Abstract
Background
Vorapaxar, a protease‐activated receptor‐1 antagonist, is approved for secondary prevention of cardiovascular events but is associated with increased intracranial hemorrhage.
Methods and Results
TRACER (Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome) was a trial of vorapaxar versus placebo among patients with acute coronary syndrome. Strokes were adjudicated by a central events committee. Of 12 944 patients, 199 (1.5%) had ≥1 stroke during the study period (median follow‐up, 477 days). Four patients had a single stroke of unknown type; 195 patients had ≥1 stroke classified as hemorrhagic or nonhemorrhagic (165 nonhemorrhagic, 28 hemorrhagic, and 2 both). Strokes occurred in 96 of 6473 patients (1.5%) assigned vorapaxar and 103 of 6471 patients (1.6%) assigned placebo. Kaplan‐Meier incidence of stroke for vorapaxar versus placebo was higher for hemorrhagic stroke (0.45% versus 0.14% [hazard ratio, 2.74; 95% confidence interval, 1.22–6.15]), lower but not significantly different for nonhemorrhagic stroke (1.53% versus 1.98% at 2 years [hazard ratio, 0.79; 95% confidence interval, 0.58–1.07]), and similar for stroke overall (1.93% versus 2.13% at 2 years [hazard ratio, 0.94; 95% confidence interval, 0.71–1.24]).
Conclusions
Stroke occurred in <2% of patients. Vorapaxar‐assigned patients had increased hemorrhagic stroke but a nonsignificant trend toward lower nonhemorrhagic stroke. Overall stroke frequency was similar with vorapaxar versus placebo.
Keywords: acute coronary syndrome, stroke, vorapaxar
Subject Categories: Clinical Studies, Platelets, Myocardial Infarction, Cerebrovascular Disease/Stroke, Acute Coronary Syndromes
Clinical Perspective
What Is New?
Vorapaxar is a novel antiplatelet that is approved for secondary prevention of cardiovascular events but has been associated with increased intracranial hemorrhage.
The overall stroke rate was <2% in patients with acute coronary syndrome enrolled in the TRACER (Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome) trial; although the overall stroke frequency was similar between vorapaxar‐ and placebo‐assigned patients, those randomized to vorapaxar had more hemorrhagic strokes.
Patients with a history of stroke were at greater risk for any new stroke or new ischemic stroke during follow‐up, but the risk of new hemorrhagic stroke was not significant.
What Are the Clinical Implications?
Greater than 90% of patients enrolled in the TRACER trial were receiving aspirin and a P2Y12 agent, such as clopidogrel, resulting in triple antiplatelet therapy during the course of the study; other schemes, such as combinations of vorapaxar with either aspirin or a P2Y12 agent, may have less bleeding risk and similar or more benefit.
Further research is needed to do the following: (1) identify factors associated with hemorrhagic stroke and other bleeding events with vorapaxar; and (2) evaluate the role of vorapaxar as monotherapy for secondary stroke prevention.
Introduction
Vorapaxar is a protease‐activated receptor‐1 antagonist approved for secondary prevention of cardiovascular events in patients with a history of myocardial infarction (MI) or peripheral arterial disease who have not had a stroke or transient ischemic attack (TIA).1 Although vorapaxar was approved by the US Food and Drug Administration (FDA) in 2014,2, 3 clinical use of this antiplatelet agent has been modest.4, 5
A barrier to more widespread vorapaxar use may be its association with increased intracranial hemorrhage (ICH) in 2 phase 3 trials: TRACER (Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome) and TRA2P‐TIMI 50 (Thrombin Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events–Thrombolysis in Myocardial Infarction 50).6, 7 The TRACER trial, which examined vorapaxar in acute coronary syndromes (ACSs), was stopped after the requisite number of events per protocol had been achieved but earlier than planned when the data safety monitoring board observed a higher ICH rate with vorapaxar.6 The TRA2P‐TIMI 50 trial, which examined vorapaxar for secondary prevention, was allowed to continue, but patients with previous stroke or incident stroke during the trial (in whom bleeding risk was greater) were discontinued from the study.7
Secondary analyses of the TRA2P‐TIMI 50 trial have further characterized vorapaxar's hemorrhagic and overall stroke risk. One analysis demonstrated that vorapaxar‐assigned patients with a history of ischemic stroke had a higher risk of bleeding and ICH but no lower risk of ischemic events.8 Another analysis, limited to the FDA‐approved patient population, which excludes patients with a history of stroke or TIA, demonstrated that although vorapaxar‐assigned patients had a higher rate of ICH, they also had a lower rate of ischemic stroke and of stroke overall.9
The aim of this analysis is to further characterize stroke subtypes and outcomes with vorapaxar using data from the TRACER trial.
Methods
Transparency and Openness Promotion
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
The TRACER Trial
The design of the TRACER trial has been described previously.6 In brief, the TRACER trial was a phase 3, randomized, double‐blinded, placebo‐controlled, multicenter trial of vorapaxar versus placebo in ACS. The TRACER trial was approved by the Duke Institutional Review Board, as the coordinating center, and by local institutional review boards. All participants provided written informed consent. Altogether, 12 944 patients were enrolled in >800 centers in 37 countries. Patients were randomized within 24 hours of presentation to the hospital with ischemic symptoms, if they had either positive cardiac biomarkers (troponin and creatine kinase‐muscle/brain [CK‐MB]) or had ST‐segment depression/transient elevation and an additional risk‐enhancing characteristic (aged >55 years, prior percutaneous coronary intervention, prior coronary artery bypass graft surgery, prior MI, peripheral arterial disease, or diabetes mellitus). Exclusion criteria included an indication for systemic anticoagulation. Patients with a history of atrial fibrillation were, therefore, only enrolled if they were not receiving anticoagulation or did not have an indication to be receiving anticoagulation. Patients were randomized to either vorapaxar, which included a 40‐mg loading dose, followed by a 2.5‐mg/d maintenance dose, or placebo. Concomitant antiplatelet therapy was left to the discretion of the treating physician. Most patients in the trial were receiving dual antiplatelet therapy in addition to study drug, with administration of aspirin to 99% and clopidogrel to 91.8% (91.7% in the placebo arm and 91.9% in the vorapaxar arm) of patients, resulting in triple antiplatelet therapy in most patients in the vorapaxar arm and dual antiplatelet therapy plus placebo in most patients in the placebo arm.6 Duration of planned treatment was the entire length of the study and a minimum of 1 year.
