity and mortality, the study demonstrated that treatment responders (>30% decrease in baseline BNP levels) had significantly better outcomes compared with nonresponders. Moreover, the GUIDE‐IT (Guiding Evidence Based Therapy Using Biomarker Intensified Treatment in Heart Failure) study,21 which enrolled 894 high‐risk HF patients with reduced LVEF who were randomized to either an NT‐proBNP‐guided strategy or usual care, also failed to show superiority of an NP‐based approach and was terminated for futility.
NPs in AS and Aortic Valve Replacement
NPs have been shown to correlate with AS severity, symptom‐free survival, optimal timing of aortic valve replacement, and mortality.22, 23, 24, 25 BNP is recognized in the current European Society of Cardiology and European Association for Cardio‐Thoracic Surgery guidelines for the management of valvular heart disease2 as the only biomarker with prognostic value in AS. Regarding risk stratification, elevated NPs at baseline have been identified as predictors of postoperative mortality in patients undergoing surgical aortic valve replacement.9, 10
Consequently, NPs have also been evaluated for their prognostic implications in the context of TAVI. In a study comprising 340 TAVI patients, O'Sullivan et al26 showed that patients in the upper tertile group (BNP ≥596 pg/mL) had higher rates of all‐cause mortality and major adverse cardiac and cerebrovascular events (death, major stroke, and myocardial infarction) at 30 days compared with patients in the lower tertile group (BNP ≤201 pg/mL). With respect to long‐term outcome, Koskinas et al27 demonstrated in a study of 340 TAVI patients that high preprocedural BNP levels predict all‐cause mortality and cardiovascular death at 2 years. In 219 of those patients, both BNP and NT‐pro‐BNP were measured serially before and after the procedure. In their study, NT‐proBNP levels after TAVI showed the highest prognostic discrimination for 2‐year mortality. In a study of 333 TAVI patients by Ribeiro et al,28 elevated NT‐proBNP at baseline (cutoff value ≥2000 pg/mL) emerged as a predictor of all‐cause mortality, cardiovascular death, and rehospitalization for HF at a median follow‐up of 2 years. The authors concluded that NT‐proBNP levels should be included in the decision‐making process and in clinical patient follow‐up.
The prognostic implications of periprocedural changes of BNP were investigated by O'Neill et al. Based on data from the PARTNER (Placement of Aortic Transcatheter Valve Trial Edwards SAPIEN Transcatheter Heart Valve) trial,11 comprising 933 patients with baseline and serial BNP measurements, the authors demonstrated that an increase of BNP level at 30 days was associated with death and the combined end point of death or rehospitalization. Furthermore, data from the multicenter OCEAN‐TAVI (Optimized Catheter valvular intervention) registry13 presented by Mizutani et al showed that elevated BNP levels at discharge were significantly associated with 2‐year mortality and thus might aid in risk stratification of TAVI patients. In addition, a study of 504 patients regarding the prognostic value of periprocedural NT‐proBNP levels after TAVI29 has been reported recently by Liebetrau et al. The authors showed that elevated postprocedural NT‐proBNP is a predictor of mortality if interpreted in the context of transapical access with concomitant aortic regurgitation and in transfemoral patients with concomitant aortic regurgitation and reduced LVEF.
In conclusion, our results are in line with previously published data emphasizing that both pre‐ and postprocedural NPs including NT‐proBNP are of predictive value in patients undergoing TAVI and thus might be incorporated into clinical practice.
NT‐proBNP Ratio and Variables Associated With Responder and Nonresponder Status
To the best of our knowledge, this study is the first to investigate the prognostic value of a biomarker ratio based on NT‐proBNP levels at discharge and at baseline in patients undergoing TAVI.
Although we acknowledge the important aforementioned scientific data demonstrating that NPs aid in risk stratification of TAVI patients during follow‐up (eg, at 30 days or 1 year), we strongly support a strategy of early risk stratification. Implementation of an NT‐proBNP ratio as a simple tool might be beneficial in identifying those patients in need of closer follow‐up or optimized medical treatment. Notably, in our analysis, there was no statistically significant difference in HF medication at discharge between groups, perhaps suggesting that a large proportion of TAVI patients did not receive optimal therapy. This may be attributed in part to the fact that medical treatment of TAVI patients was not routinely guided by NT‐proBNP; however, the best explanation seems to be the well‐known underuse of recommended HF therapy in elderly patients.30 Notably, responders had higher preprocedural NT‐proBNP levels compared with nonresponders, and that finding seems to be attributed mainly to the higher rates of chronic HF and more severe AS in this group.
Our data also show that highly elevated NT‐proBNP levels—both at baseline and at discharge (upper quartile, respectively)—are significantly associated with survival regardless of a postprocedural decrease of NT‐proBNP after TAVI.
