Abstract
Background
Several randomized controlled trials (RCTs) have already shown that paclitaxel‐coated balloons and stents significantly reduce the rates of vessel restenosis and target lesion revascularization after lower extremity interventions.
Methods and Results
A systematic review and meta‐analysis of RCTs investigating paclitaxel‐coated devices in the femoral and/or popliteal arteries was performed. The primary safety measure was all‐cause patient death. Risk ratios and risk differences were pooled with a random effects model. In all, 28 RCTs with 4663 patients (89% intermittent claudication) were analyzed. All‐cause patient death at 1 year (28 RCTs with 4432 cases) was similar between paclitaxel‐coated devices and control arms (2.3% versus 2.3% crude risk of death; risk ratio, 1.08; 95% CI, 0.72–1.61). All‐cause death at 2 years (12 RCTs with 2316 cases) was significantly increased in the case of paclitaxel versus control (7.2% versus 3.8% crude risk of death; risk ratio, 1.68; 95% CI, 1.15–2.47; —number‐needed‐to‐harm, 29 patients [95% CI, 19–59]). All‐cause death up to 5 years (3 RCTs with 863 cases) increased further in the case of paclitaxel (14.7% versus 8.1% crude risk of death; risk ratio, 1.93; 95% CI, 1.27–2.93; —number‐needed‐to‐harm, 14 patients [95% CI, 9–32]). Meta‐regression showed a significant relationship between exposure to paclitaxel (dose‐time product) and absolute risk of death (0.4±0.1% excess risk of death per paclitaxel mg‐year; P<0.001). Trial sequential analysis excluded false‐positive findings with 99% certainty (2‐sided α, 1.0%).
Conclusions
There is increased risk of death following application of paclitaxel‐coated balloons and stents in the femoropopliteal artery of the lower limbs. Further investigations are urgently warranted.
Clinical Trial Registration
URL: www.crd.york.ac.uk/PROSPERO. Unique identifier: CRD42018099447.
Keywords: balloon angioplasty, paclitaxel, paclitaxel‐coated balloon, paclitaxel‐eluting stent
Subject Categories: Peripheral Vascular Disease, Meta Analysis, Mortality/Survival
Clinical Perspective
What Is New?
There is strong evidence from statistical inference that the risk of death is significantly increased beyond the first year following application of paclitaxel‐coated balloons and stents in the femoropopliteal artery of the leg in patients with intermittent claudication.
Actual causes remain unknown and further clinical investigations are urgently warranted.
What Are the Clinical Implications?
Collection and reporting of longer‐term follow‐up (beyond 1 year) in case of all commercial clinical studies is recommended to confirm or refute the present findings.
Pharmacological studies may also help understand the potential biological mechanisms behind the association of paclitaxel in the lower limbs and patient mortality.
Introduction
To date, percutaneous transluminal angioplasty and stenting have developed to the mainstream treatment of symptomatic peripheral arterial disease with constantly increasing numbers of procedures worldwide.1, 2 The femoropopliteal artery is the most common site of involvement in atherosclerosis of the lower limb and is typically characterized by multilevel steno‐occlusive disease, often complex calcified morphology, and aggressive postangioplasty neointimal hyperplasia associated with high rates of early vessel restenosis and failure.3 Drug‐eluting stents (DESs) and drug‐coated balloons (DCBs) have been extensively investigated as a potential solution to inhibit vessel restenosis and improve clinical outcomes after endovascular revascularization of the femoropopliteal artery.4
Following testing in numerous randomized controlled trials (RCTs) and various commercial coating formulations, paclitaxel has emerged as the single potent and proven antirestenotic agent for the infrainguinal vessels.3, 4, 5 Recent meta‐analyses of several RCTs with low risk of bias have amassed strong evidence about the clinical effectiveness of paclitaxel DES and DCB in significantly reducing restenosis and thereby reducing the risk of recurrent limb ischemia and target lesion/limb revascularization.4, 6, 7 Consequently, after extensive preclinical testing and having demonstrated strong clinical efficacy combined with a good safety profile, a number of paclitaxel DES and DCB devices have gradually received the CE mark and Food and Drug Administration approval for use in the femoropopliteal segment of the leg.
However, the INPACT‐DEEP (Study of IN.PACT AmphirionTM Drug Eluting Balloon vs. Standard PTA for the Treatment of Below the Knee Critical Limb Ischemia) randomized study has shown higher rates of major amputations in the active paclitaxel arm compared with control.8 In addition, a couple of RCTs with longer‐term follow‐up have shown hints of increased late patient mortality with the use of paclitaxel DESs9 or DCBs.10, 11 In the absence of obvious causal links, these findings have been dismissed by expert review panels as statistical artifacts or anomalies, and both devices are currently under clinical use with extended on label indications. Moreover, coronary drug‐eluting stents have been long incriminated for late stent thrombosis with an associated risk of death.12 Hence, we conducted an updated systematic review and quantitative meta‐analysis of RCTs investigating paclitaxel‐coated balloons and stents in the femoropopliteal artery, in order to analyze the early and late risk of death associated with these novel endovascular technologies that deliver paclitaxel to the vessel wall of the lower limbs.
Material and Methods
Literature Search
The authors declare that all supporting data are available within the tables, figures, and supplemental material of the present article. This systematic review has been registered in the PROSPERO public database (CRD42018099447; http://www.crd.york.ac.uk/PROSPERO). We performed electronic searches of PubMed (Medline), EMBASE (Excerpta Medical Database), AMED (Allied and Complementary medicine Database), Scopus, CENTRAL (Cochrane Central Register of Controlled Trials), archived online content, public filings of regulatory bodies (Food and Drug Administration and European Medicines Agency) and published abstracts from international vascular meetings for eligible RCTs. There were no restrictions on publication language, publication date, or publication status. The literature was screened for randomized studies investigating paclitaxel‐coated DESs or DCBs in the femoropopliteal artery of the lower limbs. Search terms included Cochrane, femoral artery, popliteal artery, femoropopliteal artery, late lumen loss, restenosis, target lesion revascularization, peripheral angioplasty, stent, randomized, balloon angioplasty, paclitaxel‐eluting balloons, paclitaxel‐coated balloons, paclitaxel‐eluting stents, paclitaxel‐coated stents, paclitaxel‐eluting stents, drug‐coated balloons, and drug‐eluting stents, as well as the corresponding Medical Subjects Headings with Boolean syntax (ie, the logic terms AND and/or OR). The literature search was last updated in August 2018. Trials were considered for inclusion in the present meta−analysis if they fulfilled the following inclusion criteria: (1) Randomized controlled study design, (2) investigation of a paclitaxel‐coated/paclitaxel‐eluting stent or balloon in the femoropopliteal artery, (3) patient population with peripheral arterial disease of the femoral and/or popliteal artery and symptoms of intermittent claudication and/or critical limb ischemia, (4) clinical follow‐up of at least 1 year available.
Evaluation of the quality and risk of bias of the selected RCTs was performed independently by two of the authors (K.K., D.K.) using the Cochrane Collaboration's tool for assessing risk of bias,13 which evaluates 7 key design items of an RCT: (1) random sequence generation, (2) allocation concealment, (3) blinding of participants and personnel, (4) blinding of outcome assessment, (5) incomplete outcome data, (6) selective outcome reporting, and (7) other potential sources of bias. Each aforementioned domain was evaluated as high, low, or unclear risk of bias according to Cochrane. Disagreements were resolved by consensus.
Data Extraction and Outcome Measures
The trial selection process complied with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses statement.14 The reference lists of all selected articles were also queried for potential study candidates. Three of the authors (K.K., S.S., and P.K.) selected the trials to be included in further quantitative synthesis and independently extracted relevant raw data in duplicate. Information was abstracted from the main text and tables of published manuscripts or other archived online material as cited. Again, any disagreements were resolved by consensus among the investigators. Data extracted from each RCT included baseline patient demographics, procedural variables, follow‐up time period, prescribed antiplatelet therapy, and outcome data on patient mortality during different time periods. Our meta‐analysis concentrated only on patient mortality because metrics of clinical effectiveness have been previously thoroughly reported.4, 15 The outcome measure was set at all‐cause patient death and adjudicated/reported as such in the studies. All‐cause patient death was analyzed at different time points following paclitaxel‐coated balloon and/or stent angioplasty of the leg.
Statistical Methods
Quantitative synthesis of the included RCTs was performed in R language environment (version 3.4.1) with the “meta” package (version 4.9‐2). Categorical variables were expressed as counts and percentages, and continuous variables as means ± SD if normally distributed. Patient mortality rates were pooled with a random effects model to account for any clinical and study design heterogeneity. Summary statistics were expressed both as risk ratios and risk differences and the associated 95% CI. To help clinical judgment, the number‐needed‐to‐harm (NNH) with corresponding 95% CIs were also calculated in cases of statistical significance. Publication bias was assessed (1) qualitatively by visual inspection of inverted funnel plot asymmetry, and (2) quantitatively with the Horbold‐Egger test.16
Prespecified sensitivity and subgroup analyses were performed to test the validity and stability of the results. Analyses included fixed versus random effects models, bayesian models to address rare events, omission of one study at a time (leave‐one‐out meta‐analysis), and subgroups of different paclitaxel dosages. Meta‐regression was employed to further explore the relationship of treatment effects and exposure to paclitaxel. Trial sequential analysis (TSA, Copenhagen Trial Unit) was further used to adjust for between‐trial diversity and reduce the risk of false‐positive findings. TSA was applied to ensure enough statistical power to detect the relevant effect size, better control type I and II errors, and calculate the diversity‐adjusted information size in the context of the present meta‐analysis.17 We also conducted sensitivity analyses on different TSA settings (Trial Sequential Analysis Version 0.9.5.5, Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, 2016). The level of statistical significance was set at α=0.05.