Patients were assessed at 30 days, 4 months, 8 months, 12 months, and every 6 months thereafter. The primary efficacy end point was a composite that included death from cardiovascular causes, MI, stroke, recurrent ischemia, rehospitalization, or coronary revascularization. Primary safety end points were moderate or severe bleeding, according to the Global Use of Strategies to Open Occluded Arteries classification, and clinically significant bleeding, according to the TIMI classification.10, 11
Stroke End Points
Suspected strokes in the TRACER trial were adjudicated and categorized by a central events committee, which included a vascular neurologist. Site investigators provided clinical information on standard case report forms and clinical information such as consult notes, procedure reports, admission and discharge notes, imaging reports, and autopsy information, if available.
Statistical Analysis
Baseline characteristics were summarized among patients with no stroke events during the trial and among patients who had at least one stroke, as well as by stroke type (ICH/nonhemorrhagic) among patients experiencing an event. Baseline characteristics were further summarized by treatment group within each stroke type. Stroke attributes and outcomes/actions after stroke were summarized by treatment group. Categorical variables were presented as counts and proportions, and continuous variables were presented as medians with interquartile ranges. All analyses of patient and stroke characteristics were descriptive, and no statistical tests were performed.
The distribution of time to event for each type of stroke (ischemic, hemorrhagic, and any), overall and within vorapaxar and placebo groups, was assessed and 2‐year rates were estimated by the Kaplan‐Meier (KM) method. Comparisons were made by means of the log‐rank test. The KM method was also used to analyze whether there was a difference in rates of stroke at 2 years in patients with versus without history of stroke before trial enrollment. Baseline characteristics were balanced between treatment arms by randomization and, therefore, no adjustment for baseline confounders was used in modeling outcomes on treatment. All statistical analyses were performed using SAS software, version 9.4 (SAS Institute Inc, Cary, NC).
Results
Baseline Characteristics
Of the 12 944 patients enrolled in the TRACER trial, a total of 199 (1.5%) had ≥1 stroke (Table 1). Compared with patients without stroke, those with strokes tended to be older (median age, 69 years [quartile 1–quartile 3, 60–74 years] versus 64 years [quartile 1–quartile 3, 58–71 years]) and were more likely to have a history of hypertension (80.9% versus 70.4%), diabetes mellitus (39.2% versus 31.3%), and comorbidities before study enrollment, such as stroke (11.1% versus 4.2%), TIA (8.5% versus 2.4%), MI (31.7% versus 29.3%), peripheral arterial disease (12.6% versus 7.2%), and atrial fibrillation (10.1% versus 4.3%).
Table 1.
Baseline Demographic and Clinical Characteristics of the TRACER Trial Patients With and Without at Least 1 Episode of Stroke During the Trial
| Variable | No Stroke (N=12 745) | At Least 1 Stroke (N=199) | Total (N=12 944) |
|---|---|---|---|
| Patient characteristics | |||
| Age, y | 64 (58–71) | 69 (60–74) | 64 (58–72) |
| Female sex | 3576 (28.1) | 56 (28.1) | 3632 (28.1) |
| Race | |||
| White | 10 873 (85.5) | 166 (83.8) | 11 039 (85.5) |
| Black or African American | 305 (2.4) | 7 (3.5) | 312 (2.4) |
| Asian | 1042 (8.2) | 14 (7.1) | 1056 (8.2) |
| Native Hawaiian or other Pacific Islander | 32 (0.3) | 0 (0.0) | 32 (0.2) |
| Multiracial | 425 (3.3) | 10 (5.1) | 435 (3.4) |
| Body mass index, kg/m2 | 28 (25–31) | 27 (25–31) | 28 (25–31) |
| Region of enrollment | |||
| North America | 3339 (26.2) | 65 (32.7) | 3404 (26.3) |
| Latin America | 824 (6.5) | 24 (12.1) | 848 (6.6) |
| Europe 1 | 5758 (45.2) | 81 (40.7) | 5839 (45.1) |
| Europe 2 | 1471 (11.5) | 16 (8.0) | 1487 (11.5) |
| Asia/Pacific | 925 (7.3) | 11 (5.5) | 936 (7.2) |
| Australia/New Zealand | 428 (3.4) | 2 (1.0) | 430 (3.3) |
| Cardiovascular risk factors | |||
| Hypertension | 8967 (70.4) | 161 (80.9) | 9128 (70.5) |
| Hypercholesterolemia | 7941 (62.3) | 121 (60.8) | 8062 (62.3) |
| Diabetes mellitus | 3992 (31.3) | 78 (39.2) | 4070 (31.5) |
| Smoker at enrollment | 3491 (27.4) | 45 (22.6) | 3536 (27.3) |
| Comorbidities | |||
| Stroke | 531 (4.2) | 22 (11.1) | 553 (4.3) |
| History of TIA | 308 (2.4) | 17 (8.5) | 325 (2.5) |
| Myocardial infarction | 3728 (29.3) | 63 (31.7) | 3791 (29.3) |
| Peripheral arterial vascular disease | 911 (7.2) | 25 (12.6) | 936 (7.2) |
| History of atrial fibrillation | 548 (4.3) | 20 (10.1) | 568 (4.4) |
| Status at time of presentation | |||
| Killip class ≤2 at enrollment | 12 513 (99.0) | 189 (95.5) | 12 702 (99.0) |
| Creatinine clearance, mL/min | 95 (73–122) | 82 (64–103) | 95 (73–121) |
| ECG findings | |||
| ST‐segment elevation at enrollment | 725 (5.7) | 11 (5.5) | 736 (5.7) |
| ST‐segment depression at enrollment | 4129 (32.4) | 70 (35.2) | 4199 (32.4) |
Data presented as median (quartile 1–quartile 3) for continuous variables and as number (percentage) for discrete variables. TIA indicates transient ischemic attack; TRACER, Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome.
