Abstract
Background
Exposure to green vegetation has been linked to positive health, but the pathophysiological processes affected by exposure to vegetation remain unclear. To study the relationship between greenness and cardiovascular disease, we examined the association between residential greenness and biomarkers of cardiovascular injury and disease risk in susceptible individuals.
Methods and Results
In this cross‐sectional study of 408 individuals recruited from a preventive cardiology clinic, we measured biomarkers of cardiovascular injury and risk in participant blood and urine. We estimated greenness from satellite‐derived normalized difference vegetation index (NDVI) in zones with radii of 250 m and 1 km surrounding the participants’ residences. We used generalized estimating equations to examine associations between greenness and cardiovascular disease biomarkers. We adjusted for residential clustering, demographic, clinical, and environmental variables. In fully adjusted models, contemporaneous NDVI within 250 m of participant residence was inversely associated with urinary levels of epinephrine (−6.9%; 95% confidence interval, −11.5, −2.0/0.1 NDVI) and F2‐isoprostane (−9.0%; 95% confidence interval, −15.1, −2.5/0.1 NDVI). We found stronger associations between NDVI and urinary epinephrine in women, those not on β‐blockers, and those who had not previously experienced a myocardial infarction. Of the 15 subtypes of circulating angiogenic cells examined, 11 were inversely associated (8.0–15.6% decrease/0.1 NDVI), whereas 2 were positively associated (37.6–45.8% increase/0.1 NDVI) with contemporaneous NDVI.
Conclusions
Independent of age, sex, race, smoking status, neighborhood deprivation, statin use, and roadway exposure, residential greenness is associated with lower levels of sympathetic activation, reduced oxidative stress, and higher angiogenic capacity.
Keywords: cardiovascular disease risk factors, catecholamine, endothelial progenitor cells, environment, greenness, normalized difference vegetation index, oxidative stress
Subject Categories: Angiogenesis, Biomarkers, Mechanisms, Cardiovascular Disease
Clinical Perspective
What Is New?
Individuals who live in greener areas have lower levels of sympathetic activation, oxidative stress, and a better angiogenic profile.
Living in green spaces is associated with lower stress and diminished cardiovascular disease risk.
Female participants, participants not on β‐blockers, or those who had not previously experienced a myocardial infarction show significantly stronger associations between greenness and urinary epinephrine levels.
What Are the Clinical Implications?
Persistent exposure to greenness is conducive to cardiovascular health.
Evaluation of exposure to green spaces may be informative in assessing cardiovascular disease risk.
In particular, women or those without a history of myocardial infarction may benefit by proximity to green spaces.
Introduction
Extensive evidence supports the notion that cardiovascular disease (CVD) risk is affected by many features of the built environment, such as residential characteristics and location, proximity to major roadways, availability of healthy food, and neighborhood walkability.1, 2, 3 In comparison, the health effects of neighborhood green spaces have received less attention. However, there are emerging data to suggest that green spaces are important features of urban environments conducive to human health. Several recent studies have reported that individuals living in areas of high vegetation display fewer depressive symptoms, but greater neighborhood satisfaction, better social interactions,4 and higher social support.5 There is also evidence to suggest that exposure to vegetation could have beneficial effects on cardiovascular health. For instance, in an analysis of the entire population of England, it was found that the rates of cardiovascular mortality were lower in areas with higher levels of greenness.6 It has also been reported that increased residential green space is associated with the reduction in both cardiovascular and respiratory mortality,7 and the odds of hospitalization for heart disease and stroke are lower among adults who live in neighborhoods with highly variable greenness.8 Individuals with ischemic stroke residing in close proximity to green spaces have survival rates higher than those who live in less green areas.9 In addition, a temporal decrease in tree canopy (due to emerald ash borer infestation) was found to be associated with a progressive increase in CVD mortality.10 Taken together, the results of these studies support the view that exposure to greenness affects CVD risk. Nonetheless, the link between vegetation exposure and cardiovascular health remains weak, in particular, because it is unclear how exposure to green spaces and vegetation influences cardiovascular health and which pathophysiological processes and mechanisms mediate the relationship between green spaces and CVD risk.
Several mechanisms have been proposed to account for the relationship between cardiovascular health and green spaces.11 Neighborhood greenness and parks can encourage physical activity and potentially shield residents from heat and ambient pollutants, as well as lessen their exposure to noise and roadway traffic emissions.12, 13, 14, 15 Green spaces can also foster social contacts and social cohesion, and thereby promote a sense of well‐being. Because physical inactivity, air pollution, noise, and lack of social cohesion are important determinants of CVD risk,1 it appears likely that these factors mediate some of the beneficial health effects of greenness. However, a leading hypothesis linking green spaces to better health relates to a reduction in mental stress. Residents living in greener neighborhoods have been reported to be at a lower risk of psychological distress,16 decreased treatment for anxiety and mood disorders,17 better mental health, and lower levels of mental stress.18 Although these studies provide a plausible link between green spaces and cardiovascular health, such data are derived mostly from questionnaires and evaluations, and objective evaluation of the underlying biological processes that link stress to CVD are lacking. Direct measurements of changes in stress levels, such as the activation of the sympathetic nervous system (SNS) are preferred not only because they are more objective and quantitative,19 but also because they can directly link environmental exposures to CVD risk and susceptibility.
In humans, the activity of the SNS could be assessed by measuring levels of catecholamines in blood or urine.19 While plasma levels of catecholamines show high variability and sporadic changes, the levels of these amines in urine could be used to reliably assess basal levels of SNS activation. Previous studies have shown that catecholamine levels in urine are associated with depression and anxiety,20, 21, 22 as well as perceived,23 chronic, and acute stress.24, 25, 26 High activity of the SNS has also been related to changes in blood pressure, thrombosis, and inflammation—conditions that by themselves could increase CVD risk.27 In this study, we tested the hypothesis that residential exposure to green spaces reduces CVD risk by decreasing SNS activation. To test this hypothesis, we evaluated potential pathophysiological links between neighborhood greenness and urinary levels of catecholamines and their metabolites and other biomarkers of CVD risk. Because preexisting CVD risk increases susceptibility to environmental exposures,1, 28 we studied this relationship in a cohort of individuals with moderate‐to‐severe CVD risk.
Materials and Methods
Study Population
We screened and recruited study participants from the outpatient cardiology clinic of the University of Louisville between October 2009 and December 2014 irrespective of age, sex, or race. The clinic treats mostly individuals with existing CVD or those at high risk for developing CVD. All study activities were approved by the University of Louisville Institutional Review Board (IRB 09.0174 and 10.0350). To minimize circadian variability, we enrolled the participants and acquired their blood and urine samples only between 1:00 and 4:00 pm Eastern Standard Time. Prior to study procedures, each participant provided written informed consent. We excluded participants who were unable to provide written informed consent, pregnant or lactating women, prisoners, and those with lung, liver, or kidney disease; coagulopathies; substance abuse; chronic cachexia; and severe comorbidities. After enrollment, one participant chose to withdraw from the study and was excluded from all data analysis.