Results
Included RCTs
The literature search yielded 386 articles eligible for potential inclusion based on their title and content of abstract. Of those, 48 items were found to be relevant and were included in further full‐text analysis. Another 20 articles were excluded because they did not meet the predefined inclusion criteria. In all, 28 RCTs with 4663 patients were finally included in the present meta‐analysis (Figure S1). Full citation information for all studies, as well as the properties of the 12 different devices tested that were coated with 2.0‐, 3.0‐ or 3.5‐μg/mm2 paclitaxel, are outlined in Tables S1 and S2. There were 4 RCTs with a paclitaxel DES9, 18, 19, 20, 21, 22 and 24 RCTs testing different paclitaxel DCB devices. Out of the 24 DCB studies, 16 involved sole application of a paclitaxel‐coated balloon (versus balloon PTA)23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48; 4 combined the paclitaxel balloon with bare metal stent (versus PTA and BMS)49, 50, 51, 52; and 3 studies investigated use of a DCB for the treatment of in‐stent restenosis (versus PTA).53, 54, 55 Baseline patient demographics and morphologic lesion variables were largely homogeneously distributed across all studies and in line with previous meta‐analyses. The design characteristics of the 28 selected RCTs are shown in Table 1.
Table 1.
Design Characteristics of the Included Randomized Controlled Trialsa
| Study and Sources | Year and Study Design | Allocation in Study Arms | Paclitaxel‐Coated Device | Primary Study End Point | Maximum Follow‐Up Period | Study Registration | Dual Antiplatelet Therapy |
|---|---|---|---|---|---|---|---|
| ZILVER PTX9, 19, 23 |
2011 Multi‐center Open label (1:1) |
DES (n=241) vs PTA (n=238) | ZILVER‐PTX Stent by Cook Medical | Primary patency at 1 y | 5 y | NCT00120406 | >2 mo |
| THUNDER28, 57 |
2008 Multi‐center Single‐blind (1:1:1) |
DCB (n=48) vs PTA (n=54) | Cotavance Balloon by Bavaria Medizin | Late lumen loss at 6 mo | 5 y | NCT00156624 | 1 mo |
| INPACT SFA10, 11, 25, 56, 82 |
2015 Multi‐center Single‐blind (2:1) |
DCB (n=220) vs PTA (n=111) | IN.PACT Admiral by Medtronic | Primary patency at 1 y | 3 y |
NCT01175850 NCT01566461 |
1 mo (3 mo if bail‐out stenting) |
| FEMPAC29 |
2008 Multicenter Single‐blind (1:1) |
DCB (n=45) vs PTA (n=42) | Paccocath Balloon by Bavaria Medizin | Late lumen loss at 6 mo | 2 y | NCT00472472 | Long‐term (not specified) |
| LEVANT I27 |
2012 Multicenter Single‐blind (1:1) |
DCB (n=49) vs PTA (n=52) | Lutonix by CR Bard | Late lumen loss at 6 mo | 2 y | NCT00930813 | 1 mo (3 mo if bailout stenting) |
| LEVANT II24, 26, 31, 33 |
2015 Multi‐center Single‐blind (2:1) |
DCB (n=316) vs PTA (n=160) | Lutonix by CR Bard | Primary patency at 1 y | 2 y | NCT01412541 | 1 mo |
| ILLUMENATE EU32, 35 |
2017 Multicenter Single‐blind (3:1) |
DCB (n=222) vs PTA (n=72) | Stellarex by Spectranetics | Primary patency at 1 y | 2 y | NCT01858363 | 1 mo (3 mo if bailout stenting) |
| CONSEQUENT30, 36 |
2017 Multicenter Single‐blind (1:1) |
DCB (n=78) vs PTA (n=75) | SeQuent Please by B. Braun | Late lumen loss at 6 mo | 2 y | NCT01970579 | 2 mo |
| ISAR‐STATH51 |
2017 Two‐center Open label (1:1:1) |
DCB+BMS (n=48) vs PTA+BMS (n=52) | IN.PACT Admiral by Medtronic | Diameter Stenosis at 6 mo | 2 y | NCT00986752 | 6 mo |
| ISAR‐PEBIS55 |
2017 Two‐center Open label (1:1) for ISR |
DCB (n=36) vs PTA (n=34) | IN.PACT Admiral by Medtronic | Diameter stenosis at 6 to 8 mo | 2 y | NCT01083394 | >6 mo |
| IN.PACT SFA JAPAN34, 41 |
2018 Multi‐center Single‐blind (2:1) |
DCB (n=68) vs PTA (n=32) | IN.PACT Admiral by Medtronic | Primary patency at 1 y | 2 y | NCT01947478 | 1 mo (3 mo if bailout stenting) |
| ACOART I40, 42 |
2016 Multicenter Single‐blind (1:1) |
DCB (n=100) vs PTA (n=100) | Orchid by Acotec Scientific | Late lumen loss at 6 mo | 2 y | Not registered | 6 mo |
| FINN‐PTX18 |
2018 Multi‐center Open label (2:1) |
DES (n=28) vs PTFE (n=18) | ZILVER‐PTX Stent by Cook Medical | Secondary Patency at 2 y | 2 y | NCT01450722 | 3 mo (Aspirin in control group) |
| BATTLE20, 21 |
2018 Multicenter Open label (1:1) |
DES (n=86) vs BMS (n=85) | ZILVER‐PTX Stent by Cook Medical | In‐stent binary restenosis at 1 y | 1 y | NCT02004951 | >2 mo (clopidogrel to continue for 2 y) |
| DEBATE in SFA22 |
2018 Multi‐center Open label (1:1:1) |
DES (n=85) vs BMS (n=170) | ZILVER‐PTX Stent by Cook Medical | In‐stent binary restenosis at 1 y | 1 y | UMIN000010071 | >2 mo (Aspirin to continue lifelong) |
| DEBELLUM38, 39 |
2014 Single‐center Open (1:1) |
DCB (n=25) vs PTA (n=25) | IN.PACT Admiral by Medtronic | Late lumen loss at 6 mo | 1 y | Not registered | 1 mo |
| PACIFIER45 |
2012 Multicenter Single‐blind (1:1) |
DCB (n=41) vs PTA (n=44) | IN.PACT Pacific by Medtronic | Late lumen loss at 6 mo | 1 y | NCT01083030 | >2 mo |
| FAIR54 |
2015 Multicenter Single‐blind (1:1) for ISR |
DCB (n=62) vs PTA (n=57) | IN.PACT Admiral by Medtronic | 6‐mo binary restenosis | 1 y | NCT01305070 | >6 mo |
| BIOLUX P‐I44 |
2015 Multicenter Single‐blind (1:1) |
DCB (n=30) vs PTA (n=30) | Passeo‐18 Lux by Biotronik | Late lumen loss at 6 mo | 1 y | NCT01056120 | 1 mo (3 mo if bailout stenting) |
| RANGER‐SFA37 |
2018 Multicenter Single‐blind (2:1) |
DCB (n=71) vs PTA (n=34) | Ranger by Boston Scientific | Primary patency at 1 y | 1 y | NCT02013193 | >1 mo |
| ILLUMENATE pivotal43 |
2017 Multicenter Single‐blind (2:1) |
DCB (n=200) vs PTA (n=100) | Stellarex by Spectranetics | Primary patency at 1 y | 1 y | NCT01858428 & NCT01912937 | 1 mo |
| DEBATE‐SFA50 |
2013 Single‐center Open (1:1) |
DCB+BMS (n=53) vs PTA+BMS (n=51) | IN.PACT Admiral by Medtronic | 1‐y binary restenosis | 1 y | NCT01556542 | 3 mo |
| LUTONIX JAPAN48 |
2018 Multicenter Japan (1:1) |
DCB (n=71) vs PTA (n=38) | Lutonix by CR BARD | Primary patency at 1 y | 1 y | Not registered | 1 mo |
| RAPID49 |
2017 Multicenter Double‐blind (1:1) |
DCB+BMS (n=80) vs PTA+BMS (n=80) | LegFlow by Cardionovum | Primary patency at 1 y | 1 y | ISRCTN47846578 | 3 mo |
| EFFPAC47 |
2018 Multicenter Single‐blind (1:1) |
DCB (n=85) vs PTA (n=86) | Luminor by iVascular | Late lumen loss at 6 mo | 1 y | NCT02540018 | >1 mo |
| PACUBA53 |
2016 Dual‐center Single‐blind (1:1) for ISR |
DCB (n=85) vs PTA (n=86) | FREEWAY by Eurocor | Primary patency at 1 y | 1 y | NCT01247402 | 3 mo |
| FREEWAY52 |
2017 Multi‐center Single‐blind (1:1) |
DCB+BMS (n=105) vs PTA+BMS (n=99) | FREEWAY by Eurocor | Target lesion revascularization | 1 y | NCT01960647 | Not specified |
| DRECOREST46 |
2018 Single‐center Open (1:1) |
DCB (n=30) vs PTA (n=30) | IN.PACT by Medtronic for failing bypass | Target lesion revascularization | 1 y | NCT03023098 | 3 mo |
BMS indicates bare metal stent; DCB, drug‐coated balloon; DES, drug‐eluting stent; ISR, in‐stent restenosis; PTA, percutaneous transluminal angioplasty; PTFE, polytetrafluoroethylene.