Stroke Frequency and Type
Any stroke
Of the 199 patients who experienced stroke during the study (Table 2), 4 had a single stroke of unknown type and 195 had at least one stroke that was classified as hemorrhagic or nonhemorrhagic. Of the patients with at least one stroke with a classified type, 165 experienced only nonhemorrhagic stroke, 28 experienced only hemorrhagic stroke, and 2 experienced both a hemorrhagic and a nonhemorrhagic stroke. Hemorrhagic conversion was seen in 3 (3.1%) of the 96 primary nonhemorrhagic strokes in the vorapaxar arm and in 5 (4.9%) of the 103 primary nonhemorrhagic strokes in the placebo arm (Table 2).
Table 2.
Stroke Characteristics, Among Patients With at Least 1 CEC‐Adjudicated Stroke, by Treatment Group and Overall
| Variable | Vorapaxar (N=96) | Placebo (N=103) | Total (N=199) |
|---|---|---|---|
| No. of stroke events | |||
| 1 | 92 (95.8) | 93 (90.3) | 185 (93.0) |
| 2 | 4 (4.2) | 7 (6.8) | 11 (5.5) |
| 3 | 0 (0.0) | 3 (2.9) | 3 (1.5) |
| First event: type of stroke | |||
| Nonhemorrhagic | |||
| Suspected embolic | 38 (39.6) | 54 (52.4) | 92 (46.2) |
| Other | 36 (37.5) | 39 (37.9) | 75 (37.7) |
| With hemorrhagic conversion | 3 (3.1) | 4 (3.9) | 7 (3.5) |
| Primary intracerebral hemorrhage | 15 (15.6) | 3 (2.9) | 18 (9.0) |
| Subarachnoid hemorrhage | 3 (3.1) | 0 (0.0) | 3 (1.5) |
| Uncertain | 1 (1.0) | 3 (2.9) | 4 (2.0) |
| Any nonhemorrhagic stroke | 74 (77.1) | 93 (90.3) | 167 (83.9) |
| Any hemorrhagic stroke | 22 (22.9) | 8 (7.8) | 30 (15.1) |
| Any hemorrhagic conversion | 3 (3.1) | 5 (4.9) | 8 (4.0) |
| Any intracerebral hemorrhage | 16 (16.7) | 3 (2.9) | 19 (9.5) |
| Any subarachnoid hemorrhage | 3 (3.1) | 0 (0.0) | 3 (1.5) |
| Any stroke of uncertain type | 1 (1.0) | 3 (2.9) | 4 (2.0) |
Data are presented as number (percentage) for discrete variables. CEC indicates central clinical events committee.
At least one stroke during the study period occurred in 96 (1.5%) of 6473 patients assigned vorapaxar and in 103 (1.6%) of 6471 patients assigned placebo (Table 2). KM rates at 2 years for overall stroke in vorapaxar‐ versus placebo‐assigned patients were similar: 1.93% versus 2.13% (hazard ratio [HR], 0.94; 95% confidence interval [CI], 0.71–1.24) (Figure [A]).
Figure 1.
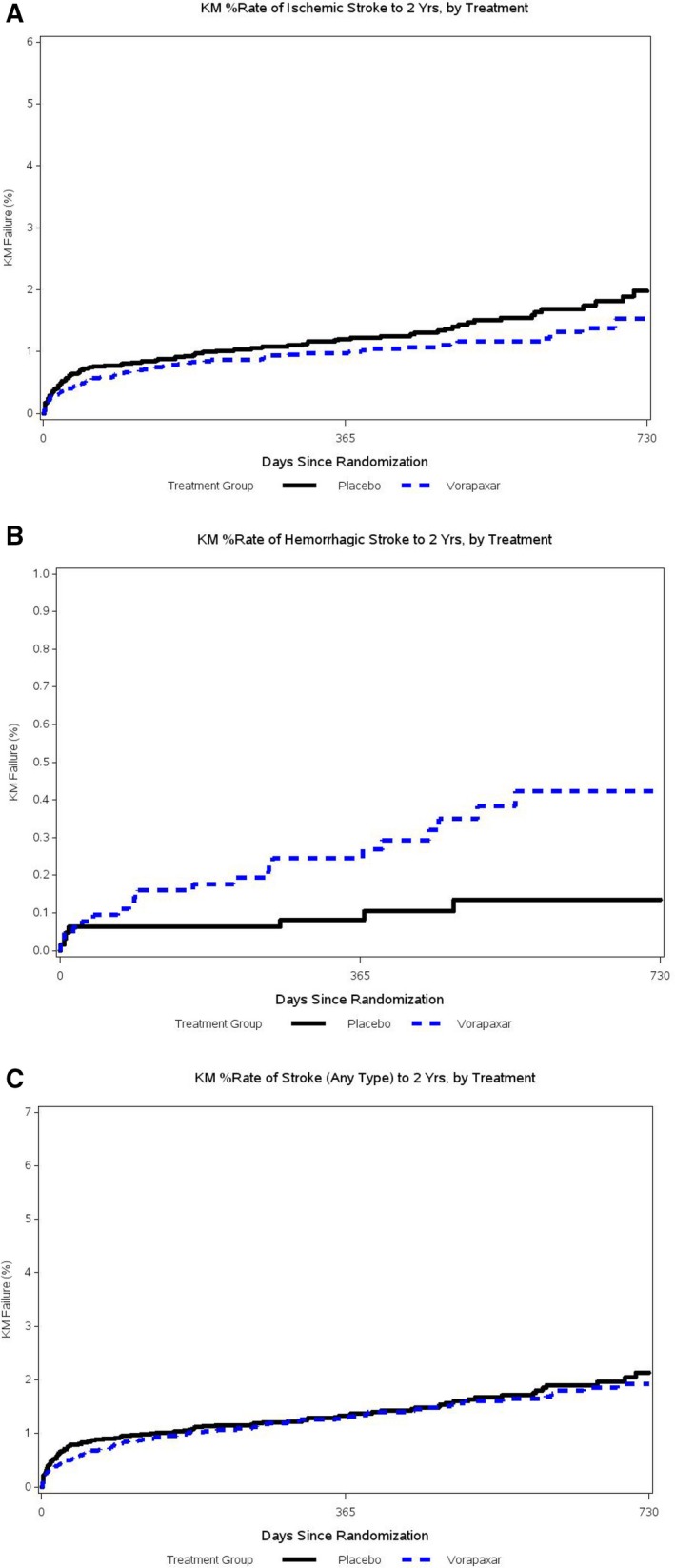
Kaplan‐Meier percentage rate to 2 years in vorapaxar vs placebo in any stroke (A), hemorrhagic stroke (B), and nonhemorrhagic stroke (C). CI indicates confidence interval; HR, hazard ratio.