The total study cohort included 508 participants. We identified residential location of participants by geocoding home addresses collected during enrollment. We corrected addresses for spelling errors, invalid characters in the data, and invalid or erroneous formats. We used the ArcMap9.3+ (ESRI, Redlands, CA) Geographic Information Systems software to geocode participants’ addresses using street and address data provided by the Louisville/Jefferson County Information Consortium. Addresses not assigned to actual residential locations (eg, apartment complexes and mobile home communities) were manually located, if possible, through zip codes, street names, and specific addresses of surrounding residences. The data were available to study investigators, but because these data sets consist of identifiable participant characteristics and residential information, they cannot be publicly shared, and no approval process was established to broadly share participant data.
Of those enrolled in the study, we could not obtain the residential location of 51 participants due to invalid addresses provided. An additional 49 participants did not have valid urinary catecholamine measurements. Smokers were not excluded from the analysis, as previous evidence suggests that smokers may be particularly sensitive to environmental influences.29 Municipal property records show that less than 10% of single‐family residential homes at participant addresses (56% of participants) changed ownership within 6 months before enrollment, indicating low residential mobility among participants. However, similar data for apartment renters were not available.
Covariates such as age, sex, ethnicity, body mass index (calculated from self‐reported height and weight variables), and tobacco exposure (verified by urinary cotinine concentration) were collected through the participant questionnaires. Information relevant to CVD risk factors, cardiovascular history, and medication use was collected from both questionnaire and medical records. Median household income, a proxy for neighborhood socioeconomic status, was collected from the US Census Bureau for 2010 at the block group level.30 To account for the effects of area characteristics, independent of green spaces, we adjusted our analyses for the area deprivation index for each census block group encompassing each participant's residence. This deprivation index is an area‐based measure of neighborhood socioeconomic deprivation that includes 17 census‐derived variables pertaining to income, education, occupation, disparity, household makeup, and housing characteristics.31
To estimate exposure to air pollutants, we measured roadway exposure and daily fine particulate matter (PM2.5). For roadway exposure, we measured distance from participant homes to the nearest major roadway using Geographic Information Systems. Major roadways were defined as any roadway segments that are traversed by >5000 vehicles per day, as measured by the Kentucky Transportation Cabinet.32 Roadway density was defined as the total length of all roadways within 50 m of each participant's geocoded residential location. The average of daily PM2.5 values for all monitors in the Louisville Metropolitan Area were collected from the US Environmental Protection Agency and utilized to estimate daily ambient PM2.5.33 These daily ambient levels were then applied to estimate PM2.5 exposure on the day of participant enrollment. Daily between‐monitor variation of PM2.5 was minimal, indicating that there is little detectible intra‐urban variation in PM2.5 levels.
Residential Greenness
To estimate vegetation exposure, we used the satellite‐derived normalized difference vegetation index (NDVI) data from the US Geological Survey.34 The NDVI is derived from the ratio of red and infrared light reflected from sunlight into space, which is indicative of photosynthetic activity.35, 36 NDVI was quantified using remotely sensed satellite imagery, collected utilizing the Moderate Resolution Imaging Spectroradiometer by the National Aeronautics and Space Administration's Earth Observing System. Summertime NDVI values in the Louisville area at 250‐m resolution range typically from −0.1 (representing no vegetation) to 0.9 (representing high‐density forest cover). This range of values is consistent across most cities in the eastern United States but is highly variable between urban areas located in arid regions. However, undeveloped rural areas surrounding urban locations rarely harbor areas with <0.5 NDVI.
We quantified residential greenness by using the GIS software to calculate NDVI at geographic residential locations at the time of individual participant enrollment (contemporaneous NDVI). Both peak9, 37 and cumulative NDVI38 have been used to examine the relationship between greenness and long‐term outcomes such as mortality or birth outcomes. However, for our analysis, we used contemporaneous NDVI (at the time of study enrollment) because in Louisville, NDVI differs significantly between summer and winter months (due to loss of leaves in winter), and because we expected a temporal relationship between NDVI and cardiovascular biomarkers measured in the study. To minimize atmospheric interference common in single‐point‐in‐time imagery, we collected composite 14‐day imagery at 250‐m resolution, available through the US Geological Survey EarthExplorer remote sensing data repository. For our analysis, we selected representative 14‐day composite NDVI data sets for each month during the enrollment period and then applied to each participant based on the date of enrollment to delineate contemporaneous NDVI. To quantify greenness at and around participant residences that represents both localized influences and neighborhood scales, we calculated the value of contemporaneous mean NDVI for a circular zone with a radius of 250 and 1000 m around the residential addresses. We clipped the NDVI images to remove areas of pixels falling partially outside the zone. Areas containing water, as identified by the National Landcover Database, were excluded and not considered in the circular zone calculations.39
Quantification of Urinary Catecholamines, Monoamines, and Their Metabolites
To obtain reliable estimates of stress, we measured urinary levels of catecholamines, monoamines, and their metabolites. Urinary levels of catecholamines and monoamines and their metabolites were measured using ultra performance liquid chromatography–tandem mass spectrometry analysis.40 For this procedure, urine samples were thawed on ice, vortexed, and diluted 1:50 with 0.2% formic acid containing isotopic labeled internal standards. The samples were then applied to Waters Acquity Class‐H ultra performance liquid chromatography coupled with a Waters Xevo TQ‐Sμ mass spectrometer and subjected to multiple reaction monitoring analysis. The procedure measured norepinephrine, dopamine, serotonin, normetanephrine, 3‐methoxytyramine, metanephrine, epinephrine, 5‐hydroxyindole‐3‐acetic acid, homovanillic acid, and vanillylmandelic acid concentrations. The analytes in urine samples were quantified using peak area ratio based on 8 point‐standard curves, run before and after the urine samples using TargetLynx software (Waters Inc., Milford, MA). The concentration of each analyte was normalized to the level of creatinine in the sample. Creatinine was measured on a COBAS MIRA‐plus analyzer (Roche, Branchburg, NJ) with Infinity Creatinine Reagent (Thermo Fisher Scientific, Waltham, MA).
Quantification of Isoprostanes
For assessment of oxidative stress, participant urine samples were analyzed by the Vanderbilt University Eicosanoid Core Laboratory to measure urinary F2‐isoprostane and F2‐isoprostane metabolite among a subset of participants with dynamic CVD risk.41 Phospholipids were first extracted from urine samples and were subjected to alkaline hydrolysis to release free isoprostane. These free isoprostanes were then quantified using a high‐sensitivity gas chromatography–mass spectrometry assay described before.41
Measurements of Circulating Angiogenic Cells
To assess the endothelial repair capacity and to capture the large heterogeneity in circulating angiogenic cells (CACs), we measured 15 different subtypes of CACs in peripheral blood. However, because of technical reasons and because CACs cannot be measured in stored samples, we measured CAC levels only in a subgroup of 255 participants. These cells were identified and quantified using a 7‐color flow cytometry procedure on fresh blood specimens as previously described.42 We used the cell surface markers CD31, CD34, CD45, and AC133 to quantify phenotypically distinct subpopulations.43, 44 The surface marker CD34 identifies stem/progenitor cells, AC133 identifies early progenitor cells and CD31 identifies endothelial cells. CD45+ cells are of hematopoietic origin. We used a combination of these markers to identify cell subpopulations within these main categories.