The design of the ZILVER PTX study included a primary randomization (optimal PTA vs primary DES) and a secondary randomization in the case of PTA failure (bailout BMS vs bail‐out DES)—results of the 2 randomization levels were pooled for the purposes of the present meta‐analysis. For the Thunder trial (3‐arm trial), the arm of paclitaxel dissolved in the contrast medium was excluded. For the ISAR‐STATH trial (3‐arm trial), the arm of directional atherectomy was excluded from the present analysis. For the DEBATE in SFA trial, the 2 BMS arms (with or without cilostazol) were pooled against the ZILVER PTX arm. For the DEBELLUM trial, only femoropopliteal lesions were analyzed. In the LEVANT I trial, randomization between plain BA and PCB was performed after provisional stent placement in a quarter of the cases (26 of 101). In the RAPID study, the Supera biomimetic nitinol stent was used in both arms. Data extraction was supplemented by online archived material from international meetings or regulatory authority filings as cited (US Food and Drug Administration—Japan Pharmaceuticals and Medical Devices Agency).
Briefly, paclitaxel‐coated balloons and stents were used primarily for the treatment of short‐distance intermittent claudication (n=4133 of 4663 subjects; 89%) in the majority of the study population and infrequently for a critical limb ischemia indication (n=530). A detailed overview of baseline patient and lesion characteristics is provided in Table S3 for all included studies. Overall, approximately two thirds of the patients were men; the mean age ranged from 67 to 76 years; and there was a high incidence of smoking, hypertension, and hyperlipidemia across all studies. The crude incidence of diabetes mellitus ranged from 21% to 77%. A wide range of intermediate to higher‐length lesions was enlisted. With few exceptions, most protocols recommended a short period of 1 to 3 months of dual antiplatelet therapy. The median RCT follow‐up period was 2 years (range, 1–5 years). Fifteen studies had 1 year, 10 studies had 2 years, 1 study had 4 years, and 2 studies had 5 years of clinical follow‐up available (Table 1). A majority of the RCTs were executed as randomized multicenter studies except for 3 single‐center studies and 3 dual‐center studies. Randomization and allocation concealment were performed adequately and methodological quality was high for all trials with the exception of an inherently high risk of performance bias in all 28 RCTs because of the universal absence of systematic blinding of the operators during application of the devices (Figure S2).
All‐Cause Death at 1 Year
All‐cause patient death up to 1 year was reported by all included RCTs for a total of 4432 subjects. There was good evidence that the pooled risk of death did not differ significantly between the active use of paclitaxel‐coated balloons or stents versus the control arms. There were 58 deaths out of 2506 patients in the paclitaxel arms (2.3% crude risk of death) compared with 45 deaths in 1926 patients in the control arms (2.3% crude risk of death) with a calculated pooled risk ratio of 1.08 (95% CI, 0.72–1.61; Figure 1. There was no statistically significant heterogeneity between studies (P=0.98).
Figure 1.
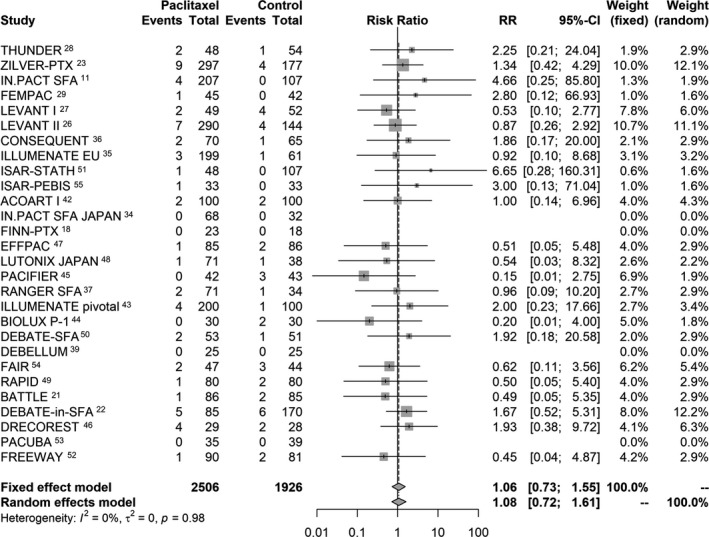
Random effects forest plot of all‐cause patient death at 1 year. Pooled point estimate was expressed as risk ratio (RR).
All‐Cause Death at 2 Years
In all, 12 studies out of the 28 RCTs reported the incidence of all‐cause patient death up to 2 years in a total of 2316 patients. There was good evidence that application of paclitaxel‐coated devices in the femoropopliteal artery was related to significantly increased risk of death. There were 101 deaths out of 1397 patients in the paclitaxel arms (7.2% crude risk of death) compared with 35 deaths in 919 patients in the control arms (3.8% crude risk of death) with a calculated risk ratio of 1.68 (95% CI, 1.15–2.47). Absolute risk difference was 3.5% (95% CI, 1.7–5.3%) with a corresponding NNH of 29 patients (95% CI, 19–59). There was no statistically significant heterogeneity between studies (P=0.80; Figure 2.
Figure 2.
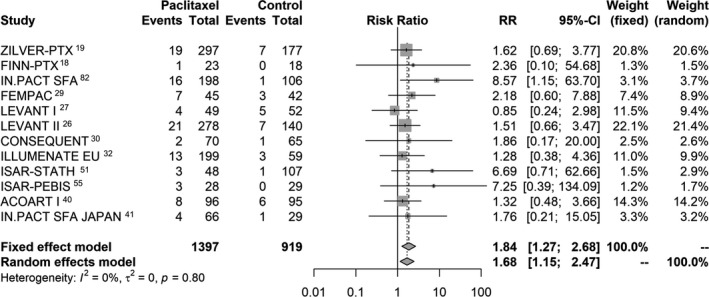
Random effects forest plot of all‐cause death at 2 years. Pooled point estimate was expressed as risk ratio (RR).
All‐Cause Death Up to 5 Years
Long‐term analysis of all‐cause death up to 5 years was informed by 3 RCTs including 863 cases. One study had 4 years56 and 2 had 5 years of follow‐up.9, 57 Some 78 deaths out of 529 cases occurred in the paclitaxel arms (14.7% crude risk of death) versus 27 deaths out of 334 cases in the control (8.1% crude risk of death) with a pooled risk ratio of 1.93 (95% CI, 1.27–2.93). Absolute risk difference was 7.2% (95% CI, 3.1–11.3%) and NNH was 14 patients (95% CI, 9–32). There was no statistically significant heterogeneity between studies (P=0.92; Figure 3.
Figure 3.
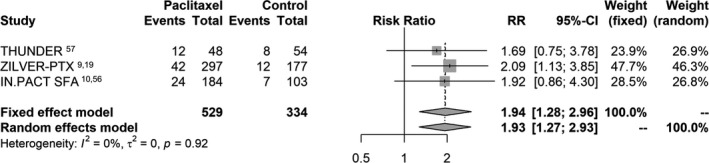
Random effects forest plot of all‐cause death at 4 to 5 years. Pooled point estimate was expressed as risk ratio (RR).
Sensitivity and Subgroup Analyses
There was no visual asymmetry of the respective funnel plots to suggest publication bias at 1 year (Horbold‐Egger test, 0.27; P=0.66), 2 years (Horbold‐Egger test, 0.47; P=0.55), or 4 to 5 years of follow‐up (Horbold‐Egger test, −0.43; P=0.11; Figure S3). We opted to report summary estimates from a frequentist random effects model to account for conceptual and study design differences among different RCTs. The randomized studies included here tested numerous designs of paclitaxel‐coated devices with variable drug dosages and different drug excipients in slightly different patient populations. Hence, a different, but similar treatment effect was assumed as the basis for the random effects modeling.58 In addition, different methods of analyses (with or without continuity correction and Bayesian methods) were employed to interrogate potential bias and uncertainty arising from meta‐analysis of low event rates as recommended elsewhere.59 Bayesian methods generally increased the size of treatment effect (Table S4). The pooled point estimates remained consistent across sensitivity and subgroup analyses with some variation in the magnitude of effect size. There were also differences in the estimated long‐term risk of death when examining different paclitaxel doses, although those results are underpowered, with variable follow‐up periods, and informed by few studies in each case (Table 2).
Table 2.
Sensitivity and Subgroup Analyses of All‐Cause Patient Death
| Risk Ratio (95% CI) | |
|---|---|
| All‐cause death at 2 y | |
| Fixed effects model | 1.84 (1.27–2.68) |
| Random effects model | 1.68 (1.15–2.47) |
| All‐cause death at 4 to 5 y | |
| Fixed effects model | 1.94 (1.28–2.96) |
| Random effects model | 1.93 (1.27–2.93) |
| Subgroups (random effects) | |
| Paclitaxel DES only | 1.87 (1.11–3.15) |
| Paclitaxel DCB only | 1.44 (1.04–2.00) |
| Multicenter studies only | 1.48 (1.11–1.97) |
| Dose subgroups (beyond 1 y) | |
| 3.5 μg/mm2 paclitaxel balloon | 2.31 (1.15–4.63) |
| 3.0 μg/mm2 paclitaxel stent | 2.10 (1.15–3.83) |
| 3.0 μg/mm2 paclitaxel balloon | 1.65 (0.95–2.87) |
| 2.0 μg/mm2 paclitaxel balloon | 1.27 (0.70–2.32) |
| Trial sequential analysis (TSA; random effects at 2 y) | |
| TSA diversity adjusted (α=5%, β=20%) | 1.70 (1.19–2.43) |
| TSA diversity adjusted (α=5%, β=10%) | 1.70 (1.24–2.33) |
| TSA diversity adjusted (α=1%, β=10%) | 1.70 (1.08–2.69) |
CI indicates confidence interval; DCB, drug‐coated balloon; DES, drug‐eluting stent.
