Abstract
Background
Spontaneous coronary artery dissection (SCAD) is a cause of acute coronary syndrome predominantly in women without usual cardiovascular risk factors. Many have a history of migraine headaches, but this association is poorly understood. This study aimed to determine migraine prevalence among SCAD patients and assess differences in clinical factors based on migraine history.
Methods and Results
A cohort study was conducted using the Mayo Clinic SCAD “Virtual” Multi‐Center Registry composed of patients with SCAD as confirmed on coronary angiography. Participant‐provided data and records were reviewed for migraine history, risk factors, SCAD details, therapies, and outcomes. Among 585 patients (96% women), 236 had migraine history; the lifetime and 1‐year prevalence of migraine were 40% and 26%, respectively. Migraine was more common in SCAD women than comparable literature‐reported female populations (42% versus 24%, P<0.0001; 42% versus 33%, P<0.0001). Among all SCAD patients, those with migraine history were more likely to be female (99.6% versus 94%; P=0.0002); have SCAD at a younger age (45.2±9.0 years versus 47.6±9.9 years; P=0.0027); have depression (27% versus 17%; P=0.025); have recurrent post‐SCAD chest pain at 1 month (50% versus 39%; P=0.035); and, among those assessed, have aneurysms, pseudoaneurysms, or dissections (28% versus 18%; P=0.018). There was no difference in recurrent SCAD at 5 years for those with versus without migraine (15% versus 19%; P=0.39).
Conclusions
Many SCAD patients have a history of migraine. SCAD patients with migraine are younger at the time of SCAD; have more aneurysms, pseudoaneurysms, and dissections among those imaged; and more often report a history of depression and post‐SCAD chest pain.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov. Unique identifiers: NCT01429727, NCT01427179.
Keywords: cardiovascular disease, dissection, migraine, myocardial infarction, women
Subject Categories: Myocardial Infarction, Women, Cardiovascular Disease
Clinical Perspective
What Is New?
Migraines are common among those who have had acute coronary syndrome due to spontaneous coronary artery dissection (SCAD), especially women.
Within our SCAD cohort, those with migraines were younger at the time of SCAD; more often reported depression and post‐SCAD chest pain at 1 month; and, among those imaged, had more arterial aneurysms, pseudoaneurysms, and dissections.
What Are the Clinical Implications?
All SCAD patients should be assessed for comorbid conditions such as migraines, depression, and anxiety, and undergo at least one‐time vascular imaging.
A history of migraines can guide medication decisions, as migraines may be exacerbated by nitrates and be improved with β blockade.
The association of SCAD, migraines, and vascular abnormalities such as fibromuscular dysplasia provides insight about possible SCAD pathophysiology, which likely represents a spectrum of arteriopathy.
Introduction
Spontaneous coronary artery dissection (SCAD) is an important cause of nonatherosclerotic myocardial infarction (MI) and sudden cardiac arrest, particularly among young and middle‐aged women.1 SCAD may account for as many as 4.0% of all acute coronary syndromes and 30% of acute coronary syndromes in women under 50 years of age.2, 3, 4 SCAD occurs due to an intramural hematoma±intimal/medial tear in a coronary artery, which can impair blood flow to the myocardium with consequent MI and/or sudden cardiac arrest.5 While the majority of SCAD occurs in a single coronary artery, it can also occur in several coronary arteries concurrently.6 It may also recur weeks to years following an initial SCAD, the risk factors for which are not well identified.2
The recent evolution of social media networking7 and diagnostic imaging techniques8 has accelerated research efforts and improved SCAD recognition and awareness. Although many SCAD patients do not have classic atherosclerotic risk factors, recent studies have associated SCAD with fibromuscular dysplasia (FMD), pregnancy, severe emotional/mental stress, extreme physical exertion, coronary tortuosity, and connective tissue disorders.5, 6, 9, 10, 11, 12 However, the precise etiology of SCAD, along with effective preventative measures and long‐term outcomes, remain unknown.
Migraine headaches have emerged as a condition found in SCAD patients.11, 13 SCAD cohort studies report migraine in 37% to 46% of patients,6, 8, 11, 13 as compared with a lifetime prevalence of migraine of 24% in US women with stable angina from the WISE (Women's Ischemia Syndrome Evaluation) cohort.14 In generally healthy populations, such as the GEM (Genetic Epidemiology of Migraine) cohort from the Netherlands, the lifetime prevalence of migraine is estimated at 25% to 33% in women and 8% to 13% for men.15, 16 Migraine headaches, based on patient self‐report or headache criteria, are associated with an increased risk of cardiovascular events, such as ischemic stroke and MI.17, 18, 19, 20 Migraine is also increased in patients with other vascular abnormalities, such as intracranial aneurysms, cervical or vertebral artery dissections, and FMD.21, 22, 23, 24 In FMD cohorts, over 30% have migraine.23 As migraine is frequently noted in these vascular conditions, it bears further investigation in SCAD. The primary objective of this study was to assess prevalence, presentation, clinical factors, and characteristics of patients with a history of both SCAD and migraine and compare them with patients who have had SCAD without a history of migraine.
Methods
This study was approved by the Mayo Clinic Institutional Review Board. The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. The ongoing Mayo Clinic SCAD “Virtual” Multi‐Center Registry, established in 2010, is an international registry of patients who experienced nonatherosclerotic SCAD as confirmed on review of coronary angiography images by 2 interventional cardiologists (RG, PB).7, 25 People with coronary artery atherosclerosis–related dissection or isolated iatrogenic dissection or who do not provide written informed consent are not included. Participants are recruited to the Mayo Clinic SCAD Registry by presenting with acute SCAD at a Mayo Clinic hospital during outpatient evaluation for SCAD in the dedicated Mayo Clinic SCAD Clinic, or by physician referral, social media networking, or patient word‐of‐mouth. Written informed consent, extensive questionnaires including a personal narrative of the SCAD event, medical records, and images are collected from participants and thoroughly reviewed. Patients are followed through clinic visits and/or intermittent questionnaires after registry enrollment. Of 718 patients enrolled from January 2010 to January 2017, 585 patients had complete questionnaires and records to be included in this study.
Patients with a history of migraine were identified (from self‐report on questionnaires, documentation in medical records, and/or diagnosis from provider visit) and lifetime prevalence of migraine was calculated. Patients with ≤1 lifetime “migraine” event were not considered to have a migraine history.26 Rare reports of “ocular” migraine were included (n=4). Demographic information, SCAD details, comorbidities, and treatments were compared between the 2 cohorts.
In addition to assessing lifetime migraine prevalence among all participants, we estimated the crude and age‐adjusted cohort 1‐year prevalence of active migraine, as is frequently reported in headache literature.15, 16, 27 These data were extracted from follow‐up questionnaires with specific questions about migraines (Figure 1) which were available for 231 participants in the study cohort. Those who did not have this additional data available (including patients not yet due for the follow‐up questionnaire and those who did not yet return a follow‐up questionnaire) were excluded from this portion of the analysis. Regarding frequency, most patients responded with a numerical response (eg, number of headaches per month), but nonspecific responses such as “rare” or “occasional” were assigned a rate of once per year (n=4). Responses were assumed to apply to the previous year to allow for age adjustment. Active migraine was defined as ≥1 migraine per year for the 1‐year prevalence estimate.28
Figure 1.

Excerpts from SCAD follow‐up questionnaire referencing migraines.
Another subset of participants had appropriate imaging available to assess for extracoronary vascular abnormalities (EVAs; n=335) defined as invasive angiography, computed tomography angiography, and/or magnetic resonance angiography of vascular beds from brain to pelvis,11, 29 82 of whom had partial imaging. EVAs were defined as FMD, aneurysms, pseudoaneurysms, or dissections. Prevalence of EVAs, including FMD, was compared between those with and without migraine history. Those who did not have images available were excluded. Iatrogenic dissections were excluded.
Migraine‐related medications at the time of enrollment in the SCAD Registry were also reviewed, including the following vitamins and supplements: magnesium, riboflavin (and B complexes), and coenzyme Q‐10.30 When available, migraine medication details immediately before and after the first SCAD hospitalization were collected retrospectively from contemporaneous medical records. Any available post‐SCAD neurology referrals for migraine, at either the Mayo Clinic or other institutions, were reviewed.