Statistical Analysis
Participant characteristics are expressed as n (%) for categorical variables and mean (SD) for continuous variables in Table. Residential NDVI values were divided into high (>0.55), medium (0.36–0.54), and low (<0.36) categories based on tertiles of contemporaneous NDVI values within a circular zone of 250 m around the participant's residence. We compared participant demographics, CVD risk factors, and biomarkers between high, medium, and low NDVI using ANOVA or chi‐squared tests as appropriate. For each modeled outcome and NDVI metric, we grouped NDVI values into tertiles, and tested participant characteristics from Table for differences between tertiles. Based on significant differences between tertiles and biological plausibility, we selected covariates of sex, race, age, smoking status, area deprivation index, statin use, and the density of roadways within 50 m of the residence for all adjusted statistical models. While daily PM2.5 was significantly associated with greenness in the overall cohort, it was not significantly associated among the cohort subgroups and was subsequently not included as a covariate in the analyses.
Table 1.
Overall Cohort Demographics and Cardiovascular Disease Stratified by Low/Medium/High Contemporaneous NDVI Values (n=408) Within 250‐m‐Radius Circular Zone Surrounding the Participant Residences
| Categorical Variable, n (%) | Total, n=408 | Low Green 0.0 to 0.36 NDVI, n=136 | Medium Green 0.36 to 0.54 NDVI, n=136 | High Green 0.54 to 0.86 NDVI, n=136 | P Value |
|---|---|---|---|---|---|
| Sex | 0.078 | ||||
| Male | 210 (52%) | 60 (44%) | 72 (54%) | 78 (57%) | |
| Race | 0.013a | ||||
| White | 228 (56%) | 65 (48%) | 72 (53%) | 91 (67%) | |
| Black | 159 (39%) | 64 (47%) | 58 (43%) | 37 (27%) | |
| Other | 21 (5%) | 7 (5%) | 6 (4%) | 8 (6%) | |
| CVD risk factors | |||||
| Hypertension | 300 (75%) | 103 (76%) | 98 (74%) | 99 (74%) | 0.926 |
| Hyperlipidemia | 240 (60%) | 81 (60%) | 73 (55%) | 86 (64%) | 0.374 |
| Diabetes mellitus | 121 (30%) | 39 (29%) | 47 (35%) | 35 (26%) | 0.225 |
| Current smoker | 145 (36%) | 58 (43%) | 34 (26%) | 53 (39%) | 0.009a |
| High CVD riskb | 167 (61%) | 65 (61%) | 58 (60%) | 44 (64%) | 0.895 |
| Cardiovascular history | |||||
| Myocardial infarction | 128 (32%) | 37 (27%) | 47 (36%) | 44 (33%) | 0.325 |
| Stroke | 43 (8%) | 14 (10%) | 18 (14%) | 11 (8%) | 0.357 |
| CABG/PCI/stents | 113 (28%) | 33 (24%) | 39 (29%) | 41 (30%) | 0.488 |
| Heart failure | 78 (20%) | 23 (17%) | 30 (23%) | 25 (19%) | 0.452 |
| Medications | |||||
| β‐Blocker | 218 (55%) | 82 (62%) | 72 (55%) | 64 (48%) | 0.074 |
| ACE/ARB | 223 (56%) | 70 (53%) | 72 (55%) | 81 (60%) | 0.426 |
| Diuretic | 156 (39%) | 59 (44%) | 49 (38%) | 48 (36%) | 0.325 |
| Statin | 198 (50%) | 64 (48%) | 69 (53%) | 65 (49%) | 0.617 |
| Aspirin | 203 (51%) | 74 (56%) | 58 (44%) | 71 (53%) | 0.155 |
| Continuous variable, mean (SD) | |||||
| Age, y | 51.4 (10.8) | 52.5 (11.1) | 49.4 (10.5) | 52.3 (10.6) | 0.033a |
| BMI | 32.9 (8.2) | 33.0 (8.5) | 33.6 (8.6) | 32.0 (7.4) | 0.284 |
| Systolic blood pressure | 131.0 (20.5) | 131.8 (19.3) | 129.7 (19.8) | 131.4 (22.6) | 0.710 |
| Diastolic blood pressure | 80.7 (11.8) | 80.1 (11.0) | 80.8 (11.9) | 81.3 (12.5) | 0.747 |
| Lipid levels, mg/dL | |||||
| Cholesterol | 192.8 (53.0) | 191.3 (45.5) | 195.3 (62.2) | 191.4 (49.2) | 0.861 |
| HDL | 44.6 (13.0) | 47.6 (14.0) | 43.2 (13.4) | 43.0 (10.7) | 0.042a |
| LDL | 106.7 (40.9) | 107.0 (41.0) | 109.0 (41.2) | 103.4 (40.9) | 0.707 |
| Household income, ×10−3 | 36.9 (22.3) | 27.2 (15.7) | 36.5 (21.8) | 48.4 (24.0) | <0.001a |
| Area deprivation index | 109.2 (10.9) | 114.8 (8.0) | 108.7 (10.6) | 104.1 (11.0) | <0.001a |
| Roads within 50 m | 109.6 (49.2) | 114.1 (52.7) | 108.3 (51.8) | 106.3 (42.6) | 0.404 |
| PM2.5 | 13.8 (5.6) | 13.6 (5.6) | 13.1 (5.1) | 14.8 (6.1) | 0.045a |
ACE indicates angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; CABG, coronary artery bypass graft; CVD, cardiovascular disease; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; NDVI, normalized difference vegetation index; PCI, percutaneous coronary intervention; PM2.5, particulate matter <2.5 μm.
Significant difference between tertiles based on ANOVA or Chi‐squared analysis (P<0.05).
Estimated on the basis of a Framingham Risk Score (FRS) >20 or prior cardiovascular event.
To minimize spatial confounding, we performed a cluster analysis to geographically define and account for 9 clusters of participant residences based on census block group boundaries (Figure S1). To define each cluster, we aggregated census block groups and identified a first cluster of 46 addresses in the central business district of Louisville. Subsequent clusters were drawn by aggregating block groups radially around the first cluster, beginning with participants residing to the northwest of the central business district, with nearly equal numbers of participants. Eight clusters were within Jefferson County and each cluster contained 45 to 47 participants. The final cluster (cluster 9) contained all participants residing outside of Jefferson County. These were segregated into a different cluster from residents of Jefferson County because of differences in local governance, land cover, residential characteristics, and so on.
We used generalized estimating equations for clustered data to estimate the associations between plasma and urinary biomarkers with contemporaneous NDVI values within a circular zone of 250 m and 1 km around the participant's residence. Because the plasma and urinary biomarkers had positive values and were positively skewed, we used the gamma distribution with the log‐link function. For each model, % change (and 95% confidence interval) for each biomarker was calculated per 0.1 NDVI. We also calculated predicted values from the generalized estimating equation for the epinephrine model with contemporaneous NDVI at 250 m. We then log‐transformed and determined the best fits of the data by linear regression with 95% confidence limits and 95% predictions limits. We performed all statistical analyses using SAS version 9.4 software (SAS Institute, Inc., Cary, NC) and Graphpad Prism, version 7 (Graphpad Software, La Jolla, CA).
