Abstract
Background
Over 6000 children have an in‐hospital cardiac arrest in the United States annually. Most will not survive to discharge, with significant variability in survival across hospitals suggesting improvement in resuscitation performance can save lives.
Methods and Results
A prospective observational study of quality of chest compressions (CC) during pediatric in‐hospital cardiac arrest associated with development and implementation of a resuscitation quality bundle. Objectives were to: 1) implement a debriefing program, 2) identify impediments to delivering high quality CC, 3) develop a resuscitation quality bundle, and 4) measure the impact of the resuscitation quality bundle on compliance with American Heart Association (AHA) Pediatric Advanced Life Support CC guidelines over time. Logistic regression was used to assess the relationship between compliance and year of event, adjusting for age and weight. Over 3 years, 317 consecutive cardiac arrests were debriefed, 38% (119/317) had CC data captured via defibrillator‐based accelerometer pads, data capture increasing over time: (2013:13% [12/92] versus 2014:43% [44/102] versus 2015:51% [63/123], P<0.001). There were 2135 1‐minute cardiopulmonary resuscitation (CPR) epoch data available for analysis, (2013:152 versus 2014:922 versus 2015:1061, P<0.001). Performance mitigating themes were identified and evolved into the resuscitation quality bundle entitled CPR Coaching, Objective‐Data Evaluation, Action‐linked‐phrases, Choreography, Ergonomics, Structured debriefing and Simulation (CODE ACES2). The adjusted marginal probability of a CC epoch meeting the criteria for excellent CPR (compliant for rate, depth, and chest compression fraction) in 2015, after CPR Coaching, Objective‐Data Evaluation, Action‐linked‐phrases, Choreography, Ergonomics, Structured debriefing and Simulation was developed and implemented, was 44.3% (35.3–53.3) versus 19.9%(6.9–32.9) in 2013; (odds ratio 3.2 [95% confidence interval:1.3–8.1], P=0.01).
Conclusions
CODE ACES2 was associated with progressively increased compliance with AHA CPR guidelines during in‐hospital cardiac arrest.
Keywords: cardiopulmonary arrest, cardiopulmonary resuscitation (CPR), emergency cardiac care, pediatrics, quality and outcomes, quality improvement, sudden cardiac death
Subject Categories: Cardiopulmonary Arrest, Cardiopulmonary Resuscitation and Emergency Cardiac Care, Quality and Outcomes, Pediatrics
Clinical Perspective
What Is New?
On January of 2013, we started a weekly cardiac arrest debriefing program and over the next 36 months we debriefed 317 consecutive events, from which 105 had cardiopulmonary resuscitation (CPR) metrics captured with 2135 60‐second epochs of data available for analysis.
What was unique was that high and low performance examples were rigorously explored to identify mediating themes; lessons learned iteratively evolved into the Resuscitation Quality Bundle entitled CPR Coaching, Objective‐Data Evaluation, Action‐linked‐phrases, Choreography, Ergonomics, Structured debriefing and Simulation (CODE ACES2).
Implementation of the debriefing program and CODE ACES2 was associated with a 3‐fold increase in compliance with the American Heart Association Guidelines for CPR over the 3‐year period.
What Are the Clinical Implications?
The CODE ACES2 framework can be used to debrief and investigate recurrent patterns that can lead to system change.
Strategies to optimize resuscitation team performance include assessment of room ergonomics, using action‐linked‐phrases to minimize delays to time‐sensitive actions and developing standard institutional choreography for common events.
Finally, we introduce the “CPR coach,” a team member who coaches compressors to achieve high quality CPR, cognitively unloading the leader so they can focus on advanced life support and reversing the underlying cause of the arrest.
Introduction
More than 6000 pediatric in‐hospital cardiac arrests (IHCA) occur in the United States annually.1 Although published survival to discharge rates following pediatric IHCA have recently increased to 43% to 47% in some centers, most children experiencing IHCA will not survive.2, 3 There is significant variability in survival from pediatric IHCA across hospitals, suggesting the opportunity to improve survival rates by implementing strategies that decrease errors and optimize resuscitation performance.4, 5
Promising research suggests post‐resuscitation debriefings are associated with subsequent improved resuscitation performance and perhaps even acute survival and neurologic outcomes.6, 7, 8, 9, 10 Dine reported a synergistic effect on chest compression (CC) performance between debriefing and audiovisual cardiopulmonary resuscitation (CPR) feedback devices.7 We have not identified any reports detailing specific approaches to debriefing targeted at enabling identification of challenges to resuscitation performance and solutions. Important steps for continuous quality improvement in resuscitation include identification of high and low IHCA skill performance, use of debriefings to identify performance mediating factors, and development, testing, and dissemination of identified solutions.
Our objectives were to: (1) implement a weekly cardiac arrest debriefing program; (2) identify recurring impediments to high quality CC in debriefings; (3) develop strategies to address impediments and iteratively create a “Resuscitation Quality Bundle” (RQB); and (4) measure the impact of the RQB on resuscitation performance as measured by compliance to American Heart Association (AHA) Pediatric Advanced Life Support (PALS) Guidelines over the study period.
Methods
Study Design, Population, and Unit of Analysis
This was a prospective observational single‐site study of the quality of CC delivered to children during a 3‐year period simultaneous with development and implementation of a RQB. The patient study population included any child who received CC at the Johns Hopkins Children's Center, a university‐based children's hospital. The units of analysis included 60‐second epochs of CC data recorded between January 1, 2013 and December 31, 2015. The study was focused on the quality of CC performance and not on survival. Resuscitation events were eligible if the patient was aged ≤21 years and if a complete defibrillator data file was successfully retrieved after the event. The Johns Hopkins Hospital Institutional Review Board approved use of these data as a Quality Improvement (QI) program. This was not deemed human subjects research, as such there was no requirement for consent to be obtained.
Phases of Resuscitation Quality Bundle Creation and Implementation
Creation and implementation of the RQB occurred in 5 phases: design of surveillance program, data capture, debriefing program, creation of RQB, and program analysis.
Phase 1—Surveillance program
We used an informatics‐based, active surveillance program, (ie, the “Resuscitation Event Analysis Clearinghouse (REACH) Surveillance System”), to identify all cardiac arrest events; this system has been described in detail elsewhere.11 Briefly, this program uses organizational Information Technology (IT) system signals to identify any “potential IHCA,” (ie, code button activation, rapid response team pages, electronic medical record, IHCA flow sheets, CPR billing codes) and catalogs based on event details, such as date, time and location, for future analysis.
Phase 2—Data capture
Team members investigate cataloged signals to verify actual IHCA events associated with provision of CC and/or defibrillation. Once an IHCA was identified all available objective data were collected, including demographic and resuscitation performance data required as part of participation with the AHA Get With The Guidelines‐Resuscitation database as well as data from the electronic medical record, the defibrillator record of CC captured with an accelerometer embedded in the defibrillator pads via the Zoll R series (Chelmsford, MA) and bedside monitor vital sign numeric values and waveform data.
Before implementation of this QI program, although the defibrillators could give real‐time CC feedback and capture data for post‐event review, clinicians placed defibrillator pads on a patient only if the patient had a shockable rhythm. In May of 2013, the Federal Drug Administration approved use of pediatric defibrillator pads to capture and report CC data in children aged <8 years, clearing the way for incorporating the use of the defibrillator in every pediatric IHCA. A key goal of this program was to ensure that the defibrillator be turned on and pads applied within 120 seconds for every pediatric IHCA.
Defibrillator data were reviewed using the manufacturer metrics. Objective CPR performance data were assessed for compliance with the 2010 AHA pediatric Basic Life Support (PBLS) and PALS Guidelines for CC depth and rate and the 2013 AHA consensus recommendation for chest compression fraction (CCF).12, 13 Event data were abstracted and entered into the REACH surveillance database as part of the QI program. CC data were then processed using software developed by the QI team to quantify additional aspects of performance not available in the commercially available manufacturer software. These standardized objective data were transformed into our resuscitation performance debriefing tool and used during debriefings described in Phase 3 (Figure 1).
Figure 1.