In our study, parameters associated with nonresponder status included atrial fibrillation, arterial hypertension, peripheral artery disease, and impaired renal function, all of which are conditions known to be associated with increased levels of baseline NT‐proBNP.19, 31 It seems obvious that these patients may display persistently elevated NT‐proBNP levels, reflecting myocardial damage that is not fully reversible by TAVI. In contrast, the responder group had higher mean pressure gradients and smaller aortic valve areas as echocardiographic parameters of AS severity. This finding supports the idea that TAVI in this patient group yields immediate effects in terms of ventricular unloading and decrease of myocardial stretch, leading to an acute postprocedural decrease of circulating NT‐proBNP levels. Our findings also suggest the intuitive assumption that patients whose elevated NT‐proBNP levels are driven mainly by severe AS benefit the most from TAVI.
Interestingly, the responder group in our study included more patients with impaired LVEF. An adequate explanation seems to be beyond the scope of our study, but these patients might constitute an important subgroup for future studies.
Limitations
Our study is limited mainly by its retrospective single‐center design with the focus on survival. End points such as HF symptoms, physical capacity, cardiac‐related rehospitalization, and quality of life are not accounted for. In addition, both measured and unmeasured confounding factors may limit the conclusions that can be drawn from our analysis. Echocardiography data were partially analyzed in retrospect, and follow‐up echo data were not sufficiently available, which may under‐ and overestimate differences between groups. However, our study cohort represents a typical, unselected, real‐world TAVI population with a considerable sample size and a relatively long follow‐up period. We acknowledge that ≥1 might not be the perfect cutoff value, but we chose to dichotomize the ratio because, from a clinical point of view, this approach seems to be the most reasonable and keeps our analysis simple. Because risk stratification of TAVI patients constitutes a major clinical challenge, implementing an NT‐proBNP ratio might be of great value.
Conclusion
Periprocedural changes of NT‐proBNP are significantly associated with survival in patients undergoing TAVI. Patients who fail to respond to TAVI with a postprocedural decrease of NT‐proBNP should be carefully monitored and evaluated for optimized medical treatment. Future studies are necessary to determine the potential role of NT‐proBNP in risk stratification and therapeutic management of patients after TAVI.
Disclosures
Kuhn received speaker's honoraria from Medtronic. D. Frank works as a proctor for and has received speaker's honoraria as well as travel support from Medtronic and Edwards. The remaining authors have no disclosures to report.
Supporting information
Figure S1. Distribution of the NT‐proBNP (N‐terminal pro–B‐type natriuretic peptide) ratio.
(J Am Heart Assoc. 2019;8:e010876 DOI: 10.1161/JAHA.118.010876)
References
- 1. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez‐Sarano M. Burden of valvular heart diseases: a population‐based study. Lancet. 2006;368:1005–1011. [DOI] [PubMed] [Google Scholar]
- 2. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Muñoz D, Rosenhek R, Sjögren J, Tornos Mas P, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL; ESC Scientific Document Group . 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–2791. [DOI] [PubMed] [Google Scholar]
- 3. Redfors B, Furer A, Lindman BR, Burkhoff D, Marquis‐Gravel G, Francese DP, Ben‐Yehuda O, Pibarot P, Gillam LD, Leon MB, Généreux P. Biomarkers in aortic stenosis: a systematic review. Struct Heart. 2017;1:18–30. [Google Scholar]
- 4. Frank D, Stark S, Lutz M, Weissbrodt A, Freitag‐Wolf S, Petzina R, Rosenberg M, Lutter G, Frey N. Preprocedural high‐sensitive troponin predicts survival after transcatheter aortic valve implantation (TAVR). Int J Cardiol. 2013;169:e38–e39. [DOI] [PubMed] [Google Scholar]
- 5. Krau NC, Lünstedt NS, Freitag‐Wolf S, Brehm D, Petzina R, Lutter G, Bramlage P, Dempfle A, Frey N, Frank D. Elevated growth differentiation factor 15 levels predict outcome in patients undergoing transcatheter aortic valve implantation. Eur J Heart Fail. 2015;17:945–955. [DOI] [PubMed] [Google Scholar]