Statistical Analysis
The data were analyzed using Excel version 14.0 (Microsoft Corporation, Redmond, WA), JMP version 13.0.0 (SAS Institute Inc, Cary, NC), and the R Foundation for Statistical Computing (Vienna, Austria). Ordinal and nominal variables were compared using Pearson's chi‐squared test and continuous variables were compared using the Student's t test. Kaplan–Meier curves were generated using log‐rank analysis to compare SCAD recurrence at 5 years, the primary outcome of interest. Linear regression for continuous variables, logistic regression for categorical variables, and Cox proportional hazard analyses were used for multivariable analyses with 95% CIs. Variables included in the multivariable analyses were age and sex, as those were significantly different among those with and without migraines, and hypertension, which is associated with increased SCAD recurrence in 1 observational study.31 A post hoc, 2‐sided power analysis was conducted using an α of 0.05, power of 0.8, assumed SCAD recurrence of 0.2 based on published data,1 and hazard ratio of 2.31 A 1‐sample proportion test was used to compare the migraine prevalence values (both lifetime and 1‐year) in our cohort with literature reported values.14, 15, 16, 27 The observed 1‐year prevalence of migraine by age was compared with published 1‐year female prevalence values by comparing observed and expected values. An age‐adjusted standardized incidence ratio of migraines in SCAD patients was calculated by comparing observed migraines in SCAD patients to expected values of migraines in the general population using literature‐reported values.27 The standardized incidence ratio and 95% CIs were calculated using an exact Poisson test. Statistical significance was set at P<0.05.
Results
Patient Characteristics
The mean age of the total cohort (n=585) was 46.6±9.6; 96% of the patients were female and 94% were white. Although some patients had hypertension (n=215; 37%) and hyperlipidemia (n=207; 35%), the majority did not have traditional atherosclerotic risk factors (Table 1). Pregnancy and female hormone‐related factors are detailed in Table 2.
Table 1.
Baseline and Clinical Characteristics
| Total Cohort | No History of Migraine | History of Migraine | Unadjusted P Value | Adjusted P Value | |
|---|---|---|---|---|---|
| n=585 | n=349 (60%) | n=236 (40%) | |||
| Female | 564 (96) | 329 (94) | 235 (99.6) | 0.0007 | 0.0002 |
| Age, y | 46.6±9.6 | 47.6±9.9 | 45.2±9.0 | 0.0023 | 0.0027 |
| White | 549 (94) | 329 (94) | 220 (93) | 0.60 | 0.87 |
| Hypertension | 215 (37) | 127 (36) | 88 (37) | 0.83 | 0.57 |
| Diabetes mellitus | 22 (3.8) | 14 (4.0) | 8 (3.4) | 0.70 | 0.55 |
| Hyperlipidemia | 207 (35) | 130 (37) | 77 (33) | 0.25 | 0.45 |
| Hx of smoking | 160 (27) | 102 (29) | 58 (25) | 0.22 | 0.35 |
| Marfan or Ehlers‐Danlos | 15 (2.6) | 7 (2.0) | 8 (3.4) | 0.30 | 0.30 |
| History of dissection of other artery | 74 (13) | 39 (11) | 35 (15) | 0.19 | 0.30 |
| History of stroke/TIA | 18 (3.1) | 9 (2.6) | 9 (3.8) | 0.40 | 0.48 |
| Significant family hx of cardiovascular disorders | 79 (14) | 44 (13) | 35 (15) | 0.44 | 0.47 |
| Family hx of aneurysm | 121 (21) | 68 (19) | 53 (22) | 0.38 | 0.44 |
| Family hx head/neck aneurysm | 48 (8.2) | 29 (8.3) | 19 (8.0) | 0.91 | 0.84 |
| Family hx of dissection | 17 (2.9) | 10 (2.9) | 7 (3.0) | 0.94 | 0.66 |
| Family hx head/neck dissection | 4 (0.68) | 1 (0.29) | 3 (1.3) | 0.16 | 0.18 |
| Presentation and management | |||||
| Cardiac arrest | 64 (11) | 39 (11) | 25 (11) | 0.83 | 0.67 |
| PCI | 256 (44) | 153 (44) | 103 (44) | 0.96 | 0.72 |
| CABG | 58 (9.9) | 34 (9.7) | 24 (10) | 0.87 | 0.76 |
| Tortuous coronary vessels, n=504 | 426 (85) | 241 (83) | 185 (86) | 0.36 | 0.15 |
| Coronary territories | |||||
| Multivessel | 115 (20) | 73 (21) | 42 (18) | 0.35 | 0.14 |
| Left main | 42 (7.2) | 25 (7.2) | 17 (7.2) | 0.99 | 0.71 |
| Left anterior descending | 353 (60) | 209 (60) | 144 (61) | 0.78 | 0.81 |
| Diagonal | 20 (3.4) | 13 (3.7) | 7 (3.0) | 0.62 | 0.49 |
| Ramus | 18 (3.1) | 13 (3.7) | 5 (2.1) | 0.27 | 0.36 |
| Left circumflex | 91 (16) | 61 (17) | 30 (13) | 0.12 | 0.12 |
| Obtuse marginal | 88 (15) | 54 (15) | 34 (14) | 0.72 | 0.90 |
| Right coronary | 80 (14) | 46 (13) | 34 (14) | 0.67 | 0.65 |
| Right posterior descending | 44 (7.5) | 27 (7.7) | 17 (7.2) | 0.81 | 0.95 |
| Right posterolateral | 19 (3.2) | 12 (3.4) | 7 (3.0) | 0.75 | 0.37 |
| Outcomes | |||||
| Chest pain during month following SCAD | 252 (43) | 135 (39) | 117 (50) | 0.009 | 0.035 |
| Recurrent SCAD, KM* 5‐y estimate | 17% | 19% | 15% | 0.39 | |
Values presented as n (%) or mean±SD. CABG indicates coronary artery bypass graft; Hx, history; PCI, percutaneous coronary intervention; SCAD, spontaneous coronary artery dissection; TIA, transient ischemic attack.
Kaplan–Meier method analysis.
Table 2.
Pregnancy and Hormonal Factors in the Female Cohort
| Female Cohort | No History of Migraine | History of Migraine | P Value | |
|---|---|---|---|---|
| N=563* | N=329 (58%) | N=234 (42%) | ||
| Pregnancy‐associated SCAD | 74 (13) | 40 (12) | 34 (15) | 0.41 |
| Hx of gestational hypertension | 83 (15) | 45 (14) | 38 (16) | 0.40 |
| Hx of preeclampsia/eclampsia | 45 (8.0) | 26 (7.9) | 19 (8.1) | 0.93 |
| SCAD while menstruating | 62 (11) | 36 (11) | 26 (11) | 0.95 |
| SCAD on exogenous hormones† | 95 (17) | 51 (16) | 44 (19) | 0.30 |
| Postmenopausal SCAD, % | 206 (37) | 123 (37) | 83 (35) | 0.64 |
Values presented as n (%). Hx indicates history; SCAD, spontaneous coronary artery dissection.
One woman was excluded from this analysis because she did not know reproductive or hormonal details about her SCAD.
Includes hormonal birth control and postmenopausal hormonal therapy (including topical therapies).
Prevalence of Migraine Headaches
The lifetime prevalence of migraine in our SCAD cohort of 585 patients was 40% (female lifetime prevalence=42%). These patients were identified from self‐report on SCAD registry questionnaires (n=178, 75%) and review of records (n=58, 25%).
Of those with completed follow‐up questionnaires that included migraine frequency data, 60 of 231 had active migraine headaches (Figure 2), yielding a 1‐year prevalence of migraine among SCAD patients of 26% (27% among women). The age‐adjusted standardized incidence ratio for migraine in patients with SCAD compared with the literature‐reported female population migraine prevalence was 1.37 (95% CI, 1.05–1.76; P=0.019),27 indicating an estimated 37% higher age‐adjusted 1‐year migraine prevalence in SCAD patients (Table 3).
