Abstract
Background
The influence of familial factors on the prognosis of heart failure (HF) is unknown. This nationwide follow‐up study aimed to determine familial mortality risks of HF among Swedish siblings hospitalized for HF.
Methods and Results
We linked several Swedish nationwide registers for individuals aged 0 to 80 years. The study population consisted of 373 people hospitalized for HF for the first time between 2000 and 2012 with 1 proband sibling previously hospitalized for HF for the first time between 2000 and 2007. Families with congenital heart disease were excluded. Familial hazard ratios (HRs) for mortality after first HF hospitalization were determined with Cox regression. The influence of proband survival was categorized as short survival (<5 years) or long survival (≥5 years) and determined continuously for the initial 5 years of proband survival. Adjustments were made for age, sex, time period, and common HF comorbidities. Short proband survival was associated with a HR of 2.02 (95% confidence interval, 1.32–3.09) for overall mortality. This HR was 2.35 (95% confidence interval, 1.18–4.67) in patients without preceding coronary heart disease, whereas patients with ischemic HF had an HR of 1.84 (95% confidence interval, 1.05–3.23). For each year of proband survival, the risk of death decreased, with a HR of 0.86 (95% confidence interval, 0.77–0.98).
Conclusions
Our results suggest that family history of poor survival in specific relation to HF is an important risk factor for death in HF patients. Additional studies are needed to characterize the molecular underpinnings and detailed phenotypic characteristics of such patients.
Keywords: family study, heart failure, mortality, prognosis
Subject Categories: Heart Failure, Mortality/Survival, Epidemiology, Genetics
Clinical Perspective
What Is New?
Few genetic polymorphisms have been confirmed to influence the progression or prognosis of heart failure, and the net effect of familial factors relating to survival in heart failure is unknown.
This study determined the risk of death due to familial factors in heart failure.
Measured from time of first hospitalization for heart failure, having an affected sibling with short survival was associated with an increased risk of death, also after adjusting for other important covariates.
What Are the Clinical Implications?
The results of this study identify heart failure patients with an increased risk of death.
Further studies are warranted on genetic polymorphisms important for the prognosis of heart failure.
Introduction
Family history of heart failure (HF), independent of common comorbidities, has been associated with an increased risk of HF.1, 2, 3 It is largely unknown to what extent familial and possibly genetic factors influence the prognosis of HF. Several candidate genetic polymorphisms have been proposed to influence HF progression or prognosis.4, 5, 6, 7, 8 More recently, genome‐wide association studies have explored genetic determinants of survival in HF patients with the finding of a single nucleotide polymorphism (rs9885413) on chromosome 5q22 associated with a 36% increased mortality risk.9 However, as with the incidence of HF,10 the prognosis of HF likely depends on the cumulative effect of several contributing factors including shared environment and epigenetic and genetic factors; all of these factors may be reflected by family studies, providing important tools for determining clinical risk as well as direction for future genetic studies.11, 12 To the best of our knowledge, no population study has assessed familial prognosis of HF. In this sibling study, we aimed to determine the effect of poor HF survival in family members on the prognosis of HF patients.
Methods
Registers
The data, analytic methods, and study materials will not be made available to researchers for purposes of reproducing the results or replicating the procedure. The cohort data used were compiled from several national registers maintained by Statistics Sweden and the Swedish National Board of Health and Welfare. The registers used included the Hospital Discharge Register (HDR), which contains nationwide coverage of hospital discharge diagnoses since 1987 with a missing main discharge diagnosis for <1% of patients. The positive predictive values for many diagnoses in the HDR vary between 85% and 95%, although the validity of many cardiovascular diseases tends to be higher (90–95%).13 HF registered as a main diagnosis, as used in this study, has been found to carry a positive predictive value of >95%.13, 14, 15 The Multigeneration Register contains information on maternal parentage (97% coverage) and paternal parentage (95% coverage) of all individuals born in Sweden since 1932 who did not emigrate or die between 1932 and 1960.16 The Cause of Death Register, which is considered to be valid for use in epidemiological studies for several diagnoses, including ischemic heart disease, contains data from 1964 and onward.17, 18
This study was conducted in compliance with the Helsinki Declaration and was approved by the ethics committee of Lund University, Sweden, which waived the requirement of informed consent because of the use of anonymized register data.
Study Sample
The cohort consisted of patients (referred to as subjects) hospitalized for HF for the first time (HF registered as a main diagnosis in the HDR) between calendar years 2000 and 2012, all of whom also had 1 proband sibling (referred to as a proband) who was previously hospitalized for HF for the first time between 2000 and 2007. With survival data available until 2012 for both subject and proband, to obtain 5 year records of proband survival, only subjects with a proband first hospitalized for HF no later than 2007 were included.
Only a main diagnosis of HF was used to ensure that primary and not secondary HF cases were included and to increase the validity of HF diagnosis. International Classification of Diseases (ICD) code 428 (ICD, Ninth Revision [ICD‐9]; 1987–1996) and code I50 (ICD, 10th Revisions [ICD‐10]; 1997–2012) were used to define HF.
To reduce the risk of registering a recurrent HF hospitalization event as the first hospitalization for HF, all families recorded with a main HF diagnosis in HDR between calendar years 1987 and 1999 were excluded.
We excluded all families with a member recorded as having emigrated or immigrated to Sweden during the period 1987–2012. Half siblings (ie, individuals born of either the same mother or father only) were not counted as eligible sibling relatives. We also excluded patients diagnosed with malignant (in situ excluded) cancer, main and secondary diagnoses 140 to 208 (ICD‐9) or C00‐C97 (ICD‐10) during the period of 5 years before and 2 years after the event of HF hospitalization.