Trial Sequential Analysis
TSA was performed to test availability of adequate patient sample size and adjust positive findings after the first year in order to minimize the risk of statistical errors because none of the included studies were designed or powered to investigate differences in patient mortality. The information size required for a valid meta‐analysis may be assumed to be at least as large as the sample size of a single well‐powered RCT designed to confirm or reject the null hypothesis.60 TSA included 13 RCTs with clinical follow‐up >1 year (weighted mean follow‐up of 3 years; range, 2–5 years) from 2401 cases in total. TSA is illustrated in Figure 4, which shows the cumulative curve of the Z score statistic and the O'Brien‐Fleming trial sequential monitoring boundaries to control statistical errors against the available sample size. Clearly, the cumulative Z curve crosses the external alpha‐spending boundaries, and the required information size (cumulative patient sample) has been achieved. Using a type II error threshold of β=10% (power 90%) and by varying the threshold of type I error (alpha range, 1–5%), TSA confirmed accumulation of adequate information size to refute a type I error (ie, false‐positive results) with up to 99% certainty (two‐sided α, 1.0%).
Figure 4.
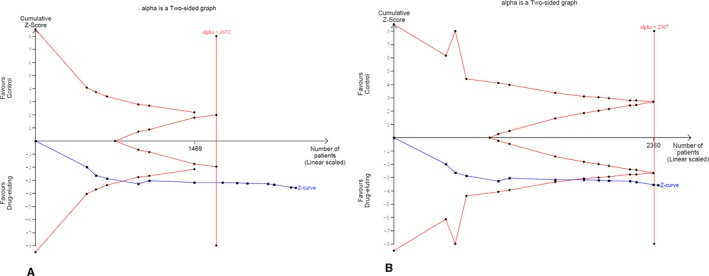
Trial sequential analysis of all‐cause death. External red lines denote the O'Brien‐Fleming alpha spending trial sequential monitoring boundaries. Internal red wedge lines denote the futility O'Brien‐Fleming beta spending lines. Cumulative Z curve (blue line) crossed the alpha monitoring boundaries and the required information size (patient sample) has been reached in both illustrative scenarios; (A) α=5%, β=10%; and (B) α=1%, β=10%). Vertical red line denotes the calculated required sample size, whereas the Z value is the test statistic (|Z|=1.96 corresponds to a P value of 0.05; the higher the Z value, the lower the P value).
Meta‐Regression Analysis
In line with Bradford Hill's criteria61 for establishing epidemiological evidence for causation, we explored presence of a biological gradient, that is, whether greater exposure leads to greater incidence of the effect. To that end, we performed meta‐regression of the absolute risk difference of all‐cause death against exposure to paclitaxel in all 28 RCTs. Considering that crystalline paclitaxel delivered by paclitaxel‐coated devices has a half‐life of weeks to months,62, 63, 64 we calculated exposure to paclitaxel as the dose‐time product after treatment. To account for nominal device dose and treated vessel surface area, the following equation was used for calculation of paclitaxel dose‐time product expressed in milligram‐years for each individual study (i):
where, Dosei is the nominal paclitaxel dose loaded on the balloon or stent (μg/mm2), Di is the reference vessel diameter (mm), Lengthi is the treated lesion length (mm), and Timei indicates the available follow‐up time period (years). Random effects meta‐regression identified a highly significant association between paclitaxel dose‐time product and absolute risk of death; there was a 0.4±0.1% excess risk of death for every paclitaxel milligram‐year (95% CI, 0.1–0.6%; P<0.001; Figure 5). The result was stable on a resampling permutation test (1000 iterations; P=0.013).
Figure 5.
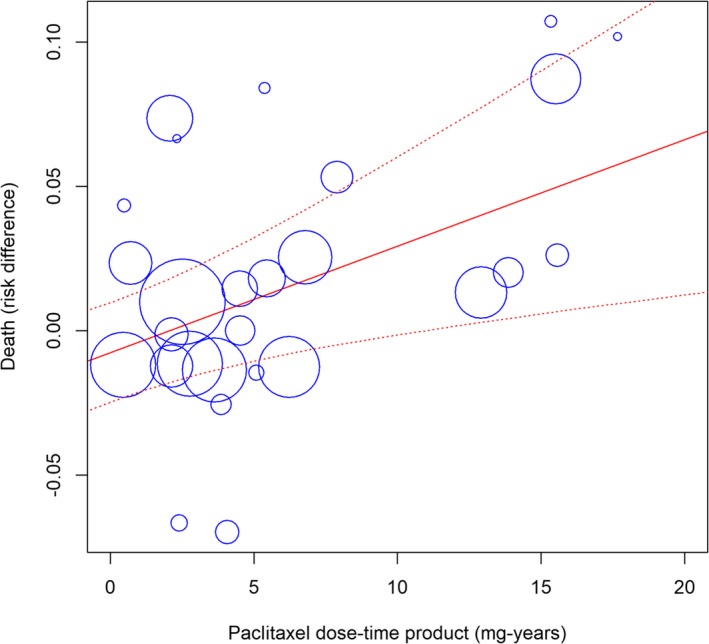
Meta‐regression (mixed effects model) of all‐cause death against paclitaxel exposure (dose‐time product calculated in milligram‐years). The size of the blue symbols is inversely proportional to the variance of the estimated treatment effect for each study. Solid and dotted red lines indicate the regression line with its corresponding 95% confidence bands. Intercept is −0.8±0.9% and coefficient of the regression line is 0.4±0.1% (95% confidence interval, 0.1–0.6%; P<0.001). The equation of the regression line is Y=(−0.008)+0.004X. The “metareg” function of the “meta” library was employed in R language.
Discussion
Paclitaxel‐coated balloon and stents have emerged as the most promising strategy for the inhibition of neointimal hyperplasia following angioplasty of the femoropopliteal artery of the leg. Several randomized controlled studies have already demonstrated strong evidence of clinical effectiveness enrolling predominantly patients suffering from intermittent claudication (≈90% of the sample size).3, 4 Following the progressive release of longer‐term clinical outcomes, a comprehensive updated systematic review and meta‐analysis was undertaken to compare the all‐cause patient mortality associated with the use of those devices. Overall, there was good evidence that all‐cause death at 1 year was equivalent between paclitaxel and control arms. However, the risk of all‐cause death appeared to increase dramatically after the first year in the case of paclitaxel arms. Synthesis of study‐level outcomes at 2 years documented a significant 68% relative risk increase of all‐cause death with a corresponding NNH of 29 patients. Risk of death increased further in the long‐term analysis (at 5 years) with a 93% relative risk increase and an NNH of 14. Overall, the present statistical inference appeared to be credible and stable on various sensitivity tests. Furthermore, we found neither any statistical heterogeneity nor any major diversity between the included studies. We employed trial sequential analysis to address potential random sampling errors and other unknown bias because of the fact that none of the present studies was designed or adequately powered to explore the outcome of patient mortality. Interestingly, TSA meta‐analysis powered at 90% has shown accumulation of adequate information size to exclude false‐positive findings (type I error) with 99% certainty.