Results
Participant Characteristics
Of the 508 participants recruited for the study, 408 met our inclusion criteria. These participants were 52% males, 56% whites, 39% blacks, and 5% other races. A majority of the study population was hypertensive (75%), hyperlipidemic (60%), and at risk for CVD (61%), based on a Framingham Risk Score of >20% or having a previous major adverse cardiovascular event. The cohort was 51.4±10.8 years old and mostly overweight or obese with a mean body mass index of 32.9±8.2 (see Table). Most participants lived in areas of low greenness located in northwestern Jefferson County. A less dense, but relatively even, distribution of participants was observed throughout the residential areas in the rest of the county (Figure 1). However, a majority of the participants lived in neighborhoods consisting of single‐family residential homes and lots containing both grasses and trees. The distribution of the study population between areas of low, medium, and high contemporaneous greenness is shown in Table. Black participants were more likely to reside in areas with lower greenness. In comparison with those living in areas of high greenness, participants residing in areas of low greenness were more likely to live in a deprived area and have significantly lower median household income. No significant differences were observed in cardiovascular history, medication use, sex, body mass index, or the sum of CVD risk factors, although individuals living in areas of low greenness were more likely be younger, to smoke, and to have slightly lower plasma high‐density lipoprotein levels. Spearman correlations between continuous demographic variables and NDVI within circular zones of 250 m and 1 km around the participant's residence are shown in Table S1.
Figure 1.
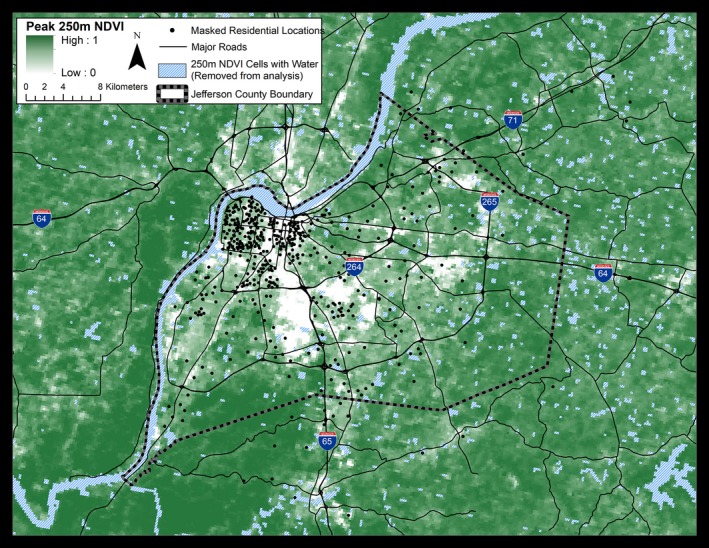
Geographic location of study participants and the distribution of greenness within Jefferson County, Kentucky. Study participants (n=408) from indicated residential areas were recruited from a preventive cardiology clinic at the University of Louisville. Values of greenness, calculated as peak normalized difference vegetation index (NDVI), are shown at a resolution of 250 m. Attributes of land cover and greenness within the county vary between −0.1 and 1 NDVI unit. Low NDVI values are associated with business districts, industrial areas, and transportation zones. Residential areas show moderate NDVI, whereas urban parks, forests, and underdeveloped areas show high NDVI values. To protect participant privacy, exact residential locations are geographically masked and do not represent residential addresses.
Association Between Greenness and Catecholamine Levels
In fully adjusted models, the NDVI levels within a circular zone of 250 m radius around the residence were inversely associated with urinary levels of epinephrine (−6.9% change/0.1 unit NDVI difference; 95% confidence interval, −11.5; −2.0, P=0.006), but not with the urinary levels of norepinephrine or other catecholamines, monoamines, or their metabolites (Figure 2A). The association between urinary epinephrine and NDVI remained significant even when the radius of the residential zone was increased to 1 km (Figure 2B). The urinary levels of epinephrine displayed a log‐linear dose dependence with NDVI (Figure 2C). Subgroup analysis showed a stronger relationship between NDVI and epinephrine among females, those not using β‐blockers, and those who had not previously experienced a MI (Figure 2D).
Figure 2.
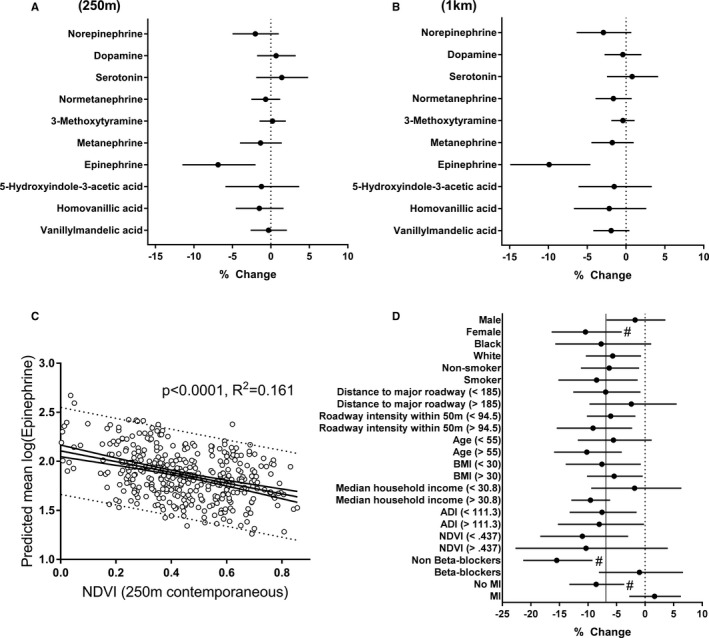
Adjusted associations between urinary levels of catecholamines and residential greenness. Mean NDVI values were calculated for circular zones surrounding the participant (n=408) residences with a radius of (A) 250 m, or (B) 1 km. C, Urinary epinephrine levels vs NDVI levels within a 250‐m‐radius zone around the participant residences. Open circles are individual predicted mean values of urinary epinephrine, and the continuous line is the best fit of a linear relationship to the data with 95% confidence limits. Dotted lines show 95% prediction limits. D, Subgroup analysis of the association between epinephrine and NDVI. Vertical solid line represents values obtained from the full model, and # represents significant interaction. All estimates represent percent change (and 95% confidence intervals) per 0.1 unit increase in NDVI. ADI indicates Area Deprivation Index; BMI, body mass index; MI, myocardial infarction; NDVI, normalized difference vegetation index.
Association Between Greenness and Isoprostanes
Given our observation that residential proximity to greenness is associated with lower levels of urinary epinephrine, we next examined changes in oxidative stress among a subset of participants with dynamic CVD risk (n=82) with demographic features similar to the parent cohort (Table S2). In this subgroup, we found that greenness, in fully adjusted models, was inversely associated with urinary levels of F2‐isoprostane, a sensitive and robust indicator of lipid peroxidation and systemic oxidative stress45 (Figure 3). F2‐isoprostane levels were inversely associated with 250 m NDVI, −9% (95% confidence interval, −15.1, −2.5; P=0.007) per 0.1 unit. This association remained significant within a 1‐km‐radius circular zone around the participant's residence.
Figure 3.