Families with a member recorded with main or secondary diagnoses of congenital heart disease 745 to 747 (ICD‐9) or Q20‐Q28 (ICD‐10) in 1987–2012 were excluded throughout the study. Families with any member with a main or secondary diagnosis of cardiomyopathy 428 (ICD‐9) or I42 (ICD‐10) in 1987–2012 were included in the analyses if not explicitly stated otherwise. For descriptive purposes, the first recorded diagnosis of cardiomyopathy in 1987–2012 was further stratified: alcoholic cardiomyopathy 425F (ICD‐9) or I426 (ICD‐10); dilated cardiomyopathy (not a standalone diagnosis in ICD‐9) or I420 (ICD‐10); hypertrophic cardiomyopathy, including hypertrophic obstructive cardiomyopathy 425B (ICD‐9) or I421–I422 (ICD‐10); restrictive cardiomyopathy 425D (ICD‐9) or I423–I425 (ICD‐10). The category other causes or unspecified phenotypes were defined as cardiomyopathies recorded with other 428 (ICD‐9) or I42 (ICD‐10) diagnoses.
Predictor Variable
The predictor variable was survival time of the proband after first hospitalization for HF in 2000–2007, which allowed for a minimum range of measure of 5 years at the end of follow‐up on December 31, 2012. Proband survival was used as both a continuous predictor and a categorized predictor with survival dichotomized as <5 years or longer (≥5 years).
Outcome Variable and Subjects Studied
Subject mortality was calculated from the date of hospital admission for HF and measured as both overall mortality (main outcome) and death from cardiovascular causes (secondary outcome), as registered in the Swedish Cause of Death Register.
Other Covariates
Additional variables used in all analyses were subject sex, age, and calendar year at onset (time of first recorded main diagnosis) of HF. The following comorbidities (main and secondary diagnoses), recorded previous to (earliest January 1, 1987) or at the time of HF admission, were also used: coronary heart disease (CHD), code 410 to 414 (ICD‐9) or I20 to I25 (ICD‐10); diabetes mellitus, code 250 (ICD‐9) or E10 to E14 (ICD‐10); hypertension, code 401 to 405 (ICD‐9) or I10 to I15 (ICD‐10); nonrheumatic valvular disease, code 424 (ICD‐9) or I34 to I37 (ICD‐10); stroke (430, 431, 434–436, 438 (ICD‐9) or I60 to I64, I69 (ICD‐10).
Statistical Analysis
Subject mortality was analyzed using survival analysis with Cox regression and relative risks expressed as hazard ratios (HRs). The smaller models were adjusted for subject sex, age at onset, and year of onset of HF. The variables age at onset and year of onset were calculated as the number of years deviated from the subject mean. The full models also included adjustments made for subject comorbidities, as specified earlier.
All covariates were tested for interaction, and assumptions of proportional hazards were tested with Schoenfeld tests. Log linearity was also tested graphically using log−log survival plots. In addition, overall mortality was also calculated as incidence ratios.2
The results included 95% confidence intervals (CIs), which were applied with 2‐decimal accuracy. All analyses were made using STATA v14.1 (StataCorp).
Results
A total of 96 deaths occurred among 373 eligible subjects found in 372 families. Total follow‐up time was 1195.97 years with an overall mortality incidence ratio of 80.27 (95% CI, 65.71–98.06) per 1000 person‐years. In one family, both affected siblings were hospitalized on the same day, and these patients were regarded as both subjects and probands. Limited by the event of death or regression right censoring, subject mean and median follow‐up time was 3.21 and 2.59 years, respectively.
Table 1 shows the baseline characteristics of subjects at the time of first hospitalization for HF based on whether survival time of the proband was <5 years or ≥5 years. A total of 59 (20.8%) deaths occurred among 283 subjects with a proband surviving ≥5 years, whereas 37 (41.1%) of 90 subjects died among those with a proband surviving <5 years. The majority of patients with cardiomyopathy had a dilated phenotype. Table 2 shows the same baseline characteristics for the 241 subjects in families with no registered case of cardiomyopathy.
Table 1.
Baseline Characteristics of Subjects* at First Hospitalization for HF
| Characteristic | Proband Survival After Proband First Hospitalization for HF | P Value† | |
|---|---|---|---|
| ≥5 y | <5 y | ||
| Total, n | 283 | 90 | |
| Female sex, n (%) | 102 (36.0) | 33 (36.7) | 1.00 |
| Age interval at diagnosis, y, n (%) | |||
| <50 | 12 (4.2) | 1 (1.1) | 0.016 |
| 50–59 | 45 (15.9) | 8 (8.9) | |
| 60–69 | 98 (34.6) | 47 (52.2) | |
| ≥70 | 128 (45.2) | 34 (37.8) | |
| Mean | 66.4 | 67.1 | 0.47 |
| Median | 68.6 | 68.1 | 0.78 |
| Time period of first hospitalization, n (%) | |||
| 2000–2007 | 100 (35.3) | 38 (42.2) | 0.26 |
| 2008–2012 | 183 (64.7) | 52 (57.8) | |
| Hospital record of comorbidities, n (%) | |||
| CHD | 136 (48.1) | 53 (58.9) | 0.090 |
| Diabetes mellitus | 84 (29.7) | 31 (34.4) | 0.43 |
| Hypertension | 149 (52.7) | 50 (55.6) | 0.72 |
| Nonrheumatic valvular disease | 43 (15.2) | 10 (11.1) | 0.39 |
| Stroke | 52 (18.4) | 17 (18.9) | 0.88 |
| Cardiomyopathy, n (%) | |||
| Alcoholic | 1 (0.4) | 0 | 0.98 |
| Dilated | 34 (12.0) | 11 (12.2) | |
| Hypertrophic | 4 (1.4) | 1 (1.1) | |
| Restrictive | 1 (0.4) | 0 | |
| Other causes or unspecified phenotype | 11 (3.9) | 2 (2.2) | |
CHD indicates coronary heart disease; HF, heart failure.