The authors consider the herein reported findings of particular concern because most of the interrogated devices have already received clearance by regulatory authorities and are currently under routine clinical use. The potential causes of this alarming late increased incidence of death remain unknown. Experience with paclitaxel‐coated devices has been previously limited to the coronary TAXUS stents (Boston Scientific, Marlborough, MA), which allow for prolonged release of paclitaxel from a polymer‐based stent coating. Of note, long‐term results of the safety of the TAXUS paclitaxel stent in the heart (patient‐level analysis of 2797 randomized patients receiving TAXUS versus bare stents) have long shown a significant increase of long‐term death and myocardial infarction after 1 year following implantation (6.7% versus 4.5%, P<0.01).65 Likewise, long‐term results from the SYNTAX (TAXUS Drug‐Eluting Stent Versus Coronary Artery Bypass Surgery for the Treatment of Narrowed Arteries) study (1800 patients with complex coronary artery disease randomized between TAXUS paclitaxel stent and coronary artery bypass surgery) have shown a significantly higher cardiovascular mortality up to 5 years in the case of paclitaxel coronary interventions compared with bypass grafting (9.6% versus 5.6%; P=0.008) to be explained mostly by late myocardial infarctions. The latter was further confirmed in the nested nonrandomized SYNTAX registries extending to involve both a cardiovascular (12.1% versus 4.7%) and an inexplicable noncardiovascular (14.9% versus 5.3%) mortality difference.66 Consequently, the coronary field has gradually moved away from paclitaxel DES also because of the well‐recognized issues of vessel wall tissue inflammation, aneurysm formation, and late stent thrombosis.62, 63
In addition, most drug‐coated balloons and stents for the femoropopliteal artery contain at least an order of magnitude higher payload of paclitaxel in comparison with paclitaxel‐eluting coronary stents (a 3.5×32 mm coronary TAXUS stent contains around 200 μg paclitaxel compared with around 1.2 mg for the ZILVER‐PTX 6.0×120 stent, 4.5 mg for the LUTONIX 6.0×120 drug‐coated balloon and 8.5 mg for the IN.PACT 6.0×120 balloon). From a pharmacological viewpoint, paclitaxel‐coated balloons and stents have been engineered to deliver prolonged levels of paclitaxel into the vessel tissues so as to inhibit smooth muscle cell proliferation and avert vessel restenosis. Paclitaxel is a lipophilic cytotoxic agent that blocks the cell cycle during mitosis by interfering with the spindle disassembly.67 To enable sustained tissue bioavailability without a polymer, most modern balloons and stents (Table S2) are coated with a mostly solid crystalline form of paclitaxel combined with a unique paclitaxel spacer or excipient that modulates drug transfer into the vessel wall.5, 68 Preclinical animal studies have shown prolonged vessel wall retention of paclitaxel in the iliofemoral arteries after DES‐ or DCB‐mediated delivery with therapeutic tissue levels (>1 ng/mg) maintained up to 2 months after delivery.63, 69, 70, 71
The authors postulate that late paclitaxel toxicity may be the reason for the observed increased death rate. Contrary to solvent‐based (eg, cremophor) intravenous paclitaxel used in cancer chemotherapy that has a half‐life of around 6 hours,72 paclitaxel crystals loaded on DCB or DES for the peripheral arteries have a half‐life of weeks to months, depending on the exact chemical properties of the applied paclitaxel formulation.62, 63, 64 Increased paclitaxel crystallinity helps achieve higher tissue uptake and retention and improved biologic effect, however, at the expense of microparticle formation that may embolize in the downstream systemic circulation.63 Worrisome rates of potential downstream embolization of the skeletal muscles of the lower limbs have been confirmed on the bench and animal studies62, 73 and are postulated to be responsible for the significantly higher rates of major amputations noted in the active paclitaxel arm of the randomized INPACT‐DEEP study.8, 71 Preclinical follow‐up studies have shown that in the case of paclitaxel‐coated balloons, ≈1% to 10% of the paclitaxel dose gets transferred into the target vessel wall, and as much as 90% (or as much as 8.5 mg of paclitaxel on a 6.0×120 mm 3.5 μg/mm2 device) gets lost into the systemic circulation with unknown consequences.64, 74 Rates of distal paclitaxel embolization, if any, in the case of paclitaxel DES remain unknown.70
Within the modern epidemiologic framework of structural causal modeling developed by Judea Pearl,75, 76 the present work shows strong signals of biomedical causality between paclitaxel and mortality within multiple controlled randomized trials. According to the more traditional Bradford Hill criteria61 for establishing a causal relationship between a presumed cause and an observed health effect, the present work has shown evidence of strength, consistency, temporality, and biological gradient. Risk of death was identical at 1 year but more than doubled during the second year following intervention. Twelve of 13 studies showed increased mortality between 1 and 2 years after intervention. In addition, a significant relationship between dose‐time exposure to paclitaxel and incidence of deaths was identified; risk of death increased by 0.4±0.1% per paclitaxel milligram‐year. Interestingly, the risk of death beyond 1 year also seemed to vary among different paclitaxel dosages, being significantly higher in the 3.5 μg/mm2 devices compared with the lower‐dose devices (Table 2). Still, the present meta‐analysis is underpowered to discern outcome differences between the different paclitaxel devices as some devices are supported by a single trial and follow‐up beyond 2 years is missing in most cases. The authors would therefore encourage collection of longer‐term follow‐up (beyond 1 year) in case of all studies to help confirm or refute the present findings.
Two‐year clinical outcomes from large‐scale phase IV registries have been recently released for 2 DCB and 1 DES device in the femoropopliteal artery. The ZILVER‐PTX postmarket single‐arm DES surveillance registry reported a 2.6% annualized risk of death (41 of 787 subjects at 2 years),19 the Lutonix Global SFA registry stated a 3.0% risk (38 of 637 died at 2 years),77 and the IN.PACT Global postmarket DCB study a 3.5% annualized risk of death (89 of 1269 at 2 years).78 The aforementioned rates of patient death appear to be consistent with an annualized 3.1% incidence of death in the case of paclitaxel DES and DCB documented in the present meta‐analysis (compared with 1.9% in the control angioplasty arms). Population‐based contemporary series of intermittent claudication that constituted 89% of the patient population included here have documented a 1.0% to 2.0% annual mortality rate,79, 80, 81 which is consistent with the 1.9% incidence of all‐cause death in the control arms of the current analysis.
The present meta‐analysis has several limitations. First, we excluded studies with DCB or DES in the below‐knee infrapopliteal arteries as they pertain to a distinctively different patient population mostly with critical limb ischemia associated with high morbidity and mortality rates. Second, some study protocols did not include an independent blinded clinical events committee for event adjudication, and nearly universally a single‐blind or open‐label study design was applied that may have introduced detection or performance bias, respectively. Third, unfortunately and with few exceptions,9, 10, 19, 82 most studies did not report the actual causes of deaths to help infer potential causal links with paclitaxel use. An association of paclitaxel with more cardiovascular deaths, but also of infectious, gastrointestinal, and pulmonary origin, was noted in the INPACT SFA (Randomized Trial of IN.PACT AdmiralTM Drug Coated Balloon vs Standard PTA for the Treatment of SFA and Proximal Popliteal Arterial Disease) and ZILVER PTX (Evaluation of the Zilver PTX Drug‐Eluting Peripheral Stent) studies (Table 3). Although baseline demographics were generally well balanced, in a few studies a numerically greater incidence of patient comorbidities—eg, smoking, hyperlipidemia, hypertension, or diabetes mellitus—were noted in the paclitaxel arms, for example, in the ZILVER PTX study. Hence, undetected sources of heterogeneity could not be explored in depth in the absence of individual patient data. Finally, we could not establish a plausible mechanism between paclitaxel and deaths, but as Sir Bradford Hill noted, knowledge of the mechanism may be limited by current knowledge.61
Table 3.
Causes of Death
| Paclitaxel‐Coated Balloon (IN.PACT SFA) at 3 Years10, 82 | Paclitaxel‐Coated Stent (ZILVER PTX) at 2 Years19, 23 | |||
|---|---|---|---|---|
| Paclitaxel | Control | Paclitaxel | Control | |
| Cardiovascular | 9 | 0 | 18 | 8 |
| Cancer | 2 | 2 | ||
| Infectious | 5 | 0 | ||
| Pulmonary | 3 | 0 | ||
| Other | 3 | 0 | NA | NA |
NA indicates not applicable.
In conclusion, there seems to be an increased long‐term risk of death beyond the first year following femoropopliteal application of paclitaxel‐coated balloons and stents in the lower limbs. Actual causes for this serious late side effect remain unknown, and further investigations with longer‐term follow‐up are urgently warranted.
Disclosures
For the purposes of this project no author received any payment or other support by a commercial company. Dr Katsanos reports personal fees from Medtronic, grants from Medtronic, and personal fees from Boston Scientific outside the submitted work. Dr Kitrou reports personal fees from Bard outside the submitted work. Dr Spiliopoulos reports personal fees from Medtronic outside the submitted work. Dr Karnabatidis reports personal fees and grants from Bard and Rontis outside the submitted work. The remaining authors have no disclosures to report.
Supporting information
Table S1. Design Characteristics of the Included Randomized Controlled Trials*
Table S2. Design Characteristics of the Tested Paclitaxel DES and DCB Devices
Table S3. Baseline Patient Characteristics of Included Randomized Clinical Trials
Table S4. Sensitivity Analyses of Rare Events (Risk Ratio; 95% CI or CrI)41 (R “meta” Package (Version 4.9‐2—Bayesian With https://gemtc.drugis.org)
Figure S1. Literature search and study selection process following the PRISMA statement.
Figure S2. Evaluation of risk of bias of each RCT according to the Cochrane Collaboration Tool.
Figure S3. Funnel plots of all‐cause death analyses at (A) 1 year, (B) 2 years, and (C) 4 to 5 years of follow‐up.
Acknowledgments
Data sharing: All authors had unrestricted access to the data sets and can take responsibility for the integrity of the data and the accuracy of the data analysis. The lead and corresponding author (Katsanos) performed all statistical analyses and has final overall responsibility for the submitted version of the manuscript (study guarantor). The lead and corresponding author (Katsanos) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained. Raw data are presented in the submitted tables and figures.
(J Am Heart Assoc. 2018;7:e011245 DOI: 10.1161/JAHA.118.011245.)