Figure 2.
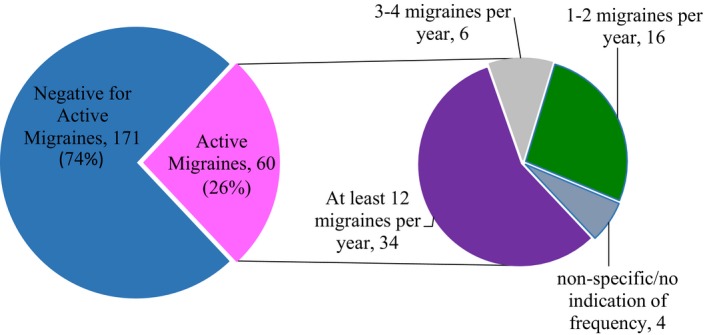
Figure diagramming the frequency of migraine headaches per year among patients with spontaneous coronary artery dissection (SCAD) and active migraine among 231 persons who responded to dedicated migraine survey questions.
Table 3.
Age‐Adjusted Standardized Incidence Ratio of Migraine Headaches in Patients With SCAD
| Age, in Years, by Decade | Total With SCAD, n | Total SCAD With Migraine, n | Expected % With Migraine From Literature27 | Expected Total in SCAD With Migraine, n | SIR | 95% CI | P Value |
|---|---|---|---|---|---|---|---|
| All | 231 | 60 | 44 | 1.37 | (1.05–1.76) | 0.019 | |
| 30 to 39* | 23 | 10 | 28.4 | 7 | |||
| 40 to 49 | 67 | 20 | 25.8 | 17 | |||
| 50 to 59 | 90 | 22 | 18.5 | 17 | |||
| 60+ | 51 | 8 | 6.5 | 3 |
Age‐adjusted standardized incident ratio of migraine in SCAD patients compared with published literature values by age.27 SCAD indicates spontaneous coronary artery dissection; SIR, standardized incidence ratio.
One patient was 23, and she was included in the 30 to 39 age cohort. She had migraine headaches.
Comparison of Migraine Prevalence Among SCAD Patients to Other Non‐SCAD Cohorts
We noted a higher prevalence of migraine among SCAD patients compared with the WISE cohort, some of whom had atherosclerotic disease and were of older age (40% in all/42% in females versus 24%; P<0.0001),14 and the GEM cohort from the Netherlands (42% versus 33%; P<0.0001).16 The 1‐year migraine prevalence among SCAD patients in our cohort was more frequent in comparison to the 1‐year migraine prevalence of 17.6% (P<0.001) among women from a US cohort,27 but similar in frequency to the prevalence among females in the GEM cohort16 (26% versus 25%, P=0.73). Demographic and other relevant data comparing these cohorts with our SCAD cohort are presented in Table 4. As compared with SCAD patients without migraine, SCAD patients with migraine were more likely to be female and less likely to be male in both unadjusted and adjusted analyses (0.4% versus 6%; P=0.0002). They were also younger at the time of dissection (45.2±9.0 years versus 47.6±9.9 years; P=0.0027), even when assessing the women only (P=0.0031) (Table 1, Figure 3).
Table 4.
Comparison With Literature‐Reported Cohorts Regarding Migraine Prevalence
| SCAD Cohort | WISE Cohort14 | GEM Cohort16 | AMMP Cohort27 | P Value | |
|---|---|---|---|---|---|
| Year | 2018 | 2006 | 1999 | 2013 | |
| Country | Primarily US | US | Netherlands | US | |
| Cohort size, n | 585 | 905 | 6491 | 162 756 | |
| White, % | 94 | 82 | 96* | 87 | |
| Female, % | 96 | 100 | 54 | 53 | |
| Mean age, y±SD | 46.6±9.6 | 58† | 39.8±0.15 | NR | 46.6 vs 39.8, P≤0.0001 |
| Age range, y | 20–73 | 12–79 | 20–65 | NR | |
| Major comorbidities | SCAD | 94% with chest pain, 4.5% CAD | None | None | |
| Primary migraine assessment method | Self‐report, general questionnaire | Self‐report, general questionnaire | Self‐report, headache specific questionnaires‡ | Self‐report, headache specific questionnaire | |
| Lifetime prevalence of migraine, % |
All: 40 Female: 42 |
All/Female: 24 | NE |
All: 40% vs 24%, Female: 42% vs 24%, P≤0.0001 |
|
|
All: 40 Female: 42 |
All: NR Female: 33 |
NE |
Female: 42% vs 33% P≤0.0001 |
||
| 1‐y prevalence of migraine, % |
All: 26 Female: 27 |
NE | Female: 25 |
All: 26% vs 25%, P=0.73 Female: 27% vs 25%, P=0.51 |
|
|
All: 12 Female: 17.3 |
All: 26% vs 12% P≤0.0001 All: 26% vs 17.3% P≤0.0005 Female: 27% vs 17.3% P≤0.0001 |
AAMP indicates American Migraine Prevalence and Prevention; CAD, coronary artery disease; F, female; GEM, Genetic Epidemiology of Migraine; NE, not evaluated; NR, not reported; SCAD, spontaneous coronary artery dissection; SD, standard deviation; WISE, Women's Ischemia Syndrome Evaluation.
Ethnically Dutch population percent of the Netherlands in 1999 (available at https://opendata.cbs.nl/statline/#/CBS/en/dataset/03743eng/table?ts=15248 48061950; accessed on April 27, 2018) as the authors stated “the overwhelming majority of Netherlanders are white.”16
Standard deviation was not reported.
Participants were first evaluated with a mailed brief headache screen (stage 1), positive screens completed a more comprehensive migraine questionnaire (stage 2), then a random subset of screen positives from stage 2 was clinically interviewed (stage 3).16
Figure 3.
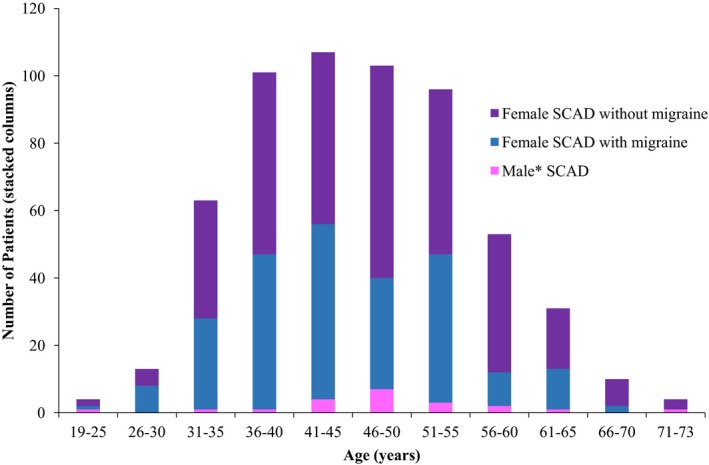
Distribution of patients based on age, sex, and migraine status. Spontaneous coronary artery dissection (SCAD) predominantly occurs from 30 to 60 years of age, with SCAD in those with migraine history tending to occur at a younger age compared with patients with SCAD but no migraine headache history. *One man had SCAD and history of migraine; he was age 50 at time of SCAD.
Among the subset of patients screened for EVAs (n=335), 58% had FMD (n=195), 23% had additional non‐FMD EVAs (aneurysms, pseudoaneurysms, and dissections; n=76), and 65% had any EVAs (n=219) (Table 5). Non‐FMD EVAs were more common among SCAD patients with migraine compared with those without migraine in the unadjusted analysis (28% versus 18%; P=0.025) (Figure 4); this difference remained after adjusting for age, sex, and history of hypertension (P=0.018). FMD or any EVA were not significantly different between the 2 groups on unadjusted analysis; however, any EVA was statistically significant when adjusting for age, sex, and history of hypertension (P=0.035).
Table 5.