Only families with 2 cases of HF and no case of congenital heart disease included.
Two independent‐samples t test, Wilcoxon rank sum test, and Fisher exact test were performed for calculations of mean, median, and expected frequencies of categorical variables, respectively.
Table 2.
Baseline Characteristics of Subjects at First Hospitalization for HF, Cardiomyopathies Excluded*
| Characteristic | Proband Survival After First Hospitalization for HF | P Value† | |
|---|---|---|---|
| >5 y | <5 y | ||
| Total, n | 175 | 66 | |
| Female sex, n (%) | 65 (37.1) | 24 (36.4) | 1.00 |
| Age interval at diagnosis, y, n (%) | |||
| <50 | 3 (1.7) | 0 | 0.074 |
| 50–59 | 20 (11.4) | 6 (9.1) | |
| 60–69 | 56 (32.0) | 33 (50.0) | |
| ≥70 | 96 (54.9) | 27 (40.9) | |
| Mean | 68.5 | 67.6 | 0.34 |
| Median | 70.3 | 68.2 | 0.080 |
| Time period of first hospitalization, n (%) | |||
| 2000–2007 | 49 (28.0) | 28 (42.4) | 0.044 |
| 2008–2012 | 126 (72.0) | 38 (57.6) | |
| Hospital record of comorbidities, n (%) | |||
| CHD | 88 (50.3) | 41 (62.1) | 0.11 |
| Diabetes mellitus | 54 (30.9) | 22 (33.3) | 0.76 |
| Hypertension | 97 (55.4) | 40 (60.6) | 0.56 |
| Nonrheumatic valvular disease | 31 (17.7) | 9 (13.6) | 0.56 |
| Stroke | 37 (21.1) | 12 (18.2) | 0.72 |
CHD indicates coronary heart disease; HF, heart failure.
Only families with 2 cases of HF and no case of cardiomyopathy or congenital heart disease included.
Two independent‐samples t test, Wilcoxon rank sum test, and Fisher exact test were performed for calculations of mean, median, and expected frequencies of categorical variables, respectively.
HF Mortality Risk According to Sibling HF Survival Time Status
Figures 1 and 2 depict unadjusted Kaplan–Meier survival functions of overall mortality and cardiovascular mortality, respectively, from the time of subject first hospitalization for HF in 2000–2012, as determined by proband survival time after first HF hospitalization in 2000–2007. Proband survival time was categorized as <5 years and ≥5 years.
Figure 1.
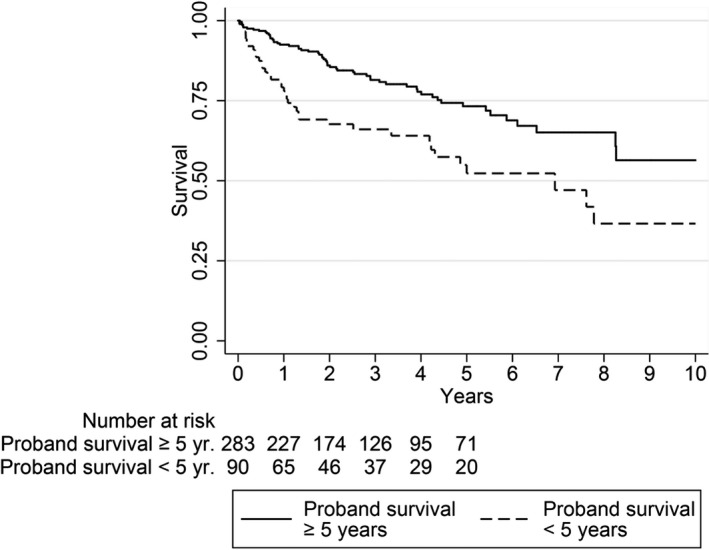
Unadjusted Kaplan–Meier survival functions for overall mortality from first hospitalization for heart failure 2000–2012, as determined by proband heart failure survival.
Figure 2.
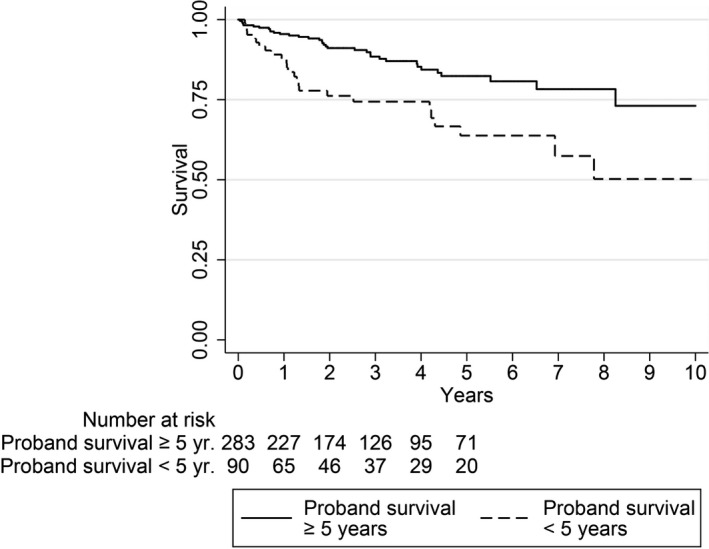
Unadjusted Kaplan–Meier survival functions for cardiovascular mortality from first hospitalization for heart failure 2000–2012, as determined by proband heart failure survival.
The overall mortality incidence rate (137.15 cases per 1000 person‐years) among subjects having a proband that survived <5 years was >2 times the incidence rate (63.70 per 1000 person years) of subjects with longer proband survival, conferring an incidence ratio of 2.15 (95% CI, 1.39–3.30). Stratified by subject sex, this incidence ratio was 2.08 (95% CI, 1.29–3.54) for male subjects and 2.32 (95% CI, 1.06–4.90) for female subjects.