- 6. Lutz M, von Ingersleben N, Lambers M, Rosenberg M, Freitag‐Wolf S, Dempfle A, Lutter G, Frank J, Bramlage P, Frey N, Frank D. Osteopontin predicts clinical outcome in patients after treatment of severe aortic stenosis with transcatheter aortic valve implantation (TAVI). Open Heart. 2017;4:e000633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stundl A, Lünstedt N, Courtz F, Freitag‐Wolf S, Frey N, Holdenrieder S, Zur B, Grube E, Nickenig G, Werner N, Frank D, Sinning JM. Soluble ST2 for risk stratification and the prediction of mortality in patients undergoing transcatheter aortic valve implantation. Am J Cardiol. 2017;120:986–993. [DOI] [PubMed] [Google Scholar]
- 8. Roberts E, Ludman AJ, Dworzynski K, Al‐Mohammad A, Cowie MR, McMurray JJ, Mant J; NICE Guideline Development Group for Acute Heart Failure . The diagnostic accuracy of the natriuretic peptides in heart failure: systematic review and diagnostic meta‐analysis in the acute care setting. BMJ. 2015;350:h910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bergler‐Klein J, Klaar U, Heger M, Rosenhek R, Mundigler G, Gabriel H, Binder T, Pacher R, Maurer G, Baumgartner H. Natriuretic peptides predict symptom‐free survival and postoperative outcome in severe aortic stenosis. Circulation. 2004;109:2302–2308. [DOI] [PubMed] [Google Scholar]
- 10. Pedrazzini GB, Masson S, Latini R, Klersy C, Rossi MG, Pasotti E, Faletra FF, Siclari F, Minervini F, Moccetti T, Auricchio A. Comparison of brain natriuretic peptide plasma levels versus logistic EuroSCORE in predicting in‐hospital and late postoperative mortality in patients undergoing aortic valve replacement for symptomatic aortic stenosis. Am J Cardiol. 2008;102:749–754. [DOI] [PubMed] [Google Scholar]
- 11. O'Neill BP, Guerrero M, Thourani VH, Kodali S, Heldman A, Williams M, Xu K, Pichard A, Mack M, Babaliaros V, Herrmann HC, Webb J, Douglas PS, Leon MB, O'Neill WW. Prognostic value of serial B‐type natriuretic peptide measurement in transcatheter aortic valve replacement (from the PARTNER Trial). Am J Cardiol. 2015;115:1265–1272. [DOI] [PubMed] [Google Scholar]
- 12. Stähli BE, Gebhard C, Saleh L, Falk V, Landmesser U, Nietlispach F, Maisano F, Lüscher TF, Maier W, Binder RK. N‐terminal pro‐B‐type natriuretic peptide‐ratio predicts mortality after transcatheter aortic valve replacement. Catheter Cardiovasc Interv. 2015;85:1240–1247. [DOI] [PubMed] [Google Scholar]
- 13. Mizutani K, Hara M, Iwata S, Murakami T, Shibata T, Yoshiyama M, Naganuma T, Yamanaka F, Higashimori A, Tada N, Takagi K, Araki M, Ueno H, Tabata M, Shirai S, Watanabe Y, Yamamoto M, Hayashida K. Elevation of B‐type natriuretic peptide at discharge is associated with 2‐year mortality after transcatheter aortic valve replacement in patients with severe aortic stenosis: insights from a multicenter prospective OCEAN‐TAVI (Optimized Transcatheter Valvular Intervention‐Transcatheter Aortic Valve Implantation) Registry. J Am Heart Assoc. 2017;6:e006112 DOI: 10.1161/JAHA.117.006112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem NM, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE, van Es GA, Hahn RT, Kirtane AJ, Krucoff MW, Kodali S, Mack MJ, Mehran R, Rodés‐Cabau J, Vranckx P, Webb JG, Windecker S, Serruys PW, Leon MB; Valve Academic Research Consortium‐2 . Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium‐2 consensus document. J Thorac Cardiovasc Surg. 2013;145:6–23. [DOI] [PubMed] [Google Scholar]
- 15. Cowie MR, Struthers AD, Wood DA, Coats AJ, Thompson SG, Poole‐Wilson PA, Sutton GC. Value of natriuretic peptides in assessment of patients with possible new heart failure in primary care. Lancet. 1997;350:1349–1353. [DOI] [PubMed] [Google Scholar]
- 16. Anand IS, Fisher LD, Chiang YT, Latini R, Masson S, Maggioni AP, Glazer RD, Tognoni G, Cohn JN; Val‐HeFT Investigators . Changes in brain natriuretic peptide and norepinephrine over time and mortality and morbidity in the Valsartan Heart Failure Trial (Val‐HeFT). Circulation. 2003;107:1278–1283. [DOI] [PubMed] [Google Scholar]