Results in Cohort Screened for Extracoronary Vascular Abnormalities
| Total Screened Cohort | No History of Migraine | History of Migraine* | Unadjusted P Value | Adjusted P Value | |
|---|---|---|---|---|---|
| n=335 | n=170 (51) | n=165 (49%) | |||
| Any EVA† | 219 (65) | 104 (61) | 115 (70) | 0.10 | 0.035‡ |
| Any FMD | 195 (58) | 96 (56) | 99 (60) | 0.51 | 0.32 |
| Body FMD§ | 161 (52) | 80 (52) | 81 (52) | 0.91 | 0.65 |
| H/N FMDǁ | 77 (28) | 32 (23) | 45 (32) | 0.10 | 0.08 |
| Non‐FMD EVA | 76 (23) | 30 (18) | 46 (28) | 0.025 | 0.018 |
| H/N non‐FMD EVA | 40 (14) | 17 (12) | 23 (16) | 0.33 | 0.34 |
| H/N aneurysms/pseudoaneurysms | 30 (11) | 12 (8.7) | 18 (13) | 0.26 | 0.35 |
| H/N dissections | 18 (6.5) | 10 (7.3) | 8 (5.7) | 0.60 | 0.77 |
| Body non‐FMD EVA | 40ǁ (13) | 17 (11) | 23 (15) | 0.31 | 0.24 |
| Body aneurysms/pseudoaneurysms | 25 (8.1) | 12 (7.7) | 13 (8.4) | 0.83 | 0.82 |
| Body dissections | 20 (6.5) | 8 (5.2) | 12 (7.7) | 0.36 | 0.21 |
Values presented as n (%). EVA indicates extracoronary vascular abnormalities; FMD, fibromuscular dysplasia; H/N, head and/or neck.
Proportionally more of those with migraine history underwent vascular screening compared with those without migraine history (70% [55% full/15% partial] vs 48% [35% full/13% partial], respectively; P=0.02 for both any imaging and full imaging).
Extracoronary vascular abnormalities including aneurysm, pseudoaneurysm, fibromuscular dysplasia, or dissection on imaging.
The other covariates may be driving the relationship between any EVA and migraine on the multivariable analysis.
n=310 screened.
n=278 screened; four patients had both H/N non‐FMD EVA and body non‐FMD EVA, and were included in each group.
Figure 4.
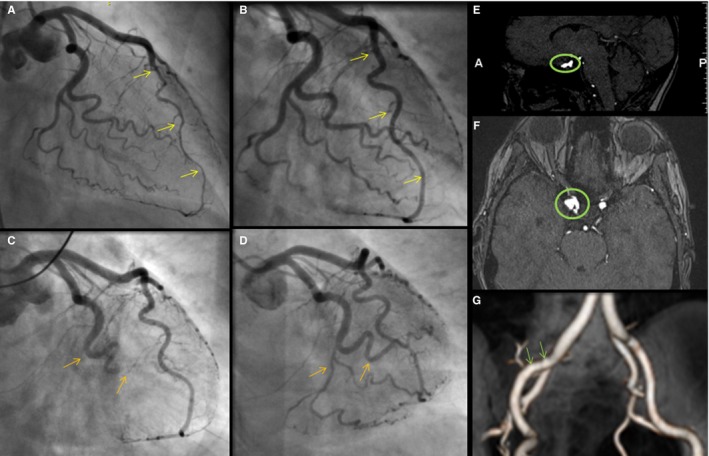
Imaging of vascular abnormalities and recurrent SCAD in a patient with migraine. This 55‐year‐old female's initial spontaneous coronary artery dissection (SCAD) caused an intramural hematoma of the left anterior descending coronary artery (A, arrows); follow‐up coronary angiography demonstrated interval healing (B, arrows). Several years later, she presented with SCAD of the left circumflex with occlusion of the first obtuse marginal, distal circumflex and its branches (C, arrows). Despite an unsuccessful percutaneous intervention attempt, follow‐up coronary angiography showed interval healing (D, arrows). She also was found to have a 7‐mm right periophthalmic cavernous carotid aneurysm (E and F), 3‐mm left cavernous internal carotid artery aneurysm, 2‐ to 3‐mm right cavernous internal carotid aneurysm, and mild fibromuscular dysplasia of the right external iliac artery (G).
History of depression (27% versus 17%; P=0.0053) and occurrence of recurrent chest pain during the month following SCAD (50% versus 39%; P=0.009) were higher among SCAD patients with migraine history compared with those without migraine history, respectively, and these results remained significant after adjusting for age, sex, and hypertension (P=0.025 and 0.035, respectively) (Tables 1 and 6). History of anxiety and concern for recurrent SCAD were significantly higher among those with migraines on unadjusted analysis (32% versus 24%, P=0.040; and 46% versus 37%, P=0.031, respectively), but these associations did not remain significant when adjusting for age, sex, and hypertension. By Kaplan–Meier methods, the combined 5‐year SCAD recurrence incidence was 17.2%. There was no statistically significant difference in 5‐year recurrent SCAD incidence (15% versus 19%; P=0.39) between those with migraine and those without migraine (Figure 5) even when adjusting for sex, age, and history of hypertension (risk ratio, 0.74; 95% CI, 0.44–1.23). Post hoc power analysis assuming a recurrence of 20% and hazard ratio of 2 determined 69 required events for 80% power, which was less than the 88 recurrent SCAD events observed in the cohort.
Table 6.
Mood and Psychological Characteristics
| Total Cohort | No History of Migraine | History of Migraine | Unadjusted P Value | Adjusted P Value | |
|---|---|---|---|---|---|
| n=585 | n=349 (60%) | n=236 (40%) | |||
| History of depression | 125 (21) | 61 (17) | 64 (27) | 0.0053 | 0.025 |
| History of anxiety | 159 (27) | 84 (24) | 75 (32) | 0.040 | 0.14 |
| Effective at stress management | 409 (70) | 250 (72) | 159 (67) | 0.27 | 0.33 |
| Stress enough to affect health | 239 (41) | 134 (38) | 105 (44) | 0.14 | 0.18 |
| Stress enough to affect QOL | 204 (35) | 112 (32) | 92 (39) | 0.086 | 0.15 |
| Concern for recurrent SCAD | 239 (41) | 130 (37) | 109 (46) | 0.031 | 0.17 |
| Concern for sudden cardiac death | 165 (28) | 94 (27) | 71 (30) | 0.41 | 0.72 |
Values presented as n (%). QOL indicates quality of life; SCAD, spontaneous coronary artery dissection.
Figure 5.
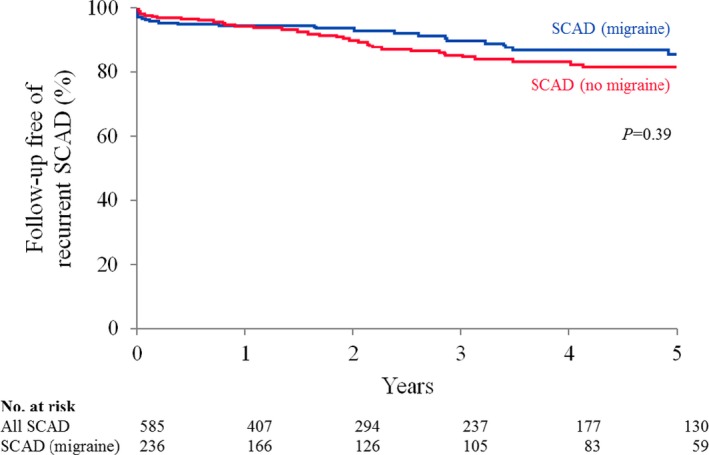
5‐year incidence of SCAD recurrence among patients with and without migraine history. No statistically significant difference was found in the Kaplan–Meier survival curve for SCAD patients with migraine (blue line) and that of SCAD patients without migraine (red line) (P=0.39).
Migraine Management
A total of 161 patients with migraine history (68% of the cohort with SCAD and migraine) had comprehensive information available on migraine medications immediately before and after SCAD. Of these, 33 (20%) were taking triptans before SCAD. After SCAD MI hospitalization, triptans were discontinued in 39% of patients (n=13).
The most common migraine‐related medications taken by patients with SCAD and migraine history at time of enrollment in the SCAD Registry were β‐blockers (n=140; 59%); <1% were on vasoconstrictors. At the time of enrollment in the SCAD registry, a majority were on aspirin therapy (n=210; 89%), most frequently dosed at 81 mg/day. Incidentally, among migraineurs, headache‐related concern about nitrate medications was sometimes documented or reported by patients (n=29) and some migraineurs (n=16) commented on subjective improvement in headaches following SCAD.