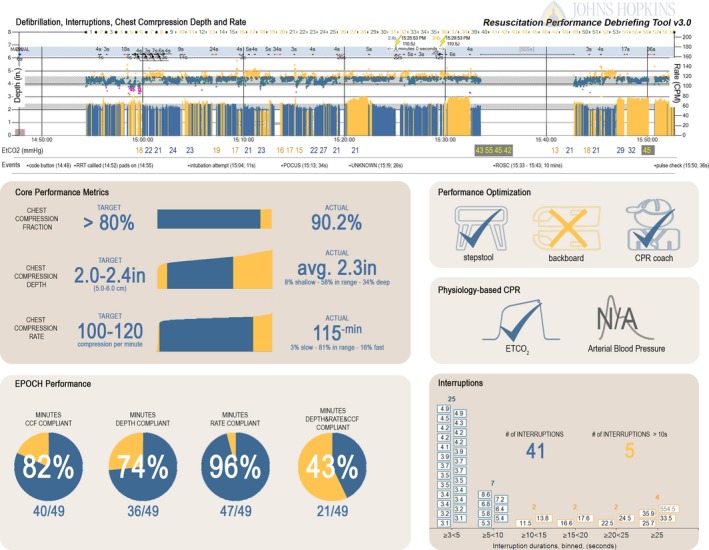
Johns Hopkins Resuscitation Performance Debriefing tool. Using data from the defibrillator, electronic health record, and bedside monitor, the performance debriefing tool is used to comprehensively evaluate performance. A timeline visualizes quality of the resuscitation in terms of: (1) excellent epochs of CPR; (2) depth, rate, and interruptions in chest compressions; (3) defibrillation timing and peri‐shock pause durations; (4) defibrillator‐based ETCO 2 values achieved throughout; and (5) key events (associated with chest compression interruptions). Core performance metrics are presented numerically and graphically with reference targets. Optimization methods and physiology‐based CPR techniques are presented including the use of backboard, stepstool, CPR coach, ETCO 2 and arterial blood pressure (ie, ETCO 2 and/or diastolic blood pressure goals were stated and used to guide chest compression quality). Proportions of minutes compliant for depth, rate, and chest compression fraction are depicted as pie charts; interruptions are presented as a histogram. CPR indicates cardiopulmonary resuscitation; ETCO 2, end‐tidal carbon dioxide; ROSC, Return of Spontaneous Circulation.
Clinical volunteers from each discipline used peer‐to‐peer subjective data collection tools to capture the experience of those who participated in the resuscitation, ie, pharmacist to pharmacist, respiratory therapist to respiratory therapist, nurse to nurse, etc. The forms asked domain specific team elements (ex. Who was the Charge Nurse? Who was the Medication Nurse?), as well as open ended questions related to perceived performance, including anything the rescuer wanted discussed at the debriefing. (Please see Table S1 for example of Pharmacy form.)
Phase 3—Debriefing
We began weekly IHCA debriefings on January 1, 2013. Every Wednesday morning, a 90‐minute meeting occurred, locally named “Code Busters.” Through trial and error, the catchment period for IHCAs to be discussed at a Wednesday debriefing ended at midnight on the preceding Sunday and included any event in the preceding Sunday to Sunday 7‐day period. This provided sufficient time to collect and analyze data as well as to allow attendees to plan to attend the debriefing. The CPR project coordinator sent electronic invitations (including calendar meeting requests) to all clinicians present for IHCA event from all disciplines. For example, if an IHCA occurred in the cardiac catheterization suite and included extracorporeal cardiopulmonary resuscitation (ECPR), the debriefing included representatives from cardiology, cardiac anesthesia, cardiac surgery, catheter laboratory nursing personnel, perfusionists, and any intensive care unit responders. Debriefings started with a statement to create a psychologically safe environment followed by a facilitator exploring the team's memories of their performance and challenges they experienced.14 At the beginning of our program, little objective data were available, so most debriefings were based on discussion of the subjective experiences of those involved. As our data collection became more robust, review of objective data became the bedrock of the program.
Phase 4—Resuscitation quality bundle
The primary objective of the weekly IHCA debriefings was to help team members identify any errors or deviations from AHA Guidelines that occurred during the resuscitation, explore contributing factors and identify potential preventative solutions. As the program progressed, the secondary objective was to identify recurrent performance‐mediating themes. These themes included errors with associated stressors, as well as exemplars of outstanding performance. Strategies were developed and refined to systematically address identified issues. This iterative approach was used to cluster solutions into themes and actionable processes comprising the RQB.
Phase 5—Program analysis
We analyzed and compared CC performance data across years to quantify changes in adherence to AHA PBLS and PALS Guidelines after exposure to the evolving QI Program and RQB.
Data Capture
Before the launch of this QI program, all defibrillators in the Johns Hopkins Children's Center were standardized to 1 model, (ZOLL R Series Plus defibrillator (ZOLL Corp. Chelmsford, MA) with One‐step CPR electrodes), and deployed with identical settings configured by our clinical engineering department.
Epochs
Each ZOLL record was partitioned into epochs and analyzed for compliance with AHA PBLS and PALS Guidelines. Epochs were defined as a 60‐second period of resuscitation. An event could have ≥1 associated epochs. Time‐zero for event epochs began with the detection of the first CC after the defibrillator was turned on and electrodes were placed on the patient.
Compliance With CPR Performance Goals
Thresholds were selected defining CC rate, depth and interruption duration compliance based on AHA 2010 guidelines for pediatric patients.12 CC rate was compliant if between 100 and 120 CC/min.12 For patients aged <1 year CC depth was considered compliant if >3.8 cm, and >5 cm for those aged >1 year.12 Interruptions >10 seconds were non‐compliant/excessively long.12 CCF ≥80% was compliant.13
Epoch‐Level Variables
Performance variables recorded for each epoch included: CC rate, depth, interruptions and chest compression fraction (CCF), and a composite variable we refer to as “excellent CPR,” ie, compliance with all 3 measures (rate+depth+CCF). Average CC rate and depth for the epoch determined compliance for those variables. CCF was defined as the proportion of time CC were performed during a resuscitation (and for each epoch) relative to total time that CC were indicated (ie, because of loss of pulse or Heart Rate <60 and indicators of poor perfusion). Ongoing CC were CC without interruption. Interruptions were defined as any pause in CCs >3 seconds; these contributed to non‐compression time for use in calculation of the CCF and for analysis of interruptions. The frequency of interruptions longer than 3 seconds and those longer than 10 seconds were determined.15, 16 We compared the proportion of epochs compliant with the AHA 2010 PBLS and PALS Guidelines for all CC quality metrics across the study period.12 Our primary outcome measure was the proportion of CC epochs per year that met the criteria for “excellent CPR.”
Statistical Analysis
Demographics were reported at the patient‐event level. Performance data were reported at the epoch level. Both were reported in aggregate as well as stratified by year. Median values and interquartile ranges were reported for continuous variables with comparisons made using the Kruskal‐Wallis non‐parametric test. For categorical variables, frequencies and proportions were reported, and differences were analyzed using Fisher exact test or Chi‐square statistic. To assess the percentage of epochs by year that achieved compliance by rate, depth, CCF, as well as excellent CPR, we used logistic regression adjusting for patient age and weight. Models included robust cluster variance estimators to adjust for the potential correlation of epochs within events. We report odds ratios and marginal probabilities with 95% confidence intervals. P values ≤0.05 were considered significant. Statistics were performed with Stata/SE 15.1 for Windows (64‐bit x86‐64), (StataCorp LLC, College Station, TX). To protect confidential patient information, the data, analytic methods, and study materials will not be readily available for purposes of reproducing the results or replicating the procedure. However, it may be possible to make the deidentified data set available for review with appropriate institutional review board and institutional approval.
Results
Events
Over the 3‐year study period, 241 children had 317 cardiac arrest events that were debriefed. Defibrillator records that contained CPR quality data were captured in 38% (119/317) of events with an increasing proportion over time (2013:13% [12/92] versus 2014:43% [44/102] versus 2015:51% [63/123], P<0.001). Fourteen records contained <1 minute of quality data and were not included in the analysis. Thus, there were 93 children who had 105 events that contributed CPR quality records with ≥1 complete epochs of data. There were 2135 60‐second epochs of quality data captured by the defibrillator.
Fifty‐eight percent of patients were male and the weights and ages of the children with IHCA data captured varied significantly across the 3 years of the study (see Table 1); children in 2013 were older and heavier. This may be explained by the fact that the Federal Drug Administration had not approved the use of the defibrillator pads with embedded accelerometer for children aged <8 years until May 2013. The median (interquartile range) event duration was 15 (5.0, 29.0) minutes with no significant changes by year. More events took place during the 7 am to 11 pm time period (DAY:76% versus NIGHT:24%) although given the distribution of hours (7 am–11 pm: 66%; 11 pm–7 am 33%) the difference is not statistically significant (P=0.145). Similarly, more events occurred during the week than on the weekend (WEEK:78% versus WEEKEND: 22%; P=0.256). Most events (72%) occurred entirely within the hospital rather than beginning with OHCA with resuscitation continued in the hospital (28%), with no significant changes in distribution across years. More than 95% of events occurred in a “critical care area,” ie, Pediatric Intensive Care Unit 54% (57/105) or Pediatric Emergency Department 39% (41/105), with 2% (2/105) of events occurring while under the supervision of an anesthesiologist (ie, Operating Room, Magnetic Resonance Imaging suite). There were 3 IHCA events that occurred on the general wards over the 3‐year period, representing only 3% of events. The most frequent first documented rhythm was pulseless electrical activity 58% (61/105) followed by bradycardia with poor perfusion 20% (21/105), asystole 12% (13/105), pulseless ventricular tachycardia, or ventricular fibrillation 8% (8/105) (and one unknown), with no significant changes in the distribution over the 3‐year study period.
Table 1.