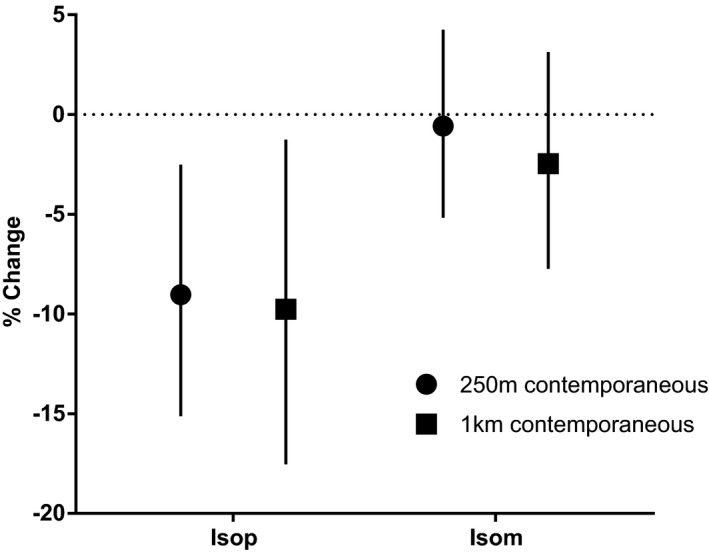
Adjusted associations between residential greenness and oxidative stress. Urinary levels of isoprostanes were measured as an index of oxidative stress in a subset of participants (n=82) to assess their relationship with contemporaneous residential greenness within a circular zone of indicated radii surrounding the participant residences. The results are presented as percent change (with 95% confidence intervals) in the urinary levels of the parent F2‐isoprostane (IsoP) and F2‐isoprostane metabolites (IsoM) per 0.1 unit higher normalized difference vegetation index (NDVI). Estimates are derived from generalized estimating equations using the gamma distribution and log link function.
Association Between Greenness and Circulating Angiogenic Cells
To measure endothelial repair capacity, we examined changes in circulating angiogenic cells, a subpopulation of blood cells that are recruited from the bone marrow to promote and facilitate tissue angiogenesis,46 in a subgroup similar to the parent cohort (Table S3, n=255). As shown in Figure 4A, in fully adjusted models, CAC subtypes 1, 3, 5, 6, 7, 9, 10, 11 12, 13, and 15 were inversely associated with NDVI values at 250 m, whereas subtypes 4 and 14 were positively associated with NDVI. These associations were particularly robust, with the effect size of inverse associations ranging from −8.0% to −15.6%, while positive associations were 45.8% and 37.6%. Within a 1‐km radius, CAC subtypes 1, 3, 6, 7, 9, 10, 12, and 15 remained inversely associated with effect sizes of −6.9% to −10.1%, as shown in Figure 4B. Positive associations were again found within a 1‐km radius for CAC 4 and 14, with similarly attenuated effect sizes of 31.1% and 29%, respectively.
Figure 4.
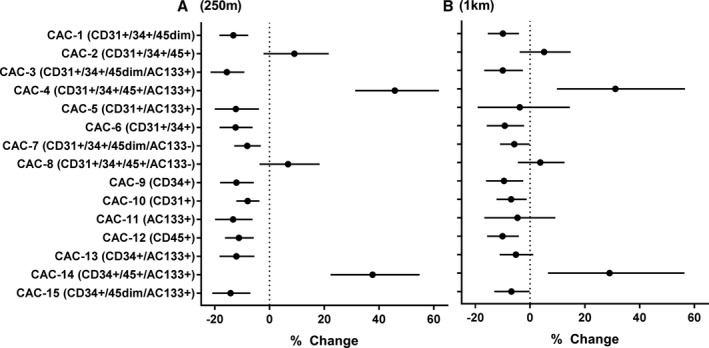
Adjusted associations between circulating angiogenic cell (CAC) levels with residential greenness. The levels of CACs in peripheral blood were measured in a subset of participants (n=255) for associations with resident greenness as measured by average normalized difference vegetation index (NDVI) in a circular zone of (A) 250‐m and (B) 1‐km‐radius surrounding the participant residences. Model estimates represent percent change (and 95% confidence intervals) per 0.1 unit higher NDVI.
Discussion
In this study, we found that the residential proximity to vegetation was associated with cardiovascular health as reflected by a range of biomarkers of cardiovascular injury and disease risk. Our results show that those who lived in greener surroundings had lower levels of sympathetic activation, oxidative stress, and a better angiogenic profile than those living in less green areas. This relationship was independent of sex, race, age, smoking status, neighborhood deprivation index, statin use, and roadway exposure. Although the mechanisms underlying the beneficial vascular effects of greenness remain obscure, these observations support the notion that exposure to green spaces could have salutary effects on cardiovascular health.
Previous studies have reported that environments rich in vegetation are associated with lower CVD mortality in that area.6, 7 Similarly, greenness has been reported to be associated with improved recovery from major adverse cardiovascular events9 and lower all‐cause mortality.38 However, despite this evidence, the mechanisms underlying the association between greenness and cardiovascular health have remained largely speculative. Some investigators suggest that green surroundings support cardiovascular health by decreasing exposure to air pollution and encouraging physical activity, while others have found a link between residential proximity to greenness and better mental health.11 Adding to this body of knowledge, our results suggest that the beneficial effects of greenness may be attributable, at least in part, to a favorable neurohormonal profile associated with diminished oxidative stress, and a pro‐angiogenic state. These findings are consistent with the results of previous studies showing that residential greenness is inversely associated with stress, anxiety, and mood disorders—a spectrum of mental states that are associated with the activation of the SNS and the hypothalamus‐pituitary axis.11, 47 In prior work, chronic conditions such as depression and anxiety have been linked to an increase in urinary norepinephrine levels,20 whereas an increase in both urinary epinephrine and norepinephrine has been found to be associated with low socioeconomic status48 and stress attributable to unemployment.49 Because in our study greenness was associated with reduced levels of epinephrine, but not norepinephrine, it seems unlikely that exposure to greenness is associated with lower levels of anxiety and depression or reflective of chronic stress associated with low socioeconomic status.
Elevated levels of catecholamines in the urine have been linked to high CVD burden,50 and given the CVD risk of our cohort, it is possible that the association between urinary epinephrine levels and greenness is simply a reflection of a difference in CVD burden. However, this seems unlikely because patients with CVD risk have higher norepinephrine and lower epinephrine levels,50 a profile inconsistent with our results. Similarly, association between epinephrine and greenness could not be explained by difference in traditional cardiovascular risk factors, such as blood pressure51 or lipids,52 which are not usually associated with a sole increase in urinary epinephrine levels. Nonetheless, our results are consistent with the view that exposure to greenness results in either lower levels of perceived stress23 or better sleep53 and circadian alignment54—conditions that are associated with lower levels of urinary epinephrine but not norepinephrine.
The results of our study show that the association between greenness and reduced epinephrine levels were more pronounced in females than in males. This is consistent with previous work showing an association between exposure to greenness and mortality in women.38 We also found that the influence of greenness on epinephrine levels was attenuated in those taking β‐blockers and those who had previously experienced an MI. These findings suggest that greenness has stronger effects on catecholamine levels in those who are healthier or with low to moderate CVD risk, but that these effects may be attenuated in individuals with frank CVD.