References
- 1. Gerhard‐Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FG, Hamburg NM, Kinlay S, Lookstein R, Misra S, Mureebe L, Olin JW, Patel RA, Regensteiner JG, Schanzer A, Shishehbor MH, Stewart KJ, Treat‐Jacobson D, Walsh ME. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e686–e725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Olin JW, Allie DE, Belkin M, Bonow RO, Casey DE Jr, Creager MA, Gerber TC, Hirsch AT, Jaff MR, Kaufman JA, Lewis CA, Martin ET, Martin LG, Sheehan P, Stewart KJ, Treat‐Jacobson D, White CJ, Zheng ZJ, Masoudi FA, Bonow RO, DeLong E, Erwin JP III, Goff DC Jr, Grady K, Green LA, Heidenreich PA, Jenkins KJ, Loth AR, Peterson ED, Shahian DM; American College of Cardiology Foundation; American Heart Association; American College of Radiology; Society for Cardiac Angiography Interventions; Society for Interventional Radiology; Society for Vascular Medicine; Society for Vascular Nursing; Society for Vascular Surgery . ACCF/AHA/ACR/SCAI/SIR/SVM/SVN/SVS 2010 performance measures for adults with peripheral artery disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures, the American College of Radiology, the Society for Cardiac Angiography and Interventions, the Society for Interventional Radiology, the Society for Vascular Medicine, the Society for Vascular Nursing, and the Society for Vascular Surgery (Writing Committee to Develop Clinical Performance Measures for Peripheral Artery Disease). J Am Coll Cardiol. 2010;56:2147–2181. [DOI] [PubMed] [Google Scholar]
- 3. Katsanos K, Tepe G, Tsetis D, Fanelli F. Standards of practice for superficial femoral and popliteal artery angioplasty and stenting. Cardiovasc Intervent Radiol. 2014;37:592–603. [DOI] [PubMed] [Google Scholar]
- 4. Katsanos K, Spiliopoulos S, Karunanithy N, Krokidis M, Sabharwal T, Taylor P. Bayesian network meta‐analysis of nitinol stents, covered stents, drug‐eluting stents, and drug‐coated balloons in the femoropopliteal artery. J Vasc Surg. 2014;59:1123–1133.e8. [DOI] [PubMed] [Google Scholar]
- 5. Ng VG, Mena C, Pietras C, Lansky AJ. Local delivery of paclitaxel in the treatment of peripheral arterial disease. Eur J Clin Invest. 2015;45:333–345. [DOI] [PubMed] [Google Scholar]
- 6. Fusaro M, Cassese S, Ndrepepa G, King LA, Tada T, Ott I, Kastrati A. Paclitaxel‐coated balloon or primary bare nitinol stent for revascularization of femoropopliteal artery: a meta‐analysis of randomized trials versus uncoated balloon and an adjusted indirect comparison. Int J Cardiol. 2013;168:4002–4009. [DOI] [PubMed] [Google Scholar]
- 7. Kayssi A, Al‐Atassi T, Oreopoulos G, Roche‐Nagle G, Tan KT, Rajan DK. Drug‐eluting balloon angioplasty versus uncoated balloon angioplasty for peripheral arterial disease of the lower limbs. Cochrane Database Syst Rev. 2016:CD011319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zeller T, Baumgartner I, Scheinert D, Brodmann M, Bosiers M, Micari A, Peeters P, Vermassen F, Landini M, Snead DB, Kent KC, Rocha‐Singh KJ. Drug‐eluting balloon versus standard balloon angioplasty for infrapopliteal arterial revascularization in critical limb ischemia: 12‐month results from the IN.PACT DEEP randomized trial. J Am Coll Cardiol. 2014;64:1568–1576. [DOI] [PubMed] [Google Scholar]
- 9. Dake MD, Ansel GM, Jaff MR, Ohki T, Saxon RR, Smouse HB, Machan LS, Snyder SA, O'Leary EE, Ragheb AO, Zeller T; Zilver PTXI . Durable clinical effectiveness with paclitaxel‐eluting stents in the femoropopliteal artery: 5‐year results of the Zilver PTX randomized trial. Circulation. 2016;133:1472–1483; discussion 1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schneider PA, Laird JR, Tepe G, Brodmann M, Zeller T, Scheinert D, Metzger C, Micari A, Sachar R, Jaff MR, Wang H, Hasenbank MS, Krishnan P; IN.PACT SFA Trial Investigators . Treatment effect of drug‐coated balloons is durable to 3 years in the femoropopliteal arteries: long‐term results of the IN.PACT SFA randomized trial. Circ Cardiovasc Interv. 2018;11:e005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tepe G, Laird J, Schneider P, Brodmann M, Krishnan P, Micari A, Metzger C, Scheinert D, Zeller T, Cohen DJ, Snead DB, Alexander B, Landini M, Jaff MR; IN.PACT SFA Trial Investigators . Drug‐coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12‐month results from the IN.PACT SFA randomized trial. Circulation. 2015;131:495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stone GW, Ellis SG, Colombo A, Dawkins KD, Grube E, Cutlip DE, Friedman M, Baim DS, Koglin J. Offsetting impact of thrombosis and restenosis on the occurrence of death and myocardial infarction after paclitaxel‐eluting and bare metal stent implantation. Circulation. 2007;115:2842–2847. [DOI] [PubMed] [Google Scholar]
- 13. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cassese S, Byrne RA, Ott I, Ndrepepa G, Nerad M, Kastrati A, Fusaro M. Paclitaxel‐coated versus uncoated balloon angioplasty reduces target lesion revascularization in patients with femoropopliteal arterial disease: a meta‐analysis of randomized trials. Circ Cardiovasc Interv. 2012;5:582–589. [DOI] [PubMed] [Google Scholar]
- 16. Begg CB. Publication bias In: Cooper H, Hedges LV, eds. The Handbook of Research Synthesis. New York, NY, US: Russell Sage Foundation; 1994:399–409. [Google Scholar]
- 17. Wetterslev J, Jakobsen JC, Gluud C. Trial Sequential Analysis in systematic reviews with meta‐analysis. BMC Med Res Methodol. 2017;17:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bjorkman P, Auvinen T, Hakovirta H, Romsi P, Turtiainen J, Manninen H, Venermo M; Finn PTXsg , Lappalainen K, Alback A, Korpela A, Makinen K, Saari P, Perala J. Drug‐eluting stent shows similar patency results as prosthetic bypass in patients with femoropopliteal occlusion in a randomized trial. Ann Vasc Surg. 2018;53:165–170. [DOI] [PubMed] [Google Scholar]
- 19. Dake MD, Ansel GM, Jaff MR, Ohki T, Saxon RR, Smouse HB, Snyder SA, O'Leary EE, Tepe G, Scheinert D, Zeller T; Zilver PTXI . Sustained safety and effectiveness of paclitaxel‐eluting stents for femoropopliteal lesions: 2‐year follow‐up from the Zilver PTX randomized and single‐arm clinical studies. J Am Coll Cardiol. 2013;61:2417–2427. [DOI] [PubMed] [Google Scholar]
- 20. Goueffic Y. BATTLE trial: 1 year outcomes in a multicenter randomized trial comparing MISAGO® vs. ZILVER® PTX® for the treatment of intermediate length femoropopliteal lesions. Charing Cross Symposium April 2018; Oral presentation.
- 21. Goueffic Y, Kaladji A, Guyomarch B, Montagne C, Fairier D, Gestin S, Riche VP, Vent PA, Chaillou P, Costargent A, Patra P. Bare metal stent versus paclitaxel eluting stent for intermediate length femoropopliteal arterial lesions (BATTLE trial): study protocol for a randomized controlled trial. Trials. 2014;15:423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miura T, Miyashita Y, Soga Y, Hozawa K, Doijiri T, Ikeda U, Kuwahara K; DEBATE in SFA Investigators . Drug‐eluting versus bare‐metal stent implantation with or without cilostazol in the treatment of the superficial femoral artery. The DEBATE in SFA Study. Circ Cardiovasc Interv. 2018;11:e006564. [DOI] [PubMed] [Google Scholar]
- 23. Dake MD, Ansel GM, Jaff MR, Ohki T, Saxon RR, Smouse HB, Zeller T, Roubin GS, Burket MW, Khatib Y, Snyder SA, Ragheb AO, White JK, Machan LS; Zilver PTX Investigators . Paclitaxel‐eluting stents show superiority to balloon angioplasty and bare metal stents in femoropopliteal disease: twelve‐month Zilver PTX randomized study results. Circ Cardiovasc Interv. 2011;4:495–504. [DOI] [PubMed] [Google Scholar]
- 24. FDA . Available at: https://www.accessdata.fda.gov/cdrh_docs/pdf13/P130024S009B.pdf. Accessed August 30, 2018.
- 25. FDA . Available at: https://www.accessdata.fda.gov/cdrh_docs/pdf14/P140010S015B.pdf. Accessed August 25, 2018.
- 26. Rosenfield K, Jaff MR, White CJ, Rocha‐Singh K, Mena‐Hurtado C, Metzger DC, Brodmann M, Pilger E, Zeller T, Krishnan P, Gammon R, Muller‐Hulsbeck S, Nehler MR, Benenati JF, Scheinert D; LEVANT 2 Investigators . Trial of a paclitaxel‐coated balloon for femoropopliteal artery disease. N Engl J Med. 2015;373:145–153. [DOI] [PubMed] [Google Scholar]
- 27. Scheinert D, Duda S, Zeller T, Krankenberg H, Ricke J, Bosiers M, Tepe G, Naisbitt S, Rosenfield K. The LEVANT I (Lutonix paclitaxel‐coated balloon for the prevention of femoropopliteal restenosis) trial for femoropopliteal revascularization: first‐in‐human randomized trial of low‐dose drug‐coated balloon versus uncoated balloon angioplasty. JACC Cardiovasc Interv. 2014;7:10–19. [DOI] [PubMed] [Google Scholar]
- 28. Tepe G, Zeller T, Albrecht T, Heller S, Schwarzwalder U, Beregi JP, Claussen CD, Oldenburg A, Scheller B, Speck U. Local delivery of paclitaxel to inhibit restenosis during angioplasty of the leg. N Engl J Med. 2008;358:689–699. [DOI] [PubMed] [Google Scholar]
- 29. Werk M, Langner S, Reinkensmeier B, Boettcher HF, Tepe G, Dietz U, Hosten N, Hamm B, Speck U, Ricke J. Inhibition of restenosis in femoropopliteal arteries: paclitaxel‐coated versus uncoated balloon: femoral paclitaxel randomized pilot trial. Circulation. 2008;118:1358–1365. [DOI] [PubMed] [Google Scholar]
- 30. Albrecht T, Waliszewski M, Roca C, Redlich U, Tautenhahn J, Pech M, Halloul Z, Gogebakan O, Meyer DR, Gemeinhardt I, Zeller T, Muller‐Hulsbeck S, Ott I, Tepe G. Two‐year clinical outcomes of the CONSEQUENT trial: can femoropopliteal lesions be treated with sustainable clinical results that are economically sound? Cardiovasc Intervent Radiol. 2018;41:1008–1014. [DOI] [PubMed] [Google Scholar]
- 31. Banyai M. Lutonix SFA long lesion study two year results. LINC symposium January 2018; Oral presentation.