Demographic Characteristics of Patients With Chest Compression Quality Data Captured During In‐Hospital Cardiac Arrests Between 2013 and 2015
| 2013 | 2014 | 2015 | Total | ||
|---|---|---|---|---|---|
| No. of patients | 11 | 34 | 48 | 93 | |
| No. of cardiac arrest events | 11 | 40 | 54 | 105 | |
| Age, y | |||||
| Median, IQR | 8.3 (3.7–15.2) | 1.4 (0.4–7.3) | 1.5 (0.5–7.0) | 1.8 (0.44–8.9) | P=0.03 |
| Min‐max | 0.07 to 20.05 | 0.01 to 17.5 | 0.04 to 17.6 | 0.01 to 20.05 | |
| <1 y, n (%) | 1 (9%) | 14 (41%) | 20 (42%) | 35 (38%) | P=0.1 |
| ≥1 y, n (%) | 10 (91%) | 20 (59%) | 28 (58%) | 58 (62%) | |
| Weight, kg | |||||
| Median, IQR | 30.0 (13.2–40.0) | 9.8 (6.4–23.6) | 9.3 (6.0–18.1) | 10.0 (6.3–28.0) | P=0.03 |
| Min‐max | 3.0 to 70.0 | 2.9 to 93.1 | 3.2 to 106.0 | 2.9 to 106.0 | |
| Sex | |||||
| Female n (%) | 5 (45%) | 17 (50%) | 22 (46%) | 44 (42%) | P=0.93 |
| Male n (%) | 6 (55%) | 17 (50%) | 26 (54%) | 49 (58%) | |
| Arrest duration, min | |||||
| Median, IQR | 13.0 (3.0–22.0) | 19.0 (4.5–35.0) | 14.5 (5.0–26.0) | 15.0 (5.0–29.0) | P=0.47 |
IQR indicates interquartile range.
Chest Compression and Interruption Quality
Over the 3‐year study period, there was a marked increase in the proportion of epochs that were compliant with the AHA PBLS and PALS Guideline (see Table 2).12 Our primary outcome measure is the proportion of epochs compliant with all 3 chest compression quality measures (rate+depth+CCF), ie, “excellent CPR” epochs. Adjusting for age category and weight, there was a 3.2 increase in the odds that CC epochs would meet the criteria for excellent CPR in 2015, after CPR Coaching, Objective‐Data Evaluation, Action‐linked‐phrases, Choreography, Ergonomics, Structured debriefing and Simulation (CODE ACES2) was developed and implemented, than in 2013: (odds ratio 3.2 [95% confidence interval (CI): 1.3–8.1], P=0.01). The adjusted marginal probability of excellent CPR in 2013 was 19.9% (95% CI: 6.9, 32.9) versus 41.8% (30.5, 53.0) in 2014 and 44.3% (35.2, 53.3) in 2015.
Table 2.
Year‐to‐Year Chest CC of all AHA Quality Metrics
| Total | 2013 | 2014 | 2015 | P Value | |
|---|---|---|---|---|---|
| CC quality measures | |||||
| CC events (n) | 105 | 11 | 40 | 54 | |
| All CC epochs (n) | 2135 | 152 | 922 | 1061 | |
| Event average CC metrics—median, IQR | |||||
| CC Rate (per min) | 114 (108–120) | 114 (106–125) | 116 (113–128) | 110 (107–115) | <0.001a |
| CC depth (cm): aged ≤1 y | 4.2 (3.3–4.7) | 2.2 (2.2–2.2) | 4.4 (2.8–4.8) | 4.0 (3.6–4.7) | 0.35 |
| CC depth (cm): aged >1 y | 5.4 (4.4–6.0) | 5.9 (4.2–6.6) | 5.4 (4.6–6.0) | 5.3 (4.2–5.8) | 0.43 |
| CCF | 0.93 (0.85–0.96) | 0.91 (0.76–0.97) | 0.94 (0.88–0.98) | 0.93 (0.84–0.95) | 0.19 |
| Cumulative epoch cc metricsb | |||||
| Rate compliant: n (%) | 1130 (69%) | 30.2 (10.8, 49.7) | 63.4 (52.8, 74.1) | 78.8 (72.4, 85.2) | <0.001a |
| Depth compliant: n (%) | 1349 (63%) | 55.2 (23.7, 86.7) | 67.1 (55.6, 78.7) | 60.9 (50.3, 71.5) | 0.65 |
| CCF compliant: n (%) | 1718 (81%) | 66.2 (50.8, 81.6) | 83.0 (75.2, 90.9) | 79.8 (75.4, 84.2) | 0.13 |
| Rate+depth+CCF compliant: n (%) | 884 (41%) | 19.9 (6.9, 32.9) | 41.8 (30.5, 53.0) | 44.3 (35.3, 53.3) | 0.04a |
AHA indicates American Heart Association; CC, chest compression; CCF, chest compression fraction; IQR, interquartile range.
Indicates statistical significance at P<0.05.
Marginal probabilities with 95% confidence interval from logistic regression models adjusting for age and weight.
When evaluating individual metrics, the most substantial gains were made in compliance with the CC rate metric (100–120/min) (adjusted marginal probabilities in 2013:30.2% [95% CI: 10.8, 49.7] versus 2014:63.4% [52.8, 74.1] versus 2015:78.8% [72.4, 85.2], P<0.001). Compliance with CCF >80% increased, though not significantly: 2013:66.2% (95% CI: 50.8, 81.6) versus 2014:83.0 (75.2, 90.9) versus 2015:79.8 (75.4, 84.2), P=0.13. Depth compliance increased then declined, but these were also not significant: 2013:55.2% (95% CI: 23.7, 86.7) versus 2014:67.1% (55.6, 78.7) versus 2015:60.9% (50.3, 71.5), P=0.65.
When stratified by age, CCF increased significantly for children aged <1 year over the 3 years (up to 73.5%; P<0.001), but was never as high as that of children aged >1 year, which was high at the start (82.3%) with no significant change throughout (85.5%).13 Notably, when stratified by age, compliance with AHA PBLS Guidelines for chest compression depth improved for children aged <1 year but for children aged >1 year showed a small decline (though not significant).12 The debriefings identified factors contributing to this decline in compliance with CC depth (see Discussion).
Resuscitation Quality Bundle Elements
In addition to reviewing whether the CC were compliant with the AHA Guidelines, providers also identified and reviewed instances of errors or breaches in best practices per expert consensus or institutional goals12, 13 (See Table 3). Performance mediating strategies were developed based on behaviors observed to enhance high performance or to mitigate low performance. These strategies were categorized into elements of a multifactorial RQB. The 7 elements identified were: 1) CPR coach, 2) objective date evaluation, 3) action‐linked phrases, 4) choreography, 5) ergonomics 6) structured debriefing, and 7) simulation. Our program is now referred to as “CODE ACES2.”17
Table 3.
Types of Errors Discussed During Weekly In‐Hospital Cardiac Arrest Debriefings
| Type of Error | Examples |
|---|---|
| Delays in care | Delay in defibrillation (goal of ≤180 s)Delay in delivery of first dose of epinephrine for non‐shockable rhythm (goal of <5 min)Delay in starting chest compressions (breeched institutional goal of starting chest compressions in ≤10 s of loss of pulse or heart rate <60 with poor perfusion) |
| Pauses | Prolonged pause in chest compressions for the use of point‐of‐care ultrasoundProlonged pause during procedures (rhythm check, defibrillation, intubation, chest tube, surgical dissection for placement of ECMO catheters, etc.)Inadequate pause when unable to move chest with BMV and unable to intubate without pausingInadequate pause to assess initial rhythm and determine if defibrillation is indicated |
| Other | Defibrillating a non‐shockable rhythmUse of sodium bicarbonate or calcium with no clear indicationNeglect to use backboardNeglect to use stepstoolNeglect to place defibrillator pads to enable real‐time feedbackEpinephrine given <every 3 minEpinephrine given >every 5 min |
| Institutionally defined error, based on new standards | No Quality CPR coach assignedDefibrillator not placed directly across from the compressorDefibrillator not placed on the same side as the patient monitorTurning patient >1 time (ie, do not coordinate placement of backboard and placement of back pad)Delay in use of end‐tidal carbon dioxide (within 30 s of turning on defibrillator that has ETCO2)Delay in activation of ECMO (goal of 5 min after chest compressions started, if ROSC not yet achieved)Prolonged pause in chest compression when moving patient from Emergency Medical Services gurney to Emergency Department bed |
BMV indicates bag‐mask ventilation; ECMO, extracorporeal membrane oxygenation; ROSC, return of spontaneous circulation.
Discussion
This study describes the development of a RQB now called CODE ACES2, associated with improved compliance with the AHA PBLS and PALS Guidelines during IHCA. The magnitude of improvement measured is notable when put in the context of resuscitation performance reported in the literature. Niles et al recently reported pediatric IHCA metrics captured by pediRES‐Q, an International Resuscitation Collaborative, with data from 12 hospitals across North America and Europe (including Johns Hopkins).18 That multicenter data set is remarkably similar in size to ours, ie, 112 events (versus our 105 events) and 2046 60‐second epochs (versus our 2135 epochs). Of their data set, 384 (19%) were considered “excellent epochs,” ie, meeting guidelines for CC rate, depth, and CCF, which is similar to our baseline in 2013 of 22%. This highlights the important progress we made in our single‐site cohort, ie, the proportion of “excellent epochs” of CPR increased from 22% to 45% over the 3‐year study period. The pediRES‐Q network noted particularly low compliance with guidelines for the younger children.18 Infants aged <1 year received “excellent epochs” of CPR only 5% of the time, compared with 46% at Johns Hopkins in 2015 after implementation of our CODE ACES2 program.