Hemorrhagic stroke
Among patients having strokes during the study period, there were proportionally more hemorrhagic strokes among vorapaxar‐assigned patients: 22 (22.9%) of 96 patients versus 8 (7.8%) of 103 patients (Table 2). Likewise, the KM percentage rate to 2 years for hemorrhagic stroke was significantly higher in vorapaxar‐ versus placebo‐assigned patients: 0.45% versus 0.13% (HR, 2.74; 95% CI, 1.22–6.15) (Figure [B]).
Nonhemorrhagic stroke
Among patients having strokes during the study period, there were proportionally fewer nonhemorrhagic strokes among vorapaxar‐assigned patients: 74 (77.1%) of 96 patients versus 93 (90.3%) of 103 patients (Table 2). KM percentage rates to 2 years for nonhemorrhagic stroke were lower, but not significantly different, in vorapaxar‐ versus placebo‐assigned patients: 1.53% versus 1.98% (HR, 0.79; 95% CI, 0.58–1.07) (Figure [C]).
Baseline Characteristics of Patients by Type of Stroke (Hemorrhagic Versus Nonhemorrhagic)
We examined baseline characteristics of patients whose first stroke was hemorrhagic versus nonhemorrhagic, both overall (Table 3) and by treatment assignment (Table 4). Compared with patients whose first stroke was nonhemorrhagic stroke, those with an initial hemorrhagic stroke were more likely to be women (39.3% versus 25.1%) and less likely to have hypertension (67.9% versus 82.6%) and a history of prior stroke (3.6% versus 12.6%).
Table 3.
Baseline Demographic and Clinical Characteristics of the TRACER Trial Patients With Hemorrhagic Versus Nonhemorrhagic Stroke Overall
| Variable | Patients With Hemorrhagic Stroke (N=28) | Patients With Nonhemorrhagic Stroke (N=167) |
|---|---|---|
| Patient characteristics | ||
| Age, y | 70 (62–76) | 68 (60–73) |
| Female sex | 11 (39.3) | 42 (25.1) |
| Race | ||
| White | 24 (85.7) | 138 (83.1) |
| Black or African American | 0 (0.0) | 7 (4.2) |
| Asian | 1 (3.6) | 13 (7.8) |
| Native Hawaiian or other Pacific Islander | 0 (0.0) | 0 (0.0) |
| Multiracial | 3 (10.7) | 7 (4.2) |
| Body mass index, kg/m2 | 29 (25–31) | 27 (25–32)a |
| Region of enrollment | ||
| North America | 6 (21.4) | 58 (34.7) |
| Latin America | 4 (14.3) | 20 (12.0) |
| Europe 1 | 14 (50.0) | 65 (38.9) |
| Europe 2 | 3 (10.7) | 12 (7.2) |
| Asia/Pacific | 1 (3.6) | 10 (6.0) |
| Australia/New Zealand | 0 (0.0) | 2 (1.2) |
| Cardiovascular risk factors | ||
| Hypertension | 19 (67.9) | 138 (82.6) |
| Hypercholesterolemia | 14 (50.0) | 104 (62.3) |
| Diabetes mellitus | 11 (39.3) | 65 (38.9) |
| Smoker at enrollment | 3 (10.7) | 41 (24.6) |
| Comorbidities | ||
| Stroke | 1 (3.6) | 21 (12.6) |
| History of TIA | 3 (10.7) | 14 (8.4) |
| Myocardial infarction | 9 (32.1) | 54 (32.3) |
| Peripheral arterial vascular disease | 2 (7.1) | 22 (13.2) |
| History of atrial fibrillation | 2 (7.1) | 17 (10.2) |
| Status at time of presentation | ||
| Killip class ≤2 at enrollment | 27 (96.4) | 158 (95.2) |
| Creatinine clearance, mL/minb | 82 (60–102) | 82 (65–105) |
| ECG findings | ||
| ST‐segment elevation at enrollment | 1 (3.6) | 10 (6.0) |
| ST‐segment depression at enrollment | 11 (39.3) | 57 (34.1) |
Data are presented as median (quartile 1–quartile 3) for continuous variables and number (percentage) for discrete variables. TIA indicates transient ischemic attack; TRACER, Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome.
For body mass index, N=165 for patients with nonhemorrhagic stroke.
For creatinine clearance, N=27 for patients with hemorrhagic stroke and N=154 for patients with nonhemorrhagic stroke.
Table 4.