With the same basis, Table 3 analogously describes small and full multivariable Cox regression models, with covariates as specified earlier, of subject overall mortality after the first admission for HF. With reference to proband HF survival ≥5 years, shorter proband HF survival was associated with an increased risk of death, both in the small model (HR: 1.98; 95% CI, 1.31–3.00) and in the fully adjusted model (HR: 2.02; 95% CI, 1.32–3.09). Similar results were obtained when we restricted the outcome to registered cardiovascular death only (Table 4).
Table 3.
Overall Mortality of Subjects* First Hospitalized for HF, as Determined by Survival of Previously Affected Sibling (Proband)
| Small Model, HR (95% CI)† | P Value | Fully Adjusted Model, HR (95% CI)† | P Value | |
|---|---|---|---|---|
| Proband HF survival <5 y (yes/no)‡ | 1.98 (1.31–3.00) | 0.001 | 2.02 (1.32–3.09) | 0.001 |
| Subject characteristics | ||||
| Sex (female/male) | 0.92 (0.60–1.41) | 0.69 | 0.98 (0.63–1.51) | 0.91 |
| Age at onset§ | 2.14 (1.52–3.02) | <0.001 | 2.11 (1.44–3.08) | <0.001 |
| Year of onset§ | 0.97 (0.88–1.06) | 0.46 | ||
| CHD (yes/no) | 0.90 (0.58–1.42) | 0.66 | ||
| Diabetes mellitus (yes/no) | 2.68 (1.73–4.16) | <0.001 | ||
| Hypertension (yes/no) | 0.80 (0.53–1.20) | 0.30 | ||
| Stroke (yes/no) | 2.18 (1.40–3.39) | 0.001 | ||
| VHD (yes/no) | 1.75 (1.01–3.00) | 0.044 | ||
CHD indicates coronary heart disease; CI, confidence interval; HF, heart failure; HR, hazard ratio; VHD, valvular heart disease.
Only families with 2 cases of HF and no case of congenital heart disease included.
Cox regression HRs, adjustments as specified below.
37 deaths among 90 subjects with proband survival <5 y, from a total of 96 deaths among 373 subjects at risk.
Age at onset and year of onset presented as per 10‐ and 1‐y deviations from mean, respectively.
Table 4.
Cardiovascular Mortality of Subjects* First Hospitalized for HF, as Determined by Survival of Previously Affected Sibling (Proband)
| Small Model, HR (95% CI)† | P Value | Fully Adjusted Model, HR (95% CI)† | P Value | |
|---|---|---|---|---|
| Proband HF survival <5 y (yes/no)‡ | 2.24 (1.34–3.76) | 0.002 | 2.35 (1.38–4.03) | 0.002 |
| Subject characteristics | ||||
| Sex (female/male) | 0.92 (0.54–1.58) | 0.76 | 1.16 (0.66–2.03) | 0.61 |
| Age at onset§ | 2.45 (1.56–3.85) | <0.001 | 2.16 (1.30–3.59) | 0.003 |
| Year of onset§ | 0.99 (0.88–1.11) | 0.89 | ||
| CHD (yes/no) | 1.42 (0.79–2.56) | 0.24 | ||
| Diabetes mellitus (yes/no) | 1.70 (0.97–2.97) | 0.065 | ||
| Hypertension (yes/no) | 0.48 (0.28–0.81) | 0.007 | ||
| Stroke (yes/no) | 2.94 (1.70–5.08) | <0.001 | ||
| VHD (yes/no) | 1.62 (0.83–3.18) | 0.16 | ||
CHD indicates coronary heart disease; CI, confidence interval; HF, heart failure; HR, hazard ratio; VHD, valvular heart disease.
Only families with 2 cases of HF and no case of congenital heart disease included.
Cox regression HRs, adjustments as specified below.
25 cardiovascular deaths among 90 subjects with proband survival <5 y, from a total of 60 cardiovascular deaths among 373 subjects at risk.
Age at onset and year of onset presented as per 10‐ and 1‐y deviations from mean, respectively.
After excluding families with any cardiomyopathy, short proband survival <5 years was still similarly associated with an increased relative risk of subject overall mortality (Table 5), with a fully adjusted HR of 2.22 (95% CI, 1.33–3.71). Likewise, the risk of cardiovascular death also remained significantly increased in this cohort (Table 6).
Table 5.
Overall Mortality of Subjects First Hospitalized for HF, as Determined by Survival of Previously Affected Sibling (Proband)—Cardiomyopathies Excluded*
| Small Model, HR (95% CI)† | P Value | Fully Adjusted Model, HR (95% CI)† | P Value | |
|---|---|---|---|---|
| Proband HF survival <5 y (yes/no)‡ | 2.04 (1.24–3.36) | 0.005 | 2.22 (1.33–3.71) | 0.002 |
| Subject characteristics | ||||
| Sex (female/male) | 1.03 (0.61–1.74) | 0.90 | 1.19 (0.69–2.07) | 0.53 |
| Age at onset§ | 1.68 (1.07–2.64) | 0.025 | 1.65 (1.01–2.70) | 0.046 |
| Year of onset§ | 1.01 (0.90–1.13) | 0.88 | ||
| CHD (yes/no) | 0.93 (0.54–1.59) | 0.78 | ||
| Diabetes mellitus (yes/no) | 1.96 (1.14–3.36) | 0.014 | ||
| Hypertension (yes/no) | 0.86 (0.52–1.42) | 0.55 | ||
| Stroke (yes/no) | 2.44 (1.42–4.21) | 0.001 | ||
| VHD (yes/no) | 1.34 (0.70–2.60) | 0.38 | ||
CHD indicates coronary heart disease; CI, confidence interval; HF, heart failure; HR, hazard ratio; VHD, valvular heart disease.