Neurology referral information was available on 44 patients with SCAD and migraine; of these, 23 patients were seen at 28 visits for headache management. The other 21 patients were referred for other complaints, such as transient ischemic attack, head and/or neck aneurysms or dissections, or dizziness. Recommendations to avoid vasoconstrictors, such as triptans, were made in 10 of 28 visits (36%). Gabapentin was most often recommended for migraine prophylaxis (n=9; 32%). Acute management medications most often suggested were nonsteroidal anti‐inflammatory drugs (n=7; 25%) and antiemetics (n=7; 25%).
Discussion
The primary findings in our study are as follows: (1) both the lifetime prevalence and 1‐year prevalence of migraine were higher in this cohort of patients with SCAD as compared with literature‐reported prevalence values14, 16, 27; (2) among those assessed, patients with SCAD and migraine more commonly have non‐FMD EVA compared with patients without migraine history; (3) migraine history does not appear to incur greater risk of recurrent SCAD at 5 years; (4) when compared with those without migraine history, SCAD patients with migraine history are more often female, younger at the time of SCAD, and more commonly have recurrent chest pain at 1 month following SCAD and history of depression.
We report the first, to our knowledge, large study to investigate migraine in those with SCAD. Smaller SCAD cohorts have previously noted migraine headaches in 37% to 46% of patients.6, 11, 13 However, these cohorts do not distinguish lifetime or 1‐year prevalence of migraine or compare SCAD patients with migraine to SCAD patients without migraine. The Australian SCAD cohort does not specify 1‐year versus lifetime prevalence and reports a migraine prevalence of 43% among 40 patients with SCAD,13 which is similar to the lifetime migraine prevalence in this large and comprehensive SCAD cohort of 585 patients.
As migraine represents an important comorbid condition among SCAD patients, consideration of the link between migraine and other vascular conditions may yield insights into SCAD pathophysiology. Migraine headaches are associated with vascular phenomena such as aneurysms, retinal vasculopathy, reversible cerebral vasoconstriction, cervical and vertebral artery dissections, myocardial infarction, and stroke.17, 18, 19, 20, 21, 22, 24, 28, 32, 33, 34 Systemic vascular changes in migraineurs include increased aortic stiffness indicating large‐artery dysfunction35 and increased activity of extracellular proteins such as elastase and matrix metalloproteinases.36, 37 Some studies suggest that endothelial dysfunction may contribute to conditions such as stroke and cervical artery dissection in migraineurs.36, 38, 39, 40 Supporting this association, a recent migraine genome study identified multiple loci related to vascular and endothelial genes.41 Cervical artery dissection genomic studies have also identified loci that overlap with ones associated with migraine, such as PHACTR1 (also associated with FMD) and LRP1, both of which code for vascular function–related proteins.13, 41, 42
In the context of SCAD and migraine, these considerations are particularly intriguing given the finding that non‐FMD EVAs (and all EVAs on adjusted analyses) were more common in SCAD patients with migraine. Upon stratification based on location or type of EVA, statistically significant differences were lost, likely due to insufficient sample sizes. Nevertheless, this strengthens the supposition that migraine, SCAD, and arteriopathies including EVAs and FMD, are indicative of an underlying systemic propensity to vascular injury and/or dysfunction, and in some may include genetic predispositions.13, 23, 36, 38, 43
Whatever the baseline characteristics of the vasculature, it stands to reason that other factors must also be involved. One potential factor may be hormonal changes in women.18 Migraine headaches are associated with the menstrual cycle in over 50% of women.44, 45 A peak in frequency of migraine without aura has been correlated with the fall in estrogen (and progesterone) that triggers menstruation.44, 46, 47 Hormonal decline has also been suggested as a possible inciting trigger for menstrual‐related post‐SCAD chest pain48 and pregnancy‐associated SCAD, which occurs most frequently during the first weeks postpartum.5, 12
At present, although exogenous hormones can be safely used to control some types of migraine,49, 50 they are often discouraged post‐SCAD due to uncertainty about risk. As estrogen is known to have multiple vascular effects,51 further research into hormonal influences in migraine and SCAD is critical to identify shared mechanisms of disease and clarify the potential harms and benefits of hormonal substances in both conditions.49
Interestingly, we found an increased incidence of recurrent chest pain among those with SCAD and migraine compared with other SCAD patients (although overall both groups had frequent chest pain following SCAD). This may be related to increased global sensitivity to pain, which has been noted among migraineurs.52, 53 Endothelial dysfunction, coronary vasospasm, and microvascular disease can cause nonatherosclerotic chest pain,54 but the role of these processes in SCAD is poorly understood. Even though post‐SCAD chest pain often responds to antianginal medication, such as nitrates,5 the use of nitrates may exacerbate headaches. Such limitation in therapeutic options could contribute to more prevalent recurrent chest pain among patients with SCAD and migraine.
Depression, known to be common among those who have experienced SCAD,55 was more frequent among SCAD patients with migraine. Migraine is associated with an increased prevalence of mood disorders,56 and the coexistence of depression with migraine is associated with greater migraine‐related disability.57 Both anxiety and depression are known to increase cardiovascular disease risk.58 Our findings stress the importance of identification and appropriate management of mood disorders in patients with SCAD, especially migraineurs, in an effort to reduce the risk of future disability and harm.
In patients with migraine, triptans were infrequently discontinued at time of SCAD. Although only case reports have linked SCAD to vasoconstricting migraine abortives,59 the use of such medications in those with cardiovascular disease is generally contraindicated.60, 61, 62, 63 Providers who care for patients with SCAD and migraine should review migraine medications and consider discontinuing triptans. Subsequent referral to neurology may be necessary, as many migraineurs rely on these medications for acute headache relief. Of note, migraine headaches have been noted to improve after SCAD, possibly related to medications frequently added following SCAD such as β‐blockers, which also have migraine prophylactic effects.61 A similar phenomenon has been noted following cervical artery dissection.64
There are no established guidelines for managing migraine in patients with a history of cardiovascular disease. Generally speaking, risk factors such as smoking should be addressed, β‐blockers or angiotensin receptor blockers should be used as indicated following MI,34, 61 and vasoconstricting abortives should be avoided.60, 61, 62, 63 Figure 6 includes our brief migraine management recommendations.
Figure 6.
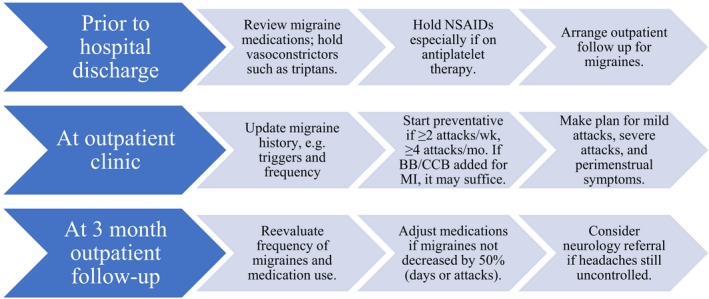
Recommendations for management of migraine post‐spontaneous coronary artery dissection (SCAD). This general approach, based on this study's observations, the Mayo Clinic SCAD Clinic experiences, and recommendations from neurology literature, is not meant to be comprehensive and individualization of treatment is required.60, 61, 62, 63 BB indicates β‐blocker; CCB, calcium‐channel blocker; NSAIDs, nonsteroidal anti‐inflammatory drugs.
Limitations
Limitations of this study include selection and recall bias due to use of a registry and the cohort study design. Regardless, this is a substantial cohort of SCAD patients encompassing a large geographic representation, which includes information that would not otherwise be known in this population.
Another potential limitation is that migraines in this study were defined both subjectively as self‐report on surveys and objectively as recorded in the medical record; this variation may lead to an inaccurate overall prevalence of migraines. However, self‐report of migraines commonly occurs in the medical literature,14, 23, 28, 65 and less than half of patients meeting criteria for migraine obtain a medical diagnosis.66, 67 Patient self‐report of migraine and International Classification of Headache Disorders II headache criteria have been shown to have fairly good agreement of 72%.65 Therefore, we used both subjective and objective approaches to migraine diagnosis in an attempt to capture the most complete compilation of migraineurs.