Baseline Demographic and Clinical Characteristics of the TRACER Trial Patients With Hemorrhagic Versus Nonhemorrhagic Stroke by Treatment Assignment
| Variable | Patients With Hemorrhagic Stroke | Patients With Nonhemorrhagic Stroke | ||
|---|---|---|---|---|
| Vorapaxar (N=21) | Placebo (N=7) | Vorapaxar (N=74) | Placebo (N=93) | |
| Patient characteristics | ||||
| Age, y | 69 (61–75) | 74 (64–77) | 66 (60–73) | 69 (61–74) |
| Female sex | 9 (42.9) | 2 (28.6) | 20 (27.0) | 22 (23.7) |
| Race | ||||
| White | 17 (81.0) | 7 (100.0) | 58 (78.4) | 80 (87.0) |
| Black or African American | 0 (0.0) | 0 (0.0) | 1 (1.4) | 6 (6.5) |
| Asian | 1 (4.8) | 0 (0.0) | 8 (10.8) | 5 (5.4) |
| Native Hawaiian or other Pacific Islander | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Multiracial | 3 (14.3) | 0 (0.0) | 6 (8.1) | 1 (1.1) |
| Body mass index, kg/m2 a | 30 (25–31) | 27 (25–31) | 28 (24–31) | 27 (25–32) |
| Region of enrollment | ||||
| North America | 4 (19.0) | 2 (28.6) | 23 (31.1) | 35 (37.6) |
| Latin America | 4 (19.0) | 0 (0.0) | 12 (16.2) | 8 (8.6) |
| Europe 1 | 11 (52.4) | 3 (42.9) | 26 (35.1) | 39 (41.9) |
| Europe 2 | 1 (4.8) | 2 (28.6) | 6 (8.1) | 6 (6.5) |
| Asia/Pacific | 1 (4.8) | 0 (0.0) | 7 (9.5) | 3 (3.2) |
| Australia/New Zealand | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (2.2) |
| Cardiovascular risk factors | ||||
| Hypertension | 13 (61.9) | 6 (85.7) | 60 (81.1) | 78 (83.9) |
| Hypercholesterolemia | 11 (52.4) | 3 (42.9) | 46 (62.2) | 58 (62.4) |
| Diabetes mellitus | 10 (47.6) | 1 (14.3) | 31 (41.9) | 34 (36.6) |
| Smoker at enrollment | 3 (14.3) | 0 (0.0) | 17 (23.0) | 24 (25.8) |
| Comorbidities | ||||
| Stroke | 0 (0.0) | 1 (14.3) | 8 (10.8) | 13 (14.0) |
| History of TIA | 2 (9.5) | 1 (14.3) | 9 (12.2) | 5 (5.4) |
| Myocardial infarction | 7 (33.3) | 2 (28.6) | 20 (27.0) | 34 (36.6) |
| Peripheral arterial vascular disease | 1 (4.8) | 1 (14.3) | 6 (8.1) | 16 (17.2) |
| History of atrial fibrillation | 1 (4.8) | 1 (14.3) | 9 (12.2) | 8 (8.6) |
| Status at time of presentation | ||||
| Killip class ≤2 at enrollment | 20 (95.2) | 7 (100.0) | 68 (91.9) | 90 (97.8) |
| Creatinine clearance, mL/minb | 75 (47–108) | 86 (82–89) | 78 (65–100) | 90 (65–106) |
| ECG findings | ||||
| ST‐segment elevation at enrollment | 1 (4.8) | 0 (0.0) | 3 (4.1) | 7 (7.5) |
| ST‐segment depression at enrollment | 9 (42.9) | 2 (28.6) | 26 (35.1) | 31 (33.3) |
Data are presented as median (quartile 1–quartile 3) for continuous variables and number (percentage) for discrete variables. TIA indicates transient ischemic attack; TRACER, Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome.
For body mass index for patients with nonhemorrhagic stroke, N=73 for the vorapaxar group and N=92 for the placebo group.
For creatinine clearance for patients with hemorrhagic stroke, N=20 for the vorapaxar group; for patients with nonhemorrhagic stroke, N=69 for the vorapaxar group and N=85 for the placebo group.
We also examined and found an association between increased blood pressure at presentation and risk of stroke, with an HR (95% CI) per 10–mm Hg increase in systolic blood pressure calculated at 1.07 (1.00–1.52, P=0.051) for ischemic stroke, 1.23 (1.05–1.43, P=0.009) for hemorrhagic stroke, and 1.10 (1.03–1.17, P=0.030) for any stroke.
Stroke Frequency in Patients With a History of Stroke
Table 5 displays KM rates of stroke (hemorrhagic, nonhemorrhagic, and any) at 2 years for patients with and without stroke history. The KM rates in patients with stroke history were significantly higher for ischemic stroke (5.95 versus 1.58 [HR, 3.29; 95% CI, 2.06–5.25]) and for any stroke (6.58 versus 1.84 [HR, 2.95; 95% CI, 1.89–4.59]), but not significantly different for hemorrhagic stroke (0.66 versus 0.27 [HR, 1.73; 95% CI, 0.41–7.25]).
Table 5.
Unadjusted Incidence of Stroke (Ischemic, Hemorrhagic, and Any) After 2 Years, Among All Patients and in Patients With or Without Prior Stroke
| End Point | No History of Stroke | History of Stroke | Total |
|---|---|---|---|
| Ischemic stroke | 144 (1.579) | 20 (5.953) | 164 (1.755) |
| Hemorrhagic stroke | 26 (0.265) | 2 (0.658) | 28 (0.279) |
| Any stroke | 175 (1.843) | 22 (6.577) | 197 (2.032) |
Data are given as event count at 2 years (percentage Kaplan‐Meier rate). Log‐rank test results: ischemic stroke, P<0.0001; hemorrhagic stroke, P=0.4498; and any stroke, P<0.0001.
Events After Stroke
Events after first strokes are displayed in Figure S1 (A and B). The figure subgroups patients on the basis of whether they were assigned vorapaxar or placebo and by what type of stroke they had: any (hemorrhagic plus nonhemorrhagic), hemorrhagic, and nonhemorrhagic. Patients were further subdivided on the basis of whether study drug was continued or discontinued after their stroke, to illustrate the impact of different management strategies on outcomes. The number of events after stroke was small and relatively comparable across all groups.
Discussion
Stroke occurred in <2% of patients with ACS during long‐term follow‐up in the TRACER trial. Patients assigned to vorapaxar had more hemorrhagic strokes and fewer nonhemorrhagic strokes. Overall stroke frequency was similar between vorapaxar‐ and placebo‐assigned patients. Patients with a history of stroke were 6 to 10 times more likely to have an ischemic stroke than a hemorrhagic stroke. A small number of adverse events occurred after stroke across all groups.