- 32. Brodmann M. ILLUMENATE European randomized trial: 2‐year results. LINC symposium January 2018; Oral presentation.
- 33. Fanelli F. Optimal DCB performance LEVANT 2 and global SFA real‐world registry. LINC symposium January 2017; Oral presentation.
- 34. Iida O, Soga Y, Urasawa K, Saito S, Jaff MR, Wang H, Ookubo H, Yokoi H; MDT‐2113 SFA Japan Investigators . Drug‐coated balloon vs standard percutaneous transluminal angioplasty for the treatment of atherosclerotic lesions in the superficial femoral and proximal popliteal arteries: one‐year results of the MDT‐2113 SFA Japan randomized trial. J Endovasc Ther. 2018;25:109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schroeder H, Werner M, Meyer DR, Reimer P, Kruger K, Jaff MR, Brodmann M; ILLUMENATE EU RCT Investigators . Low‐dose paclitaxel‐coated versus uncoated percutaneous transluminal balloon angioplasty for femoropopliteal peripheral artery disease: one‐year results of the ILLUMENATE European Randomized Clinical Trial (randomized trial of a novel paclitaxel‐coated percutaneous angioplasty balloon). Circulation. 2017;135:2227–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tepe G, Gogebakan O, Redlich U, Tautenhahn J, Ricke J, Halloul Z, Meyer DR, Waliszewski M, Schnorr B, Zeller T, Muller‐Hulsbeck S, Ott I, Albrecht T. Angiographic and clinical outcomes after treatment of femoro‐popliteal lesions with a novel paclitaxel‐matrix‐coated balloon catheter. Cardiovasc Intervent Radiol. 2017;40:1535–1544. [DOI] [PubMed] [Google Scholar]
- 37. Bausback Y, Willfort‐Ehringer A, Sievert H, Geist V, Lichtenberg M, Del Giudice C, Sauguet A, Diaz‐Cartelle J, Marx C, Strobel A, Schult I, Scheinert D; RANGER SFA Investigators . Six‐month results from the initial randomized study of the Ranger paclitaxel‐coated balloon in the femoropopliteal segment. J Endovasc Ther. 2017;24:459–467. [DOI] [PubMed] [Google Scholar]
- 38. Fanelli F, Cannavale A, Boatta E, Corona M, Lucatelli P, Wlderk A, Cirelli C, Salvatori FM. Lower limb multilevel treatment with drug‐eluting balloons: 6‐month results from the DEBELLUM randomized trial. J Endovasc Ther. 2012;19:571–580. [DOI] [PubMed] [Google Scholar]
- 39. Fanelli F, Cannavale A, Corona M, Lucatelli P, Wlderk A, Salvatori FM. The “DEBELLUM”—lower limb multilevel treatment with drug eluting balloon—randomized trial: 1‐year results. J Cardiovasc Surg (Torino). 2014;55:207–216. [PubMed] [Google Scholar]
- 40. Guo W. 2‐year results of the ACOART‐I. LINC symposium January 2018; Oral presentation.
- 41. Iida O. 2 year results from the MDT‐2113 SFA Japan trial—DCB vs.standard PTA for the treatment of atherosclerotic lesions in the SFA/PPA. LINC symposium January 2018; Oral presentation.
- 42. Jia X, Zhang J, Zhuang B, Fu W, Wu D, Wang F, Zhao Y, Guo P, Bi W, Wang S, Guo W. Acotec drug‐coated balloon catheter: randomized, multicenter, controlled clinical study in femoropopliteal arteries: evidence from the AcoArt I trial. JACC Cardiovasc Interv. 2016;9:1941–1949. [DOI] [PubMed] [Google Scholar]
- 43. Krishnan P, Faries P, Niazi K, Jain A, Sachar R, Bachinsky WB, Cardenas J, Werner M, Brodmann M, Mustapha JA, Mena‐Hurtado C, Jaff MR, Holden AH, Lyden SP. Stellarex drug‐coated balloon for treatment of femoropopliteal disease: twelve‐month outcomes from the randomized ILLUMENATE pivotal and pharmacokinetic studies. Circulation. 2017;136:1102–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Scheinert D, Schulte KL, Zeller T, Lammer J, Tepe G. Paclitaxel‐releasing balloon in femoropopliteal lesions using a BTHC excipient: twelve‐month results from the BIOLUX P‐I randomized trial. J Endovasc Ther. 2015;22:14–21. [DOI] [PubMed] [Google Scholar]
- 45. Werk M, Albrecht T, Meyer DR, Ahmed MN, Behne A, Dietz U, Eschenbach G, Hartmann H, Lange C, Schnorr B, Stiepani H, Zoccai GB, Hanninen EL. Paclitaxel‐coated balloons reduce restenosis after femoro‐popliteal angioplasty: evidence from the randomized PACIFIER trial. Circ Cardiovasc Interv. 2012;5:831–840. [DOI] [PubMed] [Google Scholar]
- 46. Bjorkman P, Kokkonen T, Alback A, Venermo M. Drug‐coated vs. plain balloon angioplasty in bypass vein grafts (the DRECOREST I‐study). Ann Vasc Surg. 2018. Available at: https://www.sciencedirect.com/science/article/pii/S089050961830565X. Accessed November 28, 2018. [DOI] [PubMed] [Google Scholar]
- 47. Teichgraber U, Lehmann T, Aschenbach R, Scheinert D, Zeller T, Brechtel K, Blessing E, Lichtenberg M, Thieme M. EffPac‐Trial: effectiveness of LUMINOR® DCB versus POBA in the SFA: 12 months results. Charing Cross Symposium April 2018; Oral presentation.
- 48. PMDA . Available at: http://www.pmda.go.jp/medical_devices/2017/M20170830001/780045000_22900BZX00252000_A100_1.pdf. Accessed May 30, 2018.
- 49. de Boer SW, van den Heuvel DAF, de Vries‐Werson DAB, Vos JA, Fioole B, Vroegindeweij D, Elgersma OE, Tutein Nolthenius RP, Heyligers JMM, Bosma GPT, de Leeuw B, Bouwman LH, Bockler D, Dovzhanskiy DI, Vos FWF, Vink TWF, Hooijboer PGA, Hissink RJ, de Vries JPM. Short‐term results of the RAPID randomized trial of the legflow paclitaxel‐eluting balloon with Supera stenting vs Supera stenting alone for the treatment of intermediate and long superficial femoral artery lesions. J Endovasc Ther. 2017;24:783–792. [DOI] [PubMed] [Google Scholar]
- 50. Liistro F, Grotti S, Porto I, Angioli P, Ricci L, Ducci K, Falsini G, Ventoruzzo G, Turini F, Bellandi G, Bolognese L. Drug‐eluting balloon in peripheral intervention for the superficial femoral artery: the DEBATE‐SFA randomized trial (drug eluting balloon in peripheral intervention for the superficial femoral artery). JACC Cardiovasc Interv. 2013;6:1295–1302. [DOI] [PubMed] [Google Scholar]
- 51. Ott I, Cassese S, Groha P, Steppich B, Hadamitzky M, Ibrahim T, Kufner S, Dewitz K, Hiendlmayer R, Laugwitz KL, Schunkert H, Kastrati A, Fusaro M. Randomized comparison of paclitaxel‐eluting balloon and stenting versus plain balloon plus stenting versus directional atherectomy for femoral artery disease (ISAR‐STATH). Circulation. 2017;135:2218–2226. [DOI] [PubMed] [Google Scholar]
- 52. Tacke J. The randomized Freeway Stent Study completed the 12‐month follow‐up and favored the use of drug‐eluting balloons over plain balloons for the postdilatation of nitinol stents in the SFA and PI segments to lower restenosis rate. CIRSE symposium September 2017; Oral presentation.
- 53. Kinstner CM, Lammer J, Willfort‐Ehringer A, Matzek W, Gschwandtner M, Javor D, Funovics M, Schoder M, Koppensteiner R, Loewe C, Ristl R, Wolf F. Paclitaxel‐eluting balloon versus standard balloon angioplasty in in‐stent restenosis of the superficial femoral and proximal popliteal artery: 1‐year results of the PACUBA trial. JACC Cardiovasc Interv. 2016;9:1386–1392. [DOI] [PubMed] [Google Scholar]
- 54. Krankenberg H, Tubler T, Ingwersen M, Schluter M, Scheinert D, Blessing E, Sixt S, Kieback A, Beschorner U, Zeller T. Drug‐coated balloon versus standard balloon for superficial femoral artery in‐stent restenosis: the randomized femoral artery in‐stent restenosis (FAIR) trial. Circulation. 2015;132:2230–2236. [DOI] [PubMed] [Google Scholar]
- 55. Ott I, Cassese S, Groha P, Steppich B, Voll F, Hadamitzky M, Ibrahim T, Kufner S, Dewitz K, Wittmann T, Kasel AM, Laugwitz KL, Schunkert H, Kastrati A, Fusaro M. ISAR‐PEBIS (Paclitaxel‐Eluting Balloon Versus Conventional Balloon Angioplasty for In‐Stent Restenosis of Superficial Femoral Artery): a randomized trial. J Am Heart Assoc. 2017;6:e006321 DOI: 10.1161/JAHA.117.006321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Laird J. Long term durability of DCB treatment in the SFA: 4‐year results of the IN.PACT SFA study. LINC symposium January 2018; Oral presentation.