- 17. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; Authors/Task Force Members; Document Reviewers . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 18. Maisel A, Mueller C, Adams K Jr, Anker SD, Aspromonte N, Cleland JG, Cohen‐Solal A, Dahlstrom U, DeMaria A, Di Somma S, Filippatos GS, Fonarow GC, Jourdain P, Komajda M, Liu PP, McDonagh T, McDonald K, Mebazaa A, Nieminen MS, Peacock WF, Tubaro M, Valle R, Vanderhyden M, Yancy CW, Zannad F, Braunwald E. State of the art: using natriuretic peptide levels in clinical practice. Eur J Heart Fail. 2008;10:824–839. [DOI] [PubMed] [Google Scholar]
- 19. Zois NE, Bartels ED, Hunter I, Kousholt BS, Olsen LH, Goetze JP. Natriuretic peptides in cardiometabolic regulation and disease. Nat Rev Cardiol. 2014;11:403–412. [DOI] [PubMed] [Google Scholar]
- 20. Karlström P, Alehagen U, Boman K, Dahlström U; UPSTEP‐Study Group . Brain natriuretic peptide‐guided treatment does not improve morbidity and mortality in extensively treated patients with chronic heart failure: responders to treatment have a significantly better outcome. Eur J Heart Fail. 2011;13:1096–1103. [DOI] [PubMed] [Google Scholar]
- 21. Felker GM, Anstrom KJ, Adams KF, Ezekowitz JA, Fiuzat M, Houston‐Miller N, Januzzi JL Jr, Mark DB, Piña IL, Passmore G, Whellan DJ, Yang H, Cooper LS, Leifer ES, Desvigne‐Nickens P, O'Connor CM. Effect of natriuretic peptide‐guided therapy on hospitalization or cardiovascular mortality in high‐risk patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. 2017;318:713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gerber IL, Stewart RA, Legget ME, West TM, French RL, Sutton TM, Yandle TG, French JK, Richards AM, White HD. Increased plasma natriuretic peptide levels reflect symptom onset in aortic stenosis. Circulation. 2003;107:1884–1890. [DOI] [PubMed] [Google Scholar]
- 23. Weber M, Arnold R, Rau M, Brandt R, Berkovitsch A, Mitrovic V, Hamm C. Relation of N‐terminal pro‐B‐type natriuretic peptide to severity of valvular aortic stenosis. Am J Cardiol. 2004;94:740–745. [DOI] [PubMed] [Google Scholar]
- 24. Steadman CD, Ray S, Ng LL, McCann GP. Natriuretic peptides in common valvular heart disease. J Am Coll Cardiol. 2010;55:2034–2048. [DOI] [PubMed] [Google Scholar]
- 25. Clavel MA, Malouf J, Michelena HI, Suri RM, Jaffe AS, Mahoney DW, Enriquez‐Sarano M. B‐type natriuretic peptide clinical activation in aortic stenosis: impact on long‐term survival. J Am Coll Cardiol. 2014;63:2016–2025. [DOI] [PubMed] [Google Scholar]
- 26. O'Sullivan CJ, Stortecky S, Heg D, Jüni P, Windecker S, Wenaweser P. Impact of B‐type natriuretic peptide on short‐term clinical outcomes following transcatheter aortic valve implantation. EuroIntervention. 2015;10:e1–e8. [DOI] [PubMed] [Google Scholar]
- 27. Koskinas KC, O'Sullivan CJ, Heg D, Praz F, Stortecky S, Pilgrim T, Buellesfeld L, Jüni P, Windecker S, Wenaweser P. Effect of B‐type natriuretic peptides on long‐term outcomes after transcatheter aortic valve implantation. Am J Cardiol. 2015;116:1560–1565. [DOI] [PubMed] [Google Scholar]
- 28. Ribeiro HB, Urena M, Le Ven F, Nombela‐Franco L, Allende R, Clavel MA, Dahou A, Côté M, Laflamme J, Laflamme L, DeLarochellière H, DeLarochellière R, Doyle D, Dumont E, Bergeron S, Pibarot P, Rodés‐Cabau J. Long‐term prognostic value and serial changes of plasma N‐terminal prohormone B‐type natriuretic peptide in patients undergoing transcatheter aortic valve implantation. Am J Cardiol. 2014;113:851–859. [DOI] [PubMed] [Google Scholar]
- 29. Liebetrau C, Gaede L, Kim WK, Arsalan M, Blumenstein JM, Fischer‐Rasokat U, Wolter JS, Kriechbaum S, Huber MT, van Linden A, Berkowitsch A, Dörr O, Nef H, Hamm CW, Walther T, Möllmann H. Early changes in N‐terminal pro‐B‐type natriuretic peptide levels after transcatheter aortic valve replacement and its impact on long‐term mortality. Int J Cardiol. 2018;265:40–46. [DOI] [PubMed] [Google Scholar]
- 30. Komajda M, Hanon O, Hochadel M, Lopez‐Sendon JL, Follath F, Ponikowski P, Harjola VP, Drexler H, Dickstein K, Tavazzi L, Nieminen M. Contemporary management of octogenarians hospitalized for heart failure in Europe: Euro Heart Failure Survey II. Eur Heart J. 2009;30:478–486. [DOI] [PubMed] [Google Scholar]
- 31. Gupta DK, Wang TJ. Natriuretic peptides and cardiometabolic health. Circ J. 2015;79:1647–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Distribution of the NT‐proBNP (N‐terminal pro–B‐type natriuretic peptide) ratio.