After the TRACER6 and TRA2P‐TIMI 507 trials demonstrated an increased rate of hemorrhagic stroke in vorapaxar‐treated patients, the need to better characterize vorapaxar's stroke and bleeding risk has been emphasized. Our results build on previous studies of stroke outcomes with vorapaxar. Earlier analyses from the TRA2P‐TIMI 50 trial demonstrated that vorapaxar‐assigned patients with a history of ischemic stroke have a higher rate of bleeding and ICH without a lower rate of ischemic events8 and that vorapaxar‐assigned patients without a history of stroke have higher rates of hemorrhagic stroke but lower rates of ischemic stroke and of stroke overall.9 The TARDIS (Triple Antiplatelets for Reducing Dependency after Ischemic Stroke) trial, which compared intense triple therapy with guideline‐based therapy (1 or 2 antiplatelet agents) in patients with ischemic stroke, has similarly demonstrated increased bleeding with a triple antiplatelet therapy strategy without an advantage in reducing the incidence or severity of stroke.12 Our study expands on these results by examining stroke type and outcomes in the TRACER trial population, including all patients in the TRACER trial regardless of prior stroke history, and reporting on adverse events after stroke.
Although our findings are consistent with those of the TRA2P‐TIMI 50 trial substudies, there are several notable differences. Although both our and prior studies demonstrate a significantly higher rate of hemorrhagic stroke among vorapaxar‐assigned patients, we found a nonsignificantly lower rate of ischemic stroke and a similar rate of stroke overall, whereas the TRA2P‐TIMI 50 trial substudy found a significantly lower rate of both these outcomes. The higher overall stroke rate in our study may be because of a higher‐risk patient population. Although the TRA2P‐TIMI 50 trial substudy excluded patients with a history of cerebrovascular accident or TIA, who are at especially high risk of ICH with vorapaxar,8 we included patients with this higher‐risk history. In addition, patients in the TRACER trial started receiving vorapaxar during ACS presentations as opposed to at least 2 weeks after their events, as in the TRA2P‐TIMI 50 trial. Patients with ACS are more likely to be platelet naïve and, thus, may carry higher bleeding risk with initiation of triple antiplatelet therapy. Despite studying patients who were at higher risk, we still found a similar overall stroke rate with vorapaxar versus placebo.
Our data on stroke severity also resemble and expand on data from the TRA2P‐TIMI 50 trial. The TRA2P‐TIMI 50 trial substudy also found that the rate of hemorrhagic conversion of nonhemorrhagic stroke was not higher among vorapaxar‐treated patients.9 We build on these results by reporting on other adverse events after stroke, stratifying whether vorapaxar was continued or discontinued, and assessing whether the initial stroke was hemorrhagic or nonhemorrhagic. Our finding of no large numerical differences in adverse events after stroke may suggest that strokes among vorapaxar‐treated patients are of similar clinical significance. Alternatively, the apparent similarities could be because of the small number of strokes and small number of adverse events. Larger studies with more stroke outcomes and longer longitudinal follow‐up would be required to differentiate between these possibilities.
These results address a key question about vorapaxar on its risk‐benefit profile with regard to stroke. Although vorapaxar was shown in the TRACER6 and TRA2P‐TIMI 507 trials to reduce rates of cardiovascular events, concern arising from vorapaxar's association with ICH has resulted in limited vorapaxar use. To evaluate the risk‐benefit of vorapaxar with regard to stroke, however, it is important not only to consider vorapaxar's association with hemorrhagic stroke, but also its effect on nonhemorrhagic stroke and stroke overall. The prior TRA2P‐TIMI 50 trial data8, 9 have suggested and our data seem to confirm that although vorapaxar is associated with higher rates of hemorrhagic stroke, it may also be associated with similar stroke rates overall. These results should not be overinterpreted, but it is reasonable to consider whether this observation of similar overall stroke rates should mitigate initial concern relating to vorapaxar's ICH association and whether it may shift vorapaxar's overall risk‐benefit profile toward benefit.
Further research is needed to identify factors associated with hemorrhagic stroke and other bleeding events with vorapaxar. A recent meta‐analysis of patients with ACS enrolled in clinical trials identified older age, prior TIA or cerebrovascular accident, higher systolic blood pressure, and more antithrombotic agents as risk factors for ICH.13 We also found an association between higher systolic blood pressure and hemorrhagic stroke, as well as nonhemorrhagic and all types of stroke. A next step could be developing a clinical predictor similar to those that exist to help risk stratify patients in other settings (eg, HAS‐BLED14 and CHA2DS2‐VASc15 for anticoagulation in atrial fibrillation, the DAPT Score16 for dual antiplatelet therapy after percutaneous coronary intervention, and the Intracranial‐B2LEED3S Score17 for risk of ICH with other antiplatelets). Another goal could be identifying novel bleeding risk factors, including genetic and other biomarkers. These could potentially include neuroimaging biomarkers that may be associated with increased risk of hemorrhagic stroke. In addition, clinical studies should be performed that examine alternative antiplatelet combinations. In the TRACER trial, vorapaxar was combined with standard therapy per the treating physician's discretion, which in >90% of patients included aspirin and a P2Y12 agent, such as clopidogrel, resulting in triple antiplatelet therapy.6 Other schemes, such as combinations of vorapaxar with either aspirin or a P2Y12 agent, may have less bleeding risk and similar or more benefit. In addition, the role of vorapaxar as monotherapy for secondary stroke prevention has not been assessed, and future trials could compare it with current standard of care (eg, aspirin monotherapy or aspirin plus a P2Y12 agent) for this indication.
Limitations
This study has some limitations. First, the overall number of strokes in the TRACER trial was small, limiting sample size and statistical power. Second, Rankin scores (a validated stroke severity rating scale) were not rigorously collected on all subjects. The lack of these data limits this study's ability to assess the clinical significance of the strokes that occurred. Finally, the study's generalizability should be interpreted in the context of differences between the study population and the FDA‐approved patient population. In this study population, vorapaxar was started during ACS, as opposed to after a minimum of 2 weeks after an ischemic event, as is mandated by the FDA. This analysis also included patients with a history of stroke or TIA, and the FDA strongly recommends against starting vorapaxar in patients with this history.