There are 3 key findings to highlight. The first is that our data reinforce previous published reports that a post‐event debriefing program, in combination with real‐time defibrillator‐based CC feedback during IHCA events, is associated with measurable improvements in actual resuscitation performance.6, 7, 8, 9, 10 The second is that our Resuscitation Quality Improvement Program has created novel approaches to capture, analyze and visually depict IHCA CC to more fully characterize CC performance during resuscitation. The third finding is that by systematically focusing resuscitation debriefings on variables associated with high and low performance and identifying recurring themes we have developed standardized solutions to impediments and structured these solutions into a data driven resuscitation quality bundle.
CODE‐ACES2 is now an ongoing performance improvement program that focuses on techniques and strategies to improve resuscitation compliance with AHA PBLS and PALS Guidelines. Benchmarking of metrics over time and creation of an institutional shared mental model of key components of a RQB have been integral to this process. Although each of the CODE‐ACES2 elements can stand‐alone, they are all synergistic and require reinforcement through training, education, and debriefing.
CPR coach
When the 2005 AHA Adult BLS and PBLS Guidelines emphasized the importance of high‐quality CPR, we found that such emphasis, while warranted, can produce unintended consequences.19 In 2007, we noted frequent delays in defibrillation and intubation as well as failure to identify and treat reversible causes. Through debriefings and observations of simulated cardiac arrests, the resuscitation leadership recognized that the code team leader cannot simultaneously focus on high quality CPR, early defibrillation, ALS algorithms and identification of reversible causes of arrest—≥1 of these foci is inevitably compromised. To maximize resource effectiveness through division of labor we introduced a role that was initially called the quality CPR (QCPR) leader. This person was instructed to focus on directing high quality CPR while the code team leader focused on the higher level problem‐solving of managing the patient according to the appropriate PALS algorithm and diagnosing reversible causes. Ultimately, we recognized the CPR coach can cognitively unload the code team leader so that instead of spending mental energy on monitoring quality of CC, they can run through H's and T's earlier in the resuscitation. While this QCPR leader role was easily accepted and incorporated into our Pediatric Intensive Care Unit culture, it caused confusion when used during resuscitations performed outside of the Intensive Care Unit, particularly when the QCPR leader stood at the foot of the bed, in a position near the code team leader. To prevent such confusion, we needed to more clearly differentiate the QCPR and team leader roles and clarify the chain of command. We changed the title of this role from the QCPR leader to the QCPR coach and, ultimately, to CPR coach. We made it clear that the CPR coach's function is independent from that of the code team leader and can reduce the responsibilities of the code team leader. However, the CPR coach ultimately reports to the code team leader and tries to achieve the goals delineated by the code team leader (see additional discussion below). We then attempted to identify the ideal physical position of the CPR coach, ultimately placed directly across from the chest compressor. This allowed the compressor and the CPR coach to make eye contact and ensured that the compressor and airway manager could clearly hear the CPR coach with no need for loud voices. In this position, the CPR coach can point to the CC data on the defibrillator screen, to assist in the coaching. The CPR coach focuses on coaching those resuscitation team members who are performing CC and bag‐mask‐ventilation to ensure they perform excellent chest compressions, appropriate (and not excessive) ventilation, and rapid defibrillation with minimal peri‐shock pause. We have now incorporated this CPR coach role into our formal institutional resuscitation curricula for training pediatric residents,20 introducing first‐year medical students to in‐hospital BLS21 and for nursing annual competencies.
The CPR coach initially performed subjective assessments of chest compression quality, while providing encouragement, and switching out compressors as needed. Now, the CPR coach focuses on objective data to coach compressors, using several methods to drive performance including verbal guidance and modeling best practices as they orient rescuers to and direct optimization of displayed CC performance data. Examples of CC performance data include “external metrics,” ie, CC metrics displayed by the defibrillator (CC depth and rate), and “internal metrics,” ie, arterial or venous pressure measurements displayed by the bedside monitor, hand‐held end‐tidal carbon dioxide (ETCO2) device, etc…). If an arterial catheter is present, the arterial diastolic pressure can assist in evaluation of effectiveness of chest compression depth. The relaxation pressure can serve as a surrogate for aortic end‐diastolic pressure that helps determine coronary perfusion pressure. If a central venous or right atrial catheter is in place, the arterial relaxation pressure minus the central venous pressure provides an estimate of coronary perfusion pressure, with a goal of >20 mm Hg.13 The end‐tidal carbon dioxide (ETCO2) will trend with pulmonary blood flow ETCO2 as a surrogate for cardiac output and quality of chest compressions. If the ETCO2 is low, the CPR coach will encourage the compressor to improve CC quality, such as depth and rate, providing data and goals to the compressor. Chest compressions are considered optimized if: 1) the teams is achieving age‐appropriate diastolic blood pressure (>30 mm Hg for children, >25 for infants)3, 2) ETCO2 >20 and as close to 25 as possible,13, and simultaneously 3) rescuers attempt to comply with “external” parameters guidelines, ie, age appropriate CC depth and rate with no leaning—yet monitor actual rate and depth needed to achieve the internal physiologic goals.
In general, CPR coaches will attempt to guide rescuers to optimize all mechanics of resuscitation simultaneously. However, the experienced CPR coach, in conjunction with the code team leader, can select the most important monitoring variables to emphasize, tailored to the patient's age and factors such as presence of heart defects or open chest. At this point we have not incorporated elements such as cerebral near‐infared spectroscopy goals into the CPR coach goals but will watch for data to support doing so at some point. We created cognitive aids listing the parameters noted above (eg, pocket cards and information placed on each defibrillator cart (Figure S1). We also created curricula training the code team leaders and the CPR coaches on these parameters, the hierarchy of these parameters and how to communicate these goals, (ie, how the team leader states the goals to the CPR coach and the CPR coach guides the compressors to achieve those goals).
From the science of teams perspective, the CPR coach performs 2 functions that may go unfulfilled when under time pressure and high stakes outcomes.22, 23 First, the role is dedicated to performance monitoring and backup or supporting behavior (ie, detecting and correcting performance issues in fellow team members).24 Second, the role maximizes use of available resources, a key team leadership function,25, 26 in real time to support adherence to guidelines. Codifying these functions into the CPR coach role improves role clarity during a code and helps balance workload across the team by offloading responsibility from the primary team leader. Leary et al previously reported about code leader offloading, using explicitly defined “physician/nurse code leadership dyads,” with physician leaders focused on medical aspects of IHCAs and nurses focused on organization of the room, reporting success in decreasing overcrowding associated with IHCA arrests.27 Infinger et al described the role of CPR performance coaching for out‐of‐hospital cardiac arrests with improvement in CC depth and shortening time to defibrillation.28 Pilot data at our institution on the CPR coach role have been encouraging and was used in designing a recently published multicenter, randomized, controlled trial of simulated cardiac arrests to analyze the impact of the CPR coach on compliance with BLS Guidelines.29 Cheng et al demonstrated a significant improvement in compliance with excellent CPR, chest compression depth, and fraction as well as a significant decrease in pre‐shock pause, post‐shock pause, and peri‐shock pause in simulated cardiac arrests for those with a CPR coach versus without one.29 Next steps are to study the impact of the CPR coach on survival and optimize how the code team leader leverages the cognitive unloading provided by the CPR coach.
Objective data evaluation
Following every cardiac arrest, our CODE ACES2 team gathers all available data from the bedside monitor, defibrillator, electronic health record, and emergency alerting systems. These data are rigorously analyzed to characterize and benchmark CPR technical performance and select the metrics to be evaluated (see Table 4 for a list of common metrics given monitor and/or defibrillator data availability). Each event is assessed for age‐based compliance with AHA CC targets, process of care exceptions, defibrillation timing and appropriateness, physiologic markers of cardiac output/perfusion such as diastolic blood pressure and ETCO2, and pre‐arrest vital signs. All data are visually displayed and presented in a standardized format during weekly post‐event debriefings. Areas of high and low guidelines compliance are discussed during the debriefing to identify event factors that hinder or enhance performance. Raw CC data from the defibrillator are transformed into the Johns Hopkins Resuscitation Performance Debriefing Tool for each event (See Figure 1), including a breakdown of compliance with AHA guidelines at the minute epoch level.
Table 4.