The observed associations between greenness and circulating angiogenic cells suggest that individuals living in greener areas are likely to have better wound healing response and higher capacity to repair blood vessels than those who live in less green areas. This is consistent with previous work reporting better recovery from cardiovascular events in individuals who live near more green spaces.9 Nevertheless, higher levels of angiogenesis are likely also to promote tumor growth, and therefore the association between greenness and angiogenesis needs further exploration and better assessment for its overall contribution to the risk of chronic diseases such as cancer and heart disease.
Our analyses showed that the NDVI levels were positively associated with circulating hemangiogenic (CD45+/CD34+) cells but inversely associated with endothelial progenitors (CD45dim/CD34+/CD31+) (Figure 5). In comparison with CD45dim/CD34+ cells, the CD45+/CD34+/CD31+ cells are more primitive, demonstrate strong paracrine signaling,55 and have superior regeneration properties.56 Classified by some investigators as hematopoietic stem cells or hematopoietic stem progenitor cells,57, 58 these cells are neither hematopoietic nor mesenchymal in origin, as they can differentiate into endothelial cells, osteoblasts, muscle, and neural cells.59 Although these cells do not populate the bone marrow,59 they express Oct4 and Nanog in abundance similar to that of undifferentiated embryonic stem cells.60 Therefore, the positive association of CD45+/CD34+/CD133+ cells with residential greenness further supports the notion that living next to green spaces is associated with an improved wound‐healing response and reparative capacity.
Figure 5.
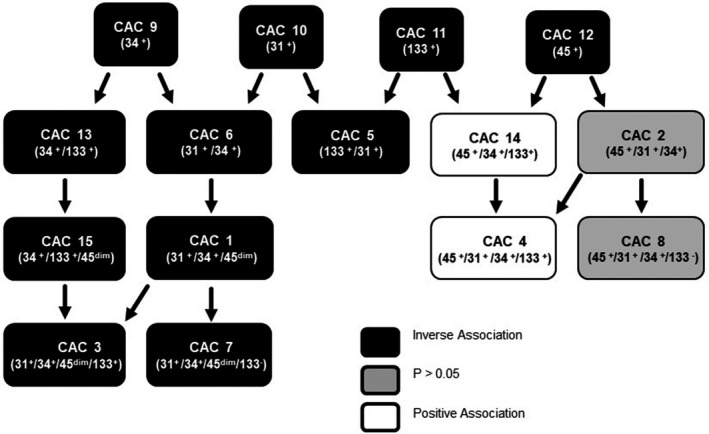
Interrelationships between circulating angiogenic cells (CAC) subtypes associated with residential proximity to greenness. Circulating levels of 15 antigentically defined subpopulations of CACs were measured in 255 participants and examined for their association with levels of greenness (as estimated by normalized difference vegetation index [NDVI]) within a circular zone of a 250‐m radius of their residence while adjusting for covariates. CACs inversely associated with NDVI values are shown in filled black boxes, whereas those positively associated with NDVI are shown in white boxes. Cells showing no significant association are in gray boxes.
Strengths and Limitations
This study is the first of its kind to assess the impact of residential greenness with individual‐level data on biomarkers of CVD risk in a moderate‐sized cohort of participants. Our analysis focused on comprehensive evaluation of risk factors as well as identification of underlying mechanistic changes in catecholamines, circulating angiogenic cells, and oxidative stress. For exposure assessment, we used NDVI, a common satellite‐derived metric of greenness, which is highly indicative of the coverage and density of photosynthetically active vegetation. Moreover, we examined residential area average NDVI values in circular zones with both 250‐m and 1‐km radii that were contemporaneous to participant enrollment. Furthermore, the study cohort included participants with a wide range of greenness exposure. Major covariates were accounted for via questionnaire, as well as clinical, demographic, and environmental assessments to account for socioeconomic and environmental factors known to be highly associated with greenness.
Despite these strengths, the study has some limitations. First, we did not estimate specific environmental exposures. However, our analysis was adjusted for density of roadways near participant residence, a metric of roadway‐based pollution and noise. Other influences on health that may be affected by nearby greenness, such as psychosocial stress, health behaviors, and heat regulation, were not accounted for in the study. Our findings are generalizable to a large portion of the overall population with elevated risk for CVD but may not be applicable to healthier populations. Although potential confounders such as race, smoking, and sex were considered, these variables cannot fully account for nuances of socioeconomic status, environmental exposures, and genetic variation, which likely could lead to residual confounding. We addressed issues of spatial autocorrelation and residual confounding by using generalized estimating equations to account for potential clustering of participants and environmental features. This cluster area adjustment partially accounts for spatially correlated but unavailable factors that could potentially confound the associations between NDVI and study outcomes. Some covariate data including height and weight relied solely on self‐reported data and may be subject to inaccurate reporting. While the study did utilize NDVI, a measure highly indicative of vegetation, it cannot account for leaf area, biomass density, height, overlapping vegetation layers, speciation, and other important characteristics of greenness. NDVI also cannot account for differences in perceptions of nearby residents of greenness quality or utility. Furthermore, only greenness around residences was assessed, accounting for a large but incomplete portion of participants’ overall whereabouts. For assessing changes in catecholamine levels, we measured urinary levels of catecholamines and their metabolites only at one time during the day. In most such studies, 24‐hour urine collection is used to estimate total catecholamine production. However, a spot urine collection was preferred to prevent “averaging” out of the levels of catecholamines such as epinephrine, which show large circadian variations.54 Urine was collected during a similar time window (afternoon) for most participants, to minimize circadian variation. Finally, because of the cross‐sectional design of this study, causality of the link between greenness and cardiovascular health cannot be established. To further understand these associations, prospective longitudinal studies are needed to assess the role of hypothesized mediators of relationships between greenness and cardiovascular outcomes.
Sources of Funding
This work was supported in part by grants from the WellPoint Foundation and the National Institute of Environmental Health Sciences (ES019217, ES023716, and ES029846).
Disclosures
None.
Supporting information
Table S1. Spearman Correlations Between Vegetation Metrics and Continuous Variables
Table S2. Plasma Biomarker Subgroup (n=82) Analysis Demographics and Cardiovascular Disease Stratified by Low/High 250‐m Contemporaneous NDVI Values Within 250‐m‐Radius Circular Zone Surrounding the Participants’ Residence
Table S3. Circulating Angiogenic Cells Subgroup Analysis Demographics and Cardiovascular Disease Stratified by Low/Medium/High 250‐m Contemporaneous NDVI Values (n=255) Within 250‐m‐Radius Circular Zone Surrounding the Participants’ Residence
Figure S1. Residential area block group clusters.
Acknowledgments
We thank the phlebotomists at the UofL Ambulatory Care and University Medical Associates for biological sample collection and Dave Young, Wes Abplanalp, Deirdre Higdon, Sumanth D. Prabhu, for their assistance with this study.
(J Am Heart Assoc. 2018;7:e009117 DOI: 10.1161/JAHA.118.009117.)