Conclusion
We found that although vorapaxar was associated with an increased rate of hemorrhagic stroke, it was also associated with a nonsignificant trend toward lower ischemic stroke and a similar rate of stroke overall. There were few adverse events after strokes in both treatment groups. These results contribute to our understanding of stroke types and outcomes in vorapaxar‐treated patients. Further research is needed to identify factors associated with stroke with vorapaxar, which could include both clinical predictors and novel biomarkers.
Sources of Funding
The TRACER (Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome) trial was funded by Merck & Co.
Disclosures
Armstrong's full list of disclosures is given at http://thecvc.ca/about-us/relationships-with-industry/. He reports research grants or contracts from Sanofi Aventis Recherche & Développement, Merck, Boehringer Ingelheim, Bayer, and CSL Limited; and consulting for AstraZeneca, Merck, Bayer, Novartis, and Boehringer Ingelheim. Aylward reports research grants from Merck & Co, AstraZeneca, Sanofi, and GSK; honoraria (speaker's bureau and advisory board) from AstraZeneca, Eli Lilly, Boehringer Ingelheim, Bayer J&J, Servier, and Bristol Myers Squibb. Held reports institutional research grants from AstraZeneca, GlaxoSmithKline, Pfizer/Bristol Myers Squibb, Roche, and Schering‐Plough (now Merck); and consulting for AstraZeneca. Moliterno reports grants from Merck, Inc, during the conduct of the study. Strony is a former employee of Merck & Co and a current employee of Johnson & Johnson. Van de Werf reports research grants, honoraria for lectures, and advisory board membership for Merck. Wallentin reports grants from Merck & Co and Roche Diagnostics; grants and personal fees from Bristol‐Myers Squibb/Pfizer, AstraZeneca, GlaxoSmithKline, and Boehringer Ingelheim; and personal fees from Abbott. White reports grants from Sanofi Aventis, Eli Lilly and Company, National Institutes of Health (NIH), and DalGen Products and Services; personal fees and nonfinancial support from AstraZeneca; grants and personal fees from Omthera Pharmaceuticals, Pfizer, and Elsai Inc; and personal fees from Sirtex and Acetelion. Tricoci reports a consultant agreement and research grant from Merck. All disclosures are available at https://dcri.org/about-us/conflict-of-interest/. Harrington reports consultant fees/honoraria from Adverse Events, Amgen Inc, Daiichi‐Lilly, Gilead Sciences, Janssen Research and Development, Medtronic, Merck, Novartis Corporation, The Medicines Company, Vida Health, Vox Media, and WebMD; research/research grants from AstraZeneca, BMS, CSL Behring, GSK, Merck, Portola, Sanofi‐Aventis, and The Medicines Company; ownership interest/partnership/principal from Element Science and MyoKardia; officer, director, trustee, or other fiduciary role for Evidint and Scanadu; Data Safety Monitoring Board for Regado; and other: American Heart Association. Mahaffey reports financial disclosures available at http://med.stanford.edu/profiles/kenneth-mahaffey; research grant or contract from Afferent, Amgen, Apple Inc, AstraZeneca, Cardiva Medical Inc, Daiichi, Ferring, Google (Verily), Johnson & Johnson, Luitpold, Medtronic, Merck, NIH, Novartis, Sanofi, St Jude, and Tenax; consulting for Abbott, Ablynx, AstraZeneca, Baim Institute, Boehringer Ingelheim, Bristol Myers Squibb, Cardiometabolic Health Congress, Elsevier, GlaxoSmithKline, Johnson & Johnson, Medergy, Medscape, Merck, Mitsubishi, Myokardia, NIH, Novartis, Novo Nordisk, Oculeve, Portola, Radiometer, Springer Publishing, Theravance, University of California, San Francisco, and WebMD; and equity in BioPrint Fitness. Melloni reports research grants or contracts from Abbott Laboratories, Amgen Inc, Duke Clinical Research Institute, Genzyme Corporation, Janssen Research & Development, Lundbeck Pharmaceuticals, Pfizer, and TESARO Inc; and consulting for Amgen Inc, Genetech, and Shire. A full list of disclosures for Dr Melloni is available at https://dcri.org/about-us/conflict-of-interest/. The remaining authors have no disclosures to report.
Supporting information
Figure S1. Events by management of vorapaxar and placebo after first stroke. A, Overall (=any stroke). B, By stroke type.
Acknowledgments
Melloni had full access to all the data in the study and takes responsibility for its integrity and the data analysis.
(J Am Heart Assoc. 2018;7:e009609 DOI: 10.1161/JAHA.118.009609.)
An oral presentation of the results of this work was given at the American Heart Association Scientific Sessions, November 10 to 12, 2018, in Chicago, IL.
References
- 1. Ungar L, Rodriguez F, Mahaffey KW. Vorapaxar: emerging evidence and clinical questions in a new era of PAR‐1 inhibition. Coron Artery Dis. 2016;27:604–615. [DOI] [PubMed] [Google Scholar]
- 2. Baker NC, Lipinski MJ, Lhermusier T, Waksman R. Overview of the 2014 Food and Drug Administration Cardiovascular and Renal Drugs Advisory Committee meeting about vorapaxar. Circulation. 2014;130:1287–1294. [DOI] [PubMed] [Google Scholar]
- 3. Magnani G, Bonaca MP, Braunwald E, Dalby AJ, Fox KA, Murphy SA, Nicolau JC, Oude Ophuis T, Scirica BM, Spinar J, Theroux P, Morrow DA. Efficacy and safety of vorapaxar as approved for clinical use in the United States. J Am Heart Assoc. 2015;4:e001505 DOI: 10.1161/JAHA.114.001505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Staton T. Merck cuts 148 jobs after giving up on ex‐blockbuster hopeful Zontivity. 2016. http://www.fiercepharma.com/marketing/merck-cuts-148-jobs-after-giving-up-ex-blockbuster-hopeful-zontivity. Accessed December 28, 2016.
- 5. Staton T. With $25M‐plus Aralez deal, Merck finds a buyer for abandoned clot‐fighter Zontivity. 2016. http://www.fiercepharma.com/marketing/25m-plus-aralez-deal-merck-finds-a-buyer-for-abandoned-zontivity. Accessed December 28, 2016.