Metrics Used to Facilitate Objective Data Evaluation
| Resuscitation performance metrics |
| Time from pulselessness to initiation of compressions |
| (For ventricular fibrillation/pulseless ventricular tachycardia ‐ Time from shockable rhythm to defibrillation/was <180 s |
| Frequency, duration and timing of interruptions (binned by <5, 5 to 9.9, 10 to 14.9, 15 to 19.9, ≥20 s |
| Overall chest compression fraction/was CCF >90% |
| Average CC depth/was CC depth guideline compliant for age appropriate guideline |
| Average CC rate/was CC rate guideline compliant |
| Percent compressions compliant for depth |
| Depth, rate, CCF, # of interruptions >10 s per each 1 min of CPR (ie, values for 60s epochs) |
| Number and patterns of epoch compliance for resuscitation |
| Ex. Percent of “excellent epochs,” 60 s epochs compliant for depth, rate and CCF |
| ETCO2 achieved throughout resuscitation, timing, and duration >20 mm Hg |
| Diastolic blood pressure achieved throughout resuscitation, timing, and duration |
| >30 mm Hg (children) |
| >25 mm Hg (infants) |
CC indicates chest compression; CCF, chest compression fraction; ETCO 2, end‐tidal carbon dioxide.
When our resuscitation quality program began, we used reports available from the defibrillator manufacturer after each cardiac arrest to objectively ground our weekly debriefings. While these reports were found to be an excellent starting point for the debriefing, we soon realized averaging of metrics such as CC depth, CC rate, and total CCF did not provide sufficient information about the resuscitation quality. In fact, it can result in a false sense of security. For example, if half of the CC were delivered at 80/minute and the remaining half were delivered at 140/minute, the average CC rate would fall within the 100 to 120/minute rate considered to be compliant, even though none of the CC were actually compliant for rate. To emphasize a goal of delivering CC precisely compliant with the AHA Guidelines, we needed a new approach, with a more refined method of data presentation and analysis. Histograms were developed, visually highlighting what proportion of individual CCs were compliant with AHA Guidelines for CC rate and depth, making it efficient for the facilitator to immediately highlight performance gaps. We ultimately found if we divided every resuscitation into 60‐second epochs and analyzed and depicted CC quality metrics for each epoch we could more rapidly identify compliance/non‐compliance patterns. It also helped us identify specific resuscitation events, such as the arrival of the CPR coach or switch compressors that influenced CC performance. This visual comparison of minute‐to‐minute performance during the arrest facilitated the debriefing discussion and also increased our statistical power when analyzing our institutional performance over time. This performance evaluation approach is now used during all post‐resuscitation debriefings including those that take place after in‐situ or our “Sim Hospital” based mock codes.
Action‐linked phrases
We have previously reported the performance advantages that result when rescuers speak observations aloud, and link them directly with a resuscitation action.17 Several key examples include: 1) “There's no pulse, I'm starting compressions”—will decrease time to starting compressions, 2) “That's ventricular fibrillation, start compressions and get a defibrillator”—will decrease time to shock, and 3) “Shock delivered, resume CPR”—will reduce duration of post‐shock pause in CC.
During the weekly debriefings, the facilitator listens closely to the initial interventions as they are reported by the rescuer who discovered that the patient was pulseless. The facilitator solicits overlapping details from as many team members as possible to develop a complete and accurate report of these crucial preliminary events. For example, if a nurse says “I was suctioning and noticed his heart rate suddenly dropped from 90 to 50, so I pressed the Code Button and went to get the step stool and epinephrine,” we will highlight how in the hospital many of us have paradoxically forgotten our “first responder instincts.”30 In a previous study, we observed first responders in the hospital setting had essentially lost instincts to open the airway or initiate chest compressions, but rather reported feeling a responsibility to “prepare the room” for the Code Team. In debriefings, we point out if we pulled a limp and blue child from a pool, we would never run away from that child but would immediately start chest compressions, which usually creates an “aha moment” for those being debriefed, helping them to simplify their priorities in the future.
In all of our simulation trainings and weekly IHCA debriefings, if someone notices the patient has lost a pulse or the heart rate has dropped <60, we reinforce the next action must be to start CC (unless the patient has a “Do not resuscitate” order). We review the data captured on the bedside monitor (either from electrocardiogram, pulse oximetry waveform, or central/arterial line pulsations) to determine the time elapsed between loss of pulse (or Heart Rate <60/min) and initiation of CC. We reinforce that our institutional standard that providers should begin CC within 10 seconds of pulseless arrest (or Heart Rate <60/min with signs of poor perfusion) and then discuss strategies to increase likelihood of success the next time. Training in the use of action‐linked phrases is now incorporated into monthly simulation‐based training for rapid response teams, and annual “First Few Minutes” training for ward nurses.
Action‐linked phrases combine 2 critical behaviors of high‐performance teams,31 ie, 1) situational awareness update or “call out” with 2) task delegation. Situational awareness is defined in 3 levels: possessing timely and accurate information, correct interpretation of the information, and projecting of the current state into likely future states.32 By linking those call outs to specific actions, the interpretation or meaning of that information is made explicit and is a red flag to speak up if a team member has a different mental model.
Choreography
This element includes intentional structured plans promoting a shared mental model on the way in which a team physically interacts with the room, the equipment, the patient and one another to reduce error and time to task completion. We observed prolonged delays in CC and even dislodgement of vascular catheters and endotracheal tubes associated with activities such as placement of defibrillator pads, switching compressors, placing a backboard and moving a patient. In addition, we also observed that team members often stopped the tasks they were performing when a teammate attempted to organize the team. We used simulation, in the laboratory and in situ, to identify the ideal choreography and CPR priorities for any given maneuver.33 We now refer to this as a variant of our previously described teaching style “rapid cycle deliberate practice;”20 now described as “rapid cycle deliberate prototyping.” We now train leaders to specifically direct team members to continue their current tasks while next steps are discussed or directed. For more complex actions, this can take the form of the code team leader or the CPR coach making a statement that alerts the entire team that something is about to happen. The CPR coach will state to the compressor “Continue CPR while I organize the team,” followed by the detailed instruction of the upcoming action.
We created a video of our institution's “gold standard” approach and choreography of the first 3 minutes of a cardiac arrest and use it during training sessions. There are different videos for staff depending on staff roles and locations in the hospital, ie, ward nurses and security.34 We implemented an institutional standard choreography for common and recurrent actions such as switching of compressors, (See Figure 2) and for defibrillation to minimize pre‐shock pause and maximize coronary perfusion pressure.34 Either predetermined (passive) or just‐in‐time (active) choreography reinforces shared mental models that allow for dynamic and random teams to work together more effectively and is emphasized heavily during institutional training sessions and in weekly cardiac arrest debriefings.
Figure 2.
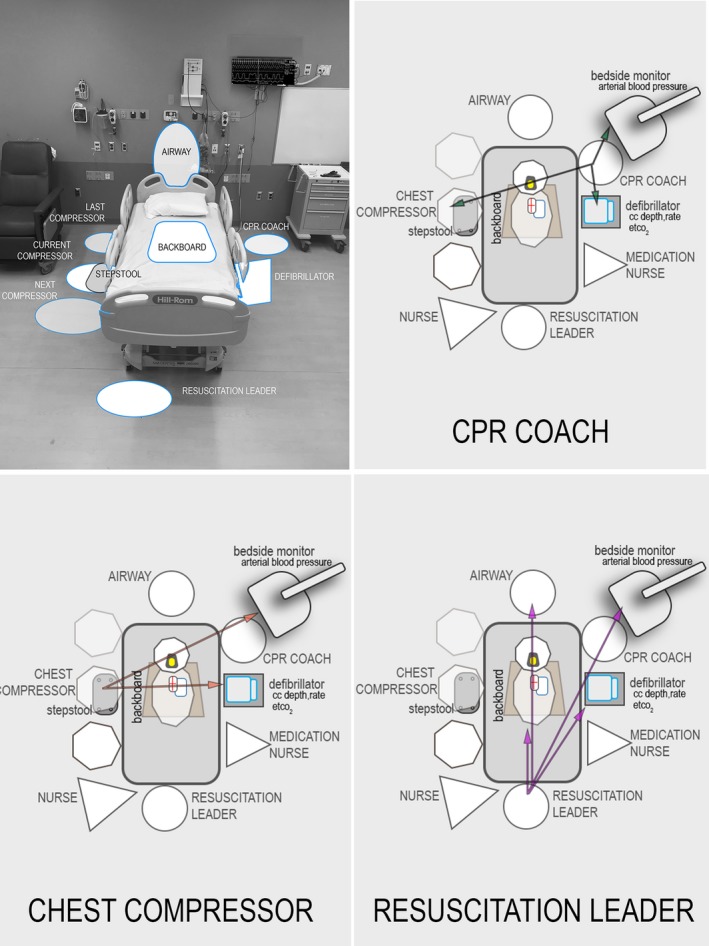
Introduction of CPR coach, choreography and ergonomics to optimize resuscitation performance. Introduction of CPR coach role to optimize compliance with AHA guidelines and cognitively unload the resuscitation leader; choreography of key roles during an in‐hospital cardiac arrest to enhance communication and ergonomics, with important “sight lines” from perspective of the compressor, the CPR coach and the leader are highlighted. CC indicates chest compression; CPR, cardiopulmonary resuscitation; ETCO2, end‐tidal carbon dioxide.