Aura status is associated with worse outcomes among those with migraines68, 69 but was not consistently collected in this cohort. Even though this is a common limitation among published migraine studies,14, 17, 70 we intend to incorporate aura‐related data and additional composite outcomes of interest into future studies.
Only a subset of patients was imaged for systemic vascular abnormalities, and some patients had incomplete imaging limiting the data available for comparison among the 2 groups. A greater proportion of those with migraine underwent vascular imaging, which was likely clinically driven, although comprehensive vascular imaging is recommended for all patients following SCAD.1, 5 Patients diagnosed with FMD should be cared for clinically according to current recommendations.70
Conclusions
Migraine headaches are frequent in SCAD, and this observation raises the question of a possible underlying vascular propensity to injury, potentially modified by hormonal or genetic factors, as other vascular studies have hypothesized. Migraineurs with SCAD are more likely to report a history of depression and recurrent chest pain at 1 month but do not appear to be at higher risk for recurrent SCAD at 5 years. As SCAD research continues, further investigation into this newly characterized association between migraine and SCAD may significantly impact our understanding of mechanisms of disease and clinical management decisions, particularly related to systemic vascular abnormalities, recurrent angina, mood disorders, and migraine medications.
Sources of Funding
This project was funded by the Department of Cardiovascular Diseases, Mayo Clinic, Rochester, Minnesota; and SCAD Research, Inc., Scottsdale, Arizona. Dr Tweet is supported by the Office of Research on Women's Health (NIH HD65987). This publication was made possible by CTSA Grant Number UL1 TR002377 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of National Institutes of Health. No funding sources had any role in the study's design, conduct, or reporting.
Disclosures
None.
Acknowledgments
The authors sincerely thank the Mayo SCAD Registry participants who volunteered their data for this study, Jill Boyum for her assistance with the Mayo Clinic SCAD Registry, and Megan O'Byrne for her statistical assistance.
(J Am Heart Assoc. 2018;7:e010140 DOI: 10.1161/JAHA.118.010140)
References
- 1. Hayes SN, Kim ESH, Saw J, Adlam D, Arslanian‐Engoren C, Economy KE, Ganesh SK, Gulati R, Lindsay ME, Mieres JH, Naderi S, Shah S, Thaler DE, Tweet MS, Wood MJ. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American Heart Association. Circulation. 2018;137:e523–e557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nakashima T, Noguchi T, Haruta S, Yamamoto Y, Oshima S, Nakao K, Taniguchi Y, Yamaguchi J, Tsuchihashi K, Seki A, Kawasaki T, Uchida T, Omura N, Kikuchi M, Kimura K, Ogawa H, Miyazaki S, Yasuda S. Prognostic impact of spontaneous coronary artery dissection in young female patients with acute myocardial infarction: a report from the angina pectoris‐myocardial infarction multicenter investigators in Japan. Int J Cardiol. 2016;207:341–348. [DOI] [PubMed] [Google Scholar]
- 3. Nishiguchi T, Tanaka A, Ozaki Y, Taruya A, Fukuda S, Taguchi H, Iwaguro T, Ueno S, Okumoto Y, Akasaka T. Prevalence of spontaneous coronary artery dissection in patients with acute coronary syndrome. Eur Heart J Acute Cardiovasc Care. 2016;5:263–270. [DOI] [PubMed] [Google Scholar]
- 4. Saw J, Aymong E, Mancini GB, Sedlak T, Starovoytov A, Ricci D. Nonatherosclerotic coronary artery disease in young women. Can J Cardiol. 2014;30:814–819. [DOI] [PubMed] [Google Scholar]
- 5. Tweet MS, Gulati R, Hayes SN. Spontaneous coronary artery dissection. Curr Cardiol Rep. 2016;18:60. [DOI] [PubMed] [Google Scholar]
- 6. Saw J, Aymong E, Sedlak T, Buller CE, Starovoytov A, Ricci D, Robinson S, Vuurmans T, Gao M, Humphries K, Mancini GB. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv. 2014;7:645–655. [DOI] [PubMed] [Google Scholar]
- 7. Tweet MS, Gulati R, Aase LA, Hayes SN. Spontaneous coronary artery dissection: a disease‐specific, social networking community‐initiated study. Mayo Clin Proc. 2011;86:845–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tweet MS, Gulati R, Williamson EE, Vrtiska TJ, Hayes SN. Multimodality imaging for spontaneous coronary artery dissection in women. JACC Cardiovasc Imaging. 2016;9:436–450. [DOI] [PubMed] [Google Scholar]
- 9. Eleid MF, Guddeti RR, Tweet MS, Lerman A, Singh M, Best PJ, Vrtiska TJ, Prasad M, Rihal CS, Hayes SN, Gulati R. Coronary artery tortuosity in spontaneous coronary artery dissection: angiographic characteristics and clinical implications. Circ Cardiovasc Interv. 2014;7:656–662. [DOI] [PubMed] [Google Scholar]
- 10. Henkin S, Negrotto SM, Tweet MS, Kirmani S, Deyle DR, Gulati R, Olson TM, Hayes SN. Spontaneous coronary artery dissection and its association with heritable connective tissue disorders. Heart. 2016;102:876–881. [DOI] [PubMed] [Google Scholar]
- 11. Prasad M, Tweet MS, Hayes SN, Leng S, Liang JJ, Eleid MF, Gulati R, Vrtiska TJ. Prevalence of extracoronary vascular abnormalities and fibromuscular dysplasia in patients with spontaneous coronary artery dissection. Am J Cardiol. 2015;115:1672–1677. [DOI] [PubMed] [Google Scholar]
- 12. Tweet MS, Hayes SN, Codsi E, Gulati R, Rose CH, Best PJM. Spontaneous coronary artery dissection associated with pregnancy. J Am Coll Cardiol. 2017;70:426–435. [DOI] [PubMed] [Google Scholar]
- 13. McGrath‐Cadell L, McKenzie P, Emmanuel S, Muller DW, Graham RM, Holloway CJ. Outcomes of patients with spontaneous coronary artery dissection. Open Heart. 2016;3:e000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ahmed B, Bairey Merz CN, McClure C, Johnson BD, Reis SE, Bittner V, Pepine CJ, Sharaf BL, Sopko G, Kelsey SF, Shaw L. Migraines, angiographic coronary artery disease and cardiovascular outcomes in women. Am J Med. 2006;119:670–675. [DOI] [PubMed] [Google Scholar]
- 15. Bigal ME, Lipton RB, Stewart WF. The epidemiology and impact of migraine. Curr Neurol Neurosci Rep. 2004;4:98–104. [DOI] [PubMed] [Google Scholar]
- 16. Launer LJ, Terwindt GM, Ferrari MD. The prevalence and characteristics of migraine in a population‐based cohort: the GEM study. Neurology. 1999;53:537–542. [DOI] [PubMed] [Google Scholar]
- 17. Kurth T, Winter AC, Eliassen AH, Dushkes R, Mukamal KJ, Rimm EB, Willett WC, Manson JE, Rexrode KM. Migraine and risk of cardiovascular disease in women: prospective cohort study. BMJ. 2016;353:i2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Linstra KM, Ibrahimi K, Terwindt GM, Wermer MJ, MaassenVanDenBrink A. Migraine and cardiovascular disease in women. Maturitas. 2017;97:28–31. [DOI] [PubMed] [Google Scholar]