Only families with 2 cases of HF and no case of cardiomyopathy or congenital heart disease included.
Cox regression HRs, adjustments as specified below.
28 deaths among 66 subjects with proband survival <5 y, from a total of 64 deaths among 241 subjects at risk.
Age at onset and year of onset presented as per 10‐ and 1‐y deviations from mean, respectively.
Table 6.
Cardiovascular Mortality of Subjects First Hospitalized for HF, as Determined by Survival of Previously Affected Sibling (Proband)—Cardiomyopathies Excluded*
| Small Model, HR (95% CI)† | P Value | Fully Adjusted Model, HR (95% CI)† | P Value | |
|---|---|---|---|---|
| Proband HF survival <5 y (yes/no)‡ | 1.98 (1.05–3.73) | 0.034 | 2.30 (1.19–4.45) | 0.013 |
| Subject characteristics | ||||
| Sex (female/male) | 0.85 (0.43–1.68) | 0.65 | 1.26 (0.61–2.62) | 0.54 |
| Age at onset§ | 2.16 (1.18–3.97) | 0.013 | 1.81 (0.94–3.47) | 0.076 |
| Year of onset§ | 1.06 (0.91–1.24) | 0.42 | ||
| CHD (yes/no) | 1.55 (0.76–3.16) | 0.23 | ||
| Diabetes mellitus (yes/no) | 1.25 (0.62–2.50) | 0.54 | ||
| Hypertension (yes/no) | 0.53 (0.28–1.01) | 0.054 | ||
| Stroke (yes/no) | 3.18 (1.61–6.26) | 0.001 | ||
| VHD (yes/no) | 1.15 (0.51–2.60) | 0.74 | ||
CHD indicates coronary heart disease; CI, confidence interval; HF, heart failure; HR, hazard ratio; VHD, valvular heart disease.
Only families with 2 cases of HF and no case of cardiomyopathy or congenital heart disease included.
Cox regression HRs, adjustments as specified below.
17 deaths among 66 subjects with proband survival <5 y, from a total of 40 deaths among 241 subjects at risk.
Age at onset and year of onset presented as per 10‐ and 1‐y deviations from mean, respectively.
HF Overall Mortality Risk in Stratified Models
Table 7 shows the results of analogous analyses, with the inclusion of cardiomyopathies, for overall mortality stratified by subject median age at first HF hospitalization, by sex, and by occurrence of previous diagnosis of CHD. Fewer deaths occurred among younger subjects, and among this half at or below the median age (68.3 years) of first hospitalization for HF, the increased risk associated with short proband HF survival <5 years was not significant, with a fully adjusted HR of 1.76 (95% CI, 0.88–3.54). However, short proband survival was significantly associated with an increased risk of death among older subjects as well as among male and female subjects.
Table 7.
Overall Mortality of Subjects* First Hospitalized for HF, as Determined by Survival of Previously Affected Sibling (Proband), Stratified Analyses
| Small Model, HR (95% CI)† | P Value | Fully Adjusted Model, HR (95% CI)‡ | P Value | |
|---|---|---|---|---|
| Subjects with median age of onset ≤68.3 y (total at risk, n=187; deaths, n=39) | ||||
| Proband HF survival <5 y (yes/no) | 1.87 (0.97–3.61) | 0.061 | 1.76 (0.88–3.54) | 0.11 |
| Subjects with median age of onset ≥68.3 y (total at risk, n=186; deaths, n=57) | ||||
| Proband HF survival <5 y (yes/no) | 1.99 (1.16–3.43) | 0.013 | 2.41 (1.35–4.29) | 0.003 |
| Male subjects (total at risk, n=238; deaths, n=63) | ||||
| Proband HF survival <5 y (yes/no) | 1.81 (1.09–3.01) | 0.023 | 1.74 (1.03–2.97) | 0.040 |
| Female subjects. (total at risk, n=135; deaths, n=33) | ||||
| Proband HF survival <5 y (yes/no) | 2.27 (1.12–4.59) | 0.022 | 2.53 (1.21–5.29) | 0.014 |
| Subjects without CHD (total at risk, n=184; deaths, n=38)§ | ||||
| Proband HF survival <5 y (yes/no) | 2.19 (1.12–4.25) | 0.021 | 2.35 (1.18–4.67) | 0.015 |
| Subjects with CHD (Total at risk, n=189; deaths, n=58)§ | ||||
| Proband HF survival <5 y (yes/no) | 1.87 (1.10–3.18) | 0.021 | 1.84 (1.05–3.23) | 0.033 |
CHD indicates coronary heart disease; CI, confidence interval; HF, heart failure; HR, hazard ratio; VHD, valvular heart disease.
Only families with 2 cases of HF and no case of congenital heart disease included.
Cox regression HRs, adjusted for subject age at onset and sex within appropriate strata.
Cox regression HRs, adjusted for subject age at onset, sex, coronary heart disease, diabetes mellitus, hypertension, nonrheumatic valvular heart disease, and stroke, within appropriate strata.
Status at time of first hospitalization for HF.
Subjects were also stratified by nonischemic and ischemic HF, the latter defined as the presence of a recorded diagnosis (main or secondary) of CHD from 1987 to the day of first hospitalization for HF. Occurrence of a CHD diagnosis only after first hospitalization for HF would consequently be classified as nonischemic HF. Among subjects with nonischemic HF, proband survival <5 years was associated with a full‐model HR of 2.35 (95% CI, 1.18–4.67). As for subjects with ischemic HF, this association was slightly lower, with a fully adjusted HR of 1.84 (1.05–3.23, 95% CI).