Ergonomics
While coaching targets psychomotor performance improvement (ie, better CC resulting in optimal perfusion) and choreography targets task execution through shared mental models and coordinated action, ergonomics focuses on optimizing the interaction of environment with these and other human factors driven behavior. During this intervention, we used 2 techniques to understand environmental factors that inhibit performance. We used a method we call “Room Diagramming.” Room diagramming took place either pre‐or post‐event. Pre‐event room diagrams mapped how the patient bed would be oriented for ECPR cannulations, and where surgeons, Operating Room nurses, compressors, defibrillator, etc… would be and how this would vary depending on femoral versus neck cannulation, room size, configuration, etc… to optimize ergonomics. These plans were practiced during monthly ECPR simulations. Post‐event room diagrams were used during our weekly debriefings. When sub‐par performance (eg, a long interruption as measured by the defibrillator or bedside monitor) was identified, participants would draw a diagram of the resuscitation. These included the location of the patient (with his/her orientation in the environment), individuals including their role in the resuscitation (active/passive/observer), and equipment. These diagrams facilitated discussion and identification of potential causes for the poor performance. Then using table‐top simulation methods,35 solutions for that particular problem were explored by modifying the diagram, creating configuration permutations, and determining the likely impact on the problem; we call this method a room diagram enhanced table top exercise (rdTTX) and use the Johns Hopkins Resuscitation Room Diagram Tool (Figure 3). Through the use of this technique we evolved the concept of Quality of CPR Sightlines, (see Figure 2). This ergonomic approach ensures the code team leader, the CPR coach, the Airway/head of bed provider, and active chest compressor all have visual access to crucial internal and external quality feedback, patient physiologic data, and one another; all elements when inaccessible had been identified during our debriefings as contributing factors to poor performance in multiple resuscitations.
Figure 3.
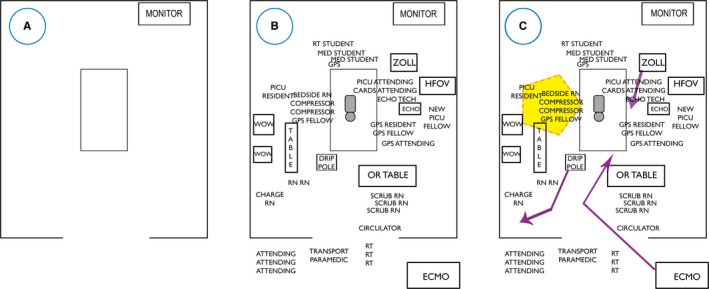
Johns Hopkins Resuscitation Room Diagram Tool. Facilitates understanding of the spatial issues of a cardiac arrest event, should also capture the dynamic nature of every resuscitation. Use of the Room Diagram Tool emphasizes: the number of people in the room, sight lines, ergonomics, and communication. A, A blank tool, (B) initial phase of a resuscitation with patient, team and equipment drawn in, and (C) tool after areas of concern were discussed (eg, orange circle indicating source of noise) as well as how equipment was moved based on priority (eg, red arrow indicating ECMO equipment moving from hallway to bedside). Can be used for an event that happened to debrief retrospectively or can be used to design an the “ideal event” prospectively. CARDS indicates cardiology; DRIP, IV pole with multiple medication pump; ECHO, Echocardiogram machine; ECMO, extracorporeal membrane oxygenation; GPS, General Pediatric Surgery; HFOV, High Frequency Oscillatory Ventilator; MED, medical; PICU, Pediatric Intensive Care Unit; RN, Registered Nurse.
During our study of each patient room, we assessed sight lines and evaluated what we now refer to as “sound channels.” This helped us understand why someone could not see the real‐time feedback on the defibrillator screen and could not hear medication orders. We recognized that at nearly every event resuscitation team members consistently worked around large pieces of equipment or furniture in the room, many of which were not necessary to the resuscitation. While a ventilator might need to stay in the room, a commode or reclining chair did not. In addition, the team often left the defibrillator wherever it was delivered into the room even if it faced away from the compressor and other team members. To optimize the ability of a compressor to use the information from the defibrillator to inform their quality of CC, our institutional standard has become for the defibrillator to be at the bedside directly across from the compressor. To increase the likelihood this will occur, we determined the need for modification of the defibrillator cart. We chose one with a slim profile, that does not get in the way of team members and is tall such that the screen is elevated above the patient and can easily be seen from across the bed. The cart has multiple drawers labeled to hold adult pads, pediatric pads, gel, ETCO2 cuvettes (plastic adapters connecting bag to mask or endotracheal tube), and sterile gowns for compressors when we activate ECPR. Finally, this cart holds a backboard on it so that when an emergency alert is triggered the person who grabs it is able to simultaneously bring in the backboard, defibrillator pads/paddles, and ETCO2 supplies—all key elements to initiate and optimize high‐quality BLS.
Structured debriefing
During this project, we determined that a comprehensive debriefing of a cardiac arrest event requires 45 minutes, in addition to the time necessary to collect, analyze, and prepare CPR performance and patient physiology data for review before the meeting. As described above, in addition to the patient data, the CODE‐ACES2 team solicits role‐based data and information to be included during debriefing through a set of survey instruments we call peer‐to‐peer debriefing forms, ie role‐relevant (physician/fellow, pharmacy, nursing, and respiratory therapy), questions related to non‐technical skills36 and quality of CPR concerns to inform the discussion.
The debriefing begins with a psychological safety, privacy, and confidentiality acknowledgement14, 37, 38 (Data S1) and introduction of participants by name and role. After completing those elements targeted at creating a safe environment, we move into a case‐specific discussion.
The event debriefing begins when the physician or nurse who was caring for the patient before the cardiac arrest gives an overview of the patient's history. Next, the rescuer who initiated CC describes events immediately preceding the need for compressions and their perception of the indication for CC. We identify other key roles, usually who was acting as the code team leader, CPR coach, compressors, airway manager, medication nurse, documenter, pharmacist, and potentially other roles. We then review patient monitor data beginning with the few minutes preceding the cardiac arrest and through the first few minutes of the resuscitation—we may review additional data, based on the questions that arise during the debriefing. We review the cardiac rhythm at the time of the cardiac arrest and documentation in the electronic medical record. During this review and debriefing process, clarification questions are asked. The core team members leading the debriefing sessions primarily use the debriefing with good judgement methodology.39, 40 This method allows a debriefer to safely and directly identify a performance gap and then fill that gap based on the participants mental model/frame and experiences.
We review the Johns Hopkins Resuscitation Performance Debriefing Tool (v3.0 Johns Hopkins University, Baltimore MD) (Figure 1). We focus much of the discussion on the age appropriate AHA PBLS metrics and PALS Guidelines and assess compliance and focusing the discussion on any impediments to compliance, and what can be done to remove those impediments. We review any electronic medical record data about timing of defibrillation, epinephrine and/or other medications, intubation, and ECPR documentation. At that time, we verify the data that will be entered into the Get With The Guidelines—Resuscitation database and determine if the team has committed any of what Get With The Guidelines—Resuscitation identifies as Process of Care Exceptions, or breaches of evidence based best practices. At the end of the discussion, we synthesize lessons learned and action items which then trigger institutional QI mechanisms (eg, reporting to the pediatric CPR committee, providing case‐based data for ECPR simulation, etc.).
Attendance at the weekly structured debriefings varies. There is a core group that attends every week to ensure that QI work will continue regardless of staff attendance. For debriefing of some arrests, all clinical staff that attended the resuscitation may be present but on occasion, there may be only 1 or 2 clinical staff members present as the result of vacations or having other clinical conflicts. Typically, the “system” still benefits because of the work done in gathering the peer‐to‐peer debriefing forms and review of the objective data from the defibrillator, etc… We use a combination of the Epic Code Narrator attendee list (in combination with our REACH This was previously defined email list where people write a list of who they remember to be in attendance) with the Outlook email invitation list. In addition, we have a designated clinical champion for each area, so that if no one is able to be present for an arrest in that area, at least the clinical champion in that area will come and bring the details they have ascertained and take back lessons learned from the discussion, ie, (Radiology, PACU, etc…).
These structured debriefings have the added benefit of functioning as a source of accurate and timely data for organizational systems integration and regulatory reporting processes. For example, as participants of the AHA's Get With The Guidelines—Resuscitation national registry, we use the weekly debriefings to discuss and finalize any incomplete data elements from the resuscitation and rapidly identify any core measures (eg, time to shock, time to first epinephrine, Endotracheal Tube confirmation) that lie outside recommended parameters. These data are then presented at the monthly CPR Advisory Committee meeting. The detailed discussion of the factors contributing to any performance gaps enables identification of recommendations needed to improve processes or modify policies. Recommendations for equipment updates are presented to the Capital Budget Committee for funding. This ensures we are in full compliance with all regulatory requirements regarding data capture and review for emergency events and have all necessary equipment for best practices. An 11‐item debriefing and discussion guide that describes this process is located in Table S2.