- 6. Tricoci P, Huang Z, Held C, Moliterno DJ, Armstrong PW, Van de Werf F, White HD, Aylward PE, Wallentin L, Chen E, Lokhnygina Y, Pei J, Leonardi S, Rorick TL, Kilian AM, Jennings LH, Ambrosio G, Bode C, Cequier A, Cornel JH, Diaz R, Erkan A, Huber K, Hudson MP, Jiang L, Jukema JW, Lewis BS, Lincoff AM, Montalescot G, Nicolau JC, Ogawa H, Pfisterer M, Prieto JC, Ruzyllo W, Sinnaeve PR, Storey RF, Valgimigli M, Whellan DJ, Widimsky P, Strony J, Harrington RA, Mahaffey KW; TRACER Investigators . Thrombin‐receptor antagonist vorapaxar in acute coronary syndromes. N Engl J Med. 2012;366:20–33. [DOI] [PubMed] [Google Scholar]
- 7. Morrow DA, Braunwald E, Bonaca MP, Ameriso SF, Dalby AJ, Fish MP, Fox KA, Lipka LJ, Liu X, Nicolau JC, Ophuis AJ, Paolasso E, Scirica BM, Spinar J, Theroux P, Wiviott SD, Strony J, Murphy SA; TRA 2P–TIMI 50 Steering Committee and Investigators . Vorapaxar in the secondary prevention of atherothrombotic events. N Engl J Med. 2012;366:1404–1413. [DOI] [PubMed] [Google Scholar]
- 8. Morrow DA, Alberts MJ, Mohr JP, Ameriso SF, Bonaca MP, Goto S, Hankey GJ, Murphy SA, Scirica BM, Braunwald E; Thrombin Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events–TIMI 50 Steering Committee and Investigators . Efficacy and safety of vorapaxar in patients with prior ischemic stroke. Stroke. 2013;44:691–698. [DOI] [PubMed] [Google Scholar]
- 9. Bonaca MP, Scirica BM, Braunwald E, Wiviott SD, Goto S, Nilsen DW, Bonarjee V, Murphy SA, Morrow DA. New ischemic stroke and outcomes with vorapaxar versus placebo: results from the TRA 2 degrees P‐TIMI 50 trial. J Am Coll Cardiol. 2014;64:2318–2326. [DOI] [PubMed] [Google Scholar]
- 10. Giugliano RP, Newby LK, Harrington RA, Gibson CM, Van de Werf F, Armstrong P, Montalescot G, Gilbert J, Strony JT, Califf RM, Braunwald E; EARLY ACS Steering Committee . The early glycoprotein IIb/IIIa inhibition in non‐ST‐segment elevation acute coronary syndrome (EARLY ACS) trial: a randomized placebo‐controlled trial evaluating the clinical benefits of early front‐loaded eptifibatide in the treatment of patients with non‐ST‐segment elevation acute coronary syndrome—study design and rationale. Am Heart J. 2005;149:994–1002. [DOI] [PubMed] [Google Scholar]
- 11. Mega JL, Braunwald E, Mohanavelu S, Burton P, Poulter R, Misselwitz F, Hricak V, Barnathan ES, Bordes P, Witkowski A, Markov V, Oppenheimer L, Gibson CM; ATLAS ACS‐TIMI 46 study group . Rivaroxaban versus placebo in patients with acute coronary syndromes (ATLAS ACS‐TIMI 46): a randomised, double‐blind, phase II trial. Lancet. 2009;374:29–38. [DOI] [PubMed] [Google Scholar]
- 12. Bath PM, Woodhouse LJ, Appleton JP, Beridze M, Christensen H, Dineen RA, Duley L, England TJ, Flaherty K, Havard D, Heptinstall S, James M, Krishnan K, Markus HS, Montgomery AA, Pocock SJ, Randall M, Ranta A, Robinson TG, Scutt P, Venables GS, Sprigg N. Antiplatelet therapy with aspirin, clopidogrel, and dipyridamole versus clopidogrel alone or aspirin and dipyridamole in patients with acute cerebral ischaemia (TARDIS): a randomised, open‐label, phase 3 superiority trial. Lancet. 2018;391:850–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mahaffey KW, Hager R, Wojdyla D, White HD, Armstrong PW, Alexander JH, Tricoci P, Lopes RD, Ohman EM, Roe MT, Harrington RA, Wallentin L. Meta‐analysis of intracranial hemorrhage in acute coronary syndromes: incidence, predictors, and clinical outcomes. J Am Heart Assoc. 2015;4:e001512 DOI: 10.1161/JAHA.114.001512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user‐friendly score (HAS‐BLED) to assess 1‐year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. [DOI] [PubMed] [Google Scholar]
- 15. Olesen JB, Torp‐Pedersen C, Hansen ML, Lip GY. The value of the CHA2DS2‐VASc score for refining stroke risk stratification in patients with atrial fibrillation with a CHADS2 score 0–1: a nationwide cohort study. Thromb Haemost. 2012;107:1172–1179. [DOI] [PubMed] [Google Scholar]
- 16. Kereiakes DJ, Yeh RW, Massaro JM, Cutlip DE, Steg PG, Wiviott SD, Mauri L; DAPT Study Investigators . DAPT score utility for risk prediction in patients with or without previous myocardial infarction. J Am Coll Cardiol. 2016;67:2492–2502. [DOI] [PubMed] [Google Scholar]
- 17. Amarenco P, Sissani L, Labreuche J, Vicaut E, Bousser MG, Chamorro A, Fisher M, Ford I, Fox KM, Hennerici MG, Mattle H, Rothwell PM, Steg PG, Diener HC, Sacco RL, Greving JP, Algra A. The intracranial‐B2LEED3S score and the risk of intracranial hemorrhage in ischemic stroke patients under antiplatelet treatment. Cerebrovasc Dis. 2017;43:145–151. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Events by management of vorapaxar and placebo after first stroke. A, Overall (=any stroke). B, By stroke type.