HF Overall Mortality as Determined by Proband Per‐Year Survival
The per‐year prognostic effect of proband survival on subject overall mortality was calculated during the first 5 years after initial proband hospitalization (Table 8. Proband survival time was thus viewed as a continuous predictor during this minimum of 5 years of proband follow‐up. The fully adjusted model rendered an annual HR of 0.86 (95% CI, 0.77–0.98). Thus for every year of proband survival, there was an associated ≈14% reduction in subject mortality risk.
Table 8.
Overall Mortality of Subjects* First Hospitalized for HF, as Determined by Yearly Survival of Previously Affected Sibling (Proband), Calculated for First 5 Years of Proband Survival
| Small Model, HR (95% CI)† | P Value | Fully Adjusted Model, HR (95% CI)† | P Value | |
|---|---|---|---|---|
| Effect on subject mortality conferred by proband per‐year survival‡ | 0.88 (0.78–0.99) | 0.031 | 0.86 (0.77–0.98) | 0.018 |
| Subject characteristics | ||||
| Sex (female/male) | 0.90 (0.58–1.38) | 0.62 | 0.94 (0.61–1.46) | 0.79 |
| Age at onset§ | 2.16 (1.54–3.02) | <0.001 | 2.14 (1.47–3.12) | <0.001 |
| Year of onset§ | 0.96 (0.87–1.05) | 0.34 | ||
| CHD (yes/no) | 0.90 (0.57–1.41) | 0.64 | ||
| Diabetes mellitus (yes/no) | 2.69 (1.74–4.17) | <0.001 | ||
| Hypertension (yes/no) | 0.79 (0.53–1.20) | 0.27 | ||
| Stroke (yes/no) | 2.26 (1.46–3.51) | <0.001 | ||
| VHD (yes/no) | 1.61 (0.94–2.76) | 0.082 | ||
CHD indicates coronary heart disease; CI, confidence interval; HF, heart failure; HR, hazard ratio; VHD, valvular heart disease.
Only families with 2 cases of HF and no case of congenital heart disease included.
Cox regression HRs, adjustments as specified below.
37 deaths among 90 subjects with proband survival <5 y, from a total of 96 deaths among 373 subjects at risk.
Age at onset and year of onset presented as per 10‐ and 1‐y deviations from mean, respectively.
Secondary Analyses
To consider differences in time of hospitalization between the study subject and the proband, which could suggest differences in HF therapeutics and management, the cohort was stratified at the median sibling pair hospitalization time difference (1726 days, ≈ 4.72 years; Table 9. For both strata, the relative risk of death associated with proband survival <5 years remained significant and was approximately 2 times that of longer proband survival.
Table 9.
Overall Mortality of Subjects* First Hospitalized for HF, as Determined by Survival of Previously Affected Sibling (Proband), Stratified by Median Difference in Time Between Subject and Proband Time of Hospitalization
| Small Model, HR (95% CI)† | P Value | Fully Adjusted Model, HR (95% CI)‡ | P Value | |
|---|---|---|---|---|
| Occurrence of subject hospitalization within median 1726 d after that of proband | ||||
| Proband HF survival <5 y (yes/no)§ | 1.93 (1.16–3.20) | 0.012 | 2.17 (1.25–3.78) | 0.006 |
| Occurrence of subject hospitalization median >1726 d after that of proband (total at risk, n=186; deaths, n=33) | ||||
| Proband HF survival <5 y (yes/no)ǁ | 2.07 (1.02–4.22) | 0.044 | 2.98 (1.34–6.62) | 0.008 |
CHD indicates coronary heart disease; CI, confidence interval; HF, heart failure; HR, hazard ratio; VHD, valvular heart disease.
Only families with 2 cases of HF and no case of congenital heart disease included.
Cox regression HRs, adjusted for subject age at onset and sex, within appropriate strata.
Cox regression HRs, adjusted for subject age at onset, sex, CHD, diabetes mellitus, hypertension, nonrheumatic VHD, and stroke, within appropriate strata.
25 deaths among 47 subjects with proband survival <5 y, from a total of 63 deaths among 187 subjects at risk.
12 deaths among 43 subjects with proband survival <5 y, from a total of 33 deaths among 186 subjects at risk.
To recognize the effect of family survival on subject overall mortality not specifically related to HF per se, the incidence ratio19 of the death of siblings without HF (non‐HF siblings) was calculated and used to adjust the model. All families with no non‐HF sibling alive on January 1, 1987, were excluded, leaving 69 deaths associated with 276 subjects with HF at risk, all of whom had at least 1 non‐HF sibling. The non‐HF sibling cohort was categorized by birth year (starting in 1932) into 5‐year strata, for which incidence of death was calculated as number of deaths occurring in the period 1987–2012 divided by the total number of years each non‐HF sibling contributed until time of death or end of follow‐up on December 31, 2012. The mean incidence for non‐HF siblings in each family was divided by the corresponding incidence by age stratum of the cohort, creating a family incidence ratio as a measure of family survival not pertaining to HF. In addition, besides the adjustment made for subject CHD in the main analysis, adjustments were also made for familial risk of CHD by introducing a covariate calculated as the number of siblings recorded with CHD during the specified period 1987–2012 or until time of death, divided by number of siblings alive on January 1, 1987. Overall mortality of subjects with HF proband survival time <5 years was approximately 2 times that of subjects with longer proband HF survival time and remained significant when these 2 covariates were added to the models (Table 10).
Table 10.