Simulation
We use simulation to support our resuscitation quality improvement program beyond the traditional BLS and PALS courses, frequently through the Rapid Cycle Deliberate Prototyping approach. As mentioned above, we conducted a series of simulations to determine the optimal location for the CPR coach to stand relative to the compressor, the defibrillator/CPR feedback device and the Code team leader. We also have simulated whether they can and should be responsible for related tasks including operating the defibrillator, monitoring ventilation quality, or administering medication. Ultimately, in our institution we decided that having them operate the defibrillator made sense as they were handling the device with CPR feedback, but giving medications introduced too much cognitive load. We have used simulation to determine the ideal choreography to place the backboard and the defibrillator pads at the same time to minimize interruptions, now reliably being able to do both simultaneously in <3 seconds.33, 34 In summary, we use simulation to understand the problem, to find an ideal solution and then to train our team until they have a shared mental model of the solution.
In summary, we have developed a Resuscitation Quality Bundle associated with improved compliance with AHA PBLS and PALS Guidelines that can serve as a template for other hospitals. This program has several key components. The first involved development and implementation of an active surveillance program that successfully increased capture of cardiac arrest events. The second was implementation of a weekly, structured cardiac arrest debriefing program associated with a progressive increase in capture of defibrillator accelerometer‐based data, enabling objective data evaluation. The third was to systematically capture lessons learned from the debriefing exercises, as well as tools developed to facilitate the debriefings. These elements were tied together into a resuscitation quality bundle we now call CODE ACES2. The development of this program has enabled our institution to have a shared mental model of the choreography and scripting of key elements of pediatric resuscitation, as well as factors that mediate performance. Ultimately, we will be measuring the impact on long‐term clinical outcomes.
Limitations
First, in this manuscript, we share our institution's approach to the complex problem of IHCA. The solutions we present may not be generalizable to all programs, nor have we studied each one in a controlled fashion. However, we are hopeful that describing the CODE ACES2 elements supplemented by our tools, aids, examples, and previous work will be useful to those starting a debriefing program and those attempting to improve compliance with AHA guidelines in their institution. Second, the proportion of cardiac arrest event data that were collected via the defibrillator during 2013 was only a small fraction of that captured in 2015. It is possible that there was selection bias and that the compressions we did not capture differed in quality from those that were captured, and that we are over‐representing the improvement in compliance. Third, we have not reported survival data in this study. To measure improvement compliance with guidelines across our Children's Center and have the power to detect a difference, we included all cardiac arrest events where chest compressions were performed within the walls of the hospital, including events starting outside the walls of the hospital; we also included repeat events. This means we are not able to explore the impact of the CODE ACES2 program on survival to discharge.
Finally, we observed a biphasic response in compliance with CC depth guidelines. We believe this is multifactorial in nature. The use of arterial catheters allows a code team leader to monitor, prioritize and target the diastolic blood pressure over CC depth, and this would not be reflected in the defibrillator record. Also, there is growing concern at our institution that the AHA recommended CC depth is not appropriate for some children (ie, for example that 3.8 cm may be too deep for a 4 kg 2‐month‐old), thus the code team leader would define a depth goal different than the AHA goal based on patient‐specific physiology. These are important issues to consider as institutions develop quality programs.
Conclusions
Over a 3‐year period, we debriefed >300 IHCAs, created a culture of capturing and using CC quality data to refine resuscitation performance and identified recurring debriefing themes that were transformed into a resuscitation quality bundle. CODE‐ACES2 focuses on strategies that mediate performance, ultimately driving improved compliance with PBLS and PALS Guidelines. Benchmarking of metrics over time and creation of an institutional shared mental model regarding key components of a resuscitation have been integral to improved resuscitation performance.
Disclosures
Dr Hunt has received honoraria and reimbursement of travel expenses from Zoll Medical Corporation for speaking engagements unrelated to this study (modest relationship). Dr Duval‐Arnould has received unrestricted funding for resuscitation‐related work from Zoll Medical (modest relationship). Zoll Medical Corporation has a non‐exclusive license for the use of educational technology on which Dr Hunt and Dr Duval‐Arnould have patents (modest relationship). Dr Hunt has grant funding from the National Institutes of Health that is unrelated to this study. The remaining authors have no disclosures to report.
Supporting information
Data S1. Johns Hopkins Resuscitation Debriefing Privacy and Confidentiality
Table S1. Johns Hopkins Pharmacy Acute Event Peer‐to‐Peer Debriefing Form
Table S2. Johns Hopkins Resuscitation Debriefing and Discussion Guide
Figure S1. Johns Hopkins Kids Kard Pediatric Acute Emergencies Cognitive Aid. Cognitive Aid for pediatric emergencies with area designed to support cardiac arrest management, includes AHA CPR depth and rate targets, and expert consensus‐based targets.
Acknowledgments
We would like to acknowledge the participants of the weekly “Code Busters” debriefing for being brave enough to make themselves vulnerable, as we explored the strengths and weaknesses of our collective performance week after week, to improve the care of our patients. We would like to thank the team that created the Johns Hopkins Hospital gold standard choreography First Few Minutes video: Nancy Sullivan for leading, Marida Twilley, Deborah Aksamit, Lynne Farrow, Pamela Boone‐Guercio and Sarah Smith for participation, Julianne Perretta for Instructional Design, and Andrew Stella for video production. As always, we would like to thank the staff of the Johns Hopkins Medicine Simulation Center and the Johns Hopkins CPR Office for their tireless support of these Quality Assurance efforts.
(J Am Heart Assoc. 2018;7:e009860 DOI: 10.1161/JAHA.118.009860)
References
- 1. Knudson JD, Neish SR, Cabrera AG, Lowry AW, Shamszad P, Morales DL, Graves DE, Williams EA, Rossano JW. Prevalence and outcomes of pediatric in‐hospital cardiopulmonary resuscitation in the United States: an analysis of the Kids’ Inpatient Database. Crit Care Med. 2012;40:2940–2944. [DOI] [PubMed] [Google Scholar]
- 2. Girotra S, Spertus JA, Li Y, Berg RA, Nadkarni VM, Chan PS; American Heart Association Get With the Guidelines–Resuscitation Investigators . Survival trends in pediatric in‐hospital cardiac arrests: an analysis from Get With the Guidelines‐Resuscitation. Circ Cardiovasc Qual Outcomes. 2013;6:42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Berg RA, Sutton RM, Reeder RW, Berger JT, Newth CJ, Carcillo JA, McQuillen PS, Meert KL, Yates AR, Harrison RE, Moler FW, Pollack MM, Carpenter TC, Wessel DL, Jenkins TL, Notterman DA, Holubkov R, Tamburro RF, Dean JM, Nadkarni VM; Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network (CPCCRN) Pediatric Intensive Care Quality of Cardio‐Pulmonary Resuscitation (PICqCPR) investigators . Association between diastolic blood pressure during pediatric in‐hospital cardiopulmonary resuscitation and survival. Circulation. 2018;137:1784–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jayaram N, Spertus JA, Nadkarni V, Berg RA, Tang F, Raymond T, Guerguerian AM, Chan PS; American Heart Association's Get with the Guidelines‐Resuscitation Investigators . Hospital variation in survival after pediatric in‐hospital cardiac arrest. Circ Cardiovasc Qual Outcomes. 2014;7:517–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ornato JP, Peberdy MA, Reid RD, Feeser VR, Dhindsa HS; NRCPR Investigators . Impact of resuscitation system errors on survival from in‐hospital cardiac arrest. Resuscitation. 2012;83:63–69. [DOI] [PubMed] [Google Scholar]
- 6. Edelson DP, Litzinger B, Arora V, Walsh D, Kim S, Lauderdale DS, Vanden Hoek TL, Becker L, Abella BS. Improving in‐hospital cardiac arrest process and outcomes with performance debriefing. Arch Intern Med. 2008;168:1063–1069. [DOI] [PubMed] [Google Scholar]