References
- 1. Bhatnagar A. Environmental determinants of cardiovascular disease. Circ Res. 2017;121:162–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Oakes JM, Andrade KE, Biyoow IM, Cowan LT. Twenty years of neighborhood effect research: an assessment. Curr Epidemiol Rep. 2015;2:80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Riggs DW, Yeager RA, Bhatnagar A. Defining the human envirome. Circ Res. 2018;122:1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Orban E, Sutcliffe R, Dragano N, Jockel KH, Moebus S. Residential surrounding greenness, self‐rated health and interrelations with aspects of neighborhood environment and social relations. J Urban Health. 2017;94:158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fan Y, Das KV, Chen Q. Neighborhood green, social support, physical activity, and stress: assessing the cumulative impact. Health Place. 2011;17:1202–1211. [DOI] [PubMed] [Google Scholar]
- 6. Mitchell R, Popham F. Effect of exposure to natural environment on health inequalities: an observational population study. Lancet. 2008;372:1655–1660. [DOI] [PubMed] [Google Scholar]
- 7. Villeneuve PJ, Jerrett M, Su JG, Burnett RT, Chen H, Wheeler AJ, Goldberg MS. A cohort study relating urban green space with mortality in Ontario, Canada. Environ Res. 2012;115:51–58. [DOI] [PubMed] [Google Scholar]
- 8. Pereira G, Foster S, Martin K, Christian H, Boruff BJ, Knuiman M, Giles‐Corti B. The association between neighborhood greenness and cardiovascular disease: an observational study. BMC Public Health. 2012;12:466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wilker EH, Wu CD, McNeely E, Mostofsky E, Spengler J, Wellenius GA, Mittleman MA. Green space and mortality following ischemic stroke. Environ Res. 2014;133:42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Donovan GH, Butry DT, Michael YL, Prestemon JP, Liebhold AM, Gatziolis D, Mao MY. The relationship between trees and human health: evidence from the spread of the emerald ash borer. Am J Prev Med. 2013;44:139–145. [DOI] [PubMed] [Google Scholar]
- 11. James P, Banay RF, Hart JE, Laden F. A review of the health benefits of greenness. Curr Epidemiol Rep. 2015;2:131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dadvand P, Rivas I, Basagaña X, Alvarez‐Pedrerol M, Su J, De Castro Pascual M, Amato F, Jerret M, Querol X, Sunyer J, Nieuwenhuijsen MJ. The association between greenness and traffic‐related air pollution at schools. Sci Total Environ. 2015;523:59–63. [DOI] [PubMed] [Google Scholar]
- 13. Lafortezza R, Carrus G, Sanesi G, Davies C. Benefits and well‐being perceived by people visiting green spaces in periods of heat stress. Urban For Urban Green. 2009;8:97–108. [Google Scholar]
- 14. Dzhambov AM, Dimitrova DD. Green spaces and environmental noise perception. Urban For Urban Green. 2015;14:1000–1008. [Google Scholar]
- 15. Browning M, Lee K. Within what distance does “greenness” best predict physical health? A systematic review of articles with GIS buffer analyses across the lifespan. Int J Environ Res Public Health. 2017;14:E675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Astell‐Burt T, Feng X, Kolt GS. Mental health benefits of neighbourhood green space are stronger among physically active adults in middle‐to‐older age: evidence from 260,061 Australians. Prev Med. 2013;57:601–606. [DOI] [PubMed] [Google Scholar]
- 17. Nutsford D, Pearson AL, Kingham S. An ecological study investigating the association between access to urban green space and mental health. Public Health. 2013;127:1005–1011. [DOI] [PubMed] [Google Scholar]
- 18. Triguero‐Mas M, Dadvand P, Cirach M, Martinez D, Medina A, Mompart A, Basagana X, Grazuleviciene R, Nieuwenhuijsen MJ. Natural outdoor environments and mental and physical health: relationships and mechanisms. Environ Int. 2015;77:35–41. [DOI] [PubMed] [Google Scholar]
- 19. Axelrod J, Reisine TD. Stress hormones: their interaction and regulation. Science. 1984;224:452–459. [DOI] [PubMed] [Google Scholar]
- 20. Hughes JW, Watkins L, Blumenthal JA, Kuhn C, Sherwood A. Depression and anxiety symptoms are related to increased 24‐hour urinary norepinephrine excretion among healthy middle‐aged women. J Psychosom Res. 2004;57:353–358. [DOI] [PubMed] [Google Scholar]
- 21. Tsigos C, Chrousos GP. Hypothalamic‐pituitary‐adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53:865–871. [DOI] [PubMed] [Google Scholar]
- 22. Paine NJ, Watkins LL, Blumenthal JA, Kuhn CM, Sherwood A. Association of depressive and anxiety symptoms with 24‐hour urinary catecholamines in individuals with untreated high blood pressure. Psychosom Med. 2015;77:136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Parks CG, Miller DB, McCanlies EC, Cawthon RM, Andrew ME, DeRoo LA, Sandler DP. Telomere length, current perceived stress, and urinary stress hormones in women. Cancer Epidemiol Biomarkers Prev. 2009;18:551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ghaddar A, Omar KH, Dokmak M, Kansour NA, Jbara Z, Laham S, Ali S. Work‐related stress and urinary catecholamines among laboratory technicians. J Occup Health. 2014;55:398–404. [DOI] [PubMed] [Google Scholar]
- 25. Elfering A, Grebner S, Gerber H, Semmer NK. Workplace observation of work stressors, catecholamines and musculoskeletal pain among male employees. Scand J Work Environ Health. 2008;34:337–344. [DOI] [PubMed] [Google Scholar]
- 26. Akerstedt T, Gillberg M, Hjemdahl P, Sigurdson K, Gustavsson I, Daleskog M, Pollare T. Comparison of urinary and plasma catecholamine responses to mental stress. Acta Physiol Scand. 1983;117:19–26. [DOI] [PubMed] [Google Scholar]
- 27. Black PH, Garbutt LD. Stress, inflammation and cardiovascular disease. J Psychosom Res. 2002;52:1–23. [DOI] [PubMed] [Google Scholar]
- 28. Bhatnagar A. Environmental cardiology: studying mechanistic links between pollution and heart disease. Circ Res. 2006;99:692–705. [DOI] [PubMed] [Google Scholar]
- 29. Pope CA, Burnett RT, Krewski D, Jerrett M, Shi Y, Calle EE, Thun MJ. Cardiovascular mortality and exposure to airborne fine particulate matter and cigarette smoke. Circulation. 2009;120:941–948. [DOI] [PubMed] [Google Scholar]
- 30. United States Census Bureau . American Factfinder. 2017.
- 31. University of Wisconsin Health Innovation Program . Area deprivation index. 2014.
- 32. Kentucky Transportation Cabinet . KYTC Datamart. 2014.
- 33. United States Environmental Protection Agency . Outdoor air quality data. 2016.
- 34. United States Geological Survey . Earthexplorer. 2016.
- 35. Tucker CJ. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens Environ. 1979;8:127–150. [Google Scholar]
- 36. Pettorelli N, Vik JO, Mysterud A, Gaillard J‐M, Tucker CJ, Stenseth NC. Using the satellite‐derived NDVI to assess ecological responses to environmental change. Trends Ecol Evol. 2005;20:503–510. [DOI] [PubMed] [Google Scholar]
- 37. de Keijzer C, Agis D, Ambros A, Arevalo G, Baldasano JM, Bande S, Barrera‐Gomez J, Benach J, Cirach M, Dadvand P, Ghigo S, Martinez‐Solanas E, Nieuwenhuijsen M, Cadum E, Basagana X; Group M‐HS . The association of air pollution and greenness with mortality and life expectancy in Spain: a small‐area study. Environ Int. 2017;99:170–176. [DOI] [PubMed] [Google Scholar]
- 38. James P, Hart JE, Banay RF, Laden F. Exposure to greenness and mortality in a nationwide prospective cohort study of women. Environ Health Perspect. 2016;124:1344–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Consortium M‐RLC . National land cover database 2011. 2016.