Overall Mortality After First Hospitalization for HF, as Determined by Survival Time of the First Affected Sibling (Proband), Related to CHD in Family and to Mortality of Siblings Without HF*
| Small Model, HR (95% CI)† | P Value | Fully Adjusted Model, HR (95% CI)‡ | P Value | |
|---|---|---|---|---|
| Regular Cox regression calculation | ||||
| Proband HF survival <5 y (yes/no)§ | 1.78 (1.09–2.89) | 0.021 | 1.79 (1.08–2.96) | 0.024 |
| Cox regression calculation with added adjustment in both models for the relative number of siblings with CHD and for the effect of mortality incidence ratio of non‐HF siblings | ||||
| Proband HF survival <5 y (yes/no)§ | 1.80 (1.08–3.02) | 0.025 | 1.91 (1.12–3.25) | 0.017 |
CHD indicates coronary heart disease; CI, confidence interval; HF, heart failure; HR, hazard ratio; VHD, valvular heart disease.
Only families with 2 cases of HF and with at least 1 sibling free of HF included. Families with a recorded case of congenital heart disease excluded.
Cox regression HRs, adjusted for subject age at onset and sex.
Cox regression HRs, adjusted for subject age at onset, sex, CHD, diabetes mellitus, hypertension, nonrheumatic VHD, and stroke.
27 deaths among 70 subjects with proband survival <5 y, from a total of 69 deaths among 276 subjects at risk.
Discussion
In subjects first hospitalized for HF, poor proband (ie, sibling) HF survival <5 years was associated with overall mortality and risk of cardiovascular death >2 times those of subjects with longer proband survival. These associations remained significant after excluding families with any recorded cardiomyopathy. Overall mortality decreased with every year of proband survival during the 5‐year observation period; this finding is congruent with an underlying familial association with HF survival. The HF overall mortality risk remained significantly associated with short proband HF survival when considering survival of non‐HF siblings, which suggests a familial association of HF survival per se. Such a familial, and perhaps hereditary, association may be related to either the severity of the condition triggering HF or, in turn, long‐term maladaptive factors influencing the progression of HF, in conjunction with the neurohormonal hypothesis.20, 21 The hypothesis of severity of comorbidities, however, is not suggested by the results of increased familial risk with both ischemic and nonischemic HF and with adjustment for potential etiologies such as diabetes mellitus and hypertension. In addition, the observed mortality risks were not ameliorated when we adjusted for familial aggregation of CHD. Risks were not predominantly elevated among younger subjects, possibly reflecting factors affecting progression and prognosis first when the processes of HF are triggered. However, fewer deaths occurred among younger subjects in general and may account for the results not reaching significant levels in this group. The overall results could also, at least in part, be reflected by a familial susceptibility to malignant arrhythmias triggered by HF progression.22
The nondichotomized phenotype with a continuous pattern of decreasing subject mortality risk with the length of proband survival is suggestive of a complex trait caused by multifactorial determinants, as in a polygenic model, potentially with environmental interactions.23
Strengths and Limitations
As a nationwide register study with almost complete coverage of hospital records,13 there was an inherently reduced risk of recall and selection bias that was further ameliorated by the structure of the Swedish healthcare system, a low‐cost all‐embracing system readily available to the entire population. Consequently, this study also represents the morbidity and mortality of female HF patients, who are often disproportionally studied in relation to prevalence.24 Furthermore, the diagnoses studied have been found to have validity of 90% to 95%.13, 14, 15 This study concerns prognostic effects of familial survival in HF—a strength from a clinical perspective, taking into account genetic, epigenetic, and environmental factors.
Limitations of this study design include the lack of genetic data and information about other variable parameters with the potential to influence the risk of HF and death, including left ventricular ejection fraction and precise reading of blood pressure. No comorbidities diagnosed only after first hospitalization for HF were recorded, allowing, for example, the presence of insidious CHD not yet diagnosed at first HF hospitalization to still contribute to the etiology of HF and mortality of HF. However, when adjusting for CHD in a family, as an additional proxy of familial risk of CHD, the results remained essentially unchanged. Patients may have been diagnosed with but not hospitalized for HF before being included in this study. However, with relatively evenly distributed prerequisites for health care and with HF being underdiagnosed in its earlier stages25 and being a progressive condition,21 survival measured from the first event requiring hospitalization for HF would be an important point of reference. Although follow‐up time was limited, there were no signs of risk decreasing with time elapsed from the date of hospitalization. The numbers of participants and failures were relatively few, thus limiting the number of adjusting variables.
Conclusions
In conclusion, these results suggest that family history of poor survival in specific relation to HF is an important risk factor for death in HF patients. Further genetic analyses of individuals in families with poor HF survival are needed to explore the molecular underpinnings of our findings. Careful monitoring may be considered for HF patients with a history of poor familial HF survival. Additional studies are needed to identify whether a specific phenotypic presentation is characterized by increased progression or susceptibility to arrhythmias to be able to offer optimized treatment.
Sources of Funding
This work was supported by grants from the Swedish Research Council and Avtal om Läkarutbildning och Forskning funding (Zöller, Sundquist, Smith, and Sundquist), by the Swedish Heart–Lung Foundation (Zöller, Smith), by the European Research Council (Smith) and by the Crafoord Foundation (Smith).
Disclosures
None.
Acknowledgments
The authors wish to thank science editor Patrick Reilly for his useful comments on the text.
(J Am Heart Assoc. 2018;7:e010181 DOI: 10.1161/JAHA.118.010181.)