- 19. Rambarat CA, Elgendy IY, Johnson BD, Reis SE, Thompson DV, Sharaf BL, Bittner V, Sopko G, Bairey Merz CN, Pepine CJ, Ahmed B. Migraine headache and long‐term cardiovascular outcomes: an extended follow‐up of the women's ischemia syndrome evaluation. Am J Med. 2017;130:738–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sacco S, Ornello R, Ripa P, Tiseo C, Degan D, Pistoia F, Carolei A. Migraine and risk of ischaemic heart disease: a systematic review and meta‐analysis of observational studies. Eur J Neurol. 2015;22:1001–1011. [DOI] [PubMed] [Google Scholar]
- 21. De Giuli V, Grassi M, Lodigiani C, Patella R, Zedde M, Gandolfo C, Zini A, DeLodovici ML, Paciaroni M, Del Sette M, Azzini C, Toriello A, Musolino R, Calabro RS, Bovi P, Sessa M, Adami A, Silvestrelli G, Cavallini A, Marcheselli S, Bonifati DM, Checcarelli N, Tancredi L, Chiti A, Lotti EM, Del Zotto E, Tomelleri G, Spalloni A, Giorli E, Costa P, Poli L, Morotti A, Caria F, Lanari A, Giacalone G, Ferrazzi P, Giossi A, Piras V, Massucco D, D'Amore C, Di Lisi F, Casetta I, Cucurachi L, Cotroneo M, De Vito A, Coloberti E, Rasura M, Simone AM, Gamba M, Cerrato P, Micieli G, Malferrari G, Melis M, Iacoviello L, Padovani A, Pezzini A. Association between migraine and cervical artery dissection: the Italian project on stroke in young adults. JAMA Neurol. 2017;74:512–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lebedeva ER, Gurary NM, Sakovich VP, Olesen J. Migraine before rupture of intracranial aneurysms. J Headache Pain. 2013;14:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Olin JW, Froehlich J, Gu X, Bacharach JM, Eagle K, Gray BH, Jaff MR, Kim ES, Mace P, Matsumoto AH, McBane RD, Kline‐Rogers E, White CJ, Gornik HL. The United States registry for fibromuscular dysplasia: results in the first 447 patients. Circulation. 2012;125:3182–3190. [DOI] [PubMed] [Google Scholar]
- 24. Zhou M, Zheng H, Gong S, Guo J, Chen N, Zhou D, Yang R, Zhu C, He L. Vertebral artery hypoplasia and vertebral artery dissection: a hospital‐based cohort study. Neurology. 2015;84:818–824. [DOI] [PubMed] [Google Scholar]
- 25. Goel K, Tweet M, Olson TM, Maleszewski JJ, Gulati R, Hayes SN. Familial spontaneous coronary artery dissection: evidence for genetic susceptibility. JAMA Intern Med. 2015;175:821–826. [DOI] [PubMed] [Google Scholar]
- 26. Headache Classification Committee of the International Headache Society (IHS) . The International Classification of Headache Disorders 3rd edition (beta version). Cephalalgia. 2013;33:629–808. [DOI] [PubMed] [Google Scholar]
- 27. Buse DC, Loder EW, Gorman JA, Stewart WF, Reed ML, Fanning KM, Serrano D, Lipton RB. Sex differences in the prevalence, symptoms, and associated features of migraine, probable migraine and other severe headache: results of the American Migraine Prevalence and Prevention (AMPP) Study. Headache. 2013;53:1278–1299. [DOI] [PubMed] [Google Scholar]
- 28. Kurth T, Gaziano JM, Cook NR, Logroscino G, Diener HC, Buring JE. Migraine and risk of cardiovascular disease in women. JAMA. 2006;296:283–291. [DOI] [PubMed] [Google Scholar]
- 29. Liang JJ, Prasad M, Tweet MS, Hayes SN, Gulati R, Breen JF, Leng S, Vrtiska TJ. A novel application of CT angiography to detect extracoronary vascular abnormalities in patients with spontaneous coronary artery dissection. J Cardiovasc Comput Tomogr. 2014;8:189–197. [DOI] [PubMed] [Google Scholar]
- 30. Holland S, Silberstein SD, Freitag F, Dodick DW, Argoff C, Ashman E. Evidence‐based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1346–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Saw J, Humphries K, Aymong E, Sedlak T, Prakash R, Starovoytov A, Mancini GBJ. Spontaneous coronary artery dissection: clinical outcomes and risk of recurrence. J Am Coll Cardiol. 2017;70:1148–1158. [DOI] [PubMed] [Google Scholar]
- 32. Mawet J, Debette S, Bousser MG, Ducros A. The link between migraine, reversible cerebral vasoconstriction syndrome and cervical artery dissection. Headache. 2016;56:645–656. [DOI] [PubMed] [Google Scholar]
- 33. Rose KM, Wong TY, Carson AP, Couper DJ, Klein R, Sharrett AR. Migraine and retinal microvascular abnormalities: the atherosclerosis risk in communities study. Neurology. 2007;68:1694–1700. [DOI] [PubMed] [Google Scholar]
- 34. Sacco S, Ricci S, Carolei A. Migraine and vascular diseases: a review of the evidence and potential implications for management. Cephalalgia. 2012;32:785–795. [DOI] [PubMed] [Google Scholar]
- 35. Schillaci G, Sarchielli P, Corbelli I, Pucci G, Settimi L, Mannarino MR, Calabresi P, Mannarino E. Aortic stiffness and pulse wave reflection in young subjects with migraine: a case‐control study. Neurology. 2010;75:960–966. [DOI] [PubMed] [Google Scholar]
- 36. Mawet J, Kurth T, Ayata C. Migraine and stroke: in search of shared mechanisms. Cephalalgia. 2015;35:165–181. [DOI] [PubMed] [Google Scholar]
- 37. Tzourio C, El Amrani M, Robert L, Alperovitch A. Serum elastase activity is elevated in migraine. Ann Neurol. 2000;47:648–651. [PubMed] [Google Scholar]
- 38. Metso TM, Tatlisumak T, Debette S, Dallongeville J, Engelter ST, Lyrer PA, Thijs V, Bersano A, Abboud S, Leys D, Grond‐Ginsbach C, Kloss M, Touze E, Pezzini A, Metso AJ. Migraine in cervical artery dissection and ischemic stroke patients. Neurology. 2012;78:1221–1228. [DOI] [PubMed] [Google Scholar]
- 39. Tietjen GE. Migraine as a systemic vasculopathy. Cephalalgia. 2009;29:987–996. [DOI] [PubMed] [Google Scholar]
- 40. González‐Quintanilla V, Toriello M, Palacio E, González‐Gay MA, Castillo J, Montes S, Martínez‐Nieto R, Fernandez J, Rojo A, Gutiérrez S, Pons E, Oterino A. Systemic and cerebral endothelial dysfunction in chronic migraine. A case‐control study with an active comparator. Cephalalgia. 2016;36:552–560. [DOI] [PubMed] [Google Scholar]
- 41. Gormley P, Anttila V, Winsvold BS, Palta P, Esko T, Pers TH, Farh KH, Cuenca‐Leon E, Muona M, Furlotte NA, Kurth T, Ingason A, McMahon G, Ligthart L, Terwindt GM, Kallela M, Freilinger TM, Ran C, Gordon SG, Stam AH, Steinberg S, Borck G, Koiranen M, Quaye L, Adams HH, Lehtimaki T, Sarin AP, Wedenoja J, Hinds DA, Buring JE, Schurks M, Ridker PM, Hrafnsdottir MG, Stefansson H, Ring SM, Hottenga JJ, Penninx BW, Farkkila M, Artto V, Kaunisto M, Vepsalainen S, Malik R, Heath AC, Madden PA, Martin NG, Montgomery GW, Kurki MI, Kals M, Magi R, Parn K, Hamalainen E, Huang H, Byrnes AE, Franke L, Huang J, Stergiakouli E, Lee PH, Sandor C, Webber C, Cader Z, Muller‐Myhsok B, Schreiber S, Meitinger T, Eriksson JG, Salomaa V, Heikkila K, Loehrer E, Uitterlinden AG, Hofman A, van Duijn CM, Cherkas L, Pedersen LM, Stubhaug A, Nielsen CS, Mannikko M, Mihailov E, Milani L, Gobel H, Esserlind AL, Christensen AF, Hansen TF, Werge T, Kaprio J, Aromaa AJ, Raitakari O, Ikram MA, Spector T, Jarvelin MR, Metspalu A, Kubisch C, Strachan DP, Ferrari MD, Belin AC, Dichgans M, Wessman M, van den Maagdenberg AM, Zwart JA, Boomsma DI, Smith GD, Stefansson K, Eriksson N, Daly MJ, Neale BM, Olesen J, Chasman DI, Nyholt DR, Palotie A. Meta‐analysis of 375,000 individuals identifies 38 susceptibility loci for migraine. Nat Genet. 2016;48:856–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kiando SR, Tucker NR, Castro‐Vega LJ, Katz A, D'Escamard V, Treard C, Fraher D, Albuisson J, Kadian‐Dodov D, Ye Z, Austin E, Yang ML, Hunker K, Barlassina C, Cusi D, Galan P, Empana JP, Jouven X, Gimenez‐Roqueplo AP, Bruneval P, Hyun Kim ES, Olin JW, Gornik HL, Azizi M, Plouin PF, Ellinor PT, Kullo IJ, Milan DJ, Ganesh SK, Boutouyrie P, Kovacic JC, Jeunemaitre X, Bouatia‐Naji N. PHACTR1 is a genetic susceptibility locus for fibromuscular dysplasia supporting its complex genetic pattern of inheritance. PLoS Genet. 2016;12:e1006367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pezzini A, Del Zotto E, Giossi A, Volonghi I, Grassi M, Padovani A. The migraine‐ischemic stroke connection: potential pathogenic mechanisms. Curr Mol Med. 2009;9:215–226. [DOI] [PubMed] [Google Scholar]