- 40. Xie Z, Lorkiewicz P, Riggs DW, Bhatnagar A, Srivastava S. Comprehensive, robust, and sensitive UPLC‐MS/MS analysis of free biogenic monoamines and their metabolites in urine. J Chromatogr B Analyt Technol Biomed Life Sci. 2018;1099:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Milne GL, Yin H, Brooks JD, Sanchez S, Roberts LJ, Morrow JD. Quantification of F2‐isoprostanes in biological fluids and tissues as a measure of oxidant stress. Methods Enzymol. 2007;433:113–126. [DOI] [PubMed] [Google Scholar]
- 42. Duda DG, Cohen KS, Scadden DT, Jain RK. A protocol for phenotypic detection and enumeration of circulating endothelial cells and circulating progenitor cells in human blood. Nat Protoc. 2007;2:805–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. O'Toole TE, Hellmann J, Wheat L, Haberzettl P, Lee J, Conklin DJ, Bhatnagar A, Pope CA III. Episodic exposure to fine particulate air pollution decreases circulating levels of endothelial progenitor cells. Circ Res. 2010;107:200–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. DeJarnett N, Yeager R, Conklin DJ, Lee J, O'Toole TE, McCracken J, Abplanalp W, Srivastava S, Riggs DW, Hamzeh I, Wagner S, Chugh A, DeFilippis A, Ciszewski T, Wyatt B, Becher C, Higdon D, Ramos KS, Tollerud DJ, Myers JA, Rai SN, Shah J, Zafar N, Krishnasamy SS, Prabhu SD, Bhatnagar A. Residential proximity to major roadways is associated with increased levels of AC133+ circulating angiogenic cells. Arterioscler Thromb Vasc Biol. 2015;35:2468–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Griendling KK, Touyz RM, Zweier JL, Dikalov S, Chilian W, Chen YR, Harrison DG, Bhatnagar A; American Heart Association Council on Basic Cardiovascular S . Measurement of reactive oxygen species, reactive nitrogen species, and redox‐dependent signaling in the cardiovascular system: a scientific statement from the American Heart Association. Circ Res. 2016;119:e39–e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fadini GP, Losordo D, Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res. 2012;110:624–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Maas J, Verheij RA, de Vries S, Spreeuwenberg P, Schellevis FG, Groenewegen PP. Morbidity is related to a green living environment. J Epidemiol Community Health. 2009;63:967. [DOI] [PubMed] [Google Scholar]
- 48. Castro‐Diehl C, Diez Roux AV, Seeman T, Shea S, Shrager S, Tadros S. Associations of socioeconomic and psychosocial factors with urinary measures of cortisol and catecholamines in the Multi‐Ethnic Study of Atherosclerosis (MESA). Psychoneuroendocrinology. 2014;41:132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fleming R, Baum A, Reddy D, Gatchel RJ. Behavioral and biochemical effects of job loss and unemployment stress. J Human Stress. 1984;10:12–17. [DOI] [PubMed] [Google Scholar]
- 50. Lee ZS, Critchley JA, Tomlinson B, Young RP, Thomas GN, Cockram CS, Chan TY, Chan JC. Urinary epinephrine and norepinephrine interrelations with obesity, insulin, and the metabolic syndrome in Hong Kong Chinese. Metabolism. 2001;50:135–143. [DOI] [PubMed] [Google Scholar]
- 51. Saxena AR, Chamarthi B, Williams GH, Hopkins PN, Seely EW. Predictors of plasma and urinary catecholamine levels in normotensive and hypertensive men and women. J Hum Hypertens. 2014;28:292–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ward KD, Sparrow D, Landsberg L, Young JB, Vokonas PS, Weiss ST. The relationship of epinephrine excretion to serum lipid levels: the Normative Aging Study. Metabolism. 1994;43:509–513. [DOI] [PubMed] [Google Scholar]
- 53. Nishihara K, Mori K, Endo S, Ohta T, Ohara K. Relationship between sleep efficiency and urinary excretion of catecholamines in bed‐rested humans. Sleep. 1985;8:110–117. [DOI] [PubMed] [Google Scholar]
- 54. Morris CJ, Purvis TE, Hu K, Scheer FA. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc Natl Acad Sci U S A. 2016;113:E1402–E1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Luo YH, Tseng PC, Lee YC, Perng RP, Whang‐Peng J, Chen YM. A prospective study of the use of circulating markers as predictors for epidermal growth factor receptor‐tyrosine kinase inhibitor treatment in pulmonary adenocarcinoma. Cancer Biomark. 2016;16:19–29. [DOI] [PubMed] [Google Scholar]
- 56. Whiteley J, Bielecki R, Li M, Chua S, Ward MR, Yamanaka N, Stewart DJ, Casper RF, Rogers IM. An expanded population of CD34+ cells from frozen banked umbilical cord blood demonstrate tissue repair mechanisms of mesenchymal stromal cells and circulating angiogenic cells in an ischemic hind limb model. Stem Cell Rev. 2014;10:338–350. [DOI] [PubMed] [Google Scholar]
- 57. Niemiro GM, Edwards T, Barfield JP, Beals JW, Broad EM, Motl RW, Burd NA, Pilutti LA, De Lisio M. Circulating progenitor cell response to exercise in wheelchair racing athletes. Med Sci Sports Exerc. 2018;50:88–97. [DOI] [PubMed] [Google Scholar]
- 58. Schwandt S, Liedtke S, Kogler G. The influence of temperature treatment before cryopreservation on the viability and potency of cryopreserved and thawed CD34+ and CD45+ cord blood cells. Cytotherapy. 2017;19:962–977. [DOI] [PubMed] [Google Scholar]
- 59. Rogers I, Yamanaka N, Bielecki R, Wong CJ, Chua S, Yuen S, Casper RF. Identification and analysis of in vitro cultured CD45‐positive cells capable of multi‐lineage differentiation. Exp Cell Res. 2007;313:1839–1852. [DOI] [PubMed] [Google Scholar]
- 60. Wong CJ, Casper RF, Rogers IM. Epigenetic changes to human umbilical cord blood cells cultured with three proteins indicate partial reprogramming to a pluripotent state. Exp Cell Res. 2010;316:927–939. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Spearman Correlations Between Vegetation Metrics and Continuous Variables
Table S2. Plasma Biomarker Subgroup (n=82) Analysis Demographics and Cardiovascular Disease Stratified by Low/High 250‐m Contemporaneous NDVI Values Within 250‐m‐Radius Circular Zone Surrounding the Participants’ Residence
Table S3. Circulating Angiogenic Cells Subgroup Analysis Demographics and Cardiovascular Disease Stratified by Low/Medium/High 250‐m Contemporaneous NDVI Values (n=255) Within 250‐m‐Radius Circular Zone Surrounding the Participants’ Residence
Figure S1. Residential area block group clusters.