- 57. Tepe G, Schnorr B, Albrecht T, Brechtel K, Claussen CD, Scheller B, Speck U, Zeller T. Angioplasty of femoral‐popliteal arteries with drug‐coated balloons: 5‐year follow‐up of the THUNDER trial. JACC Cardiovasc Interv. 2015;8:102–108. [DOI] [PubMed] [Google Scholar]
- 58. Higgins JP, Thompson SG, Spiegelhalter DJ. A re‐evaluation of random‐effects meta‐analysis. J R Stat Soc Ser A Stat Soc. 2009;172:137–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Efthimiou O. Practical guide to the meta‐analysis of rare events. Evid Based Ment Health. 2018;21:72–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random‐effects model meta‐analyses. BMC Med Res Methodol. 2009;9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965;58:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gongora CA, Shibuya M, Wessler JD, McGregor J, Tellez A, Cheng Y, Conditt GB, Kaluza GL, Granada JF. Impact of paclitaxel dose on tissue pharmacokinetics and vascular healing: a comparative drug‐coated balloon study in the familial hypercholesterolemic swine model of superficial femoral in‐stent restenosis. JACC Cardiovasc Interv. 2015;8:1115–1123. [DOI] [PubMed] [Google Scholar]
- 63. Granada JF, Stenoien M, Buszman PP, Tellez A, Langanki D, Kaluza GL, Leon MB, Gray W, Jaff MR, Schwartz RS. Mechanisms of tissue uptake and retention of paclitaxel‐coated balloons: impact on neointimal proliferation and healing. Open Heart. 2014;1:e000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Speck U, Cremers B, Kelsch B, Biedermann M, Clever YP, Schaffner S, Mahnkopf D, Hanisch U, Bohm M, Scheller B. Do pharmacokinetics explain persistent restenosis inhibition by a single dose of paclitaxel? Circ Cardiovasc Interv. 2012;5:392–400. [DOI] [PubMed] [Google Scholar]
- 65. Stone GW, Ellis SG, Colombo A, Grube E, Popma JJ, Uchida T, Bleuit JS, Dawkins KD, Russell ME. Long‐term safety and efficacy of paclitaxel‐eluting stents final 5‐year analysis from the TAXUS clinical trial program. JACC Cardiovasc Interv. 2011;4:530–542. [DOI] [PubMed] [Google Scholar]
- 66. Milojevic M, Head SJ, Parasca CA, Serruys PW, Mohr FW, Morice MC, Mack MJ, Stahle E, Feldman TE, Dawkins KD, Colombo A, Kappetein AP, Holmes DR Jr. Causes of death following PCI versus CABG in complex CAD: 5‐year follow‐up of SYNTAX. J Am Coll Cardiol. 2016;67:42–55. [DOI] [PubMed] [Google Scholar]
- 67. Scheller B, Speck U, Abramjuk C, Bernhardt U, Bohm M, Nickenig G. Paclitaxel balloon coating, a novel method for prevention and therapy of restenosis. Circulation. 2004;110:810–814. [DOI] [PubMed] [Google Scholar]
- 68. Posa A, Nyolczas N, Hemetsberger R, Pavo N, Petnehazy O, Petrasi Z, Sangiorgi G, Gyongyosi M. Optimization of drug‐eluting balloon use for safety and efficacy: evaluation of the 2nd generation paclitaxel‐eluting DIOR‐balloon in porcine coronary arteries. Catheter Cardiovasc Interv. 2010;76:395–403. [DOI] [PubMed] [Google Scholar]
- 69. Axel DI, Kunert W, Goggelmann C, Oberhoff M, Herdeg C, Kuttner A, Wild DH, Brehm BR, Riessen R, Koveker G, Karsch KR. Paclitaxel inhibits arterial smooth muscle cell proliferation and migration in vitro and in vivo using local drug delivery. Circulation. 1997;96:636–645. [DOI] [PubMed] [Google Scholar]
- 70. Dake MD, Van Alstine WG, Zhou Q, Ragheb AO. Polymer‐free paclitaxel‐coated Zilver PTX stents—evaluation of pharmacokinetics and comparative safety in porcine arteries. J Vasc Interv Radiol. 2011;22:603–610. [DOI] [PubMed] [Google Scholar]
- 71. Schorn I, Malinoff H, Anderson S, Lecy C, Wang J, Giorgianni J, Papandreou G. The Lutonix(R) drug‐coated balloon: a novel drug delivery technology for the treatment of vascular disease. Adv Drug Deliv Rev. 2017;112:78–87. [DOI] [PubMed] [Google Scholar]
- 72. Walle T, Walle UK, Kumar GN, Bhalla KN. Taxol metabolism and disposition in cancer patients. Drug Metab Dispos. 1995;23:506–512. [PubMed] [Google Scholar]
- 73. Yazdani SK, Pacheco E, Nakano M, Otsuka F, Naisbitt S, Kolodgie FD, Ladich E, Rousselle S, Virmani R. Vascular, downstream, and pharmacokinetic responses to treatment with a low dose drug‐coated balloon in a swine femoral artery model. Catheter Cardiovasc Interv. 2014;83:132–140. [DOI] [PubMed] [Google Scholar]
- 74. Stolzenburg N, Breinl J, Bienek S, Jaguszewski M, Lochel M, Taupitz M, Speck U, Wagner S, Schnorr J. Paclitaxel‐coated balloons: investigation of drug transfer in healthy and atherosclerotic arteries—first experimental results in rabbits at low inflation pressure. Cardiovasc Drugs Ther. 2016;30:263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Pearl J. Causality: Models, Reasoning and Inference. 2nd ed Cambridge, UK: Cambridge University Press; 2009. [Google Scholar]
- 76. Pearce N, Lawlor DA. Causal inference‐so much more than statistics. Int J Epidemiol. 2016;45:1895–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Thieme M, Von Bilderling P, Paetzel C, Karnabatidis D, Perez Delgado J, Lichtenberg M; Lutonix Global SFA Registry Investigators . The 24‐month results of the Lutonix Global SFA Registry: worldwide experience with Lutonix drug‐coated balloon. JACC Cardiovasc Interv. 2017;10:1682–1690. [DOI] [PubMed] [Google Scholar]
- 78. Micari A, Brodmann M, Keirse K, Peeters P, Tepe G, Frost M, Wang H, Zeller T; IN.PACT Global Study Investigators . Drug‐coated balloon treatment of femoropopliteal lesions for patients with intermittent claudication and ischemic rest pain: 2‐year results from the IN.PACT global study. JACC Cardiovasc Interv. 2018;11:945–953. [DOI] [PubMed] [Google Scholar]
- 79. Rantner B, Kollerits B, Pohlhammer J, Stadler M, Lamina C, Peric S, Klein‐Weigel P, Muhlthaler H, Fraedrich G, Kronenberg F. The fate of patients with intermittent claudication in the 21st century revisited—results from the CAVASIC study. Sci Rep. 2017;8:45833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hageman D, Fokkenrood HJ, Gommans LN, van den Houten MM, Teijink JA. Supervised exercise therapy versus home‐based exercise therapy versus walking advice for intermittent claudication. Cochrane Database Syst Rev. 2018;4:CD005263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Fakhry F, Spronk S, van der Laan L, Wever JJ, Teijink JA, Hoffmann WH, Smits TM, van Brussel JP, Stultiens GN, Derom A, den Hoed PT, Ho GH, van Dijk LC, Verhofstad N, Orsini M, van Petersen A, Woltman K, Hulst I, van Sambeek MR, Rizopoulos D, Rouwet EV, Hunink MG. Endovascular revascularization and supervised exercise for peripheral artery disease and intermittent claudication: a randomized clinical trial. JAMA. 2015;314:1936–1944. [DOI] [PubMed] [Google Scholar]
- 82. Laird JR, Schneider PA, Tepe G, Brodmann M, Zeller T, Metzger C, Krishnan P, Scheinert D, Micari A, Cohen DJ, Wang H, Hasenbank MS, Jaff MR; IN.PACT SFA Trial Investigators . Durability of treatment effect using a drug‐coated balloon for femoropopliteal lesions: 24‐month results of IN.PACT SFA. J Am Coll Cardiol. 2015;66:2329–2338. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Design Characteristics of the Included Randomized Controlled Trials*
Table S2. Design Characteristics of the Tested Paclitaxel DES and DCB Devices
Table S3. Baseline Patient Characteristics of Included Randomized Clinical Trials
Table S4. Sensitivity Analyses of Rare Events (Risk Ratio; 95% CI or CrI)41 (R “meta” Package (Version 4.9‐2—Bayesian With https://gemtc.drugis.org)
Figure S1. Literature search and study selection process following the PRISMA statement.
Figure S2. Evaluation of risk of bias of each RCT according to the Cochrane Collaboration Tool.
Figure S3. Funnel plots of all‐cause death analyses at (A) 1 year, (B) 2 years, and (C) 4 to 5 years of follow‐up.