References
- 1. Lee DS, Pencina MJ, Benjamin EJ, Wang TJ, Levy D, O'Donnell CJ, Nam BH, Larson MG, D'Agostino RB, Vasan RS. Association of parental heart failure with risk of heart failure in offspring. N Engl J Med. 2006;355:138–147. [DOI] [PubMed] [Google Scholar]
- 2. Lindgren MP, Smith JG, Li X, Sundquist J, Sundquist K, Zoller B. Sibling risk of hospitalization for heart failure—a nationwide study. Int J Cardiol. 2016;223:379–384. [DOI] [PubMed] [Google Scholar]
- 3. Lindgren MP, PirouziFard M, Smith JG, Sundquist J, Sundquist K, Zoller B. A swedish nationwide adoption study of the heritability of heart failure. JAMA Cardiol. 2018;3:703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Andersson B, Sylven C. The DD genotype of the angiotensin‐converting enzyme gene is associated with increased mortality in idiopathic heart failure. J Am Coll Cardiol. 1996;28:162–167. [DOI] [PubMed] [Google Scholar]
- 5. Palmer BR, Pilbrow AP, Yandle TG, Frampton CM, Richards AM, Nicholls MG, Cameron VA. Angiotensin‐converting enzyme gene polymorphism interacts with left ventricular ejection fraction and brain natriuretic peptide levels to predict mortality after myocardial infarction. J Am Coll Cardiol. 2003;41:729–736. [DOI] [PubMed] [Google Scholar]
- 6. McNamara DM, Holubkov R, Postava L, Janosko K, MacGowan GA, Mathier M, Murali S, Feldman AM, London B. Pharmacogenetic interactions between angiotensin‐converting enzyme inhibitor therapy and the angiotensin‐converting enzyme deletion polymorphism in patients with congestive heart failure. J Am Coll Cardiol. 2004;44:2019–2026. [DOI] [PubMed] [Google Scholar]
- 7. McNamara DM, Holubkov R, Janosko K, Palmer A, Wang JJ, MacGowan GA, Murali S, Rosenblum WD, London B, Feldman AM. Pharmacogenetic interactions between beta‐blocker therapy and the angiotensin‐converting enzyme deletion polymorphism in patients with congestive heart failure. Circulation. 2001;103:1644–1648. [DOI] [PubMed] [Google Scholar]
- 8. Andersson B, Blange I, Sylven C. Angiotensin‐II type 1 receptor gene polymorphism and long‐term survival in patients with idiopathic congestive heart failure. Eur J Heart Fail. 1999;1:363–369. [DOI] [PubMed] [Google Scholar]
- 9. Smith JG, Felix JF, Morrison AC, Kalogeropoulos A, Trompet S, Wilk JB, Gidlof O, Wang X, Morley M, Mendelson M, Joehanes R, Ligthart S, Shan X, Bis JC, Wang YA, Sjogren M, Ngwa J, Brandimarto J, Stott DJ, Aguilar D, Rice KM, Sesso HD, Demissie S, Buckley BM, Taylor KD, Ford I, Yao C, Liu C; consortium C‐S, EchoGen c, consortium Q‐I, consortium C‐Q , Sotoodehnia N, van der Harst P, Stricker BH, Kritchevsky SB, Liu Y, Gaziano JM, Hofman A, Moravec CS, Uitterlinden AG, Kellis M, van Meurs JB, Margulies KB, Dehghan A, Levy D, Olde B, Psaty BM, Cupples LA, Jukema JW, Djousse L, Franco OH, Boerwinkle E, Boyer LA, Newton‐Cheh C, Butler J, Vasan RS, Cappola TP, Smith NL. Discovery of genetic variation on chromosome 5q22 associated with mortality in heart failure. PLoS Genet. 2016;12:e1006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dorn GW III. The genomic architecture of sporadic heart failure. Circ Res. 2011;108:1270–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burton PR, Tobin MD, Hopper JL. Key concepts in genetic epidemiology. Lancet. 2005;366:941–951. [DOI] [PubMed] [Google Scholar]
- 12. Guttmacher AE, Collins FS, Carmona RH. The family history—more important than ever. N Engl J Med. 2004;351:2333–2336. [DOI] [PubMed] [Google Scholar]
- 13. Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, Heurgren M, Olausson PO. External review and validation of the Swedish National Inpatient Register. BMC Public Health. 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ingelsson E, Arnlov J, Sundstrom J, Lind L. The validity of a diagnosis of heart failure in a hospital discharge register. Eur J Heart Fail. 2005;7:787–791. [DOI] [PubMed] [Google Scholar]
- 15. Nilsson AC, Spetz CL, Carsjo K, Nightingale R, Smedby B. [Reliability of the hospital registry. The diagnostic data are better than their reputation]. Läkartidningen. 1994;91:598, 603–605. [PubMed] [Google Scholar]
- 16. Ekbom A. The Swedish multi‐generation register. Methods Mol Biol. 2011;675:215–220. [DOI] [PubMed] [Google Scholar]
- 17. de Faire U, Friberg L, Lorich U, Lundman T. A validation of cause‐of‐death certification in 1,156 deaths. Acta Med Scand. 1976;200:223–228. [DOI] [PubMed] [Google Scholar]
- 18. Johansson LA, Westerling R. Comparing Swedish hospital discharge records with death certificates: implications for mortality statistics. Int J Epidemiol. 2000;29:495–502. [PubMed] [Google Scholar]
- 19. Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 20. Packer M. The neurohormonal hypothesis: a theory to explain the mechanism of disease progression in heart failure. J Am Coll Cardiol. 1992;20:248–254. [DOI] [PubMed] [Google Scholar]
- 21. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; Authors/Task Force Members; Document Reviewers . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 22. Darbar D. Genomics, heart failure and sudden cardiac death. Heart Fail Rev. 2010;15:229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lander ES, Schork NJ. Genetic dissection of complex traits. Science. 1994;265:2037–2048. [DOI] [PubMed] [Google Scholar]
- 24. Pressler SJ. Women with heart failure are disproportionately studied as compared with prevalence: a review of published studies from 2013. J Cardiovasc Nurs. 2016;31:84–88. [DOI] [PubMed] [Google Scholar]
- 25. Davies M, Hobbs F, Davis R, Kenkre J, Roalfe AK, Hare R, Wosornu D, Lancashire RJ. Prevalence of left‐ventricular systolic dysfunction and heart failure in the echocardiographic heart of England screening study: a population based study. Lancet. 2001;358:439–444. [DOI] [PubMed] [Google Scholar]