- 44. MacGregor EA. Migraine headache in perimenopausal and menopausal women. Curr Pain Headache Rep. 2009;13:399–403. [DOI] [PubMed] [Google Scholar]
- 45. Martin VT, Lipton RB. Epidemiology and biology of menstrual migraine. Headache. 2008;48(suppl 3):S124–S130. [DOI] [PubMed] [Google Scholar]
- 46. Mathew PG, Dun EC, Luo JJ. A cyclic pain: the pathophysiology and treatment of menstrual migraine. Obstet Gynecol Surv. 2013;68:130–140. [DOI] [PubMed] [Google Scholar]
- 47. Stewart WF, Lipton RB, Chee E, Sawyer J, Silberstein SD. Menstrual cycle and headache in a population sample of migraineurs. Neurology. 2000;55:1517–1523. [DOI] [PubMed] [Google Scholar]
- 48. Tweet MS, Codsi E, Best PJM, Gulati R, Rose CH, Hayes SN. Menstrual chest pain in women with history of spontaneous coronary artery dissection. J Am Coll Cardiol. 2017;70:2308–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Calhoun AH. Hormonal contraceptives and migraine with aura‐is there still a risk? Headache. 2017;57:184–193. [DOI] [PubMed] [Google Scholar]
- 50. Silberstein S, Patel S. Menstrual migraine: an updated review on hormonal causes, prophylaxis and treatment. Expert Opin Pharmacother. 2014;15:2063–2070. [DOI] [PubMed] [Google Scholar]
- 51. Miller VM, Duckles SP. Vascular actions of estrogens: functional implications. Pharmacol Rev. 2008;60:210–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. de Tommaso M, Sciruicchio V. Migraine and central sensitization: clinical features, main comorbidities and therapeutic perspectives. Curr Rheumatol Rev. 2016;12:113–126. [DOI] [PubMed] [Google Scholar]
- 53. Palacios‐Cena M, Lima Florencio L, Natalia Ferracini G, Baron J, Guerrero AL, Ordas‐Bandera C, Arendt‐Nielsen L, Fernandez‐de‐Las‐Penas C. Women with chronic and episodic migraine exhibit similar widespread pressure pain sensitivity. Pain Med. 2016;17:2127–2133. [DOI] [PubMed] [Google Scholar]
- 54. Shaw J, Anderson T. Coronary endothelial dysfunction in non‐obstructive coronary artery disease: risk, pathogenesis, diagnosis and therapy. Vasc Med. 2016;21:146–155. [DOI] [PubMed] [Google Scholar]
- 55. Liang JJ, Tweet MS, Hayes SE, Gulati R, Hayes SN. Prevalence and predictors of depression and anxiety among survivors of myocardial infarction due to spontaneous coronary artery dissection. J Cardiopulm Rehabil Prev. 2014;34:138–142. [DOI] [PubMed] [Google Scholar]
- 56. Antonaci F, Nappi G, Galli F, Manzoni GC, Calabresi P, Costa A. Migraine and psychiatric comorbidity: a review of clinical findings. J Headache Pain. 2011;12:115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Seng EK, Buse DC, Klepper JE, S JM, Grinberg AS, Grosberg BM, Pavlovic JM, Robbins MS, Vollbracht SE, Lipton RB. Psychological factors associated with chronic migraine and severe migraine‐related disability: an observational study in a tertiary headache center. Headache. 2017;57:593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cohen BE, Edmondson D, Kronish IM. State of the art review: depression, stress, anxiety, and cardiovascular disease. Am J Hypertens. 2015;28:1295–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Garcia Garcia C, Casanovas N, Recasens L, Miranda F, Bruguera J. Spontaneous coronary artery dissection in ergotamine abuse. Int J Cardiol. 2007;118:410–411. [DOI] [PubMed] [Google Scholar]
- 60. Becker WJ. Acute migraine treatment in adults. Headache. 2015;55:778–793. [DOI] [PubMed] [Google Scholar]
- 61. Diener HC, Kurth T, Holle D. Practical implications of the migraine cardio‐ and cerebrovascular association: unmet needs of patients. Cephalalgia. 2015;35:140–145. [DOI] [PubMed] [Google Scholar]
- 62. Hill SE, Kirsten L. Triptan‐induced torsades de pointes and ventricular fibrillation cardiac arrest: case report and review of the literature. Curr Drug Saf. 2014;9:236–239. [DOI] [PubMed] [Google Scholar]
- 63. Pringsheim T, Davenport WJ, Marmura MJ, Schwedt TJ, Silberstein S. How to apply the AHS evidence assessment of the acute treatment of migraine in adults to your patient with migraine. Headache. 2016;56:1194–1200. [DOI] [PubMed] [Google Scholar]
- 64. Artto V, Metso TM, Metso AJ, Putaala J, Haapaniemi E, Wessman M, Farkkila M, Kallela M, Tatlisumak T. Migraine with aura is a risk factor for cervical artery dissection: a case‐control study. Cerebrovasc Dis. 2010;30:36–40. [DOI] [PubMed] [Google Scholar]
- 65. Schurks M, Buring JE, Kurth T. Agreement of self‐reported migraine with ICHD‐II criteria in the women's health study. Cephalalgia. 2009;29:1086–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dodick DW, Loder EW, Manack Adams A, Buse DC, Fanning KM, Reed ML, Lipton RB. Assessing barriers to chronic migraine consultation, diagnosis, and treatment: results from the chronic migraine epidemiology and outcomes (CAMEO) study. Headache. 2016;56:821–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lipton RB, Diamond S, Reed M, Diamond ML, Stewart WF. Migraine diagnosis and treatment: results from the American Migraine Study II. Headache. 2001;41:638–645. [DOI] [PubMed] [Google Scholar]
- 68. Schurks M, Rist PM, Bigal ME, Buring JE, Lipton RB, Kurth T. Migraine and cardiovascular disease: systematic review and meta‐analysis. BMJ. 2009;339:b3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mahmoud AN, Mentias A, Elgendy AY, Qazi A, Barakat AF, Saad M, Mohsen A, Abuzaid A, Mansoor H, Mojadidi MK, Elgendy IY. Migraine and the risk of cardiovascular and cerebrovascular events: a meta‐analysis of 16 cohort studies including 1 152 407 subjects. BMJ Open. 2018;8:e020498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Olin JW, Gornik HL, Bacharach JM, Biller J, Fine LJ, Gray BH, Gray WA, Gupta R, Hamburg NM, Katzen BT, Lookstein RA, Lumsden AB, Newburger JW, Rundek T, Sperati CJ, Stanley JC. Fibromuscular dysplasia: state of the science and critical unanswered questions: a scientific statement from the American Heart Association. Circulation. 2014;129:1048–1078. [DOI] [PubMed] [Google Scholar]