- 7. Dine CJ, Gersh RE, Leary M, Riegel BJ, Bellini LM, Abella BS. Improving cardiopulmonary resuscitation quality and resuscitation training by combining audiovisual feedback and debriefing. Crit Care Med. 2008;36:2817–2822. [DOI] [PubMed] [Google Scholar]
- 8. Jiang C, Zhao Y, Chen Z, Chen S, Yang X. Improving cardiopulmonary resuscitation in the emergency department by real‐time video recording and regular feedback learning. Resuscitation. 2010;81:1664–1669. [DOI] [PubMed] [Google Scholar]
- 9. Zebuhr C, Sutton RM, Morrison W, Niles D, Boyle L, Nishisaki A, Meaney P, Leffelman J, Berg RA, Nadkarni VM. Evaluation of quantitative debriefing after pediatric cardiac arrest. Resuscitation. 2012;83:1124–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wolfe H, Zebuhr C, Topjian AA, Nishisaki A, Niles DE, Meaney PA, Boyle L, Giordano RT, Davis D, Priestley M, Apkon M, Berg RA, Nadkarni VM, Sutton RM. Interdisciplinary ICU cardiac arrest debriefing improves survival outcomes. Crit Care Med. 2014;42:1688–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Duval‐Arnould JM, Newton HM, McNamara L, Engorn BM, Jones K, Bernier M, Dodge P, Salamone C, Bhalala U, Jeffers JM, Engineer L, Diener‐West M, Hunt EA. Design and deployment of a pediatric cardiac arrest surveillance system. Crit Care Res Pract. 2018;2018:9187962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berg MD, Schexnayder SM, Chameides L, Terry M, Donoghue A, Hickey RW, Berg RA, Sutton RM, Hazinski MF. Part 13: pediatric basic life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122:S862–S875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meaney PA, Bobrow BJ, Mancini ME, Christenson J, de Caen AR, Bhanji F, Abella BS, Kleinman ME, Edelson DP, Berg RA, Aufderheide TP, Menon V, Leary M; CPR Quality Summit Investigators, the American Heart Association Emergency Cardiovascular Care Committee, and the Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation . Cardiopulmonary resuscitation quality: [corrected] improving cardiac resuscitation outcomes both inside and outside the hospital: a consensus statement from the American Heart Association. Circulation. 2013;128:417–435. [DOI] [PubMed] [Google Scholar]
- 14. Edmondson A. Psychological safety and learning behavior in work teams. Adm Sci Q. 1999;44:350–383. [Google Scholar]
- 15. Abella BS, Alvarado JP, Myklebust H, Edelson DP, Barry A, O'Hearn N, Vanden Hoek TL, Becker LB. Quality of cardiopulmonary resuscitation during in‐hospital cardiac arrest. J Am Med Assoc. 2005;293:305–310. [DOI] [PubMed] [Google Scholar]
- 16. Kleinman ME, Brennan EE, Goldberger ZD, Swor RA, Terry M, Bobrow BJ, Gazmuri RJ, Travers AH, Rea T. Part 5: Adult Basic Life Support and Cardiopulmonary Resuscitation Quality: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132:S414–S435. [DOI] [PubMed] [Google Scholar]
- 17. Hunt EA, Cruz‐Eng H, Bradshaw JH, Hodge M, Bortner T, Mulvey CL, McMillan KN, Galvan H, Duval‐Arnould JM, Jones K, Shilkofski NA, Rodgers DL, Sinz EH. A novel approach to life support training using “action‐linked phrases”. Resuscitation. 2015;86:1–5. [DOI] [PubMed] [Google Scholar]
- 18. Niles DE, Duval‐Arnould J, Skellett S, Knight L, Su F, Raymond TT, Sweberg T, Sen AI, Atkins DL, Friess SH, de Caen AR, Kurosawa H, Sutton RM, Wolfe H, Berg RA, Silver A, Hunt EA, Nadkarni VM; pediatric Resuscitation Quality (pediRES‐Q) Collaborative Investigators . Characterization of pediatric in‐hospital cardiopulmonary resuscitation quality metrics across an international resuscitation collaborative. Pediatr Crit Care Med. 2018;19:421–432. [DOI] [PubMed] [Google Scholar]
- 19. Hazinski MF, Nadkarni VM, Hickey RW, O'Connor R, Becker LB, Zaritsky A. Major changes in the 2005 AHA Guidelines for CPR and ECC: reaching the tipping point for change. Circulation. 2005;112:IV206–IV211. [DOI] [PubMed] [Google Scholar]
- 20. Hunt EA, Duval‐Arnould JM, Nelson‐McMillan KL, Bradshaw JH, Diener‐West M, Perretta JS, Shilkofski NA. Pediatric resident resuscitation skills improve after “rapid cycle deliberate practice” training. Resuscitation. 2014;85:945–951. [DOI] [PubMed] [Google Scholar]
- 21. Hunt EA, Duval‐Arnould JM, Chime NO, Jones K, Rosen M, Hollingsworth M, Aksamit D, Twilley M, Camacho C, Nogee DP, Jung J, Nelson‐McMillan K, Shilkofski N, Perretta JS. Integration of in‐hospital cardiac arrest contextual curriculum into a basic life support course: a randomized, controlled simulation study. Resuscitation. 2017;114:127–132. [DOI] [PubMed] [Google Scholar]
- 22. Driskell JE, Salas E, Johnston J. Does stress lead to a loss of team perspective? Group Dyn Theory Res Pract. 1999;3:291. [Google Scholar]
- 23. Ellis AP. System breakdown: the role of mental models and transactive memory in the relationship between acute stress and team performance. Acad Manag J. 2006;49:576–589. [Google Scholar]
- 24. Porter CO, Hollenbeck JR, Ilgen DR, Ellis AP, West BJ, Moon H. Backing up behaviors in teams: the role of personality and legitimacy of need. J Appl Psychol. 2003;88:391. [DOI] [PubMed] [Google Scholar]
- 25. Morgeson FP, DeRue DS, Karam EP. Leadership in teams: a functional approach to understanding leadership structures and processes. J Manage. 2010;36:5–39. [Google Scholar]
- 26. Burke CS, Stagl KC, Klein C, Goodwin GF, Salas E, Halpin SM. What type of leadership behaviors are functional in teams? A meta‐analysis Leadersh Q. 2006;17:288–307. [Google Scholar]
- 27. Leary M, Schweickert W, Neefe S, Tsypenyuk B, Falk SA, Holena DN. Improving providers’ role definitions to decrease overcrowding and improve in‐hospital cardiac arrest response. Am J Crit Care. 2016;25:335–339. [DOI] [PubMed] [Google Scholar]
- 28. Infinger AE, Vandeventer S, Studnek JR. Introduction of performance coaching during cardiopulmonary resuscitation improves compression depth and time to defibrillation in out‐of‐hospital cardiac arrest. Resuscitation. 2014;85:1752–1758. [DOI] [PubMed] [Google Scholar]
- 29. Cheng A, Duff JP, Kessler D, Tofil NM, Davidson J, Lin Y, Chatfield J, Brown LL, Hunt EA; International Network for Simulation‐based Pediatric Innovation Research and Education (INSPIRE) CPR . Optimizing CPR performance with CPR coaching for pediatric cardiac arrest: A randomized simulation‐based clinical trial. Resuscitation. 2018;132:33–40. [DOI] [PubMed] [Google Scholar]
- 30. Hunt EA, Walker AR, Shaffner DH, Miller MR, Pronovost PJ. Simulation of in‐hospital pediatric medical emergencies and cardiopulmonary arrests: highlighting the importance of the first 5 minutes. Pediatrics. 2008;121:e34–e43. [DOI] [PubMed] [Google Scholar]
- 31. Salas E, Rosen MA, Burke CS, Goodwin GF. The wisdom of collectives in organizations: an update of the teamwork competencies In: Team Effectiveness in Complex Organizations: Cross‐Disciplinary Perspectives and Approaches. New York, NY: Routledge; 2009:39–79. [Google Scholar]
- 32. Endsley MR. Toward a theory of situation awareness in dynamic systems. Hum Factors. 1995;37:32–64. [Google Scholar]
- 33. Sullivan N, Duval‐Arnould JM, Perretta JS, Rosen M, Hunt EA. Using simulation to design choreography for the first few minutes of a cardiac arrest. Clin Simul Nurs. 2015;11:489–493. [Google Scholar]
- 34. Johns Hopkins Medicine Simulation Center, Resuscitation Website, Resuscitation Tools: Johns Hopkins Hospital institutional standardized choreography for clinical responders for the First Few Minutes of an adult cardiac arrest. Available at: https://www.hopkinsmedicine.org/simulation_center/resuscitation/ffm_training.html. Accessed October 26, 2018.
- 35. Dausey DJ, Buehler JW, Lurie N. Designing and conducting tabletop exercises to assess public health preparedness for manmade and naturally occurring biological threats. BMC Public Health. 2007;29:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jones KR, Rosen M, Duval‐Arnould J, Hunt EA. Development of a cardiopulmonary resuscitation non‐technical skills scoring tool (CPR‐NTS). Crit Care Med. 2014;42:A1427. [Google Scholar]
- 37. Rudolph JW, Raemer DB, Simon R. Establishing a safe container for learning in simulation. Simul Healthc. 2014;9:339–349. [DOI] [PubMed] [Google Scholar]
- 38. Fanning RM, Gaba DM. The role of debriefing in simulation‐based learning. Simul Healthc. 2007;2:115–125. [DOI] [PubMed] [Google Scholar]
- 39. Rudolph JW, Simon R, Raemer DB, Eppich WJ. Debriefing as formative assessment: closing performance gaps in medical education. Acad Emerg Med. 2008;15:1010–1016. [DOI] [PubMed] [Google Scholar]
- 40. Rudolph JW, Simon R, Rivard P, Dufresne RL, Raemer DB. Debriefing with good judgment: combining rigorous feedback with genuine inquiry. Anesthesiol Clin. 2007;25:361–376. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Johns Hopkins Resuscitation Debriefing Privacy and Confidentiality
Table S1. Johns Hopkins Pharmacy Acute Event Peer‐to‐Peer Debriefing Form
Table S2. Johns Hopkins Resuscitation Debriefing and Discussion Guide
Figure S1. Johns Hopkins Kids Kard Pediatric Acute Emergencies Cognitive Aid. Cognitive Aid for pediatric emergencies with area designed to support cardiac arrest management, includes AHA CPR depth and rate targets, and expert consensus‐based targets.


